Effects of Epibiotic Diatoms on the Productivity of the Calanoid Copepod Acartia tonsa (Dana) in Intensive Aquaculture Systems
- 1Department of Aquaculture, National Taiwan Ocean University, Keelung, Taiwan
- 2Center of Excellence for the Oceans, National Taiwan Ocean University, Keelung, Taiwan
- 3Department of Biology, National Changhua University of Education, Changhua City, Taiwan
- 4Institute of Marine Biology, National Taiwan Ocean University, Keelung, Taiwan
- 5Center of Excellence for Ocean Engineering, National Taiwan Ocean University, Keelung, Taiwan
- 6Université de Lille, CNRS, Université du Littoral Côte d’Opale, UMR 8187 LOG, Laboratoire d’Océanologie et de Géosciences, Station Marine de Wimereux, Lille, France
We evaluated here the effects of the epibiotic diatom Tabularia sp. on the productivity of the calanoid copepod Acartia tonsa (Dana) for assessing their risk on copepod intensive aquaculture industry for the provision of live feed. In the first experiment, uninfested and intensively infested females were cultivated individually for the assessment of egg production. Intensively infested females appeared to have a significantly lower egg production (5.0–9.0 eggs/female/d) than uninfested females (22.0–26.0 eggs/female/d) during 5 consecutive days. In the second experiment, effects of culture densities on diatom epibiosis were investigated in 9 L cultures at three different densities (200, 400, and 600 ind. L–1). Another culture at higher volume (250 L) and lowest density (200 ind. L–1) was also carried out to test the effect of culture volume on diatom epibiosis. The infestation rate (%), infestation intensity (ratio of surface diatom coverage levels, classified as levels 0–3) and daily egg harvest rate (number of harvested eggs per day per liter) were evaluated among the four culture populations. The copepods had higher infestation rate (53.69–60.14%) and intensity rate (high ratios at level 2 and 3) when the densities were increased from 200 ind./L to 400 and 600 ind./L. Although egg harvest increased with increasing culture density, it seemed that the diatom-infested A. tonsa population reach a saturated egg production when the density was higher than 400 ind./L. Nevertheless, the differences of culture volumes (250 and 9 L) appeared to be not to have any effect when the copepods were cultivated at the same density (200 ind./L). This study reveals for the first time that the epibiosis of the diatom Tabularia sp. reduces the individual egg production, and egg harvest rate in high-density culture of the copepod A. tonsa. Our findings implicate that diatom epibiosis should be avoid in copepod intensive culture systems.
Introduction
Copepods represent important trophic linkages in marine food webs (Støttrup, 2000; Hwang et al., 2004; Turner, 2004). They provide nutritional benefits (van der Meeren et al., 2008; Rayner et al., 2015; Pan et al., 2018) and great palatability (Chesney, 2005; Højgaard et al., 2017) for feeding aquatic larvae. Based on the emerging developments of intensive culture techniques, different copepod species are meanwhile cultivated and used as live feed in marine larviculture (Lee et al., 2005; Drillet et al., 2011; Blanda et al., 2015; Hansen, 2017). The indoor intensive culture system could be consistently maintained at optimal culture conditions facilitating higher copepod productivity than extensive outdoor culture systems. Furthermore, they should be managed sustainably and cost-effective (Abate et al., 2016). Laboratory studies on the optimal culture conditions of several copepod species have been accomplished, and resulting evidence suggests that these can be upgraded to mass culture systems. However, a complete removal of microorganisms that provide potential health risks to copepods in intensive culture systems remains challenging (Paerl and Tucker, 1995; Petkeviciute et al., 2015; Rurangwa and Verdegem, 2015).
Copepods are common hosts of epibiotic microorganisms such as bacteria, protists and microalgae (Carman and Dobbs, 1997; Utz and Costs, 2005; Mantha et al., 2013; Burris and Dam, 2014; Romano et al., 2021). The effects of epibiotic ciliates and bacteria were the focus point of several studies (Nagasawa, 1987; Puckett and Carman, 2002; Bickel et al., 2012; Souissi et al., 2013; Burris and Dam, 2014; Jones et al., 2016). Their infestations are considered to be adverse for copepod reproduction. Epibionts are mostly found on the surface of crustacean zooplankton during their growth phase which results in the dispersion of epibionts to a new basibiont (de Souza Santos et al., 2020). The constitution of the substratum dictates the number of species that can establish themselves on the basibiont to the point of bringing about a high level of adaptability in epibiotic communities. Locating a suitable substratum is of utmost importance to an epibiont (Purushothaman et al., 2021).
Recent studies have shown host preference among epibionts (Silver-Gorges et al., 2021). Epibionts were found on planktonic crustaceans in eutrophic water conditions, but the relation between the physical parameters of water and epibiosis have not yet been studied in detail (Nayak et al., 2021). Ecologically, epibiosis is an important phenomenon as it is a direct reflection of the level of pollution since absence or presence of certain epibiont-basibiont associations can be used for bioindication (Purushothaman et al., 2021). Ciliate epibionts are frequently found on crustacean species, such as copepods (Souissi et al., 2013; Burris and Dam, 2014).
However, occurrences of microalga epibiosis on copepods were mostly documented with particular focus on epibiont morphology, phylogeny, and distribution (Carman and Dobbs, 1997; Fernandes and Calixto-Feres, 2012; Li et al., 2014; Gómez et al., 2018; Nayak et al., 2021). The impacts of algal epibiosis on copepod reproduction were rarely investigated as yet. Hakimzadeh and Bradley (1990) and Petkeviciute et al. (2015) noted higher expressions of stress-related proteins and genes in the algal-infested calanoid copepods Eurytemora affinis (Poppe) and Acartia tonsa. A field study showed that the algal infestation seemed not to affect copepod survival and egg production (Møhlenberg and Kaas, 1990). Nevertheless, the impact of epibiotic microalgae on copepod productivity remains unclear and should be further investigated.
The calanoid copepod A. tonsa has been considered as a suitable live prey in several larviculture studies (Wilcox et al., 2006; Øie et al., 2017; Vanacor-Barroso et al., 2017). Intensive mass cultures of A. tonsa has been established in a pre-industrial culture facility affiliated to the University of Lille, France since late 2014. In December 2015, epibiotic diatom infestations occurred in A. tonsa cultures calling for an investigation of its effects on copepod mass culture. Two independent experiments were performed to verify the following questions: (1) the effects of epibiotic diatoms on the egg production of individual copepods, (2) the effects of culture volume and copepod density on the epibiotic diatom infestation rate, infestation intensity and copepod egg harvest rate in the mass cultures. Our study aimed to assess the consequences of diatom epibiosis on the reproduction and mass culture productivity of A. tonsa.
Materials and Methods
Microalgae and Copepod Stock Cultures
Copepod and microalgae cultures were maintained in an indoor and pre-industrial culture facility programmed at 18 ± 1°C and 12L:12D light:dark cycle. The culture line of microalga Rhodomonas baltica (RCC350) as copepod food was obtained from the Roscoff Culture Collection, France. Batch cultures were maintained in 10 L flasks with treated natural seawater (1-μm-filtered, UV-radiated, chlorine-sterilized, at salinity 34) enriched with Walne’s medium (Walne, 1970). The algae were used for feeding the copepods at exponential growth phase (2–3 days after inoculation), and the cultures were re-inoculated every 6–7 days. The copepod A. tonsa culture strain (DFH.AT1) was obtained from Roskilde University, Denmark, and reared in 250 L culture columns containing the treated seawater where the microalgae R. baltica was added as feed (2 × 104 cells mL–1). The water of the batch cultures was completely replaced every 2 weeks.
