- Department of Radiation Oncology, Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
Objective: This systematic review and meta-analysis aimed to investigate the role of neoadjuvant immunochemotherapy with or without radiotherapy [NIC(R)T] compared to traditional neoadjuvant therapies, without immunotherapy [NC(R)T].
Summary background data: NCRT followed by surgical resection is recommended for patients with early-stage esophageal cancer. However, it is uncertain whether adding immunotherapy to preoperative neoadjuvant therapy would improve patient outcomes when radical surgery is performed following neoadjuvant therapy.
Methods: We searched PubMed, Web of Science, Embase, and Cochrane Central databases, as well as international conference abstracts. Outcomes included R0, pathological complete response (pCR), major pathological response (mPR), overall survival (OS) and disease-free survival (DFS) rates.
Results: We included data from 5,034 patients from 86 studies published between 2019 and 2022. We found no significant differences between NICRT and NCRT in pCR or mPR rates. Both were better than NICT, with NCT showing the lowest response rate. Neoadjuvant immunotherapy has a significant advantage over traditional neoadjuvant therapy in terms of 1-year OS and DFS, with NICT having better outcomes than any of the other three treatments. There were no significant differences among the four neoadjuvant treatments in terms of R0 rates.
Conclusions: Among the four neoadjuvant treatment modalities, NICRT and NCRT had the highest pCR and mPR rates. There were no significant differences in the R0 rates among the four treatments. Adding immunotherapy to neoadjuvant therapy improved 1-year OS and DFS, with NICT having the highest rates compared to the other three modalities.
Systematic Review Registration: https://inplasy.com/inplasy-2022-12-0060/, identifier INPLASY2022120060.
1 Introduction
Esophageal cancer is the seventh most common malignant tumor and the sixth leading cause of cancer-related mortality worldwide (1). Surgical resection has advocated for the treatment of early-stage esophageal cancer (2). The CROSS trial showed that neoadjuvant chemoradiation followed by surgical resection was more beneficial for esophageal cancer (3). Accordingly, the National Comprehensive Cancer Network guidelines recommend it as the standard therapy (4). Nevertheless, the treatment efficacy for esophageal cancer remains poor, with a 5-year survival rate of approximately 20% (5, 6).
Immunotherapy has become an effective treatment for many malignancies including esophageal cancer (7–9). By rescuing the immune checkpoint pathway to resist carcinoma, the anti-tumor action of T cells is blocked by immune checkpoint blockade. Immunotherapy has proven beneficial as a third-, second-, and even first-line treatment for patients with esophageal cancer. However, it remains unclear whether adding immunotherapy therapy to preoperative neoadjuvant confers an overall benefit to patient outcomes when radical surgery is performed after neoadjuvant therapy. Several studies have documented benefits when immunotherapy is added to neoadjuvant therapy (10, 11); on the other hand, adding immunotherapy to neoadjuvant therapy increases the severity of toxic side effects (12, 13).
Therefore, this systematic review and meta-analysis was conducted to evaluate the outcomes of patients treated with either of two neoadjuvant immunotherapies – neoadjuvant immunotherapy combined with chemoradiotherapy (NICRT) and neoadjuvant immunochemotherapy (NICT) – compared with two traditional neoadjuvant therapies – neoadjuvant chemoradiotherapy (NCRT) and neoadjuvant chemotherapy (NCT).
2 Methods
This study was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 (14). The present study was registered in the INPLASY (identifier: INPLASY2022120060).
2.1 Search strategy and eligibility criteria
We searched PubMed, Web of Science, Embase, and Cochrane Central databases, as well as international conference abstracts from American Society of Clinical Oncology, European Society for Medical Oncology and American Association for Cancer Research, along with various other resources, until December 16, 2022. The detailed search strategies are summarized in Supplementary Table 1. We searched for studies that explored patients with histologically confirmed-, resectable-, esophageal carcinoma who received either NICRT or NICT followed by surgery. Meanwhile, the patients treated with traditional neoadjuvant therapy (NCRT or NCT) were all derived from control patients in these studies, rather than from other studies that did not involve NICRT or NICT. We followed the Population, Intervention, Comparison, Outcome, and Study Design (PICOS) principles (Supplementary Table 2). The detailed inclusion and exclusion criteria are shown in Supplementary Table 3.
2.2 Study selection and data extraction
Two authors (CS and XZ) independently assessed each study and extracted the pertinent information therefrom. Another author (WD) resolved any differences that might have arisen in the process. Relevant parameters were extracted from each included study: author, year, country, study type, registration number, intervention model, type of article, treatment modalities and side effects, sample size, age, sex, histologic subtype, relevant clinical characteristics, and outcome data of interest.
2.3 Outcomes
The outcome indicators in this study included direct measures of treatment efficacy – R0, pathological complete response (pCR), and major pathological response (mPR) rates – as well as survival-related indicators, including overall survival (OS), disease-free survival (DFS), and death within 30 days after surgery. We did not include treatment-related adverse events during neoadjuvant therapy or post-operative complications, as the evaluation criteria used to evaluate these indicators were not uniform across different studies. The primary goal of our study was to explore immediate post-treatment efficacy and subsequent survival outcomes, to investigate the effectiveness of neoadjuvant immunotherapy.
2.4 Quality assessment
Two authors (CS and XZ) independently evaluated the quality of each study. If there were any disagreements in the process, another author (JD) settled it. The Methodological Index for Non-randomized Studies (MINORS) was used to assess single-arm and retrospective dual-arm studies (15, 16). Each item was scored from 0 to 2. There were 8 items for non-comparative studies and 12 items for comparative studies. For non-comparative studies, an overall score > 12 was considered high, between 8 and 12 was considered intermediate, and < 8 was considered low. The Cochrane Risk of Bias tool was used to assess randomized controlled trials (RCT) (17, 18). The tool scores RCT studies according to five items. The overall bias included low risk of bias, some concerns, and high risk of bias. The quality of this systematic review and meta-analysis was evaluated according to the PRISMA 2020 Checklist (14) and the AMSTAR-2 Checklist (19).
2.5 Statistical analysis
All analyses were performed by STATA (STATA, version 14.0, College, TX),. Survival curve data from included studies which were not reported were extracted by Engauge Digitizer, version 12.1 (http://markummitchell.github.io/engauge-digitizer/). We performed a single-group meta-analysis of all included studies. In order to compare the four different neoadjuvant treatment modalities with each other and to rank their respective efficacies, we performed a network meta-analysis of the comparative studies among them. The significance level of the results was set at P <0.05, as per the convention. The combined risk ratio (RR) and 95% confidence interval (CI) were used as the outcome indicators. For OS and DFS rates, the number of events used to calculate the RR value was the number of survivors rather than the number of deaths. Therefore, in the present study, RR and 95% CI > 1 indicated that treatment was more conducive to survival, whereas RR and 95% CI < 1 indicated that treatment was more detrimental to survival (Detailed data synthesis are shown in Supplementary Table 4).
Subsequently, we merged NICRT and NICT into the neoadjuvant immunotherapy group and NCRT and NCT into the traditional neoadjuvant therapy group. We performed a traditional pairwise meta-analysis of these two groups, with head-to-head studies to explore the comparative advantages of neoadjuvant immunotherapy vs. traditional neoadjuvant therapy. Exploratory subgroup analyses were performed based on the study type (prospective or retrospective), intervention model (single-arm or dual-arm), immunotherapy drugs (PD-1 or PD-L1 inhibitors), and cancer type (squamous cell carcinoma [SCC] or adenocarcinoma [AC]). Additionally, sensitivity analyses were performed by omitting each study to evaluate the stability of the results. Publication bias was assessed using the Begg’s funnel plot (20).
