- 1State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products, Ningbo University, Ningbo, Zhejiang, China
- 2Laboratory of Biochemistry and Molecular Biology, School of Marine Sciences, Ningbo University, Ningbo, China
- 3Key Laboratory of Aquacultural Biotechnology Ministry of Education, Ningbo University, Ningbo, China
Leukocyte cell-derived chemotaxin-2 (LECT2, also named ChM-II), initially identified as a chemokine mediating neutrophil migration, is a multifunctional secreted factor involved in diverse physiological and pathological processes. The high sequence similarity of LECT2 among different vertebrates makes it possible to explore its functions by using comparative biology. LECT2 is associated with many immune processes and immune-related diseases via its binding to cell surface receptors such as CD209a, Tie1, and Met in various cell types. In addition, the misfolding LECT2 leads to the amyloidosis of several crucial tissues (kidney, liver, and lung, etc.) by inducing the formation of insoluble fibrils. However, the mechanisms of LECT2-mediated diverse immune pathogenic conditions in various tissues remain to be fully elucidated due to the functional and signaling heterogeneity. Here, we provide a comprehensive summary of the structure, the “double-edged sword” function, and the extensive signaling pathways of LECT2 in immune diseases, as well as the potential applications of LECT2 in therapeutic interventions in preclinical or clinical trials. This review provides an integrated perspective on the current understanding of how LECT2 is associated with immune diseases, with the aim of facilitating the development of drugs or probes against LECT2 for the theranostics of immune-related diseases.
1 Introduction
LECT2 (leucocyte cell-derived chemotaxin 2) is a hormone-like protein that was originally identified as a chemokine mediating neutrophil migration (1). Subsequently, it has also been defined as chondromodulin II (CHM2 or ChM-II) due to its function in promoting chondrocyte proteoglycan synthesis and cartilage growth (2). In fact, as a member of chondromodulin family, although CHM2 shares lower sequence similarity with its family member CHM-1, both of them function as anti-angiogenic factors (3, 4). CHM2 suppresses angiogenesis by blocking VEGF165-VEGFR2 signaling in liver cancer (5) and reduces endothelial cell migration and tube formations by activating LECT2-Tie1 signaling in liver fibrosis (6).
LECT2 is also identified as a hepatokine. Hepatocyte-derived LECT2 not only regulates hepatocyte cells in an autocrine mode, but can also be secreted into the bloodstream to act on cells of other tissues in a paracrine way to modulate multiple metabolic homeostasis or disorders such as glucose metabolism, non-alcoholic fatty liver disease (NAFLD) (7, 8), alcohol-induced liver cirrhosis (9), obesity (10), diabetes (11, 12), and atherosclerosis (13). LECT2 is also positively correlated with diet-induced weight cycling in mice and humans, suggesting that LECT2 is a sensing hepatokine for nutritional regulation-mediated metabolic homeostasis and functions as an indicator for management of obesity in the clinic (14, 15).
Besides its soluble functioning as a cytokine or hepatokine, LECT2 also exists in the form of amyloidosis (aLECT2) and is involved in renal and hepatic amyloid lesions (16–18). aLECT2 has been found to deposit in vessels, interstitial, and glomeruli of renal biopsies (19–21). The mutation or genetic variations of LECT2 are responsible for the formation of aLECT2, while the misfolding LECT2, which leads to insoluble fibrils aggregated in cells and tissues, might be the potential pathogenesis of LECT2-mediated amyloidosis (19, 20). However, the specific role of aLECT2 in amyloidosis is still unclear, and there is also a disputation about whether this protein is feasible as a diagnosis and treatment for aLECT2-mediated amyloidosis.
Furthermore, more recent research has revealed the link between LECT2 and the development of multiple immunological diseases such as sepsis (22–24), atherosclerosis (13, 25–27), osteoporosis (28, 29), arthritis (30–33), diabetes (10–12, 34), atopic dermatitis (35), and non-alcoholic steatohepatitis (NASH) (7, 8). Nonetheless, it is disease-dependent for the action mechanisms and signaling of LECT2. Thus, herein, we have analyzed the expression and roles of LECT2 and its ligand proteins in various inflammatory diseases to provide a comprehensive review that will help researchers examining these processes and determining the bioavailability of LECT2 in the future.
2 The molecular structure and tissue distribution of LECT2
The human LECT2 gene is located at chromosome 5q31.1–32 and consists of three introns and four exons (36). Its cDNA is 456 nucleotides (nts) in length, containing an open reading frame encoding a polypeptide of 151 amino acid (aa) residues with a calculated molecular weight of 16.39 kDa and a isoelectric point of 9.42 (36). The LECT2 protein is the only member of the zinc-dependent metalloendopeptidases M23 family in vertebrates, which contains a zinc ion as a cofactor and prefers peptides containing polyglycine residues (37, 38). The phylogenic analysis shows that LECT2 is highly conserved from teleosts to Mammalia (Figure 1A). All of them have a signal peptide, three conserved disulfide bonds, and three metal-binding sites (Figure 1B). These conserved sequences or sites are crucial for the functioning of LECT2, and they also provide the theoretical basis to explore the functions of LECT2 by comparative biology.
