- 1Research Unit of Biology and Applied Ecology, Faculty of Science, University of Dschang, Dschang, Cameroon
- 2Laboratory of the Leishmaniasis Research Project, Mokolo Annex Regional Hospital, Mokolo, Cameroon
- 3Department of Animal Biology and Physiology, University of Yaoundé I, Yaoundé, Cameroon
- 4Faculty of Health Sciences, University of Bamenda, Bamenda, Cameroon
- 5Department of Medical Entomology, Centre for Research in Infectious Diseases (CRID), Yaoundé, Cameroon
- 6Faculty of Medicine and Pharmaceutical Sciences, University of Douala, Douala, Cameroon
- 7Vector Borne Diseases Laboratory of the Research Unit of Biology and Applied Ecology (VBID-RUBAE), Department of Animal Biology, Faculty of Science of the University of Dschang, Dschang, Cameroon
- 8Laboratoire de Recherche sur le Paludisme, Institut de Recherche de Yaoundé (IRY), Organisation de Coordination pour la lutte contre les Endémies en Afrique Centrale (OCEAC), Yaoundé, Cameroon
- 9Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Rockville, MD, United States
- 10Laboratory of Cellular Immunology and Parasitology, Central Washington University, Ellensburg, WA, United States
Introduction: Kousseri in the Far North Region of Cameroon has long been known as an endemic focus of visceral leishmaniasis (VL), although the study on sand flies in this focus is scarce. The present study investigates the spatial distribution, seasonality, and ecological aspects of sand flies from Kousseri. This study is based on the need to optimize the effectiveness of leishmaniasis control programs in the northern part of the country.
Methods: Sand flies were sampled monthly over 12 months in five selected sites using CDC light traps. Only captured females were morphologically identified at species level based on valid keys.
Results and discussion: Overall, 4,214 sand fly specimens were collected during 360 trapping nights. The male/female sex ratio slightly favored females (1:1.04). The eudominant Sergentomyia antennata followed by both dominant Sergentomyia schwetzi and Sergentomyia squamipleuris were the most common and abundant species, accounting for 76.1% of the collection. Phlebotomus duboscqi, the confirmed vector of cutaneous leishmaniasis (CL) in West African foci, although rare in the collection, was found in four of the five surveyed sites. This sand fly species with Se. schwetzi abounded in peri-urban areas and, respectively, in animal shed and in uninhabited house biotopes, while Se. antennata and Se. squamipleuris prevailed in rural areas and, respectively, in animal shelter biotopes and outside dwellings. All caught sand fly species except Se. schwetzi, Sergentomyia clydei, Sergentomyia inermis, and Sergentomyia adleri prevailed during the dry season. The highest Shannon–Wiener index of sand flies due to the maximal richness and evenness was found in the urban area, in outdoor biotopes, and during the rainy season (H′ = 1.68, 1.80, and 1.74, respectively). These data provide evidence that less urbanized areas, animal shelters around the compounds, and the absence of precipitation (dry season) favored the dispersion of abundant sand fly species in Kousseri. Based on previous reports on Leishmania transmission, a surveillance plan is required to prevent an outbreak of VL or an establishment of CL or canine leishmaniasis (CnL) in this focus. Further research identifying the blood meal source and the Leishmania parasites in these insects is critical for providing insightful data to fight leishmaniasis in Northern Cameroon.
1 Introduction
Leishmaniasis is a parasitic disease caused by flagellated unicellular protozoa belonging to the genus Leishmania and transmitted to animals and humans through the bite of hematophagous female phlebotomine sand flies (1, 2). This parasitic infection is among the neglected tropical diseases (NTDs), although it causes the ninth largest disease burden among infectious diseases worldwide (3). Owing to the insignificant interest shown by pharmaceutical companies and governmental entities of the countries concerned, this disease is still poorly known, even among medical personnel in some endemic regions, causing the death of infected patients without proper care and treatment (4). Although not preventable because of the absence of an efficacious vaccine, leishmaniasis remains curable through chemotherapies (5, 6). However, the efficacy of these alternatives varies as current antileishmanial treatments are long, toxic, resistant, not always available in some endemic areas (7–9), and expensive ($60–120 per patient, which often exceeds the overall budget for primary healthcare in some countries) (10). To overwhelm these gaps, prevention strategies involving protection against the bites of the adult sand fly using long-lasting impregnated mosquito nets (LLINs), repellents, and insecticide spraying are therefore recommended. Nevertheless, these interventions require a sound knowledge of the biology and ecology of the vectors (11, 12).
In insect ecology, species diversity is one of the most significant aspects (13). It can be evaluated in a community in general by using the following three main types of measurements among the various indices and models that have been established (14): (1) species richness, which counts the total number of species in a given area or environment; (2) species abundance model, which shows how abundant each species is; and (3) indices based on proportionate abundances of species. In the latter category, there are numerous mathematical diversity indices but the commonly used ones are Shannon–Wiener and Simpsons indices, which aim to combine both richness and evenness into a single figure (15).
Many studies on the ecological distribution (12, 16–20) and seasonality of sand flies (21–24) showed that several species have distinct ecological and behavioral ranges of distribution in areas of recurrent leishmaniasis transmission. In general, sand fly distribution often relies on ecotype, climate, and season. In Cameroon, the only available data on the ecological site distribution of sand fly species found that the number of Phlebotomus duboscqi, Sergentomyia distincta, and Sergentomyia affinis vorax increases with human population density while others such as Sergentomyia coronula or Sergentomyia thomsoni mandarai are totally absent in inhabited environments (25). This scarcity of data in Cameroon compromises a better knowledge of the epidemiology of leishmaniasis and the implementation of an effective national program in the country (3).
Kousseri, a locality in Northern Cameroon, is no exception to this lack of data, although it has long been described as an endemic focus of visceral leishmaniasis (VL) (26, 27). Indeed, between 1987 and 1988, a survey conducted among 120 individuals, of whom 46 had clinical symptoms of VL, confirmed, parasitologically and/or serologically, that 9 of them have the disease (26). Another seroepidemiological survey conducted in 2001 using an indirect immunofluorescent antibody test on 223 healthy students living in Kousseri has revealed 9 students (4%) seropositive for VL (28). Because of the shortage of studies on the epidemiology of VL in Kousseri, the only available data on sand fly species composition dating back more than half a century are outdated (29). This earlier study reported five sand fly species, including Sergentomyia africana, Sergentomyia antennata, Sergentomyia bedfordi, Sergentomyia logonensis, and Ph. duboscqi, all collected in a toilet room and in a dwelling using sticky traps (29). Characterizing sand fly species composition in a leishmaniasis endemic region is merely not sufficient for implementing a control program. New ways for disease management and designing effective control strategies rely on investigating the sand flies’ ecology (12, 17). This knowledge can inform targeted interventions, such as insecticide spraying or the use of bed nets, to reduce the population of a particular species and minimize disease transmission. Additionally, it can aid in prioritizing areas for surveillance and intervention efforts, maximizing the impact of limited resources in endemic regions.
The present study was carried out with the overall objective of acquiring knowledge on the spatial distribution, seasonality, and ecological aspects of sand fly species in order to provide reliable entomological data needed for the development and implementation of successful control strategies against leishmaniasis in the northern region of Cameroon.
2 Materials and methods
2.1 Study area
This study was carried out in Kousseri town and its surroundings. Kousseri is located in the Far North Region of Cameroon (Figure 1A), Logone-et-Chari Division (Figure 1B). The city is located between latitude 12° 08 009” N and longitude 15° 03 236” E, at 317 m elevation above sea level. The town is bounded to the North and East by the Logone River, to the South by the Logone-Birni Subdivision, and to the West by the Makary Subdivision (Figure 1C). It covers a total area of about 160 km2 with a total population of about 101,246 inhabitants (30). The climate is of the semiarid Sahelian type with two seasons: a prolonged dry season lasting 8 months (from October to May) and a short rainy season lasting approximately 4 months (from June to September). The temperature fluctuates monthly, dropping to 11°C from December to February and rising to 45°C in the shade from April to May. The yearly rainfall ranges from 500 to 600 mm. A clear, shrubby, and thorny savannah dominates the area. Acacias (Nelotica senegalensis, Sigal, and Seyol), Borabus, and stunted shrubs are the dominating tree species (30). Animal husbandry, fishing, and agriculture are the main activities of the population. The selection of this city for the study is based on the fact that it was previously reported as a human VL focus in the country (26, 27, 31).

Figure 1 Map showing the sand fly collection sites in Kousseri. (A) Cameroon map showing the Far North Region. (B) Far North Region Division showing Logone-et-Chari. (C) Logone-et-Chari Subdivision showing Kousseri. (D) Kousseri sand fly sampling sites (A–E).
2.2 Selection of sand fly sampling sites
Five capture sites (Figure 1D) of three ecological areas comprising urban areas (sites A and E), semi-urban areas (sites B and C), and one rural area (site E) were selected in the town and its surroundings. The site selection criteria were as follows: (i) the level of urbanization and socio-economic status of the inhabitants; (ii) the presence of suitable environments for insect vector breeding (presence of animals, shaded, and humid shelters); (iii) ecotopes found across the area; and (iv) the accessibility, security, and availability of minimal accommodation facilities.
The geographic coordinates of each sampling site were recorded using a Garmin eTrex 12-channel handheld GPS (Garmin Ltd., Schaffhausen, Switzerland). The characteristics of each collection site are described in Table 1. A photograph of each site (Figure 2) was also taken using a digital camera.

Figure 2 Photographs of different sand fly collection sites. Urban (A, B); suburban (C, D); rural (E).
2.3 Processing sand fly sampling and morphological identification
Sand flies were collected monthly from September 2020 to August 2021 for five consecutive days using both miniature Centers for Disease Control and Prevention (CDC) black light (UV) traps (Model 1212, John W. Hock Co., Gainesville, FL) and incandescent light traps (BioQuip #2836BQ-6VDC, Rancho Dominguez, CA). The CDC black light traps mimic the natural UV light that sand flies are naturally drawn to, making them an effective tool for capturing these insects. In contrast, the incandescent light traps emit a different spectrum of light that may attract sand flies that are not as responsive to UV light (32). This combination of trap types helps in studying the population dynamics of sand flies more accurately and provides a better understanding of their behavior and distribution.
Prior to sand fly trapping, two compounds were randomly chosen for each collection site. In one of the compounds, two traps (one of each trap type) were displayed in the human dwellings (bedroom or parlor), and one black light trap outdoors (in the courtyard). In the other compounds, one black light trap was set in an animal shed, one incandescent light trap was set in an uninhabited house (including a ruined house or storeroom), and another incandescent trap was set outdoors (in the courtyard or hung under a tree depending on the situation). The purpose of this setup was to provide valuable insights into the preferred habitats and behaviors of these insects. A total of six traps (three traps per compound) were deployed during 360 trapping nights. These traps were installed from sunset (6:00 to 7:00 p.m.) to sunrise (5:00 to 6:00 a.m.) at about 50–80 cm above ground level.
