- Department of Dermatology, Teikyo University School of Medicine, Tokyo, Japan
In the past, psoriasis was considered a skin disease caused only by keratinocyte disorders. However, the efficacy of immunosuppressive drugs and biologics used to treat psoriasis proves that psoriasis is an immune-mediated disease. Indeed, a variety of immune cells are involved in the pathogenesis of psoriasis, including dendritic cells, Th17 cells, and resident memory T cells. Furthermore, keratinocytes play a role in the development of psoriasis as immune cells by secreting antibacterial peptides, chemokines, tumor necrosis factor-α, interleukin (IL)-36, and IL-23. These immune cells and skin cells interact and drive the aberrant differentiation and proliferation of keratinocytes. This crosstalk between keratinocytes and immune cells critical in the pathogenesis of psoriasis forms an inflammatory loop, resulting in the persistence or exacerbation of psoriasis plaques.
1 Introduction
Psoriasis is a chronic inflammatory skin disease clinically characterized by indurated scaly erythema and pathologically by abnormal differentiation and proliferation of keratinocytes. Therefore, in the past, psoriasis was considered a skin disease caused only by keratinocyte disorders. However, reports of psoriasis successfully treated with cyclosporine have altered our understanding of the pathogenesis of psoriasis. In addition, the efficacy of immunosuppressive drugs and biologics used to treat psoriasis proves that psoriasis is an immune-mediated disease (1, 2).
To date, many studies have revealed how a variety of immune cells are involved in the pathogenesis of psoriasis. Furthermore, keratinocytes are not only the consequences of immune reactions (namely, phenotype), but also themselves play a role in the development of psoriasis as immune cells. These immune cells and keratinocytes interact, consequently driving the aberrant differentiation and proliferation of keratinocytes.
In this review article, we focus on this crosstalk mechanism and discuss its importance in the pathogenesis of psoriasis.
2 Crosstalk: immune cells to keratinocytes
In the pathogenesis of psoriasis, interleukin (IL)-17 plays a key role. Moreover, IL-17 induces the proliferation and abnormal differentiation of keratinocytes (3). Keratinocytes simulated with IL-17 and tumor necrosis factor (TNF)-α produce various inflammatory cytokines, chemokines, and antibacterial peptides (AMPs) (4–6), as discussed later. IL-22 also activates keratinocytes, resulting in the proliferation and production of these inflammatory substances (7–10). In this section, we focus on immune cells that affect keratinocytes in psoriasis (Figure 1).
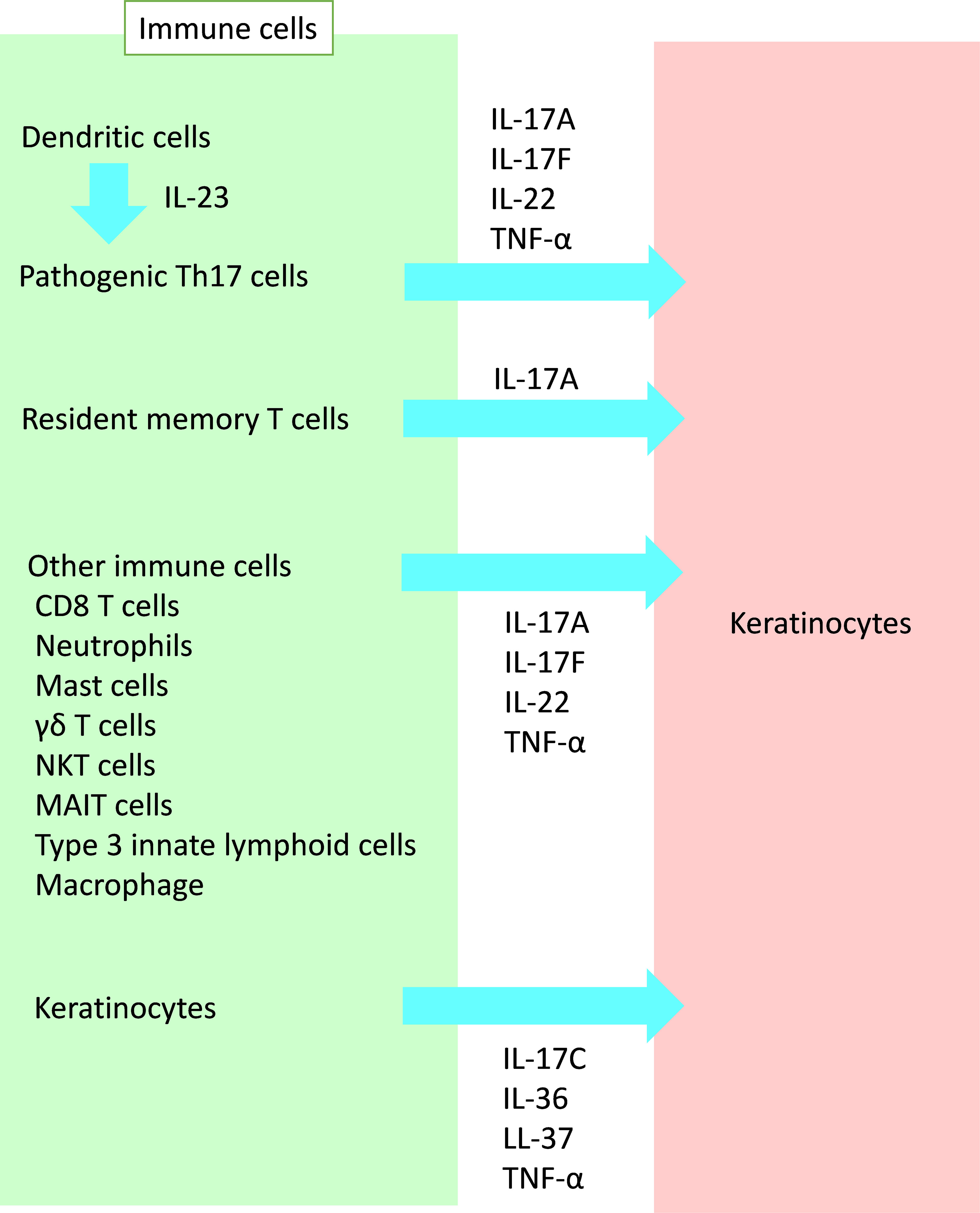
Figure 1 Crosstalk from immune cells to keratinocytes in psoriasis. A variety of immune cells affect keratinocytes in psoriasis. IL, interleukin; TNF, tumor necrosis factor; NKT, natural killer T; MAIT, mucosal-associated invariant T.
