- 1Institute of Allergology, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany
- 2Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP), Immunology and Allergology, Berlin, Germany
- 3Departement for Dermatology and Venerology, Kepler University Hospital, Linz, Austria
- 4GV20 Therapeutics, Cambridge, MA, United States
- 5Department of Dermatology, Heidelberg University, Heidelberg, Germany
Background: Mycosis fungoides (MF) is an indolent T-cell lymphoma that mainly affects the skin and presents with itch in more than half of the patients. Recently, the expression of Mas-related G protein-coupled receptor X2 (MRGPRX2), a receptor of mast cell (MC) responsible for the IgE-independent non-histaminergic itch, has been shown in lesional skin of patients with pruritic skin diseases, including chronic urticaria, prurigo, and mastocytosis. As of yet, limited knowledge exists regarding the MRGPRX2 expression in the skin of patients with MF.
Objectives: To investigate the number of MRGPRX2-expressing (MRGPRX2+) cells in the skin of patients with MF and its correlation with clinical and laboratory characteristics of the disease.
Methods: MRGPRX2 was analyzed in lesional and non-lesional skin of MF patients and healthy skin tissues by immunohistochemistry. Co-localization of MRGPRX2 with the MC marker tryptase was assessed by immunofluorescence. Public single-cell RNAseq data was reanalyzed to identify the MRGPRX2 expression on the distinct cell types.
Results: In lesional skin of MF patients, MRGPRX2+ cell number was higher than in non-lesional skin and healthy control skin (mean:15.12 vs. 6.84 vs. 5.51 cells/mm2, p=0.04), and correlated with MC numbers (r=0.73, p=0.02). MC was the primary cell type expressing MRGPRX2 in MF patients. The ratio of MRGPRX2+ MCs to MRGPRX2+ cells in lesional and non-lesional skin correlated with the severity of disease (r=0.71, p=0.02 and r=0.67, p=0.03, respectively).
Conclusions: Our findings point to the role of MRGPRX2 and MC in the pathogenesis of MF that should be investigated in further studies.
Introduction
Mycosis fungoides (MF) is a type of peripheral non-Hodgkin T-cell lymphoma characterized by its predominant manifestation in the skin. It accounts for 60% of all cutaneous T-cell lymphomas (CTCL) and nearly 50% of all primary cutaneous lymphomas. The disease typically progresses through three stages: patch, plaque, and tumor stage (1). In patients diagnosed with MF, those in the early-stage (stage I or IIA) typically exhibit a relatively indolent disease course. However, individuals with advanced-stage MF (stage IIB or higher) have a considerably poorer prognosis, characterized by a median survival of less than 5 years (2).
MF significantly affects patients’ quality of life (QoL), with itch being one of the most troublesome symptoms (3), occurring in up to 61% of MF patients (4). We have recently reported an association of chronic itch with increased disease severity, a more extensive involvement of the body surface area (BSA), and a pronounced impairment of QoL (5). The mechanisms for itch development in MF patients are not clear. Itch in many MF patients remains refractory to treatment including topical corticosteroids, ultraviolet light, and antihistamines (6).
Recently, Mas-related G protein-coupled receptor X2 (MRGRPX2) has been identified as a mast cell (MC) receptor responsible for IgE-independent MC activation and non-histaminergic itch (7). The MRGPRX2 can cause MCs to release their granules upon binding to a wide range of cationic substances, including neuropeptides, quorum sensing molecules from bacteria, venom peptides, host defense peptides, and FDA-approved drugs (8). Increased numbers of MRGPRX2-expressing (MRGPRX2+) cells have been reported in lesional skin of patients with various skin disorders including mastocytosis (9, 10), chronic urticaria (11) and chronic prurigo (12). As of yet, almost nothing is known about the role and relevance of MRGPRX2 in patients with MF. Here, we investigated the number of MRGPRX2+ cells in the skin of patients with MF and its correlation with itch and other clinical and laboratory characteristics.
Methods
Study population
The study was reviewed and approved by the Ethics Committee of the Charité - Universitätsmedizin Berlin (EA4/124/10). The patients/participants provided their written informed consent to participate in this study.
Ten patients with MF (1 female and 9 male, mean age 66.2 years) and 8 healthy controls (3 female and 5 male, mean age: 49.0 years) were included in the study. The patients’ demographic characteristics have been collected and reported in eTable 1 and elsewhere (5). Briefly, disease severity was assessed by visual analogue scale (VAS), BSA scales, modified severity-weighted assessment tool (mSWAT), and a Likert scale (0–3). Average itch in the last 24h, last week and last month was also assessed by VAS, ranging from 0 (no itch) to 10 (worst imaginable itch). Pruritus‐specific and skin-specific QoL impairments were assessed using Itch-specific quality of life questionnaire (ItchyQol) and the Dermatological Life Quality Index (DLQI), respectively. Patients were also asked about the presence of fatigue, fever, or insomnia. Laboratory parameters, including total serum IgE and serum tryptase levels, were determined at a central laboratory (Labor Berlin GmbH, Berlin, Germany). Eosinophilic cationic protein, major basic protein, IL-31, and substance P were measured using commercial ELISA kits, following the manufacturer’s instructions. Two 6mm diameter skin punch biopsies were taken from MF patients (one from lesional skin and one from non-lesional skin), and one biopsy was taken from healthy controls for histological analysis. In 10 MF patients and 8 healthy controls, biopsies were taken from the upper arm (n=5 and n=7), the trunk (n=3 and n=0), the shoulder (n=2 and n=0) and the lower extremities (n=0 and n=1).