Light Microscopy, Scanning Electron Microscopy Examinations, and Terminology of Epibiotic Diatoms
Copepods infested by epibiotic diatom were randomly collected from the stock cultures. Alive samples were observed and photographed under an inverted light microscope (IX71, OLYMPUS, Tokyo, Japan). Finally, the copepods were fixed with 4% buffered glutaraldehyde for SEM analysis. For SEM preparation, individual copepods were dehydrated in an ethanol gradient (70% → 85% → 95% → 100% → 100%), and were transferred to aluminum stubs. Then a drop of hexamethyldisilazane (HMDS) was added for critical point drying. The stubs were sputter-coated with Gold-Paladium (E1010, Hitachi Ltd., Tokyo, Japan), and observed using a Hitachi TM3000 SEM (Hitachi Ltd., Tokyo, Japan) at an accelerated voltage of 20 KV.
Colonies of epibiotic diatoms were collected and lyophilized, then treated with KMnO4 and HCl to eliminate organic matter. The acid-washed diatom valves were conserved in Milli-Q filtered water. Fifty microliters of the specimen were placed and air-dried on an aluminum stub, then as for the copepods sputter-coated with the Gold-Paladium. Specimens were observed using a Hitachi S-4800 field emission scanning electron microscope (Hitachi Ltd., Tokyo, Japan). The morphometric measurements of the diatoms were performed using imagery software Image J (v 1.41, National Institutes of Health, United States).
Experimental Design
Individual Experiment
To evaluate the impact of diatom epibiosis on the egg production of A. tonsa at individual scale, 6 uninfested and 6 intensively infested (> 70% epibiotic diatom coverage) adult females were sorted from the 250 L culture columns and cultured individually in 6-well culture plates containing treated natural seawater (10 mL/well) and R. baltica were added as feed. The environmental conditions were maintained as aforementioned (section “Microalgae and Copepod Stock Cultures”), and the culture medium was replaced every day. The daily egg production was documented during 5 consecutive days using a stereomicroscope (SZX9, OLYMPUS, Tokyo, Japan).
Population Experiment
The copepods collected from the same batch culture were divided into 4 separate culture populations to immediately initiate the experiment after a volumetric density estimation. The volumes and densities of the 4 populations (one culture each) were designed as follows: (A) 200 individuals L–1, 250 L; (B) 200 individuals L–1, 9 L culture; (C) 400 individuals L–1, 9 L culture; (D) 600 individuals L–1, 9 L culture. Population B was designated as the positive control, which examined the effects of culture volume and copepod density when the results were compared to population A, and populations C, D, respectively.
The culture conditions of the 4 populations were maintained as aforementioned (section “Microalgae and Copepod Stock Cultures”). Daily egg production was documented in all populations during 5 consecutive days as analytical replicates. After 15 min of no aeration, the eggs were siphoned from the bottom and isolated by sieving through 120 μm (to retain the copepods) and 70 μm meshes (to collect eggs). The copepods and the water were returned to the cultures, and the number of eggs was volumetrically estimated under the stereomicroscope. At the 5th day (endpoint), all the copepods were collected and volumetrically counted to estimate the final density. Around 200 each adult male and female were randomly sorted from the population, then fixed in 4% formaldehyde for further analysis. The copepod specimens were examined under inverted microscope (IX71, Olympus, Japan), and the infestation rate (%) was calculated as: number of infested copepods/total number of collected copepods. Subsequently, all the infested copepods were visually classified under the microscope into four levels of diatom exoskeleton coverage (Møhlenberg and Kaas, 1990). Meaning of the different coverages levels as level 0: 0% coverage; level 1: < 10% coverage; level 2: 10–50% coverage; level 3: > 50% coverage (Figure 1). Infestation intensity (ratio% of different coverage levels) was calculated as: number of copepods at various infestation levels/total number of infested copepods.
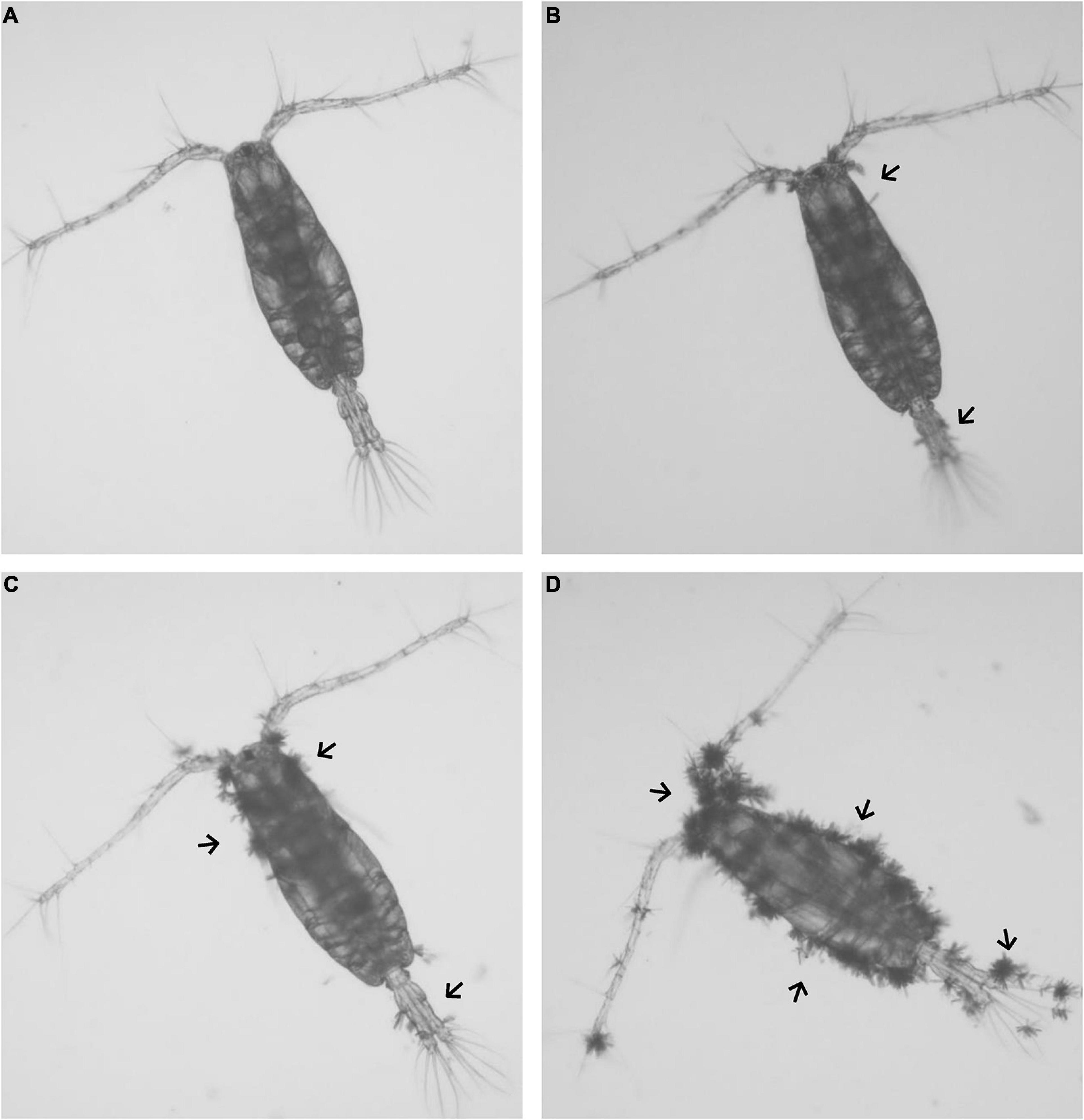
Figure 1. Illustration of different epibiont infestation levels on copepod A. tonsa. (A) Level 0: 0% coverage; (B) level 1: < 10% coverage; (C) level 2: 10–50% coverage; (D) level 3: > 50% coverage.
Data Analysis
Statistical analysis was carried out using the SPSS program (Version 17.0). In the individual experiment, Student’s t-test was used to compare the mean values of the egg production number between uninfested and intensively infested females (n = 6 each) on a daily basis. In the population experiment, the daily egg harvest number (daily egg production per liter) was estimated during 5 consecutive days in each treatment. We first confirmed the absence of interaction between treatment (population) × time (replicate) by using a repeated measure ANOVA. Then, a one-way ANOVA test was applied to the average data of daily egg production per liter obtained over 5 days in each population. Once the significant differences were detected among populations (p < 0.05), Tukey’s multiple comparison test was used to analyze specific differences between pairs of populations.