2.1 Pathogenic Th17 cells induced by dendritic cells
Th17 cells play a pivotal role in the pathogenesis of psoriasis. Murine studies have revealed that transforming growth factor (TFG)-β and IL-6 are required to activate a unique transcription factor known as retinoid-related orphan receptor-γt (RORγt). RORγt in association with other transcription factors, increases both IL-23R and IL-17A in Th17 cells. Subsequent exposure of IL-23 to developing Th17 cells enhances Th17 cytokines, including IL-17 (11). Human Th17 cells produce mainly IL-17A, IL-17F, and IL-22 in addition to TNF-α (12, 13). Their cytokines drive keratinocytes to their aberrant differentiation and proliferation, as well as producing pro-inflammatory substances. IL-23 promotes Th17 cells to become highly pathogenic. It also regulates the development and maintenance of the Th17 population (14–16). The main source of IL-23 is thought to be inflammatory dendritic cells (DC), as described in our previous review article (16), including TNF-α and inducible nitric oxide synthase (iNOS)-producing DC (Tip-DC) and slanDC.
2.2 Resident memory T cells
Recently, skin resident memory T (Trm) cells have recieved attention, especially as the cells contributing to relapse or Köbner phenomenon (3, 17–20). In resolved psoriatic skin lesions, a population of Trm cells are observed, which are responsible for local relapse of psoriasis (17–19, 21–23). Epidermal CD8+CD103+ Trm cells are considered to be one of the major immune cells in resolved skin and are capable of IL-17A (22, 24–26). Gallais Sérézal et al. confirmed through NanoString analysis that CD49a−CD103+CD8+ Trm cells were capable of triggering psoriasiform tissue response (27). These results suggest that IL-17−producing CD49a−CD103+CD8+ Trm cells are responsible for psoriasis relapse (20, 28, 29).
In addition to skin Trm cells, memory-like γδT cells (30), and skin structural cells with inflammatory memory (31, 32) could be involved in psoriasis relapse (20).
2.3 Other immune cells producing IL-17A
In addition to Th17 cells, IL-17A is produced by various cells of the innate and adaptive immune systems (11). CD8+ IL-17-producing T cells are observed in psoriatic lesions, and they produce both Th1- and Th17-related cytokines, including interferon (IFN)-γ, TNF-α, IL-17A, IL-21, and IL-22 (33–35).
Since neutrophils and mast cells staining positive for IL-17 were identified at higher densities than IL-17+ T cells in psoriatic lesions, neutrophils and mast cells are considered other significant potential sources of IL-17A in psoriasis (36–38). However, whether these cells synthesize and secrete IL-17A or whether positive staining represents cytokine uptake has yet to be determined (11). Mashiko et al. reported that human mast cells are major IL-22 producers in patients with psoriasis (39). Further investigation is needed to elucidate the role of mast cells and neutrophils in the pathogenesis of psoriasis.
IL-17A and IL-17F are also secreted by innate immune cells, such as group 3 innate lymphoid cells (ILC3s), and innate-like lymphocytes (ILLs), such as γδT cells, mucosal-associated invariant T (MAIT) cells, and natural killer T (NKT) cells (40–45).
Under the condition of abundant IL-23 in psoriasis lesional skin, some macrophages may produce IL-17A, IL-22 and IFN-γ in addition to TNF-α as described in our previous review article (16).