Following each trapping night, the net box of each trap was kept at −20°C for at least 2 h. This freezing process was necessary to immobilize all trapped insects. Thereafter, the caught sand flies were immediately separated from other insects under a field dissecting microscope. Later, they were sexed, counted, and preserved in labeled cryogenic vial tubes containing 90° alcohol and transported to the Laboratory of the Leishmaniasis Research Project located at the Mokolo Annex Regional Hospital, Mayo Tsanaga Division, Far North Region, for subsequent dissection and identification.
2.4 Identification of sand flies
Only female sand fly specimens were dissected and identified since they are the only ones that require a blood meal source and consequently involved in disease transmission. Prior to morphologic identification, the head and genitalia of each female were dissected with microneedles and sterilized forceps and individually slide-mounted in Hoyer’s medium (Hemstead Halide TM). The slides were left to dry for at least 7 days at room temperature. Thereafter, the slides were carefully examined under a stereomicroscope (Olympus BX50) at 10–40× magnification, and identified based on the morphological characteristics of pharyngeal teeth, the cibarial armature of the head, and spermathecae features of the abdomen as described by Abonnenc and Minter (33) and Abonnenc (34).
2.5 Studies parameters
Sand fly populations were assessed by calculating composition and structural diversity indices. Species richness (total count of species collected in each area), sex ratio (the number of collected males divided by the number of collected females), density of the collection (number of sand flies per light trap per night), and abundance (number of individuals of each species in each area) of sand flies were recorded for each capture site, habitat, and season. The relative abundance (RA) was calculated by dividing the number of specimens of a given species (ni) by the total number of collected specimens (N) multiplied by 100 (35), and the species dominance structure was evaluated using Heydemann’s classification. This classification has five degrees of dominance: eudominant species—those that make up >30% of all the specimens caught; dominant species (10%–30%); subdominant species (5%–10%); rare species (1%–5%); and subrare species (1%) (36).
The degree of occurrence (Deg Occ) was computed by dividing the number of sites where sand flies were caught by the total number of sites studied to assess the distribution pattern of sand flies at a spatial scale according to the method adopted by Rydzanicz and Lonc (37). According to their occurrence value, sand flies were classified into five categories:
If 0< Deg Occ ≤ 20%, the distribution pattern of the species is sporadic (S).
20< Deg Occ ≤ 40%, the distribution pattern of the species is infrequent (IF).
40< Deg Occ ≤ 60%, the distribution pattern of the species is moderate (M).
60< Deg Occ ≤ 80%, the distribution pattern of the species is frequent (F).
80< Deg Occ ≤ 100%, the distribution pattern of the species is constant (C).
2.5.1 Ecological indices
Ecological diversity indices including the Shannon–Wiener index (), the Simpson index of diversity (), and Pielou’s evenness [] were assessed to characterize the sand fly population (14). In these formulas,
- N represents the total number of individuals in the sample,
- s is the total number of species in the sample,
- Pi is the proportion of the total sample belonging to the ith species, and
- , with ni being the number of individuals in taxon i.
2.6 Statistical analysis
All the collected data were transferred into a Microsoft Excel Office 365 spreadsheet and coded appropriately. The coded data were liable to descriptive analysis and results were expressed in percentages. Prior to statistical analysis, the Shapiro–Wilk test was applied to test the normality of variables (38). As the assumption of normality was not valid (p > 0.05), nonparametric Kruskal–Wallis and Dunn pairwise (used for multiple pairwise comparisons) tests were applied to test statistically significant differences in sand fly species RA. Accordingly, the Kruskal–Wallis test analysis was followed for comparing the RA of collected sand fly species among sites (A, B, C, D, and E), habitats (outdoors, animal shed, uninhabited/ruined house, and human dwellings), and seasons (dry and rainy). Differences in sex ratios was determined using the chi-square test (χ2). Non-metric multidimensional scaling (NMDS) based on Bray–Curtis dissimilarities was used to verify the similarity of species composition among the collection sites. All the statistical analyses were performed with the aid of R software version 4.2.0 (39) using the MASS package (for abundance data) and VEGAN package (for diversity data). A p-value of less than 0.05 (p< 0.05) was considered to be statistically significant.
To estimate sand flies’ richness and the adequacy of sampling efforts, species accumulation rarefaction and extrapolation curves generated by the iNEXT software (Online Version 2022) and expressed by the following formula were used.
where N = total number of individuals in the sample (40),
3 Results
3.1 Sand fly species composition and community structure
From September 2020 to August 2021, a total of 4,214 sand fly specimens, namely, 2,150 (51.02%) females and 2,064 (48.98%) males, were caught during 360 trapping nights. The mean captures account for 11.70 sand flies per night per trap (5.97 female sand flies per night per trap and 5.73 male sand flies per night per trap). Trapping success originated from the Kawagi capture site was represented by 152 sand flies (31 males and 121 females) from an animal shelter biotope and by 142 sand flies (64 males and 78 females) from the outdoor collection.
Only female sand flies were identified of morphological basis yielding 11 species from two genera of the OW: Sergentomyia (n = 2,127, 98.93%) and Phlebotomus (n = 23, 1.07%). Among the collected species, Sergentomyia (Sergentomyia) antennata was the most abundant and eudominant species (n = 875; 40.7%), followed by both dominant Sergentomyia (Sergentomyia) schwetzi (n = 482; 22.42%) and Sergentomyia (Grassomyia) squamipleuris (n = 279; 12.48%). These three species were the most prevalent, representing 76.1% of the collection. They also showed the greatest density compared to the other species (2.43, 1.34, and 0.78 ind/trap/night, respectively). Phlebotomus (Phlebotomus) duboscqi, the only representative of the Phlebotomus genus, was rare in the capture (n = 23; 1.07%) and densely sparse (density = 0.06 ind/trap night). The composition, number, percentages, density, and dominance structure of the other female sand fly species are presented in Table 2.
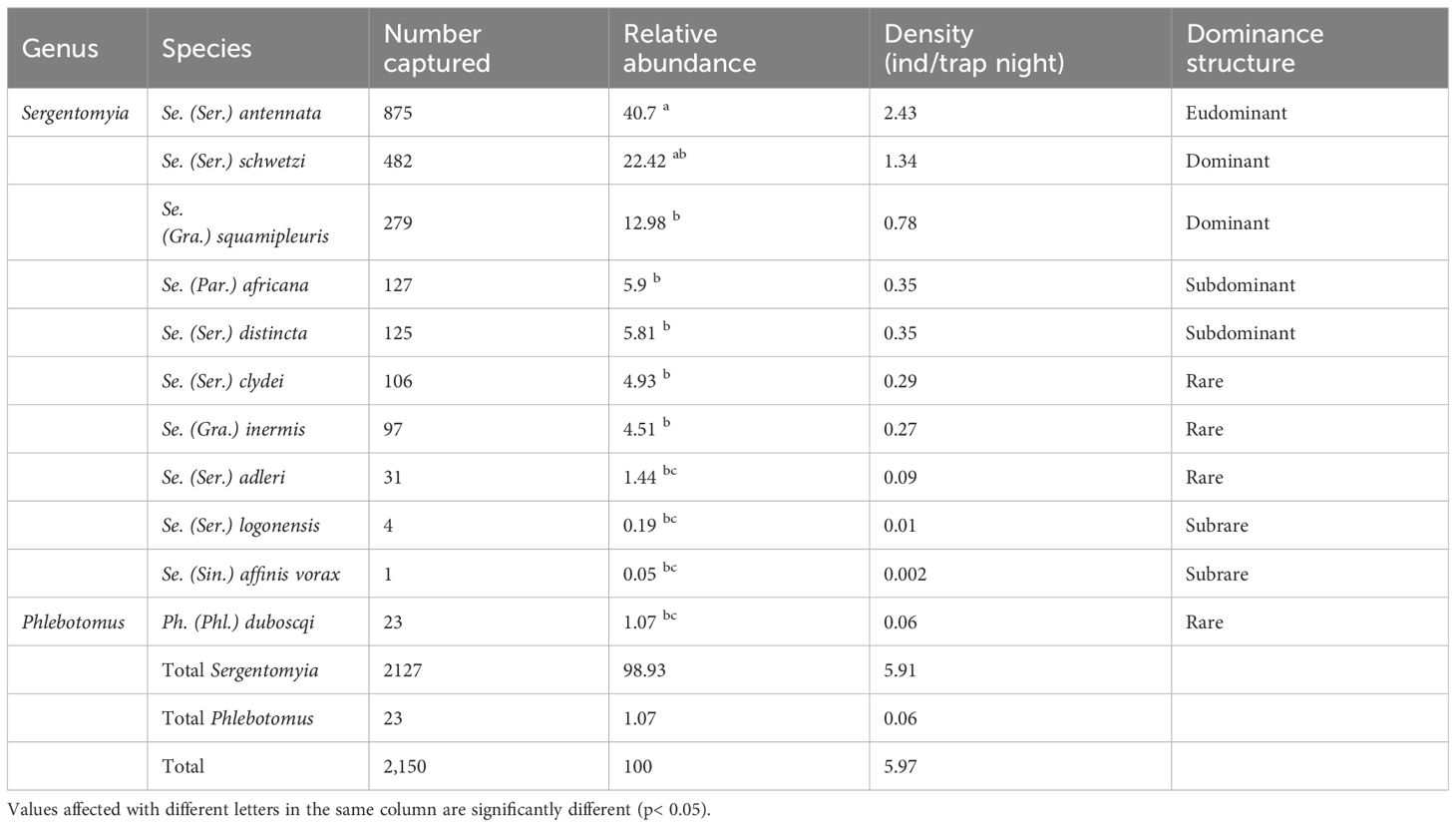
Table 2 Species composition, number, percentages, density, and dominance structure of female sand flies collected in Kousseri from September 2020 to August 2021.