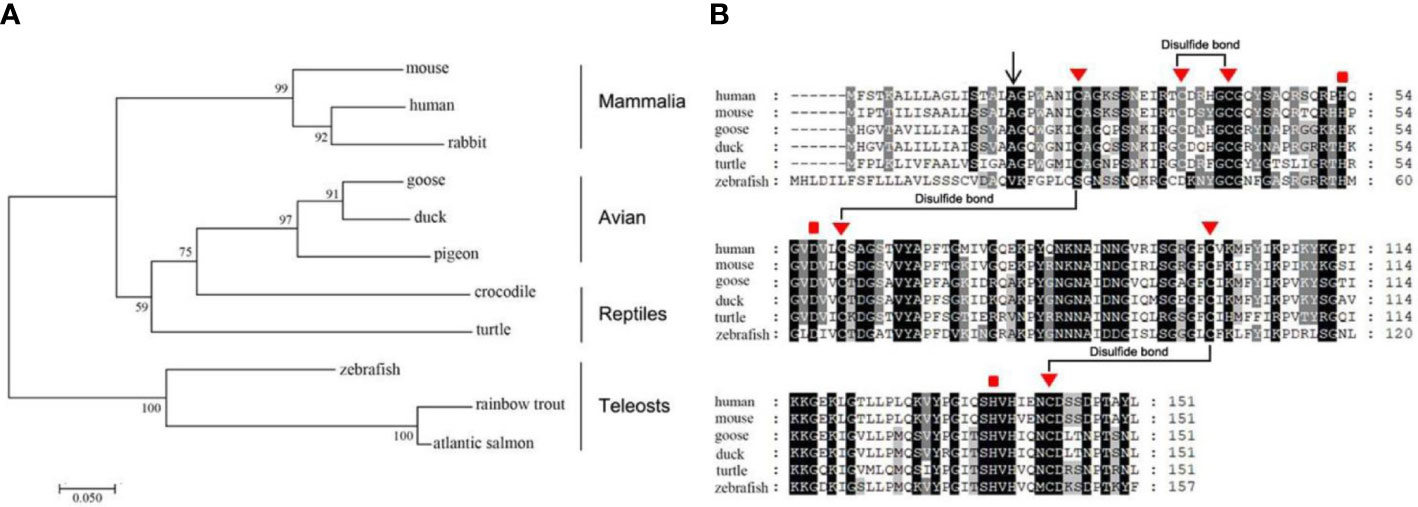
Figure 1 Analysis of the evolution and conservation of LECT2 in vertebrates. The phylogenetic analysis (neighbor-joining) (A) and multiple alignments (B) of the amino acid sequences of LECT2 in a variety of vertebrates. “▮” represents metal-binding sites.
LECT2 is primarily produced in hepatocytes and mainly secreted to the bloodstream (16), but it is also found in other tissues or cells, such as macrophages (8, 13), parathyroid cells (39), adipocytes (10, 40), cerebral nerve cells (39), and vascular endothelial cells (6, 26).
3 LECT2-mediated signaling
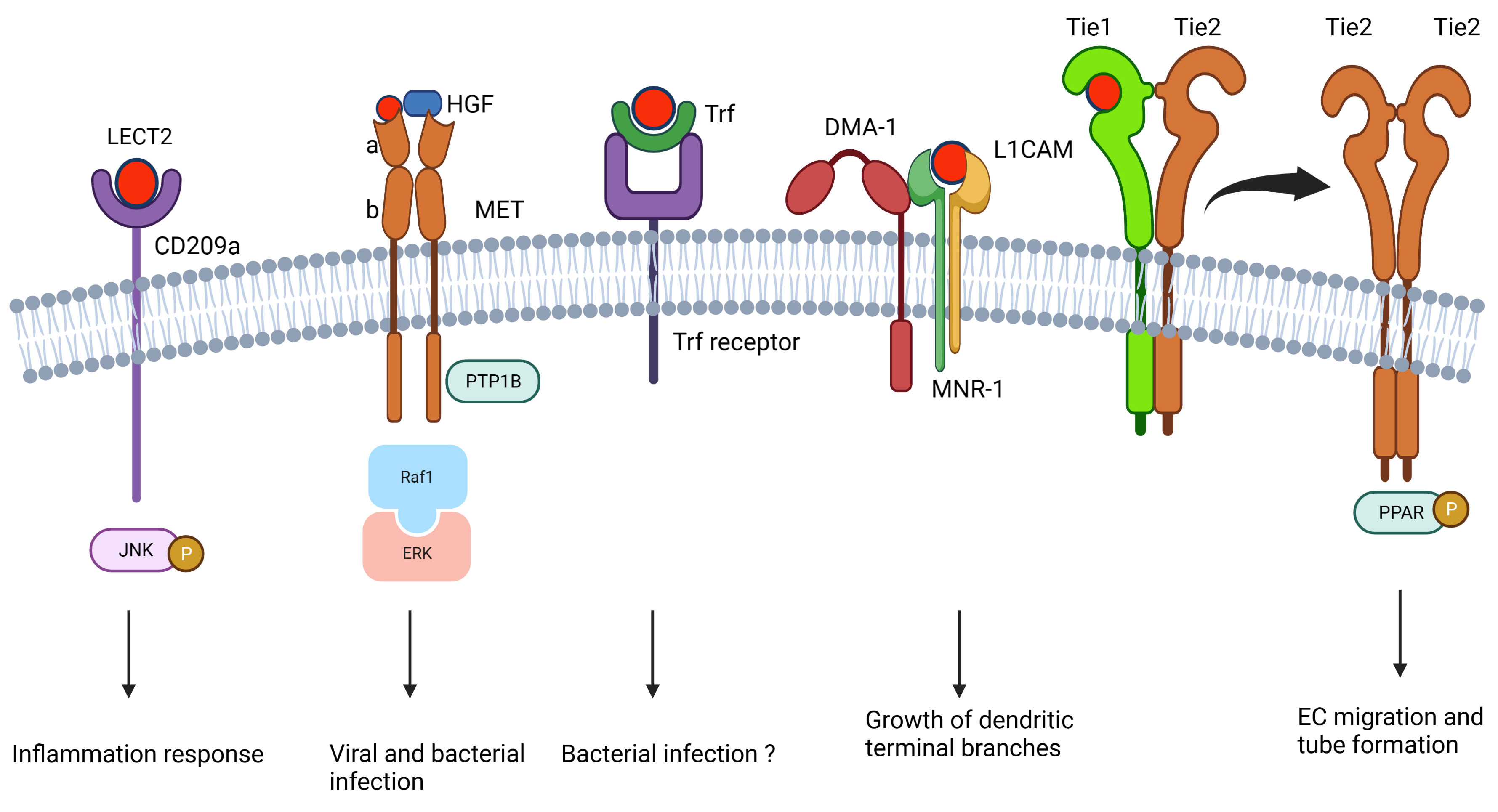
LECT2 exhibits its pleiotropic functions via its receptors, including CD209 antigen-like protein A (CD209a) (22, 41), tyrosine kinase with immunoglobulin-like and EGF-like domains 1 (Tie1) (6), MET (tyrosine protein kinase Met, also called c‐Met) (37, 42, 43), L1 cell adhesion molecule (L1CAM or SAX-7) (44), MNR-1 (44), and transferrin (Trf) (45) (Figure 2). CD209a is the first identified LECT2 receptor discovered by our group (41), and it contributes to enhancing the bacterial clearance ability of macrophages by phosphorylating the c-Jun N-terminal kinase (JNC) (Figure 2A) (22, 41). c-Met is the other receptor of LECT2, and the c-Met-LECT2 protein–protein interaction (PPI) impedes MET receptor activation to inhibit vascular invasion, metastasis, proliferation, and stemness of several cancers by antagonizing different cancer activation pathways (Figure 2B) (43, 46–48). In addition, Shirasaki et al. found that LECT2 functions as an anti-viral protein against lymphocytic choriomeningitis virus (LCMV) by binding to c-Met and thus competes with HGF-MET signaling (Figure 2B) (42). Tie1, a well-known angiopoietin receptor in many angiogenesis-related physiological and pathological processes (49), was also found to interact with LECT2. LECT2 promotes the dissociation of Tie1-Tie2 heterodimerization and the formation of Tie1-Tie2 homodimerization, which surpasses the invasion and metastasis of endothelial cells by activating PARP signaling (Figure 2E) (6). In Caenorhabditis elegans, muscle-secreted LECT-2 is an orthologue of vertebrate LECT2. It forms a multiprotein receptor–ligand complex with two skin transmembrane ligands, L1CAM and MNR-1, and a neuronal transmembrane receptor, DMA-1, which guides the growth of dendritic terminal branches (Figure 2D) (44). Trf is a glycoprotein with iron-binding and anti-microbial activity in vertebrates (Figure 2C) (50). Our group revealed that LECT2 interacts with Trf, and this interaction is highly conserved from fish to mouse (45).
4 The roles LECT2 in immune diseases
4.1 Tumor immunity