3 Results
3.1 Characteristics
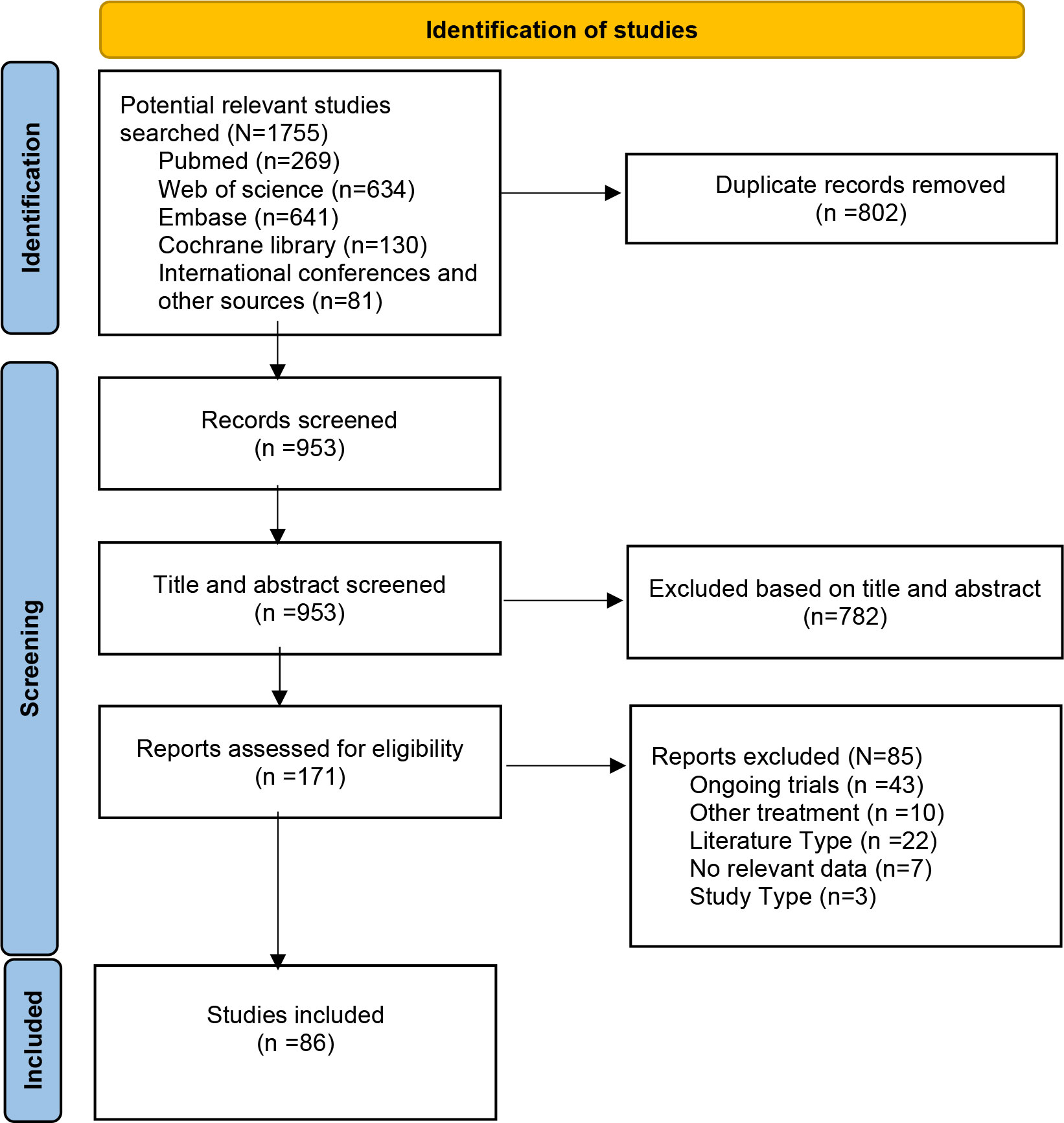
From the 1,755 considered studies, we eventually selected 86 studies (10–13, 21–102) describing a total of 5,034 patients (Figure 1). This number consisted of 16 dual-arm studies and 70 single-arm studies, five RCTs and 81 non-RCTs.
All studies were published between 2019 and 2022, most of which were conducted in China. Among these studies, the number of patients who received NICRT, NICT, NCRT, and NCT were 427, 3508, 701, and 398, respectively. The median age of all patients ranged from 42.7 to 68.8. For cancer type, the studies included SCC only (n=73), AC only (n=6), mixed SCC and AC (n=5), and undetailed pathology (n=2). For neoadjuvant immunotherapy, PD-1 inhibitors were the most common, with only 6 studies using PD-L1 inhibitors. The radiation doses ranged from 30 Gy to 56 Gy. All neoadjuvant chemotherapy regimens were conventional treatment regimens. Detailed characteristics of each study are shown in Table 1 and Supplementary Tablea 5-8.
3.2 Clinical outcomes of NICRT, NICT, NCRT and NCT
A total of 56 trials reported R0 rates (pooled R0 rate and 95% CI: NICRT - 95.6% [91.8%-99.3%]; NICT - 97.5% [96.9%-98.2%]; NCRT - 94.9% [90.3%-99.5%]; NCT - 96.6% [93.5%-99.6%]) (Figure 2). Overall, 80 trials provided pCR rates (pooled pCR rate and 95% CI: NICRT - 38.9% [32.1%-45.6%]; NICT -27.2% [24.8%-29.6%]; NCRT - 35.5% [21.3%-49.7%]; NCT - 8.6% [2.9%-14.3%]) (Figure 2). Totally, 51 trials analyzed mPR rates (pooled mPR rate and 95% CI: NICRT - 64.2% [53.8%-74.7%]; NICT - 51.8% [46.7%-56.8%]; NCRT - 47.8% [10.5%-85.1%]; NCT - 43.6% [9.5%-77.7%]) (Figure 3). In terms of survival outcomes, 28 trials reported death within 30 days after surgery (pooled rate and 95% CI: NICRT - 2.0% [0.0%-4.2%]; NICT - 0.3% [0.0%-0.6%]; NCRT - 1.7% [0.6%-2.8%]; NCT - 1.3% [0.0%-2.7%]) (Figure 3). Thirteen trials provided 1-year OS rates (pooled 1-year OS rate and 95% CI: NICRT - 87.3% [80.9%-93.6%]; NICT - 96.2% [94.2%-98.1%]; NCRT - 86.2% [79.2%-93.1%]; NCT - 85.1% [74.9%-95.3%]) (Figure 4). And a total of 16 trials analyzed 1-year DFS rates (pooled 1-year DFS rate and 95% CI: NICRT - 77.7% [70.9%-84.6%]; NICT - 90.0% [86.2%-93.7%]; NCRT - 73.2% [64.4%-82.0%]; NCT - 76.6% [64.5%-88.7%]) (Figure 4).
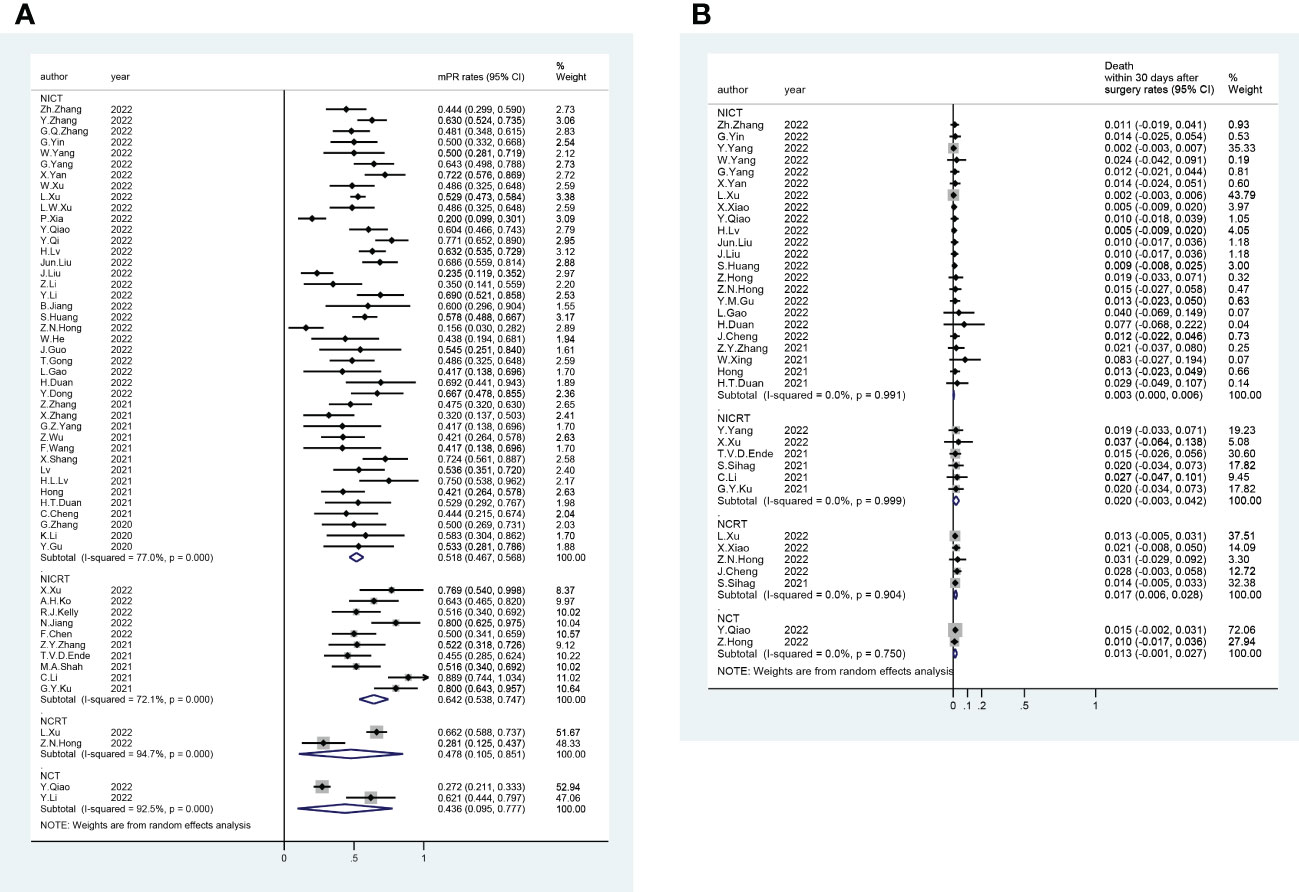
Figure 3 Forest Plot of (A) Major Pathological Response (mPR) and (B) Death within 30 Days after Surgery.
