- 1Department of Pharmacy, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2School of Pharmaceutical Science and Technology, Tianjin University, Tianjin, China
- 3Department of Pharmacy and Evidence-Based Pharmacy Center, West China Second University Hospital, Sichuan University, Chengdu, China
- 4Graduate School of Pharmaceutical Sciences, University of Tokyo, Tokyo, Japan
- 5Department of Pharmacy, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: Sintilimab plus chemotherapy significantly prolongs overall survival (OS) for patients with advanced or metastatic oesophageal squamous cell carcinoma (OSCC). However, the cost-effectiveness of this high-priced therapy is currently unknown. We evaluated the cost-effectiveness of sintilimab plus chemotherapy vs chemotherapy alone as fist-line therapy in patients with advanced or metastatic OSCC from the perspective of Chinese healthcare system.
Methods: A partitioned survival model consisting of 3 discrete health states was constructed to assess the cost and effectiveness of sintilimab plus chemotherapy vs chemotherapy as first-line treatment of OSCC. Key clinical data in the model came from the ORIENT-15 trial. Costs and utilities were collected from published sources. Life-years, quality-adjusted life-years (QALYs), incremental cost-effectiveness ratio (ICER), incremental net health benefits (INHB), and incremental net monetary benefits (INMB) were calculated for the two treatment strategies. One-way and probabilistic sensitivity analyses were conducted to account for uncertainty and model stability. Additional subgroup and scenario analyses were performed.
Results: Treatment with sintilimab plus chemotherapy provided an additional 0.37 QALYs and an incremental cost of $8,046.58 compared with chemotherapy, which resulted in an ICER of $21,782.24 per QALY gained. One-way sensitivity analysis revealed that the model was most sensitive to utility of progression-free survival (PFS) and the cost of sintilimab. The probabilistic sensitivity analysis indicated that the probability of sintilimab plus chemotherapy being cost-effective was 0.01%, 76.80% and 98.60% at the threshold of 1, 2 or 3 times GDP per capita per QALY, respectively. Subgroup analysis found that all subgroups other than PD-L1 expression combined positive scores < 1 subgroup favored sintilimab plus chemotherapy treatment due to its association with positive INHBs by varying the hazard ratios for OS and PFS. The scenario analyses showed altering the time horizon of the model or fitting survival curves separately did not reverse results of the model.
Conclusion: Sintilimab plus chemotherapy was associated with improved QALYs and an additional cost but was estimated to be cost-effective compared with chemotherapy alone as a first-line treatment for patients with advanced or metastatic OSCC at the commonly adopted willingness-to-pay threshold of 3 times GDP per capita per QALY in China.
Introduction
Oesophageal squamous cell carcinoma (OSCC) is the predominant histological subtype of oesophageal cancer in Asian populations (approximately 90%), whereas oesophageal adenocarcinoma is more common in North America and western European countries (1, 2). According to the survey statistics of the International Agency for Research on Cancer in 2020, there were 324,422 new cases of oesophageal cancer and 301,135 deaths in China, which accounted for 53.70% and 55.35% of the global incidence and mortality of esophageal cancer, respectively (3).
Currently, platinum doublet chemotherapy is commonly the most recommended first-line therapy for patients with unresectable advanced or metastatic OSCC (4, 5). However, the overall survival (OS) remains limited with a median of less than 12 months, and the 5-year survival rate is only 8% or less for patients diagnosed with advanced stages (6). Accumulating studies have shown that immune checkpoint inhibitors (ICIs) combined with chemotherapy demonstrated superior anti-tumor effects compared with chemotherapy alone (7–9). Sintilimab is a fully recombinant human IgG4 anti-PD-1 monoclonal antibody that inhibits binding of the PD-1 receptor to its ligands (PD-L1 and PD-L2), thereby restoring T-cell immune activity and reversing the immune evasion of cancer (10). Compared with pembrolizumab or nivolumab, sintilimab has a different binding site and potentially greater affinity against PD-1 (11).
