Industrial Fishing Near West African Marine Protected Areas and Its Potential Effects on Mobile Marine Predators
- 1Conservation Ecology Group, Groningen Institute for Evolutionary Life Sciences, University of Groningen, Groningen, Netherlands
- 2Department of Coastal Systems, Royal Netherlands, Institute for Sea Research (NIOZ), Den Burg, Netherlands
- 3Institut Mauritanien de Recherches Océanographiques et de Pêches, Nouadhibou, Mauritania
- 4Centro de Investigação Pesqueira Aplicada, Bissau, Guinea-Bissau
- 5Instituto da Biodiversidade e das Áreas Protegidas, Bissau, Guinea-Bissau
Marine Protected Areas (MPAs) are increasingly implemented to facilitate the conservation of marine biodiversity and key habitats. However, these areas are often less effective to conserve mobile marine species like elasmobranchs (i.e., sharks and rays). Industrial fishing near MPA borders possibly impacts vulnerable species utilizing these protected areas. Hence, we aimed to study spatiotemporal patterns of industrial fisheries near MPAs, in relation to the bycatch of elasmobranchs. Specifically, we analyzed the spatiotemporal fishing effort within the West African region, mapped fishing effort in the direct vicinity of the Parc National du Banc d’Arguin (PNBA, Mauritania) and the Bijagós Archipelago (BA, Guinea Bissau), and compared the seasonal overlap between elasmobranch bycatch and fishing effort near these MPAs. We combined Automatic Identification System (AIS) data and local fisheries observer data, and determined fishing effort for each gear type and compared this with bycatch of elasmobranchs. We found that industrial fishing effort was dominated by trawling, drifting longlines, and fixed gear types. Although no industrial fishing was observed within both MPAs, 72 and 78% of the buffer zones surrounding the MPAs were fished for the Banc d’Arguin and Bijagós, respectively. Within the Banc d’Arguin buffer zone, trawling and drifting longlines dominated, with longlines mainly being deployed in fall. In the Bijagós buffer zone, trawling and fixed gears were most prevalent. Fisheries observer data for Mauritania showed that elasmobranch catches increased during the most recent sampling years (2016–2018). Elasmobranch catches within the waters of Guinea Bissau peaked in 2016 and decreased in the following two years. Seasonal patterns in elasmobranch bycatch within the waters of both countries are likely caused by increased catches of migratory species. Catches of rays peaked in May and June for Mauritania, and in October for Guinea Bissau. Shark catches were highest in February and July in Mauritanian waters, and in May and October in the waters of Guinea Bissau. Our study indicates that industrial fisheries near the border of ecologically important MPAs may have potentially major implications for ecosystem functioning by the removal of (migratory) predatory species.
Introduction
To halt the degradation of marine ecosystems and to counter overexploitation of marine resources, an increasing number of Marine Protected Areas (MPAs) have been implemented over the last two decades (Watson et al., 2014; McDermott et al., 2018). The majority of these implemented MPAs cover coastal areas, like vegetated wetlands and coastal reefs, which can be important for marine megafauna species (Fox et al., 2012; Sievers et al., 2019). Megafaunal species (e.g., sharks, rays, sirenians, cetaceans, and sea turtles) frequently utilize coastal areas as nursery grounds in early life stages (e.g., Bangley et al., 2018), or as breeding areas (e.g., Van Waerebeek and Read, 2014), foraging areas (e.g., Eckert et al., 2006; Sievers et al., 2019), and as predator-free refuge areas later in life (e.g., Heithaus et al., 2009). However, megafauna species generally have large home ranges and are often migratory (Lewison et al., 2014). They therefore only spend a limited, but essential proportion of their life cycle in such areas. Within these coastal areas, megafaunal species exhibit essential ecological roles, including as (top) predators (Ferreira et al., 2017). In addition, due to their migratory nature, these species form important functional links (e.g., transferring nutrients) between coastal areas and other systems, such as the pelagic zone (Williams et al., 2018; Sievers et al., 2019).
Coastal areas like seagrass meadows, rocky shores, tidal flats, and mangroves also provide an essential nursery habitat for pelagic and commercial fish species (Stål et al., 2008; Binet et al., 2013; Honda et al., 2013). Designating such vital areas as MPAs can result in increased species richness and biomass of commercial fish species in surrounding areas; the so-called spillover effects (Polunin and Roberts, 1993; Stobart et al., 2009). Consequently, fisheries might be attracted to the borders of MPAs (Di Lorenzo et al., 2016). Although this phenomenon may not be problematic for highly productive species with small home ranges (i.e., small teleosts), concentrated fishing activities might pose threats to vulnerable species with large home ranges, migratory behavior, or species that only utilize the protected areas during a certain life stage (Burgess et al., 2013; Dulvy et al., 2014; Lewison et al., 2014).
Elasmobranchs (i.e., sharks and rays) are a species group susceptible to bycatch, and with their low recruitments rates, high maturity ages, and other K-selected life history characteristics, many species of this group are particularly vulnerable to any non-natural mortality rates (MacKeracher et al., 2018). In addition, the status of many elasmobranch species remains unknown and many species have wide home ranges, which challenges effective conservation of this species group (Dulvy et al., 2014; MacKeracher et al., 2018).
As a consequence of stricter fishing regulations in many developed countries, distant-water fleets of these nations moved to the territorial waters of developing countries, including many countries in West Africa (Balmford et al., 2004; Worm et al., 2009). The high productivity of these waters, caused by the upwelling of the Canary current, attracts fishing fleets from nations all over the world (Belhabib et al., 2019). Consequently, fishing effort within this region is among the highest in the world (Pauly and Christensen, 1995; Grecian et al., 2016). The region also contains highly diverse marine ecosystems which are threatened by habitat degradation, overexploitation, and pollution (Tittensor et al., 2010; Stuart-Smith et al., 2013). Furthermore, the West African region is known for its data deficiency and high prevalence of endangered marine species, in particular species like hammerhead sharks (Sphyrna spp.), Lusitanian cownose rays (Rhinoptera marginata), and blackchin guitarfishes (Glaucostegus cemiculus).
There are two large intertidal MPAs of high ecological importance within the region: Parc National du Banc d’Arguin (PNBA) in Mauritania and the Bijagós Archipelago (BA) in Guinea Bissau (Figure 1). Both areas are considered to play an important role as spawning and nursery area for commercial fish species, and for migratory species, including elasmobranchs (Jager, 1993; Valadou et al., 2006). Declines of the annual catch per unit effort of rays and sharks within the boundaries of these MPAs have sparked concerns among park managers, conservationists, scientists, and the local communities about the status of these species groups within the region (Cheikna Lemrabott et al., unpub. data; Leurs, pers. obs.). Although fishing pressure through artisanal practices and bycatch rates within the MPAs are also substantial (Campredon and Cuq, 2001; Valadou et al., 2006; Diop and Dossa, 2011), fishing effort of industrial fleets at the borders of these MPAs could potentially have negative effects on the population status of marine megafauna utilizing these coastal areas (Guénette et al., 2014; Di Lorenzo et al., 2016). Herein we describe the industrial fishing activity within the West African region between 2012 and 2018 with three main objectives: (1) to analyze the spatiotemporal extent of gear-specific fishing efforts within the region, (2) to map fishing activity in the direct vicinity of the two largest West African MPAs, PNBA and the BA, and (3) to link the industrial fishing effort with seasonal bycatch of elasmobranchs (i.e., sharks and rays) to estimate its effect on nature conservation goals of coastal MPAs.