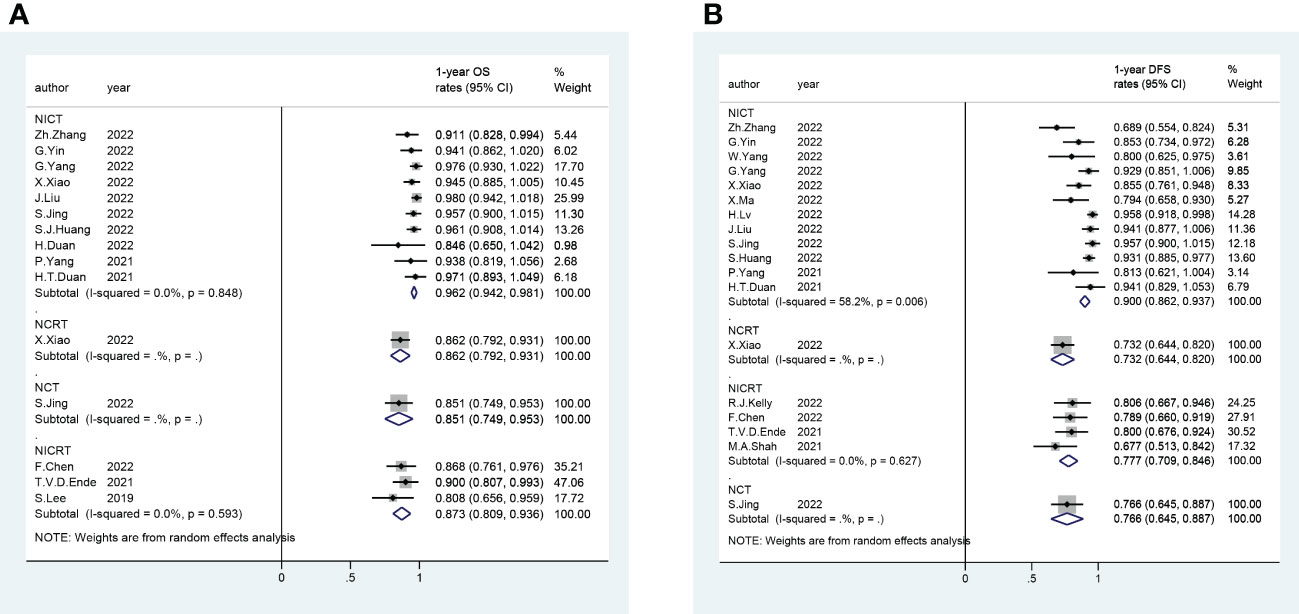
Figure 4 Forest Plot of (A) 1-year Overall Survival (OS) and (B) 1-year Disease Free Survival (DFS).
To compare different neoadjuvant treatment modalities with each other, we included 16 dual-arm trials in the network meta-analysis. Network evidence plots and contribution plots are shown in Supplementary Figures 1, 2. The network estimates are shown in Figure 5 and Supplementary Figures 3, 4. There were no significant differences in pCR and mPR rates between NICRT and NCRT (pooled RR and 95% CI of pCR rate: NICRT vs. NCRT - 1.39 [0.82,2.37]; pooled RR and 95% of mPR rate: NICRT vs. NCRT - 1.02[0.87,1.19]). Both were superior to NICT (pooled RR and 95% CI of pCR rate: NICRT vs. NICT - 1.83 [1.10,3.05], NCRT vs. NICT - 1.32 [1.00,1.74]; pooled RR and 95% CI of mPR rate: NICRT vs. NICT - 1.17[1.05,1.31], NCRT vs. NICT - 1.15[1.01,1.31]), and NCT had the poorest results (pooled RR and 95% CI of pCR rate: NICRT vs. NCT - 5.43 [2.80,10.51], NCRT vs. NCT - 3.90 [2.36,6.47], NICT vs. NCT - 2.96 [1.93,4.54]; pooled RR and 95% CI of mPR rate: NICRT vs. NCT - 1.93 [1.56,2.39], NCRT vs. NCT - 1.90 [1.52,2.37], NICT vs. NCT - 1.65[1.35,2.00]). For 1-year OS and DFS rates, NICT showed the best rates compared to other three treatments (pooled RR and 95% CI of 1-year OS rate: NICT vs. NICRT - 1.10 [1.01,1.19], NICT vs. NCRT - 1.10 [1.01,1.20], NICT vs. NCT - 1.11[1.00,1.26]; pooled RR and 95% CI of 1-year DFS rate: NICT vs. NICRT - 1.16 [1.05,1.27], NICT vs. NCRT - 1.22 [1.08,1.38], NICT vs. NCT - 1.16 [1.00,1.37]), with the other three treatments not having any statistically significant difference in these parameters amongst each other. None of the treatment modalities stood out from the others in terms of R0 rates or death within 30 days after surgery.
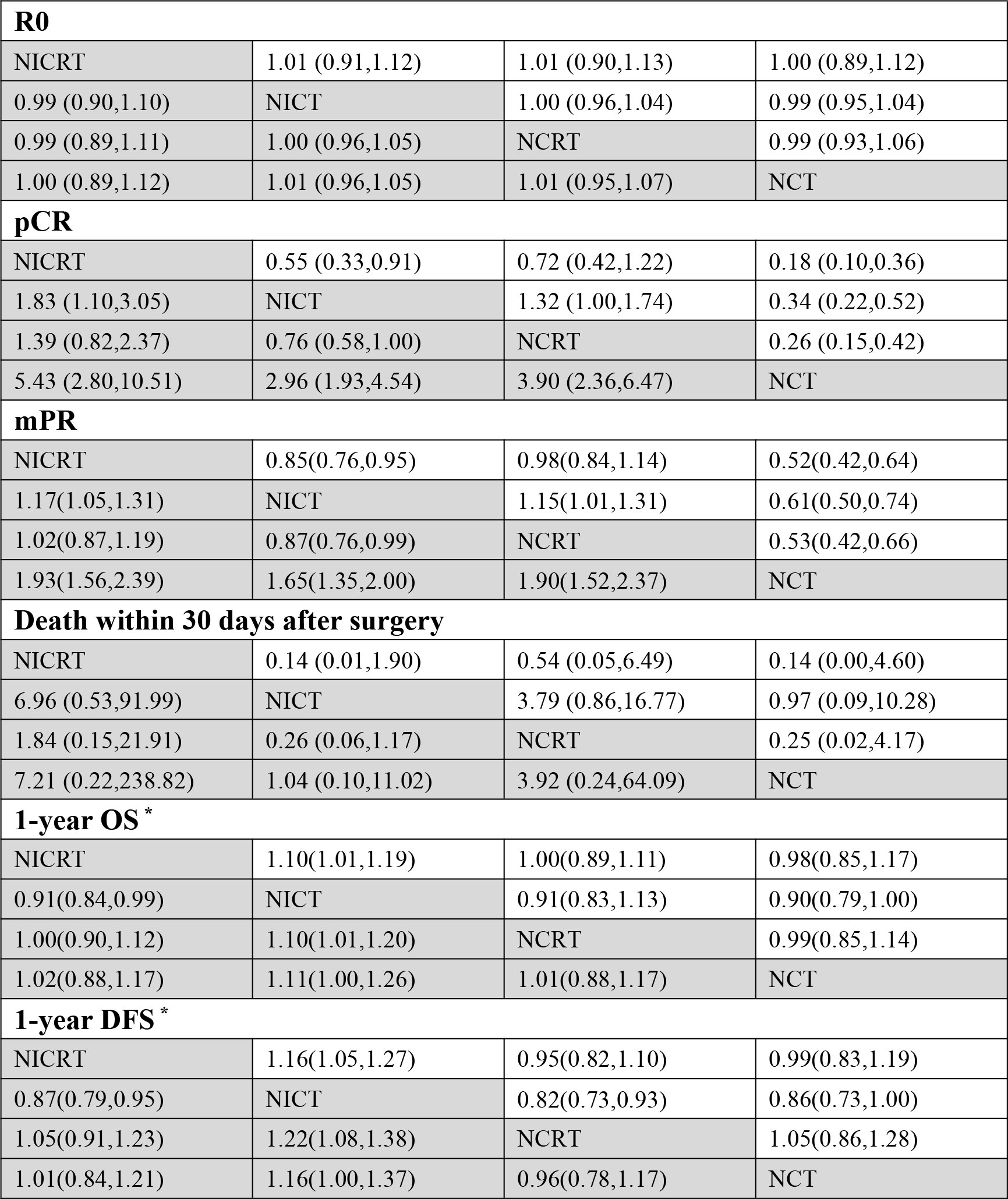
Figure 5 Results of Comparisons by Risk Ratio (RR) and 95% Confidence Interval (CI) among Four Neoadjuvant Therapies. (*The number of events in the calculation of the RR value is the number of survivors rather than the number of deaths. RR and 95% CI > 1 indicates that treatment is more conducive to survival, while RR and 95% CI < 1 indicates that treatment is more detrimental to survival.) Pathological Complete Response (pCR), Major Pathological Response (mPR), Overall Survival (OS), Disease Free Survival (DFS).
3.3 Neoadjuvant immunotherapy (NICRT and NICT) versus traditional neoadjuvant therapy (NCRT and NCT)
Next, we pooled the data for the NICRT and NICT cases into the neoadjuvant immunotherapy group and the NCRT and NCT cases into the traditional neoadjuvant therapy group. A total of 16 trials were included in this head-to-head pairwise meta-analysis. Patients in the neoadjuvant immunotherapy group exhibited significantly higher 1-year OS and DFS rates than those in the traditional neoadjuvant therapy group (pooled RR and 95% CI of the traditional group vs. immunotherapy group: 1-year OS rate - 0.90 [0.83-0.98]; 1-year DFS rate - 0.83 [0.74-0.93]) (Figure 6). However, there were no significant differences between the two groups in terms of R0, pCR, mPR, or death within 30 days after surgery (Figure 6).