2.4 Keratinocytes
Keratinocytes also act as immune cells. Some cytokines secreted by keratinocytes, including IL-17C and IL-36, act on keratinocytes in an autocrine way (46). IL-36 cytokines, such as IL-36α/β/γ, are produced by keratinocytes following stimulation by TNF-α, IL-17A, IL-22, and IL-1β. IL-36 stimulates keratinocytes to produce TNF-α and IL-17C (47, 48). IL-17C is expressed by (and acts on) epithelial cells (49). Keratinocytes, the main producers of (and responders to) IL-17C in the skin, contribute to psoriatic inflammation (50–52). IL-17C has been identified as a functional regulator of the initial psoriatic cytokine network, suggesting its role during the early stages of psoriatic inflammation, or the “priming” for plaque formation (53).
Cathelicidins are a class of AMPs. LL-37, one of cathelicidins, produced by skin injury and bacterial infection, activates toll-like receptor (TLR)8 in keratinocytes and induces IL-17C through the induction of IL-36γ (47). Inhibition of IL-17 results in normalization of IL-36γ and IL-17C to levels associated with non-lesional skin (54).
3 Crosstalk: keratinocytes to immune cells
Reciprocally, keratinocytes also produce various substances that affect immune cells. In this section, we focus on these substances and their effects on immune cells (Figure 2).
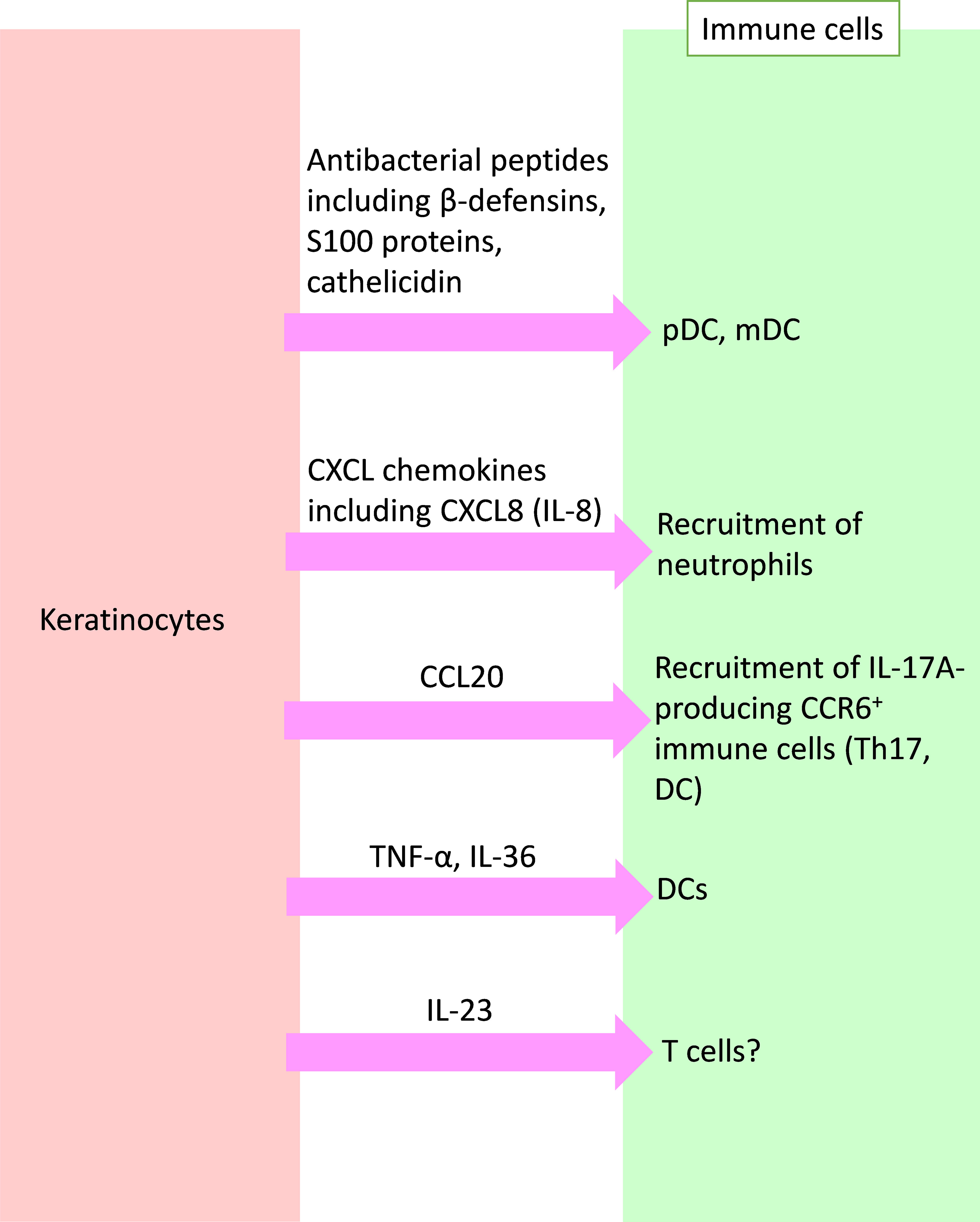
Figure 2 Crosstalk from keratinocytes to immune cells in psoriasis. Activated keratinocytes produce antibacterial peptides, chemokines, and inflammatory cytokines, and affect immune cells in psoriasis. pDC, plasmacytoid dendritic cell; mDC, myeloid DC; CXCL, C-X-C motif chemokine ligand; CCL, C-C motif chemokine ligand; IL, interleukin; TNF, tumor necrosis factor.