3.2 Influence of sex ratio and abundance on the distribution of caught sand flies
Table 3 presents the male-to-female (M/F) sex ratio distribution and the abundance of sand flies using parameters such as capture site, collection area, biotopes, and season. Cumulatively, the M/F sex ratio was slightly in favor of females (1:1.04), although no significant differences between the overall sex ratio M/F of sand flies in this study were found (p > 0.05). More male sand flies were collected in three of the five surveyed sites, namely, sites A, C, and E, from outdoors and human-dwelling biotopes, and during the dry season, while female sand flies were more abundant than males in sites D and B, the rural and urban area, respectively. Animal sheds and uninhabited houses’ biotopes had the greatest number of females during the dry season. As aforementioned, sites A and E are urban with densely plastered human dwellings, while sites B and C are peri-urban settlements with unplastered dwellings and animal sheds in the compound. Site E with animal sheds and agricultural fields around the compound was the only site in the rural area. Overall, the highest number of sand flies was found in the rural area, outdoors, and during the dry season.
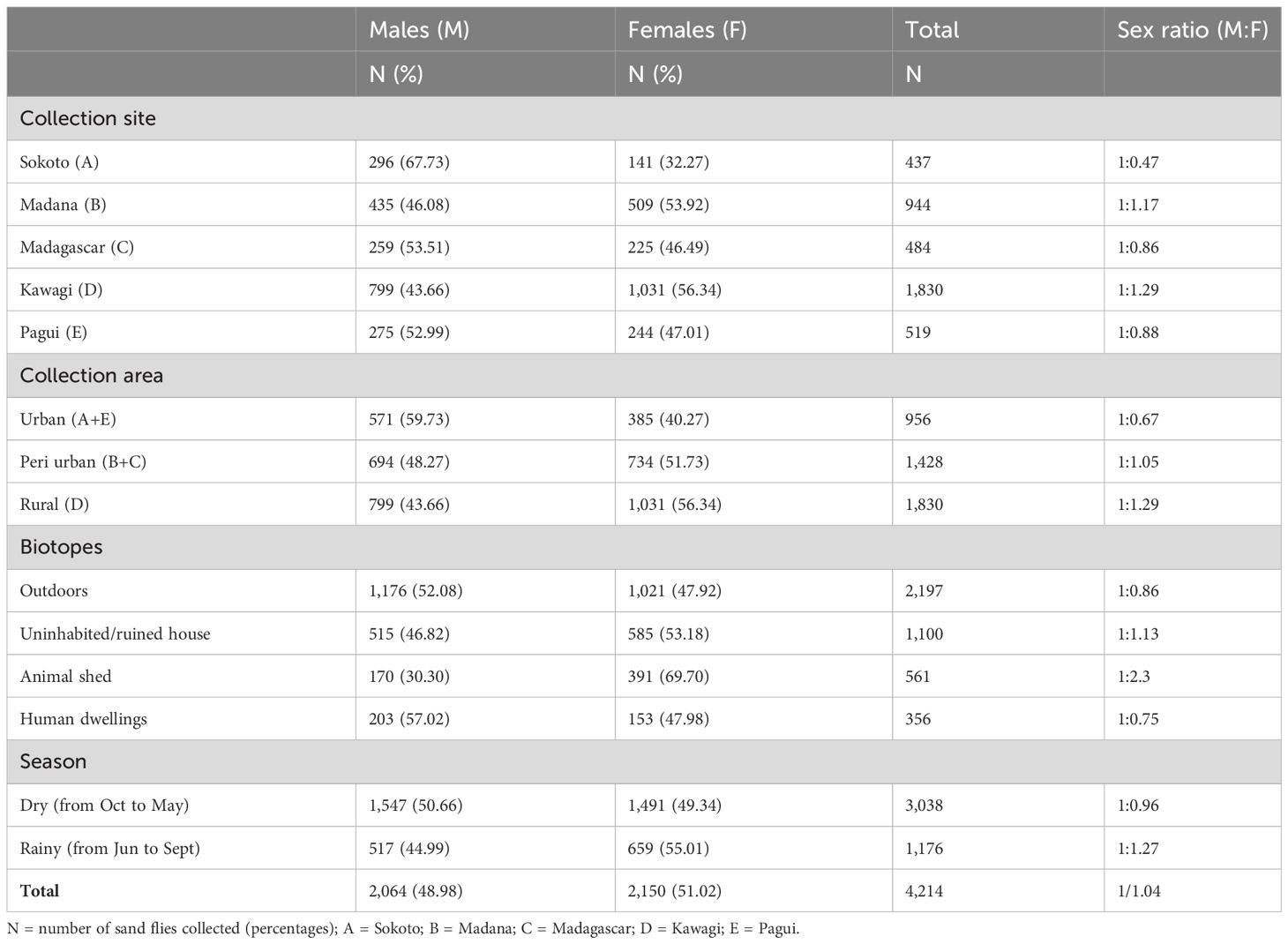
Table 3 Sex ratio distribution of caught sand flies according to collection site, area, biotopes, and season.
3.3 Influence of capture sites on female sand fly species distribution
Table 4 illustrates the RA, occurrence degree, and diversity indices of caught female sand fly species in the capture sites. Except for Sergentomyia adleri and Ph. duboscqi, which are frequent (Deg Occ = 80%), and both Sergentomyia logonensis and Sergentomyia affinis vorax, which are sporadic (Deg Occ = 20%) in captured sites, all other sand fly species in the surveyed sites were constant (Deg Occ = 100%), indicating their cosmopolitan behavior. Sergentomyia distincta in site A, Sergentomyia schwetzi in site B, and Se. antennata in sites C, D, and E were the most abundant sand fly species. Indeed, Se. distincta represents 35.46% of the captures in the site A versus only 0.97% in the rural area. It is clear that its relative number increased from urban to rural areas, suggesting that it is an urban species that might prefer humans as a blood source rather than animals. None of the sand fly species captured in site A showed a statistically significant difference (Kruskal–Wallis chi-square = 7.0321, df = 7, p-value = 0.4255). Se. schwetzi represents 54.74% of the catches in site B, a suburban site with densely populated and unplastered human dwellings, while representing only 7.47% and 22.95% in sites D and E, which are rural and urban sites, respectively. Statistically significant differences in the RA of caught sand flies were found in the species of this site (Kruskal–Wallis chi-square = 39.542, df = 9, p-value = 9.197e-06). Greatest proportions of Se. antennata and Ph. duboscqi were found in site C when compared to the other capture sites. Indeed, they represented 52.44% and 2.22% in this surveyed site versus only 18.86% and 0.68% in sites A and E, respectively. The sand fly species trapped in site C also showed statistically significant differences (Kruskal–Wallis chi-square = 15.911, df = 8, p-value = 0.043). Sergentomyia squamipleuris, Sergentomyia africana, Sergentomyia clydei, and Sergentomyia adleri showed their greatest proportion in site D compared to other captured sites, with their proportions decreasing from rural to urban areas, which showed that these species are well adapted to the rural areas where there is a wide range of domestic animal surrounding human dwellings. A statistically significant difference in the RA of caught species within site D was observed (Kruskal–Wallis chi-square = 31.376, df = 8, p-value = 0.00012). Finally, in site E, none of the sand fly species showed a relatively great number when compared to their proportions in other capture sites, but a significant difference was found among species (Kruskal–Wallis chi-square = 19.575, df = 8, p-value = 0.012).
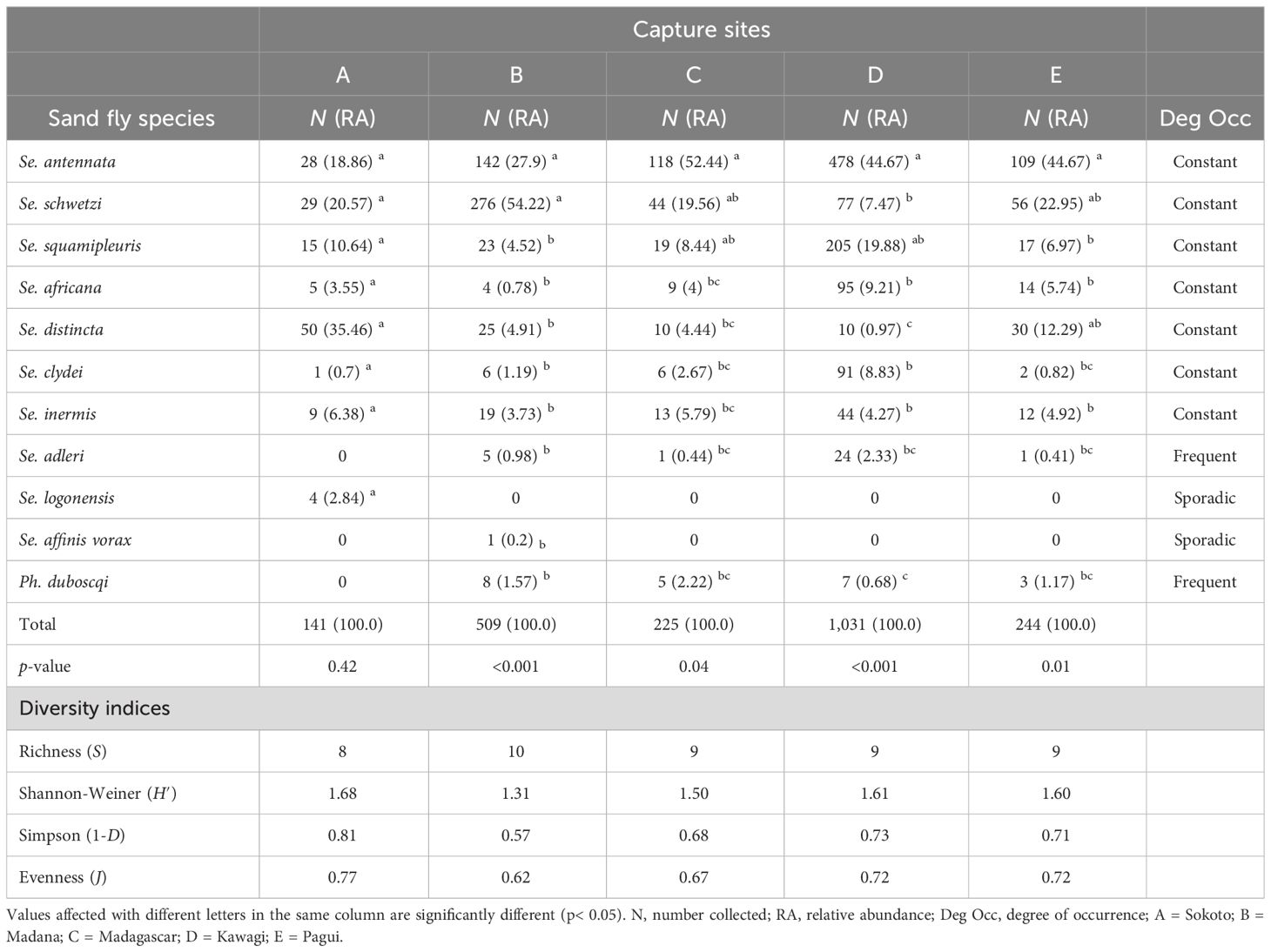
Table 4 Number, relative abundance, and occurrence degree of collected female sand fly species of Kousseri from September 2020 to August 2021 according to capture sites.