The multicentre, double blind, randomized phase 3 clinical trial ORIENT-15 conducted at 79 sites in five countries, reported the efficacy and safety of sintilimab plus chemotherapy compared with placebo plus chemotherapy for the first-line treatment of locally advanced or metastatic OSCC (12). Results indicated that sintilimab plus chemotherapy significantly prolonged the progression-free survival (PFS) (7.2 vs 5.7 months; hazard ratio [HR], 0.56; 95% CI, 0.46-0.68; P<0.001) and OS (median 16.7 vs 12.5 months; HR, 0.63; 95% CI, 0.51-0.78; P<0.001) compared with placebo plus chemotherapy. Treatment-related adverse events (AEs) of grade 3 or higher were comparable between the 2 groups (60% vs 55%). Overall, the current data support sintilimab plus chemotherapy as an attractive first-line treatment option for patients with advanced OSCC. Excitedly, the guideline of Chinese Society of Clinical Oncology (CSCO) for the diagnosis and treatment of esophageal cancer (2022 edition) published on April, 2022, has recommended sintilimab in combination with paclitaxel and cisplatin as a first-line treatment option for advanced or metastatic OSCC (13).
Notwithstanding the excellent clinical benefits of sintilimab in first-line OSCC therapy, its cost-effectiveness has not, to our knowledge, been evaluated in China and other countries. The economic evaluation of sintilimab is critical and helpful for clinicians and decision-makers to optimally allocate limited health resources. The objective of our study is to estimate the cost-effectiveness of sintilimab plus chemotherapy vs chemotherapy alone as first-line treatment for patients with advanced or metastatic OSCC based on the ORIENT-15 trial data from the perspective of Chinese healthcare system.