3.1 Antibacterial peptides, including β-defensins, S100 proteins and cathelicidin
In non-lesional skin in psoriasis patients, trauma, injury, infection, or medication causes the production of various autoantigens from stressed or damaged keratinocytes (20, 55, 56). Among them, cationic AMPs [including LL-37, human beta-defensin (hBD)-2, and hBD-3], develop with DNA or RNA to form multimeric AMP−nucleic acid complexes, which induce the production of interferon (IFN)-α and IFN-β through TLR7 or 8 in plasmacytoid dendritic cells (pDCs) or increase the amounts of IL-6 and TNF-α by myeloid dendritic cells (mDCs) (20, 57, 58). IL-6, together with TGF-β, drives naïve T cell differentiation into Th17 cells, as described above. IFN-α and TNF-α further activate mDCs to produce IL-12 and IL-23 (16, 20, 59, 60). This process could be involved in the mechanism underlying Köbner phenomenon in psoriasis (3, 61).
In psoriatic lesions, various AMPs such as hBD-2, hBD-3, S100 proteins, and cathelicidin, are also highly expressed (15, 62, 63). hBD-2 and hBD-3 are induced by TNF-α and IFN-γ in keratinocytes (64, 65). hBD-2 is also induced by IL-17A and IL-22 (66). S100 proteins, such as S100A7 (psoriasin), S100A8 (calgranulin A), S100A9 (calgranulin B), S100A12 (calgranulin C), and S100A15, are abundantly expressed in psoriatic lesions, and some are elevated in the serum of psoriatic patients (67).
In a study by Liang et al., IL-22 in conjunction with IL-17A or IL-17F synergistically induced the expression of hBD-2 and S100A9 and additively enhanced the expression of S100A7 and S100A8 in keratinocytes (68). S100A7 may also havechemotactic potential in psoriasis (15, 69). The LL-37 high expression in the psoriatic epidermis may also accelerate inflammation through its capacity to enable pDC to recognize self-DNA via TLR9 (58). These AMPs affect various immune cells resulting in triggering, sustaining, and/or exacerbating psoriatic inflammation.
3.2 Chemokines, including CXCL1, CXCL2, CXCL8 (IL-8), and CCL20
Keratinocytes stimulated with IL-17 showed increased expression of multiple chemokines, including C-X-C motif chemokine ligand (CXCL)1, CXCL2, CXCL3, CXCL5, CXCL8 (IL-8), and C-C motif chemokine ligand (CCL)20 (4, 70–72). The CXCL chemokines likely attract neutrophils to the psoriatic epidermis (3, 11). CCL20 may recruit CCR6+ cells, including Th17 and dendritic cells, to the skin (70). Inhibition of IL-17 normalizes expression of CXCL1, CXCL8 (IL-8), and CCL20 to the levels associated with non-lesional skin (54).
3.3 TNF-α, and IL-36
Keratinocytes stimulated with TNF-α, IL-17A, IL-22, and IL-1β produce IL-36 (46). IL-36 stimulates keratinocytes themselves to produce TNF-α and IL-17C (47, 48). TNF-α activates mDCs, leading to production of IL-23. IL-36 drives IFN-α production in pDCs, as well as IL-1β, IL-6, and IL-23 production in mDCs (46). These cytokines secreted by keratinocytes form an aggravating inflammatory loop in the pathogenesis of psoriasis.
3.4 IL-23
Several reports indicate that keratinocytes produce IL-23. Moreover, immunostaining of psoriatic lesions revealed enhanced expression of IL-23 in keratinocytes (73, 74). Park et al., using publicly available single-cell RNA sequencing data from human samples, revealed that IL-23 expression was detectable in psoriatic keratinocytes as well as DCs (75). Kelemen et al. reported that psoriasis-associated inflammatory conditions induced IL-23 mRNA expression in normal huma epidermal keratinocytes (76). Li et al., using a genetic mouse model, showed that keratinocyte-produced IL-23 was sufficient to cause chronic skin inflammation with an IL-17 profile and that epigenetic control of IL-23 expression in keratinocytes was important for chronic skin inflammation (77). However, whether the expression of IL-23 in keratinocytes in psoriasis contributes to the development of psoriasis remains to be elucidated.
4 Effect of IL-17 or IL-23 inhibition on immune cells and keratinocytes in psoriasis
As mentioned above, IL-23 and IL-17 play important roles in the pathogenesis of psoriasis. Indeed, biologics, including IL-23 inhibitors and IL-17 inhibitors, greatly impact keratinocytes and immune cells in psoriasis.