The species richness ranged from 8 to 10 species in the samples. The lowest species richness but highest values of Shannon index, evenness, and Simpson index of diversity were found in site A (S = 8 species; H′ = 1.65; J = 0.65, and 1-D = 0.75, respectively) followed by site D (H′ = 1.59, J = 0.55, and λ =0.72 respectively), while the highest value of species richness but lowest diversity indices were found in site B (S = 10 species, H′ = 1.36, J = 038, 1-D = 0.63).
The rarefaction curve (Figure 3) shows the stability of the number of species in each sample (the horizontal axis shows the number of specimens and the vertical axis shows the number of expected species yielded). The curves indicate reaching the asymptotic line and tend to stabilize with 10 species in Madana. In Sokoto, both species richness and abundance were lower (S = 8 species; N = 141). In Madagascar, Kawagi, and Pagui, species richness was the same (S = 9 species), but higher abundance was found in Kawagi (N = 1,031). More sampling efforts are likely to be required in Sokoto to increase the richness (Figure 3).
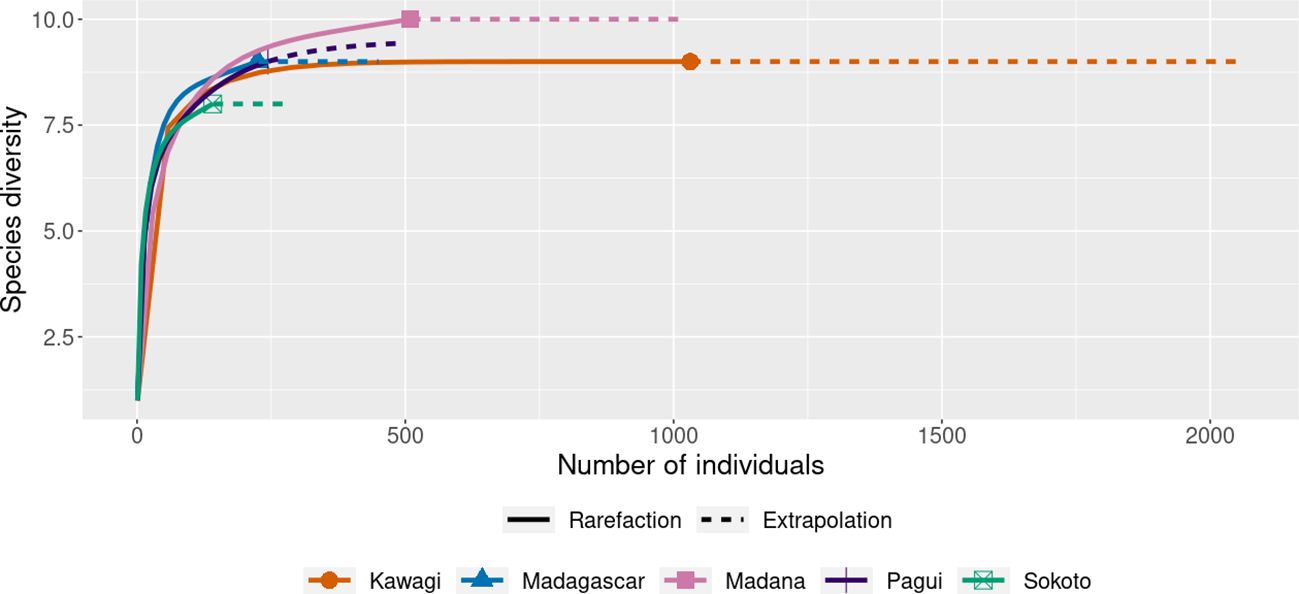
Figure 3 Rarefaction curve at 95% confidence interval, based on species richness in captured sites. Solid line = observed species (rarefaction); dashed line (extrapolation species richness).
The NMDS plot of the sample sites is shown in Figure 4. The Bray–Curtis dissimilarity is reflected in the distance between each point, which represents a site. Given their dispersion across the ordination space, the figure implies that the species composition of site A differs significantly from that of other capture sites. The resulting stress of the NMDS ordination (stress = 0) indicates an excellent representation of the original distance matrix. In other words, the distances between points on the plot closely reflect the actual dissimilarities between the sites.
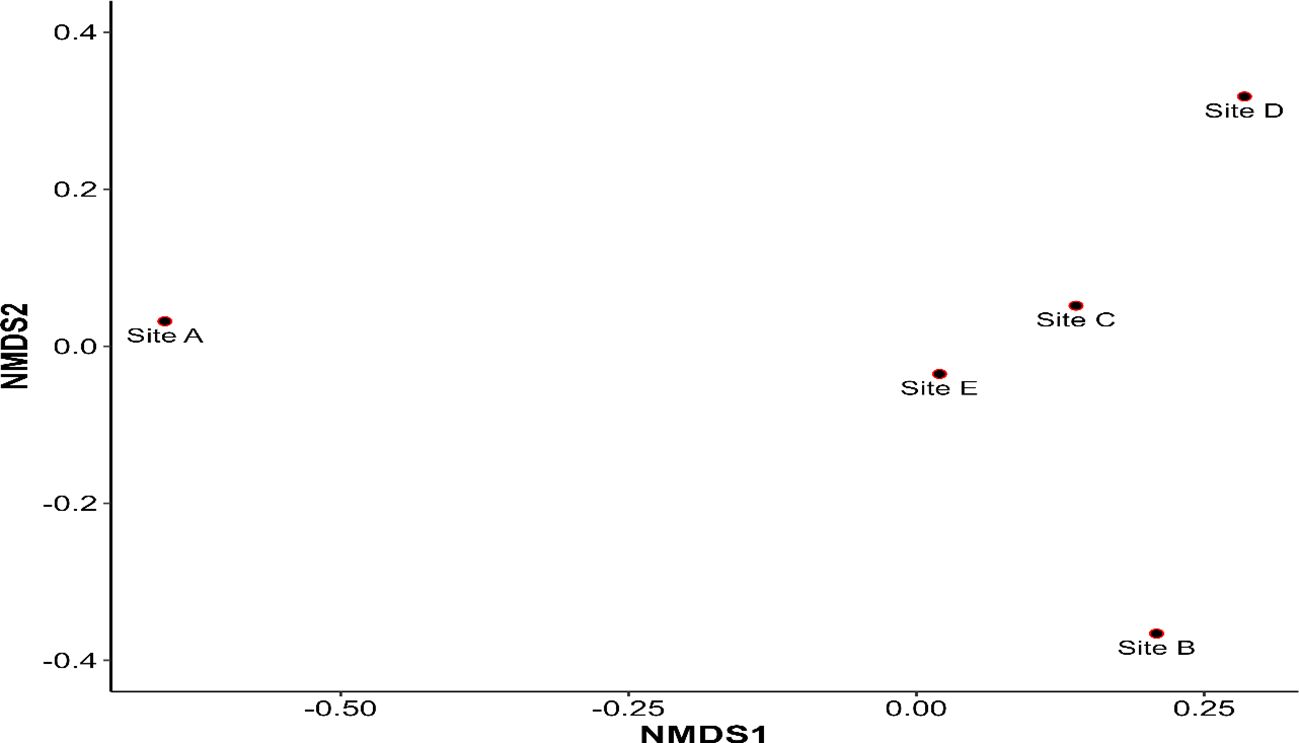
Figure 4 A non-metric multidimensional scaling (NMDS) plot on Bray–Curtis dissimilarities showing the female sand fly species composition between capture sites in Kousseri.
3.4 Influence of biotopes on female sand fly species distribution
RA of caught female sand flies varies with biotype. Se. antennata was the most abundant species in outdoors, human dwellings, and animal sheds, while Se. schwetzi was dominant in uninhabited houses (Table 5). Se. squamipleuris, Se. clydei, Se. inermis, and Se. adleri showed their greatest relative number from outdoors as they represent, respectively, 23.21%, 7.84%, 7.34%, and 2.15% of the outdoor catches in comparison to their lower proportions in animal sheds, uninhabited houses, and human dwelling biotopes. All sand fly species collected from outdoors showed significant statistical differences (Kruskal–Wallis chi-square = 23.447, df = 9, p-value = 0.005268). Se. schwetzi, mostly found in uninhabited houses, accounts for 45.64% of catches, while Se. africana and Se. distincta were the most anthropophilic species due to their higher indoor human dwelling proportions, which account for 8.5% and 17.65%, respectively, in comparison to their uninhabited houses’ proportions and animal sheds, which account for 2.05% and 1.28, respectively. A statistically significant difference was found in sand fly species’ RA collected from uninhabited/ruined houses (Kruskal–Wallis chi-square = 33.095, df = 9, p-value = 0.0001) and human dwellings (Kruskal–Wallis chi-square = 19.649, df = 8, p-value = 0.01175). Se. antennata and Ph. duboscqi both prevailed in animal sheds, accounting for 60.61% and 1.79% of the captures, respectively, while only 35.65% and 0.69% were collected outdoors, respectively. A statistically significant difference was also found in sand fly species collected in animal sheds (Kruskal–Wallis chi-square = 18.095, df = 8, p-value = 0.020). Se. schwetzi accounts for 45.64% of the catches from uninhabited houses while representing only 10.93% of the outdoor collection. A statistically significant difference was also found in sand fly species collected from uninhabited/ruined houses/storerooms (Kruskal–Wallis chi-square = 33.095, df = 9, p-value = 0.0001) and human dwellings (Kruskal–Wallis chi-square = 19.649, df = 8, p-value = 0.01175).
