- 1Department of Paediatrics, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
- 2Clinical Immunology Unit, Department of Paediatrics, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Malaysia
- 3Primary Immunodeficiency Diseases Group, Department of Clinical Medicine, Institut Perubatan dan Pergigian Termaju, Universiti Sains Malaysia, Bertam, Pulau Pinang, Malaysia
- 4Malaysian Patient Organisation for Primary Immunodeficiencies (MyPOPI), Kuala Lumpur, Malaysia
- 5Hospital Tunku Ampuan Besar Tuanku Aishah Rohani, UKM Specialist Children’s Hospital, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
- 6Department of Biochemistry, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
Background: Primary Immunodeficiency Disease (PID), also known as Inborn Errors of Immunity (IEI), comprises a group of rare genetic disorders that impair the body’s immune responses. These conditions result from monogenic germline mutations that affect the function of genes governing the innate and adaptive immune system. Therefore, individuals with PID are more susceptible to infectious diseases, allergies, and autoimmune and autoinflammatory conditions. The prevalence of PID has been on the rise, with the number of classified diseases reaching 404, and 430 genetic defects reported to cause these conditions. However, in Malaysia, genetic testing for PID is currently limited and needs to be outsourced to overseas laboratories, posing financial challenges for families. Moreover, limited research has focused on the knowledge and awareness of genetic testing among parents of children with PID in Malaysia. This study aims to address this gap and provide valuable insights into the knowledge, awareness, and perception of genetic testing among this specific population.
Method: This qualitative cross-sectional study utilised online open-ended, semi-structured focus group interviews to explore the perceptions and experiences of parents of children with Primary Immunodeficiency (PID). Participants were recruited through convenience sampling from the Malaysian Patient Organisation for Primary Immunodeficiencies (MyPOPI), a non-governmental organisation dedicated to providing support and raising awareness about PID. The study spanned from May 2023 to July 2023 and included participants from diverse regions of Malaysia who had undergone different diagnostic journeys in various hospitals.
Result: The focus group discussions yielded 11 sub-themes that highlighted the experiences, understanding and challenges of the participants regarding genetic testing based on the semi-structured questions. These sub-themes were then grouped into four main themes that are awareness and understanding of genetic testing, the journey towards diagnosis and treatment, emotional impact and psychological factors, and the importance of medical experts in diagnosing and managing PID, as well as public perception and awareness.
Conclusion: In conclusion, this study highlights the diverse knowledge, awareness, and perception surrounding genetic testing for PID. Factors such as access to services, family history, and personal circumstances shape individuals’ understanding of genetic testing. The importance of healthcare professionals, along with the need for improved accessibility and targeted communication strategies, is underscored to enhance understanding and reduce stigma surrounding genetic testing for rare diseases like PID.
1 Introduction
Primary Immunodeficiency Disease (PID) or also known as Inborn Errors of Immunity (IEI), encompasses a group of rare genetic disorders leading to the impairment of the body’s immune responses. PIDs arise from monogenic germline mutations, resulting in loss of expression, loss-of-function, or gain-of-function from the encoded proteins. These variants of the functional genes that govern the innate and adaptive immune system cause immune defects, increasing susceptibility to infectious diseases, allergies, autoimmune, and autoinflammatory diseases (1). Previous publication from the International Union of Immunological Societies Expert Committee in 2019 has reported additional classification of PIDs into 404 diseases with 430 genetic defects linked to these conditions (1, 2). The current prevalence of PID is likely to increase by 10%, shifting from 1/10,000 – 1/50,000 births to 1/1,000 – 1/5,000 births (1, 3).
There is a wide range of clinical manifestation for patients with PID, ranging from increased susceptibility to infections such as recurrent pneumonia, sinus infections to significant immune dysregulation which often lead to multiple autoimmune manifestations, delayed growth and development, lymphoproliferation and malignancy (4–6). Early diagnosis of PID diagnosis is crucial, as it correlates with effective treatment delivery and improved survival outcomes. For instance, patients with severe combined immunodeficiency (SCID) face a grim prognosis if diagnosed too late, without receiving allogeneic hematopoietic stem cell transplantation; resulting in a survival rate beyond their first year (5, 7). In 2019, Abd Hamid and colleagues reported a prevalence rate of 0.37 cases per 100,000 population for PIDs in Malaysia. Their review of PID literature identified a total of 119 PID cases, with only 26 patients received molecular diagnosis.
Since the establishment of medical genetic services in Kuala Lumpur dated back in 1994, the overall aspect of genetic services has improved significantly with the availability of genetic counselling, genetic testing, and diagnosis for paediatric and adult-onset rare diseases (8). As important as other components of genetic services, genetic testing has emerged as a powerful tool for patients affected with rare diseases. It serves as a key instrument in concluding their diagnostic odyssey journey. Additionally, for clinicians, genetic testing enables the formulation of disease management plans and provides insights into available treatment options. Furthermore, leveraging current knowledge and technology, genetic testing can identify specific genetic variants associated with rare diseases through the analysis of an individual’s DNA, enabling a more accurate molecular diagnosis.
However, genetic testing while invaluable in patient-centered care, presents both advantages and challenges. In general, the potential benefits for genetic testing are (i) to provide molecular diagnosis and identify disease causing variants, (ii) for early detection and prevention of secondary conditions, (iii) to limit the need for more intrusive and expensive testing (9, 10), (iv) to come out with a management plan and treatment options (11), (v) to provide genetic risk assessment for family members and for future pregnancy planning (12) and most importantly (vi) to give autonomy to patients in making informed medical and lifestyle decisions (13). In contrast to that, there are also challenges associated with genetic testing which may involve ethical, legal, and social issues. These include psychological burden on parents and families, such as issues of blame and genetic guilt (14), genetic discrimination from the community (15), financial issues, and the uncertainty stemming from the identification of variants of uncertain significance (VUS) results (16). VUS is among the potential outcomes of a genetic test, and it fails to meet the criteria for classification as either pathogenic or benign, and the existing evidence for the variant is conflicting (17). With the progression of genetic testing methods, a larger number of genes are under analysis, leading to a rise in the identification of VUS results. Nonetheless, comprehending VUS proves to be complex since this outcome is not suitable for diagnostic purposes and does not influence medical management, where parents of children receiving VUS results often struggle to comprehend them, which can result in adverse outcomes such as feelings of frustration and helplessness (18).
