- 1Department of Pharmacy, The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, China
- 2Department of Rhinology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
The E2F family of transcription factors plays a crucial role in the regulation of cell cycle progression and cell proliferation. Accumulative evidence indicates that aberrant expression or activation of E2F2 is a common phenomenon in malignances. E2F2 has emerged as a key player in the development and progression of various types of tumors. A wealth of research has substantiated that E2F2 could contribute to the enhancement of tumor cell proliferation, angiogenesis, and invasiveness. Moreover, E2F2 exerts its influence on a myriad of cellular processes by engaging with a spectrum of auxiliary factors and downstream targets, including apoptosis and DNA repair. The dysregulation of E2F2 in the context of carcinogenesis may be attributable to a multitude of mechanisms, which encompass modifications in upstream regulatory elements or epigenetic alterations. This review explores the function of E2F2 in cancer progression and both established and emerging therapeutic strategies aiming at targeting this oncogenic pathway, while also providing a strong basis for further research on the biological function and clinical applications of E2F2.
1 Introduction
The E2F family is an important transcription factor (TF) which plays a critical regulatory role in the cell cycle. E2F family members are defined by their DNA binding domain (DBD), and to date, eight members, contain E2F1- E2F8, have been identified, which encode for nine different gene products (1, 2). E2F transcription factor 2 (E2F2) is a transcription factor belonging to the E2F family. E2F2 is involved in biological processes such as cell cycle regulation, cell differentiation and apoptosis. When the cell enters the S phase, E2F2 can combine with DP1 to form a complex that promotes DNA synthesis and the normal progression of the cell cycle (3). In addition, E2F2 is also involved in regulating the transcriptional activities of many other genes, such as BAX, p73, p16INK4a, etc. (4). E2F2 plays an important role in a variety of physiological and pathological conditions, including biochemistry, molecular biology, cell and developmental biology, and oncology and so an. In cancer, overexpression of E2F2 has been found in various types of cancer, and the abnormal expression of E2F2 is often associated with the occurrence, metastasis and recurrence of tumors.
Overall, E2F2 is a multifaceted regulatory factor that participates in numerous physiological and pathological processes and involves various types of signaling pathways. In recent years, extensive research has confirmed the significant role of E2F2 in tumor progression, which can exert pro-cancerous or anti-cancerous effects depending on its specific context. Additionally, E2F2 has been shown to be associated with the efficacy of some anti-tumor drugs, indicating that it may serve as a new candidate for targeted cancer therapy. Therefore, this review summarizes the functions and roles of E2F2 in cancer progression, further exploring its contributions to tumor development and identifying potential therapeutic targets.
2 Discovery, structure, and distribution of E2F2
E2F2 is a member of the E2F family of transcription factors, which play important roles in the regulation of cell cycle, DNA replication, and differentiation. The discovery of E2F2 dates back to 1992 when it was first identified as a protein that binds to the adenovirus E2 promoter. E2F2 belongs to the E2F family of transcription factors, which contain a highly conserved DNA-binding domain known as the E2F DNA-binding domain. This domain consists of a pair of zinc fingers that specifically recognize and bind to DNA sequences in target gene promoters. E2F2 also contains a transactivation domain, which allows it to activate or repress gene expression. E2F2 is ubiquitously expressed in mammalian tissues, with high levels detected in proliferating cells such as embryonic tissues and cancer cells. In humans, the E2F2 gene is located on chromosome 1p36.13, and mutations in this gene have been associated with various types of cancer. E2F2 has been shown to interact with a variety of proteins involved in cell cycle regulation and DNA repair, including Rb, p53, and BRCA1 (5–8). E2F2 forms complexes with DP1 and DP2, members of the DP family of transcription factors, to regulate gene expression (9). The E2F2/DP1 or E2F2/DP2 complex can function either as a transcriptional activator or a transcriptional repressor, depending on the specific context and interacting partner proteins (10, 11). For example, the E2F2/DP1 complex activates the expression of genes required for the G1-to-S phase transition, while the E2F2/HDAC1 complex represses the expression of genes involved in cell cycle progression (12–14).
In summary, E2F2 is a ubiquitously expressed transcription factor that plays important roles in the regulation of cell cycle progression, DNA replication, and differentiation. Its structure and distribution have been well studied, and it forms complex interactions with multiple partner proteins to orchestrate gene expression programs in the cell.
3 Functions of E2F2 in specific cancer types
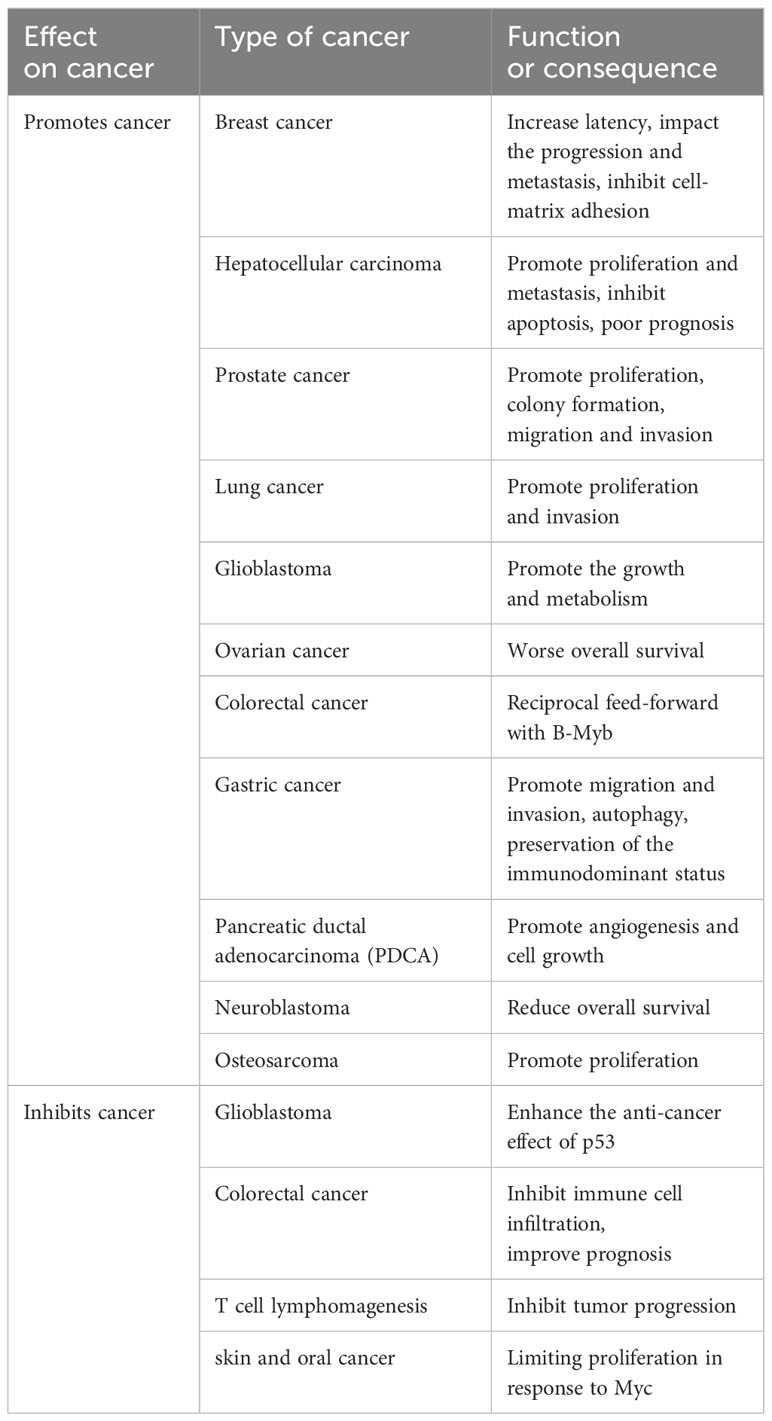
In the process of cancer progression, malignant proliferation of tumor cells is the main factor for its occurrence and development, and the disorder of cell cycle process is the root cause of malignant proliferation of cancer. Transcription factor E2F2 plays an important role in regulating cell cycle progression. Besides the limited evidence demonstrating that E2F2 could suppress tumor progression, an increasing number of experiments indicated that E2F2 was closely related to the occurrence of multiple cancer types. Based on the key role of E2F2 in the occurrence of cancer through the regulation of cell cycle process, further research on the regulatory mechanism of E2F2 in the process of tumor formation may provide new research ideas in the control of tumor growth and the development of novel anti-tumor drugs. In recent decades, in-depth research on E2F2 in cancer has elucidated E2F2 functions in tumor-cell metabolism, anti-angiogenesis, metastasis, therapy resistance, and tumor immunoediting (Table 1).