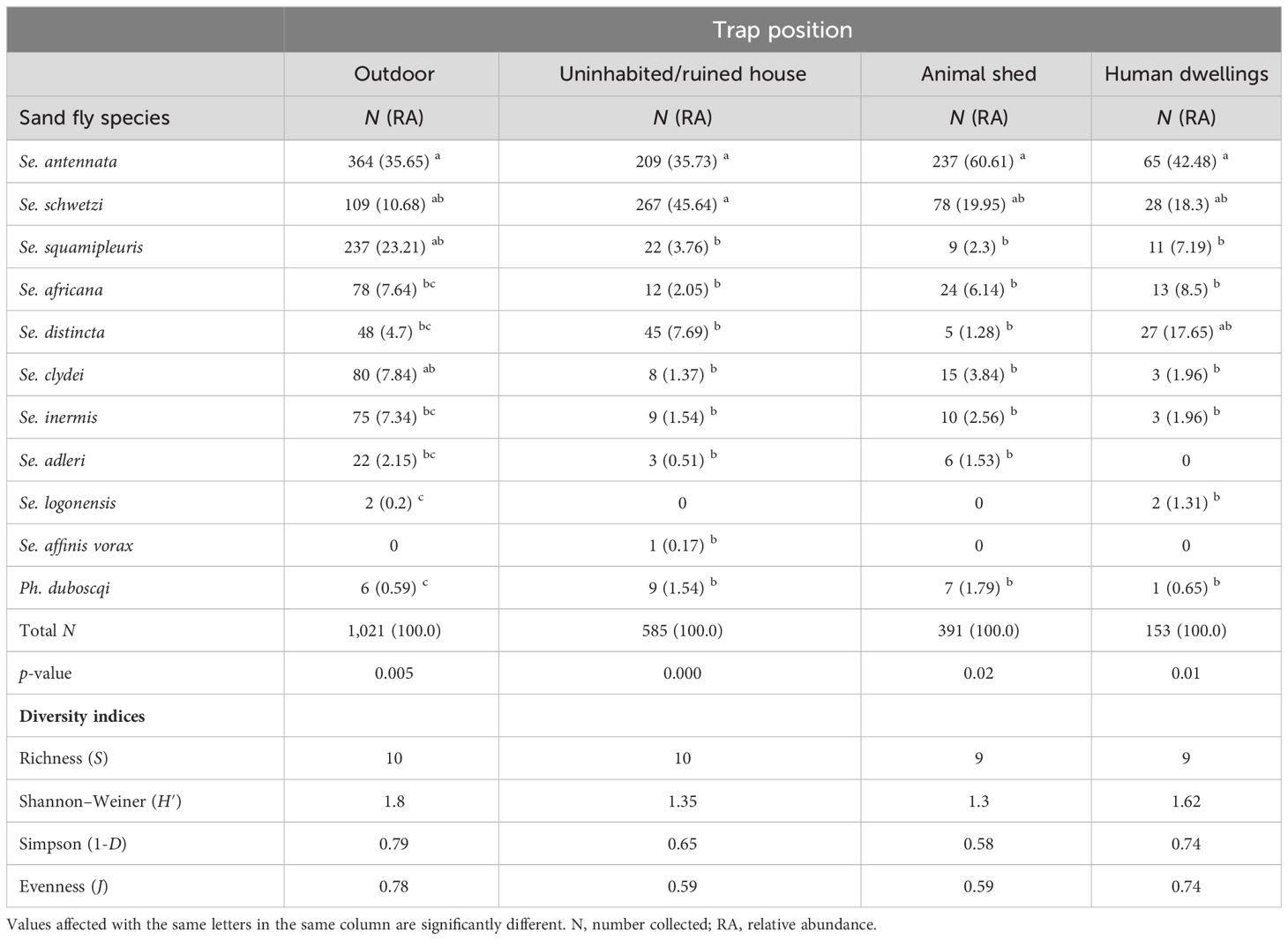
Table 5 Number, relative abundance, and diversity estimates of female sand fly species according to trap position.
The species richness was slightly higher (S = 10 species) from outdoors and in uninhabited house biotopes than from animal sheds and from human dwellings (S = 9 species). Highest species diversity indices were found in outdoors (H′ = 1.80, 1-D = 0.79; J = 0.78), followed by human dwellings (H′ = 1.62; 1-D = 0.74, J = 0.74), indicating the highest species diversity and an even species distribution. However, the lowest species diversity due to the strong dominance of Se. schwetzi and Se. antennata was recorded in the animal sheds (H′ = 1.3, 1-D = 0.58 and J = 0.59).
3.5 Influence of seasons on female sand fly species distribution
According to Table 6, Se. schwetzi, Se. clydei, Se. inermis, and Se. adleri were mostly caught in the rainy season with the greatest RA as they represented 35.20%, 8.95%, 6.37%, and 2.28%, respectively, of the total sand fly caught against only 16.78%, 3.69%, 3.69%, and 1.07% captured during the dry season. Other sand fly species including Se. antennata, Se. squamipleuris, Se. africana, Se. distincta, and Ph. duboscqi were mostly collected during the dry season with significant differences (Kruskal–Wallis chi-square = 33.789, df = 9, p-value =0.000). Se. antennata was the most abundant species captured during the dry season and represented 46.54% (n = 694) of the catches followed by Se. schwetzi, which accounts for only 14.02%. In addition, Ph. duboscqi accounted for 1.34% (n = 20) in the dry season and only 0.46% (n = 3) in the rainy season. A few species such as Se. logonensis and Se. affinis vorax were collected in extremely low RA in this study in only one season each. All sand fly species collected in this survey cannot be treated as true seasonal species even with their presence in only one season or with their RA higher in one season compared to the other. Statistically significant differences in sand fly species’ RA were also observed during the rainy season (Kruskal–Wallis chi-square = 19.107, df = 9, p-value = 0.0243).
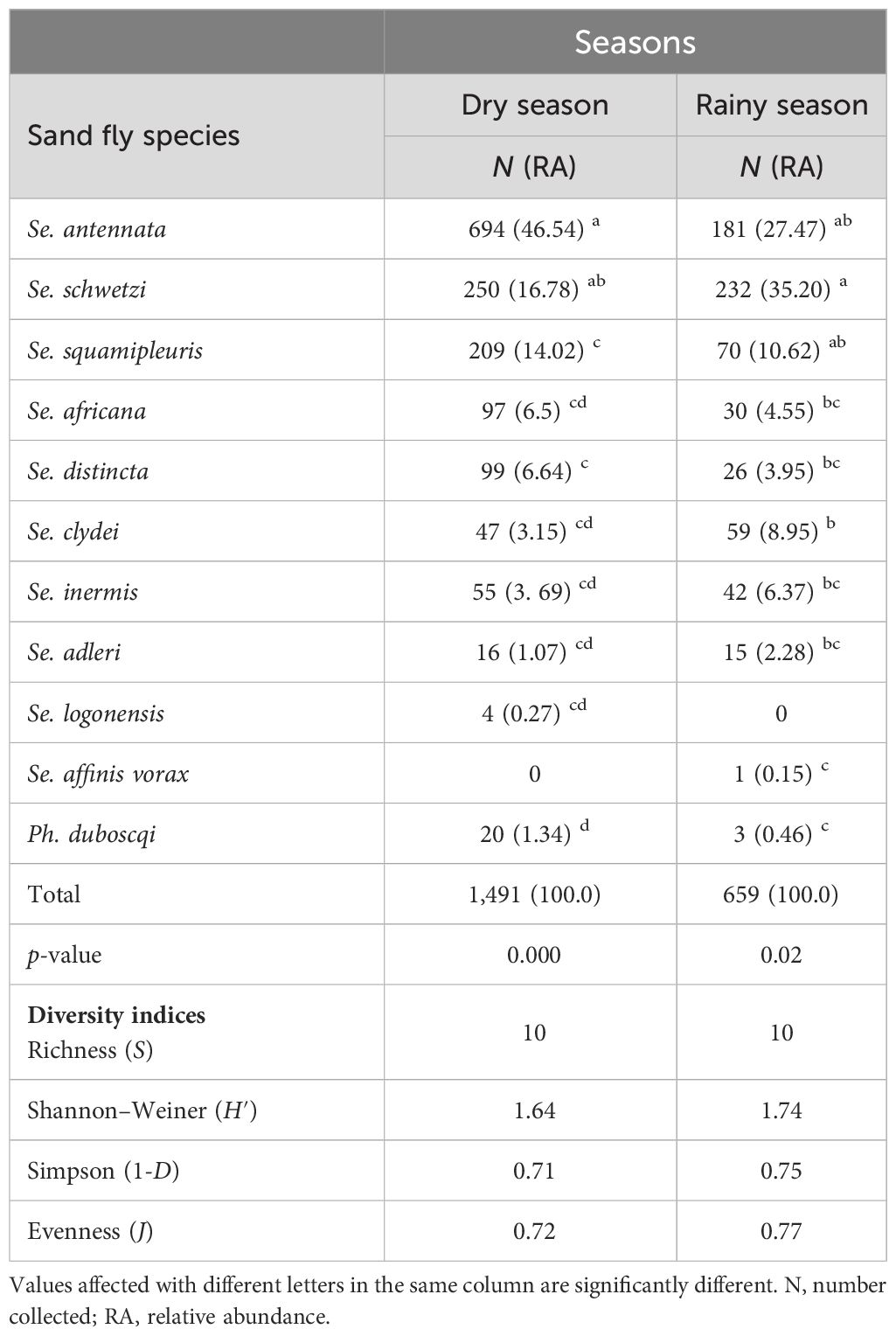
Table 6 Number, relative abundance, and diversity estimates of collected female sand fly species collected in Kousseri according to seasons.
The species richness was the same in both seasons (S = 10 species). The Shannon diversity, Simpson dominance, and evenness indices were slightly higher in the rainy season in comparison to the dry season (H′ = 1.74; 1-D = 0.75, J = 0.77 and H′ = 1.64; 1-D = 0.71, J = 0.72, respectively), emphasizing a slightly high diversity and an even distribution of species during rainy seasons due to the strong dominance of Se. antennata during the dry season, representing approximately 47% of the capture.
4 Discussion
Understanding the ecological behavior of sand fly species at a spatial and temporal scale, including diversity, distribution pattern, resting site, and seasonality, is critical for estimating the possible risk of leishmaniasis transmission and executing any control strategy. Although Kousseri has long been considered an endemic focus of VL in Cameroon (26, 28), inconsistent attention has been paid to the sand fly species involved in the transmission of the disease (29). The present study was carried out to fill this information gap.
Even though the recently published checklist of sand fly composition in Cameroon has documented 32 species and subspecies belonging to four genera of the Old World (OW), namely, 3 Phlebotomus, 1 Spelaeophlebotomus, 2 Grassomyia, and 26 Sergentomyia species (41), only 11 sand fly species, namely, 10 Sergentomyia and only 1 Phlebotomus, were identified in the present work, accounting for less than half the species reported in the country. The predominance of the genus Sergentomyia (n = 2,127, 98.93%) over the genus Phlebotomus (n = 23, 1.07%) agrees with the fact that it is usually rich in number and species diversity in tropical areas and often overwhelms Phlebotomus species (2, 42). This observation corroborates earlier findings in Cameroon (29, 31, 41, 43), Senegal (44), Mali (45), Ghana (46), and Chad (19). The present study found that Se. antennata was the most abundant and eudominant species, followed by both dominant Se. schwetzi and Se. squamipleuris, as they constituted 76.1% of the captures. This observation is in line with earlier findings in the country, which presented at least two of these sand fly species as the most abundant (31, 41). According to our results, Ph. (Phl.) duboscqi was the only representative of the Phlebotomus genus and was rare in the capture. Our findings confirm that species of this genus are less abundant in the sub-Saharan region where the Sergentomyia fauna is more abundant and diverse (42). Low percentages of Ph. duboscqi have been recorded in other entomological studies carried out in Cameroon (25, 31, 41) and other sub-Saharan countries (19, 44, 45, 47).