The role of genetic testing in the diagnosis of numerous PID is significant. It can establish a conclusive diagnosis together with clinical and immunological information, predict prognosis through genotype-phenotype correlations, utilise targeted therapies, and guide decisions related to family planning. As part of future pregnancy planning, parents may be presented with the option of prenatal diagnosis or preimplantation genetic diagnosis (19–21). Another crucial component in the diagnosis of PID is the expert management provided by clinical immunologists. Unfortunately, the availability of clinical immunologists in Malaysia is insufficient, causing delays in the detection and diagnosis of PID cases. Furthermore, clinical immunology is not officially recognised by the National Specialist Register of Malaysia as a distinct subspecialty field. Consequently, patients encounter restricted access to such services. Within the Ministry of Health (MOH) in Malaysia, many PID patients are managed by other pediatric subspecialties, such as Pediatric Respiratory Specialists, based on their availability within MOH facilities (5).
Genetic testing for PID is often offered to individuals suspected of fulfilling the criteria for PID, mainly during childhood. Currently, this testing is not available in Malaysia and is outsourced to overseas labs, with families self-funding the expenses or seeking financial aid. This test is usually decided by the parents or legal guardians since children are unable to give their consent for genetic testing. Hence, this lies the importance of addressing the perception and awareness of genetic testing among parents of children affected by PID. To date, there is limited study focusing on the knowledge, awareness, and perception on genetic testing within the rare diseases community, such as PID, in Malaysia. Existing studies available primarily explore cancer genetics (22), autism spectrum disorder (ASD) (23), and hereditary disorders (24). To the best of our knowledge, this study represents the first attempt to examine parental perceptions of genetic testing for PID in Malaysia specifically.
2 Materials and methods
2.1 Study design, participants, and data collection
In this qualitative cross-sectional study, online open-ended, semi structured focus group interviews were conducted among parents of children with PID from May 2023 to July 2023. Participants were recruited using a convenience sampling method from Malaysian Patient Organisation for Primary Immunodeficiencies (MyPOPI). MyPOPI is a non-governmental organisation that has been providing support and raising awareness about PID in Malaysia. Individuals registered with the MyPOPI group are either patients or parents of patients who have been clinically or molecularly diagnosed with PID. The members in MyPOPI group come from all part of Malaysia and their diagnostic journeys vary across different hospitals, which is why it was chosen as part of this study. Currently, MyPOPI has approximately 120 registered members from the medical community, volunteers, family members and individuals affected. The approval of this study was granted by the Research and Ethics Committee of Universiti Kebangsaan Malaysia (Project Code: JEP-2022-800).
During the recruitment process, participants for this study were selected according to the specific eligibility criteria. The criteria include that the participant can understand and read either English or Malay (Malaysian national language), and they must be parents of children with PID. The status of genetic testing is not compulsory, and parents of clinically diagnosed children are eligible to participate in this study. In assessing the adequacy of purposive samples in our study, the required samples were determined upon achieving saturation of emerging themes. In this aspect, we decided to utilise a “code meaning” approach, in which the saturation is deemed achieved in the subsequent interview when no new aspects, dimensions or nuances are identified. As documented, the number usually ranges between 9 to 17 respondents or between 4 to 8 focus group discussions (25).
An information sheet regarding the study was provided to the participants and they were recruited after giving their informed consent. Participants were then allocated into separate groups, either mothers or fathers, to ensure a homogeneous background during the interviews. To avoid researcher bias, a moderator (F.H.Z.), independent of the research team with no prior relationship with the participants, facilitated the interviews and discussions. The moderator asked a series of questions provided in the guides, with the flexibility to pose additional questions for a more comprehensive understanding of the participants’ perspectives. The focus group discussion lasted for about 1.5 to 2 hours. The interview questions were based on a previously published study and were adapted from qualitative and quantitative study (Table 1) by (26, 27), and Chin & Tham (24). The questions were validated through phases of revisions and discussions with experts in the field. The questions were translated into Malay language, and most of the discussion was conducted in Malay or in bilingual (Malay and English), given that majority of the participants were from the Malay ethnicity.
All participants recruited have or had a child diagnosed with PID and are registered members of the MyPOPI support group. A recruitment announcement was disseminated through the MyPOPI WhatsApp group. Additionally, more than thirty participants were contacted individually and invited to participate through WhatsApp for this focus group discussion.
2.2 Data analysis
All FGD sessions were recorded to ensure precise data collection with the permission of the participants, after which they were transcribed into Word documents. These transcriptions were then cross-verified against the digital recordings to confirm accuracy. The transcripts underwent multiple readings and were independently coded by the researcher (A.H.S.A.A.). Thematic analysis, following the approach outlined by Braun and Clarke (28), was analysed by the research group (A.H.S.A.A., A.A., N.A.S.I.). Based on coding analysis, the main and subthemes were identified. Final themes were decided and discussed until consensus was reached. Exemplar quotes were translated into English and presented in the results section.