3.1 Breast cancer
For women, breast cancer has the first place in cancer incidence worldwide and is the twice place in mortality worldwide by 2023 (15, 16). Several studies have shown that E2F2 is overexpressed in breast tumors compared to normal tissue (17, 18).
The mortality may always find links to the metastasis to distant sites in human breast cancer. Aliccia Bollig-Fischer found that E2F2 aberrant expression impacts the progression and metastasis of breast cancer cells, E2F2 expression regulated by human epidermal growth factor receptor 2 (HER2) oncogene could impact the cell-matrix adhesion function, with potential consequences for metastatic colonization (19). Transcriptional regulation of E2F2 plays a crucial role in HER2 overexpression, the overexpression of HER2 in breast tumors is associated with bad prognosis (20). Yuwanita reported that E2F2 loss results in increased metastasis in Myc-driven breast cancer through a PTPRD dependent mechanism potentially (21). E2F2 knockout mice have reduced expression of genes associated with the epithelial-mesenchymal transition (EMT) (22). In addition, E2F2 is related to breast cancer stem cells (BCSCs). Inhibition of E2F2 could reduce the proportion of CD24-/CD44+ cells and the expression levels of SOX2 and OCT4, thus inhibiting the self-renewal, proliferation and invasion ability of BCSCs and inhibiting the growth of breast cancer tumors (23). Moreover, E2F2 was also correlated with a patient’s response to PARP inhibition therapy (24). Therefore, E2F2 may be a potential target for breast cancer therapy.
3.2 Hepatocellular carcinoma
Liver cancer remains a global health challenge, with an estimated incidence of >1 million cases by 2025. Hepatocellular carcinoma (HCC) is the most common form of liver cancer and accounts for ~90% of cases (25). It is the sixth most common cancer in incidence and the third leading cause of cancer death among all malignancies (16). Studies have shown that the expression level of E2F2 was generally upregulated in HCC. High expression of E2F2 was associated with poor prognosis in patients (26). Similarly, Gene Expression Omnibus (GEO) data and TCGA analysis shown that E2F2 mRNA was highly expression in HCC tissue and was significantly correlated with tumor status, histological grade, and clinical stage (27). Elevated E2F2 could be a promising independent prognostic biomarker and therapeutic target for HCC (28). Therefore, E2F2 is inextricably related to the progression of HCC.
Several studies already proved that high expression of E2F2 serves as a driving force for the development of HCC (29). Down-regulating the expression of E2F2 could inhibit the proliferation of HCC cells (30). E2F2 overexpression significantly inhibited HCC cell apoptosis in a p53-dependent manner (31). Fang et al. found that HCC cell metastasis could be restrained by down-regulating of the expression of E2F2 and ECT2 (32). Activating OCT1-E2F2 signaling would intensify sorafenib and lenvatinib resistance in HCC (33). Moreover, E2F2 overexpression plays a central role in dysregulation of the cell cycle in HCC. Overexpressed E2F2 is a crucial center of cell cycle regulation and is significantly associated with patients’ poor prognosis (34).
Long non-coding RNAs (lncRNAs) have been confirmed to influence the development of HCC. Minzhen et al. found that lncRNA PRR34-AS1 could promote HCC development via modulating Wnt/β-catenin pathway by upregulating E2F2 and SOX12 (35). In addition, lipid metabolism rearrangements in nonalcoholic fatty liver disease (NAFLD) contribute to disease progression. NAFLD has emerged as a major risk for HCC. Francisco et al. found that activation of the E2F1-E2F2-CPT2 axis provides a lipid-rich environment required for hepatocarcinogenesis, E2F2 deletion confers protection against hepatocarcinogenesis by preventing lipid storage (36). Altogether, these results indicate that E2F2 might be vital to HCC development.
3.3 Prostate cancer
Prostate cancer ranks first in the number of the estimated new cases and as the second leading cause of cancer-related death in men in the America (16). The incidence and fatality rate of prostate cancer is rising continuously worldwide (37). Explore the underlying reasons and new preclinical prevention or treatment measures is urgently needed. Li et al. demonstrated that E2F2 regulated DLEU2 expression by directly binding to DLEU2 promoter. The overexpression of E2F2 in prostate cancer cells contributed to upregulation of DLEU2, then facilitated prostate cancer progression, including proliferation, colony formation, migration, and invasion (38).
E2F2 has been well-characterized as a regulator of the G1-to-S-phase transition, it has been confirmed that it may be novel therapeutic candidate for prostate cancer given its ability to induce cell-cycle arrest and inhibit cell growth. The tumor suppressor PTEN is the most frequently inactivated gene in prostate cancer. PTEN-mediated G1 cell-cycle arrest in LNCaP prostate cancer cells is associated with altered expression of E2F2 (39). Dong et al. found that let-7a acts as a tumor suppressor in prostate cancer by down-regulating E2F2 and CCND2, further study shown that the 3’UTR of E2F2 and CCND2 could directly bind to let-7a and then induce cell cycle arrest at the G1/S phase (40).