LECT2 functions as a tumor suppressor in many cancers (5, 46, 48, 51–53). Apart from directly interacting with cancer cells, LECT2 also modulates cancer progression via the tumor immune microenvironment (TIM) (51, 52). In hepatocellular carcinoma (HCC), LECT2 expression is negatively associated with the immune infiltration of monocyte, B cells, neutrophil, and myeloid dendritic cells and positively associated with hematopoietic stem cells and CD8 naive T cells. In addition, the LECT2 level is also negatively associated with multiple immune checkpoint molecules and HLA genes (51). Moreover, LECT2 is documented to prevent the recruitment of inflammatory monocytes and the acquisition of their immunosuppressive properties, and it has the ability to inhibit the EMT response and angiogenesis processes in β-catenin-activated hepatocytes (52). An oncogenic β-catenin-triggering inflammatory tumor microenvironment is indispensable for the aggressiveness of HCC in mice, and LECT2 inhibits HCC progression by blocking β-catenin-induced inflammation by interconnecting with invariant NKT (iNKT) cells (54). It has been shown that during intestinal tumorigenesis, compared to wild-type mice, LECT2-deficient mice exhibited a reduced overall survival and a significantly increased number of adenomas in the small intestine with increased severity (53). Further analysis showed that the homozygous loss of Lect2 promoted intestinal tumorigenesis by changing the tumor microenvironment, indicated by altering the balance of pro- and anti-inflammatory cytokines and key regulators of the T-cell lineage in the Wnt-activated colorectal cancer model. All the results show that LECT2 is a potential anti-tumor cytokine for cancer therapy.
4.2 Non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease (NAFLD) consists of a series of liver disorders ranging from hepatic steatosis to non-alcoholic steatohepatitis (NASH) and ultimately may lead to cirrhosis; its inflammatory responses are becoming the leading cause of liver-related morbidity and mortality worldwide (55). LECT2 is involved in all almost stages of NAFLD and is a potential diagnosis marker for this disease (7, 8, 25, 40, 56). In NAFLD, activating transcription factor 4 (ATF4) contributes to the upregulation of LECT2 transcription by binding to the LECT2 gene promoter under ER stress response (56). LECT2 is also found to promote liver steatosis by shifting the liver residual macrophage to the M1-like phenotype and to contribute to the development of liver inflammation via JNK-mediated signaling in NASH (8). Another study also found that LECT2 induces the development of NAFLD by mediating the phosphorylation level of STAT-1 and the expression of its downstream genes cluster of differentiation 36 (Cd36), chemokine (C-X-C motif), ligand 10 (Cxcl10), and unc-51-like autophagy-activating kinase 1 (Ulk1) (7). In addition, LECT2 is also reported as a non-invasive diagnostic factor for alcohol-induced liver cirrhosis (9).
4.3 Acute liver injury
Acute liver injury (ALI) is commonly caused by bacterial endotoxin/lipopolysaccharide (LPS) or drug overdose; it causes a systemic inflammatory response syndrome that is clinically much like sepsis (57, 58). In ALI mouse models, LECT2 was upregulated, and LECT2-KO mice more significantly reduced liver injuries than wild-type mice. Another study found that LECT2 knockdown alleviates liver injuries by regulating monocyte/macrophage chemotaxis (59). This result suggests that LECT2 might be used as a therapeutic target for ALI.