Secukinumab (an anti-IL-17 antibody) and guselkumab (an anti-IL-23 antibody) decrease the frequencies of inflammatory monocyte-like cells, inflammatory DC-like cells, and CD4+CD49a-CD103- T cells (78). Furthermore, bimekizumab (an anti-IL17A/F antibody) induces normalization of keratinocyte-related gene products, including CXCL1, CXCL8, CCL20, IL-36γ, and IL-17C, to levels associated with non-lesional skin (54). Krueger et al. reported that secukinumab caused reductions in critical downstream targets of IL-17A in the skin, including the AMPs (DEFB4A/β-defensin 2 and the S100 family) in addition to reductions in IL-23 and IL-17 in transcriptomic analyses (79). Inhibition of IL-23 or TNF-α also caused reductions in the gene expression of Th17-induced mediators by keratinocytes, including antimicrobial peptides (80, 81). Mehta et al. reported that inhibition of IL-23 reduced memory T cells while maintaining regulatory T cells, and vice versa for secukinumab (78). Furthermore, Whiley also revealed that clinical anti-IL-23 therapy depleted IL-17-producing Trm cells from the skin of patients with psoriasis (82).
5 Conclusion
In psoriasis, a variety of immune cells activate keratinocytes (mainly through Th17 cytokines), resulting in their abnormal differentiation and proliferation. Activated keratinocytes produce AMPs, chemokines, and various cytokines, which cause further inflammation and the recruitment of inflammatory cells. In addition, keratinocytes activate themselves by producing IL-36, IL-17C, and TNF-α. The crosstalk between immune cells and keratinocytes contributes to the development and maintenance of psoriasis.
Author contributions
MK: Conceptualization, Writing – original draft. YT: Supervision, Validation, Writing – review & editing, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kamata M, Tada Y. Efficacy and safety of biologics for psoriasis and psoriatic arthritis and their impact on comorbidities: A literature review. Int J Mol Sci (2020) 21(5):1690. doi: 10.3390/ijms21051690
2. Kamata M, Tada Y. Safety of biologics in psoriasis. J Dermatol (2018) 45:279–86. doi: 10.1111/1346-8138.14096
3. Yamanaka K, Yamamoto O, Honda T. Pathophysiology of psoriasis: A review. J Dermatol (2021) 48:722–31. doi: 10.1111/1346-8138.15913
4. Chiricozzi A, Guttman-Yassky E, Suárez-Fariñas M, Nograles KE, Tian S, Cardinale I, et al. Integrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol (2011) 131:677–87. doi: 10.1038/jid.2010.340
5. Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol (1998) 111:645–9. doi: 10.1046/j.1523-1747.1998.00347.x
6. Wang CQF, Akalu YT, Suarez-Farinas M, Gonzalez J, Mitsui H, Lowes MA, et al. IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis. J Invest Dermatol (2013) 133:2741–52. doi: 10.1038/jid.2013.237
7. Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest (2008) 118:597–607. doi: 10.1172/jci33263
8. Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med (Berl) (2009) 87:523–36. doi: 10.1007/s00109-009-0457-0
9. Wolk K, Witte E, Wallace E, Döcke WD, Kunz S, Asadullah K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol (2006) 36:1309–23. doi: 10.1002/eji.200535503
10. Wang Z, Shi D. Research progress on the neutrophil components and their interactions with immune cells in the development of psoriasis. Skin Res Technol (2023) 29:e13404. doi: 10.1111/srt.13404
11. Lynde CW, Poulin Y, Vender R, Bourcier M, Khalil S. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol (2014) 71:141–50. doi: 10.1016/j.jaad.2013.12.036
12. Annunziato F, Romagnani S. Heterogeneity of human effector CD4+ T cells. Arthritis Res Ther (2009) 11:257. doi: 10.1186/ar2843
13. Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol (2010) 130:1373–83. doi: 10.1038/jid.2009.399
14. Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem (2003) 278:1910–4. doi: 10.1074/jbc.M207577200
15. Ogawa E, Sato Y, Minagawa A, Okuyama R. Pathogenesis of psoriasis and development of treatment. J Dermatol (2018) 45:264–72. doi: 10.1111/1346-8138.14139
16. Kamata M, Tada Y. Dendritic cells and macrophages in the pathogenesis of psoriasis. Front Immunol (2022) 13:941071. doi: 10.3389/fimmu.2022.941071
17. Owczarczyk Saczonek A, Krajewska-Włodarczyk M, Kasprowicz-Furmańczyk M, Placek W. Immunological memory of psoriatic lesions. Int J Mol Sci (2020) 21. doi: 10.3390/ijms21020625