Histological analysis
MRGPRX2 staining by immunohistochemistry (10 patients and 8 healthy controls) and co-localization of MRGPRX2 with the MC marker tryptase by immunofluorescence (10 patients) were performed as described before (12) (the detailed description is provided in the Supplementary File online). Examination of the sections (MRGPRX2 staining and MRGPRX2-tryptase double staining) were carried out using a fluorescence microscope (BZ-X800; Keyence, Itasca, USA). The evaluation of the immunostained sections was done independently and blindly by two experienced investigators. The positive cells were manually counted in at least five horizontally adjacent high-power fields in the upper papillary dermis (for immunohistochemistry staining, ×200, 0.25 mm2; for immunofluorescence double staining, ×400, 0.31 mm2). Mean values per field were calculated and further converted to “per mm2”.
Eosinophil staining was performed as previously described (5). Briefly, wax blocks were cut into 5 µm sections and stained with Giemsa (Merck KG, Darmstadt, Germany) for histology. Two independent and blinded trained investigators counted eosinophils in five or more horizontally adjacent high-power fields (×400, 0.15 mm2) in each three layers of papillary dermis, and the average cell numbers per horizontal layer were calculated.
Single-cell RNAseq data analysis
In order to determine the specific cell types expressing MRGPRX2, we conducted a single-cell analysis of MRGPRX2 expression using publicly available single-cell RNAseq (scRNAseq) data from MF skin tissues (13) and from healthy human skin (14). The scRNAseq data for MF skin tissues were downloaded from the GEO database (accession code: GSE128531), while the healthy human skin data were obtained from cellxgene (Tabula Sapiens). Preprocessing of the scRNAseq data involved normalizing the raw genes counts to the library size, resulting in counts per million. Subsequently, a log transformation was applied to the normalized data. Cell annotation was performed using unknown marker genes.
Statistical analysis
The statistical analysis was performed using the SciPy (version 1.8.0) in Python 3.9.12. Differences between two independent categories of parametric and non-parametric variables were evaluated using a two-sample t-test or a Mann-Whitney U test, respectively. Differences between three or more independent categories of parametric and non-parametric variables were evaluated using one-way analysis of variance (ANOVA), with a Tukey test used as a post hoc analysis, or the Kruskal-Wallis test, with a Dunn test used as a post hoc analysis, respectively. The differences between lesional and non-lesional skin biopsy samples were compared using paired t-test or the Wilcoxon signed-rank test for parametric and non-parametric variables, respectively. The correlation between variables was analyzed using Spearman’s rank correlation. Statistical significance was set at P < 0.05. scRNAseq analysis was performed using Scanpy 1.9.3.
Results
In MF patients, the number of MRGPRX2+ cells in lesional skin was significantly higher compared to non-lesional skin and healthy skin (mean: 15.12 vs. 6.84 vs. 5.51 cells/mm2, respectively, p=0.04) (Figures 1A, B). The number of MRGPRX2+ cells correlated with MC numbers in lesional (r=0.73, p=0.02) (Figure 1C) but not non-lesional skin of MF patients. Double staining for MRGPRX2 and tryptase indicated the co-localization of MRGPRX2 with MCs (Figure 2). The number of MRGPRX2+ MCs was higher as compared to MRGPRX2+ non-MCs in both lesional (mean: 6.74 vs. 3.45 cells/mm2, p=0.09) and non-lesional skin (mean: 4.04 vs. 2.44 cells/mm2, p=0.01). scRNAseq analysis of publicly available data from the skin of MF patients (13) and healthy skin (14) showed that 5.43-5.49% of MCs express MRGPRX2, whereas lymphocytes and keratinocytes showed minimal expression at 0.01% in the skin of MF patients (Figure 3). The ratio of MRGPRX2+ MCs to MRGPRX2+ cells in lesional and non-lesional skin correlated with the severity of the disease (r=0.71, p=0.02 and r=0.67, p=0.03, respectively).