4.4 Sepsis
Bacterial or viral infection is one of the main causes of sepsis (60–62). LECT2 was firstly found to be associated with bacterial and viral infection in multiple teleosts (63–66). the anti-bacterial/anti-viral roles of LECT2 have also been verified in Aves (67, 68), mammals (22, 69), and human beings (23, 70). In vertebrates, LECT2 was firstly considered to exhibit its anti-bacterial/anti-viral activities by activating immune cells (macrophages, heterophils, and lymphocytes) (22, 68, 71) and downregulating pro-inflammatory factors such as TNF-α and IL-6 (22, 24). Several studies have revealed that LECT2 relieves both bacteria- and virus- induced sepsis in different mechanisms (22, 42, 70). In viral sepsis, LECT2 promotes retinoic acid-inducible gene I (RIG-I)-mediated anti-virus immunity by interacting with MET receptor, and this process can be antagonized by an original MET ligand hepatocyte growth factor (HGF) (42). For bacterial sepsis, LECT2 enhances the bactericidal activity of macrophages by inducing the phosphorylation of CD209a at its residue Ser28 and then leading to Raf-1 and NK-κB activation (22, 70). LECT2 has also been found to shift the development of pro-inflammatoryTh1/Th17 cells to anti-inflammatory Treg cells via the differentiation of bone marrow-derived dendritic cells (BMDCs) into dendritic cells, secretion of inflammatory cytokines, and differentiation of T cells after Helicobacter pylori infection in a CD209a receptor-dependent manner, suggesting that interrupting the LECT2-CD209a interaction may provide a promising target for H. pylori clearance (72). Moreover, LECT2 has direct anti-bacterial activity in teleosts, and this activity is conserved among vertebrates apart from humans (69). Interestingly, although LECT2 has two copies in teleosts, only LECT2-b exhibits direct anti-bacterial activity in grass carp. Fish LECT2-b not only exhibits conserved chemotactic and phagocytosis-stimulating activities but also kills Gram- and Gram+ bacteria directly in a membrane-dependent and a non-membrane-dependent manner, respectively. Additionally, LECT2-b impedes bacterial adherence to epithelial cells by inducing agglutination, which is achieved by binding peptidoglycan and lipoteichoic acid. All these results suggest that LECT2 is a potential drug for sepsis treatment.
4.5 Atherosclerosis
Atherosclerosis is a chronic, multifocal, lipid-driven immunoinflammatory disease that occurs in medium-sized and large arteries (73). LECT2 has been found to be a potential diagnosis biomarker for atherosclerosis and correlated with the developmental stage of atherosclerosis (25, 27). Another study found that LECT2 induces atherosclerotic inflammatory reaction via CD209a/JNK signaling in human endothelial cells (26). He et al. found that LECT2 administration reduces the concentrations of serum total cholesterol and low-density lipoprotein and the size of atherosclerotic lesions and thus impedes the progression of atherosclerosis (13).
4.6 Rheumatoid arthritis
Rheumatoid arthritis (RA) is a systemic autoimmune arthropathy and is characterized by a failure of inflammation to resolve automatically (74). Several studies have shown that LECT2 is a potential biomarker for RA diagnosis (30–33). Multiple clinical statistics have considered that the Val58Ile polymorphism of LECT2 is associated with the joint destruction in RA (32, 33). In a mouse arthritis model, LECT2-/- mice exhibited more severe arthritic symptoms than the wild-type controls, which were indicated by LECT2-/- mice having more severe inflammation and erosion of cartilage and bone. It was also found that exogenous expression of LECT2 can alleviate arthritis symptoms in LECT2 knockout mice (31), which strongly suggests that LECT2 treatment might be a potential strategy against inflammatory arthritis such as RA.
4.7 Osteoporosis
Osteoporosis (OS) is caused by the imbalance in the ratio between osteoblasts and osteoclasts, which is closely associated with osteogenic differentiation (OD) (28). LECT2 is low expression in mesenchymal stem cells (MSCs) with OD, and it inhibits OD in MSCs by inactivating the Wnt/β-catenin pathway. LECT2 is also found to play a role in the upregulation in serum of osteoporosis patients, is positively correlated with their bone loss, and is a potential biomarker for osteoporosis diagnosis (29). All these results suggest that LECT2 is a potential diagnosis and therapeutic target for osteoporosis.
4.8 Allergic diseases
Allergic diseases such as atopic dermatitis and parasitic infection are severe systemic hypersensitivity reactions that are rapid in onset and usually associated with skin and immune system changes, which seriously affect a patient’s health and quality of life (75, 76). Jeronimo et al. found that LECT2 is involved in modulating delayed-type hypersensitivity responses resulting from Leishmania chagasi infection, but its potential mechanism is unclear (77). In addition, Zhao et al. revealed that serum LECT2 is positively correlated with atopic dermatitis and its severity (35), but the mechanisms of how LECT2 mediates the progression of atopic dermatitis remain unknown.
5 Concluding remarks and perspectives
In its structure, LECT2 has high similarity among different vertebrates, indicated by their high conservation of three disulfide bonds and three metal-binding sites. The homology of LECT2 between different species provides the possibility to study its functions using comparative immunological methods. Currently, two of the five reported ligand proteins (CD209a and Trf) of LECT2 were firstly identified by our group in teleosts using the yeast two-hybrid system (45, 64), and the two interactions were further verified by our and the other groups. The LECT2-CD209a interaction was found to mediate bacterial clearance and obesity and drive the expansion and mobility of HSCs by modulating the macrophages and osteolineage cells (41). For LECT2-Trf interaction, although this protein–protein interaction (PPI) exists from teleosts to mice (45), its specific pathophysiologic functions are still unknown. Additionally, the complex assembled by LECT2, L1CAM, MNR-1, and DMA-1 is indispensable for the growth of dendritic terminal branches in C. elegans (44). Although all the homologous components of this complex are present in vertebrates, whether this complex exists in vertebrates and its potential roles remain unknown. These discoveries of LECT2 receptors further verify the feasibility of the digesting functions of novel proteins by comparative immunology.