Results
Light Microscopy and Scanning Electron Microscopy Examinations of Epibiotic Diatoms
LM pictures of infested copepods and the epibiotic diatoms are shown in Figure 2. The mono-species diatom colonies adhered to the A. tonsa exoskeleton without apparent preference for micro-locations on the exoskeleton (Figures 2A,B). The diatom could have 2–3 segmented chloroplast plates (Figures 2C,D) or a large chloroplast plate (Figure 2F) in the silicate valve. In accordance to LM examinations, the SEM pictures indicated a mono-specific diatom infestation on the exoskeleton of A. tonsa (Figure 3A). Detailed SEM pictures (Figure 3B) indicate that the diatoms attached to the copepods used a mucilaginous pad. The linear-lanceolate valves (Figures 3C,D) of the diatom measured (n = 15) 32.4 ± 6.2 μm in apical axis and 3.4 ± 0.3 μm in transapical axis, and the striae (10.3 ± 1.1 per 10 μm) were distributed symmetrically bilateral on the non-raphe valve with broad axial area (Figures 3E,G). Apexes were rounded but not capitate, and carried one rimoportula at each polar nodule (Figures 3F,H). A literature review was facilitated for diatom species identification based on their morphological characteristics by Snoeijs (1992); Kaczmarska et al. (2009), Totti et al. (2009); Suzuki et al. (2015), Cao et al. (2018), and Gómez et al. (2020), and the description of the genus Tabularia reported by Williams and Round (1986). The diatom was identified as Tabularia sp.

Figure 2. The copepod A. tonsa infested with the epibiotic diatom Tabularia sp. (A) adult male (B) adult female (C) infestation on an antennule (D) infestation on caudal setae (E) infestation on a spermatophore attached on female (F) diatom colony isolated from copepods. Scale bars: (A,B): 150 μm; (C–F): 20 μm.
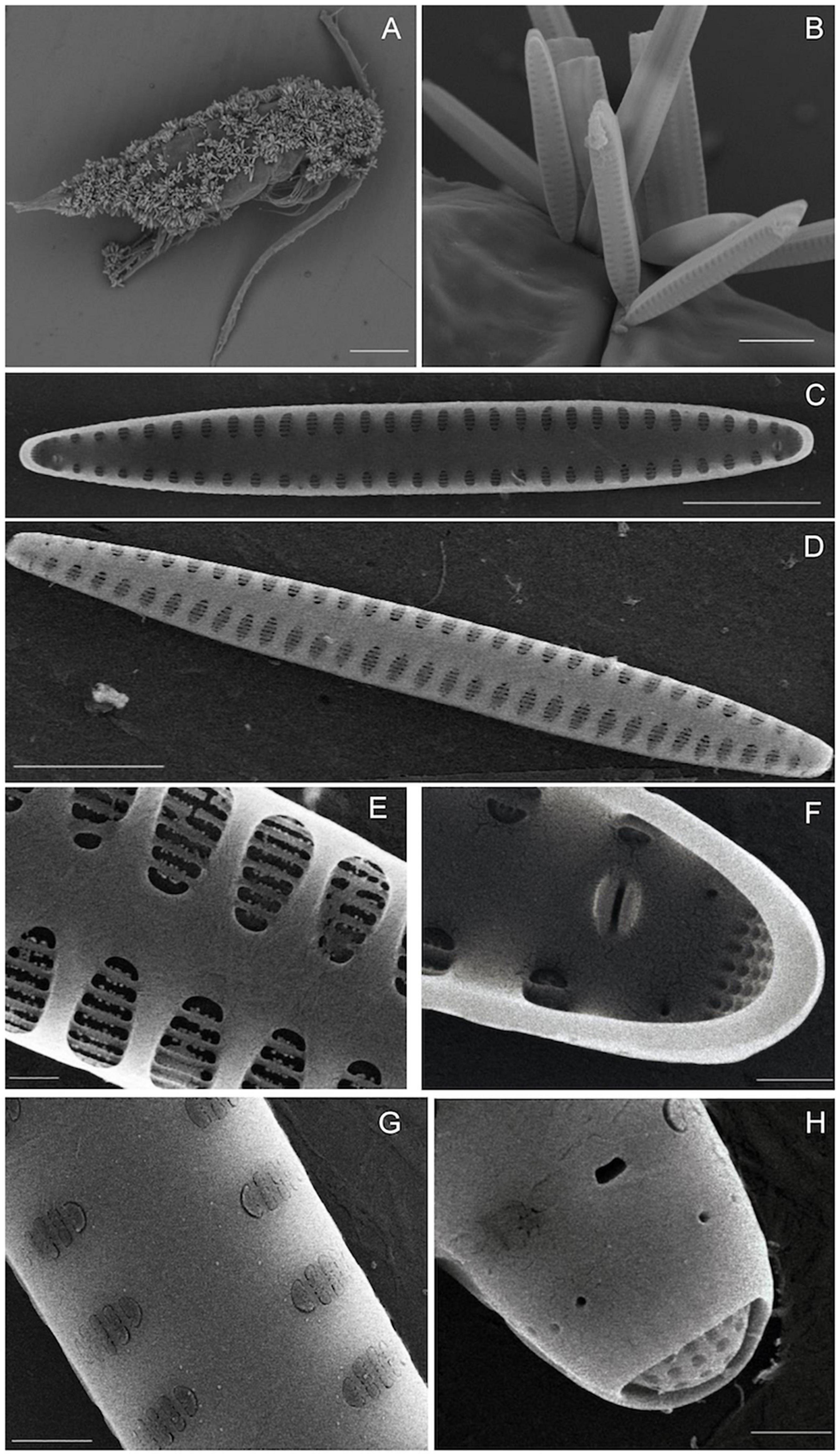
Figure 3. SEM image of the infested copepod A. tonsa and the epibiotic diatom Tabularia sp. (A) Infested A. tonsa female. (B) Colony of epibiotic diatom Tabularia sp. attached on copepod exoskeleton. (C) Interior view of entire valve. (D) External view of entire valve. (E) Interior view of striae structure. (F) Interior apex of valve showing details of rimoportula and ocellulimbus. (G) External view of striae structure. (H) External apex of valve showing details of rimoportula aperture and ocellulimbus opening. Scale bars: (A) 250 μm; (B) 15 μm; (C,D): 5 μm; (E–H) 1 μm.
Individual Culture Experiment
The individual daily egg production declined significantly (p < 0.01) when the copepods were intensively infested with epibiotic diatoms (Figure 4) during 5 consecutive days. The average egg production of uninfested females ranged from 22.0–26.0 eggs/female/day, whereas this was reduced to a range of 5.0–9.0 eggs/female/day in intensively infested individuals.