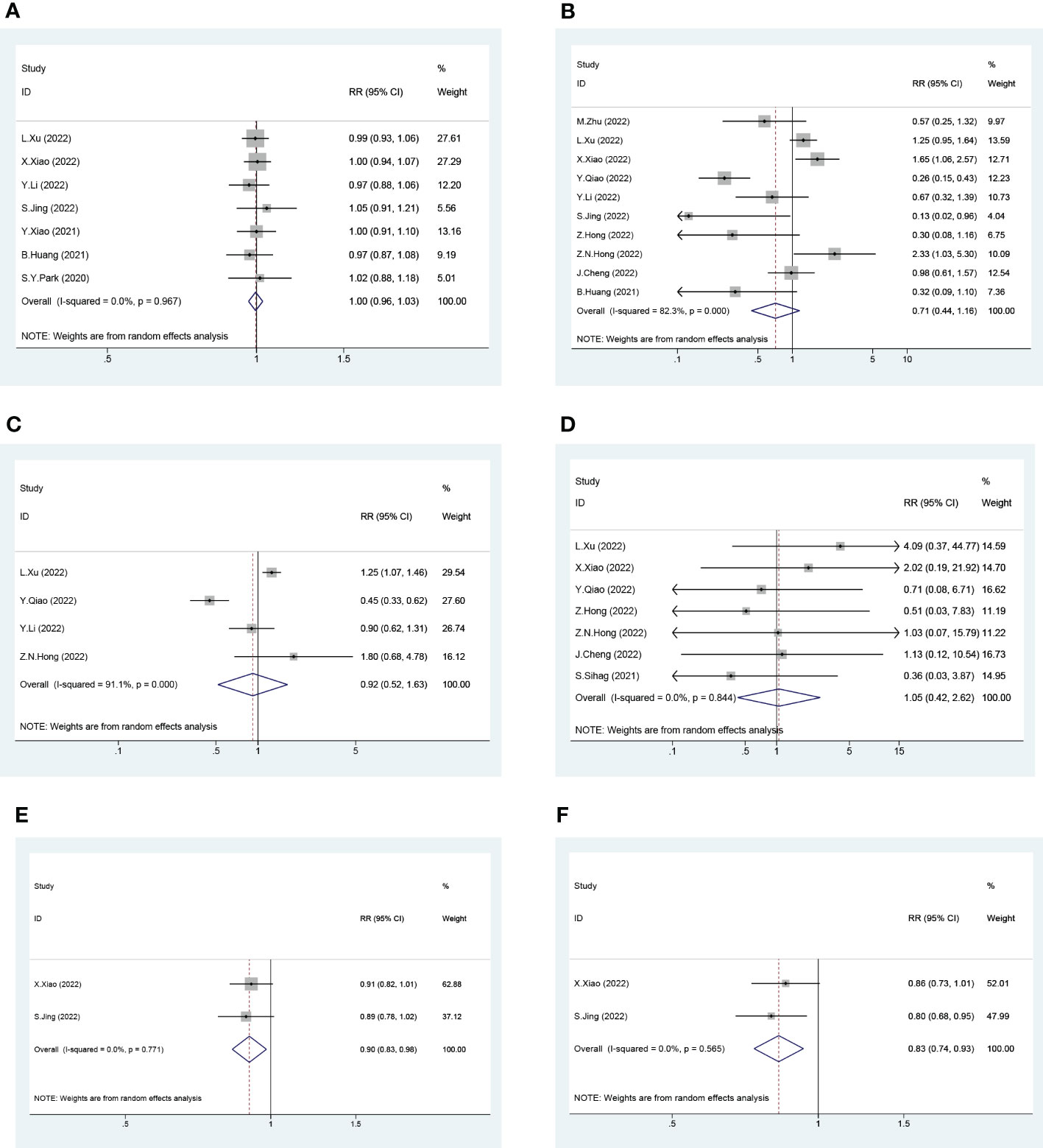
Figure 6 Forest Plot of Traditional Neoadjuvant Therapy (left) and Neoadjuvant Immunotherapy (right). (A) R0, (B) Pathological Complete Response (pCR), (C) Major Pathological Response (mPR), (D) Death within 30 Days after Surgery, (E) 1-year Overall Survival (OS) and (F) 1-year Disease Free Survival (DFS). (For 1-year OS and 1-year DFS, the number of events in the calculation of the RR value is the number of survivors rather than the number of deaths. RR and 95% CI > 1 indicates that treatment is more conducive to survival, while RR and 95% CI < 1 indicates that treatment is more detrimental to survival.).
3.4 Exploratory subgroup analysis
To explore the potential association of immunotherapy between NICRT and NICT, we conducted exploratory subgroup analysis based on study type (prospective or retrospective), intervention model (single-arm or dual-arm), immunotherapy drugs (PD-1 or PD-L1 inhibitors), and cancer type (SCC or AC), respectively. The results of the subgroup NICRT and NICT analyses were generally consistent with the above results in terms of R0, pCR, mPR, death within 30 days after surgery, 1-year OS, and 1-year DFS (Supplementary Figures 5–10).
3.5 Quality evaluation, sensitivity analysis and publication bias
The details of the risk of bias are provided in Supplementary Tables 9, 10. The MINORS was used to evaluate the 81 non-randomized studies. All the 81 studies were of high or intermediate quality. The Cochrane Risk of Bias tool was used to evaluate the five randomized studies, and it indicated that there was no high risk of bias in any of the evaluated categories among the five RCTs in our data set. Supplementary Tables 11, 12 show the quality evaluation of the present study using the PRISMA 2020 Checklist and AMSTAR-2 Checklist. Sensitivity analysis, conducted by omitting each study, indicated that all results were stable except for death within 30 days after surgery (Supplementary Figure 11). Similarly, there was no significant publication bias except for death within 30 days after surgery (Supplementary Figure 12).
4 Discussion
To the best of our knowledge, the present study is the first systematic review and meta-analysis to explore the effectiveness of four different neoadjuvant therapies (NICRT, NICT, NCRT, and NCT) followed by curative surgery for esophageal cancer, and then compare them with each other by not only postoperative outcome results but also survival-related efficacy outcomes. Drawing from data taken from 86 different studies, collectively describing 5,034 patients, we explored the comparison of R0, pCR, mPR, OS, DFS, and death within 30 days after surgery outcomes across treatment modalities. There were no significant differences in pCR and mPR rates between NICRT and NCRT; both were superior to NICT, and NCT had the poorest results. For 1-year OS and DFS rates, NICT showed the best rates compared to the other three treatments, with the other three treatments not having any statistically significant difference in these parameters amongst each other. No significant differences were observed among any of the four examined treatment modalities in terms of R0 rates or death within 30 days after surgery. As for the subgroup analyses based on the study type, intervention model, immunotherapy drugs, and cancer type, there were no significant differences between the subgroups, which is consistent with the above findings.
Although this is, to date, the largest meta-analysis to examine the role of four different neoadjuvant therapies after curative resection for esophageal cancer, previous studies on this subject have been conducted. A meta-analysis conducted by Wang et al. (103), which included 20 studies with 621 patients, explored the clinical outcomes of NICRT vs. NICT. Consistent with our findings, they reported that NICRT had an advantage over NICT in terms of mPR rates, but found no significant differences in R0 rates. However, they reported no significant differences in pCR rates between NICRT and NICT, whereas we found that NICRT had superior pCR rates to NICT (pooled RR and 95% CI: 1.83 [1.10, 3.05]). This discrepancy may be explained by Wang et al.’s smaller sample size, which included only two studies that involved NICRT. In contrast, our study included 14 studies of NICRT, including the two used by Wang et al. Additionally, the patients in the NCRT and NCT groups in their study were obtained from a meta-analysis by Li et al. (104), whereas the patients in our NCRT and NCT groups were extracted from dual-arm studies with direct head-to-head comparisons with NICRT or NICT. This significantly reduced error, increased comparability, and provided assurance of the quality of the results and conclusions. In addition, with the addition of follow-up parameters (OS and DFS), our study included more survival-related outcomes than previous studies. Our study showed greater 1-year OS and 1-year DFS rates in the NICT group, while the NICRT group showed no such results. This difference might be explained by the fact that concurrent administration of all three treatment modalities in the NICRT group significantly increased treatment-related adverse effects, resulting in patients showing no advantage in terms of survival. Wang et al. (103) reported that the incidence of preoperative grade 3-4 treatment-related adverse events was 51.2% in NICRT, which was much higher than the 19.4% in NICT. A multicenter dual-arm study conducted by Yang et al. (29) directly compared the safety of NICRT and NICT, noting that treatment-related adverse events, immune-related adverse events, and post-operative complications all had higher incidences in the NICRT group than in the NICT group. The toxicity of this treatment may ultimately result in a failure of NICRT to provide long-term survival benefits. Although this review concluded that there were no significant differences among the four different neoadjuvant treatments in terms of death within 30 days after surgery, this could be attributed to three reasons. First, the incidence of mortality within 30 days after surgery was low – close to zero, in fact – regardless of the treatment type, the differences they exhibited may not be statistically evident; second, the toxic effects of the treatment did not appear in such a short period of time and needed some time to manifest; And third, this outcome showed unstable results in both sensitivity analysis and publication bias analysis. A meta-analysis by Ge et al. (105) included 27 single-arm studies with 815 patients to explore the clinical outcomes of NICT. They reported pooled rates of R0, pCR and mPR were 98.6%, 31.4% and 48.9%, respectively, largely similar to the results obtained in our study (97.5%, 27.2%, and 51.8%, respectively). Compared to CROSS (3), which received NCRT, their R0 rate was 92.0%, which was not significantly different from the results obtained in our study and those of Ge et al. (105); however, their pCR rate was 49.0%, which was significantly higher than our results or those of Ge et al. That being said, this result is consistent with our conclusion that the pCR rate in the NCRT group was higher than that in the NICT group, while there were no significant differences in terms of R0 rate. A randomized controlled multicenter study conducted by Liu et al. (106) in 2022 reported that patients receiving NCT experienced a pCR rate of 20.8% and an mPR rate of 33.3%, consistent with our findings that the NCT group had the lowest pCR and mPR rates among the all four neoadjuvant treatments.