Additionally, E2F2 activity controls the transcriptional regulation of a myriad of genes encoding key proteins involved in nucleotide biosynthesis, DNA replication and DNA repair (41, 42). Mohaddase Hamidi et al. shown that targeting E2F1/E2F2 elicited DNA damage during S phase, leading to premature CDK1 activation and compromised prostate cancer cells viability (43).
3.4 Lung cancer
Lung cancer is one of the most aggressive tumors with very low life expectancy (44, 45). E2F2 acts as an activator is closely related to clinical stage and tumor size (46). Analysis of ONCOMINE, GEPIA, Kaplan-Meier Plotter and cBioPortal databases of E2F2 in patients with Lung cancer shown that the expression level of E2F2 was higher in lung adenocarcinoma and squamous cell lung carcinoma tissues than in lung tissues (47). The expression level of E2F2 was correlated with advanced tumor stage. Survival analysis using the Kaplan-Meier Plotter database revealed that the high transcription level of E2F2 were associated with low relapse-free survival (RFS) in all of the patients (47).
In non-small cell lung cancer (NSCLC), multiple studies have shown that up-regulation of E2F2 could promote the progression of lung cancer (48). The activating E2F2 Signaling would promote proliferation and invasion (49, 50). Mechanically, E2F2 may play a key role in the pathological mechanism of NSCLC progression through Circ_0109320/miR-595/E2F2 axis (51). In addition, Zhang et al. reported that inhibition of E2F2 transcription activity could reduce the activity of PI3K/AKT signaling pathway, and thus potentially inhibit the occurrence of lung cancer (52).
Lung adenocarcinoma causes severe cancer death worldwide. Du et al. constructed an E2F2-centered co-expression network and identified several key modules and networks overrepresented in Lung adenocarcinoma. Function analysis revealed that E2F2 overexpression accelerated cell growth, cell cycle progression and cell motility whereas E2F2 knockdown inhibited these malignant phenotypes. Mechanistic investigations uncovered that E2F2/B-Myb/FOXM1 from the Lung adenocarcinoma transcription regulatory network exhibited positive expression correlation, associated with each other, mutually transactivated each other, and regulated similar downstream gene cascades, hence constituting a consolidated core transcription regulatory circuitry (53).
3.5 Glioblastoma
Glioblastoma is a type of brain cancer that originates from the glial cells in the brain, which is the most aggressive and common form of primary brain tumor in adults. Research has shown that E2F2 may play a role in glioblastoma. Abnormal expression and activity of E2F2 have been observed in glioblastoma cells, suggesting that it may contribute to the development and progression of the disease. RNAi-mediated knockdown of E2F2 could inhibit tumorigenicity of human glioblastoma cells (54). However, for another, Mitlianga et al. found that co-expression of E2F2 and p53 enhances the anti-cancer effect of p53 in Glioblastoma cells (55). Unquestionable integrity, E2F2 is involved in gliomagenesis and could be explored as a potential therapeutic target in malignant gliomas.
Numerous microRNAs are strongly implicated in the malignancy of glioblastoma. Song et al. found that Let-7b, a member of the Let-7 microRNA family, could inhibit the malignant behavior of glioma cells and glioma stem-like cells via downregulation of E2F2 (56). Furthermore, by directly targeting E2F2, miR-125b could regulate the proliferation and miR-218 could inhibit the growth and metabolism of human glioblastoma cells (57, 58). In addition, peroxisome proliferator-activated receptor alpha (PPARalpha) is a member of the nuclear hormone receptor superfamily and functions as a transcription factor. Research has shown that PPARalpha could induce miR-214 expression by downregulation of its target E2F2. Subsequently, the overexpression of miR-214 inhibits Glioblastoma cells growth in vitro and in vivo by inducing cell cycle arrest in G0/G1 (59). Collectively, these data uncover a crucial role for microRNAs-E2F2 pathway in controlling the development of glioblastoma.
3.6 Ovarian cancer
Ovarian cancer (OC) is the fifth most common gynecological malignancy and one of the most lethal cancers in females (16). The lack of effective biomarkers is believed to be an important reason for the high lethality rate of ovarian cancer.
Previous studies indicated that E2F2 was a significantly upregulated transcription factor in ovarian cancer epithelial cells and among the OC samples, and simultaneously, high E2F2 expression was negatively associated with tumor stage and outcome among OC patients (60). E2F2 might serve as potential prognostic biomarkers and targets for OS based on the comprehensive analysis between the E2F2 and the pathogenesis and progression of OC (1).
LBX2‐AS1 was a novel lncRNA that promoted the progression of OC by inhibiting miR-455–5p and miR-491–5p. Jian Cao et al. showed that inhibition of these two miRNAs or the forced expression of E2F2 counteracted the effect of LBX2‐AS1 knockdown on the malignancy of OC cells (61). In addition, circRNAs have gained significant attention in cancer research. research has shown that circE2F2 promoted OC cell proliferation, metastasis, and glucose metabolism by stabilizing the E2F2 mRNA via binding to the HuR protein (62). These findings suggest an essential regulatory role of E2F2 on ovarian carcinogenesis.
3.7 Colorectal cancer
Emerging evidence has revealed that E2F2 participates in the tumorigenesis and progression of various tumors, although its function in colorectal cancer (CRC) is not yet fully elucidated (63, 64). On the one hand, studies have indicated that the expression of E2F2 was significantly elevated in colon cancer tissues compared to normal colon tissues, implicating E2F2 as a potential diagnostic biomarker for CRC (2). B-Myb could accelerate CRC progression via reciprocal feed-forward transactivation of E2F2 (65). On the other hand, study illustrated that E2F2 was significantly downregulated in CRC samples. Reduced E2F2 expression in CRC was prominently correlated with N, M stage and pathological stage. Decreased E2F2 expression was unfavorable for overall survival (OS), disease free survival (DFS), disease specific survival (DSS) and progress free interval (PFI) (64). Decreased E2F2 expression is closely associated with poor prognosis and immune cell infiltration in CRC, suggesting its potential as an independent prognostic biomarker and a therapeutic target for CRC (64). Furthermore, Li et al. have found that miR-31 regulated the proliferation of CRC cells by targeting E2F2, and E2F2 acted as a tumor suppressor in CRC by repressing the expression of survivin and regulating the expression of CCNA2, C-MYC, MCM4 and CDK2 (66).
3.8 Gastric cancer
Gastric cancer (GC) is a malignant tumor that originates from the epithelial cells of the stomach. It is one of the most common malignancies and a leading cause of cancer-related deaths worldwide. It can be caused by multiple factors, including genetic mutations, environmental factors, and lifestyle choices.