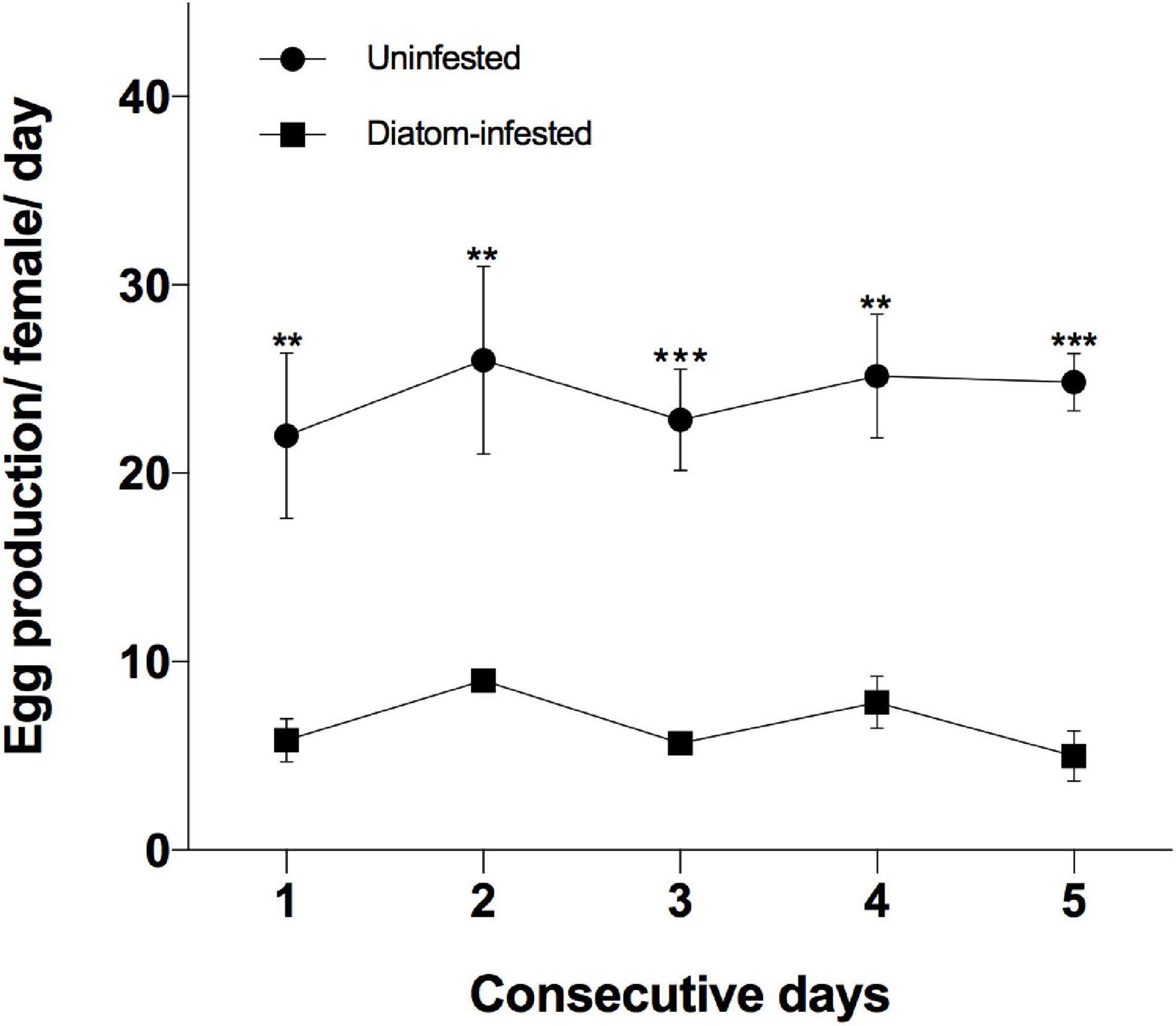
Figure 4. Egg production per female per day of the uninfested and diatom-infested copepod A. tonsa during 5 consecutive days. Data are presented as average ± standard error (n = 6), where **p < 0.01, ***p < 0.001.
Population Culture Experiment
Figure 5 shows that the daily egg production rate (eggs/L/day) averaged from the data obtained during five consecutive days. A significantly lowest egg production (496.4 ± 51.4) was found in the population A (250 L, 200 ind./L). The populations C (9 L, 400 ind./L) and D (9 L, 600 ind./L) had the top two highest egg production (3078.52 ± 524.86 and 3227.56 ± 596.81 eggs/day/L, respectively) among populations, yet the two populations were not statistical different to group B (9 L, 200 ind./L). At the same population density (200 ind./L), populations A (250 L) and B (9 L) had similar levels of infestation ratio and intensity, different in males and females (Tables 1, 2). On the other hand, both male and female infestation rate were higher when the population density increased from 200 (population B) to 400 (population C) and 600 (population D). Notably, female copepods had higher epibiotic rates (40.9–73.2%) and intensity (higher ratios in level 2 and 3) compared to males. All populations remained at similar density after the 5-day cultivation, except a remarkable decline was found in population D (Table 1).
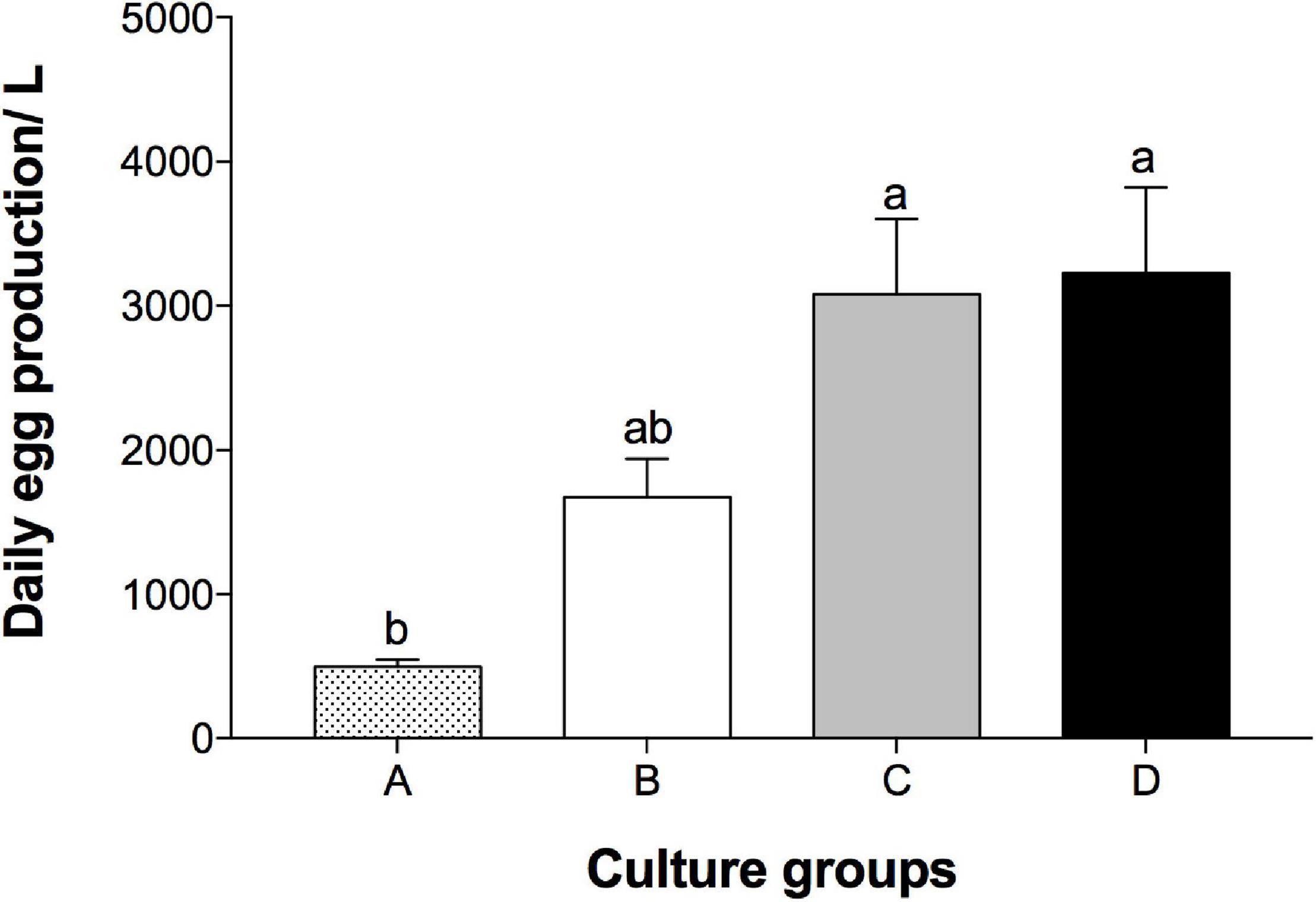
Figure 5. Daily A. tonsa egg production per liter in the culture populations at different volumes and population densities. A: 200 ind./L in 250 L, B: 200 ind./L in 9 L, C: 400 ind./L in 9 L, D: 600 ind./L in 9 L. The data were obtained during 5 consecutive days, and presented as average ± standard error. The letters (a and b) above bars indicate significant differences (p < 0.05) identified by Tukey’s post hoc test.