There was no statistically significant difference (p > 0.05) in the overall male-to-female sex ratio in this study, but females slightly predominated (1:1.04). Similarly, a sex ratio in favor of females of 1:1.1 and of 1:1.25 was also found in Mokolo cutaneous leishmaniasis (CL) focus (41) and in the Vale do Ribeira Region, Sao Paulo State, Brazil (48), respectively, while using the same trapping method. However, in other entomological studies, males predominate in sticky trap collection (31) or CDC trap (18, 49). The differences in sex ratio between these studies varied from one county to another and may be due to the climatic conditions and the trapping method. The high abundance of female sand flies rather than males observed in peri-urban and rural areas, and in animal sheds and uninhabited houses’ biotopes could be justified by the high dispersal ability of females, which are hematophagous and may be easily attracted by the light of the trap when seeking for host and mates, and searching for oviposition sites (50, 51). However, because male sand flies have a low dispersal ability (2, 42), their high abundance in urban areas and in human dwellings could suggest that this could be their breeding site. This finding is supported by Alexander (32) who found that M/F aggregations in microhabitats represent mating leks, where males intercept females descending from higher up to lay eggs in the organic litter, and by Gijón-Robles et al. (35) who reported specimens in all gonotrophic conditions inside houses.
The results found that most of the female sand fly species, namely, Se. africana, Se. antennata, Se. distincta, and Se. squamipleuris, were ubiquitous. This finding corroborates Tateng et al.’s (25) study in Mokolo and provides evidence that these sand fly species may be widely distributed independently of altitude and landscape, Mokolo being a rocky mountainous land compared to the low land of Kousseri. The absence of Ph. duboscqi and Se. adleri in certain capture sites including Sokoto, which is an urban site, could not necessarily mean that they do not exist there but could merely suggest that they may have other breeding sites than the one surveyed. The results of the current study also show that the ecotypes found across sampling areas and the level of urbanization influence the abundance of sand fly species. For instance, the RA of Se. distincta decreases from urban to rural areas. This result is consistent with Tateng et al.’s (25) findings in the Mokolo CL focus, which reported that Se. distincta, Se. vorax, and Se. schwetzi decreased from town to the sylvatic area and could take advantage of human and farmyard animals for their blood meal. Furthermore, Se. antennata, Se. clydei, Se. adleri, Se. africana, and Se. squamipleuris were mostly found in rural settlements, with their proportions decreasing from rural to urban areas. The existence of vegetation, rice paddies, and animal sheds in rural areas could provide both sugar as an energy source and blood meal for egg maturation for adult female oviposition and may explain the high abundance of these sand fly species compared to other capture sites. This result is not in agreement with Tateng et al. (25) who found high proportions of Se. antennata, Se. squamipleuris, and Se. africana in sylvatic environments, and relatively abundant Se. clydei and Se. adleri in suburban areas. Additionally, high proportions of Se. schwetzi and Ph. duboscqi observed in peri-urban areas could suggest a higher host vector exposure in this area compared to other regions. Overall observations prove that the dispersion of sand flies is much reduced, and the species composition and abundance can change from one ecotype to another close by, highlighting the importance of understanding the specific ecological factors that contribute to the distribution and abundance of female sand fly species in different settlements. Only female sand flies were identified in the current study and subjected to the analysis of the distribution of species, which may constitute a bias to our study, as the presence of male sand flies could potentially impact the overall distribution patterns observed. Future research could benefit from including both male and female sand flies in order to provide a more comprehensive understanding of species distribution.
The current study also found the preponderance of Se. squamipleuris, Se. clydei, Se. inermis, and Se. adleri from outdoor biotopes; Se. schwetzi from an uninhabited house; Se. distincta in human dwellings; and Se. antennata and Ph. duboscqi in an animal shed. This result shows that most of the sand fly species collected in Kousseri are exophilic while very few are anthroponotic. Our results differ from Dondji et al.’s (31) findings, which reported the preponderance of Se. antennata in three biotopes including human dwellings, termite mounds, and tree holes in Mokolo with the sticky trap collection. This finding also differs with Tateng et al. (25) who found a high proportion of Se. antennata, accounting for 60.10% of the catches outdoors in the light trap collection. These contrasting findings suggest that the distribution and behavior of sand fly species can vary across different regions and collection methods. Further research is needed to understand the factors influencing these differences. In addition, more studies are necessary to determine the implications of these variations for disease transmission and control. A high proportion of Se. antennata and Ph. duboscqi from animal sheds is consistent with Hassaballa et al. (17) in Kenya. This result suggests evidence that few species of sand flies may be attracted to a particular animal host species, or group, while most others tend to be opportunistic rather than specialists in their host specificity as reported by Mutinga et al. (52) in Kenya who reported an attraction of Ph. duboscqi and Phlebotomus martini to goats. Our findings differ from a study carried out by Israël et al. (53) in Chad who reported high percentages of Ph. duboscqi (76.9%) inside human houses and by Tateng et al. (25) in the Mokolo CL focus where it has been reported as one of the most anthropophilic species. The low percentages of Ph. duboscqi in human dwellings recorded in this study could suggest difficulties in access to human blood meal sources compared to animal sheds where they can easily feed on a wide range of domestic animals such as goats, sheep, dogs, and chickens predominantly found in the study area. This preference for animal hosts over humans potentially reduces the risk of disease transmission to people living in these areas. It is possible that the adoption of protective measures, such as insecticide-treated bed nets or the use of repellents, may have contributed to the lower prevalence of Ph. duboscqi and any other sand fly species in human dwellings.
Ph. duboscqi, the proven vector of CL in sub-Saharan foci due to Leishmania major transmission (54, 55), is the most likely vector of VL in this study. This result is supported by Tateng et al. (56) who recently detected Leishmania donovani DNA, the causative agent of VL, in the Mokolo CL focus. It is crucial to investigate how a seemingly rare sand fly manages to keep Leishmania transmission cycles in the Kousseri VL endemic focus. Nowadays, there is growing skepticism about the dogma that attributed Phlebotomus genus as the sole putative vector of leishmaniasis in the OW (57). Indeed, Sergentomyia sp. have been found to be infected with Leishmania, which is a public health concern. Examples include Se. schwetzi and Sergentomyia dubia as potential vectors of Leishmania infantum, the causative agent of canine leishmaniasis (CnL) (47). Moreover, Leishmania major, Leishmania donovani, and Trypanosoma DNA were detected in Se. squamipleuris in the Merti sub-county in eastern Kenya (58). Se. clydei was also found to be infected with Le. major in Tunisia (59). Se. schwetzi, Se. squamipleuris, and Se. clydei could play a secondary role in disease transmission in Kousseri. A surveillance is absolutely required to prevent spreading cases of leishmaniasis from neighboring countries, Chad (10) and Nigeria (60), where cases of CnL, VL, or CL have been reported. It is also important to note that the occurrence of CnL has been documented without parasitological confirmation in the northern region of the country by the Ministry of Livestock and Husbandry (61). Keeping this in mind, investigating the blood meal sources in these sand fly species and their role in the transmission of VL is therefore necessary. These findings could challenge the traditional understanding of Leishmania transmission and highlight the need for further research to fully understand the role of sand flies in the spread of the disease. Additionally, studying the transmission cycles in Kousseri VL endemic focus can provide valuable insights into potential alternative vectors and their impact on public health.
The seasonal dynamics of female sand fly species illustrated that Se. schwetzi, Se. clydei, Se. inermis, and Se. adleri were mostly caught in the rainy season, while the other sand fly species predominated during the dry season. This result is similar to Tateng et al. (25) in the Mokolo CL focus who also found a higher proportion of Se. antennata and Se. distincta in the dry season and of Se. affinis vorax, Se. schwetzi, and Se. clydei in the rainy season. In the current study, Ph. duboscqi represents 1.34% of the catches in the dry season, versus only 0.46% in the rainy season. This result also differs from Tateng et al. (25) who reported a slight preponderance of this sand fly species in the rainy season compared to the dry season with 0.90% and 0.70%, respectively, of total sand flies caught. Both Mokolo and Kousseri are characterized by the Soudano Sahelian climate. Several factors including temperature, precipitation, and relative humidity can also affect the seasonal distribution of sand flies (23, 24). The seasonal analysis of sand flies over 12 months may be insufficient to investigate the influence of the aforementioned factors on the distribution of species and may constitute a limitation of this survey. However, the limited duration of the study may explain at a certain level the capture of rare or sporadic species that could impact the dynamics of sand flies. Nevertheless, despite this limitation, this study still offers insightful information concerning the seasonal patterns of sand fly populations, which can serve as a foundation for future studies that aim to further investigate the climatic influences on sand fly populations over a much longer period. Even though Se. logonensis and Se. affinis vorax are present only in one season, they cannot be considered as seasonal species due to their relatively low proportion. In Paloich in Sudan, Phlebotomus orientalis, Phlebotomus heischi, Phlebotomus papatasi, Se. clydei, and Se. schwetzi are present only in the dry season and considered as seasonal (21).
A broad finding of this study is the highest Shannon index recorded in the urban area, outdoors, and during the rainy season (H′ = 1.68, 1.80, and 1.74, respectively). This index measures the degree and level of complexity of a population. In general, a high value of this index indicates a well-balanced and diversified ecosystem where there are many species, with none of the species dominating (14). Despite the large interval of this index (H′ = 0–10), which usually ranges from 1.5 to 3.5 and rarely beyond 4.5 (15), in this study, it ranged from 1.3 to 1.8, indicating a relatively high diversity. According to Ezenwa et al. (62), high biodiversity reduces the risk of vector-borne diseases and increases ecosystem resilience to disturbances such as climate change. Conversely, a low species diversity typically leads to a loss of the buffering effect of infectious diseases on humans, which favors the emergence and reemergence of vector-borne diseases (63). The lower species diversity in semi-urban and rural areas in comparison with urban areas can be attributed to the specific ecological conditions found in these areas, which may favor the growth and proliferation of dominant Se. antennata and Se. schwetzi over other species that have adapted to thrive in these environments. The highest values of the Shannon, Simpson, and evenness indices (H′ = 1.68, 1-D = 0.77, J = 0.73, respectively) due to a more diverse and even species composition were recorded in the urban area, while the lowest values of these indices were found in the semi-urban area (H′ = 1.43, 1-D = 0.68, J = 0.62). This result may be associated with the predominance of Se. schwetzi, which is a eudominant species in suburban ecotypes (Table 4). Our results also reported a higher Pielou’s index (J) in both seasons (rainy: 0.76; dry: 0.71). Indeed, J is strongly influenced by climate variation and environmental changes. When its value is high, there are no dominant species, and the dominance index (1-D) is also high. The correspondent 1-D values (rainy: 0.77; dry: 0.72) indicated that the sand fly community in this microenvironment is evenly distributed in both dry and rainy seasons. This result suggests that the sand fly population is resilient and able to maintain a relatively balanced distribution of species abundance regardless of the season. It also implies that environmental factors such as temperature, relative humidity, and rainfall do not significantly impact the distribution of sand flies in Kousseri. Further research is needed to understand the underlying factors driving these differences and their implications in sand fly diversity.