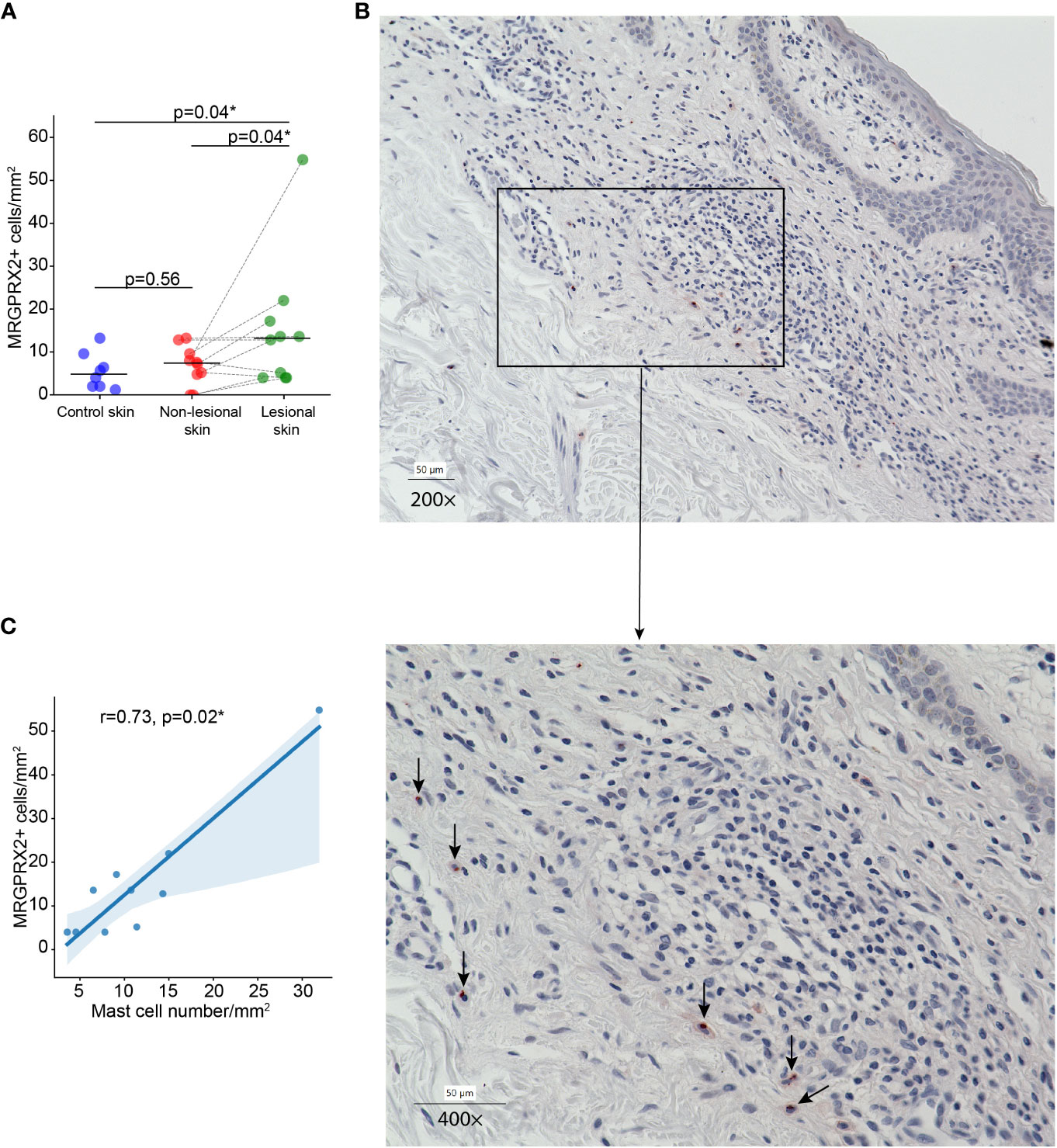
Figure 1 The number of MRGPRX2-expressing cells is increased in lesional skin of MF patients. (A) The number of MRGPRX2-expressing cells in lesional and non-lesional skin of MF patients and healthy control skin. (B) Immunohistochemical staining of MRGPRX2 in the lesional skin of patient with MF #6, ×200 (above) and ×400 (below) magnification (MRGPRX2+ cells are shown by black arrows). (C) Correlation between the number of MRGPRX2-expressing cells and mast cells in lesional skin of MF patients. MF, Mycosis fungoides; MRGPRX2, Mas-related G protein-coupled receptor X2. Mann–Whitney U test and Wilcoxon signed-rank test were used for testing the differences between unpaired data and paired data, respectively. Spearman rank correlation test was used for analyzing the correlation between two independent variables. P < 0.05 considered significant. *p<0.05.
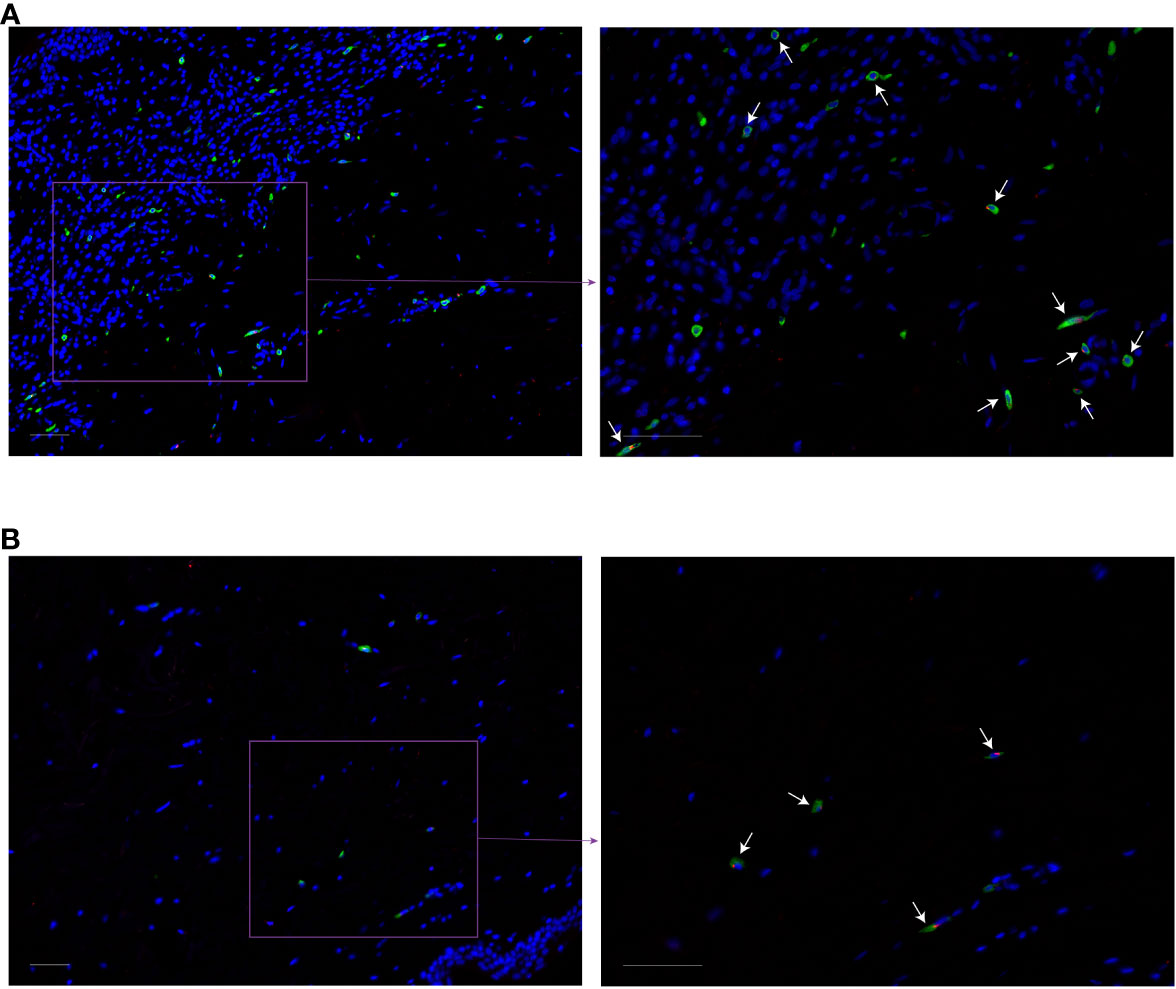
Figure 2 Co-localization of MRGPRX2 with the mast cell marker tryptase in MF patients. Immunofluorescence staining of lesional (A) and non-lesional (B) skin of the patient with MF #6 with anti-tryptase (green), anti-MRGPRX2 (red), and DAPI (blue). White arrows indicate some of tryptase-MRGPRX2 double-positive cells. ×200 (left) and ×400 (right) magnification. Bar = 50 µm. DAPI, 49-6-diamidino-2-phenylindole dihydrochloride; MF, Mycosis fungoides; MRGPRX2, Mas-related G protein-coupled receptor X2.
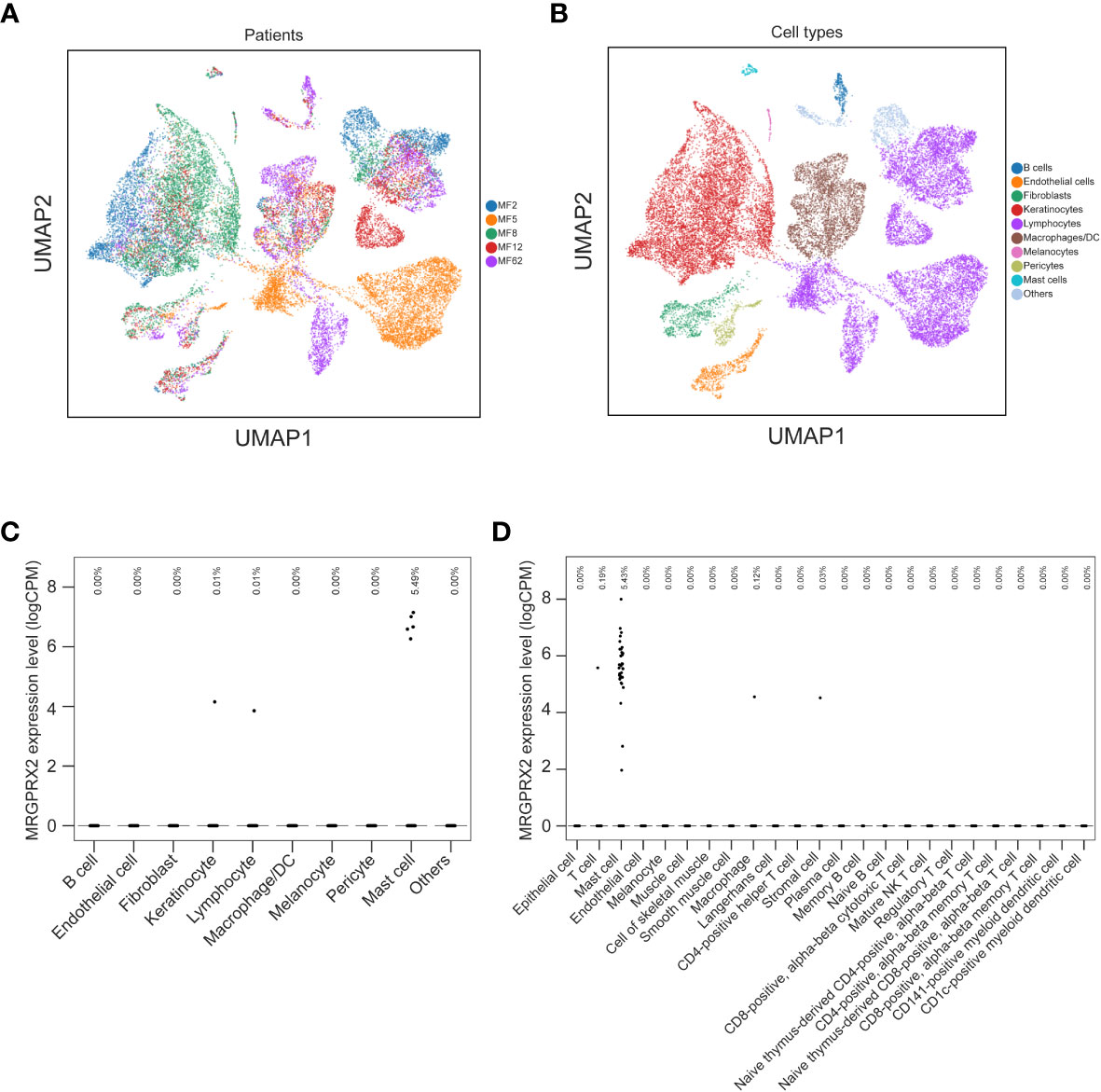
Figure 3 mRNA expression of MRGPRX2 in the skin of MF patients and the healthy skin tissues. UMAPs show the clusters of single cell transcriptomes of skin biopsies from five MF patients (A) and the cell types (B). Dot plot illustrates the mRNA expression levels of MRGPRX2 across different cell types in the skin of MF patients (C) and in healthy skin tissues from Tabula Sapiens (D). Each dot represents a single cell. Fractions of cells expressing MRGPRX2 are shown at the top of (C, D). MF, Mycosis fungoides; MRGPRX2, Mas-related G protein-coupled receptor X2; UMAP, uniform manifold approximation and projection.