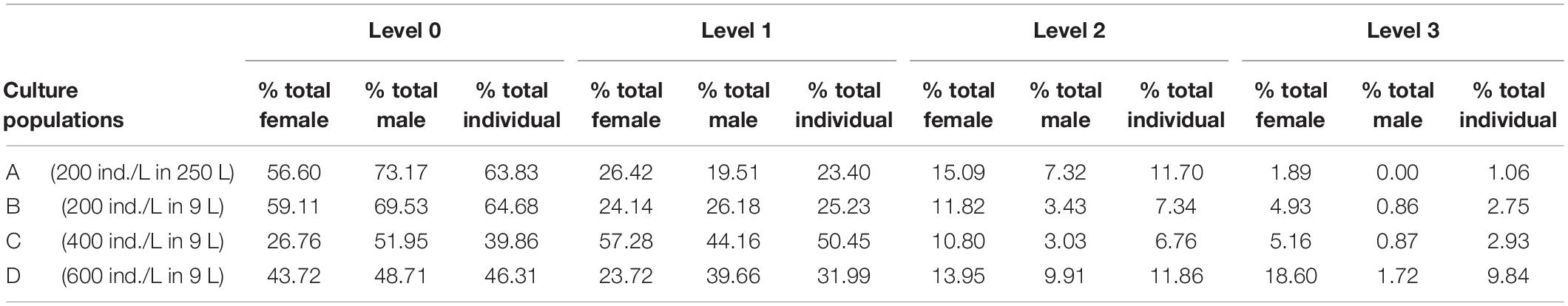
Table 2. Infestation intensity: ratio (%) of four levels of epibiont surface coverage of A. tonsa in different culture populations.
Discussion
Pennate diatoms belonging to the genus Tabularia are common components in marine benthic communities (Snoeijs, 1992). Their epibiotic associations are documented in benthic organisms such as bryozoans (Wuchter et al., 2003) and marine macroalgae (Totti et al., 2009). The occurrence of Tabularia epibiosis on copepods has been reported recently in the English Channel (Gómez et al., 2020), where it is close to the copepod culture unit in the present study. Although equipped with a well-established filtration system (sand filter, UV, and bio-filtration), the origin of the diatom contamination was likely from the inlet of natural sea water to the copepod culture environment. The epibiotic association of Tabularia with the planktonic copepod A. tonsa in aquaculture environment is reported here for the first time. Microalgal epibiosis is considered as an ecological strategy to access higher light exposure and nutritional replenishment provided by their mobile host swimming in the water column (Totti et al., 2010). In addition, the epibiotic microalgae may have higher chances to absorb the excretion released from their host as nutrients (Wahl et al., 2012). Based on the aforementioned statements, the survival and mobility of the hosts are crucially benefitting epibiotic diatoms. Thus, the infestation of epibiotic diatoms seem not to be lethal in an acute sense. Based on the analysis by light microscopy and SEM (Figures 2, 3), the reported epibiosis was a mono-specific event. This observation suggests that the diatom Tabularia sp. could outcompete other epibionts during biofouling at certain circumstances. The main object of the present work was to investigate the productivity of copepods in the events of epibiont infestation for aquaculture propose. Despite the fact that the diatom was identified as Tabularia sp. based on the morphological features examined under light and scanning electron microscopy, and a deep literature review, it should be noted that diatom molecular taxonomy or phylogeny is not the main focus in our work.
Ikeda (1977) noted a similar metabolic ratio of the diatom-infested and uninfested copepod Calanus plumchrus. However, the negative impacts of epibionts on the swimming behavior of their zooplankton hosts were confirmed (McAllen and Scott, 2000; Souissi et al., 2013; Burris and Dam, 2014). The authors suggested that the zooplankton hosts need to expend additional energy to cope with the extra burden and water drag caused by the epibiotic assemblages. Especially, the burden effect could be pronounced in the case of diatom epibiosis, where heavy silicate valves can provide a remarkable weight burden to their host (Purushothaman et al., 2021). Based on the results obtained in individual experiments, all diatom-infested A. tonsa females survived during the 5 experimental days yet produced a significantly lower quantity of eggs. This coincided with reduced egg production found in a previous study of A. tonsa females infested with ciliate epibionts (Burris and Dam, 2014). It is worthy to note that the age of copepods examined in the individual experiment of the present study, and Burris and Dam’s work were not controlled. Although we attempted to investigate the relationship between host age and diatom epibiosis by carrying out an extended experiment, it was challenging to artificially induce the diatom adherence on the copepods. Indeed, the egg production decline with increasing age of copepod (Pan et al., 2014; Rodríguez-Graña and Calliari, 2020). If the diatom epibiont increased accumulatively on the copepod across age, it is highly possible that the age could be a co-factor with diatom epibiosis reducing copepod egg production. The combined effect of age and epibiosis on the decline of egg production, if it could occur in our study, should be amplified with increasing copepod age, because the hosts become older and the epibionts accumulate more. Although the constant egg production rates were found in our 5-day individual experiment, it should be noted that the effects of age and epibiosis on the change of A. tonsa egg production may occur gradually in a time-scale of weeks. Therefore, an extended monitoring is required to verify the combined effect of age and epibiosis on the egg production of the copepod.
To better understand the risk of diatom epibiosis for A. tonsa aquaculture, we assessed diatom epibiosis and copepod egg productivity in culture populations with three densities and two volumes. The infestation rate and intensity were not different in the 9 and 250 L populations when maintained at lowest density (200 ind./L). This finding suggests that the impact of culture volume is minor with respect to diatom epibiosis. On the other hand, the diatom infestation rate and intensity of adult A. tonsa were higher in the populations with higher copepod densities (400 and 600 ind./L). Likewise, the density-dependent epibiont prevalence was compared with some Cladocera and copepod species in lake and pond waters (Barea-Arco et al., 2001; de Souza Santos et al., 2020). In the aquaculture environment, as a closed water system similar to lakes or ponds, the higher density of A. tonsa could facilitate higher encounter rates and the possibility of epibiont transmission between copepods (Burris and Dam, 2014).
The density of copepods in culture is a crucial parameter affecting copepod productivity (Jepsen et al., 2007; Mahjoub et al., 2014; Rayner et al., 2017). Jepsen et al. (2007) demonstrated that egg production (eggs L–1 d–1) increased with increasing copepod density from 100 to 600 ind. L–1 using the same A. tonsa strain (DFH-ATI). Due to the different methods of egg collection, the egg harvest per liter in the present study was lower than the result of Jepsen et al. (2007). Nevertheless, the designated densities (200–600 ind. L–1) in our study were below the limiting density threshold for A. tonsa egg production. Instead of increasing with higher copepod density, similar egg production levels (3000 eggs L–1 d–1) were measured in the culture populations C (400 ind. L–1) and D (600 ind. L–1). This finding implicates that the diatom-infested A. tonsa population, if under the same culture conditions and population origin, may reach the saturating egg production capacity at lower population density. The notable decrease of population was measured in the population D (Table 1), which had the highest ratio of infested copepods at the greatest diatom coverage (level 3). The higher intensity of infestation could be correlated to the higher encounter rate under the crowded conditions. As aforementioned, the diatom infestation is physiologically stressful to copepods, and it not only reduces individual egg production but also increases copepod mortality. Since the heavily infested copepods may die earlier, it could be expected that the infestation rate was slightly lower in population D than in population C. Overall, our findings clearly indicate the remarkable reduction of egg harvest rate in A. tonsa mass culture due to high diatom infestation intensity and copepod mortality.