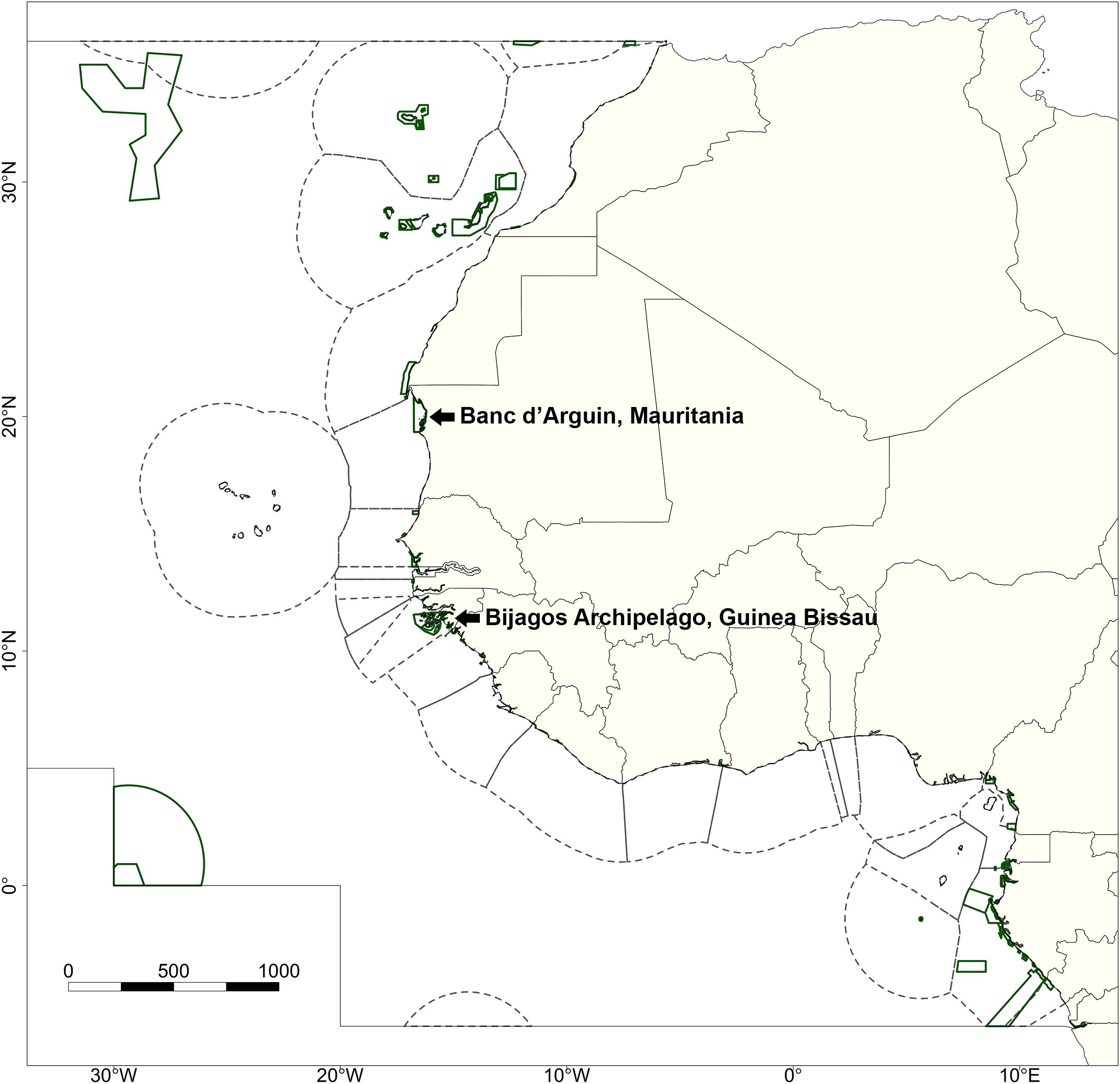
Figure 1. Defined study area indicating the Exclusive Economic Zones (EEZs; dashed lines) and Marine Protected Areas (MPAs; green lines) within the West African region. The inner gray border represents the northern and southern edges of the study area. The two focal MPAs, the Parc National du Banc d’Arguin (Mauritania) and the Bijagós Archipelago (Guinea Bissau), are specifically indicated.
Materials and Methods
Study Area
We focused on the Eastern Central Atlantic (major fishing area 34 as defined by the Food and Agriculture Organization of the United Nations, FAO) as our main study area. This study site ranges from the territorial waters of Morocco in the north to the territorial waters of the Democratic Republic of Congo in the south (Figure 1). Geographical data on the EEZs of all nations within this region were extracted from the “MarineRegions” dataset (Lonneville et al., 2019). Areas outside of any EEZ were classified as the high seas.
Within our study area, we focused on two large MPAs: PNBA (N20°14′5″, W16°6′32″) and the BA (N11°15′0″, W16°5′0″) (Figure 1), for which spatial delineation was obtained from the World Database on Protected Areas (UNEP-WCMC and IUCN., 2019). The PNBA is the largest marine park in West Africa, and was designated as a RAMSAR site in 1982 and as a UNESCO World Heritage site in 1989. The entire national park is 12,000 km2, of which 5,600 km2 marine area (Binet et al., 2013). The area comprises of a large variety of habitats, from bare tidal flats and intertidal seagrass meadows to extensive subtidal areas. The BA covers a 12,958 km2 archipelago consisting of 88 islands and islets. The archipelago was designated as a UNESCO Biosphere Reserve in 1996 and as a RAMSAR site in 2014. The Bijagós contains dense mangrove forests, tidal flats, complex gully systems, and extensive subtidal areas. Within the Bijagós Biosphere Reserve, the islands of Formosa, Orango, and Joao Vieira are designated as MPAs. Both MPAs are considered to be important for a large variety of (commercial) fish species, elasmobranchs, and migratory shorebirds.
Data Collection
Fishing effort data (2012–2018) were obtained from the Global Fishing Watch (GFW1), based on processed Automatic Identification System (AIS) transmissions of large vessels (Kroodsma et al., 2018). The GFW applied artificial neural network algorithms to the AIS-data, which determined fishing activity and gear type used based on the speed and movement pattern of the vessel. As AIS is mandatory for all vessels above 300 gross tonnage, the dataset only includes large industrial vessels.
In total, 15 different gear categories within West African waters were identified, which we reclassified into six more general categories (Table 1). In addition, the GFW linked Maritime Mobile Service Identity (MMSI) information to the AIS transmissions, providing the flag state of registration for each vessel. Fishing effort, as the total number of fishing hours (in kilohours, kh), was then determined per vessel, flag state, gear type, and year for every 0.1° longitude/latitude grid cell over 2012–2018.
Fishery-dependent data were collected as part of fisheries observer programs by the national fisheries institutes Institut Mauritanien de Recherches Océanographique et de Pêches (IMROP) and Centro de Investigação Pesqueira Aplicada (CIPA), for Mauritania and Guinea Bissau, respectively. The data from the Mauritanian EEZ are based on logbook data documented and curated by the national fisheries institute. Data for this area were reported in the total catch per functional group and the fishing effort was documented from 2012 to 2018. The data from Guinea Bissau were collected by observers, who recorded the catch (in kg) per functional group (e.g., “Rays,” “Sharks,” and “Diverse pelagics”). Observers also recorded the effort (in hours) for each vessel. The total catch per functional group and the total fishing effort were collected from 2012 to 2016 (Centro De Investigação Pesqueira Aplicada (CIPA), 2012, 2013, 2014, 2015, 2016). Vessel-based observer data were combined with fleet-wide landing data to extrapolate bycatch observations to fleet level. No information on the survey effort were recorded for these data. Presented data thus reflect non-standardized survey efforts per month.
Data Processing
A 0.1° grid (±11 × 11 km near the equator) was superimposed on the study area, and industrial fishing effort was calculated per grid cell. Fished extent was determined as the proportion of fished grid cells relative to the total number of grid cells (n = 224,926). To determine and visualize the annual, gear-specific fishing effort in direct vicinity of both MPAs, we created two buffer zones around each MPA of 1.5 and 2.0 times the surface area of the MPA. We also calculated the cumulative fishing effort over increasing distance from each MPA of each gear type specifically. Fishing effort based on the AIS data was not compared between years, as the number of vessels detected by the GFW algorithms increased every study year due to technological enhancements. For this reason, 2018 is reported for the most recent fishing effort calculations. For annual trends in fishing effort, we used the fishery-dependent data.
The fishery-dependent observer data contained information on both catches (in tons) and fishing effort (in fishing days). Catches were classified into functional groups, as limited information on species identification was available. From 2012 to 2015, both focal countries reported elasmobranch catches as part of diverse groups like, “Diverse pelagic” or “Diverse demersal.” Since 2016, catches of sharks and rays were reported separately (i.e., catches were not grouped together as elasmobranchs or grouped into other functional groups). Our data analysis only includes those catches reported as elasmobranchs, resulting in a conservative estimate of catches. Rays included all species labeled as “Raia,” and sharks included all species of hammerhead sharks (Sphyrna spp.), or species labeled as “Elasmobranchii” or “Caudo.” Fishing effort was registered as the number of hours that a vessel was actively fishing during a fishing expedition, separated per gear type. Seasonality of elasmobranch catches was investigated using catch recordings, for both countries separately. In addition, total fishing effort was determined from the registered fishing effort and was subsequently compared to the AIS-based fishing effort of the GFW. For this, seasons were determined as winter (December–February), spring (March–May), summer (June–August), and fall (September–November).