When we combined treatment modalities based on the inclusion or absence of immunotherapy (regardless of the presence of radiation therapy), we found that the neoadjuvant immunotherapy group had a significant advantage over the traditional neoadjuvant therapy group in terms of 1-year OS and DFS rates, while there were no significant differences between the two groups in other outcomes. It can be seen that the addition of immunotherapy can significantly prolong the survival of patients. This adds further evidence to the growing pile attesting to the benefit of immunotherapy in neoadjuvant therapy. As for the other results of the same, because the results obtained in this study were that there were no significant differences among the four different neoadjuvant treatments in R0 rates and death within 30 days after surgery, there were also no differences in the comparison between the combined groups. Regarding pCR and mPR rates, since the incidences were highest in the NCRT cohort and lowest in the NCT cohort, when these two were combined together in the traditional group, it canceled out the difference that had been seen when the four cohorts were being compared individually. This systematic review and meta-analysis also had limitations. First, as most of the studies included in this review were single-armed, potential bias may arise; second, since immunotherapy is still in the process of exploration, some studies have not yet released their final results. Moreover, survival endings could only be extracted for 1 year, as for the follow-up of long-term survival, follow-up studies are needed to report; third, there were only five RCTs in this review, and the lack of RCTs may potentially lead to bias; fourth, as previously noted, both sensitivity and publication bias analysis indicated instability in the data used for death within 30 days after surgery in this review, which prohibits rigorous conclusions from being drawn therefrom; more studies and data will be needed to verify the relevant findings of this study; fifth, due to the lack of available data, the role of effective biomarkers, for instance, combined positive score (CPS) and tumor proportion score (TPS), in neoadjuvant immunotherapy could not be investigated; Sixth, due to the inconsistent guidelines for and definitions of treatment-related adverse events in the different studies used in this meta-analysis, we were unable to properly compare them, instead focusing on the endpoints of efficacy and survival.
5 Conclusion
In conclusion, among the four neoadjuvant treatment modalities NICRT, NICT, NCRT, and NCT, NICRT and NCRT had the highest pCR and mPR rates. There were no significant differences in R0 rates among the four neoadjuvant treatment modalities. Adding immunotherapy to neoadjuvant therapy improved 1-year OS and DFS, with the NICT group having significantly higher longer survival according to both these metrics than any of the other three modalities. The results of this review provide a basis for future studies. Further, large multicenter RCTs and longer-term follow-ups are needed to refine these findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
All authors read and approved the final manuscript prior to submission. WS are responsible for the conception and design of the study. HW are responsible for analysis and interpretation of data, and drafting the article and revising it. CS and XZ are responsible for acquisition of data. WD and JD is responsible for data check. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to express our sincere thanks to Department of Radiation Oncology, Fourth Hospital of Hebei Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1170569/full#supplementary-material
Abbreviations
NICRT, neoadjuvant immunotherapy combined with chemoradiotherapy; NICT, neoadjuvant immunochemotherapy; NCRT, neoadjuvant chemoradiotherapy; NCT, neoadjuvant chemotherapy; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PICOS, Population, Intervention, Comparison, Outcome, and Study Design; pCR, pathological complete response; mPR, major pathological response; OS, overall survival; DFS, disease-free survival; MINORS, Methodological Index for Non-randomized Studies; RR, risk ratio; CI, confidence interval; SCC, squamous cell carcinoma; AC, adenocarcinoma; CPS, combined positive score; TPS, tumor proportion score.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Paul S, Altorki N. Outcomes in the management of esophageal cancer. J Surg Oncol (2014) 110(5):599–610. doi: 10.1002/jso.23759
3. Eyck BM, van Lanschot JJB, Hulshof M, van der Wilk BJ, Shapiro J, van Hagen P, et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol (2021) 39(18):1995–2004. doi: 10.1200/JCO.20.03614
4. Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2019) 17(7):855–83. doi: 10.6004/jnccn.2019.0033
5. Baba NY Y, Kinoshita K, Iwatsuki M, Yamashita Y-I, Chikamoto A, Watanabe M, et al. And prognostic features of patients with esophageal cancer and multiple primary cancers: a retrospective single-institution study. Ann Surg (2018) 267(3):478–83. doi: 10.1097/SLA.0000000000002118
6. Huang LF TX. The immune landscape of esophageal cancer. Cancer Commun (Lond) (2019) 39(1):79. doi: 10.1186/s40880-019-0427-z
7. Janjigian JB YY, Calvo E, Kim JW, Ascierto PA, Sharma P, Ott PA, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol (2018) 36(28):2836–44. doi: 10.1200/JCO.2017.76.6212
8. Kojima MAS T, Muro K, Francois E, Adenis A, Hsu C-H, Doi T, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol (2020) 38(35):4138–48. doi: 10.1200/JCO.20.01888
9. Ribas JDW A. Cancer immunotherapy using checkpoint blockade. Science (2018) 359(6382):1350–5. doi: 10.1126/science.aar4060
10. Li Y, Zhou A, Liu S, He M, Chen KN, Tian Z, et al. 1207P neoadjuvant PD-L1 inhibitor (socazolimab) plus chemotherapy in patients with locally advanced esophageal squamous cell carcinoma (ESCC): a multicenter, randomized, double-blind phase II study. Ann Oncol (2022) 33(S7):S1101. doi: 10.1016/j.annonc.2022.07.1325
11. Guo J, Qiao C, Lu J, Yang S, Zhang B. Neoadjuvant sintilimab combined with chemotherapy in patients with resectable esophageal squamous cell carcinoma (ESCC): preliminary results from a phase II study. J Clin Oncol (2022) 40(16):E16008–E. doi: 10.1200/JCO.2022.40.16_suppl.e16008
12. Li C, Zhao S, Zheng Y, Han Y, Chen X, Cheng Z, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer (2021) 144:232–41. doi: 10.1016/j.ejca.2020.11.039
13. Kelly RJ, Zaidi AH, Van Liere Canzoniero J, Feliciano JL, Hales RK, Voong KR, et al. Multicenter phase II study of neoadjuvant nivolumab or nivolumab plus relatlimab (antiLAG3 antibody) plus chemoradiotherapy in stage II/III esophageal/gastroesophageal junction (E/GEJ) carcinoma. J Clin Oncol (2022) 40(4 SUPPL):321. doi: 10.1200/JCO.2022.40.4_suppl.321
14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol (2021) 134:178–89. doi: 10.1016/j.jclinepi.2021.03.001
15. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi. J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg (2003) 73(9):712–6. doi: 10.1046/j.1445-2197.2003.02748.x
16. Malgie J, Schoones JW, Pijls BG. Decreased mortality in coronavirus disease 2019 patients treated with tocilizumab: a rapid systematic review and meta-analysis of observational studies. Clin Infect Dis (2021) 72(11):e742–e9. doi: 10.1093/cid/ciaa1445
17. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
18. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (2019) 366:l4898. doi: 10.1136/bmj.l4898
19. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (2017) 358:j4008. doi: 10.1136/bmj.j4008
20. Mazumdar M, Begg CB. Operating characteristics of a rank correlation test for publication bias. Biometrics (1994) 50(4):1088–101.