Regardless of culture densities and volumes, females of A. tonsa had higher infestation rate than males in all culture populations (Table 1). The higher ratio of surface coverage level 2 and 3 was found in infested females (Table 2), which indicates that the female A. tonsa (body length: 1068.36 ± 56.20 μm) can carry more diatoms on their body surface than the male (body length: 931.98 ± 18.22 μm) does. This discovery coincided with the “habitat patch size effects” hypothesis stating that large-sized host provides larger targets for epibiont adherence (de Souza Santos et al., 2020). Host behavior could also strikingly impact the host-epibiont relationship. Ramos-Rivera et al. (2021) stated that the injured host had slower swimming speed and stayed more frequently at the water surface, which facilitated a greater opportunity for epibiont adherence. It’s been noted that A. tonsa females have significantly lower swimming speeds and tended to aggregate around the tank bottom (Buskey et al., 2002; Kiørboe and Bagøien, 2005), where the benthic diatom Tabularia sp. abundantly colonized. This behavior pattern may lead to a higher encounter frequency and period to the benthic Tabularia in the culture environment, and eventually cause a higher epibiont infestation rate and intensity on female A. tonsa. On the other hand, female A. tonsa is known to live longer than male (Rodríguez-Graña et al., 2010). The greater longevity could be another explanation of female’s higher epibiotic infestation if the epibiont really increase accumulatively with copepod age.
Diatom biofouling has been extensively studied in the context of anti-fouling coating or substance applications (Molino et al., 2009; Al-Naamani et al., 2017; Wanka et al., 2018). The mechanism of diatom fouling on zooplankton was rarely studied. This may be due to the difficulty of investigating diatom life cycles and their complex interactions with their host and environmental factors. Consequently, we attempted to expose uninfested A. tonsa individuals at the same-age (i.e., hatched and grow out from the same batch of eggs) to the prior isolated Tabularia cells. However, no infestation was documented during a 14-day period. Based on our observation, the diatom cells changed their cell morphology when it was cultivated independently. This preliminary finding suggests on physiological modifications of Tabularia sp. between the free-living and epibiotic phases of its life cycle. Furthermore, Mantha et al. (2013) stated that the deterioration of water quality affects the exoskeleton of copepods. In their study this was an outcome of high epibiotic and ectoparasitic infestation. Although the analysis of water quality was excluded in the present work, higher accumulation of copepod excretions could be expected in the high-density culture populations. Under such conditions epibiosis could be triggered either by signals related to the weakened exoskeleton of copepods or simply by the chemistry of the ambient waters. Further studies are required to verify possible abiotic or biotic stimuli that trigger the settlement of the diatom, which provide implications for aquaculturists to monitor and prevent the prevalence of epibiotic diatoms on copepod mass production. Moreover, the removal protocol of epibiotic diatom using algicidal substances and bacteria (Kitaguchi et al., 2001) could be developed for epibiotic diatom control.
Conclusion
In conclusion, our study demonstrated the adverse impact of the diatom epibiont on the productivity of the copepod A. tonsa. The diatom-infested copepods decreased their egg production for about 70% at the individual basis, and they reached the saturating egg production capacity and higher mortality at lower population density. These findings implicate the risk of diatom epibiosis causing economic losses for the copepod aquaculture industry. This also means that the presence of epibionts on copepods should be regularly monitored in copepod intensive culture systems to avoid negative developments such as increased mortality and any decrease of growth and egg production.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
SS and J-SH conceived the original idea of this study. Y-JP designed and conducted the experiments and SEM analysis. W-LW performed the diatom morphology examination and species identification. Y-JP, SS, W-LW, and J-SH wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was contribution to the project CPER 2014-2020 MARCO (funded by the Europe FEDER, French Government the region Hauts-de-France and IFREMER), the International Associated Laboratory between Université de Lille and the National Taiwan Ocean University (IAL MULTIFAQUA), the Ministry of Science and Technology of Taiwan (Young Scholar Fellowship Einstein Program: 109-2636-M-019-001- to Y-JP) and the Ministry of Education of Taiwan (Higher Education Sprout Project to the CEO, NTOU). Financial supports from the Ministry of Science and Technology of Taiwan (Grant Nos. MOST 107-2621-M-019-001, MOST 108-2621-M-019-003, MOST 109-2621-M-019-002, 110-2621-M-019-001, and 110J13801-51) and the Center of Excellence for Ocean Engineering (Grant No. 109J13801-51) to J-SH was also acknowledged. This work benefitted from the French GDR “Aquatic Ecotoxicology” framework. We are very thankful to the Communauté d’Agglomération du Boulonnais (CAB) for supporting the implementation of a copepod-rearing pilot project (agreement “HALIOCAP” Université de Lille-CAB).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all past and present members of SS’s group who assisted in keeping micro-alga and copepod cultures for several years.
References
Abate, T. G., Nielsen, R., Nielsen, M., Jepsen, P. M., and Hansen, B. W. (2016). A cost-effectiveness analysis of live feeds in juvenile turbot Scophthalmus maximus (Linnaeus, 1758) farming: copepods versus Artemia. Aquac. Nutr. 22, 899–910. doi: 10.1111/anu.12307
Al-Naamani, L., Dobretsov, S., Dutta, J., and Burgess, J. G. (2017). Chitosan-zinc oxide nanocomposite coatings for the prevention of marine biofouling. Chemosphere 168, 408–417. doi: 10.1016/j.chemosphere.2016.10.033
Barea-Arco, J., Pérez-Martínez, C., and Morales-Baquero, R. (2001). Evidence of a mutualistic relationship between an algal epibiont and its host, Daphnia pulicaria. Limnol. Oceanog. 46, 871–881. doi: 10.4319/lo.2001.46.4.0871
Bickel, S., Tang, K., and Grossart, H.-P. (2012). Ciliate epibionts associated with crustacean zooplankton in german Lakes: distribution, motility, and bacterivory. Front. Microbiol. 3:243. doi: 10.3389/fmicb.2012.00243
Blanda, E., Drillet, G., Huang, C. C., Hwang, J. S., Jakobsen, H. H., Rayner, T. A., et al. (2015). Trophic interactions and productivity of copepods as live feed from tropical Taiwanese outdoor aquaculture ponds. Aquaculture 445, 11–21. doi: 10.1016/j.aquaculture.2015.04.003
Burris, Z. P., and Dam, H. G. (2014). Deleterious effects of the ciliate epibiont Zoothamnium sp. on fitness of the copepod Acartia tonsa. J. Plankton Res. 36, 788–799. doi: 10.1093/plankt/fbt137
Buskey, E. J., Lenz, P. H., and Hartline, D. K. (2002). Escape behavior of planktonic copepods in response to hydrodynamic disturbances: high speed video analysis. Mar. Ecol. Prog. Ser. 235, 135–146. doi: 10.3354/meps235135
Cao, Y., Yu, P., You, Q., Lowe, R. L., Williams, D. M., Wang, Q., et al. (2018). A new species of Tabularia (Kützing) Williams & Round from Poyang Lake, Jiangxi Province, China, with a cladistic analysis of the genus and their relatives. Phytotaxa 373, 169–183.