Bioinformatic analysis using multiple databases revealed that the level of E2F2 was highly expressed in both GC tissues and cells compared with that in normal gastric tissues and cells. High E2F2 expression was associated with the characteristics of invasive tumors and poor prognosis. Study had shown that E2F2 play a key role in the regulation of PI3K/Akt/mTOR-mediated autophagy and the downstream processes of cell migration and invasion (67). E2F2 also had potential modulatory effects on tumor immunity. CIBERSORTx analysis of the proportion of tumor-infiltrating immune cells (TICs) revealed that immune cells are correlated with E2F2 expression, suggesting that E2F2 might be responsible for the preservation of the immunodominant status for gastric cancer (68).
miRNA is a class of short chain non-coding RNA molecules that can participate in intracellular gene regulation after transcription and play an important role in the process of tumor occurrence and development (69). Researches have shown that E2F2 expression is up-regulated in GC tissues and is inversely proportional to the level of miR-31, downregulated miR-31 level associates with poor prognosis of gastric cancer and its restoration suppresses tumor cell malignant phenotypes by inhibiting E2F2 (70). Wen et al. confirmed that miR-26a could improve the sensitivity of GC cells to cisplatin-based chemotherapies through targeting NRAS and E2F2 (71). H19 could be used as a biological indicator for diagnosing GC and predicting patients’ poor prognosis. A study revealed that H19 could promote GC to proliferate and invade through miR-138/E2F2 axis (72). Therefore, miRNA and E2F2 have broad application prospects and development space in the research field of gastric cancer. In the future, with the in-depth understanding of the functional mechanism and regulatory network between miRNA and E2F2, their importance in tumor therapy and diagnosis will be further confirmed and played.
3.9 Other
E2F2 plays a significant role in a variety of other tumors as well. Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive tumors all over the world. M2 macrophage-derived exosomes promoted angiogenesis and growth of PDCA cells by targeting E2F2 (73). In mice xenografts of neuroblastoma cells stably expressing doxycycline-inducible DOT1L shRNA, ablating DOT1L expression with doxycycline significantly reduced ODC1 and E2F2 expression, reduced tumor progression, and improved overall survival (74). miR-218 suppresses the proliferation of osteosarcoma through downregulation of E2F2 (75). Gliomas are highly resistant to conventional treatment. Co-expression of E2F-2 and p53 enhances the anti-cancer effect of p53 in gliomas (55).
E2F2, in addition to its capacity to facilitate tumor progression, has also been implicated in the suppression of tumor growth through specific mechanisms. Study has demonstrated that E2F2 inactivation could cooperate with transgenic expression of Myc to enhance tumor development in the skin and oral cavity, perhaps by limiting proliferation in response to Myc (76). Rene et al. used a bitransgenic mouse model of Myc-induced T cell lymphomagenesis and analyzed tumor progression in mice deficient for E2F1, E2F2, or E2F3. Surprisingly, the targeted inactivation of E2F1 or E2F3 has no significant effect on tumor progression while the loss of E2F2 accelerates the development of lymphoma (77).
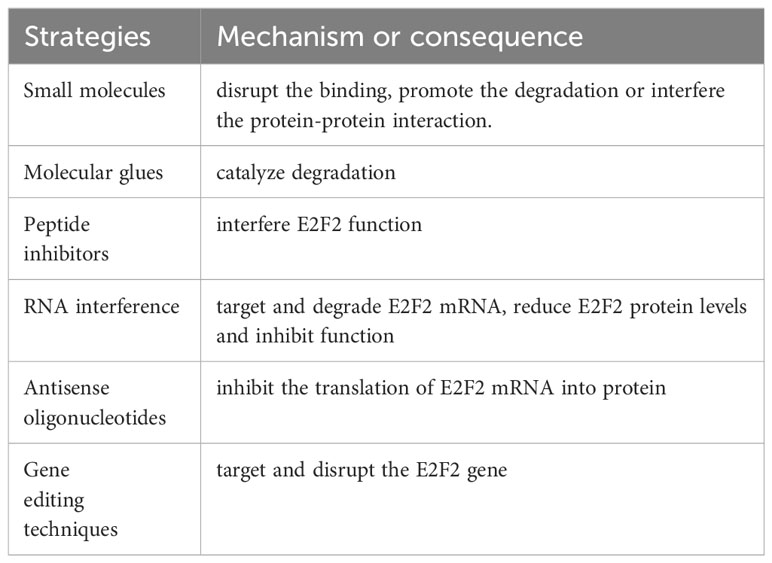
4 Strategies to inhibit E2F2 function
A multitude of studies have repeatedly shown that blocking E2F2 activity limits tumor cell proliferation and viability due to the promoting role of E2F2 in tumor progression. Here, we summarize several strategies that have been used to either alter or utilize E2F2 activity in preclinical settings to target cancer cells (Table 2).
Small molecules could be designed through high-throughput screening to specifically inhibit E2F2 function. These inhibitors could disrupt the binding of E2F2 with its target genes, promote the degradation or interfere the protein-protein interaction. Molecular glues are a class of small-molecules which could catalyze the rapid degradation of previously inaccessible proteins through a proximity-induced interaction between the target protein and E3 ligases. Study have indicated that bufalin as molecular glue markedly promoted E2F2-ZFP91 complex formation, thereby leading to E2F2 polyubiquitination via K48-linked ubiquitin chains for degradation (78). Peptide inhibitors that specifically inhibit E2F2 activity were designed using synthetic or natural peptide molecules. These peptides are able to interact with E2F2 and interfere its function. Several peptides isolated from random peptide phage display libraries have been designed to interfere with the ability of E2Fs including E2F2 to bind DNA. Incubation of human tumor cells with these peptides led to an inhibition of cell proliferation and induction of apoptosis (79). RNA interference (RNAi) could be used to specifically target and degrade E2F2 mRNA, leading to reduce E2F2 protein levels and inhibited function. Trang Nguyen-Vu et al. found that knockdown of E2F2 expression resulted in a significant disruption of estrogen receptor positive breast cancer cell proliferation (80).
In addition, there are several strategies that could be attempted to inhibit the function of E2F2. Antisense oligonucleotides (ASOs) were short synthetic strands of nucleic acids that could bind to specific RNA molecules, including E2F2 mRNA. By binding the E2F2 mRNA, ASOs could block its translation into protein, effectively inhibiting E2F2 function. Gene editing techniques such as CRISPR-Cas9 could be used to specifically target and disrupt the E2F2 gene. E2F2 activity is regulated by various upstream signaling pathways and proteins. Targeting these regulators, such as cyclin-dependent kinases (CDKs) or retinoblastoma protein (RB), can indirectly inhibit E2F2 function. Further research and validation are required to assess the efficacy and safety of these strategies before they can be utilized for therapeutic purposes.
5 Conclusion and perspectives
As a gene family associated with the regulation of many cellular functions such as cell cycle, apoptosis, differentiation, and stress response, the E2F transcription factor family plays a critical role in cell regulation. Many signals can regulate the activity of E2F family members, and E2F family members can in turn regulate multiple different target genes, thus affecting different cell functions and processes. Therefore, tight control of the expression of E2F family members plays an important role in the physiological balance of cellular tissue, and once this is disrupted, it may lead to functional disorders or even tumor occurrence. In such a regulatory network, E2F2 occupies a central position and plays an irreplaceable role in regulating intracellular signals.