5 Conclusion
Our study provides important knowledge on sand fly ecological distribution in Kousseri. Overall, our data showed that less urbanized areas, the dry season, and an outdoor environment favor the distribution of abundant sand fly species. This study also found Ph. duboscqi as the only known vector of leishmaniasis in Kousseri. Although rare in the sample, this sand fly species was caught in four of the five surveyed sites. Therefore, Kousseri should be at risk of Leishmania transmission. Solely female sand flies were identified in this study, which might constitute a bias and a limitation in accurately assessing the full extent of sand fly population dynamics. The data reported herein provide valuable information for the epidemiological surveillance of leishmaniasis and can serve as a baseline for extensive future entomological work in Kousseri. Thus, further studies should be undertaken including male sand flies in the sampling process to provide a more comprehensive understanding of the population dynamics. Additionally, investigating sand fly blood meal sources and reservoir hosts, and detecting Leishmania parasites in caught female specimens could help further assess the risk of Leishmania transmission. The data of such studies could contribute to a better assessment of the epidemiology of the disease and consequently provide additional data required for the development and implementation of a control program against leishmaniasis in Northern Cameroon as a whole and in Kousseri in particular.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
TRT-N: Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – original draft. TNA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. OBN: Conceptualization, Formal analysis, Funding acquisition, Investigation, Writing – review & editing. YC: Data curation, Methodology, Writing – review & editing. BR: Writing – review & editing. EE: Conceptualization, Investigation, Methodology, Resources, Writing – review & editing. CN: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing. VK-P: Methodology, Supervision, Writing – review & editing. DB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the PIIVec (Partnership for Increasing Impact of Vector Control) Operational Research Project (PV/OP2-02/TA) under the MRC grant MR/PO27873/1 for field study and Laboratory analysis.
Acknowledgments
We are thankful to the PIIVec and the Center for Research in Infectious Diseases (CRID) for awarding TNA grant fellowship to fund field and laboratory work. We are also incredibly grateful to the families/community in the Kousseri area and their chiefs for their assistance and consent to work in their compounds.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Akhoundi M, Kuhls K, Cannet A, Votýpka J, Marty P, Delaunay P, et al. A historical overview of the classification, evolution, and dispersion of leishmania parasites and sandflies. PloS Negl Trop Dis. (2016) 10:e0004349. doi: 10.1371/journal.pntd.0004349
2. Depaquit J, Léger N. Les phlébotomes (Diptera : Psychodidae : Phlebotominae). In: Duvallet G, Fontenille D, Robert V, editors. Entomologie Médicale et Vétérinaire. IRD Éditions, Marseille, vol. pp (2017). p. 295–320.
3. Ngouateu OB, Dondji B. Leishmaniasis in Cameroon and neighboring countries : An overview of current status and control challenges. Curr Res Parasitol Vector-Borne Dis. (2022) 2:100077. doi: 10.1016/j.crpvbd.2022.100077
4. Kimutai A, Ngure PK, Tonui WK, Gicheru MM, Nyamwamu LB. Leishmaniasis in northern and western Africa: A review. Afr J Infect Dis. (2009) 3:14–25. doi: 10.4314/ajid.v3i1.55077
5. Gillespie PM, Beaumier CM, Strych U, Hayward T, Hotez PJ, Bottazzi ME. Status of vaccine research and development of vaccines for leishmaniasis. Vaccine. (2016) 34:2992—2995. doi: 10.1016/j.vaccine.2015.12.071
6. Burgos-reyes MA, Baylón-pacheco L, Espíritu-gordillo P, Galindo-gómez S, Tsutsumi V, Rosales-encina JL. Effect of prophylactic vaccination with the membrane-bound acid phosphatase gene of leishmania Mexicana in the murine model of localized cutaneous leishmaniasis. J Immunol Res. (2021) 2021:1–13. doi: 10.1155/2021/6624246
7. Sundar S, Rai M. Advances in the treatment of leishmaniasis. Curr Opin Infect Dis. (2002) 15:593–8. doi: 10.1097/00001432-200212000-00007
8. Croft SL, Sundar S, Fairlamb AH, Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. (2006) 19:111–26. doi: 10.1128/CMR.19.1.111
9. Hefnawy A, Berg M, Dujardin J, De Muylder G. Exploiting knowledge on leishmania drug resistance to support the quest for new drugs. Trends Parasitol. (2017) 33:162–74. doi: 10.1016/j.pt.2016.11.003
10. Desjeux P. Information on the epidemiology and control of the leishmaniases by country or territory. World Health Organization (1991). Available at: http://libdoc.who.int/hq/1991/WHO_LEISH_91.30.pdf.
11. OMS. Lutte contre la leishmaniose. In: Cent Dix-Huitième Session Point 5.1 de l’ordre Du Jour Provisoire, EB118/4 (2006). p. 1–7.
12. Pereira NCL, Michalsky É.M, Lara-Silva FO, Lana RS, de Paula AJV, Pereira DM, et al. Ecology of phlebotomine sand flies in a Brazilian area with recent leishmaniasis transmission (Itaúna, in Minas Gerais state). Rev Da Sociedade Bras Med Trop. (2020) 53:e2019053. doi: 10.1590/0037-8682-2019-0538-2019
13. Gaggiotti OE, Chao A, Peres-Neto P, Chiu CH, Edwards C, Fortin MJ, et al. Diversity from genes to ecosystems: A unifying framework to study variation across biological metrics and scales. Evolution Appl. (2018) 11:1176–93. doi: 10.1111/eva.12593
14. Magurran AE. Ecological diversity and its measurement. Springer-Science+Business Media, B Y, Malden, Massachusetts (1988), 1–179. doi: 10.1007/978-94-015-7358-0
16. Senghor MW, Faye MN, Faye B, Diarra K, Elguero E, Gaye O, et al. Ecology of phlebotomine sand flies in the rural’s region, Senegal): community of mont rolland (Thies region, Senegal): area of transmission of canine leishmaniasis. PloS Negl Trop Dis. (2011) 6:e14773. doi: 10.1371/journal.pone.0014773
17. Hassaballa IB, Torto B, Sole CL, Tchouassi DP. Exploring the influence of different habitats and their volatile chemistry in modulating sand fly population structure in a leishmaniasis endemic foci, Kenya. PloS Negl Trop Dis. (2021) 15:e0009062. doi: 10.1371/journal.pntd.0009062
18. Senne NA, Vilela TS, Sanavria A, Santos HA, Rabello RS, Angelo IC. Ecology and spatial distribution of sand fly species in low endemic areas for American Tegumentary Leishmaniasis in the municipality of Seropédica, Rio de Janeiro, Brazil. Med Vet Entomol. (2021), 1–8. doi: 10.1111/mve.12505
19. Israël DK, Coulibaly CA, Sissoko IM, Wilke BB, Beier JC, Muller GC. Distribution and diversity of sand fly species (Diptera : psychodidae, phlebotominae) in two geoclimatic zones of Chad. Front Trop Dis. (2022) 2:762295. doi: 10.3389/fitd.2021.762295
20. Jalali H, Nikookar SH, Vasoukolaei NH, Jahanifard E. Ecology of sand flies (Diptera : Psychodidae, Phlebotominae) in Jajarm County, an area with high risk of cutaneous leishmaniasis. BMC Zool. (2022) 7:14. doi: 10.1186/s40850-022-00113-0
21. Quate LW. Phlebotomus sandflies of the Paloich area in the Sudan (Diptera, Psychodidae). J Med Entomol. (1964) 1:213–68. doi: 10.1093/jmedent/1.3.213
22. Panthawong A, Chareonviriyaphap T, Phasuk J. Species diversity and seasonality of phlebotomine sand flies (Diptera: Psychodidae) in Satun province, Thailand. Southeast Asian J Trop Med Public Health. (2015) 46:857–65.
23. Sawalha SS, Ramlawi A, Sansur RM, Salem IM, Amr ZS. Diversity, ecology, and seasonality of sand flies (Diptera : Psychodidae) of the Jenin District (Palestinian Territories). J Vector Ecol. (2017) 42:120–9. doi: 10.1111/jvec.2017.42.issue-1
24. Darkaoui N, Janati Idrissi A, Talbi FZ, El Fattouhi Y, El Omari H, Najy M, et al. Seasonal dynamics of sand flies (Diptera, pshycodidae), vectors of cutaneous leishmaniasis, in the city of fez, northern Morocco. Sci World J. (2022) 2022:120–129. doi: 10.1155/2022/4095129
25. Tateng NA, Ngouateu OB, Payne KV, Maurer M, von Stebut E, Krüger A, et al. Ecological site distribution of sand fly species of Mokolo, an endemic focus of cutaneous leishmaniasis in northern Cameroon. Acta Tropica. (2023) 239:106809. doi: 10.1016/j.actatropica.2022.106809
26. Kaptue L, Zekeng L, Fomekong E, Nsangou A, Tagu JP, Tchuela J. Visceral leishmaniasis in Cameroon. Report of various cases and clinical study in the region of Kousseri, Far-North of Cameroon. Bull la Societe pathol exot (1990). (1992) 85:156–8.
27. Dondji B. Leishmaniasis and phlebotomus of Cameroon: review of current data. Bull la Societe pathol exot. (2001) 94:277–9.
28. Dondji B, Dereure J, Poste B, Dedet JP. Lgeishmaniose viscérale au Cameroun. Enquête séro-épidémiologique dans la région de Kousseri, Nord-Cameroun. Bull Soc Pathol Exot. (2001) 94:418–20.
30. PCD. Plan Communal de Developpement (PCD) de Kousseri, réalise par la commune de Kousseri avec l’appui de l’Association Canal de Développement sur financement PNDP. (2013), 173p.