Methods
Analytic overview
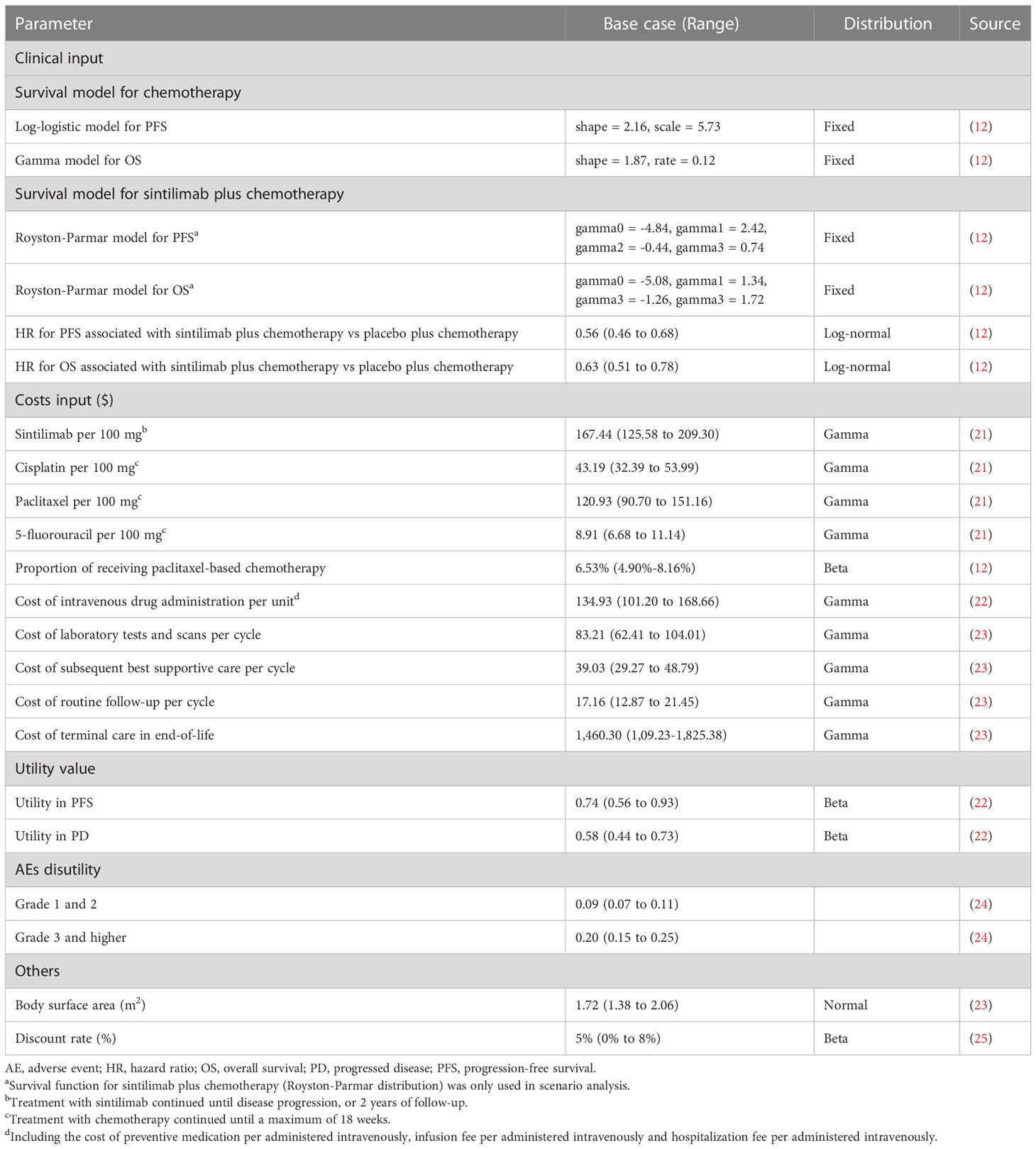
The target population for this analysis was patients who had locally advanced or metastatic oesophageal squamous cell carcinoma and did not receive previous systemic treatment, consistent with the patient characteristics in the ORIENT-15 trial (12). A partitioned survival model with 3 health states was developed to conduct the economic analyses from a Chinese healthcare system perspective, and the perspective is consistent with the objective of this study (to estimate the cost-effectiveness of sintilimab plus chemotherapy and help decision-makers in healthcare system to optimally allocate limited health resources) (14). The three mutually exclusive health states were progression-free survival (PFS), progressed disease (PD), and death (Figure 1). In the model, the proportion of patients in each health state at each cycle t was estimated from OS and PFS curves of the ORIENT-15 trial (12). The proportion of patients alive at each cycle (1-week cycle) was estimated by the area under the OS curve, and the proportion alive with PFS was estimated by the area under the PFS curve (15). The proportion of PD was estimated by the difference between the OS and PFS curves (15). The time horizon was 11 years given that more than 99% of the patients died at this time point, and 5 to 20 years were included in the scenario analyses.
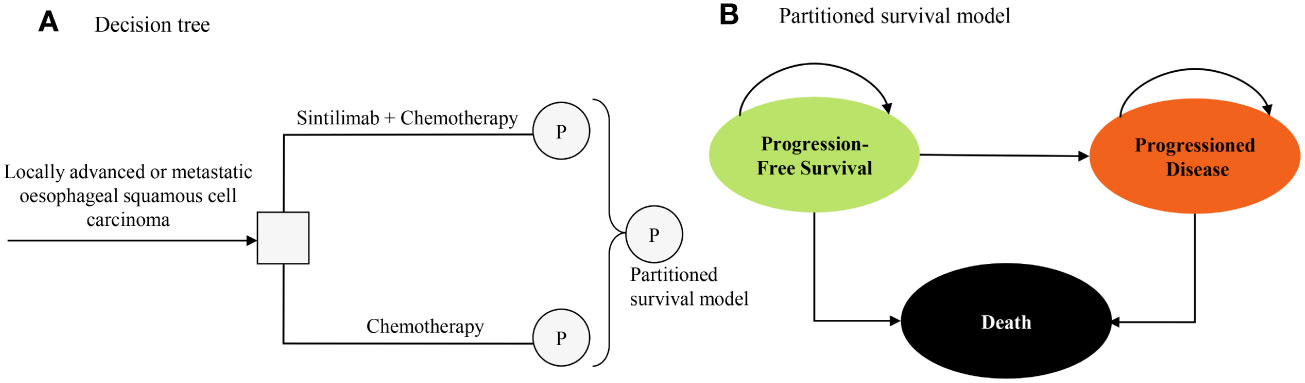
Figure 1 Model structure of a decision tree combining the partitioned survival model with the 3 health states. (A) Decision tree. (B) Partitioned survival model.