Carman, K. R., and Dobbs, F. C. (1997). Epibiotic microorganisms on copepods and other marine crustaceans. Microsc. Res. Tech. 37, 116–135. doi: 10.1002/(sici)1097-0029(19970415)37:2<116::aid-jemt2>3.0.co;2-m
Chesney, E. J. (2005). “Copepods as live prey: a review of factors that influence the feeding success of marine fish larvae,” in Copepods in Aquaculture, eds C.-S. Lee, P. J. O’Bryen, and N. H. Marcus (Oxford: Blackwell Publishing), 133–150. doi: 10.1002/9780470277522.ch11
de Souza Santos, G., Ibraim, V. R. C., Silva, E. E. C., and Eskinazi-Sant’Anna, E. M. (2020). Interaction between Epistylis sp. and copepods in tropical lakes: responses of epibiont infestation to species host density. Limnologica 84:125815. doi: 10.1016/j.limno.2020.125815
Drillet, G., Frouël, S., Sichlau, M. H., Jepsen, P. M., Højgaard, J. K., Joarder, A. K., et al. (2011). Status and recommendations on marine copepod cultivation for use as live feed. Aquaculture 315, 155–166. doi: 10.1016/j.aquaculture.2011.02.027
Fernandes, L. F., and Calixto-Feres, M. (2012). Morphology and distribution of two epizoic diatoms (Bacillariophyta) in Brazil. Acta Bot. Bras. 26, 836–841. doi: 10.1590/S0102-33062012000400012
Gómez, F., Courcot, L., and Artigas, L. F. J. C. (2020). Observations of the diatoms Sceptronema orientale Takano and Tabularia parva (Kützing) D.M. Williams & Round on the exoskeleton of copepods in the English Channel and coastal Celtic Seas. Cryptogam. Algol. 41, 25–30. doi: 10.5252/cryptogamie-algologie2020v41a4
Gómez, F., Wang, L., and Lin, S. (2018). Morphology and molecular phylogeny of epizoic araphid diatoms on marine zooplankton, including Pseudofalcula hyalina gen. & comb. nov. (Fragilariophyceae, Bacillariophyta). J. Physiol. 54, 557–570. doi: 10.1111/jpy.12760
Hakimzadeh, R., and Bradley, B. P. (1990). The heat shock response in the copepod Eurytemora affinis (POPPE). J. Therm. Biol. 15, 67–77. doi: 10.1016/0306-4565(90)90050-R
Hansen, B. W. (2017). Advances using copepods in aquaculture. J. Plankton Res. 39, 972–974. doi: 10.1093/plankt/fbx057
Højgaard, J. K., Hansen, B. W., and Hwang, J. S. (2017). Prey capture capabilities by juveniles of the false percula clownfish (Amphiprion ocellaris) fed calanoid nauplii vs. adults. Mar. Freshw. Behav. Physiol. 50, 387–396. doi: 10.1080/10236244.2017.1421047
Hwang, J. S., Ho, J. S., and Shih, C. T. (2004). Contemporary Studies of Copepoda, Proceedings of the 8th International Conference on Copepoda. Zool. Stud. 43, 165–512.
Ikeda, T. (1977). The effect of laboratory conditions on the extrapolation of experimental measurements to the ecology of marine zooplankton. IV. Changes in respiration and excretion rates of boreal zooplankton species maintained under fed and starved conditions. Mar. Biol. 41, 241–252. doi: 10.1007/BF00394910
Jepsen, P. M., Andersen, N., Holm, T., Jørgensen, A. T., Højgaard, J. K., and Hansen, B. W. (2007). Effects of adult stocking density on egg production and viability in cultures of the calanoid copepod Acartia tonsa (Dana). Aquac. Res. 38, 764–772. doi: 10.1111/j.1365-2109.2007.01730.x
Jones, S., Carrasco, N. K., Perissinotto, R., and Vosloo, A. (2016). Association of the epibiont Epistylis sp. with a calanoid copepod in the St Lucia Estuary, South Africa. J. Plankton Res. 38, 1404–1411. doi: 10.1093/plankt/fbw069
Kaczmarska, I., Ehrman, J. M., Moniz, M. B. J., and Davidovich, N. (2009). Phenotypic and genetic structure of interbreeding populations of the diatom Tabularia fasciculata (Bacillariophyta). Phycologia 48, 391–403. doi: 10.2216/08-74.1
Kiørboe, T., and Bagøien, E. (2005). Motility patterns and mate encounter rates in planktonic copepods. Limnol. Oceanogr. 50, 1999–2007. doi: 10.4319/lo.2005.50.6.1999
Kitaguchi, H., Kambara, S., Mitsutani, A., and Ishida, Y. (2001). Screening of algicidal bacteria against epiphytic diatom Tabularia affinis. Rep. Res. Inst. Mar. Biores. 12, 11–17.
Lee, C.-S., O’Bryen, P. J., and Marcus, N. H. (2005). Copepods in Aquaculture. Oxford: Blackwell Publishing.
Li, X.-S., Chen, C.-P., Liang, J.-R., Wu, W.-Z., and Gao, Y.-H. (2014). Morphology and occurrence of a marine epizoic diatom Falcula hyalina Takano (Bacillariophyta) in China. Algol. Stud. 145, 169–179. doi: 10.1127/1864-1318/2014/0158
Mahjoub, M. S., Wu, C. H., Leeper, A., Hwang, J. S., and Drillet, G. (2014). Population density and mate selection in the copepod Acartia tonsa. J. Plankton Res. 36, 872–876. doi: 10.1093/plankt/fbu017
Mantha, G., Awasthi, A. K., Al-Aidaroos, A. M., and Hwang, J. S. (2013). Diversity and abnormalities of cyclopoid copepods around hydrothermal vent fluids, Kueishantao Island, north-eastern Taiwan. J. Nat. Hist. 47, 685–697. doi: 10.1080/00222933.2012.747638
McAllen, R., and Scott, G. (2000). Behavioural effects of biofouling in a marine copepod. J. Mar. Biolog. Assoc. U.K. 80, 369–370. doi: 10.1017/s0025315499002003
Møhlenberg, F., and Kaas, H. (1990). Colacium vesiculosum Ehrenberg (Euglenophyceae), infestation of planktonic copepods in the Western Baltic. Ophelia 31, 125–132. doi: 10.1080/00785326.1990.10430856
Molino, P. J., Campbell, E., and Wetherbee, R. (2009). Development of the initial diatom microfouling layer on antifouling and fouling-release surfaces in temperate and tropical Australia. Biofouling 25, 685–694. doi: 10.1080/08927010903089912
Nagasawa, S. (1987). Exoskeletal scars caused by bacterial attachment to copepods. J. Plankton Res. 9, 749–753. doi: 10.1093/plankt/9.4.749
Nayak, A. R., Malkiel, E., McFarland, M. N., Twardowski, M. S., and Sullivan, J. M. (2021). A review of holography in the aquatic sciences: in situ characterization of particles, plankton, and small scale biophysical interactions. Front. Mar. Sci. 7:572147. doi: 10.3389/fmars.2020.572147
Øie, G., Galloway, T., Sørøy, M., Holmvaag Hansen, M., Norheim, I., Halseth, C., et al. (2017). Effect of cultivated copepods (Acartia tonsa) in first-feeding of Atlantic cod (Gadus morhua) and ballan wrasse (Labrus bergylta) larvae. Aquac. Nutr. 23, 3–17. doi: 10.1111/anu.12352
Paerl, H. W., and Tucker, C. S. (1995). Ecology of blue-green algae in aquaculture ponds. J. World Aquac. Soc. 26, 109–131. doi: 10.1111/j.1749-7345.1995.tb00235.x
Pan, Y. J., Sadovskaya, I., Hwang, J. S., and Souissi, S. (2018). Assessment of the fecundity, population growth and fatty acid composition of Apocyclops royi (Cyclopoida, Copepoda) fed on different microalgal diets. Aquac. Nutr. 24, 970–978. doi: 10.1111/anu.12633
Pan, Y. J., Souissi, S., Souissi, A., Wu, C. H., Cheng, S. H., and Hwang, J. S. (2014). Dietary effects on egg production, egg-hatching rate and female life span of the tropical calanoid copepod Acartia bilobata. Aquac. Res. 45, 1659–1671. doi: 10.1111/are.12113