3 Results
3.1 Description of participants
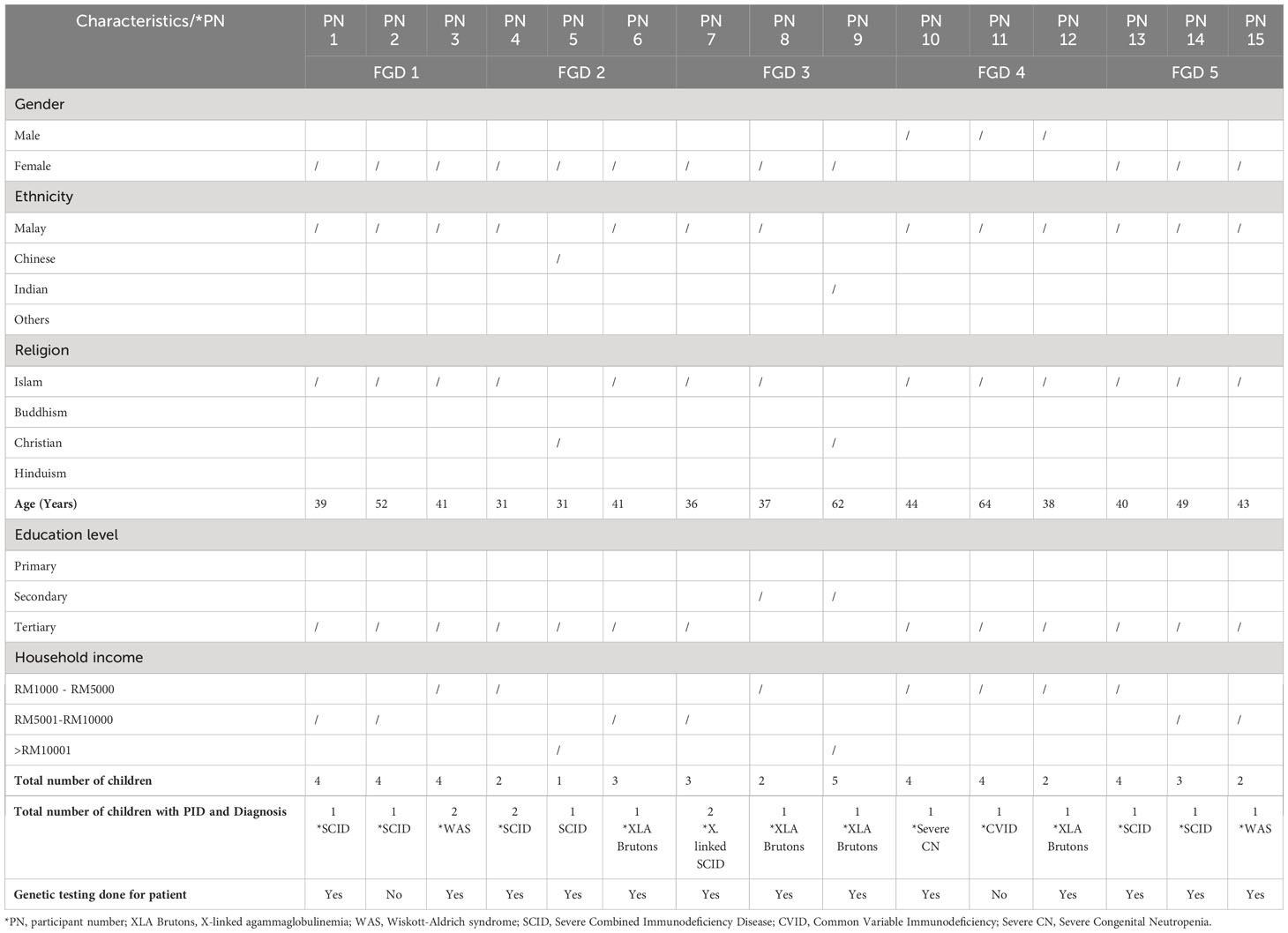
The study was conducted with a total of 15 parents that had provided consent. The demographic characteristics of the parents (interviewees) and patients’ characteristics include those of 12 mothers and three fathers (Table 2). Among those 15 participants, 13 had genetic testing done for their children and only seven participants had undergone genetic testing. Five participants who had undergone genetic testing were found to carry the same variants identified in their children while one participant did not carry the variant. Reasons for not undergoing genetic testing among other participants included cost factors, unwillingness to know the information, and not having the time yet to proceed with genetic testing.
3.2 Thematic analysis
The focus group discussions yielded 11 sub-themes that highlighted the experience, understanding and challenges of the participants on genetic testing based on the semi-structured questions. The sub-themes were then grouped into four main themes. The main themes revolve around their knowledge and understanding on genetic testing, awareness on genetic testing and services, perception towards genetic testing and challenges in the molecular diagnosis of PID (Table 3).
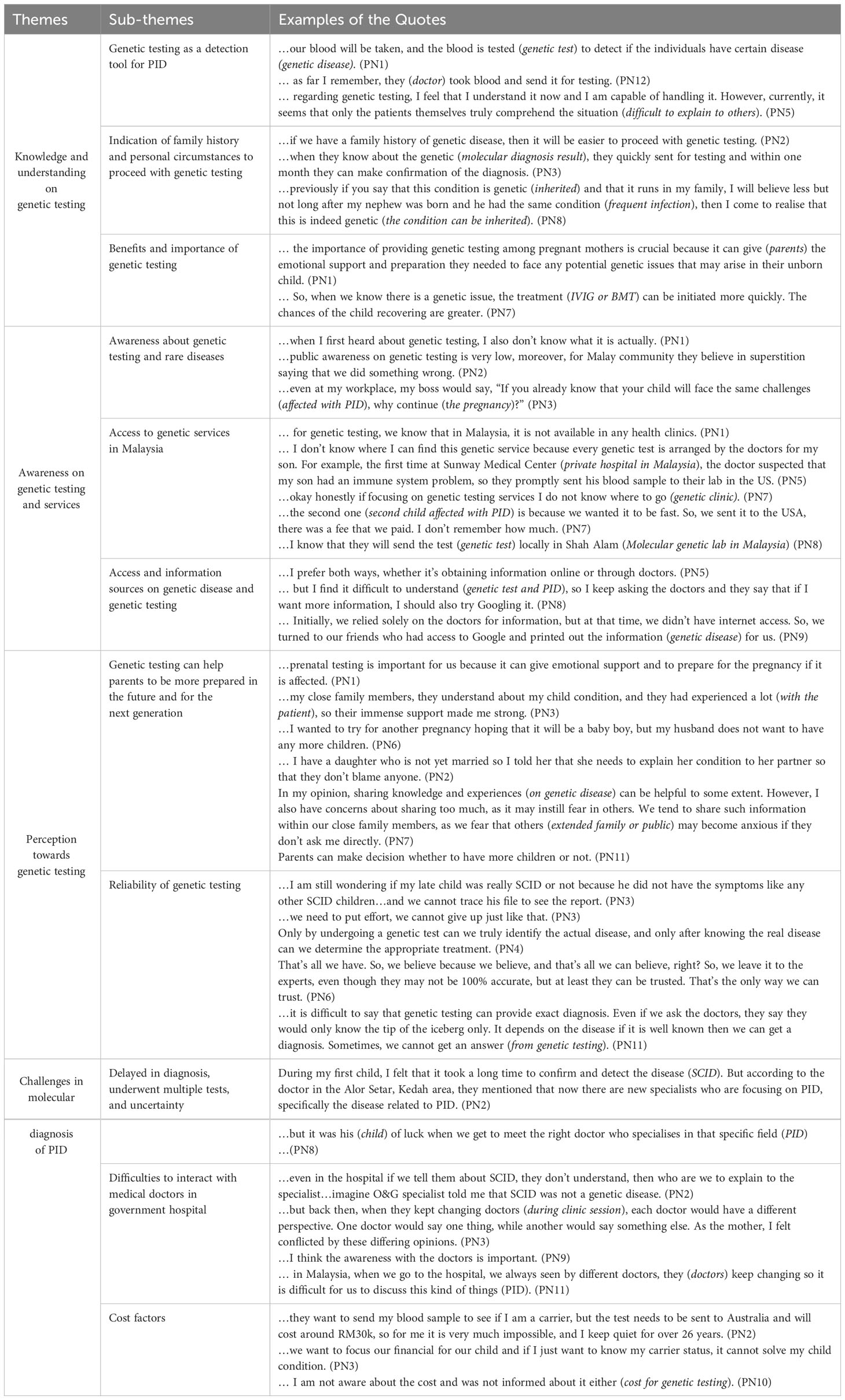
Table 3 Quotes, sub-themes, and themes identified in this study through the analysis of interview transcripts.
3.2.1 Knowledge and understanding on genetic testing
During the discussions, one of the topics that emerged was the participants’ comprehension of genetic testing. It was found that the majority of the participants’ children had undergone molecular genetic testing to verify the diagnosis of PID. However, two patients were clinically diagnosed with SCID and CVID. Next, we identified the participants’ comprehension of genetic testing, including its process, and the circumstances in which it is recommended, particularly in cases involving a family history. The participants emphasised the advantages and importance of genetic testing, further prompting discussion on these aspects. This theme is further divided into three sub-themes:
3.2.1.1 Genetic testing as a detection tool for PID
Throughout the focus group sessions, participants expressed and shared their knowledge regarding genetic testing. Surprisingly, most of them were unaware of genetic testing before undergoing the test. Their interactions with medical experts specialising in PID provided insights into the world of genetic testing. Participants came to understand that genetic testing usually involves the collection of a blood sample, which is then sent to laboratories located abroad for analysis. One parent expressed understanding about genetic testing for detecting PID but found it difficult to explain the test to others.
3.2.1.2 Indication of family history and personal circumstances to proceed with genetic testing
Most participants reached a consensus that genetic testing may be justified when there is a positive family history of recurrent infections and frequent hospitalisations. They emphasised the importance of communicating this information to doctors if they are unaware, enabling healthcare professionals to be properly informed and adjust their management accordingly. Additionally, being aware of a positive family history can assist in assessing risks during pregnancy and help avoid administering vaccines that may exacerbate the patient’s condition.