Results
Spatiotemporal Fishing Activity off West Africa
A total of 5,449 kh (0.39 h–1 km–2) of fishing effort by AIS-operating vessels was observed within the entire West African region, including the high seas, between 2012 and 2018 (Figure 2 and Supplementary Table S1), with an average annual effort of 778 ± 466 kh (mean ± sd). Over the 6-year study period, at least 42.2% of the West African region (5.9 × 106 km2) was fished at least once (at our 0.1° resolution), with a mean annual extent of 21.9 ± 6.7% (3.9 ± 0.9 × 106 km2) (Supplementary Figure S1). Fishing effort concentrated in coastal waters (70% in EEZs compared to 30% in high seas), with the EEZs of Mauritania (10%), Western Sahara (8%), Morocco (8%), and Guinea Bissau (7%) together containing over 36% of the total fishing effort (Supplementary Table S1). The spatial distribution of the fishing effort peaked between the longitudes −18.45 and −15.45 (70.3 ± 56.6 kh), and off Sierra Leone between the latitudes 3.15 and 5.65 (27.2 ± 19.6 kh) (Figure 2). From the six gear types observed within the study area, trawlers (2,625 kh; 48.2%) and drifting longlines (1,901 kh; 34.9%) were the most deployed gears. Fishing effort of other gear types was relatively low (∼200 kh combined; Supplementary Table S1). Drifting longlines mainly operated on the high seas (80.3% of the total effort by longliners). Trawlers were concentrated within the coastal zones and only covered 1.2 ± 0.3% of the entire region. Over the entire study period, vessels from 60 flag states were observed within the West African region, although only 10 flag states were responsible for 88% of the total fishing effort. The five most active flag states within the region were Spain (24%), China (15%), Japan (12%), Morocco (11%), and Ghana (6%).
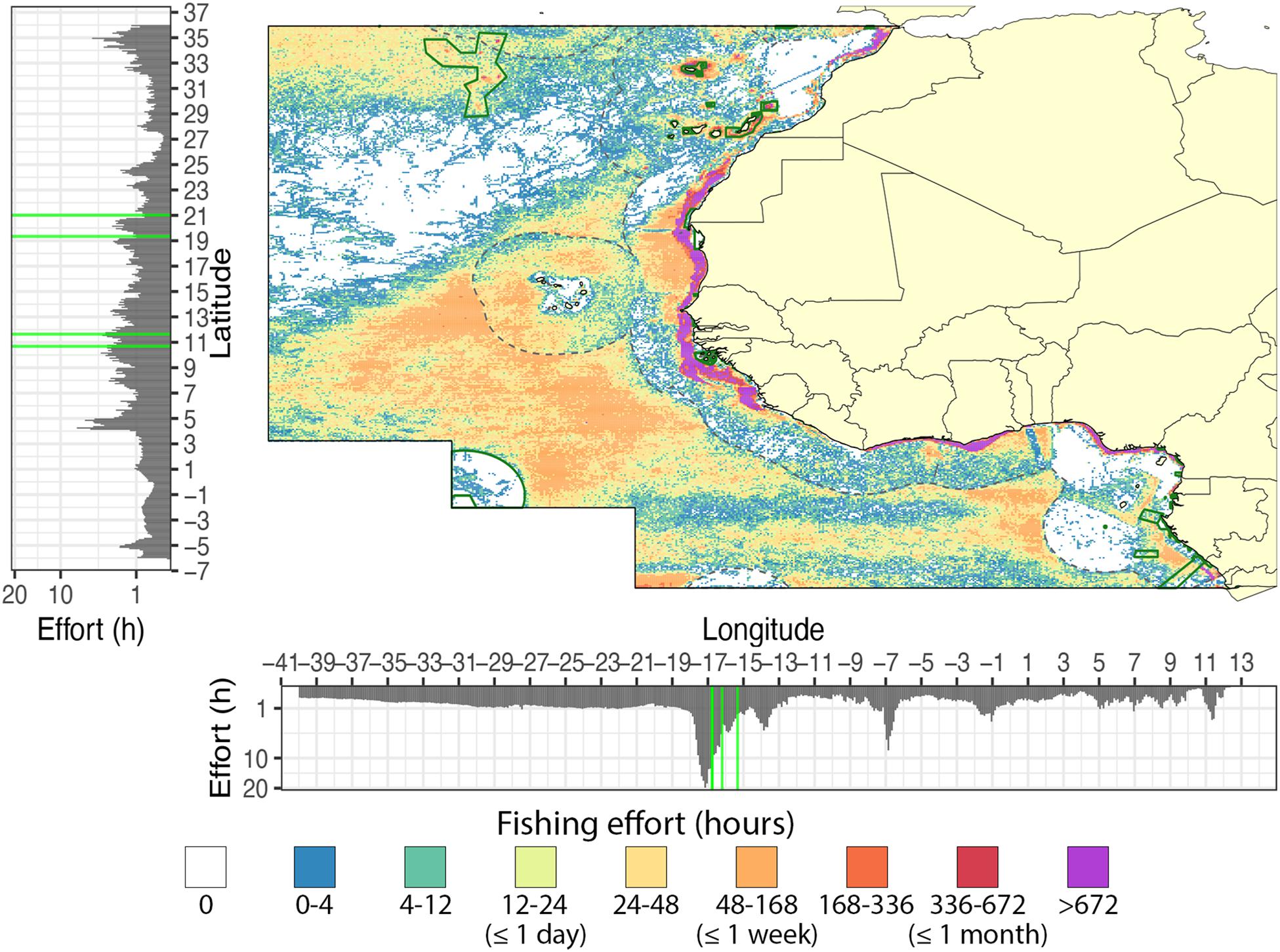
Figure 2. Total fishing effort off West Africa from 2012 to 2018. Color scale indicates the total hours of fishing within each grid cell (low = blue, moderate = yellow/orange, and high = purple). Histograms on the axis show the total fishing effort in hours over the longitudinal and latitudinal range of the region. The longitudinal and latitudinal ranges of both MPAs are indicated with green lines.
Fishing Activity Near MPAs
Parc National du Banc d’Arguin (PNBA)
Automatic Identification System-registered vessels showed a total of 560.7 kh fishing effort (3.2 h–1 km–2) within the Mauritanian EEZ over the study period, covering 95.3% of the EEZ. Based on the fishery-dependent data, fishing effort of the entire fleet operated within the Mauritanian EEZ ranged between 26.7.103 days in 2013 and 54.1.103 fishing days in 2018 (Figure 3A). No significant increase in fishing effort was found for the Mauritanian EEZ. In total, 41 flag states operated within this EEZ during the study period, with Spain (36.4%), China (30.4%), and Mauritania (7.7%) being the dominant fleets (Supplementary Table S1). Fishing vessels deployed all gear types, with trawlers as the most dominant gear type (353.3 kh; 63.0%). Because these trawlers mainly operated in coastal waters (Figure 4), the fished extent was relatively small (35.1% of the EEZ). Fishing effort increased over short distances from the PNBA, with trawlers showing the highest increase in efforts near the MPA and within the buffer zones (Supplementary Figure S2). Fishing effort within the 2.0x buffer zone around the PNBA was 117.5 kh in 2018, with no industrial fishing observed within the boundaries of the PNBA. In 2018, 42.0% of the grid cells within the buffer zone were fished at least once, with trawlers dominating in both effort (89.3 kh) and extent (33.2%).