Mounting evidence supports that LECT2 has versatile roles in immune diseases. It attenuates tumorigenesis by modulating TIM (51, 52); modulates inflammatory responses in several tissues such as liver (7, 8, 25, 40, 56, 59), bone marrow (29), joints (30–33), and blood vessels (13, 25–27); alleviates bacteria/virus-induced sepsis (22, 23, 42, 67–71); and accelerates the progression of allergic diseases (35, 77). It also mediates the Wnt/β-catenin pathway to regulate osteogenic differentiation of MSCs (28). The interaction of LECT2 with CD209a promotes the proliferation of HSCs in the bone marrow and mobilization to the blood, and it also regulates HSC homeostasis by affecting the expression of TNFα in macrophages and osteoblasts (41). Given the multifunctionality of LECT2 and its theranostical application in multiple immune-related diseases, there are many areas that are worth further investigating. Firstly, many of the LECT2-mediated pathophysiologic roles interplay with each other, and it is imperative to investigate whether and how LECT2 modulates the crosstalk among different immune diseases. For example, LECT2 is involved in the development of several liver immune diseases such as NAFLD, insulin resistance, liver regeneration, and HCC. LECT2 is the common mediator for them, and these liver immune diseases can be interchangeable or occur simultaneously. However, no related literature describes the role of LECT2 in their crosstalk. Secondly, considering the broad spectrum of LECT2-mediated liver immune diseases, it is theoretically feasible to construct an algorithm using LECT2 levels to predicate the progression of these diseases. Thirdly, LECT2 has been found to promote the progress of several immune diseases and is considered as a therapeutic target, but no agent with activity that reduces LECT2 levels has been identified for the moment. Further studies are needed to screen/identify agents with functions that lower LECT2 levels. Finally, the potentially clinical applications of LECT2 in immune diseases have been verified in a mouse model. However, further study is still imperative to shed light on their action mechanisms to avoid unpredicted risks before LECT2 is used clinically in humans. For example, recombinant LECT2 (rLECT2) administration was found to alleviate the sepsis induced by bacteria and virus in a mouse model, and LECT2 is also negatively associated with sepsis in humans, but there is a dearth of studies about whether LECT2 has similar mechanisms and efficacy. Therefore, there remains a need for studies focusing on the action mechanism and clinical applications of rLECT2 in humans. Further exploration of the role of LECT2 in varieties of immune diseases and its correlation with clinical immune-related diseases will advance the development of LECT2 as an appealing theranostical target for immune diseases.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This work is supported by the National Natural Science Foundation of China (31972821), the General Scientific Research Project of Education of Zhejiang Province (422204123), and the Starting Research Fund of Ningbo University (421912073).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yamagoe S, Yamakawa Y, Matsuo Y, Minowada J, Mizuno S, Suzuki K. Purification and primary amino acid sequence of a novel neutrophil chemotactic factor LECT2. Immunol Lett (1996) 52(1):9–13. doi: 10.1016/0165-2478(96)02572-2
2. Hiraki Y, Inoue H, Kondo J, Kamizono A, Yoshitake Y, Shukunami C, et al. A novel growth-promoting factor derived from fetal bovine cartilage, chondromodulin II: Purification and amino acid sequence. J Biol Chem (1996) 271(37):22657–62. doi: 10.1074/jbc.271.37.22657
3. Zhu S, Qiu H, Bennett S, Kuek V, Rosen V, Xu H, et al. Chondromodulin-1 in health, osteoarthritis, cancer, and heart disease. Cell Mol Life Sci (2019) 76(22):4493–502. doi: 10.1007/s00018-019-03225-y
4. Shukunami C, Hiraki Y. Role of cartilage-derived anti-angiogenic factor, chondromodulin-I, during endochondral bone formation. Osteoarthr Cartil (2001) 9:S91–101. doi: 10.1053/joca.2001.0450
5. Chen C-K, Yu W-H, Cheng T-Y, Chen M-W, Su C-Y, Yang Y-C, et al. Inhibition of VEGF165/VEGFR2-dependent signaling by LECT2 suppresses hepatocellular carcinoma angiogenesis. Sci Rep (2016) 6(1):1–12. doi: 10.1038/srep31398
6. Xu M, Xu H, Lin Y, Sun X, Wang L, Fang Z, et al. LECT2, a ligand for Tie1, plays a crucial role in liver fibrogenesis. Cell (2019) 178(6):1478–1492.e20. doi: 10.1016/j.cell.2019.07.021
7. Wang J, Chen Y, Pan R, Wu C, Chen S, Li L, et al. Leukocyte cell-derived chemotaxin 2 promotes the development of nonalcoholic fatty liver disease through STAT-1 pathway in mice. Liver Int (2021) 41(4):777–87. doi: 10.1111/liv.14816
8. Takata N, Ishii K, Takayama H, Nagashimada M, Kamoshita K, Tanaka T, et al. LECT2 as a hepatokine links liver steatosis to inflammation via activating tissue macrophages in NASH. Sci Rep (2021) 11(1):555. doi: 10.1038/s41598-020-80689-0