3.2.1.3 Benefits and importance of genetic testing
The participants underscored the significant role of genetic testing in facilitating early preparation for the arrival of a child, particularly during the prenatal stage. They recognised that early diagnosis through genetic testing enables timely and precise treatment, offering the opportunity to initiate interventions at the earliest possible stage. Additionally, the participants shared the belief that genetic testing can aid in both physical and mental preparations for their children. One mother, whose child had SCID, a severe combined immunodeficiency disorder, highlighted the resilience and determination of SCID mothers, who refuse to succumb to the disease or consider pregnancy termination. With effective treatments available for SCID, these mothers choose to fight for the necessary medical interventions. Overall, most participants agreed that they consent to genetic testing in the hope of finding answers to their children’s condition and receiving appropriate treatment.
3.2.2 Awareness on genetic testing and services
The second theme explores families and the public’s awareness of genetic testing and services. Three sub-themes covered are as follows.
3.2.2.1 Awareness about genetic testing and rare diseases
The awareness of genetic testing among the participants before having a child with genetic disease was lower compared to the present, where they have become more exposed to genetic testing. There is also a lack of public awareness regarding genetic testing for PID, resulting in limited understanding and knowledge about the benefits and implications of genetic testing for PID within the general population.
3.2.2.2 Access to genetic services in Malaysia
The participants had limited knowledge about the available options for genetic testing. Their understanding primarily stemmed from interactions with clinical immunologists who facilitated the diagnosis of their children. In cases where family members expressed interest in genetic testing, participants would advise the family members to consult these clinical immunologists. While some participants were aware of local genetic testing facilities, they acknowledged that sending samples overseas could yield faster and more precise results. One mother expressed appreciation for the presence of a clinical geneticist at the hospital where she gave birth, as the geneticist provided valuable explanations about the testing process during her hospital stay.
3.2.2.3 Access and information sources on PID and genetic test
Participants gathered information from various sources, including online resources and consultations with medical experts. They actively sought knowledge through online platforms, such as reputable websites and articles, to supplement their understanding of genetic testing. Additionally, they sought guidance from medical professionals who provided valuable insights, explanations, and personalised recommendations based on their expertise in the field of PID.