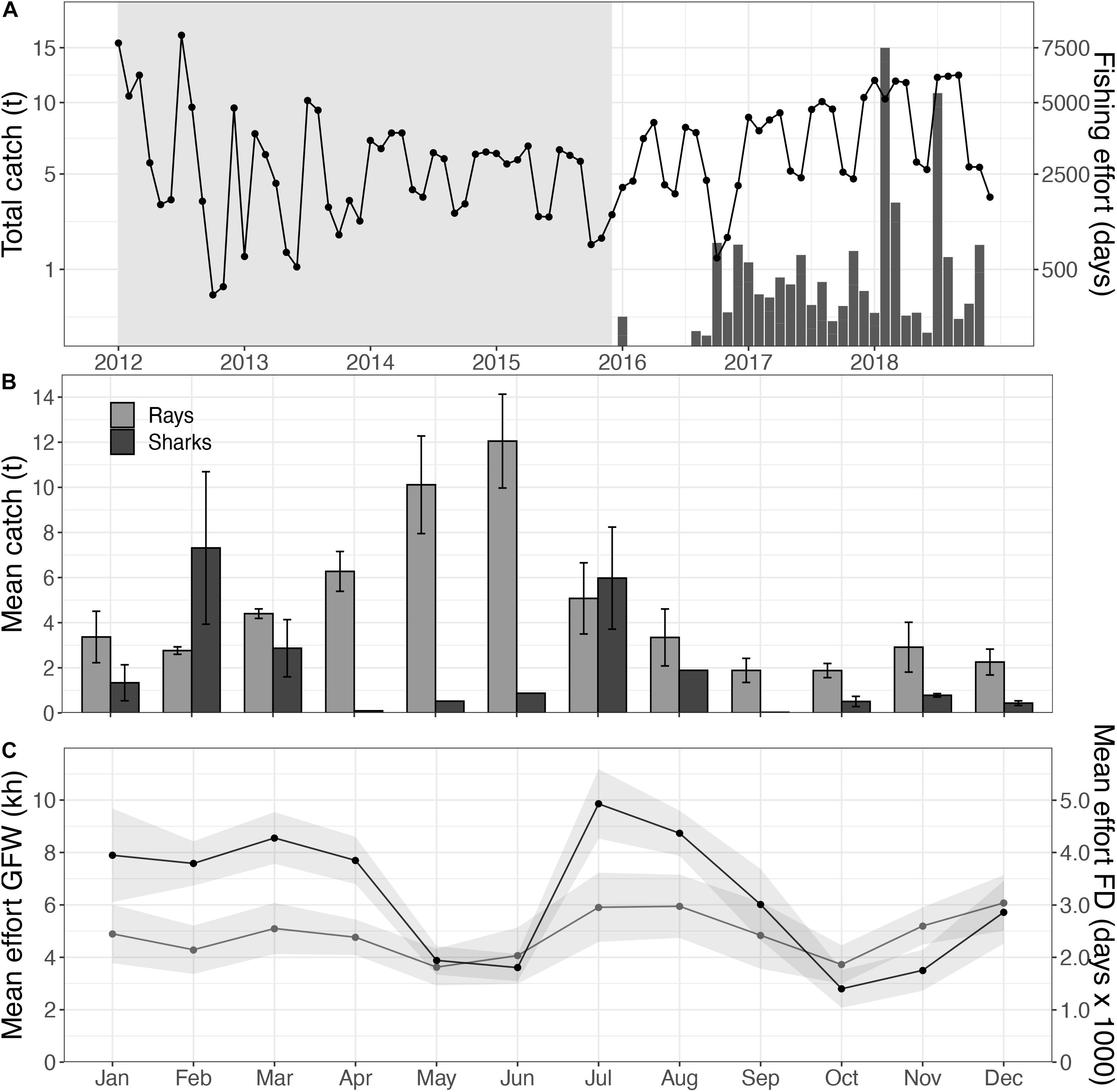
Figure 3. Total elasmobranch catches (bars) and fishing effort (line) within the Mauritanian EEZ, with no-data periods for elasmobranchs indicated in gray (A); with a close-up of the monthly mean catches, separated for sharks (black) and rays (gray), over the 2016–2018 period (B), in relation to fishing effort within the PNBA 2x buffer zone based on the AIS data (gray; in kh), and the total fishing effort in the Mauritanian EEZ as reported by the fisheries institute (black; in fishing days, FD) (C).
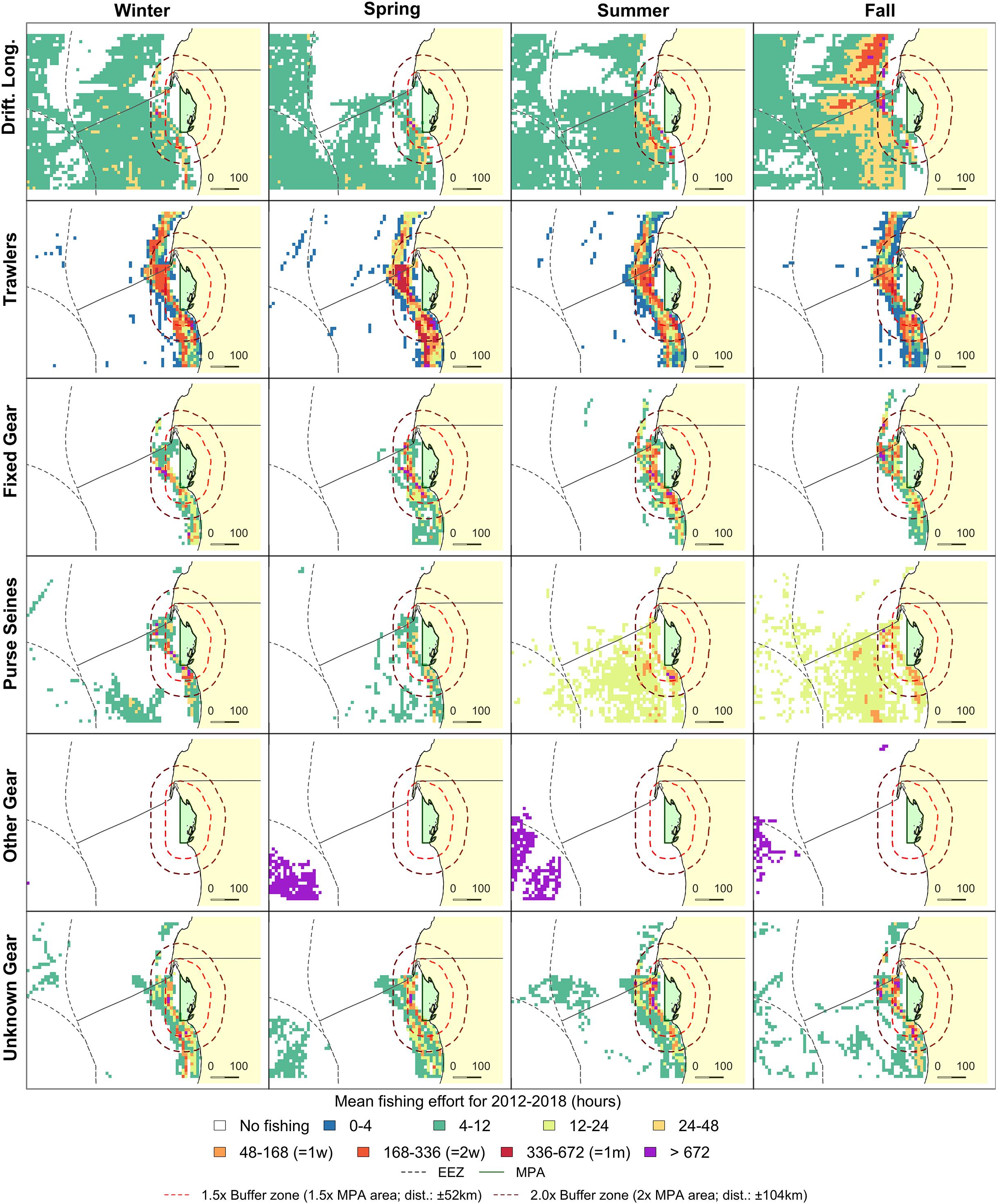
Figure 4. Fishing effort in the direct vicinity of PNBA (green) in Mauritania. Grid cell colors indicate seasonal mean fishing effort over the 2012–2018 period. Orange and red dashed lines represent 1.5x and 2.0x buffer zones of the PNBA. Exclusive Economic Zones (EEZ) are indicated as gray dashed lines.
Spatial distribution of trawlers was relatively constant throughout the year, while effort was highest in July (4.2 ± 3.8 kh) and December (4.4 ± 2.8 kh). There was a clear seasonal change in the spatial distribution of drifting longlines and fixed gears within the Mauritanian EEZ. Drifting longlines were constantly present, but gradually increased from spring (3.3 kh) to fall (8.4 kh). Fixed gear types showed higher fishing effort in fall and winter (Figure 4). Overall fishing effort within the 2.0x-buffer zone peaked in the months July, August, and December (Figure 3C). Seasonal patterns in fishing effort between the AIS data (2.0x buffer zone) and the fishery-dependent data (Mauritanian EEZ) showed similar patterns (Figure 5C).