21. Zhu M, Chen C, Foster NR, Hartley C, Mounajjed T, Salomao MA, et al. Pembrolizumab in combination with neoadjuvant chemoradiotherapy for patients with resectable adenocarcinoma of the gastroesophageal junction. Clin Cancer Res (2022) 28(14):3021–31. doi: 10.1158/1078-0432.CCR-22-0413
22. Zhou YH, Li JY, Yan JX, Guo P, He WW, Liu Y. Alleviation of neoadjuvant immunochemotherapy for esophageal squamous cell carcinoma and its relationship with expression and changes of PD-L1. Neoplasma (2022) 69(4):785–93. doi: 10.4149/neo_2022_211223N1826
23. Zhang Z, Ye J, Li H, Gu D, Du M, Ai D, et al. Neoadjuvant sintilimab and chemotherapy in patients with resectable esophageal squamous cell carcinoma: a prospective, single-arm, phase 2 trial. Front Immunol (2022) 13:1031171. doi: 10.3389/fimmu.2022.1031171
24. Zhang Y, Shen G, Xu R, Huang G, Yang S, Zheng Q, et al. Real-world effectiveness and safety of camrelizumab-based neoadjuvant therapy in resectable esophageal cancer: initial results of a prospective multicenter observational study. J Clin Oncol (2022) 40(4_suppl):250–. doi: 10.1200/JCO.2022.40.4_suppl.250
25. Zhang X, Gari A, Li M, Chen J, Qu C, Zhang L, et al. Combining serum inflammation indexes at baseline and post treatment could predict pathological efficacy to anti−PD−1 combined with neoadjuvant chemotherapy in esophageal squamous cell carcinoma. J Transl Med (2022) 20(1):61. doi: 10.1186/s12967-022-03252-7
26. Zhang G, Yuan J, Pan C, Xu Q, Cui X, Zhang J, et al. Abstract CT544: multi-omics analysis uncovers prognostic biomarkers and tumor ecosystem dynamics during toripalimab combined with nab-paclitaxel and s-1 as neoadjuvant therapy for esophageal squamous carcinoma: a single-center, open-label, single-arm phase 2 trial. Cancer Res (2022) 82(12_Supplement):CT544–CT. doi: 10.1158/1538-7445.AM2022-CT544
27. Yu Y, Wang W, Qin Z, Li H, Liu Q, Ma H, et al. A clinical nomogram for predicting tumor regression grade in esophageal squamous-cell carcinoma treated with immune neoadjuvant immunotherapy. Ann Transl Med (2022) 10(2):102. doi: 10.21037/atm-22-78
28. Yin GQ, Li ZL, Li D. The safety and efficacy of neoadjuvant camrelizumab plus chemotherapy in patients with locally advanced esophageal squamous cell carcinoma: a retrospective study. Cancer Manag Res (2022) 14:2133–41. doi: 10.2147/CMAR.S358620
29. Yang Y, Tan L, Hu J, Li Y, Mao Y, Tian Z, et al. Safety and efficacy of neoadjuvant treatment with immune checkpoint inhibitors in esophageal cancer: real-world multicenter retrospective study in China. Dis Esophagus (2022) 35(11):1–8. doi: 10.1093/dote/doac031
30. Yang W, Xing X, Yeung SJ, Wang S, Chen W, Bao Y, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer (2022) 10(1):e003497. doi: 10.1136/jitc-2021-003497
31. Yang G, Sun X, Yang H, Luo G, Zheng Y, Huang M, et al. 1256P three courses of neoadjuvant camrelizumab combined with chemotherapy in locally advanced esophageal squamous cell carcinoma (ESCC): a prospective phase II clinical trial. Ann Oncol (2022) 33(S7):S1122. doi: 10.1016/j.annonc.2022.07.1374
32. Yan X, Duan H, Ni Y, Zhou Y, Wang X, Qi H, et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: a prospective, single-arm, phase II study (TD-NICE). Int J Surg (2022) 103:106680. doi: 10.1016/j.ijsu.2022.106680
33. Xu X, Sun Z, Zhang Y, Shen L, Liu Q, Zhang C, et al. Neoadjuvant chemoradiotherapy combined with perioperative toripalimab in locally advanced esophageal cancer. J Clin Oncol (2022) 40(16):E16065–E. doi: 10.1200/JCO.2022.40.16_suppl.e16065
34. Xu W, Jiang Y, Wang C, Wu J, Li J, Hu Y, et al. The efficacy and safety of neoadjuvant camrelizumab and chemotherapy for locally advanced thoracic esophageal squamous cell carcinoma. J Clin Oncol (2022) 40(4_suppl):278–. doi: 10.1200/JCO.2022.40.4_suppl.278
35. Xu L, Wei XF, Li CJ, Yang ZY, Yu YK, Li HM, et al. Pathologic responses and surgical outcomes after neoadjuvant immunochemotherapy versus neoadjuvant chemoradiotherapy in patients with locally advanced esophageal squamous cell carcinoma. Front Immunol (2022) 13:1052542. doi: 10.3389/fimmu.2022.1052542
36. Xu L, Qi Y, Jiang Y, Ji Y, Zhao Q, Wu J, et al. Crosstalk between the gut microbiome and clinical response in locally advanced thoracic esophageal squamous cell carcinoma during neoadjuvant camrelizumab and chemotherapy. Ann Transl Med (2022) 10(6):325. doi: 10.21037/atm-22-1165
37. Xiao X, Yang YS, Zeng XX, Shang QX, Luan SY, Zhou JF, et al. The comparisons of neoadjuvant chemoimmunotherapy versus chemoradiotherapy for oesophageal squamous cancer. Eur J Cardiothorac Surg (2022) 62(1):ezac341. doi: 10.1093/ejcts/ezac341
38. Xia P, Li P, Wu S, Wang Y, Ye P, Zhang C, et al. Evaluation of the safety and effectiveness of neoadjuvant combined chemoimmunotherapy in the treatment of locally advanced esophageal squamous cell carcinoma: a retrospective single-arm cohort study. Ann Transl Med (2022) 10(18):991. doi: 10.21037/atm-22-4268
39. Wang X, Yang W, Zhou Q, Luo H, Chen W, Yeung SJ, et al. The role of (18)F-FDG PET/CT in predicting the pathological response to neoadjuvant PD-1 blockade in combination with chemotherapy for resectable esophageal squamous cell carcinoma. Eur J Nucl Med Mol Imaging (2022) 49(12):4241–51. doi: 10.1007/s00259-022-05872-z
40. Wang W, Li L. Neoadjuvant pembrolizumab plus chemotherapy for resectable locally advanced esophageal squamous cell carcinoma (ESCC): interim results. J Clin Oncol (2022) 40(16):E16011–E. doi: 10.1200/JCO.2022.40.16_suppl.e16011
41. Wang R, Zhang G, Zhu Q, Ma T, Weng C, Zhang D, et al. 1234P neoadjuvant camrelizumab plus docetaxel and carboplatin in locally advanced esophageal squamous cell carcinoma (ESCC): a prospective study. Ann Oncol (2022) 33(S7):S1113. doi: 10.1016/j.annonc.2022.07.1352
42. Uboha NV, Eickhoff JC, Maloney JD, McCarthy D, De Camp M, Deming DA, et al. Phase I/II trial of perioperative avelumab in combination with chemoradiation (CRT) in the treatment of stage II/III resectable esophageal and gastroesophageal junction (E/GEJ) cancer. J Clin Oncol (2022) 40(16):4034. doi: 10.1200/JCO.2022.40.16_suppl.4034
43. Qiao Y, Zhao C, Li X, Zhao J, Huang Q, Ding Z, et al. Efficacy and safety of camrelizumab in combination with neoadjuvant chemotherapy for ESCC and its impact on esophagectomy. Front Immunol (2022) 13:953229. doi: 10.3389/fimmu.2022.953229
44. Qi Y, Meng X, Shan Z, Fan Q, Wang F. Camrelizumab combined with chemotherapy or apatinib as neoadjuvant therapy for locally advanced esophageal squamous cell carcinoma: A phase 2 trial. Dis Esophagus (2022) 35:62. doi: 10.1093/dote/doac051.355
45. Matsuda S, Yamamoto S, Kato K, Daiko H, Kojima T, Hara H, et al. FRONTiER: a feasibility trial of nivolumab with neoadjuvant CF or DCF, FLOT therapy for locally advanced esophageal carcinoma (JCOG1804E)–short-term results for cohorts c and d. J Clin Oncol (2022) 40(4_suppl):286–. doi: 10.1200/JCO.2022.40.4_suppl.286
46. Ma X, Zhao W, Li B, Yu Y, Ma Y, Thomas M, et al. Neoadjuvant immune checkpoint inhibitors plus chemotherapy in locally advanced esophageal squamous cell carcinoma: perioperative and survival outcomes. Front Oncol (2022) 12:810898. doi: 10.3389/fonc.2022.810898
47. Lv H, Tian Y, Li J, Huang C, Sun B, Gai C, et al. Neoadjuvant sintilimab plus chemotherapy in resectable locally advanced esophageal squamous cell carcinoma. Front Oncol (2022) 12:864533. doi: 10.3389/fonc.2022.864533
48. Liu J, Yang Y, Liu Z, Fu X, Cai X, Li H, et al. Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer (2022) 10(3):e004291. doi: 10.1136/jitc-2021-004291