3.2.3 Perception towards genetic testing
The analysis of participants’ responses revealed a positive perception towards genetic testing. This optimism primarily stems from the recognition that genetic testing equips parents with improved preparedness in addressing their children’s health concerns. Participants indicated that genetic testing enhances their ability to communicate effectively with their children regarding diagnoses, enabling open and informed conversations about potential health outcomes. Furthermore, participants expressed that genetic testing plays a pivotal role in facilitating family planning decisions and future pregnancy plans. This comprehensive and favourable perspective underlines the multifaceted advantages associated with genetic testing, ranging from informed parenting to making well-considered decisions about family dynamics and future healthcare choices. Two subthemes covered in this theme:
3.2.3.1 Genetic testing can help parents to be more prepared in the future and for the next generation
The results underscore the significant role of genetic testing in enhancing parental preparedness for the future, both for their immediate family and subsequent generations. Participants consistently emphasised that genetic testing empowers parents with valuable insights into potential health risks and conditions that may impact their children and future generations. Parents have the tools and information to make smart choices about caring for their family’s health. With genetic testing, they can learn about potential health issues that could affect their children and even future generations. This helps parents to plan, take early action, and make lifestyle changes that contribute to their family’s well-being. Genetic testing serves as a means to be ready and informed, ensuring that future generations have the necessary tools to handle any health challenges that might arise.
3.2.3.2 Reliability of genetic testing
The majority of participants expressed confidence in the reliability of genetic testing. For some, the journey to diagnose their children had been filled with uncertainty and confusion, leading them to believe that genetic testing could offer definitive answers. They saw genetic testing as their only hope to bring an end to the long and challenging diagnostic journey, and thus entrusted their faith in this test. In situations where uncertainty persisted, they were willing to leave the outcome to fate, understanding that genetic testing provided the best opportunity for clarity and resolution.
3.2.4 Challenges in the molecular diagnosis of PID
The study highlighted several challenges associated with the molecular diagnosis of PID. Participants commonly reported experiencing delays in diagnosis due to the necessity for multiple tests and lingering uncertainties. Additionally, participants expressed difficulties in effectively communicating and collaborating with medical professionals in government hospitals, which could potentially impede the diagnostic process. Furthermore, cost emerged as a notable challenge, as the financial burden of molecular diagnostic procedures acted as a deterrent for some participants. These challenges collectively underscore the multifaceted nature of obstacles faced in the molecular diagnosis of PID, encompassing aspects of procedural complexity, healthcare system interactions, and economic considerations.
3.2.4.1 Delayed diagnosis, undergone multiple tests, and uncertainty
Participants expressed frustration with the lengthy diagnostic process for their children, which involved multiple clinical tests, including an HIV test. They also faced legal issues due to false accusations of rape due to an ulcer in the child’s anus. Concerns about frequent hospital visits and the mental toll of not being able to undergo prenatal testing were additional challenges. These challenges highlighted the participants’ desire for comprehensive genetic testing and timely interventions.
3.2.4.2 Difficulties to interact with medical doctors in government hospital
The study found that patients often struggled to communicate with medical doctors in government hospitals due to frequent change of doctors, making it hard to have consistent conversations about their disease. Another significant problem was the limited knowledge of many doctors and specialists in these hospitals regarding rare diseases like PID. This lack of knowledge posed difficulties for patients in obtaining the right information and help they needed. These issues highlight the challenges patients face in talking to doctors and receiving proper care in government hospitals, especially when there is a lack of continuous care and awareness about rare diseases.
3.2.4.3 Cost factors
Participants acknowledge that the cost can pose a challenge when it comes to genetic testing. They note that most tests are not sponsored, requiring them to personally cover the expenses. In the past, the price of genetic tests was exorbitant. Additionally, participants express the desire to prioritise saving money for the care of their affected child with PID.
4 Discussion
Genetic testing in Malaysia has been advancing, yet there is a notable gap in comprehensive studies addressing the knowledge, awareness, and perception regarding genetic testing, particularly concerning Primary Immunodeficiency Disease (PID). While Malaysia is making strides in genetic research and healthcare, a thorough examination of public and healthcare professionals’ understanding and perspectives regarding genetic testing is essential for informed healthcare decisions. Specific to PID, a dedicated study evaluating the knowledge and awareness of the families of children with PID would bridge the gaps and opportunities in the diagnosis and management of this group of rare genetic disorders, potentially leading to improved healthcare outcomes and enhanced genetic services in the country.
The findings of our study revealed an adequate level of awareness and understanding among participants regarding genetic testing for PID when their child was first suspected of having PID. Most participants showed improvement in knowledge and understanding of genetic testing for PID after first hearing about it and conducting further research. However, it was observed that a few participants still had a low understanding of genetic testing even after experiencing it. Some issues were raised by participants with a low understanding, believing that since only one child in the family was affected with PID, there was no inherited risk. In some cases, no specific genetic diagnosis was found. This led to confusion about whether the illness in their child was genuinely inherited or simply a random occurrence. The performance of genetic testing for PID faced obstacles concerning expenses, availability, and understanding of results. Interpreting results can be challenging due to recent advancements in identifying variations linked to PID. It is crucial to note that not every variant result in a disease, and making such assertions requires solid evidence and thorough research. Understanding genetic test results involves connecting a positive outcome with the actual disease characteristics (19). In addition, some participants demonstrated a good understanding of genetics and genetic testing, while others had limited knowledge in this area. This disparity in awareness can be attributed to differences in educational background and exposure to genetic information. Participants with a better understanding of genetics were more likely to recognise the benefits and importance of genetic testing for PID (29).
In the context of awareness, family history and personal circumstances with PID play an important role in shaping individuals’ awareness and perception of genetic testing. Participants with a family history of PID are more likely to be aware of the importance of genetic testing and the potential benefits it offers in terms of early diagnosis and appropriate treatment. On the other hand, those without a family history of PID often had limited awareness, a sense of denial towards the diagnosis and they would rely on other sources for information on the disease (30). Apart from having the strongest warning signs of PID which is a family history, knowing the signs and symptoms of PID is also important in the process of recognising the disease (31).