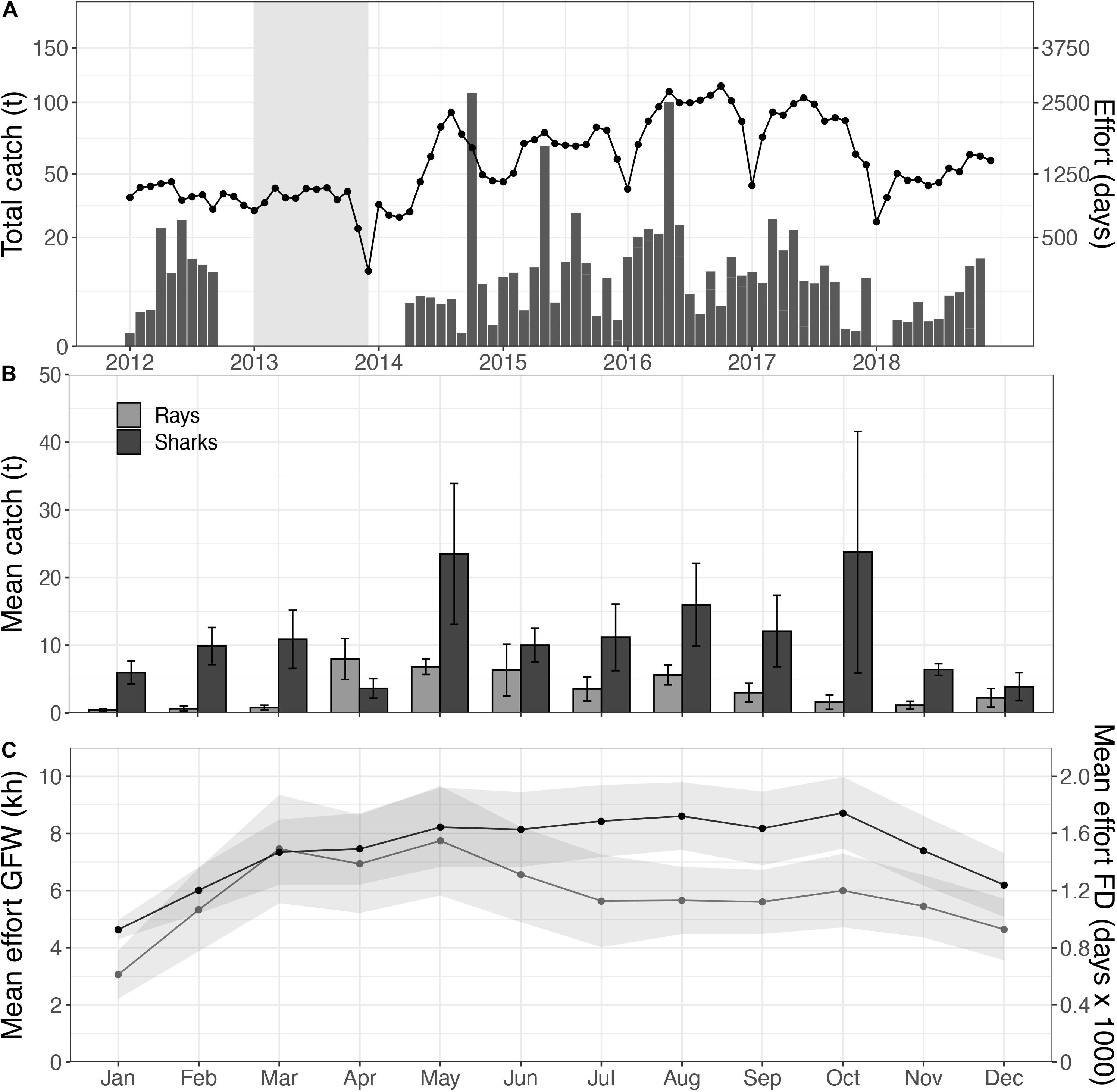
Figure 5. Total elasmobranch catches (bars) and fishing effort (line) within the Guinea-Bissau EEZ, with no-data periods for elasmobranchs indicated in gray (A), with a close-up of the monthly mean catches, separated for sharks (black) and rays (gray), over the 2014–2016 period (B), in relation to fishing effort within the BA 2x buffer zone based on the AIS data (gray; in kh), and the total fishing effort in the EEZ of Guinea Bissau as reported by the fisheries institute (black; in fishing days, FD) (C).
Traceable catches of sharks and rays were only documented in 2016, 2017, and 2018. Elasmobranch catches peaked with 85.8 tons in 2018, of which 55.5 tons were rays (64.7%) and 30.3 tons were sharks (35.3%) (Figure 3A). Ray catches were highest from April to July (8.4 ± 3.3 tons; mean ± se), whereas shark catches peaked in February (7.3 ± 3.4 tons) and July (6.0 ± 2.3 tons) (Figure 3B).
Bijagós Archipelago (BA)
Fishing effort within the EEZ of Guinea Bissau totaled to 386.0 kh (3.4 h–1 km–2) in the study period, with a total fished extent of 73.5%. Based on fishery-dependent data, the fishing effort significantly increased (ß = 12.39, t = 5.05, p < 0.01) with 12.4 days per month from 10.4.103 days in 2013 to 27.8.103 fishing days in 2016 (Figure 5A). A total of 21 flag states were active within the EEZ, dominated by mainly Spain (34.3%), China (28.8%), and Senegal (9.8%) (Supplementary Table S1). During the study period, all six gear types (Table 1) were observed. Trawlers showed highest effort (374 kh; 96.9%), and were concentrated near the coast (48.4% of EEZ) (Figure 6). Unidentified gear types were the second most dominant with a fishing activity of 8.7 kh (2.3%).
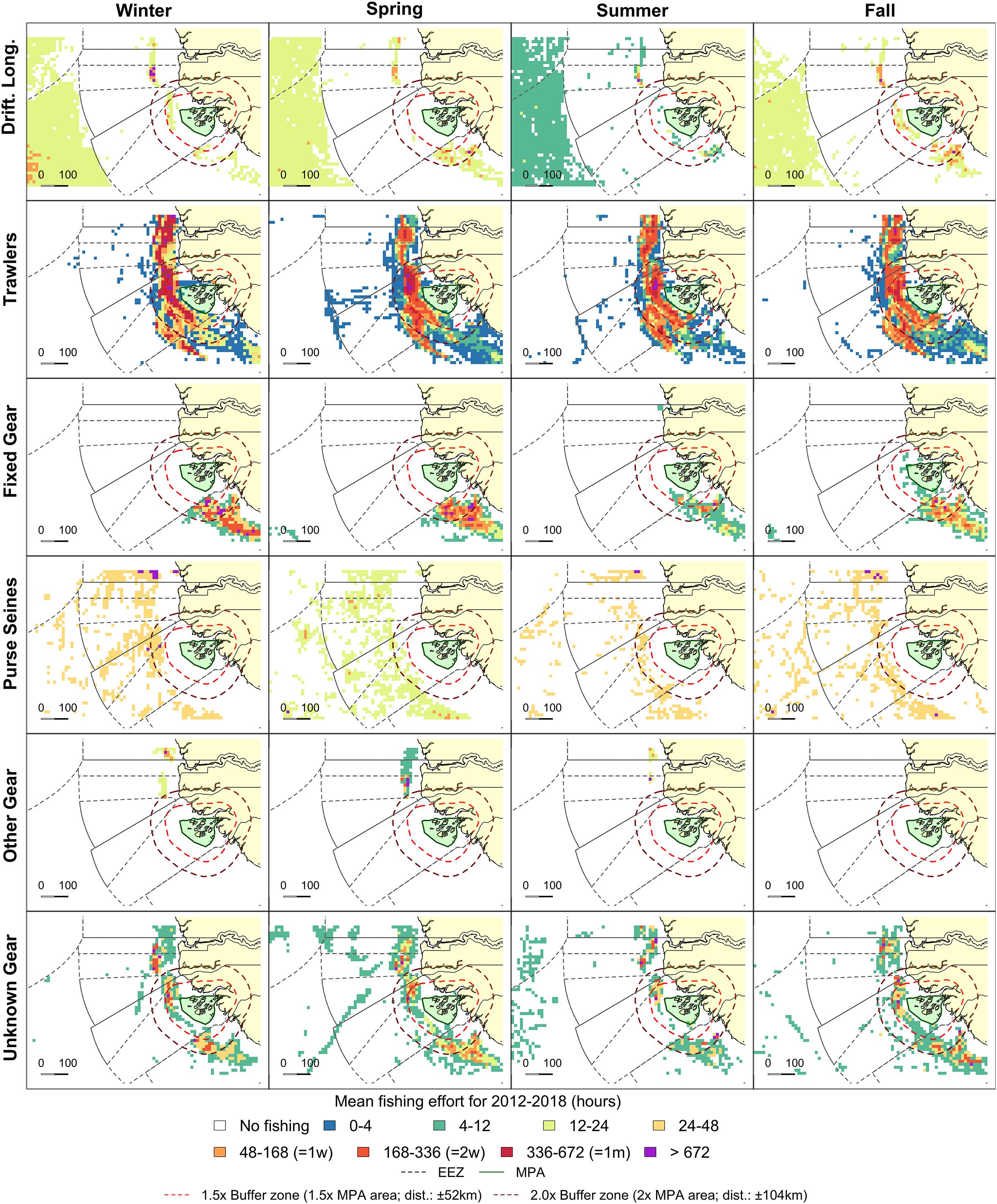
Figure 6. Fishing effort in the direct vicinity of the BA in Guinea Bissau (in green). Grid cell colors represent seasonal mean fishing effort over the 2012–2018 period. Orange and red dashed lines indicate 1.5 and 2.0 buffer zones, respectively. Exclusive Economic Zones (EEZ) are indicated as gray dashed lines.
No industrial fishing effort was observed within the BA boundaries, but high effort was observed near the MPA borders (Supplementary Figure S2). Within the 2.0x buffer zone, fishing effort was 88.3 kh in 2018 with an extent of 42.9%. Trawlers were dominant in both effort (65.4%) and extent (41.2%) in 2018 based on AIS data. Fished extent within the buffer zone remained relatively constant throughout the year for all gear types, but fishing effort peaked in spring (Figures 5C, 6). Seasonal patterns in fishing effort between the AIS data (2.0x buffer zone) and the fishery-dependent data (entire EEZ) showed similar patterns (Figure 5C).