18. Gallais Sérézal I, Classon C, Cheuk S, Barrientos-Somarribas M, Wadman E, Martini E, et al. Resident T cells in resolved psoriasis steer tissue responses that stratify clinical outcome. J Invest Dermatol (2018) 138:1754–63. doi: 10.1016/j.jid.2018.02.030
19. Matos TR, O'Malley JT, Lowry EL, Hamm D, Kirsch IR, Robins HS, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest (2017) 127:4031–41. doi: 10.1172/jci93396
20. Tian D, Lai Y. The relapse of psoriasis: mechanisms and mysteries. JID Innov (2022) 2:100116. doi: 10.1016/j.xjidi.2022.100116
21. Di Meglio P, Villanova F, Navarini AA, Mylonas A, Tosi I, Nestle FO, et al. Targeting CD8(+) T cells prevents psoriasis development. J Allergy Clin Immunol (2016) 138:274–6.e6. doi: 10.1016/j.jaci.2015.10.046
22. Cheuk S, Wikén M, Blomqvist L, Nylén S, Talme T, Ståhle M, et al. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J Immunol (2014) 192:3111–20. doi: 10.4049/jimmunol.1302313
23. Suárez-Fariñas M, Fuentes-Duculan J, Lowes MA, Krueger JG. Resolved psoriasis lesions retain expression of a subset of disease-related genes. J Invest Dermatol (2011) 131:391–400. doi: 10.1038/jid.2010.280
24. Fujiyama T, Umayahara T, Kurihara K, Shimauchi T, Ito T, Aoshima M, et al. Skin infiltration of pathogenic migratory and resident T cells is decreased by secukinumab treatment in psoriasis. J Invest Dermatol (2020) 140:2073–6.e6. doi: 10.1016/j.jid.2020.02.024
25. Kurihara K, Fujiyama T, Phadungsaksawasdi P, Ito T, Tokura Y. Significance of IL-17A-producing CD8(+)CD103(+) skin resident memory T cells in psoriasis lesion and their possible relationship to clinical course. J Dermatol Sci (2019) 95:21–7. doi: 10.1016/j.jdermsci.2019.06.002
26. Cheuk S, Schlums H, Gallais Sérézal I, Martini E, Chiang SC, Marquardt N, et al. CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity (2017) 46:287–300. doi: 10.1016/j.immuni.2017.01.009
27. Gallais Sérézal I, Hoffer E, Ignatov B, Martini E, Zitti B, Ehrström M, et al. A skewed pool of resident T cells triggers psoriasis-associated tissue responses in never-lesional skin from patients with psoriasis. J Allergy Clin Immunol (2019) 143:1444–54. doi: 10.1016/j.jaci.2018.08.048
28. Tokura Y, Phadungsaksawasdi P, Kurihara K, Fujiyama T, Honda T. Pathophysiology of skin resident memory T cells. Front Immunol (2020) 11:618897. doi: 10.3389/fimmu.2020.618897
29. Ryan GE, Harris JE, Richmond JM. Resident memory T cells in autoimmune skin diseases. Front Immunol (2021) 12:652191. doi: 10.3389/fimmu.2021.652191
30. Hartwig T, Pantelyushin S, Croxford AL, Kulig P, Becher B. Dermal IL-17-producing γδ T cells establish long-lived memory in the skin. Eur J Immunol (2015) 45:3022–33. doi: 10.1002/eji.201545883
31. Naik S, Larsen SB, Gomez NC, Alaverdyan K, Sendoel A, Yuan S, et al. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature (2017) 550:475–80. doi: 10.1038/nature24271
32. Larsen SB, Cowley CJ, Sajjath SM, Barrows D, Yang Y, Carroll TS, et al. Establishment, maintenance, and recall of inflammatory memory. Cell Stem Cell (2021) 28:1758–74.e8. doi: 10.1016/j.stem.2021.07.001
33. Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol (2008) 181:4733–41. doi: 10.4049/jimmunol.181.7.4733
34. Ortega C, Fernández AS, Carrillo JM, Romero P, Molina IJ, Moreno JC, et al. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J Leukoc Biol (2009) 86:435–43. doi: 10.1189/jlb.0109046
35. Hijnen D, Knol EF, Gent YY, Giovannone B, Beijn SJ, Kupper TS, et al. CD8(+) T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-γ, IL-13, IL-17, and IL-22. J Invest Dermatol (2013) 133:973–9. doi: 10.1038/jid.2012.456
36. Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol (2011) 187:490–500. doi: 10.4049/jimmunol.1100123
37. Res PC, Piskin G, de Boer OJ, van der Loos CM, Teeling P, Bos JD, et al. Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PloS One (2010) 5:e14108. doi: 10.1371/journal.pone.0014108
38. Reich K, Papp KA, Matheson RT, Tu JH, Bissonnette R, Bourcier M, et al. Evidence that a neutrophil-keratinocyte crosstalk is an early target of IL-17A inhibition in psoriasis. Exp Dermatol (2015) 24:529–35. doi: 10.1111/exd.12710
39. Mashiko S, Bouguermouh S, Rubio M, Baba N, Bissonnette R, Sarfati M. Human mast cells are major IL-22 producers in patients with psoriasis and atopic dermatitis. J Allergy Clin Immunol (2015) 136:351–9.e1. doi: 10.1016/j.jaci.2015.01.033