Like other members of the E2F family, E2F2 usually binds to the promoter region of genes to control their expression, playing a crucial role in the regulation of the cell cycle. In recent years, with the deepening research on the relationship between the E2F family and cancer, the function of the E2F2 is no longer limited to the cell cycle. In the human microenvironment, E2F2 exhibits a multifaceted and nuanced role, interacting with a myriad of molecular pathways and cellular processes. Its intricate involvement in the regulation of cell proliferation, apoptosis, and DNA repair mechanisms underscores its significance in maintaining genomic stability and cellular homeostasis. Furthermore, E2F2’s complex interaction with other transcription factors and its responsiveness to various signaling contribute to its diverse functions in development, tissue regeneration, and disease pathogenesis, including cancer.
However, similar to the majority of genes, E2F2 exhibits a dual functionality within living organisms. It is capable of not only promoting processes such as cell proliferation, apoptosis, and inflammation but also inhibiting these processes. E2F2 facilitates the transition of cells into the S phase, promoting DNA replication and cell proliferation. However, excessive E2F2 activation could disrupt the cell cycle and inhibit cell growth. E2F2 could both induce apoptosis, which is crucial for tissue homeostasis and cancer prevention, and suppress apoptosis, potentially leading to the survival of abnormal cells and tumor growth. Additionally, E2F2 can modulate the inflammatory response, which is a defense mechanism against infection and injury, but its overactivation can result in tissue damage and chronic diseases. E2F2 can also inhibit inflammation to prevent excessive injury.
E2F2 has been shown to play a dual role in cancer progression. On the one hand, it can act as a tumor suppressor by inhibiting the proliferation of cancer cells and promoting apoptosis. On the other hand, E2F2 can also promote tumor growth and metastasis by regulating the expression of genes involved in cell cycle progression, angiogenesis, and epithelial-to-mesenchymal transition (EMT). The context-dependent function of E2F2 in cancer makes it a challenging but promising target for therapeutic intervention.
The biological function of E2F2 in cancer is complex, with its dysregulation affecting various pathways and processes. It has been shown to influence metastasis, cancer stem cell properties, apoptosis, drug resistance, cell cycle progression, and transcriptional regulation of key cancer-related genes. In different cancer types, E2F2 interacts with factors such as PTPRD, p53, OCT1, let-7a, and B-Myb, and is involved in pathways including Wnt/β-catenin and PI3K/AKT. Its role as a tumor suppressor or oncogene appears to be context-dependent, highlighting the need for a nuanced understanding of its functions in cancer biology. However, we believe that the current findings are just the tip of the iceberg of its functions. There is still a need for further research on its extensive functions and how it regulates different genes, mutually promotes and inhibits various genes, and complex regulatory networks.
Although the specific mechanisms and roles of E2F2 in tumorigenesis have not been fully elucidated yet, the existing research results have already demonstrated the importance of E2F2 as a therapeutic target. Studies have shown that inhibiting E2F2 expression and reducing the expression level of E2F2 protein can suppress tumor occurrence, growth, metastasis, and recurrence. Therefore, screening for drugs that target E2F2 provides new evidence for the development of anti-cancer drugs. However, there are still many difficulties regarding drug development targeting E2F2. Such as how to use PROTAC technology to develop small molecule drugs that are more selective, less side effect, and stronger potency? What is the binding site of E2F2 small molecule inhibitors with E2F2? How to modify the developed drugs to reduce their side effects, improve their selectivity and efficacy? What is the antigenic epitope recognized by anti-E2F2 antibodies? Are antibody drugs better than E2F2 small molecule inhibitors? Will knocking out or inhibiting E2F2 have any adverse effects on the body? Answering these questions will help to find candidate drugs that regulate the function of E2F2 better.
With a better understanding of E2F2’s contributions to cancer development, researchers may be able to develop novel diagnostic tools, therapeutic targets, and treatment strategies. By exploring the complex interplay between E2F2 and other cellular factors, it may be possible to identify new pathways for intervention and improve patient outcomes. Ultimately, the ongoing investigation of E2F2’s role in cancer may pave the way for more effective and personalized treatment approaches, offering hope for cancer patients in the future.
Author contributions
YG: Writing – original draft, Writing – review & editing. XQ: Writing – review & editing, Formal analysis. ZL: Writing – original draft. WZ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant No. 82304534), Beijing Medical and Health Foundation (Grant No. ABS-2023–014), the Doctoral Research Start-up Foundation of Henan Cancer Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhou Q, Zhang F, He Z, Zuo MZ. E2F2/5/8 serve as potential prognostic biomarkers and targets for human ovarian cancer. Front Oncol. (2019) 9:161. doi: 10.3389/fonc.2019.00161
2. Yao H, Lu F, Shao Y. The E2F family as potential biomarkers and therapeutic targets in colon cancer. PeerJ. (2020) 8:e8562. doi: 10.7717/peerj.8562
3. Sharma N, Timmers C, Trikha P, Saavedra HI, Obery A, Leone G. Control of the p53-p21CIP1 Axis by E2f1, E2f2, and E2f3 is essential for G1/S progression and cellular transformation. J Biol Chem. (2006) 281:36124–31. doi: 10.1074/jbc.M604152200
4. Saavedra HI, Wu L, de Bruin A, Timmers C, Rosol TJ, Weinstein M, et al. Specificity of E2F1, E2F2, and E2F3 in mediating phenotypes induced by loss of Rb. Cell Growth Differ. (2002) 13:215–25.