This study followed the reporting guideline of Consolidated Health Economic Evaluation Reporting Standards (CHEERS) (Supplementary Table 1) (16). This evaluation was based on a literature review of publicly available data and on modeling techniques, which did not require institutional review board review or exemption by the ethics committee.
Clinical data inputs
To construct the partitioned survival model, graphic data from the ORIENT-15 trial were extracted by GetData Graph Digitizer, version 2.18, and time-to-event data were obtained as described in the study by Guyot et al. (17). The original and reconstructed Kaplan–Meier curves were shown in Supplementary Figure 1, and summary measures regarding median duration and survival rate in original publication and reconstructed data were shown in Supplementary Table 2. Because time horizon in this economic study was 11 years (99% of the patients died) beyond the follow-up period in ORIENT-15 trial, the OS and PFS curves of chemotherapy alone were fitted and extrapolated using the following parametric survival functions: Weibull, log-normal, log-logistic, exponential, generalized gamma, Gompertz, and Royston-Parmar spline models and parametric mixture and non-mixture cure models (18–20). The eligible survival function was chosen based on the visual inspection, and lowest value of the Akaike information criterion (AIC) and Bayesian information criterion (BIC) (18). The proportional hazards (PH) modelling method was used to estimate the parametric PFS and OS curves for the sintilimab plus chemotherapy group based on the hazard ratios (HRs) of sintilimab plus chemotherapy vs chemotherapy alone reported in ORIENT-15 trial (12). This could avoid the bias resulted from inconsistency of parametric survival distribution between two groups (18).
The final survival functions of the sintilimab plus chemotherapy vs chemotherapy alone were shown in Table 1 and the goodness-of-fit results were shown in Supplementary Table 3. The proportions of patients with PFS and OS were calculated by using the selected survival distribution. The validation plot, survival distribution, were shown in Supplementary Figure 2, 3. The key clinical inputs were listed in Table 1.
Cost and utility inputs
Only direct medical costs within the healthcare system were included in this study (Table 1), including costs of acquiring and administrating drugs, costs of subsequent treatment after disease progressed, costs for routine follow-up, laboratory tests and scans, costs for end-of-life care and costs for the management of AEs. All costs were reported in 2021 US dollars with the exchange rate in 2021: $1 = ¥6.45, and were adjusted to 2021 value using the consumer price index (26).
According to the ORIENT-15 trial report, sintilimab was given intravenously at a dose of 200 mg every 3 weeks, and it was continued until progressive disease, lost to follow-up, death, or a maximum of 2 years, whichever occurred first (12). And 6.53% patients would receive cisplatin (75 mg/m2 every 3 weeks) plus paclitaxel (87.5 mg/m2 on the first and second weeks; 175 mg/m2 every 3 weeks starting from the fourth week) as chemotherapy regimen, and other patients (93.47%) would receive cisplatin plus 5-fluorouracil (4000 mg/m2 every 3 weeks) as chemotherapy regimen (12). Chemotherapy was given within a maximum of 18 weeks (12). To calculate the dosage of paclitaxel, we assumed that the body surface area was 1.72 m2 for a typical patient in China (23). The prices of sintilimab, cisplatin, paclitaxel, and 5-fluorouracil were collected from Chinese bid-winning price (21).
After disease progression, 41.0% patients in the sintilimab plus chemotherapy group and 54% in the chemotherapy alone group received subsequent systemic medication therapy, and the proportion and costs for each subsequent therapy were shown in Supplementary Table 4, which were estimated from ORIENT-15 trial and Chinese bid-winning price (21). The costs of laboratory tests and scans ($134.93 per cycle), costs of subsequent best supportive care ($39.03 per cycle), cost of routine follow-up ($17.16 per cycle) and cost of terminal care in end-of-life ($1,460.30 per patient) were collected from an economic evaluation among patients with advanced OSCC. The analysis included the costs associated with management of grade 3 or higher AEs (probability ≥ 5%), which were extracted from the literature (Supplementary Table 5).