Access to genetic services emerged as a significant factor influencing awareness and understanding of genetic testing. Participants who had easy access to genetic services, such as genetic counsellors or specialised clinics, demonstrated a higher level of knowledge and understanding of genetic testing. However, individuals from remote areas or with limited healthcare resources faced challenges in accessing genetic services, which affected their awareness and understanding of genetic testing for PID (32). In this study, most participants had little knowledge about the access to genetic services in Malaysia, as their child was being treated by a clinical immunologist at the time of diagnosis. In addition, access to information sources may help the patient in the diagnostic journey and understanding of genetic testing. Participants who had access to reliable and up-to-date information sources, such as specialised healthcare centers or patient support groups, were able to navigate the diagnostic process more efficiently. However, individuals who lacked access to such information sources faced difficulties in obtaining accurate information about PID and genetic testing, leading to further delays in diagnosis.
The role of clinical immunologists in diagnosing and treating PID was identified in our study. The participants emphasised the necessity of healthcare providers who possess comprehensive knowledge and understanding of PID and genetic testing. Those who had positive encounters with well-informed healthcare professionals reported greater satisfaction and confidence in their diagnosis and treatment plans. These findings highlighted the crucial role of knowledgeable medical experts in effectively managing PID (5) and the need to increase the number of clinical immunologists per population in Southeast Asia (20). Correspondingly, families of PID patients may rely on the healthcare provider administering treatment to acquire knowledge and understanding regarding genetic testing. However, according to an unpublished study conducted among healthcare providers in semi-government hospitals, a significant majority of the study population exhibit inadequate knowledge and awareness regarding the use of genetic testing in PID management. This study concluded that insufficient education and hands-on experience in utilising genetic testing for PID management contribute to the lack of awareness among the study population on genetic testing. Hence, it is essential to increase awareness initiatives in society about PID, the accessibility of genetic testing, and the crucial role of genetic healthcare professionals in establishing capable and secure healthcare environments in Malaysia (Saain et al., 2022)1.
Correlates to the previous statement, poor interaction with healthcare professionals were brought up by some participants, leading to frustration and dissatisfaction throughout the diagnostic journey. Insufficient communication, lack of empathy, and limited knowledge on the part of healthcare professionals contributed to negative experiences and hindered the diagnostic process for PID. Enhancing the knowledge and understanding of healthcare professionals through continuing education and specialised training in genetic testing for PID is crucial in improving patient experiences and outcomes (33, 34).
Additionally, the participants expressed that there is limited public awareness about genetic testing and rare diseases, including PID. Participants indicated that genetic testing and rare diseases are not well understood or recognised by the public. This lack of awareness can lead to misconceptions and stigmatisation surrounding genetic testing and PID, further hindering access to appropriate healthcare services and support. In a recent 2020 study on genetic testing for hereditary disorders in Malaysia, it is found that there is a positive perception on genetic testing among 450 respondents that reside in Klang Valley (24). Further research is required to assess public opinions regarding genetic testing for rare diseases, aiming to enhance the accessibility of genetic testing services, reducing the stigma in genetic testing and early disease detection (35).
Communication emerged as a key factor in raising public awareness about genetic testing and rare diseases. Effective communication strategies, including public health campaigns, educational initiatives, and media engagement, can play a significant role in increasing awareness and understanding among the public. Collaborative efforts between healthcare professionals, patient advocacy groups, and policymakers are needed to bridge the gap in public awareness and ensure that individuals have accurate information about genetic testing for PID (36). The statement PN7 highlights the potential benefits and concerns of sharing knowledge and experiences regarding genetic diseases. While sharing such information can be beneficial to some extent, there is a worry that excessive sharing may create unnecessary fear or anxiety in others. Consequently, individuals tend to restrict the sharing of such information to their immediate family members, as they fear causing distress to extended family or the public who may not directly inquire about it (37).
The emotional impact and psychological factors associated with genetic testing for PID were evident in our study. Participants highlighted the importance of support and preparation for children with PID when considering genetic testing. Many individuals expressed feelings of anxiety and fear, anticipating the potential results and implications of genetic testing. Genetic counsellors and healthcare professionals were seen as valuable sources of support in managing these emotional concerns and providing individuals with the necessary information and resources to cope with the testing process (38). Coping and acceptance were identified as important factors in the psychological well-being of individuals undergoing genetic testing for PID. Participants reported a range of coping strategies, including seeking emotional support from family and friends, engaging in informational and emotional support groups, and accessing mental health services. Acceptance of the test results, whether positive or negative, was found to be a significant factor in the emotional well-being of individuals and their ability to adapt to the implications of genetic testing (39).
Moreover, pursuing genetic testing can present various challenges for individuals. One primary challenge mentioned by the participants in the diagnostic journey is the financial aspect, as genetic testing can be costly, and insurance coverage may be limited or unavailable. Not all individuals have the financial means to afford these tests. Additionally, there may be limited accessibility to genetic testing facilities, particularly in rural or underserved areas, leading to geographical barriers. The lack of infrastructure needed for genetic testing locally in Malaysia is a significant barrier that restricts patients from accessing affordable testing (40). The psychological impact of genetic testing, such as receiving unexpected results or confronting the potential for genetic conditions, can also pose emotional challenges. Furthermore, ethical considerations, such as concerns about privacy and the potential for discrimination based on genetic information, can add complexity to the decision-making process of pursuing genetic testing (19, 41).
5 Limitation
The present qualitative study faced several limitations. Most participants were mothers of children with PID, and there was a lack of representation from fathers. This may have limited the diversity and representativeness of the finding. The low participation of fathers’ perspectives could affect the comprehensiveness of insights, as their unique perspectives and experiences might not be adequately represented. These limitations should be considered when interpreting the results and generalising the findings to other populations or contexts.
6 Conclusion
In conclusion, this study highlights adequate knowledge, awareness, and a positive perception surrounding genetic testing for PID. Factors such as access to genetic services, family history, and personal circumstances can influence individuals’ understanding and awareness of genetic testing. The diagnostic journey for PID is often characterised by delayed diagnosis, multiple tests, and high-cost factors, emphasising the need for improved accessibility and information sources to parents on genetic testing. Emotional impact and psychological factors play a significant role in individuals’ experiences with genetic testing, underscoring the importance of support and coping strategies. The role of knowledgeable healthcare professionals in diagnosing and managing PID cannot be overstated, while public perception and awareness of genetic testing and rare diseases remain limited, calling for targeted communication strategies to enhance understanding and reduce stigma.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Research and Ethics Committee of Universiti Kebangsaan Malaysia. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AHA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. FH: Conceptualization, Formal analysis, Methodology, Writing – review & editing. II: Resources, Writing – review & editing. IA: Resources, Writing – review & editing. LB: Resources, Writing – review & editing. NI: Conceptualization, Formal analysis, Methodology, Supervision, Validation, Writing – review & editing. AA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Faculty of Medicine UKM is supporting the publication of this study. This research was conducted without any external funding or financial support.