49. Liu J, Li J, Lin W, Shao D, Depypere L, Zhang Z, et al. Neoadjuvant camrelizumab plus chemotherapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): a multicenter, phase 2 study. Int J Cancer (2022) 151(1):128–37. doi: 10.1002/ijc.33976
50. Li Z, Xu PF, Mao WM, Kuang YK, Fan HY, Zou B, et al. A study of neoadjuvant sintilimab combined with chemotherapy TP for locally advanced esophageal squamous cell carcinoma (ESCC). J Clin Oncol (2022) 40(16):e16038. doi: 10.1200/JCO.2022.40.16_suppl.e16038
51. Ko AH, Noel M, Chao J, Sohal D, Crow M, Oberstein PE, et al. 1229P a multicenter phase II study of sotigalimab (CD40 agonist) in combination with neoadjuvant chemoradiation for resectable esophageal and gastroesophageal junction (GEJ) cancers. Ann Oncol (2022) 33(S7):S1111. doi: 10.1016/j.annonc.2022.07.1347
52. Jing SW, Zhai C, Zhang W, He M, Liu QY, Yao JF, et al. Comparison of neoadjuvant immunotherapy plus chemotherapy versus chemotherapy alone for patients with locally advanced esophageal squamous cell carcinoma: a propensity score matching. Front Immunol (2022) 13:970534. doi: 10.3389/fimmu.2022.970534
53. Jiang N, Jiang M, Zhu X, Ren B, Zhang J, Guo Z, et al. SCALE-1: safety and efficacy of short course neoadjuvant chemo-radiotherapy plus toripalimab for locally advanced resectable squamous cell carcinoma of esophagus. J Clin Oncol (2022) 40(16):4063. doi: 10.1200/JCO.2022.40.16_suppl.4063
54. Jiang B, Yang X, Zhang J, Huang M. Abstract 5230: neoadjuvant programmed cell death protein 1 inhibitors combined with chemotherapy in resectable esophageal squamous carcinoma: an open-label, single-arm study. Cancer Res (2022) 82(12_Supplement):5230–. doi: 10.1158/1538-7445.AM2022-5230
55. Huang SJ, Tian D, Wang SC, Zeng RJ, Dong YJ, Hong LL, et al. Pathological responses of the primary tumor and locoregional lymph nodes after neoadjuvant immunochemotherapy in esophageal squamous cell cancer. World J Oncol (2022) 13(4):195–204. doi: 10.14740/wjon1489
56. Huang S, Wu H, Cheng C, Zhou M, Xu E, Lin W, et al. Conversion surgery following immunochemotherapy in initially unresectable locally advanced esophageal squamous cell carcinoma-a real-world multicenter study (RICE-retro). Front Immunol (2022) 13:935374. doi: 10.3389/fimmu.2022.935374
57. Hong ZN, Zhang Z, Chen Z, Weng K, Peng K, Lin J, et al. Safety and feasibility of esophagectomy following combined neoadjuvant immunotherapy and chemotherapy for locally advanced esophageal cancer: a propensity score matching. Esophagus (2022) 19(2):224–32. doi: 10.1007/s10388-021-00899-x
58. Hong ZN, Gao L, Weng K, Huang Z, Han W, Kang M. Safety and feasibility of esophagectomy following combined immunotherapy and chemotherapy for locally advanced esophageal squamous cell carcinoma: a propensity score matching analysis. Front Immunol (2022) 13:836338. doi: 10.3389/fimmu.2022.836338
59. He W, Leng X, Mao T, Luo X, Zhou L, Yan J, et al. Toripalimab plus paclitaxel and carboplatin as neoadjuvant therapy in locally advanced resectable esophageal squamous cell carcinoma. Oncologist (2022) 27(1):e18–28. doi: 10.1093/oncolo/oyab011
60. Gu YM, Shang QX, Zhang HL, Yang YS, Wang WP, Yuan Y, et al. Safety and feasibility of esophagectomy following neoadjuvant immunotherapy combined with chemotherapy for esophageal squamous cell carcinoma. Front Surg (2022) 9:851745. doi: 10.3389/fsurg.2022.851745
61. Gong T, Song W, Lu J, Liu J, Fan B, Zhao J, et al. Efficacy and safety of neoadjuvant chemotherapy and immunotherapy in locally resectable advanced esophageal squamous cell carcinoma. Dis Esophagus (2022) 35:108–9. doi: 10.1093/dote/doac051.520
62. Gao L, Lu J, Zhang P, Hong ZN, Kang M. Toripalimab combined with docetaxel and cisplatin neoadjuvant therapy for locally advanced esophageal squamous cell carcinoma: a single-center, single-arm clinical trial (ESONICT-2). J Gastrointest Oncol (2022) 13(2):478–87. doi: 10.21037/jgo-22-131
63. Feng J, Wang L, Yang X, Chen Q, Cheng X. Pathologic complete response prediction to neoadjuvant immunotherapy combined with chemotherapy in resectable locally advanced esophageal squamous cell carcinoma: real-world evidence from integrative inflammatory and nutritional scores. J Inflamm Res (2022) 15:3783–96. doi: 10.2147/JIR.S367964
64. Duan H, Shao C, Pan M, Liu H, Dong X, Zhang Y, et al. Neoadjuvant pembrolizumab and chemotherapy in resectable esophageal cancer: an open-label, single-arm study (PEN-ICE). Front Immunol (2022) 13:849984. doi: 10.3389/fimmu.2022.849984
65. Dong Y, Luo J, Liu S, Lin Y, Han H. 1225P neoadjuvant therapy of camrelizumab combined with chemotherapy in patients (pts) with resectable esophageal squamous cell cancer (ESCC): preliminary results of a preoperative phase exploratory clinical trial. Ann Oncol (2022) 33(S7):S1119. doi: 10.1016/j.annonc.2022.07.1343
66. Cheng J, Guo M, Yang Y, Liu Y, Hu W, Shang Q, et al. Perioperative outcomes of minimally invasive esophagectomy after neoadjuvant immunotherapy for patients with locally advanced esophageal squamous cell carcinoma. Front Immunol (2022) 13:848881. doi: 10.3389/fimmu.2022.848881
67. Chen F, Qiu L, Mu Y, Sun S, Yuan Y, Shang P, et al. Neoadjuvant chemoradiotherapy with camrelizumab in patients with locally advanced esophageal squamous cell carcinoma. Front Surg (2022) 9:893372. doi: 10.3389/fsurg.2022.893372
68. Zhao L, Xing W, Yang Y, Zhang Y, Ma B, Fu X, et al. The sequence of chemotherapy and anti-PD-1 antibody influence the efficacy of neoadjuvant immunochemotherapy in locally advanced esophageal squamous cell cancer: a phase II study. J Clin Oncol (2021) 39(15_suppl):4051–. doi: 10.1200/JCO.2021.39.15_suppl.4051
69. Zhang Z, Ye J, Li H, Du M, Gu D, Zhang J, et al. 1378P a single-center, prospective, open-label, single-arm trial of sintilimab with paclitaxel and carboplatin as a neoadjuvant therapy for esophageal squamous carcinoma. Ann Oncol (2021) 32:S1042–S3. doi: 10.1016/j.annonc.2021.08.1487
70. Zhang Z, Hong ZN, Xie S, Lin W, Lin Y, Zhu J, et al. Neoadjuvant sintilimab plus chemotherapy for locally advanced esophageal squamous cell carcinoma: a single-arm, single-center, phase 2 trial (ESONICT-1). Ann Transl Med (2021) 9(21):1623. doi: 10.21037/atm-21-5381
71. Zhang X, Yang G, Su X, Luo G, Cai P, Zheng Y, et al. Neoadjuvant programmed death1 blockade plus chemotherapy in locally advanced esophageal squamous cell carcinoma. J Clin Oncol (2021) 39(15 SUPPL):e16076. doi: 10.1200/JCO.2021.39.15_suppl.e16076
72. Yang P, Zhou X, Yang X, Wang Y, Sun T, Feng S, et al. Neoadjuvant camrelizumab plus chemotherapy in treating locally advanced esophageal squamous cell carcinoma patients: a pilot study. World J Surg Oncol (2021) 19(1):333. doi: 10.1186/s12957-021-02446-5
73. Yang G, Su X, Yang H, Luo G, Gao C, Zheng Y, et al. Neoadjuvant programmed death-1 blockade plus chemotherapy in locally advanced esophageal squamous cell carcinoma. Ann Transl Med (2021) 9(15):1254. doi: 10.21037/atm-21-3352
74. Yamamoto S, Kato K, Daiko H, Kojima T, Hara H, Abe T, et al. FRONTiER: a feasibility trial of nivolumab with neoadjuvant CF or DCF therapy for locally advanced esophageal carcinoma (JCOG1804E)-the short-term results of cohort a and b. J Clin Oncol (2021) 39(3):202. doi: 10.1200/JCO.2021.39.3_suppl.202
75. Xing W, Zhao L, Zheng Y, Liu B, Liu X, Li T, et al. The sequence of chemotherapy and toripalimab might influence the efficacy of neoadjuvant chemoimmunotherapy in locally advanced esophageal squamous cell cancer-a phase II study. Front Immunol (2021) 12:772450. doi: 10.3389/fimmu.2021.772450
76. Xiao Y, Huo Q, Yang Y, Lu B, Wang X. Clinical trial of carrelizumab injection combined with operation in the treatment of patients with stage II/III esophageal squamous cell carcinoma. Chin J Clin Pharmacol (2021) 37(24):3323–5.