5. Wang H, Shao N, Ding QM, Cui J, Reddy ES, Rao VN. BRCA1 proteins are transported to the nucleus in the absence of serum and splice variants BRCA1a, BRCA1b are tyrosine phosphoproteins that associate with E2F, cyclins and cyclin dependent kinases. Oncogene. (1997) 15:143–57. doi: 10.1038/sj.onc.1201252
6. Liu K, Lin FT, Ruppert JM, Lin WC. Regulation of E2F1 by BRCT domain-containing protein TopBP1. Mol Cell Biol. (2003) 23:3287–304. doi: 10.1128/MCB.23.9.3287-3304.2003
7. Raj D, Liu T, Samadashwily G, Li F, Grossman D. Survivin repression by p53, Rb and E2F2 in normal human melanocytes. Carcinogenesis. (2008) 29:194–201. doi: 10.1093/carcin/bgm219
8. DeBruhl H, Wen H, Lipsick JS. The complex containing Drosophila Myb and RB/E2F2 regulates cytokinesis in a histone H2Av-dependent manner. Mol Cell Biol. (2013) 33:1809–18. doi: 10.1128/MCB.01401-12
9. Moberg K, Starz MA, Lees JA. E2F-4 switches from p130 to p107 and pRB in response to cell cycle reentry. Mol Cell Biol. (1996) 16:1436–49. doi: 10.1128/MCB.16.4.1436
10. Dynlacht BD, Brook A, Dembski M, Yenush L, Dyson N. DNA-binding and trans-activation properties of Drosophila E2F and DP proteins. Proc Natl Acad Sci U S A. (1994) 91:6359–63. doi: 10.1073/pnas.91.14.6359
11. Magae J, Wu CL, Illenye S, Harlow E, Heintz NH. Nuclear localization of DP and E2F transcription factors by heterodimeric partners and retinoblastoma protein family members. J Cell Sci. (1996) 109:1717–26. doi: 10.1242/jcs.109.7.1717
12. Girling R, Bandara LR, Ormondroyd E, Lam EW, Kotecha S, Mohun T, et al. Molecular characterization of Xenopus laevis DP proteins. Mol Biol Cell. (1994) 5:1081–92. doi: 10.1091/mbc.5.10.1081
13. Zhang D, Vuocolo S, Masciullo V, Sava T, Giordano A, Soprano DR, et al. Cell cycle genes as targets of retinoid induced ovarian tumor cell growth suppression. Oncogene. (2001) 20:7935–44. doi: 10.1038/sj.onc.1204971
14. Reimer D, Sadr S, Wiedemair A, Concin N, Hofstetter G, Marth C, et al. Heterogeneous cross-talk of E2F family members is crucially involved in growth modulatory effects of interferon-gamma and EGF. Cancer Biol Ther. (2006) 5:771–6. doi: 10.4161/cbt.5.7.2750
15. Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, et al. Breast cancer statistic. CA Cancer J Clin. (2022) 72:524–41. doi: 10.3322/caac.21754
16. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistic. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
17. Li Y, Huang J, Yang D, Xiang S, Sun J, Li H, et al. Expression patterns of E2F transcription factors and their potential prognostic roles in breast cancer. Oncol Lett. (2018) 15:9216–30. doi: 10.3892/ol
18. Feng H, Sun SZ, Cheng F, Zhang NQ. Mediation of circ_RPPH1 on miR-146b-3p/E2F2 pathway to hinder the growth and metastasis of breast carcinoma cells. Aging (Albany NY). (2021) 13:20552–68. doi: 10.18632/aging.v13i16
19. Bollig-Fischer A, Marchetti L, Mitrea C, Wu J, Kruger A, Manca V, et al. Modeling time-dependent transcription effects of HER2 oncogene and discovery of a role for E2F2 in breast cancer cell-matrix adhesion. Bioinformatics. (2014) 30:3036–43. doi: 10.1093/bioinformatics/btu400
20. Justenhoven C, Pierl CB, Haas S, Fischer HP, Hamann U, Baisch C, et al. Polymorphic loci of E2F2, CCND1 and CCND3 are associated with HER2 status of breast tumors. Int J Cancer. (2009) 124:2077–81. doi: 10.1002/ijc.24198
21. Yuwanita I, Barnes D, Monterey MD, O’Reilly S, Andrechek ER. Increased metastasis with loss of E2F2 in Myc-driven tumors. Oncotarget. (2015) 6:38210–24. doi: 10.18632/oncotarget.v6i35
22. Fujiwara K, Yuwanita I, Hollern DP, Andrechek ER. Prediction and genetic demonstration of a role for activator E2Fs in Myc-induced tumors. Cancer Res. (2011) 71:1924–32. doi: 10.1158/0008-5472.CAN-10-2386
23. Lin QY, Wang JQ, Wu LL, Zheng WE, Chen PR. miR-638 represses the stem cell characteristics of breast cancer cells by targeting E2F2. Breast Cancer. (2020) 27:147–58. doi: 10.1007/s12282-019-01002-0
24. Rennhack JP, Andrechek ER. Low E2F2 activity is associated with high genomic instability and PARPi resistance. Sci Rep. (2020) 10:17948. doi: 10.1038/s41598-020-74877-1
25. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. (2021) 7:6. doi: 10.1038/s41572-020-00240-3
26. Shen S, Wang Y. Expression and prognostic role of E2F2 in hepatocellular carcinoma. Int J Gen Med. (2021) 14:8463–72. doi: 10.2147/IJGM.S334033
27. Zhan L, Huang C, Meng XM, Song Y, Wu XQ, Miu CG, et al. Promising roles of mammalian E2Fs in hepatocellular carcinoma. Cell Signal. (2014) 26:1075–81. doi: 10.1016/j.cellsig.2014.01.008
28. Zeng Z, Cao Z, Tang Y. Increased E2F2 predicts poor prognosis in patients with HCC based on TCGA data. BMC Cancer. (2020) 20:1037. doi: 10.1186/s12885-020-07529-2
29. Huang J, Yang J, Lei Y, Gao H, Wei T, Luo L, et al. An ANCCA/PRO2000-miR-520a-E2F2 regulatory loop as a driving force for the development of hepatocellular carcinoma. Oncogenesis. (2016) 5:e229. doi: 10.1038/oncsis.2016.22
30. Dong Y, Zou J, Su S, Huang H, Deng Y, Wang B, et al. MicroRNA-218 and microRNA-520a inhibit cell proliferation by downregulating E2F2 in hepatocellular carcinoma. Mol Med Rep. (2015) 12:1016–22. doi: 10.3892/mmr.2015.3516