31. Dondji B, Duhlinska DD, Same-Ekobo A. Species composition of the Phlebotomine sandfly fauna (Diptera: Phlebotominae) In Mokolo Region, Northern Cameroon. Insect Sci Applic. (2000) 20:221–6. doi: 10.1017/S1742758400019676
32. Alexander B. Sampling methods for phlebotomine sandflies. Med Vet Entomol. (2000) 14:109–22. doi: 10.1046/j.1365-2915.2000.00237.x
33. Abonnenc E, Minter DM. Tables d’identification bilingues des Phlébotomes de la région Ethiopienne. Cahier Office de La Recherche Scientifique et Technique d’ Outre-Mer. Entomol Medicale. (1965) 5:24–63.
34. Abonnenc E. Les Phlébotomes de la Région Ethiopienne (Diptera, Psychodidae). Off la Recherche Scientif Tech d’ Outre-Mer. (1972) 55:289.
35. Gijón-Robles P, Abattouy N, Corpas-Lopez V, El Khalfaoui N, Morillas-Marquez F, Riyad M, et al. Intra and peridomiciliary comparison of density, sex ratio,i and gonotrophic stage of Phlebotomus sergenti in an active anthroponotic cutaneous leishmaniasis focus in Morocco. Acta Tropica. (2021) 221:106005. doi: 10.1016/j.actatropica.2021.106005
36. Amao H, Idowu ET, Oyeniyim T, Otubanjo OA, Awolola TS. Relative abundance, distribution, and diversity of Culex (Diptera : Culicidae) mosquito species in Lagos, Southwest Nigeria. Nigeria J Entomol. (2018) 34:38–49. doi: 10.36108/NJE/8102/43(0150)1965
37. Rydzanicz K, Lonc E. Species composition and seasonal dynamics of mosquito larvae in the Wroclaw, Poland area. J Vector Ecol J Soc Vector Ecol. (2004) 28:255–66.
38. Sokal RR, Rohlf FJ, Company New York. Biometry : the principles and practice of statistics in biological. 4th Editio. Freeman WH, editor. W. H. Freeman and Company All, New York (2012), 1–909.
39. R Core Team. R: A language and environment for statistical computing. Vienna, Austria (2021). Available at: https://www.r-project.org.
40. Hsieh TC, Ma KH, Chao A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol. (2016) 7:1451–6. doi: 10.1111/2041-210X.12613
41. Tateng NA, Vincent PK, Ngouateu OB, Kirstein OD, Warburg A, Von Stebut E, et al. Inventory and taxonomy of phlebotomine sand flies of the Mokolo leishmaniasis focus , northern Cameroon , with description of new Sergentomyia taxa (Diptera : Psychodidae). Acta Tropica. (2019) 194:172–80. doi: 10.1016/j.actatropica.2019.04.006
42. Lane RP. Sandflies (Phlebotominae). In: Lane RP, Crosskey RW, editors. Medical Insects and Arachnids. Springer-Science+Business Media, B.V, London, UK (1993), p. 78–119. doi: 10.1007/978-94-011-1554-4
43. Rageau R, Adam JP. Note sur les phlebotomes d’Evodoula (Cameroun francais). Bull Ia Socide Palhologie Exot. (1953) 46:587–94.
44. Ba Y, Trouillet J, Thonna J, Fontenille D. Phlébotomes du Sénégal (Diptera-Psychodidae) : Peuplement et dynamique des populations. Parasites. (1998) 5:143–50. doi: 10.1051/parasite/1998052143
45. Coulibaly CA, Sissoko I, Traore B, Diallo A, Samake S, Traore SF, et al. Diversity of sand flies (Diptera : psychodidae : phlebotominae) in two different eco-climatic and endemic zones of cutaneous leishmaniasis in Mali, West Africa. J Med Entomol. (2016) 53(4):923–7. doi: 10.1093/jme/tjw060
46. Doe ED, Kwakye-nuako G, Addo SO, Egyir-yawson A. Identification of sand flies (Diptera : psychodidae) collected from cutaneous leishmaniasis endemic focus in the Ho municipality, Ghana. Int Ann Sci. (2021) 10:33–44. doi: 10.21467/ias.10.1.33-44
47. Senghor MW, Niang AA, Depaquit J, Ferté H, Faye MN, Elguero E, et al. Transmission of leishmania infantum in the canine leishmaniasis focus of mont- rolland , Senegal : ecological , parasitological and molecular evidence for a possible role of sergentomyia sand flies. PloS Negl Trop Dis. (2016) 10:e0004940. doi: 10.1371/journal.pntd.0004940
48. Oliveira BFG, Domingos M, de F, Ovallos FG, de Camargo-Neves VLF. Updating ecological and behavioral aspects of the sandfly fauna in the vale do Ribeira Region, São Paulo State, Brazil. Insects. (2021) 12:988. doi: 10.3390/insects12110988
49. Jaturas N, Vitta A, Samung Y, Apiwathnasorn C, Polseela R. Species composition and nocturnal activity of phlebotomine sand flies (Diptera : Psychodidae) inhabiting a limestone cave in Thailand. J Vector Ecol. (2018) 43:52–8. doi: 10.1111/jvec.12282
50. Service NW. Phlebotomine sandflies (Order Diptera: Family Psychodidae). In: A Guide to Medical Entomology. Macmillan Tropical & Sub-Tropical Medical Texts., Palgrave, London (1980), p. 78–82. doi: 10.1007/978-1-349-16334-2
51. Alten B, Ozbel Y, Ergunay K, Kasap OE, Cull B, Antoniou M, et al. Sampling strategies for phlebotomine sand flies (Diptera : Psychodidae) in Europe. Bull Entomol Res. (2015) 105:664–78. doi: 10.1017/S0007485315000127
52. Mutinga MJ, Kyai FM, Omogo DM. Investigations on the epidemiology of Leishmaniases in Kenya I : Studies on vectors of Leishmania Major in Marigat, Baringo District, Kenya. Insect Sci Applic. (1986) 7:181–9. doi: 10.1017/S1742758400008948
53. Israël DK, Coulibaly CA, Beier JC, Muller GC, Doumbia S. Systematic review of visceral leishmaniasis in central Africa. Asian J Med Health. (2021) 19:8–20. doi: 10.9734/AJMAH/2021/v19i730341
54. Dedet JP, Derouin F, Cornet M. Infestation spontanée de Phlebotomus duboscqi par les promastigotes de Leishmania au Senegal. C. R Séan. Acad Sci Paris Série D. (1978) 286:301–2.
55. Anderson JM, Samake S, Jaramillo-gutierrez G, Sissoko I, Cheick A, Soucko C, et al. Seasonality and Prevalence of Leishmania major Infection in Phlebotomus duboscqi Neveu-Lemaire from Two Neighboring Villages in Central Mali. PloS Negl Trop Dis. (2011) 5:e1139. doi: 10.1371/journal.pntd.0001139
56. Tateng NA, Kirstein OD, Ngouateu OB, Krüger A, von Stebut E, Maurer M, et al. First detection of Leishmania donovani in sand flies from Cameroon and its epidemiological implications. Trop Med Int Health. (2018) 23:1014–21. doi: 10.1111/tmi.13123
57. Maia C, Depaquit J. Can Sergentomyia (Diptera, Psychodidae) play a role in the transmission of mammal-infecting Leishmania? Parasites. (2016) 23. doi: 10.1051/parasite/2016062
58. Owino BO, Mwangi JM, Kiplagat S, Mwangi HN, Ingonga JM, Chebet A, et al. Molecular detection of Leishmania donovani , Leishmania major , and Trypanosoma species in Sergentomyia squamipleuris sand flies from a visceral leishmaniasis focus in Merti sub − County , eastern Kenya. Parasites Vectors. (2021) 14:53. doi: 10.1186/s13071-020-04517-0
59. Ayari C, Othman SB, Chemkhi J, Ahmed T, Fisa R, Salah AB, et al. First detection of Leishmania major DNA in Sergentomyia (Sintonius) clydei (Sinton 1928, Psychodidae: Phlebotominae), from an outbreak area of cutaneous leishmaniasis in Tunisia. J Mol Epidemiol Evolution Genet Infect Dis. (2016) 39:241–8. doi: 10.1016/j.meegid.2015.10.030
60. Adediran OA, Kolapo TU, Uwalaka EC. Seroprevalence of canine leishmaniasis in Kwara , Oyo and Ogun states of Nigeria. J Parasitic Dis. (2016) 40:510–4. doi: 10.1007/s12639-014-0535-2
61. Dondji B, Dereure J, Pratlong F, Duhlinska DD, Same-Ekobo A, Dedet JP. Characterization of Leishmania major causing cutaneous leishmaniasis in northern Cameroon. Trans R Soc Trop Med Hyg. (1998) 92:677–8. doi: 10.1016/s0035-9203(98)90810-0
62. Ezenwa VO, Godsey MS, King RJ, Guptill SC. Avian diversity and West Nile virus : testing associations between biodiversity and infectious disease risk. Proc R Soc B. (2006) 273:109–17. doi: 10.1098/rspb.2005.3284
Keywords: sand fly species, ecological distribution, seasonality, diversity index, visceral leishmaniasis, Kousseri
Citation: Tebo-Nzesseu TR, Tateng NA, Ngouateu OB, Yamssi C, Elanga NE, Ndo C, Bamou R, Khan-Payne V and Dondji B (2024) Distribution and ecological aspects of sand fly species from Kousseri, an endemic focus of visceral leishmaniasis in Northern Cameroon. Front. Trop. Dis 5:1371670. doi: 10.3389/fitd.2024.1371670
Received: 17 January 2024; Accepted: 11 April 2024;
Published: 13 May 2024.
Edited by:
Cleber Galvão, Oswaldo Cruz Foundation (Fiocruz), BrazilReviewed by:
Claudia Rios-Velásquez, Instituto Leônidas & Maria Deane (ILMD/Fiocruz Amazônia), BrazilMargarete Afonso, Oswaldo Cruz Foundation (Fiocruz), Brazil
Copyright © 2024 Tebo-Nzesseu, Tateng, Ngouateu, Yamssi, Elanga, Ndo, Bamou, Khan-Payne and Dondji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tateng Ngouateu Aimé, aime.tateng@univ-dschang.org
 Thierry Rostand Tebo-Nzesseu
Thierry Rostand Tebo-Nzesseu Ngouateu Aime Tateng
Ngouateu Aime Tateng Omer Bebe Ngouateu2,3
Omer Bebe Ngouateu2,3 Cedric Yamssi
Cedric Yamssi Cyrille Ndo
Cyrille Ndo Blaise Dondji
Blaise Dondji