Acknowledgments
We would like to thank all the participants involved in this project. We also extend our acknowledgment to MyPOPI for their partnership in our research collaboration and to the Faculty of Medicine, The National University of Malaysia (UKM) for their continuous support towards this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ Saain NF, Ali A, Ali A, Zahedi FD, Syed Omar SA. (2022). Knowledge, awareness, and practice of genetic on management of Primary Immunodeficiency Disorders (PID) [Unpublished manuscript]. Universiti Kebangsaan Malaysia (2022).
References
1. Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. Human inborn errors of immunity: 2019 update on the classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol (2020) 40:24–64.
2. Bousfiha A, Moundir A, Tangye SG, Picard C, Jeddane L, Al-Herz W, et al. The 2022 update of IUIS phenotypical classification for human inborn errors of immunity. J Clin Immunol (2022) 42(7):1508–20. doi: 10.1007/s10875-022-01352-z
3. Zhang Q, Frange P, Blanche S, Casanova JL. Pathogenesis of infections in HIV-infected individuals: insights from primary immunodeficiencies. Curr Opin Immunol (2017) 48:122–33.
4. Adli A, Wahab AA, Latiff AAA, Ismail I, Faizah MZ, Boekhren KB, et al. Clinical and laboratory observation on immunoglobulin replacement therapy switching from an intravenous to a subcutaneous route in a Malaysian X-linked agammaglobulinemia patient. Med J Malaysia (2022) 77(1):95–7.
5. Abd Hamid IJ, Zainudeen ZT, Hashim IF. Current perspectives and challenges of primary immunodeficiency diseases in Malaysia. Malays J Paediatr Child Health (2019) 25(2):1–6.
6. Rhim JW, Kim KH, Kim DS, Kim BS, Kim JS, Kim CH, et al. Prevalence of primary immunodeficiency in Korea. J Korean Med Sci (2012) 27(7):788–93.
7. Kumrah R, Vignesh P, Patra P, Singh A, Anjani G, Saini P, et al. Genetics of severe combined immunodeficiency. Genes Dis (2020) 7(1):52–61.
8. Lee JMH, Thong MK. Genetic counseling services and development of training programs in Malaysia. J Genet Couns (2013) 22(6):911–6. doi: 10.1007/s10897-013-9589-z
9. Soden SE, Farrow EG, Saunders CJ, Lantos JD. Genomic medicine: evolving science, evolving ethics. Personalized Med (2012) 9(5):523–8. doi: 10.2217/pme.12.56
10. Liu Z, Zhu L, Roberts R, Tong W. Toward clinical implementation of next-generation sequencing-based genetic testing in rare diseases: where are we? Trends Genet (2019) 35(11):852–67. doi: 10.1016/j.tig.2019.08.006
11. Wright CF, FitzPatrick DR, Firth HV. Paediatric genomics: diagnosing rare disease in children. Nat Rev Genet (2018) 19(5):253–68. doi: 10.1038/nrg.2017.116
12. Marchant G, Robert JS. Genetic testing for autism predisposition: Ethical, legal and social challenges. Hous J Health L & Pol'y (2008) 9:203.
13. Samuel GN, Dheensa S, Farsides B, Fenwick A, Lucassen A. Healthcare professionals’ and patients’ perspectives on consent to clinical genetic testing: moving towards a more relational approach. BMC Med Ethics (2017) 18(1):47. doi: 10.1186/s12910-017-0207-8
14. Clarke A. Anticipated stigma and blameless guilt: Mothers’ evaluation of life with the sex-linked disorder, hypohidrotic ectodermal dysplasia (XHED). Soc Sci Med (2016) 158:141–8. doi: 10.1016/j.socscimed.2016.04.027
15. Wauters A, Van Hoyweghen I. Global trends on fears and concerns of genetic discrimination: a systematic literature review. J Hum Genet (2016) 61(4):275–82. doi: 10.1038/jhg.2015.151
16. Hoffman-Andrews L. The known unknown: the challenges of genetic variants of uncertain significance in clinical practice. J Law Biosci (2017) 4(3):648–57. doi: 10.1093/jlb/lsx038
17. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med (2015) 17(5):405–24. doi: 10.1038/gim.2015.30
18. Makhnoon S, Shirts BH, Bowen DJ. Patients' perspectives of variants of uncertain significance and strategies for uncertainty management. J Genet Couns (2019) 28(2):313–25. doi: 10.1002/jgc4.1075
19. Heimall JR, Hagin D, Hajjar J, Henrickson SE, Hernandez-Trujillo HS, Tan Y, et al. Use of genetic testing for primary immunodeficiency patients. J Clin Immunol (2018) 38(3):320–9. doi: 10.1007/s10875-018-0489-8
20. Chan CM, Mahlaoui N, Sánchez Ramón S, Pergent M, Solis L, Prevot J, et al. Primary immunodeficiencies (PID) Life Index in Southeast Asia: A comparative analysis of PID Principles of Care (PoC). Front Immunol (2023) 14:1151335. doi: 10.3389/fimmu.2023.1151335
21. Chan CM, Abdul Latiff AH, Noh LM, Ismail IH, Abd Hamid IJ, Liew WK, et al. Transition practice for primary immunodeficiency diseases in Southeast Asia: a regional survey. Front Immunol (2023) 14:1209315. doi: 10.3389/fimmu.2023.1209315
22. Aizuddin AN, Ramdzan AR, Syed Omar SA, Mahmud Z, Latiff ZA, Amat S, et al. Genetic testing for cancer risk: is the community willing to pay for it? Int J Environ Res Public Health (2021) 18(16):8752. doi: 10.3390/ijerph18168752
23. Amini F, Yee KW, Soh SC, Alhadeethi A, Amini R, Ng ESC. Awareness and perception of medical genetic services among Malaysian parents of autism spectrum disorders children: the lessons to be learned. Adv Autism (2021) 8(1):27–38. doi: 10.1108/AIA-08-2020-0047
24. Chin JJ, Tham HW. Knowledge, awareness, and perception of genetic testing for hereditary disorders among Malaysians in klang valley. Front Genet (2020) 11. doi: 10.3389/fgene.2020.512582
25. Hennink M, Kaiser BN. Sample sizes for saturation in qualitative research: A systematic review of empirical tests. Soc Sci Med (2022) 292(1):114523. doi: 10.1016/j.socscimed.2021.114523
26. Metcalfe SA, Hickerton C, Savard J, Terrill B, Turbitt E, Gaff C, et al. Australians’ views on personal genomic testing: focus group findings from the Genioz study. Eur J Hum Genet (2018) 26(8):1101–12.