MF patients and healthy controls did not differ in terms of gender and total serum IgE levels, although healthy controls were statistically significantly younger than MF patients (eTable 1). The age of patients and controls did not correlate with the number of MRGPRX2+ cells or with MC numbers in the skin. Lesional skin and non-lesional skin of MF patients did not significantly differ in numbers of MCs, eosinophils, MRGPRX2+ MCs, the ratio of MRGPRX2+ MCs to MCs, and the ratio of MRGPRX2+ MCs to MRGPRX2+ cells (eTable 2). The number of MRGPRX2+ cells did not correlate with disease severity, disease duration, pruritus, QoL impairment, eosinophil numbers, and serum levels of tryptase, total IgE, substance P, IL-31, eosinophilic cationic protein, and major basic protein (Figure 4).
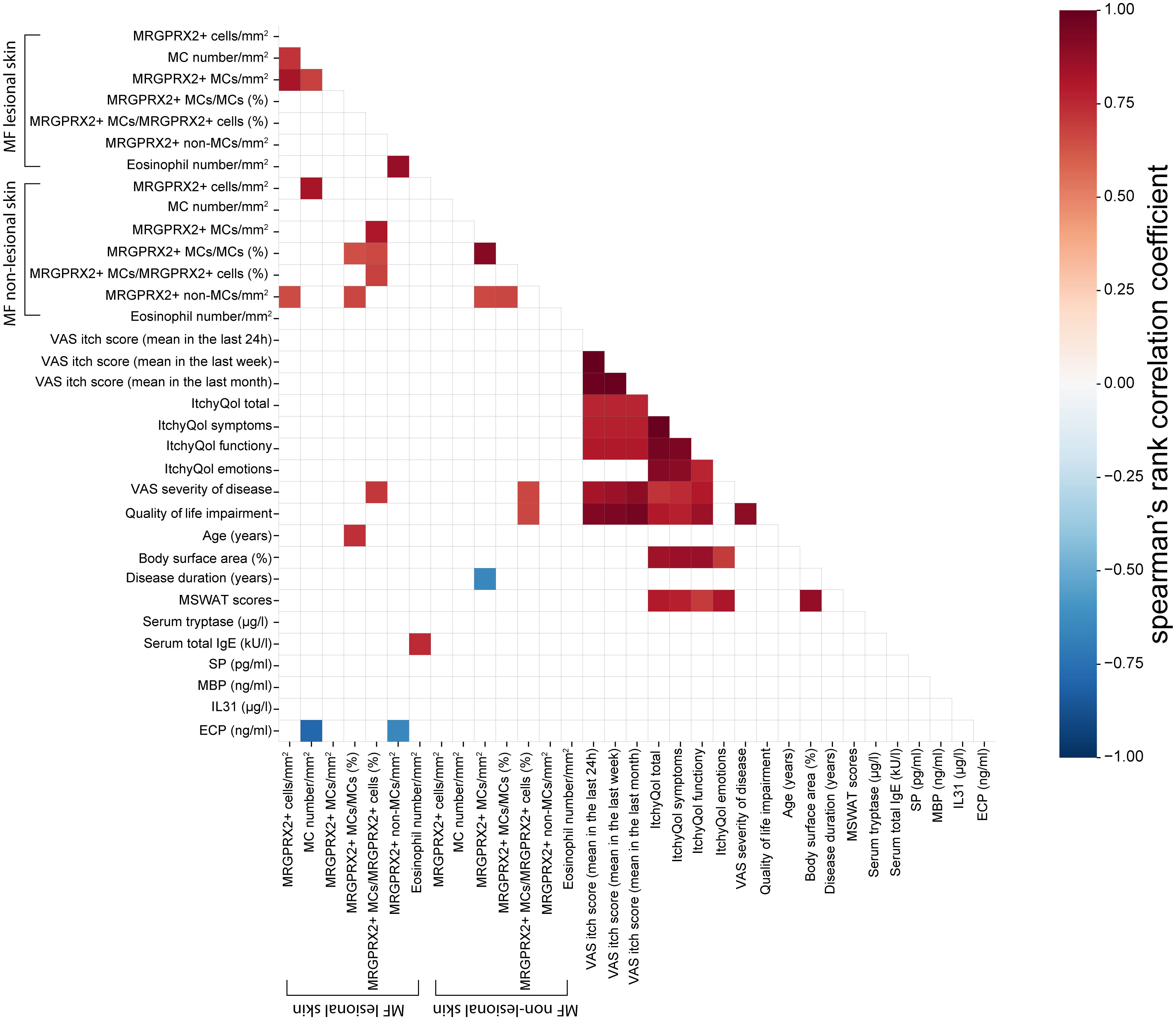
Figure 4 Spearman’s rank correlation matrix between the variables in MF patients. The color of the cells represents correlation coefficients, indicating strength and direction of the correlations, ranging from red (positive correlations) to blue (negative correlations). The strength of the correlation is indicated in the color scale (at the right of the panel). Blank space indicates the correlation was not statistically significant (Spearman correlation with P < 0.05 considered significant). The values refer to valid data only (excluding missing data). ECP, Eosinophilic cationic protein; IL31, Interlukin-31; ItchyQol, Itch-specific quality of life questionnaire; MBP, major basic protein; MC, Mast cell; MF, Mycosis fungoides; MRGPRX2, Mas-related G protein-coupled receptor X2; MSWAT score, Modified severity-weighted assessment tool score; QOL, Quality of life; SP, Substance P; VAS, Visual analogue scale.