40. Bernink JH, Ohne Y, Teunissen MBM, Wang J, Wu J, Krabbendam L, et al. c-Kit-positive ILC2s exhibit an ILC3-like signature that may contribute to IL-17-mediated pathologies. Nat Immunol (2019) 20:992–1003. doi: 10.1038/s41590-019-0423-0
41. Navarro-Compán V, Puig L, Vidal S, Ramírez J, Llamas-Velasco M, Fernández-Carballido C, et al. The paradigm of IL-23-independent production of IL-17F and IL-17A and their role in chronic inflammatory diseases. Front Immunol (2023) 14:1191782. doi: 10.3389/fimmu.2023.1191782
42. Hall AO, Towne JE, Plevy SE. Get the IL-17F outta here! Nat Immunol (2018) 19:648–50. doi: 10.1038/s41590-018-0141-z
43. Rosine N, Rowe H, Koturan S, Yahia-Cherbal H, Leloup C, Watad A, et al. Characterization of blood mucosal-associated invariant T cells in patients with axial spondyloarthritis and of resident mucosal-associated invariant T cells from the axial entheses of non-axial spondyloarthritis control patients. Arthritis Rheumatol (2022) 74:1786–95. doi: 10.1002/art.42090
44. Bielecki P, Riesenfeld SJ, Hütter JC, Torlai Triglia E, Kowalczyk MS, Ricardo-Gonzalez RR, et al. Skin-resident innate lymphoid cells converge on a pathogenic effector state. Nature (2021) 592:128–32. doi: 10.1038/s41586-021-03188-w
45. Cai Y, Shen X, Ding C, Qi C, Li K, Li X, et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity (2011) 35:596–610. doi: 10.1016/j.immuni.2011.08.001
46. Sachen KL, Arnold Greving CN, Towne JE. Role of IL-36 cytokines in psoriasis and other inflammatory skin conditions. Cytokine (2022) 156:155897. doi: 10.1016/j.cyto.2022.155897
47. Miura S, Garcet S, Li X, Cueto I, Salud-Gnilo C, Kunjravia N, et al. Cathelicidin antimicrobial peptide LL37 induces toll-like receptor 8 and amplifies IL-36γ and IL-17C in human keratinocytes. J Invest Dermatol (2023) 143:832–41.e4. doi: 10.1016/j.jid.2022.10.017
48. Miura S, Garcet S, Salud-Gnilo C, Gonzalez J, Li X, Murai-Yamamura M, et al. IL-36 and IL-17A cooperatively induce a psoriasis-like gene expression response in human keratinocytes. J Invest Dermatol (2021) 141:2086–90. doi: 10.1016/j.jid.2021.01.019
49. Brembilla NC, Boehncke WH. Revisiting the interleukin 17 family of cytokines in psoriasis: pathogenesis and potential targets for innovative therapies. Front Immunol (2023) 14:1186455. doi: 10.3389/fimmu.2023.1186455
50. Vandeghinste N, Klattig J, Jagerschmidt C, Lavazais S, Marsais F, Haas JD, et al. Neutralization of IL-17C reduces skin inflammation in mouse models of psoriasis and atopic dermatitis. J Invest Dermatol (2018) 138:1555–63. doi: 10.1016/j.jid.2018.01.036
51. Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol (2011) 12:1159–66. doi: 10.1038/ni.2156
52. Johnston A, Fritz Y, Dawes SM, Diaconu D, Al-Attar PM, Guzman AM, et al. Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation. J Immunol (2013) 190:2252–62. doi: 10.4049/jimmunol.1201505
53. Boonpethkaew S, Meephansan J, Jumlongpim O, Tangtanatakul P, Soonthornchai W, Wongpiyabovorn J, et al. Transcriptomic profiling of peripheral edge of lesions to elucidate the pathogenesis of psoriasis vulgaris. Int J Mol Sci (2022) 23(9):4983. doi: 10.3390/ijms23094983
54. Oliver R, Krueger JG, Glatt S, Vajjah P, Mistry C, Page M, et al. Bimekizumab for the treatment of moderate-to-severe plaque psoriasis: efficacy, safety, pharmacokinetics, pharmacodynamics and transcriptomics from a phase IIa, randomized, double-blind multicentre study. Br J Dermatol (2022) 186:652–63. doi: 10.1111/bjd.20827
55. Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol (2017) 140:645–53. doi: 10.1016/j.jaci.2017.07.004
56. Ni X, Lai Y. Keratinocyte: A trigger or an executor of psoriasis? J Leukoc Biol (2020) 108:485–91. doi: 10.1002/jlb.5mr0120-439r
57. Ganguly D, Chamilos G, Lande R, Gregorio J, Meller S, Facchinetti V, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med (2009) 206:1983–94. doi: 10.1084/jem.20090480
58. Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature (2007) 449:564–9. doi: 10.1038/nature06116
59. Nakajima A, Matsuki T, Komine M, Asahina A, Horai R, Nakae S, et al. TNF, but not IL-6 and IL-17, is crucial for the development of T cell-independent psoriasis-like dermatitis in Il1rn-/- mice. J Immunol (2010) 185:1887–93. doi: 10.4049/jimmunol.1001227
60. Lande R, Botti E, Jandus C, Dojcinovic D, Fanelli G, Conrad C, et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun (2014) 5:5621. doi: 10.1038/ncomms6621