Elasmobranch catches within the EEZ of Guinea Bissau were reported separately in 2012 and from 2014 to 2018 (Figure 5A). In other years, catches were integrated in other functional groups and are therefore not included here. Reported catches were highest in 2016, with 262.92 tons, of which 18.97 tons (7.2%) were ray species and 243.95 tons (92.8%) were shark species. In the most recent year of the study (2018), total elasmobranch catches were 39.46 tons, with catches existing of 35.79 tons of rays (90.7%) and 3.68 tons of sharks (9.3%). Ray catches were highest in April and May with 7.95 ± 3.04 (mean ± se) and 6.80 ± 1.13 tons, respectively (Figure 5B). Shark catches were also highest in October with a mean weight of 23.74 ± 17.86 tons and in May (23.49 ± 10.42 tons).
Discussion
In this study, we provide new insights in the recent (2012–2018) effort and spatiotemporal distribution of industrial fisheries in West Africa. In addition, we focused on fishing effort in the vicinity of two large, coastal MPAs. AIS records demonstrated that fishing activity is concentrated near the borders of MPA: PNBA (Mauritania) and the Bijagós Biosphere Reserve (BA, Guinea Bissau). Fishing effort within the Mauritanian EEZ was relatively stable, whereas effort within the EEZ of Guinea Bissau increased significantly with 12 fishing days a month. Industrial fishing activity was mainly dominated by trawlers, drifting longlines, and fixed gears. These gears mainly target mackerel (Scomber spp.), sardinella (Sardinella spp.), horse mackerels (Trachurus spp.), and cephalopods (Belhabib et al., 2013; Belhabib and Pauly, 2015; FAO, 2019), but have bycatches of sharks and rays. In the waters from both Mauritania and Guinea Bissau, the catches of elasmobranchs peaked in the most recent years of the study period. Seasonal peaks in industrial shark and ray catches were observed as well, but these did not coincide with seasonal maxima in industrial fishing effort. We showed that industrial fisheries (especially trawlers) are concentrated within a thin belt surrounding both MPAs. This concentrated fishing effort could have potential effects on mobile marine predators such as elasmobranchs and other species that utilize coastal MPAs for a part of their life cycle only. Hence, fishing concentrations near MPA borders may impair the role of coastal MPAs for the protection of endangered highly mobile marine megafauna. Inclusion of seasonal migration patterns and seasonal fishery bans near MPAs could aid in the conservation of mobile marine megafauna.
Although fishing effort near the PNBA and BA showed a seasonal pattern, a similar pattern was not visible in reported elasmobranch catches from both EEZs. The observed peaks are probably explained by temporal higher abundances of these species, indicating migratory behavior of these species. In Mauritania, sharks were caught most in February and July. These observations are congruent with Zeeberg et al. (2006), who report highest catches in August for hammerhead sharks and February for other shark species. The scalloped hammerhead shark (Sphyrna lewini), for instance, utilizes shallow coastal habitats during early life stages (e.g., mangrove areas), before it moves to more pelagic and deeper habitats (Hoyos-Padilla et al., 2014; Coiraton et al., 2020). The species migrates back to coastal, shallow habitats for parturition during the boreal summer (Capapé et al., 1998; Hazin et al., 2001). Recent findings suggest that scalloped hammerhead sharks are more dependent on coastal habitats than previously hypothesized (Coiraton et al., 2020). The PNBA is also hypothesized to be an important feeding and parturition site for the Lusitanian cownose ray (R. marginata). Within the PNBA, ray catches by artisanal fishermen peak from November to the end of February (Cheikna Lemrabott, in prep.). A similar season (September to December) is reported for industrial fisheries and scientific surveys outside the PNBA (Hofstede, 2001; Krakstad et al., 2004, 2005). Our study, on the other hand, shows that the catches of rays peak in April and July within the Mauritanian EEZ. Differences might be caused by the fact that temporal scales of these studies do not overlap with the temporal scale of this study. Alternatively, annual differences in coastal upwelling events might cause changes in catches.
For Guinea Bissau, we demonstrate increased catches of sharks and rays in May, October, and November. However, little information is available on elasmobranch abundance and habitat use. The scientific reports, based on observer data, additionally comprise limited species-specific information and have little consistence in registration. The actual numbers thus may be uncertain. However, reported bycatch of elasmobranches are supported by other studies (Belhabib and Pauly, 2015), sometimes showing much higher catch rates. We therefore argue that our estimates probably underestimate actual catches.
We demonstrated that trawlers were present during the whole year and dominated both fishing effort and spatial extent near the PNBA and BA. Drifting longlines were absent near BA, but peaked near the PNBA in fall. Both gears generally have high bycatch of sharks and rays (Zeeberg et al., 2006; Oliver et al., 2015). Drifting longlines were not present near BA, but the presence of this gear type near the PNBA peaked in fall. Trawlers have reported bycatch to mainly consist of pelagic teleosts (31%), hammerhead sharks (28%), and other shark species (19%) (Hofstede and Dickey-Collas, 2006). Similarly, Zeeberg et al. (2006) reported that 42% of all bycatch for trawlers operating off Mauritania was hammerhead sharks, with other bycatch including large teleosts (i.e., sunfish Mola mola and billfishes; 26%), reef manta rays (Manta birostris; 9%), other sharks (9%), cetaceans (8%), benthic rays (5%), and sea turtles (1%). Bycatch of longline gear types within the region is characterized by species such as the Atlantic blue marlin (Makaira nigricans), blue sharks (Prionace glauca), and smooth hammerhead sharks (Sphyrna zygaena) (Fernandez-Carvalho et al., 2015). Hence, trawlers and longliners surrounding the MPAs pose a conservation threat to elasmobranchs within the MPAs.
Our results show that overall fishing effort was mainly concentrated near the borders of both MPAs. MPAs are known to increase local fish biomass, drawing fishing vessels to their borders to target the “spillover” from these areas (Di Lorenzo et al., 2016). Another possible explanation for the concentrated fishing in this area is the local upwelling of the Canary Current, which makes the coast off the Western Sahara and Mauritania one of the richest fishing areas in the world (Goffinet, 1992). However, this does not explain why fishing effort is also concentrated near the BA, as it is located south of the upwelling’s boundary (Goffinet, 1992). This upwelling is strongest during the short period from December to March (Cushing, 1971), which could result in elevated fishing activity due to higher local production. Indeed, it partly coincides with elevated fishing effort within the Mauritanian EEZ, but not with peaks in fishing effort in the waters of Guinea Bissau, as migratory species utilize coastal areas for (parts) of their lifecycle and migrate between multiple habitats. For instance, American cownose rays (Rhinoptera bonasus) can migrate over distances of more than 1,500 km and scalloped hammerhead shark movements could be traced at 684 km from coastal areas (Diemer et al., 2011; Ogburn et al., 2018). Our results from the 2.0x buffer zones around the PNBA and BA could indicate that this concentrated fishing activity might interfere with the migratory nature of these marine megafauna species.
In this study, we revealed spatiotemporal patterns of industrial fisheries in West Africa. We showed seasonal fluctuations but overall high concentrations of effort near the borders of the Banc d’Arguin National Park and the BA MPAs. We furthermore showed seasonal patterns in elasmobranchs bycatch recordings within the EEZs of the corresponding countries, illustrating the migratory behavior of these species. We therefore conclude that the high concentration of fishing effort surrounding these important coastal areas conflicts with the migratory nature and vulnerability of elasmobranch species using these areas. This may lead to a further decrease of these vulnerable species in both pelagic and coastal habitats, and their associated ecological role in linking these habitats. The increasing removal of predatory species from marine ecosystems can cascade through the ecosystem, with consequences for (both ecological and economic) ecosystem services (Barbier et al., 2011; Estes et al., 2011). For example, the removal of top predators like cod (Gadus morhua) is assumed to be the most likely explanation for the observed increase in mid-sized fishes, which in turn has caused increases in macro-algae recruitment (ecologic) or a weakening of the biological pump of nutrients from great depths, possibly negatively influencing productivity of fisheries (economic) (Sieben et al., 2011; Hammerschlag et al., 2019). The densely concentrated fishing activity near the border of such protected areas therefore not only undermines the conservation value of these areas for these megafauna species, but might cascade into reduced functioning of coastal ecosystems and associated local livelihoods.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
GL, KR, SCL, HO, and LG outlined and drafted the study. GL coordinated the study and wrote the first draft. GL, KR, and SCL conducted data analyses. GL, KR, SCL, and LG wrote consecutive draft versions of the manuscript. GL, KR, SCL, IB, and DN collected and processed data used in this study. SLP, AR, and HO provided changes and feedback on later versions of the manuscript. All authors approved the submitted version.