Discussion
This study demonstrates a higher number of MRGPRX2+ cells in lesional skin of MF patients. The increase in lesional MRGPRX2+ cells as compared to non-lesional skin was slightly higher in MF (2.21 times higher, mean: 15.12 vs. 6.84 cells/mm2) as compared to previously reported in chronic prurigo (1.50 times higher, mean: 3.98 vs. 2.66 cells/mm2) (12) but lower than in indolent systemic mastocytosis (4.29 times higher, median: 22.3 vs. 5.2 cells/mm2) (9). Increased numbers of MRGPRX2+ MCs were seen in lesional skin of patients with MC-driven disorders, such as chronic spontaneous urticaria (11), chronic prurigo (12) and cutaneous mastocytosis (10). In line with this, the immunofluorescence analysis and reanalysis of scRNAseq datasets provided further evidence supporting the predominant expression of MRGPRX2 on MCs in both healthy donor skin and MF patients’ skin (13, 14). This finding offers a potential explanation for the positive correlation observed between the number of MRGPRX2+ cells and MCs in lesional skin of MF patients seen in our study.
Although in most MF patients the majority of MRGPRX2+ cells are MCs, other MRGPRX2-expressing cells might be relevant including sensory neurons (15), keratinocytes (15), basophils and eosinophils (16). In patients with indolent systemic mastocytosis, the number of MRGPRX2+ cells correlated with eosinophil number (9). However, we did not see such a correlation in MF patients, and eosinophils were rarely seen in skin samples. As shown by micro-array data, MRGPRX2 is expressed on T cells, which are pathogenic drivers in MF, although not confirmed by real-time PCR and further studies are needed (17). In our scRNAseq data analysis, we could see a small number of T cells expressing MRGPRX2 mRNA.