61. Takahashi T, Yamasaki K. Psoriasis and antimicrobial peptides. Int J Mol Sci (2020) 21(18):6791. doi: 10.3390/ijms21186791
62. Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol (2009) 30:131–41. doi: 10.1016/j.it.2008.12.003
63. Morizane S, Gallo RL. Antimicrobial peptides in the pathogenesis of psoriasis. J Dermatol (2012) 39:225–30. doi: 10.1111/j.1346-8138.2011.01483.x
64. Harder J, Bartels J, Christophers E, Schröder JM. A peptide antibiotic from human skin. Nature (1997) 387:861. doi: 10.1038/43088
65. Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol (2003) 171:3262–9. doi: 10.4049/jimmunol.171.6.3262
66. Hollox EJ, Huffmeier U, Zeeuwen PL, Palla R, Lascorz J, Rodijk-Olthuis D, et al. Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet (2008) 40:23–5. doi: 10.1038/ng.2007.48
67. Büchau AS, Gallo RL. Innate immunity and antimicrobial defense systems in psoriasis. Clin Dermatol (2007) 25:616–24. doi: 10.1016/j.clindermatol.2007.08.016
68. Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med (2006) 203:2271–9. doi: 10.1084/jem.20061308
69. Jinquan T, Vorum H, Larsen CG, Madsen P, Rasmussen HH, Gesser B, et al. Psoriasin: a novel chemotactic protein. J Invest Dermatol (1996) 107:5–10. doi: 10.1111/1523-1747.ep12294284
70. Harper EG, Guo C, Rizzo H, Lillis JV, Kurtz SE, Skorcheva I, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol (2009) 129:2175–83. doi: 10.1038/jid.2009.65
71. Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suárez-Fariñas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol (2008) 159:1092–102. doi: 10.1111/j.1365-2133.2008.08769.x
72. Homey B, Dieu-Nosjean MC, Wiesenborn A, Massacrier C, Pin JJ, Oldham E, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol (2000) 164:6621–32. doi: 10.4049/jimmunol.164.12.6621
73. Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J Immunol (2006) 176:1908–15. doi: 10.4049/jimmunol.176.3.1908
74. Yawalkar N, Tscharner GG, Hunger RE, Hassan AS. Increased expression of IL-12p70 and IL-23 by multiple dendritic cell and macrophage subsets in plaque psoriasis. J Dermatol Sci (2009) 54:99–105. doi: 10.1016/j.jdermsci.2009.01.003
75. Park YJ, Kim YH, Lee ES, Kim YC. Comparative analysis of single-cell transcriptome data reveals a novel role of keratinocyte-derived IL-23 in psoriasis. Front Immunol (2022) 13:905239. doi: 10.3389/fimmu.2022.905239
76. Kelemen E, Ádám É, Sági SM, Göblös A, Kemény L, Bata-Csörgő Z, et al. Psoriasis-associated inflammatory conditions induce IL-23 mRNA expression in normal human epidermal keratinocytes. Int J Mol Sci (2022) 23(1):540. doi: 10.3390/ijms23010540
77. Li H, Yao Q, Mariscal AG, Wu X, Hülse J, Pedersen E, et al. Epigenetic control of IL-23 expression in keratinocytes is important for chronic skin inflammation. Nat Commun (2018) 9:1420. doi: 10.1038/s41467-018-03704-z
78. Mehta H, Mashiko S, Angsana J, Rubio M, Hsieh YM, Maari C, et al. Differential Changes in Inflammatory Mononuclear Phagocyte and T-Cell Profiles within Psoriatic Skin during Treatment with Guselkumab vs. Secukinumab J Invest Dermatol (2021) 141:1707–18.e9. doi: 10.1016/j.jid.2021.01.005
79. Krueger JG, Wharton KA Jr., Schlitt T, Suprun M, Torene RI, Jiang X, et al. IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J Allergy Clin Immunol (2019) 144:750–63. doi: 10.1016/j.jaci.2019.04.029
80. Krueger JG, Ferris LK, Menter A, Wagner F, White A, Visvanathan S, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: Safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol (2015) 136:116–24.e7. doi: 10.1016/j.jaci.2015.01.018
81. Balato A, Schiattarella M, Di Caprio R, Lembo S, Mattii M, Balato N, et al. Effects of adalimumab therapy in adult subjects with moderate-to-severe psoriasis on Th17 pathway. J Eur Acad Dermatol Venereol (2014) 28:1016–24. doi: 10.1111/jdv.12240
Keywords: psoriasis, crosstalk, immune cell, skin cell, keratinocyte
Citation: Kamata M and Tada Y (2023) Crosstalk: keratinocytes and immune cells in psoriasis. Front. Immunol. 14:1286344. doi: 10.3389/fimmu.2023.1286344
Received: 01 September 2023; Accepted: 26 October 2023;
Published: 09 November 2023.
Edited by:
Stefan Tukaj, University of Gdansk, PolandReviewed by:
William D. Shipman, Yale University, United StatesHideki Fujita, Nihon University, Japan
Copyright © 2023 Kamata and Tada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yayoi Tada, ytada-tky@umin.ac.jp
 Masahiro Kamata
Masahiro Kamata Yayoi Tada
Yayoi Tada