31. Zeng Z, Jiang W, Kan J, Zhang D, Li R, He F, et al. Shentao Ruangan formula promotes apoptosis via the E2F2-p53 pathway in hepatocellular carcinoma. Phytomedicine. (2023) 109:154565. doi: 10.1016/j.phymed.2022.154565
32. Fang ZQ, Li MC, Zhang YQ, Liu XG. MiR-490–5p inhibits the metastasis of hepatocellular carcinoma by down-regulating E2F2 and ECT2. J Cell Biochem. (2018) 119:8317–24. doi: 10.1002/jcb.26876
33. Wang Y, Tan K, Hu W, Hou Y, Yang G. LncRNA AC026401.3 interacts with OCT1 to intensify sorafenib and lenvatinib resistance by activating E2F2 signaling in hepatocellular carcinoma. Exp Cell Res. (2022) 420:113335. doi: 10.1016/j.yexcr.2022.113335
34. Hong SH, Eun JW, Choi SK, Shen Q, Choi WS, Han JW, et al. Epigenetic reader BRD4 inhibition as a therapeutic strategy to suppress E2F2-cell cycle regulation circuit in liver cancer. Oncotarget. (2016) 7:32628–40. doi: 10.18632/oncotarget.v7i22
35. Qin M, Meng Y, Luo C, He S, Qin F, Yin Y, et al. lncRNA PRR34-AS1 promotes HCC development via modulating Wnt/beta-catenin pathway by absorbing miR-296–5p and upregulating E2F2 and SOX12. Mol Ther Nucleic Acids. (2021) 25:37–52. doi: 10.1016/j.omtn.2021.04.016
36. Gonzalez-Romero F, Mestre D, Aurrekoetxea I, O’Rourke CJ, Andersen JB, Woodhoo A, et al. E2F1 and E2F2-mediated repression of CPT2 establishes a lipid-rich tumor-promoting environment. Cancer Res. (2021) 81:2874–87. doi: 10.1158/0008-5472.CAN-20-2052
37. Wang X, Sun B, Wei L, Jian X, Shan K, He Q, et al. Cholesterol and saturated fatty acids synergistically promote the Malignant progression of prostate cancer. Neoplasia. (2022) 24:86–97. doi: 10.1016/j.neo.2021.11.004
38. Li P, Xu H, Yang L, Zhan M, Shi Y, Zhang C, et al. E2F transcription factor 2-activated DLEU2 contributes to prostate tumorigenesis by upregulating serum and glucocorticoid-induced protein kinase 1. Cell Death Dis. (2022) 13:77. doi: 10.1038/s41419-022-04525-1
39. van Duijn PW, Ziel-van der Made AC, van der Korput JA, Trapman J. PTEN-mediated G1 cell-cycle arrest in LNCaP prostate cancer cells is associated with altered expression of cell-cycle regulators. Prostate. (2010) 70:135–46. doi: 10.1002/pros.21045
40. Dong Q, Meng P, Wang T, Qin W, Qin W, Wang F, et al. MicroRNA let-7a inhibits proliferation of human prostate cancer cells in vitro and in vivo by targeting E2F2 and CCND2. PloS One. (2010) 5:e10147. doi: 10.1371/journal.pone.0010147
41. Ren B, Cam H, Takahashi Y, Volkert T, Terragni J, Young RA, et al. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev. (2002) 16:245–56. doi: 10.1101/gad.949802
42. Bertoli C, Skotheim JM, de Bruin RA. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol. (2013) 14:518–28. doi: 10.1038/nrm3629
43. Hamidi M, Eriz A, Mitxelena J, Fernandez-Ares L, Aurrekoetxea I, Aspichueta P, et al. Targeting E2F sensitizes prostate cancer cells to drug-induced replication stress by promoting unscheduled CDK1 activity. Cancers (Basel). (2022) 14(19): 4952. doi: 10.3390/cancers14194952
44. Feliciano A, Garcia-Mayea Y, Jubierre L, Mir C, Hummel M, Castellvi J, et al. miR-99a reveals two novel oncogenic proteins E2F2 and EMR2 and represses stemness in lung cancer. Cell Death Dis. (2017) 8:e3141. doi: 10.1038/cddis.2017.544
45. Li JW, Zheng G, Kaye FJ, Wu L. PROTAC therapy as a new targeted therapy for lung cancer. Mol Ther. (2023) 31:647–56. doi: 10.1016/j.ymthe.2022.11.011
46. Chen L, Yu JH, Lu ZH, Zhang W. E2F2 induction in related to cell proliferation and poor prognosis in non-small cell lung carcinoma. Int J Clin Exp Pathol. (2015) 8:10545–54
47. Sun CC, Zhou Q, Hu W, Li SJ, Zhang F, Chen ZL, et al. Transcriptional E2F1/2/5/8 as potential targets and transcriptional E2F3/6/7 as new biomarkers for the prognosis of human lung carcinoma. Aging (Albany NY). (2018) 10:973–87. doi: 10.18632/aging.v10i5
48. Su J, Zhou J, Feng Y, Zhang H, Zhang X, Zhao X, et al. circPTN Promotes the Progression of Non-Small Cell Lung Cancer through Upregulation of E2F2 by Sponging miR-432–5p. Int J Genomics. (2022) 2022:6303996. doi: 10.1155/2022/6303996
49. Li X, Zhang Z, Jiang H, Li Q, Wang R, Pan H, et al. Circular RNA circPVT1 Promotes Proliferation and Invasion Through Sponging miR-125b and Activating E2F2 Signaling in Non-Small Cell Lung Cancer. Cell Physiol Biochem. (2018) 51:2324–40. doi: 10.1159/000495876
50. Zhou X, Tao H. Overexpression of microRNA-936 suppresses non-small cell lung cancer cell proliferation and invasion via targeting E2F2. Exp Ther Med. (2018) 16:2696–702. doi: 10.3892/etm
51. Bai Q, Li L, Chen F, Zhu J, Cao L, Yang Y, et al. Suppression of circular RNA hsa_circ_0109320 attenuates non-small cell lung cancer progression via miR-595/E2F7 axis. Med Sci Monit. (2020) 26:e921200. doi: 10.12659/MSM.921200
52. Zhang H, Tulahong A, Wang W, Nuerrula Y, Zhang Y, Wu G, et al. Downregulation of microRNA-519 enhances development of lung cancer by mediating the E2F2/PI3K/AKT axis. Int J Clin Exp Pathol. (2020) 13:711–20.