9. Sak J, Prystupa A, Kiciński P, Luchowska-Kocot D, Kurys-Denis E, Bis-Wencel H. Leukocyte cell-derived chemotaxin-2 and fibroblast growth factor 21 in alcohol-induced liver cirrhosis. World J Hepatol (2021) 13(12):2071–80. doi: 10.4254/wjh.v13.i12.2071
10. Kim J, Lee S, Kim D, Lee E, Park C, Choe H, et al. Adipose tissue LECT2 expression is associated with obesity and insulin resistance in Korean women. Obes (Silver Spring Md.) (2022) 30(7):1430–41. doi: 10.1002/oby.23445
11. Qin Y, Xiao K, Zhong Z, Zhao Y, Yu T, Sun X. LECT2 ameliorates blood-retinal barrier impairment secondary to diabetes Via activation of the Tie2/Akt/mTOR signaling pathway. Invest Ophthalmol Vis Sci (2022) 63(3):7. doi: 10.1167/iovs.63.3.7
12. Misu H. Identification of hepatokines involved in pathology of type 2 diabetes and obesity. Endocrine J (2019) 66(8):659–62. doi: 10.1507/endocrj.EJ19-0255
13. He W, Dai T, Chen J, Wang J. Leukocyte cell-derived chemotaxin 2 inhibits development of atherosclerosis in mice. Zoological Res (2019) 40(4):317–23. doi: 10.24272/j.issn.2095-8137.2019.030
14. Chikamoto K, Misu H, Takayama H, Kikuchi A, Ishii K, Lan F, et al. Rapid response of the steatosis-sensing hepatokine LECT2 during diet-induced weight cycling in mice. Biochem Biophys Res Commun (2016) 478(3):1310–6. doi: 10.1016/j.bbrc.2016.08.117
15. Willis S, Sargeant J, Yates T, Takamura T, Takayama H, Gupta V, et al. High-fat feeding increases circulating FGF21, LECT2, and fetuin-a in healthy men. J Nutr (2020) 150(5):1076–85. doi: 10.1093/jn/nxz333
16. Xie Y, Fan K, Guan S, Hu Y, Gao Y, Zhou W. LECT2: A pleiotropic and promising hepatokine, from bench to bedside. J Cell Mol Med (2022) 26(13):3598–607. doi: 10.1111/jcmm.17407
17. Mann B, Bhandohal J, Cobos E, Chitturi C, Eppanapally S. LECT-2 amyloidosis: What do we know? J Invest Med (2022) 70(2):348–53. doi: 10.1136/jim-2021-002149
18. Zhu S, Bennett S, Li Y, Liu M, Xu J. The molecular structure and role of LECT2 or CHM-II in arthritis, cancer, and other diseases. J Cell Physiol (2022) 237(1):480–8. doi: 10.1002/jcp.30593
20. Benson M, James S, Scott K, Liepnieks J, Kluve-Beckerman B. Leukocyte chemotactic factor 2: A novel renal amyloid protein. Kidney Int (2008) 74(2):218–22. doi: 10.1038/ki.2008.152
21. Larsen C, Walker P, Weiss D, Solomon A. Prevalence and morphology of leukocyte chemotactic factor 2-associated amyloid in renal biopsies. Kidney Int (2010) 77(9):816–9. doi: 10.1038/ki.2010.9
22. Lu X, Chen J, Yu C, Shi Y, He Y, Zhang R, et al. LECT2 protects mice against bacterial sepsis by activating macrophages via the CD209a receptor. J Exp Med (2013) 210(1):5–13. doi: 10.1084/jem.20121466
23. Ando K, Kato H, Kotani T, Ozaki M, Arimura Y, Yagi J. Plasma leukocyte cell-derived chemotaxin 2 is associated with the severity of systemic inflammation in patients with sepsis. Microbiol Immunol (2012) 56(10):708–18. doi: 10.1111/j.1348-0421.2012.00488.x
24. Dang M, Kato H, Ueshiba H, Omori-Miyake M, Yamagoe S, Ando K, et al. Possible role of LECT2 as an intrinsic regulatory factor in SEA-induced toxicity in d-galactosamine-sensitized mice. Clin Immunol (Orlando Fla.) (2010) 137(3):311–21. doi: 10.1016/j.clim.2010.08.002
25. Yoo H, Hwang S, Choi J, Lee H, Chung H, Seo J, et al. Association of leukocyte cell-derived chemotaxin 2 (LECT2) with NAFLD, metabolic syndrome, and atherosclerosis. PloS One (2017) 12(4):e0174717. doi: 10.1371/journal.pone.0174717
26. Hwang H, Jung T, Hong H, Seo J, Kim S, Kim N, et al. LECT2 induces atherosclerotic inflammatory reaction via CD209 receptor-mediated JNK phosphorylation in human endothelial cells. Metabolism: Clin Exp (2015) 64(9):1175–82. doi: 10.1016/j.metabol.2015.06.001
27. Sonmez F, Yildiz P, Akhtar M, Aydin C, Sonmez O, Ay N, et al. New markers in atherosclerosis: Thrombospondin-2 (THBS-2) and leukocyte cell-derived chemotaxin-2 (LECT-2); An immunohistochemical study. Med Sci Monit (2016) 22:5234–9. doi: 10.12659/MSM.898889
28. Xu Z, He J, Zhou X, Zhang Y, Huang Y, Xu N, et al. Down-regulation of LECT2 promotes osteogenic differentiation of MSCs via activating wnt/β-catenin pathway. Biomed Pharmacother (2020) 130:110593. doi: 10.1016/j.biopha.2020.110593
29. Wang Q, Xu F, Chen J, Xie Y, Xu S, He W. Serum leukocyte cell-derived chemotaxin 2 (LECT2) level is associated with osteoporosis. Lab Med (2023) 54(1):106–11. doi: 10.1093/labmed/lmac080
30. Ikeda D, Ageta H, Tsuchida K, Yamada H. iTRAQ-based proteomics reveals novel biomarkers of osteoarthritis. Biomarkers (2013) 18(7):565–72. doi: 10.3109/1354750X.2013.810667
31. Okumura A, Saito T, Otani I, Kojima K, Yamada Y, Ishida-Okawara A, et al. Suppressive role of leukocyte cell-derived chemotaxin 2 in mouse anti-type II collagen antibody-induced arthritis. Arthritis Rheumatism (2008) 58(2):413–21. doi: 10.1002/art.23215
32. Graessler J, Verlohren M, Graessler A, Zeissig A, Kuhlisch E, Kopprasch S, et al. Association of chondromodulin-II Val58Ile polymorphism with radiographic joint destruction in rheumatoid arthritis. J Rheumatol (2005) 32(9):1654–61.