Petkeviciute, E., Kania, P. W., and Skovgaard, A. (2015). Genetic responses of the marine copepod Acartia tonsa (Dana) to heat shock and epibiont infestation. Aquac. Rep. 2, 10–16. doi: 10.1016/j.aqrep.2015.04.001
Puckett, G. L., and Carman, K. R. (2002). Ciliate epibiont effects on feeding, energy reserves, and sensitivity to hydrocarbon contaminants in an estuarine harpacticoid copepod. Estuaries 25, 372–381. doi: 10.1007/BF02695980
Purushothaman, A., Romagnoli, T., Francis, S. V., Thomas, L. C., and Padmakumar, K. B. (2021). First report of marine epizoic diatom, Protoraphis atlantica (Protoraphidaceae) on calanoid copepods along the southeastern Arabian Sea. Symbiosis 84, 131–140. doi: 10.1007/s13199-021-00772-776
Ramos-Rivera, B. S., Castro-Mondragon, H., Kuk-Dzul, J. G., Flores-Rodríguez, P., and Flores-Garza, R. (2021). Diversity of epibionts associated with Lepidochelys olivacea (Eschscholtz 1829) Sea Turtles Nesting in the Mexican South Pacific. Animals 11:1734. doi: 10.3390/ani11061734
Rayner, T. A., Højgaard, J. K., Hansen, B. W., and Hwang, J. S. (2017). Density effect on the ovigerous rate of the calanoid copepod Pseudodiaptomus annandalei (Sewell 1919): implications for aquaculture. Aquac. Res. 48, 4573–4577. doi: 10.1111/are.13082
Rayner, T. A., Jørgensen, N. O., Blanda, E., Wu, C. H., Huang, C. C., Mortensen, J., et al. (2015). Biochemical composition of the promising live feed tropical calanoid copepod Pseudodiaptomus annandalei (Sewell 1919) cultured in Taiwanese outdoor aquaculture ponds. Aquaculture 41, 25–34. doi: 10.1016/j.aquaculture.2015.01.034
Rodríguez-Graña, L., and Calliari, D. (2020). Senescence in Acartia tonsa (Copepoda, Calanoida): male’s reproductive performance preliminary results from a southern population. Invertebr. Reprod. Dev. 64, 237–243. doi: 10.1080/07924259.2020.1748126
Rodríguez-Graña, L., Calliari, D., Tiselius, P., Hansen, B. W., and Sköld, H. N. (2010). Gender-specific ageing and non-Mendelian inheritance of oxidative damage in marine copepods. Mar. Ecol. Prog. Ser. 401, 1–13. doi: 10.3354/meps08459
Romano, F., Symiakaki, K., and Pitta, P. (2021). Temporal variability of planktonic ciliates in a coastal oligotrophic environment: mixotrophy, size classes and vertical distribution. Front. Mar. Sci. 17:641589. doi: 10.3389/fmars.2021.641589
Rurangwa, E., and Verdegem, M. C. (2015). Microorganisms in recirculating aquaculture systems and their management. Rev. Aquac. 7, 117–130. doi: 10.1111/raq.12057
Silver-Gorges, I. M., Ingels, J., Dos Santos, G. A., Valdes, Y., Pontes, L. P., Silva, A. C., et al. (2021). Epibionts reflect spatial and foraging ecology of Gulf of Mexico loggerhead turtles (Caretta caretta). Front. Ecol. Evol. 9:696412. doi: 10.3389/fevo.2021.696412
Snoeijs, P. (1992). Studies in the Tabularia fasciculata complex. Diatom Res. 7, 313–344. doi: 10.1080/0269249X.1992.9705223
Souissi, A., Souissi, S., and Hwang, J.-S. (2013). The effect of epibiont ciliates on the behavior and mating success of the copepod Eurytemora affinis. J. Exp. Mar. Biol. Ecol. 445, 38–43. doi: 10.1016/j.jembe.2013.04.002
Støttrup, J. (2000). The elusive copepods: their production and suitability in marine aquaculture. Aquac. Res. 31, 703–711. doi: 10.1046/j.1365-2109.2000.318488.x
Suzuki, H., Mitsuishi, K., Nagumo, T., and Tanaka, J. (2015). Tabularia kobayasii: a new araphid diatom (Bacillariophyta, Fragilariaceae) from Japan. Phytotaxa 219, 87–95. doi: 10.11646/phytotaxa.219.1.7
Totti, C., Poulin, M., Romagnoli, T., Perrone, C., Pennesi, C., and De Stefano, M. (2009). Epiphytic diatom communities on intertidal seaweeds from Iceland. Polar Biol. 32, 1681–1691. doi: 10.1007/s00300-009-0668-664
Totti, C., Romagnoli, T., De Stefano, M., and Bavestrello, G. (2010). “The diversity of epizoic diatoms,” in All Flesh is Grass, eds Z. Dubinsky and J. Seckbach (Dordrecht: Springer), 323–343. doi: 10.1007/978-90-481-9316-5_15
Turner, J. T. (2004). The importance of small planktonic copepods and their roles in pelagic marine food webs. Zool. Stud. 43, 255–266.
Utz, L. R., and Costs, D. W. (2005). Spatial and temporal patterns in the occurrence of peritrich ciliates as epibionts on calanoid copepods in Chesapeake Bay, USA. J. Eukaryot. Microbiol. 52, 236–244. doi: 10.1111/j.1550-7408.2005.00025.x
van der Meeren, T., Olsen, R. E., Hamre, K., and Fyhn, H. J. (2008). Biochemical composition of copepods for evaluation of feed quality in production of juvenile marine fish. Aquaculture 274, 375–397. doi: 10.1016/j.aquaculture.2007.11.041
Vanacor-Barroso, M., Carvalho, C. V. A. D., Antoniassi, R., and Ronzani-Cerqueira, V. (2017). The copepod Acartia tonsa as live feed for fat snook (Centropomus parallelus) larvae from notochord flexion to advanced metamorphosis. Lat. Am. J. Aquat. Res. 45, 159–166. doi: 10.3856/vol45-issue1-fulltext-15
Wahl, M., Goecke, F., Labes, A., Dobretsov, S., and Weinberger, F. (2012). The second skin: ecological role of epibiotic biofilms on marine organisms. Front. Microbiol. 3:292. doi: 10.3389/fmicb.2012.00292
Walne, P. R. (1970). Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria and Mytilus. Fish. Invest. Ser. 26:85.
Wanka, R., Finlay, J. A., Nolte, K. A., Koc, J., Jakobi, V., Anderson, C., et al. (2018). Fouling-release properties of dendritic polyglycerols against marine diatoms. ACS Appl. Mater. Interfaces 10, 34965–34973. doi: 10.1021/acsami.8b12017
Wilcox, J. A., Tracy, P. L., and Marcus, N. H. (2006). Improving live feeds: effect of a mixed diet of copepod nauplii (Acartia tonsa) and rotifers on the survival and growth of first-feeding larvae of the southern flounder, Paralichthys lethostigma. J. World Aquac. Soc. 37, 113–120. doi: 10.1111/j.1749-7345.2006.00014.x
Williams, D. M., and Round, F. E. (1986). Revision of the genus Synedra ehrenb. Diatom Res. 1, 313–339. doi: 10.1080/0269249X.1986.9704976
Keywords: epibiotic diatom infestation, copepod, egg production, density-dependent effects, culture volume
Citation: Pan Y-J, Wang W-L, Hwang J-S and Souissi S (2021) Effects of Epibiotic Diatoms on the Productivity of the Calanoid Copepod Acartia tonsa (Dana) in Intensive Aquaculture Systems. Front. Mar. Sci. 8:728779. doi: 10.3389/fmars.2021.728779
Received: 22 June 2021; Accepted: 03 September 2021;
Published: 23 September 2021.
Edited by:
Ana Maulvault, Portuguese Institute for Sea and Atmosphere (IPMA), PortugalReviewed by:
Danilo Luis Calliari Cuadro, Universidad de la República, UruguayNor Azman Kasan, University of Malaysia Terengganu, Malaysia
Copyright © 2021 Pan, Wang, Hwang and Souissi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiang-Shiou Hwang, jshwang@mail.ntou.edu.tw; Sami Souissi, sami.souissi@univ-lille1.fr
 Yen-Ju Pan
Yen-Ju Pan Wei-Lung Wang3
Wei-Lung Wang3  Jiang-Shiou Hwang
Jiang-Shiou Hwang Sami Souissi
Sami Souissi