Each health state was assigned a health utility preference on a scale of 0 (death) to 1 (perfect health). The PFS and PD states associated with OSCC were 0.74 and 0.58 respectively, which were derived from a cost-effectiveness analysis considering patients with OSCC (22). The disutility values due to grade 1 or 2 and grade 3 or higher AEs were included in this analysis (24). All AEs were assumed to be incurred during the first cycle.
Base-case analysis
The incremental cost-effectiveness ratio (ICER) was calculated as the incremental cost per additional quality adjusted life-year (QALY) gained between the sintilimab plus chemotherapy group and the chemotherapy alone group. When the ICER was lower than the prespecified willingness-to-pay threshold (WTP, 3 times GDP per capita per QALY gained, $37,663.26/QALY), cost-effectiveness was assumed according to the recommendation of Guidelines for Evaluation of Chinese Pharmacoeconomics (25). Costs and QALYs were discounted at an annual rate of 5% (25).
The incremental net health benefit (INHB) and incremental net monetary benefits (INMB) were also estimated based on the following formulas: INHB (λ) = (μE1 − μE0) − (μC1 − μC0)/λ = ΔE − ΔC/λ; INMB (λ) = (μE1 − μE0) × λ − (μC1 − μC0) = ΔE × λ − ΔC, where μCi and μEi were the costs and effectiveness of sintilimab plus chemotherapy (i = 1) or chemotherapy (i = 0), respectively, and λ was the WTP threshold (27, 28).
Sensitivity, subgroup, and scenario analyses
To evaluate the robustness of the base-case results, we conducted one-way and probabilistic sensitivity analyses. One-way sensitivity analyses were conducted for all parameters, and the estimated range of each parameter was based on either the reported or estimated 95% CIs in the referenced studies or determined by assuming a 25% change from the base-case value (Table 1). In the probabilistic sensitivity analyses, a Monte Carlo simulation with 1000 iterations was generated by simultaneously sampling the key model parameters from the prespecified distributions. A gamma distribution was selected for the cost