33. Kameoka Y, Yamagoe S, Hatano Y, Kasama T, Suzuki K. Val58Ile polymorphism of the neutrophil chemoattractant LECT2 and rheumatoid arthritis in the Japanese population. Arthritis rheumatism (2000) 43(6):1419–20. doi: 10.1002/1529-0131(200006)43:6<1419::AID-ANR28>3.0.CO;2-I
34. Lan F, Misu H, Chikamoto K, Takayama H, Kikuchi A, Mohri K, et al. LECT2 functions as a hepatokine that links obesity to skeletal muscle insulin resistance. Diabetes (2014) 63(5):1649–64. doi: 10.2337/db13-0728
35. Zhao K, Xu F, Jiang X, Chen J, Zhu X, Zhou Q, et al. Serum leukocyte cell-derived chemotaxin 2 level is associated with atopic dermatitis patients. Ann Palliat Med (2021) 10(10):11006–12. doi: 10.21037/apm-21-2690
36. Yamagoe S, Kameoka Y, Hashimoto K, Mizuno S, Suzuki K. Molecular cloning, structural characterization, and chromosomal mapping of the human LECT2 gene. Genomics (1998) 48(3):324–9. doi: 10.1006/geno.1997.5198
37. Zheng H, Miyakawa T, Sawano Y, Asano A, Okumura A, Yamagoe S, et al. Crystal structure of human leukocyte cell-derived chemotaxin 2 (LECT2) reveals a mechanistic basis of functional evolution in a mammalian protein with an M23 metalloendopeptidase fold. J Biol Chem (2016) 291(33):17133–42. doi: 10.1074/jbc.M116.720375
38. Okumura A, Suzuki T, Miyatake H, Okabe T, Hashimoto Y, Miyakawa T, et al. Leukocyte cell-derived chemotaxin 2 is a zinc-binding protein. FEBS Lett (2013) 587(5):404–9. doi: 10.1016/j.febslet.2013.01.025
39. Nagai H, Hamada T, Uchida T, Yamagoe S, Suzuki K. Systemic expression of a newly recognized protein, LECT2, in the human body. Pathol Int (1998) 48(11):882–6. doi: 10.1111/j.1440-1827.1998.tb03855.x
40. Berthou F, Sobolewski C, Abegg D, Fournier M, Maeder C, Dolicka D, et al. Hepatic PTEN signaling regulates systemic metabolic homeostasis through hepatokines-mediated liver-to-Peripheral organs crosstalk. Int J Mol Sci (2022) 23(7):3959. doi: 10.3390/ijms23073959
41. Lu X, Chen Q, Rong Y, Yang G, Li C, Xu N, et al. LECT2 drives haematopoietic stem cell expansion and mobilization via regulating the macrophages and osteolineage cells. Nat Commun (2016) 7:12719. doi: 10.1038/ncomms12719
42. Shirasaki T, Yamagoe S, Shimakami T, Murai K, Imamura R, Ishii K, et al. Leukocyte cell-derived chemotaxin 2 is an antiviral regulator acting through the proto-oncogene MET. Nat Commun (2022) 13(1):3176. doi: 10.1038/s41467-022-30879-3
43. Chen C, Yang C, Hua K, Hua K, Ho M, Johansson G, et al. Leukocyte cell-derived chemotaxin 2 antagonizes MET receptor activation to suppress hepatocellular carcinoma vascular invasion by protein tyrosine phosphatase 1B recruitment. Hepatol (Baltimore Md.) (2014) 59(3):974–85. doi: 10.1002/hep.26738
44. Zou W, Shen A, Dong X, Tugizova M, Xiang Y, Shen K. A multi-protein receptor-ligand complex underlies combinatorial dendrite guidance choices in C. elegance. eLife (2016) 5:e18345. doi: 10.7554/eLife.18345
45. Chen J, Yang H, Shi Y, Li M. An interaction between leukocyte cell-derived chemotaxin 2 and transferrin of ayu, plecoglossus altivelis. Fish Shellfish Immunol (2009) 26(3):536–42. doi: 10.1016/j.fsi.2009.02.010
46. Chu T, Ko C, Tai P, Chang Y, Huang C, Wu T, et al. Leukocyte cell-derived chemotaxin 2 regulates epithelial-mesenchymal transition and cancer stemness in hepatocellular carcinoma. J Biol Chem (2022) 298(10):102442. doi: 10.1016/j.jbc.2022.102442
47. Li X, Lin P, Tao Y, Jiang X, Li T, Wang Y, et al. LECT 2 antagonizes FOXM1 signaling via inhibiting MET to retard PDAC progression. Front Cell Dev Biol (2021) 9:661122. doi: 10.3389/fcell.2021.661122
48. Hung W, Chang J, Cheng Y, Chen C, Chen J, Hua K, et al. Leukocyte cell-derived chemotaxin 2 retards non-small cell lung cancer progression through antagonizing MET and EGFR activities. Cell Physiol Biochem (2018) 51(1):337–55. doi: 10.1159/000495233
49. Woo K, Baldwin H. Role of Tie1 in shear stress and atherosclerosis. Trends Cardiovasc Med (2011) 21(4):118–23. doi: 10.1016/j.tcm.2012.03.009
50. Kawabata H. Transferrin and transferrin receptors update. Free Radical Biol Med (2019) 133:46–54. doi: 10.1016/j.freeradbiomed.2018.06.037
51. Qin J, Sun W, Zhang H, Wu Z, Shen J, Wang W, et al. Prognostic value of LECT2 and relevance to immune infiltration in hepatocellular carcinoma. Front Genet (2022) 13:951077. doi: 10.3389/fgene.2022.951077
52. L’Hermitte A, Pham S, Cadoux M, Couchy G, Caruso S, Anson M, et al. Lect2 controls inflammatory monocytes to constrain the growth and progression of hepatocellular carcinoma. Hepatol (Baltimore Md.) (2019) 69(1):160–78. doi: 10.1002/hep.30140
53. Greenow K, Zverev M, May S, Kendrick H, Williams G, Phesse T, et al. Lect2 deficiency is characterised by altered cytokine levels and promotion of intestinal tumourigenesis. Oncotarget (2018) 9(92):36430–43. doi: 10.18632/oncotarget.26335
54. Anson M, Crain-Denoyelle A, Baud V, Chereau F, Gougelet A, Terris B, et al. Oncogenic β-catenin triggers an inflammatory response that determines the aggressiveness of hepatocellular carcinoma in mice. J Clin Invest (2012) 122(2):586–99. doi: 10.1172/JCI43937
55. Powell E, Wong V, Rinella M. Non-alcoholic fatty liver disease. Lancet (London England) (2021) 397(10290):2212–24. doi: 10.1016/S0140-6736(20)32511-3
56. Park C, Lee S, Kim J, Kim D, Choe H, Jeong J, et al. Endoplasmic reticulum stress increases LECT2 expression via ATF4. Biochem Biophys Res Commun (2021) 585:169–76. doi: 10.1016/j.bbrc.2021.11.038