77. Wu Z, Zheng Q, Chen H, Xiang J, Hu H, Li H, et al. Efficacy and safety of neoadjuvant chemotherapy and immunotherapy in locally resectable advanced esophageal squamous cell carcinoma. J Thorac Dis (2021) 13(6):3518–28. doi: 10.21037/jtd-21-340
78. Wu P, Wang T, Chen B, Shi M, Zhou Y, Huang B, et al. Preoperative chemotherapy combined with PD-1 inhibitor in locally advanced operable or potentially resectable esophageal squamous cell carcinoma: a real world study. J Clin Oncol (2021) 39(15_suppl):e16010–e. doi: 10.1200/JCO.2021.39.15_suppl.e16010
79. Wang F, Qi Y, Meng X, Fan Q. Camrelizumab in combination with preoperative chemotherapy for locally advanced esophageal squamous cell carcinoma: a single-arm, open-label, phase II study. J Clin Oncol (2021) 39(15):222. doi: 10.1200/JCO.2021.39.15_suppl.e16072
80. van den Ende T, de Clercq NC, van Berge Henegouwen MI, Gisbertz SS, Geijsen ED, Verhoeven RHA, et al. Neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: a single-arm phase II feasibility trial (PERFECT). Clin Cancer Res (2021) 27(12):3351–9. doi: 10.1158/1078-0432.CCR-20-4443
81. Sihag S, Ku GY, Tan KS, Nussenzweig S, Wu A, Janjigian YY, et al. Safety and feasibility of esophagectomy following combined immunotherapy and chemoradiotherapy for esophageal cancer. J Thorac Cardiovasc Surg (2021) 161(3):836–43.e1. doi: 10.1016/j.jtcvs.2020.11.106
82. Shen D, Chen Q, Wu J, Li J, Tao K, Jiang Y. The safety and efficacy of neoadjuvant PD-1 inhibitor with chemotherapy for locally advanced esophageal squamous cell carcinoma. J Gastrointest Oncol (2021) 12(1):1–10. doi: 10.21037/jgo-20-599
83. Shang X, Zhang C, Zhao G, Zhang W, Liu L, Duan X, et al. LBA3 safety and efficacy of pembrolizumab combined with paclitaxel and cisplatin as a neoadjuvant treatment for locally advanced resectable (stage III) esophageal squamous cell carcinoma (Keystone-001): interim analysis of a prospective, single-arm, single-center, phase II trial. Ann Oncol (2021) 32:S1428–S9. doi: 10.1016/j.annonc.2021.10.218
84. Shah MA, Almhanna K, Iqbal S, Thakkar P, Schneider BJ, Yantiss R, et al. Multicenter, randomized phase II study of neoadjuvant pembrolizumab plus chemotherapy and chemoradiotherapy in esophageal adenocarcinoma (EAC). J Clin Oncol (2021) 39(15_suppl):4005–. doi: 10.1200/JCO.2021.39.15_suppl.4005
85. Ma J, Zhang J, Yang Y, Zheng D, Wang X, Liang H, et al. Camrelizumab combined with paclitaxel and nedaplatin as neoadjuvant therapy for locally advanced esophageal squamous cell carcinoma (ESPRIT): a phase II, single-arm, exploratory research. Ann Oncol (2021) 32:S1400–S. doi: 10.1016/j.annonc.2021.10.083
86. Lv H, Tian Y, Li Z, Huang C, Xu Y, Tian Z. 557 neoadjuvant camrelizumab in combination with albumin paclitaxel and cisplatin for patients with locally advanced esophageal squamous cell carcinoma (ESCC). J ImmunoTher Cancer (2021) 9(Suppl 2):A587–A. doi: 10.1136/jitc-2021-SITC2021.557
87. Lv H, Tian Y, Huang C, Sun B, Gai C, Li Z, et al. 110P neoadjuvant PD-1 blockade combined with chemotherapy for patients with resectable locally advanced esophageal squamous cell carcinoma (ESCC): a real world data analysis. Ann Oncol (2021) 32(S7):S1423. doi: 10.1016/j.annonc.2021.10.128
88. Lv H, Tian Y, Huang C, Li Z, Tian Z. Camrelizumab combined with albumin paclitaxel and cisplatin as neoadjuvant therapy for locally advanced esophageal squamous cell carcinoma (ESCC). J Clin Oncol (2021) 39(15):e16021. doi: 10.1200/JCO.2021.39.15_suppl.e16021
89. Liu D, Zhang Q, Zhu J, Qian T, Yin R, Fan Z, et al. Phase-II study of toripalimab combined with neoadjuvant chemotherapy for the treatment of resectable esophageal squamous cell carcinoma. J Clin Oncol (2021) 39(15):e16029. doi: 10.1200/JCO.2021.39.15_suppl.e16029
90. Ku GY, Wu AJ-C, Sihag S, Park BJ, Jones DR, Gu P, et al. Durvalumab (D) and PET-directed chemoradiation (CRT) after induction FOLFOX for esophageal adenocarcinoma. J Clin Oncol (2021) 39(3_suppl):226–. doi: 10.1200/JCO.2021.39.3_suppl.226
91. Huang B, Shi H, Gong X, Yu J, Xiao C, Zhou B, et al. Comparison of efficacy and safety between pembrolizumab combined with chemotherapy and simple chemotherapy in neoadjuvant therapy for esophageal squamous cell carcinoma. J Gastrointest Oncol (2021) 12(5):2013–21. doi: 10.21037/jgo-21-610
92. Hong ZN, Weng K, Peng K, Chen Z, Lin J, Kang M. Neoadjuvant immunotherapy combined chemotherapy followed by surgery versus surgery alone for locally advanced esophageal squamous cell carcinoma: a propensity score-matched study. Front Oncol (2021) 11:797426. doi: 10.3389/fonc.2021.797426
93. Duan H, Wang T, Luo Z, Wang X, Liu H, Tong L, et al. A multicenter single-arm trial of sintilimab in combination with chemotherapy for neoadjuvant treatment of resectable esophageal cancer (SIN-ICE study). Ann Transl Med (2021) 9(22):1700. doi: 10.21037/atm-21-6102
94. Cheng C, Yang W, Chen W, Yeung S-CJ, Xing X, Wang X, et al. Neoadjuvant PD-1 blockade in combination with chemotherapy for patients with resectable esophageal squamous cell carcinoma. J Clin Oncol (2021) 39(3_suppl):220–. doi: 10.1200/JCO.2021.39.3_suppl.220
95. Athauda A, Starling N, Chau I, Cunningham D, Watkins DJ, Rao S, et al. Perioperative FLOT plus anti-PD-L1 avelumab (FLOT-a) in resectable oesophagogastric adenocarcinoma (OGA): interim safety analysis results from the ICONIC trial. J Clin Oncol (2021) 39(3_suppl):201–. doi: 10.1200/JCO.2021.39.3_suppl.201
96. Zhang G, Hu Y, Yang B, Xu Q, Li J, Sun S, et al. 1058P a single-centre, prospective, open-label, single-arm trial of toripalimab with nab-paclitaxel and s-1 as a neoadjuvant therapy for esophageal squamous cell carcinoma (ESCC). Ann Oncol (2020) 31(S4):S722. doi: 10.1016/j.annonc.2020.08.1178
97. Qi WX, Zhao S, Li H, Chen J. Safety and tolerability of neoadjuvant chemoradiotherapy combined with pembrolizumab for local advanced, resectable esophageal cancer: preliminary results of a prospective phase IB trial. Int J Radiat OncologyBiologyPhysics (2020) 108(3):e576–e7. doi: 10.1016/j.ijrobp.2020.07.1773
98. Park SY, Hong MH, Kim HR, Lee CG, Cho JH, Cho BC, et al. The feasibility and safety of radical esophagectomy in patients receiving neoadjuvant chemoradiotherapy with pembrolizumab for esophageal squamous cell carcinoma. J Thorac Dis (2020) 12(11):6426–34. doi: 10.21037/jtd-20-1088
99. Li K, Yang X, Luo W, Ma Q, Wang Y, Xiong Y, et al. Toripalimab plus nab-paclitaxel and carboplatin as neoadjuvant therapy for patients with esophageal squamous cell carcinoma At clinical stage T2-T4/N0-N2/M0: a single-arm, single-center clinical study. J Immunother Cancer (2020) 8:A253–A. doi: 10.1136/jitc-2020-SITC2020.0415
100. Li H, Li C, Zheng Y, Zhao S, Chen X, Han Y, et al. Preoperative combination ofpembrolizumab withchemoradiation for patients withlocally advanced esophagealsquamous cell carcinoma:mid-termresults of NCT03604991. Dis Esophagus (2020) 33(SUPPL 1):14. doi: 10.1093/dote/doaa087.35
101. Gu Y, Chen X, Wang D, Ding M, Xue L, Zhen F, et al. 175P a study of neoadjuvant sintilimab combined with triplet chemotherapy of lipo-paclitaxel, cisplatin, and s-1 for resectable esophageal squamous cell carcinoma (ESCC). Ann Oncol (2020) 31:S1307–S8. doi: 10.1016/j.annonc.2020.10.196
102. Lee S, Ahn BC, Park SY, Kim DJ, Lee CG, Cho J, et al. A phase II trial of preoperative chemoradiotherapy and pembrolizumab for locally advanced esophageal squamous cell carcinoma (ESCC). Ann Oncol (2019) 30(Supplement 5):v754. doi: 10.1093/annonc/mdz266.018
103. Wang Z, Shao C, Wang Y, Duan H, Pan M, Zhao J, et al. Efficacy and safety of neoadjuvant immunotherapy in surgically resectable esophageal cancer: a systematic review and meta-analysis. Int J Surg (2022) 104:106767. doi: 10.1016/j.ijsu.2022.106767
104. Li F, Ding N, Zhao Y, Yuan L, Mao Y. The current optimal multimodality treatments for oesophageal squamous-cell carcinoma: a systematic review and meta-analysis. Int J Surg (2018) 60:88–100. doi: 10.1016/j.ijsu.2018.10.037
105. Ge F, Huo Z, Cai X, Hu Q, Chen W, Lin G, et al. Evaluation of clinical and safety outcomes of neoadjuvant immunotherapy combined with chemotherapy for patients with resectable esophageal cancer: a systematic review and meta-analysis. JAMA Netw Open (2022) 5(11):e2239778. doi: 10.1001/jamanetworkopen.2022.39778
106. Liu J, Wang Y, Cao B, Zhang S, Cao F, Gao L, et al. A randomized, controlled, multicenter study of nab-paclitaxel plus cisplatin followed by surgery versus surgery alone for locally advanced esophageal squamous cell carcinoma (ESCC). J Clin Oncol (2022) 40(4_suppl):310–. doi: 10.1200/JCO.2022.40.4_suppl.310
Keywords: neoadjuvant therapy, immunotherapy, neoadjuvant immunotherapy, curative resection, esophageal carcinoma, meta-analysis
Citation: Wang H, Song C, Zhao X, Deng W, Dong J and Shen W (2023) Evaluation of neoadjuvant immunotherapy and traditional neoadjuvant therapy for resectable esophageal cancer: a systematic review and single-arm and network meta-analysis. Front. Immunol. 14:1170569. doi: 10.3389/fimmu.2023.1170569
Received: 21 February 2023; Accepted: 02 May 2023;
Published: 12 May 2023.
Edited by:
Sang T. Kim, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Yee Ung, University of Toronto, CanadaJianzhong Cao, Shanxi Provincial Cancer Hospital, China
Copyright © 2023 Wang, Song, Zhao, Deng, Dong and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenbin Shen, wbshen1979@sina.com
 Hesong Wang
Hesong Wang Chunyang Song
Chunyang Song Xiaohan Zhao
Xiaohan Zhao Jing Dong
Jing Dong Wenbin Shen
Wenbin Shen