While the number of MRGPRX2+ cells was elevated, we did not observe any significant correlation with clinical or laboratory characteristics of MF in our patient’s cohort. Similarly, the number of MRGPRX2+ cells did not correlate with the clinical and laboratory characteristics of patients with indolent systemic mastocytosis (9). However, the positive correlation between the ratio of lesional MRGPRX2+ MCs to MRGPRX2+ cells and disease severity points to the clinical relevance of MRGPRX2+ MCs in MF that should be investigated in further studies.
We have not determined skin levels of MRGPRX2 ligands that could account for MCs activation and itch induction. In this context, MRGPRX2 might reflect the development of connective tissue MCs and the number of MRGPRX2+ cells may be less important than the lesional presence of MRGPRX2 agonists, e.g. neuropeptides such as substance P and cortistatin (CST). The serum levels of substance P, an agonist of MRGPRX2, were significantly increased in MF patients and positively correlated with disease severity (18). CST can activate MCs for degranulation and increased numbers of CST-expressing cells and CST-expressing MCs were observed in lesions of chronic prurigo (12). Similarly, CST expression was found in lymphomas and lymphocytic leukemias (17).
Other factors can be responsible for the lack of association between MRGPRX2 and clinical features of MF including altered expression due to receptor internalization and/or genetic polymorphisms (19, 20). Lastly, itch in MF patients might not be dominantly triggered via MRGPRX2 pathway and other mechanisms, e.g. IgE-dependent MCs activation, should be ruled out (21).
The results of our study are limited by the small number of patients. Despite the technology limitations of scRNAseq, which makes it less sensitive to lowly expressed genes like MRGPRX2, the lower detection of MRGPRX2 in scRNAseq does not introduce bias when conducting comparative analysis within the same dataset.