57. Zhang Y, Xue W, Zhang W, Yuan Y, Zhu X, Wang Q, et al. Histone methyltransferase G9a protects against acute liver injury through GSTP1. Cell Death Differ (2020) 27(4):1243–58. doi: 10.1038/s41418-019-0412-8
58. Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature (2010) 464(7285):104–7. doi: 10.1038/nature08780
59. Xie Y, Zhong K, Hu Y, Xi Y, Guan S, Xu M, et al. Liver infiltration of multiple immune cells during the process of acute liver injury and repair. World J Gastroenterol (2022) 28(46):6537–50. doi: 10.3748/wjg.v28.i46.6537
60. Delano M, Ward P. The immune system’s role in sepsis progression, resolution, and long-term outcome. Immunol Rev (2016) 274(1):330–53. doi: 10.1111/imr.12499
61. Gautier T, Deckert V, Nguyen M, Desrumaux C, Masson D, Lagrost L. New therapeutic horizons for plasma phospholipid transfer protein (PLTP): Targeting endotoxemia, infection and sepsis. Pharmacol Ther (2022) 236:108105. doi: 10.1016/j.pharmthera.2021.108105
62. Cole J, Morris P, Dickman M, Dockrell D. The therapeutic potential of epigenetic manipulation during infectious diseases. Pharmacol Ther (2016) 167:85–99. doi: 10.1016/j.pharmthera.2016.07.013
63. Lin B, Chen S, Cao Z, Lin Y, Mo D, Zhang H, et al. Acute phase response in zebrafish upon aeromonas salmonicida and staphylococcus aureus infection: Striking similarities and obvious differences with mammals. Mol Immunol (2007) 44(4):295–301. doi: 10.1016/j.molimm.2006.03.001
64. Li M, Chen J, Shi Y. Molecular cloning of leucocyte cell-derived chemotaxin-2 gene in croceine croaker (Pseudosciaena crocea). Fish Shellfish Immunol (2008) 24(2):252–6. doi: 10.1016/j.fsi.2007.09.003
65. Hansen J, Woodson J, Hershberger P, Grady C, Gregg J, Purcell M. Induction of anti-viral genes during acute infection with viral hemorrhagic septicemia virus (VHSV) genogroup IVa in pacific herring (Clupea pallasii). Fish Shellfish Immunol (2012) 32(2):259–67. doi: 10.1016/j.fsi.2011.11.010
66. Chen J, Chen Q, Lu X, Li C. LECT2 improves the outcomes in ayu with vibrio anguillarum infection via monocytes/macrophages. Fish Shellfish Immunol (2014) 41(2):586–92. doi: 10.1016/j.fsi.2014.10.012
67. Xu Q, Chen Y, Tong Y, Huang Z, Zhao W, Duan X, et al. Identification and expression analysis of the leukocyte cell-derived chemotaxin-2 (LECT2) gene in duck (Anas platyrhynchos). Gene (2014) 533(1):280–5. doi: 10.1016/j.gene.2013.09.047
68. Sekelova Z, Stepanova H, Polansky O, Varmuzova K, Faldynova M, Fedr R, et al. Differential protein expression in chicken macrophages and heterophils in vivo following infection with salmonella enteritidis. Vet Res (2017) 48(1):35. doi: 10.1186/s13567-017-0439-0
69. Hu Y, Ma Z, Wu C, Wang J, Zhang Y, Zhang X. LECT2 is a novel antibacterial protein in vertebrates. J Immunol (Baltimore Md.: 1950) (2022) 208(8):2037–53. doi: 10.4049/jimmunol.2100812
70. Shen H, Li L, Chen Q, He Y, Yu C, Chu C, et al. LECT2 association with macrophage-mediated killing of helicobacter pylori by activating NF-κB and nitric oxide production. Genet Mol Res: GMR (2016) 15(4):gmr15048889. doi: 10.4238/gmr15048889
71. Wang Z, Lu J, Li C, Li Q, Pang Y. Characterization of the LECT2 gene and its protective effects against microbial infection via large lymphocytes in lampetra japonica. Dev Comp Immunol (2018) 79:75–85. doi: 10.1016/j.dci.2017.09.018
72. Zhang X, Sun K, Tang C, Cen L, Li S, Zhu W, et al. LECT2 modulates dendritic cell function after helicobacter pylori infection via the CD209a receptor. J Gastroenterol Hepatol (2023). doi: 10.1111/jgh.16138
73. Bäck M, Yurdagul A, Tabas I, Öörni K, Kovanen P. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat Rev Cardiol (2019) 16(7):389–406. doi: 10.1038/s41569-019-0169-2
74. Finckh A, Gilbert B, Hodkinson B, Bae S, Thomas R, Deane K, et al. Global epidemiology of rheumatoid arthritis, nature reviews. Rheumatology (2022) 18(10):591–602. doi: 10.1038/s41584-022-00827-y
75. Bieber T. Atopic dermatitis: an expanding therapeutic pipeline for a complex disease, nature reviews. Drug Discov (2022) 21(1):21–40. doi: 10.1038/s41573-021-00266-6
76. Cruz A, Cooper P, Figueiredo C, Alcantara-Neves N, Rodrigues L, Barreto M. Global issues in allergy and immunology: Parasitic infections and allergy. J Allergy Clin Immunol (2017) 140(5):1217–28. doi: 10.1016/j.jaci.2017.09.005
Keywords: LECT2, LECT2 receptor, immune regulation, inflammation-related diseases, comparative immunology
Citation: Zhu M-H, Liu Y-J, Li C-Y, Tao F, Yang G-J and Chen J (2023) The emerging roles of leukocyte cell-derived chemotaxin-2 in immune diseases: From mechanisms to therapeutic potential. Front. Immunol. 14:1158083. doi: 10.3389/fimmu.2023.1158083
Received: 03 February 2023; Accepted: 27 February 2023;
Published: 09 March 2023.
Edited by:
Syed M. Faisal, University of Michigan Medical School, United StatesReviewed by:
Shadab Kazmi, University of Missouri, United StatesArundhoti Das, National Institutes of Health (NIH), United States
Atika Dhar, National Institutes of Health (NIH), United States
Copyright © 2023 Zhu, Liu, Li, Tao, Yang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiong Chen, chenjiong@nbu.edu.cn; jchen1975@163.com; Guan-Jun Yang, champion2014@126.com
 Ming-Hui Zhu1,2,3
Ming-Hui Zhu1,2,3 Yan-Jun Liu
Yan-Jun Liu Guan-Jun Yang
Guan-Jun Yang Jiong Chen
Jiong Chen