53. Du K, Sun S, Jiang T, Liu T, Zuo X, Xia X, et al. E2F2 promotes lung adenocarcinoma progression through B-Myb- and FOXM1-facilitated core transcription regulatory circuitry. Int J Biol Sci. (2022) 18:4151–70. doi: 10.7150/ijbs.72386
54. Nakahata AM, Suzuki DE, Rodini CO, Fiuza ML, Okamoto OK. RNAi-mediated knockdown of E2F2 inhibits tumorigenicity of human glioblastoma cells. Oncol Lett. (2014) 8:1487–91. doi: 10.3892/ol.2014.2369
55. Mitlianga PG, Kyritsis AP, Gomez-Manzano C, Lemoine MG, Hu M, Liu TJ, et al. Co-expression of E2F-2 enhances the p53 anti-cancer effect in human glioma cells. Int J Oncol. (2001) 18:343–7. doi: 10.3892/ijo
56. Song H, Zhang Y, Liu N, Zhang D, Wan C, Zhao S, et al. Let-7b inhibits the Malignant behavior of glioma cells and glioma stem-like cells via downregulation of E2F2. J Physiol Biochem. (2016) 72:733–44. doi: 10.1007/s13105-016-0512-6
57. Wu N, Xiao L, Zhao X, Zhao J, Wang J, Wang F, et al. miR-125b regulates the proliferation of glioblastoma stem cells by targeting E2F2. FEBS Lett. (2012) 586:3831–9. doi: 10.1016/j.febslet.2012.08.023
58. Zhang Y, Han D, Wei W, Cao W, Zhang R, Dong Q, et al. MiR-218 inhibited growth and metabolism of human glioblastoma cells by directly targeting E2F2. Cell Mol Neurobiol. (2015) 35:1165–73. doi: 10.1007/s10571-015-0210-x
59. Gao Y, Han D, Sun L, Huang Q, Gai G, Wu Z, et al. PPARalpha Regulates the Proliferation of Human Glioma Cells through miR-214 and E2F2. BioMed Res Int. (2018) 2018:3842753. doi: 10.1155/2018/3842753
60. Xie L, Li T, Yang LH. E2F2 induces MCM4, CCNE2 and WHSC1 upregulation in ovarian cancer and predicts poor overall survival. Eur Rev Med Pharmacol Sci. (2017) 21:2150–6
61. Cao J, Wang H, Liu G, Tang R, Ding Y, Xu P, et al. LBX2-AS1 promotes ovarian cancer progression by facilitating E2F2 gene expression via miR-455–5p and miR-491–5p sponging. J Cell Mol Med. (2021) 25:1178–89. doi: 10.1111/jcmm.16185
62. Zhang M, Xu Y, Zhang Y, Li B, Lou G. Circular RNA circE2F2 promotes Malignant progression of ovarian cancer cells by upregulating the expression of E2F2 protein via binding to HuR protein. Cell Signal. (2021) 84:110014. doi: 10.1016/j.cellsig.2021.110014
63. Komatsu Y, Ito I, Wayama M, Fujimura A, Akaogi K, Machida H, et al. PPARgamma ligands suppress the feedback loop between E2F2 and cyclin-E1. Biochem Biophys Res Commun. (2008) 370:145–8. doi: 10.1016/j.bbrc.2008.03.046
64. Shang Y, Zhang Y, Liu J, Chen L, Yang X, Zhu Z, et al. Decreased E2F2 expression correlates with poor prognosis and immune infiltrates in patients with colorectal cancer. J Cancer. (2022) 13:653–68. doi: 10.7150/jca.61415
65. Fan X, Wang Y, Jiang T, Liu T, Jin Y, Du K, et al. B-Myb accelerates colorectal cancer progression through reciprocal feed-forward transactivation of E2F2. Oncogene. (2021) 40:5613–25. doi: 10.1038/s41388-021-01961-9
66. Li T, Luo W, Liu K, Lv X, Xi T. miR-31 promotes proliferation of colon cancer cells by targeting E2F2. Biotechnol Lett. (2015) 37:523–32. doi: 10.1007/s10529-014-1715-y
67. Li H, Zhao S, Shen L, Wang P, Liu S, Ma Y, et al. E2F2 inhibition induces autophagy via the PI3K/Akt/mTOR pathway in gastric cancer. Aging (Albany NY). (2021) 13:13626–43. doi: 10.18632/aging.v13i10
68. Chen C, Zheng Q, Pan S, Chen W, Huang J, Cao Y, et al. The RNA-binding protein NELFE promotes gastric cancer growth and metastasis through E2F2. Front Oncol. (2021) 11:677111. doi: 10.3389/fonc.2021.677111
69. Li Z, Lang Z, Wang T, Qu G, Sui W, Liu J. LncRNA SNHG22 promotes gastric cancer progression by regulating the miR-101–3p/e2f2 axis. Cell Cycle. (2023) 22:347–60. doi: 10.1080/15384101.2022.2119515
70. Wang H, Zhang X, Liu Y, Ni Z, Lin Y, Duan Z, et al. Downregulated miR-31 level associates with poor prognosis of gastric cancer and its restoration suppresses tumor cell Malignant phenotypes by inhibiting E2F2. Oncotarget. (2016) 7:36577–89. doi: 10.18632/oncotarget.v7i24
71. Wen L, Cheng F, Zhou Y, Yin C. MiR-26a enhances the sensitivity of gastric cancer cells to cisplatin by targeting NRAS and E2F2. Saudi J Gastroenterol. (2015) 21:313–9. doi: 10.4103/1319-3767.166206
72. Yu J, Fang C, Zhang Z, Zhang G, Shi L, Qian J, et al. H19 Rises in Gastric Cancer and Exerts a Tumor-Promoting Function via miR-138/E2F2 Axis. Cancer Manag Res. (2020) 12:13033–42. doi: 10.2147/CMAR.S267357
73. Yang Y, Guo Z, Chen W, Wang X, Cao M, Han X, et al. M2 macrophage-derived exosomes promote angiogenesis and growth of pancreatic ductal adenocarcinoma by targeting E2F2. Mol Ther. (2021) 29:1226–38. doi: 10.1016/j.ymthe.2020.11.024
74. Wen L, Fu L, Shi YB. Histone methyltransferase Dot1L is a coactivator for thyroid hormone receptor during Xenopus development. FASEB J. (2017) 31:4821–31. doi: 10.1096/fj.201700131R
75. Xuan C, Jin M, Gao Y, Xu S, Wang L, Wang Y, et al. miR-218 suppresses the proliferation of osteosarcoma through downregulation of E2F2. Oncol Lett. (2019) 17:571–7. doi: 10.3892/ol.2018.9576
76. Pusapati RV, Weaks RL, Rounbehler RJ, McArthur MJ, Johnson DG. E2F2 suppresses Myc-induced proliferation and tumorigenesis. Mol Carcinog. (2010) 49:152–6. doi: 10.1002/mc.20584
77. Opavsky R, Tsai SY, Guimond M, Arora A, Opavska J, Becknell B, et al. Specific tumor suppressor function for E2F2 in Myc-induced T cell lymphomagenesis. Proc Natl Acad Sci U S A. (2007) 104:15400–5. doi: 10.1073/pnas.0706307104
78. Liu TT, Yang H, Zhuo FF, Yang Z, Zhao MM, Guo Q, et al. Atypical E3 ligase ZFP91 promotes small-molecule-induced E2F2 transcription factor degradation for cancer therapy. EBioMedicine. (2022) 86:104353. doi: 10.1016/j.ebiom.2022.104353
79. Montigiani S, Muller R, Kontermann RE. Inhibition of cell proliferation and induction of apoptosis by novel tetravalent peptides inhibiting DNA binding of E2F. Oncogene. (2003) 22:4943–52. doi: 10.1038/sj.onc.1206495
Keywords: E2F2, cancer progression, therapeutic target, function, strategy
Citation: Gao Y, Qiao X, Liu Z and Zhang W (2024) The role of E2F2 in cancer progression and its value as a therapeutic target. Front. Immunol. 15:1397303. doi: 10.3389/fimmu.2024.1397303
Received: 07 March 2024; Accepted: 30 April 2024;
Published: 14 May 2024.
Edited by:
Arumugam Jayakumar, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Vidya Gopakumar, Case Western Reserve University, United StatesMatias I. Hepp, Universidad Católica de la Santísima Concepción, Chile
Copyright © 2024 Gao, Qiao, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Gao, yxgaoyang@163.com; Wenzhou Zhang, hnzzzwzx@sina.com
 Yang Gao
Yang Gao Xinjie Qiao2
Xinjie Qiao2 Wenzhou Zhang
Wenzhou Zhang