Funding
This study was funded by the MAVA Foundation through the “Waders of the Bijagós” project. LG was funded by the Dutch Research Council (NWO016.VENI.181.087). KR was funded through a grant from the Dutch Gieskes-Strijbis Fund.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Many thanks to the Global Fishing Watch for the open access data that provide a valuable insight into these remote waters. Specifically, thanks to Tyler Clavelle and David Kroodsma for the advice and help with the newest version of the dataset. We would like to thank all fisheries observers, statisticians, and all other staff from IMROP (Mauritania) and CIPA (Guinea-Bissau) for collecting and providing the fishery-dependent data used in this study. Finally, we would like to thank Fábio Barroso and Tommaso Saccà.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.602917/full#supplementary-material
Footnotes
References
Balmford, A., Gravestock, P., Hockley, N., McClean, C. J., and Roberts, C. M. (2004). The worldwide costs of marine protected areas. Proc. Natl. Acad. Sci.U.S.A 101, 9694–9697. doi: 10.1073/pnas.0403239101
Bangley, C. W., Paramore, L., Shiffman, D. S., and Rulifson, R. A. (2018). Increased abundance and nursery habitat use of the bull shark (Carcharhinus leucas) in response to a changing environment in a warm-temperate estuary. Sci. Rep. 8:6018. doi: 10.1038/s41598-018-24510-z
Barbier, E. B., Hacker, S. D., Kennedy, C., Koch, E. W., Stier, A. C., and Silliman, B. R. (2011). The value of estuarine and coastal ecosystem services. Ecol. Monogr. 81, 169–193. doi: 10.1890/10-1510.1
Belhabib, D., and Pauly, D. (2015). Fisheries in troubled waters: a catch reconstruction for Guinea-Bissau, 1950-2010. Fish. Cent. Res. Rep. 23, 1–16.
Belhabib, D., Cheung, W. W. L., Kroodsma, D., Lam, V. W. Y., Underwood, P. J., and Virdin, J. (2019). Catching industrial fishing incursions into inshore waters of Africa from space. Fish.Fish. 21, 379–392. doi: 10.1111/faf.12436
Belhabib, D., Gascuel, D., Abou Kane, E., Harper, S., Zeller, D., and Pauly, D. (2013). Preliminary estimation of realistic fisheries removals from mauritania, 1950-2010. Fish. Cent. Res. Rep. 20, 61–78.
Binet, T., Failler, P., Chavance, P. N., and Abidine, M. (2013). First international payment for marine ecosystem services: the case of the Banc d’Arguin national park, Mauritania. Glob. Environ. Change 23, 1434–1443. doi: 10.1016/j.gloenvcha.2013.09.015
Burgess, M. G., Polasky, S., and Tilman, D. (2013). Predicting overfishing and extinction threats in multispecies fisheries. Proc. Natl. Acad. Sci.U.S.A 110, 15943–15948. doi: 10.1073/pnas.1314472110
Campredon, P., and Cuq, F. (2001). Artisanal fishing and coastal conservation in West Africa. J. Coast.Conserv. 7, 91–100. doi: 10.1007/BF02742471
Capapé, C., Diop, M., and N’Dao, M. (1998). Record of four pregnant females of the scalloped hammerhead shark, Sphyrna lewini (Sphyrnidae) in Senegalese waters. Cybium 22, 89–93.
Centro De Investigação Pesqueira Aplicada (CIPA) (2012). Estatísticas Pesca Industrial, Ano 2012 Avaliação Das Capturas. Bissau.
Centro De Investigação Pesqueira Aplicada (CIPA) (2013). Estatísticas Pesca Industrial, Ano 2013 Avaliação Das Capturas. Bissau.
Centro De Investigação Pesqueira Aplicada (CIPA) (2014). Estatísticas Pesca Industrial, Ano 2014 Avaliação Das Capturas. Bissau.
Centro De Investigação Pesqueira Aplicada (CIPA) (2015). Estatísticas Pesca Industrial, Ano 2015 Avaliação Das Capturas. Bissau.
Centro De Investigação Pesqueira Aplicada (CIPA) (2016). Estatísticas Pesca Industrial, Ano 2016 Avaliação Das Capturas. Bissau.
Coiraton, C., Amezcua, F., and Ketchum, J. T. (2020). New insights into the migration patterns of the scalloped hammerhead shark Sphyrna lewini based on vertebral microchemistry. Mar. Biol. 167, 1–18. doi: 10.1007/s00227-020-3668-0
Di Lorenzo, M., Claudet, J., and Guidetti, P. (2016). Spillover from marine protected areas to adjacent fisheries has an ecological and a fishery component. J. Nat. Conserv. 32, 62–66. doi: 10.1016/j.jnc.2016.04.004
Diemer, K. M., Mann, B. Q., and Hussey, N. E. (2011). Distribution and movement of scalloped hammerhead Sphryna lewini and smooth hammerhead Sphyrna zygaena sharks along the east coast of Southern Africa. Afr. J. Mar. Sci. 33, 229–238. doi: 10.2989/1814232X.2011.600291
Diop, M., and Dossa, J. (2011). 30 Years of shark fishing. IUCN Shark Specialist Group. Dakar. Availble online at: http://www.iucnssg.org/uploads/5/4/1/2/54120303/30years_eng.pdf (accessed June 1, 2020).
Dulvy, N. K., Fowler, S. L., Musick, J. A., Cavanagh, R. D., Kyne, P. M., Harrison, L. R., et al. (2014). Extinction risk and conservation of the world’s sharks and rays. ELife 3:e00590. doi: 10.7554/eLife.00590
Eckert, S. A., Bagley, D., Kubis, S., Ehrhart, L., Johnson, C., Stewart, K., et al. (2006). Internesting and postnesting movements and foraging habitats of leatherback sea turtles (Dermochelys coriacea) nesting in florida. Chelonian Conserv. Biol. 5, 239–248.
Estes, J. A., Terborgh, J., Brashares, J. S., Power, M. E., Berger, J., and Bond, W. J., et al. (2011). Trophic downgrading of planet Earth. Science 333, 301–306. doi: 10.1126/science.1205106
FAO (2019). Report of the FAO Working Group on the Assessment of Small Pelagic Fish off Northwest Africa. Banjul, The Gambia, 26 June - 1 July 2018. FAO Fisheries and Aquaculture Report No. 1247, Vol. 1247.
Fernandez-Carvalho, J., Coelho, R., Santos, M. N., and Amorim, S. (2015). Effects of hook and bait in a tropical northeast Atlantic pelagic longline fishery: part II-target, bycatch and discard fishes. Fish. Res. 164, 312–321. doi: 10.1016/j.fishres.2014.11.009
Ferreira, L. C., Thums, M., Heithaus, M. R., Barnett, A., Abrantes, K. G., Holmes, B. J., et al. (2017). The trophic role of a large marine predator, the tiger shark Galeocerdo cuvier. Sci. Rep. 7:7641. doi: 10.1038/s41598-017-07751-2
Fox, H. E., Soltanoff, C. S., Mascia, M. B., Haisfield, K. M., Lombana, A. V., Pyke, C. R., et al. (2012). Explaining global patterns and trends in marine protected area (MPA) development. Mar. Policy 36, 1131–1138. doi: 10.1016/j.marpol.2012.02.007
Goffinet, T. (1992). Development and fisheries management: the case of northwest africa. Ocean Coast. Manag. 17, 105–136. doi: 10.1016/0964-5691(92)90039-N
Grecian, W. J., Witt, M. J., Attrill, M. J., Bearhop, S., Becker, P. H., Egevang, C., et al. (2016). Seabird diversity hotspot linked to ocean productivity in the canary current large marine ecosystem. Biol. Lett. 12:20160024. doi: 10.1098/rsbl.2016.0024
Guénette, S., Meissa, B., and Gascuel, D. (2014). Assessing the contribution of marine protected areas to the trophic functioning of ecosystems: a model for the Banc d’Arguin and the Mauritanian shelf. PloS One 9:e94742. doi: 10.1371/journal.pone.0094742
Hammerschlag, N., Schmitz, O. J., Flecker, A. S., Lafferty, K. D., Sih, A., Atwood, T. B., et al. (2019). Ecosystem function and services of aquatic predators in the anthropocene. Trends Ecol. Evol. 34, 369–383. doi: 10.1016/j.tree.2019.01.005
Hazin, F., Fischer, A., and Broadhurst, M. (2001). Aspects of reproductive biology of the scalloped hammerhead shark, Sphyrna lewini, off northeastern Brazil. Environ. Biol. Fish. 61, 151–159.