27. Farwati M, Kumbamu A, Kochan DC, Kullo IJ. Patient and provider perspectives on a decision aid for familial hypercholesterolemia. J Personalized Med (2018) 8(4):35.
28. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol (2006) 3(2):77–101. doi: 10.1191/1478088706QP063OA
29. Chinn IK, Orange JS. A 2020 update on the use of genetic testing for patients with primary immunodeficiency. Expert Rev Clin Immunol (2020) 16(9):897–909. doi: 10.1080/1744666X.2020.1814145
30. Convers KD, Slack M, Kanarek HJ. Take a leap of faith: implement routine genetic testing in your office. J Allergy Clin Immunology: In Pract (2022) 10(7):1676–87. doi: 10.1016/j.jaip.2022.05.017
31. Meyts I, Bousfiha A, Duff C, Singh S, Lau YL, Condino-Neto A, et al. Primary immunodeficiencies: A decade of progress and a promising future. Front Immunol (2021) 11:625753. doi: 10.3389/fimmu.2020.625753
32. White S, Jacobs C, Phillips J. Mainstreaming genetics and genomics: a systematic review of the barriers and facilitators for nurses and physicians in secondary and tertiary care. Genet Med (2020) 22(7):1149–55.
33. Solis L, Nordin J, Prevot J, Mahlaoui N, Sánchez-Ramón S, Ali A, et al. The PID Life Index: an interactive tool to measure the status of the PID healthcare environment in any given country. Orphanet J Rare Dis (2022) 17(1):11. doi: 10.1186/s13023-021-02161-0
34. Nielsen EE, Owen T, Roach M, Dix A. A Patient Centred Approach to Rare Disease Technology. Extended Abstracts of the 2023 CHI Conference on Human Factors in Computing Systems (2023) (2023) pp. 1-7.
35. Tiner JC, Mechanic LE, Gallicchio L, Gillanders EM, Helzlsouer KJ. Awareness and use of genetic testing: An analysis of the Health Information National Trends Survey 2020. Genet Med (2022) 24(12):2526–34. doi: 10.1016/j.gim.2022.08.023
36. Roberts JS, Patterson AK, Uhlmann WR. Genetic testing for neurodegenerative diseases: Ethical and health communication challenges. Neurobiol Dis (2020) 141:104871. doi: 10.1016/j.nbd.2020.104871
37. Geelen E, Horstman K, Marcelis CL, Doevendans PA, Van Hoyweghen I. Unravelling fears of genetic discrimination: an exploratory study of Dutch HCM families in an era of genetic non-discrimination acts. Eur J Hum Genet (2012) 20(10):1018–23. doi: 10.1038/ejhg.2012.53
38. El Hawary RE, Meshaal SS, Abd Elaziz DS, Elsharkawy MA, Alkady RS, Lotfy S, et al. Genetic counseling in primary immunodeficiency disorders: an emerging experience in Egypt. Mol Diagnosis Ther (2017) 21(6):677–84. doi: 10.1007/s40291-017-0297-5
39. Brede KK, Wandel M, Wiig I, von der Lippe C. Primary immunodeficiency diseases and gastrointestinal distress: coping strategies and dietary experiences to relieve symptoms. Qual Health Res (2021) 31(2):361–72. doi: 10.1177/1049732320967908
40. Nordin J, Solis L, Prevot J, Mahlaoui N, Chapel H, Sánchez-Ramón S, et al. The PID principles of care: where are we now? A global status report based on the PID life index. Front Immunol (2021) 12. doi: 10.3389/fimmu.2021.780140
Keywords: knowledge, awareness, perception, genetic testing, primary immunodeficiency disease, parents
Citation: Ahmad Azahari AHS, Hakim Zada F, Ismail IH, Abd Hamid IJ, Lim BWD, Ismail NAS and Ali A (2024) Knowledge, awareness, and perception on genetic testing for primary immunodeficiency disease among parents in Malaysia: a qualitative study. Front. Immunol. 14:1308305. doi: 10.3389/fimmu.2023.1308305
Received: 06 October 2023; Accepted: 07 December 2023;
Published: 12 January 2024.
Edited by:
Esther de Vries, Tilburg University, NetherlandsReviewed by:
Aziz Bousfiha, Hassan II University Casablanca, MorroccoAyca Kiykim, Istanbul University-Cerrahpasa, Türkiye
Copyright © 2024 Ahmad Azahari, Hakim Zada, Ismail, Abd Hamid, Lim, Ismail and Ali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adli Ali, adli.ali@ppukm.ukm.edu.my
 Ahmad Hazim Syakir Ahmad Azahari
Ahmad Hazim Syakir Ahmad Azahari Farheen Hakim Zada1
Farheen Hakim Zada1 Intan Hakimah Ismail
Intan Hakimah Ismail Intan Juliana Abd Hamid
Intan Juliana Abd Hamid Noor Akmal Shareela Ismail
Noor Akmal Shareela Ismail Adli Ali
Adli Ali