parameters, a log-normal distribution for the HRs, and a beta distribution for proportion, and health utility parameters. Based on the data from 1000 iterations, a cost-effectiveness acceptability curve was created to represent the probability that sintilimab plus chemotherapy would be considered cost-effective at various WTP thresholds for health gains (QALYs). To investigate the uncertainty of economic outcomes caused by the subpopulations, exploratory subgroup analyses were performed for the prespecified subgroups that were reported in the ORIENT-15 trial by varying the HRs for OS and PFS. Lastly, additional scenario analyses were conducted: 1) time horizon was 5 or 20 years; 2) four survival curves (OS and PFS curves for sintilimab plus chemotherapy vs chemotherapy alone) were fitted separately to estimate parametric survival curve. Survival curves were fitted with flexsurv packages in R, version 4.1.1, 2021, and the partitioned survival model was developed in Microsoft Excel 2019.
Results
Base-case analysis
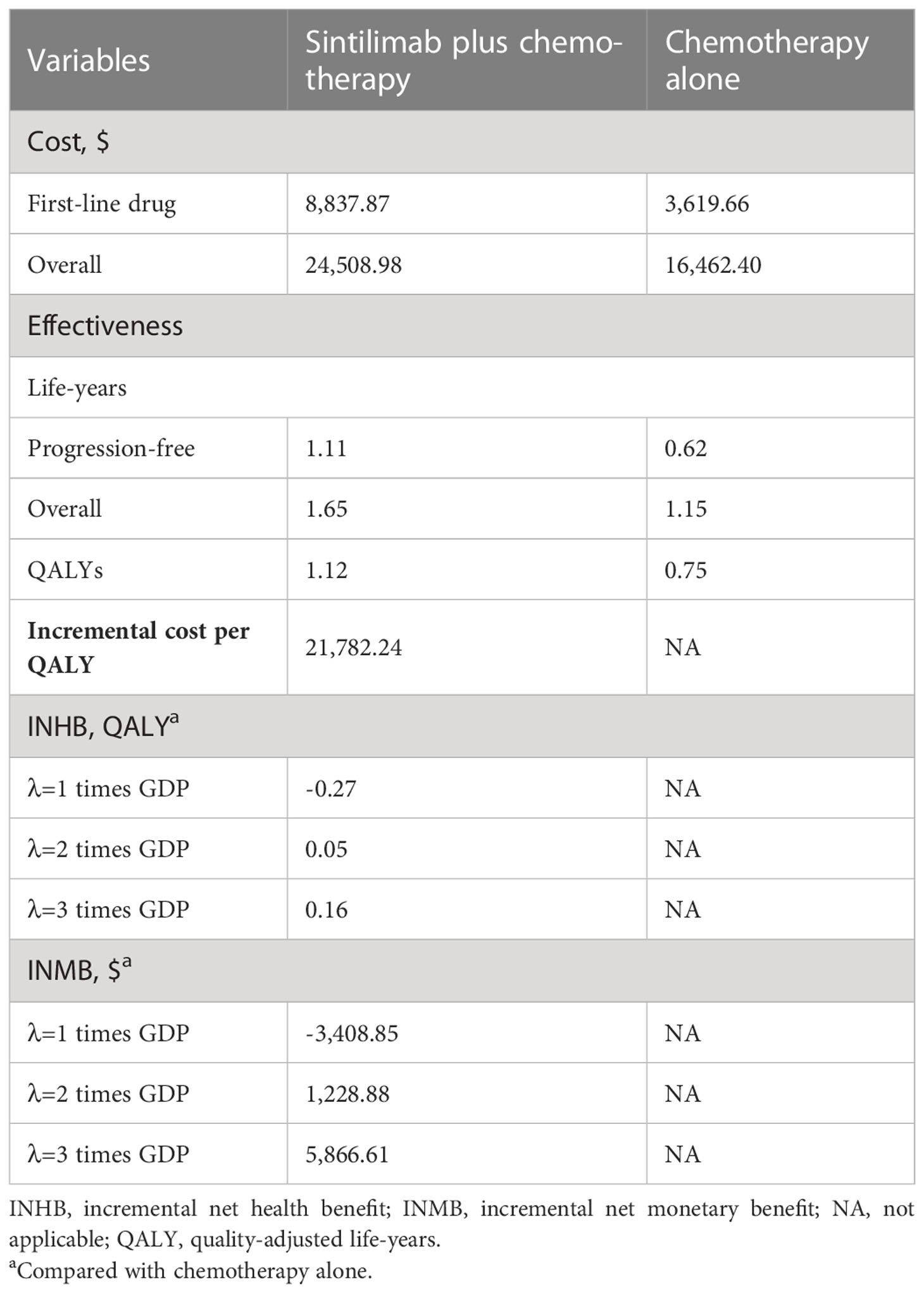
In comparison with chemotherapy alone, sintilimab plus chemotherapy provided an additional 0.37 QALYs and 0.50 overall life-years, with an incremental cost of $8,046.58, which was associated with an ICER of $21,782.24/QALY. The INHB was +0.16 QALYs, and the INMB was +$5,866.61 at a WTP threshold of 3 times GDP per capita per QALY (Table 2).
Sensitivity analysis
One-way sensitivity analyses showed that the model was particularly sensitive to utility of PFS and the cost of sintilimb (Figure 2). When the lower boundary of the utility (0.56) for PFS was adopted, the ICER of sintilimab plus chemotherapy vs chemotherapy alone was $28,892.78/QALY, and when the upper boundary (0.93) was adopted, the ICER was $17,480.31/QALY. When the cost of sintilimab was discounted by 25%, the ICER was $18,402.56/QALY. The remaining parameters, such as the HR (for PFS and OS), cost and utility related to AEs, had only moderate or low associations with the ICER. All parameters were not associated with ICERs exceeding the threshold.