In conclusion, the role and relevance of MRGPRX2, its ligands and MCs in patients with MF need further investigation. Additional studies should include larger patient cohorts and determination of levels of MRGPRX2 ligands in the skin of patients with MF to provide a rationale for MRGPRX2-targeted treatments in this disease.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Charité - Universi-tätsmedizin Berlin (EA4/124/10). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
PK, MMe, and MH designed the study and prepared the manuscript. PK, MMe, MH, PP, and CS analyzed and interpreted the data. PP, MH, and NL performed experiments. SA, DT-M, KL, KG, and VP collected the samples and clinical data. The study was supervised by PK and MMe. All coauthors critically revised and provided substantial input to the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by intramural funding. We acknowledge financial support from the Open Access Publication Fund of Charité – Universitätsmedizin Berlin and the German Research Foundation (DFG).
Conflict of interest
SA has conducted studies for/was advisor for/was speaker for AstraZeneca, Allakos, ALK, Biocryst, CSLBehring, LeoPharma, Moxie, Novartis, Pharvaris, Sanofi, Takeda, Thermofisher. MaMe received honoraria advisory board, speaker from AbbVie, Amgen, AstraZeneca, argenx, Bayer, Beiersdorf, Celldex, Escient, Galderma, gsk, Jasper, Novartis, Pharvaris, Pfizer, Sanofi-Aventis, Tevapharm, ThirdHarmonicBio, Viforpharma, outside of submitted work. PK received honoraria advisory board, speaker from Novartis, Roche and ValenzaBio, outside of submitted work. Outside of this work, MMa is or recently was a speaker and/or advisor for and/or has received research funding from Astria, Allakos, Alnylam, Amgen, Aralez, ArgenX, AstraZeneca, BioCryst, Blueprint, Celldex, Centogene, CSL Behring, Dyax, FAES, Genentech, GIInnovation, GSK, Innate Pharma, Kalvista, Kyowa Kirin, Leo Pharma, Lilly, Menarini, Moxie, Novartis, Pfizer, Pharming, Pharvaris, Roche, Sanofi/Regeneron, Shire/Takeda, Third Harmonic Bio, UCB, and Uriach. DT-M has received research funds and/or was advisor for Celldex, Moxie, Novartis and Sanofi. Outside of this work, CS is an employee of the GV20 Therapeutics, which develops drugs and research models for profit.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1197821/full#supplementary-material
Abbreviations
BSA, Body surface area; CST, Cortistatin; CTCL, Cutaneous T-cell lymphomas; DLQI, Dermatological life quality index; IL-31, Interleukin-31; ItchyQol, Itch-specific quality of life questionnaire; MC, Mast cell; MF, Mycosis fungoides; MRGPRX2, Mas-related G protein-coupled receptor X2; mSWAT, Modified severity-weighted assessment tool; QoL, Quality of life; scRNAseq, Single-cell RNA sequencing; UMAP, Uniform manifold approximation and projection; VAS, Visual analogue scale.
References
1. Kazakov DV, Burg G, Kempf W. Clinicopathological spectrum of mycosis fungoides. J Eur Acad Dermatol Venereol (2004) 18(4):397–415. doi: 10.1111/j.1468-3083.2004.00937.x
2. Kamijo H, Miyagaki T. Mycosis fungoides and sézary syndrome: updates and review of current therapy. Curr Treat Options Oncol (2021) 22(2):10. doi: 10.1007/s11864-020-00809-w
3. Ottevanger R, van Beugen S, Evers AWM, Willemze R, Vermeer MH, Quint KD. Itch in patients with cutaneous T-cell lymphoma as a quality of life indicator. JAAD Int (2022) 9:57–64. doi: 10.1016/j.jdin.2022.07.007
4. Vij A, Duvic M. Prevalence and severity of pruritus in cutaneous T cell lymphoma. Int J Dermatol (2012) 51(8):930–4. doi: 10.1111/j.1365-4632.2011.05188.x
5. Terhorst-Molawi D, Lohse K, Ginter K, Puhl V, Metz M, Hu M, et al. Mast cells and tryptase are linked to itch and disease severity in mycosis fungoides: Results of a pilot study. Front Immunol (2022) 13:930979. doi: 10.3389/fimmu.2022.930979
6. Meyer N, Paul C, Misery L. Pruritus in cutaneous T-cell lymphomas: frequent, often severe and difficult to treat. Acta Derm Venereol (2010) 90(1):12–7. doi: 10.2340/00015555-0789
7. Meixiong J, Anderson M, Limjunyawong N, Sabbagh MF, Hu E, Mack MR, et al. Activation of mast-cell-expressed mas-related G-protein-coupled receptors drives non-histaminergic itch. Immunity (2019) 50(5):1163–1171.e5. doi: 10.1016/j.immuni.2019.03.013
8. Corbière A, Loste A, Gaudenzio N. MRGPRX2 sensing of cationic compounds-A bridge between nociception and skin diseases? Exp Dermatol (2021) 30(2):193–200. doi: 10.1111/exd.14222