Heithaus, M. R., Wirsing, A. J., Burkholder, D., Thomson, J., and Dill, L. M. (2009). Towards a predictive framework for predator risk effects: the interaction of landscape features and prey escape tactics. J. Anim. Ecol. 78, 556–562. doi: 10.1111/j.1365-2656.2008.01512.x
Hofstede, R ter. (2001). Incidental Catches of Pelagic Megafauna by the EU Pelagic Fleet in the Mauritanian Exclusive Economic Zone During the Year 2001. Report No.C007/03. Wageningen: RIVO-Netherlands Institute for Fisheries Research.
Hofstede, R. ter. and Dickey-Collas, M. (2006). An investigation of seasonal and annual catches and discards of the Dutch pelagic freezer-trawlers in Mauritania, Northwest Africa. Fish. Res. 77, 184–191. doi: 10.1016/j.fishres.2005.08.012
Honda, K., Nakamura, Y., Nakaoka, M., Uy, W. H., and Fortes, M. D. (2013). Habitat use by fishes in coral reefs, seagrass beds and mangrove habitats in the philippines. PLoS ONE 8:e6573. doi: 10.1371/journal.pone.0065735
Hoyos-Padilla, E. M., Ketchum, J. T., Klimley, A. P., and Galván-Magaña, F. (2014). Ontogenetic migration of a female scalloped hammerhead shark Sphyrna lewini in the Gulf of California. Anim. Biotelemetry 2, 1–9. doi: 10.1186/2050-3385-2-17
Jager, Z. (1993). The distribution and abundance of young fish in the Banc-D’Arguin, Mauritania. Hydrobiologia 258, 185–196. doi: 10.1007/BF00006196
Krakstad, J., Olsen, M., and Wagúe, A. (2004). Survey of the Pelagic Fish Resources Off North West Africa, Part II - Mauritania. Bergen: Institute of marine research.
Krakstad, J., Sylla, S., Jallow, J., and Olsen, M. (2005). Survey of the Pelagic Fish Resources off North West Africa. Part I: Senegal - The Gambia. Bergen: Institute of marine research.
Kroodsma, D. A., Mayorga, J., Hochberg, T., Miller, N. A., Boerder, K., Ferretti, F., et al. (2018). Tracking the global footprint of fisheries. Science 359, 904–908. doi: 10.1126/science.aao5646
Lewison, R. L., Crowder, L. B., Read, A. J., and Freeman, S. A. (2004). Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol. Evol. 19, 598–604. doi: 10.1016/j.tree.2004.09.004
Lewison, R. L., Crowder, L. B., Wallace, B. P., Moore, J. E., Cox, T., Zydelis, R., et al. (2014). Global patterns of marine mammal, seabird, and sea turtle bycatch reveal taxa-specific and cumulative megafauna hotspots. Proc. Natl. Acad. Sci.U.S.A 111, 5271–5276.
Lonneville, B., Oset García, P., Schepers, L., Vanhoorne, B., Hernandez, F., and Mees, J. (2019). MarineRegions.org. Retrieved June 10, 2019. Available online at: from http://www.marineregions.org (accessed June 1, 2020).
MacKeracher, T., Diedrich, A., and Simpfendorfer, C. A. (2018). Sharks, rays and marine protected areas: a critical evaluation of current perspectives. Fish Fish. 20, 255–267. doi: 10.1111/faf.12337
McDermott, G. R., Meng, K. C., McDonald, G. G., and Costello, C. J. (2018). The blue paradox: preemptive overfishing in marine reserves. Proc. Natl. Acad. Sci.U.S.A 116, 5319–5325. doi: 10.1073/pnas.1802862115
Ogburn, M. B., Bangley, C. W., Aguilar, R., Fisher, R. A., Curran, M. C., Webb, S. F., et al. (2018). Migratory connectivity and philopatry of cownose rays Rhinoptera bonasus along the Atlantic coast, USA. Mar. Ecol. Prog. Ser. 602, 197–211. doi: 10.3354/meps12686
Oliver, S., Braccini, M., Newman, S. J., and Harvey, E. S. (2015). Global patterns in the bycatch of sharks and rays. Mar. Policy 54, 86–97. doi: 10.1016/j.marpol.2014.12.017
Pauly, D., and Christensen, V. (1995). Primary production required to sustain global fisheries. Nature 374, 255–257.
Polunin, N. V. C., and Roberts, C. M. (1993). Greater biomass and value of target coral-reef fishes in two small Caribbean marine reserves. Mar. Ecol. Prog. Ser. 100, 167–176. doi: 10.3354/meps100167
Sieben, K., Ljunggren, L., Bergström, U., and Eriksson, B. K. (2011). A meso-predator release of stickleback promotes recruitment of macroalgae in the baltic sea. J. Exp. Mar. Biol. Ecol. 397, 79–84. doi: 10.1016/j.jembe.2010.11.020
Sievers, M., Brown, C. J., Tulloch, V. J. D., Pearson, R. M., Haig, J. A., Turschwell, M. P., et al. (2019). The role of vegetated coastal wetlands for marine megafauna conservation. Trends Ecol. Evol. 34, 807–817. doi: 10.1016/j.tree.2019.04.004
Stål, J., Paulsen, S., Pihl, L., Rönnbäck, P., Söderqvist, T., and Wennhage, H. (2008). Coastal habitat support to fish and fisheries in Sweden: Integrating ecosystem functions into fisheries management. Ocean Coast. Manag. 51, 594–600. doi: 10.1016/j.ocecoaman.2008.06.006
Stobart, B., Warwick, R., González, C., Mallol, S., Díaz, D., Reñones, O., et al. (2009). Long-term and spillover effects of a marine protected area on an exploited fish community. Mar. Ecol. Prog. Ser. 384, 47–60. doi: 10.3354/meps08007
Stuart-Smith, R. D., Bates, A. E., Lefcheck, J. S., Duffy, J. E., Baker, S. C., Thomson, R. J., et al. (2013). Integrating abundance and functional traits reveals new global hotspots of fish diversity. Nature 501, 539–542. doi: 10.1038/nature12529
Tittensor, D. P., Mora, C., Jetz, W., Lotze, H. K., Ricard, D., Berghe, E. V., et al. (2010). Global patterns and predictors of marine biodiversity across taxa. Nature 466, 1098–1101. doi: 10.1038/nature09329
UNEP-WCMC and IUCN. (2019). Protected Planet: Marine Protected Areas. Retrieved from www.protectedplanet.net (accessed June 1, 2020).
Valadou, B., Brethes, J., and Inejih, C. (2006). Observations biologiques sur cinq espéces d’Élasmobranches du parc national du Banc d’Arguin (Mauritanie). Cybium 30, 313–322.
Van Waerebeek, K., and Read, A. J. (2014). American society of mammalogists reproduction of dusky dolphins, Lagenorhynchus obscurus, from Coastal Peru. J. Mamm. 75, 1054–1062.
Watson, J. E. M., Dudley, N., Segan, D. B., and Hockings, M. (2014). The performance and potential of protected areas. Nature 515, 67–73. doi: 10.1038/nature13947
Williams, J. J., Papastamatiou, Y. P., Caselle, J. E., Bradley, D., and Jacoby, D. M. P. (2018). Mobile marine predators: an understudied source of nutrients to coral reefs in an unfished atoll. Proc. R. Soc. B Biol. Sci. 285:20172456. doi: 10.1098/rspb.2017.2456
Worm, B., Hilborn, R., Baum, J. K., Branch, T. A., Collie, J. S., Costello, C., et al. (2009). Rebuilding global fisheries. Science 325, 578–585. doi: 10.1126/science.1173146
Keywords: fisheries, threatened species, coastal ecosystems, marine conservation, elasmobranchs, fisheries ecology
Citation: Leurs G, van der Reijden KJ, Cheikhna Lemrabott SY, Barry I, Nonque DM, Olff H, Ledo Pontes S, Regalla A and Govers LL (2021) Industrial Fishing Near West African Marine Protected Areas and Its Potential Effects on Mobile Marine Predators. Front. Mar. Sci. 8:602917. doi: 10.3389/fmars.2021.602917
Received: 04 September 2020; Accepted: 11 February 2021;
Published: 04 March 2021.
Edited by:
Annette Breckwoldt, Leibniz Centre for Tropical Marine Research (LG), GermanyReviewed by:
Kristian Metcalfe, University of Exeter, United KingdomYonat B. Swimmer, Pacific Islands Fisheries Science Center (NOAA), United States
Copyright © 2021 Leurs, van der Reijden, Cheikhna Lemrabott, Barry, Nonque, Olff, Ledo Pontes, Regalla and Govers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guido Leurs, g.h.l.leurs@rug.nl
 Guido Leurs
Guido Leurs Karin J. van der Reijden
Karin J. van der Reijden Sidi Yahya Cheikhna Lemrabott
Sidi Yahya Cheikhna Lemrabott Iça Barry4
Iça Barry4  Diosnes Manuel Nonque
Diosnes Manuel Nonque Han Olff
Han Olff Laura L. Govers
Laura L. Govers