9. Pyatilova P, Ashry T, Luo Y, He J, Bonnekoh H, Jiao Q, et al. The number of MRGPRX2-expressing cells is increased in skin lesions of patients with indolent systemic mastocytosis, but is not linked to symptom severity. Front Immunol (2022) 13:930945. doi: 10.3389/fimmu.2022.930945
10. Deepak V, Komarow HD, Alblaihess AA, Carter MC, Metcalfe DD, Ali H. Expression of MRGPRX2 in skin mast cells of patients with maculopapular cutaneous mastocytosis. J Allergy Clin Immunol Pract (2021) 9(10):3841–3843.e1. doi: 10.1016/j.jaip.2021.05.042
11. Fujisawa D, Kashiwakura J, Kita H, Kikukawa Y, Fujitani Y, Sasaki-Sakamoto T, et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy Clin Immunol (2014) 134(3):622–633.e9. doi: 10.1016/j.jaci.2014.05.004
12. Kolkhir P, Pyatilova P, Ashry T, Jiao Q, Abad-Perez AT, Altrichter S, et al. Mast cells, cortistatin, and its receptor, MRGPRX2, are linked to the pathogenesis of chronic prurigo. J Allergy Clin Immunol (2022) 149(6):1998–2009.e5. doi: 10.1016/j.jaci.2022.02.021
13. Gaydosik AM, Tabib T, Geskin LJ, Bayan CA, Conway JF, Lafyatis R, et al. Single-cell lymphocyte heterogeneity in advanced cutaneous T-cell lymphoma skin tumors. Clin Cancer Res (2019) 25(14):4443–54. doi: 10.1158/1078-0432.Ccr-19-0148
14. Jones RC, Karkanias J, Krasnow MA, Pisco AO, Quake SR, Salzman J, et al. The Tabula Sapiens: A multiple-organ, single-cell transcriptomic atlas of humans. Science (2022) 376(6594):eabl4896. doi: 10.1126/science.abl4896
15. Porebski G, Kwiecien K, Pawica M, Kwitniewski M. Mas-related G protein-coupled receptor-X2 (MRGPRX2) in drug hypersensitivity reactions. Front Immunol (2018) 9:3027. doi: 10.3389/fimmu.2018.03027
16. Wedi B, Gehring M, Kapp A. The pseudoallergen receptor MRGPRX2 on peripheral blood basophils and eosinophils: Expression and function. Allergy (2020) 75(9):2229–42. doi: 10.1111/all.14213
17. van Hagen PM, Dalm VA, Staal F, Hofland LJ. The role of cortistatin in the human immune system. Mol Cell Endocrinol (2008) 286(1-2):141–7. doi: 10.1016/j.mce.2008.03.007
18. Tuzova M, Conniff T, Curiel-Lewandrowski C, Chaney K, Cruikshank W, Wolpowitz D. Absence of full-length neurokinin-1 receptor protein expression by cutaneous T cells: implications for substance P-mediated signaling in mycosis fungoides. Acta Derm Venereol (2015) 95(7):852–4. doi: 10.2340/00015555-2097
19. Chompunud Na Ayudhya C, Amponnawarat A, Ali H. Substance P serves as a balanced agonist for MRGPRX2 and a single tyrosine residue is required for β-arrestin recruitment and receptor internalization. Int J Mol Sci (2021) 22(10). doi: 10.3390/ijms22105318
20. Yang S, Liu Y, Lin AA, Cavalli-Sforza LL, Zhao Z, Su B. Adaptive evolution of MRGX2, a human sensory neuron specific gene involved in nociception. Gene (2005) 352:30–5. doi: 10.1016/j.gene.2005.03.001
Keywords: cutaneous T cell lymphoma, mycosis fungoides, pruritus, MRGPRX2, mast cells
Citation: Hu M, Pyatilova P, Altrichter S, Sheng C, Liu N, Terhorst-Molawi D, Lohse K, Ginter K, Puhl V, Maurer M, Metz M and Kolkhir P (2023) In the skin lesions of patients with mycosis fungoides, the number of MRGPRX2-expressing cells is increased and correlates with mast cell numbers. Front. Immunol. 14:1197821. doi: 10.3389/fimmu.2023.1197821
Received: 31 March 2023; Accepted: 28 September 2023;
Published: 30 October 2023.
Edited by:
Andrzej Lange, Polish Academy of Sciences, PolandReviewed by:
Kanami Orihara, Tokyo Institute of Technology, JapanMiriam Margareta Düll, University Hospital Erlangen, Germany
Copyright © 2023 Hu, Pyatilova, Altrichter, Sheng, Liu, Terhorst-Molawi, Lohse, Ginter, Puhl, Maurer, Metz and Kolkhir. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pavel Kolkhir, pavel.kolkhir@charite.de
†These authors share last authorship
‡ORCID: Man Hu, orcid.org/0000-0003-2886-8023
Polina Pyatilova, orcid.org/0000-0001-5520-2900
Sabine Altrichter, orcid.org/0000-0001-9955-385X
Caibin Sheng, orcid.org/0000-0002-7706-1253
Nian Liu, orcid.org/0000-0002-4108-7937
Dorothea Terhorst-Molawi, orcid.org/0000-0001-9411-8998
Viktoria Puhl, orcid.org/0000-0002-1769-0058
Marcus Maurer, orcid.org/0000-0002-4121-481X
Martin Metz, orcid.org/0000-0002-4070-9976
Pavel Kolkhir, orcid.org/0000-0001-5380-8132
 Man Hu
Man Hu Polina Pyatilova
Polina Pyatilova Sabine Altrichter
Sabine Altrichter Caibin Sheng4‡
Caibin Sheng4‡ Katharina Ginter
Katharina Ginter Marcus Maurer
Marcus Maurer Martin Metz
Martin Metz Pavel Kolkhir
Pavel Kolkhir