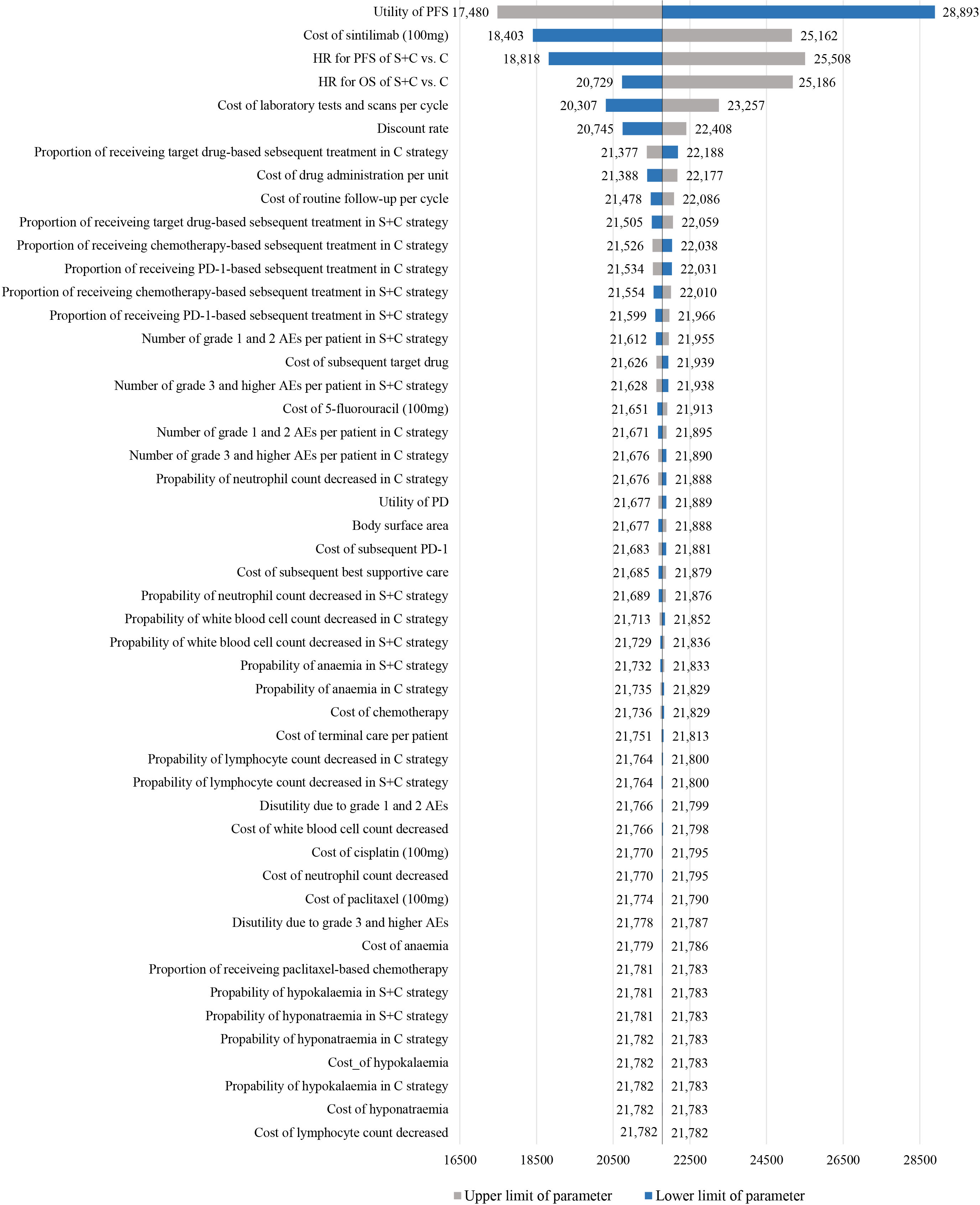
Figure 2 Tornado diagram of one-way sensitivity analyses of sintilimab plus chemotherapy vs chemotherapy alone in order of magnitude of the association.
Compared with chemotherapy alone, the probabilistic sensitivity analysis showed that sintilimab plus chemotherapy added a mean of 0.40 QALYs with an additional mean cost of $8.560.06, which resulted in a mean ICER of $21,621.89/QALY (Figure 3A). The cost-effectiveness acceptability curve showed that the probability of sintilimab plus chemotherapy being cost-effective was 0.01%, 76.80% and 98.60% at 1, 2- or 3-times GDP per capita per QALY threshold, respectively (Figure 3B).
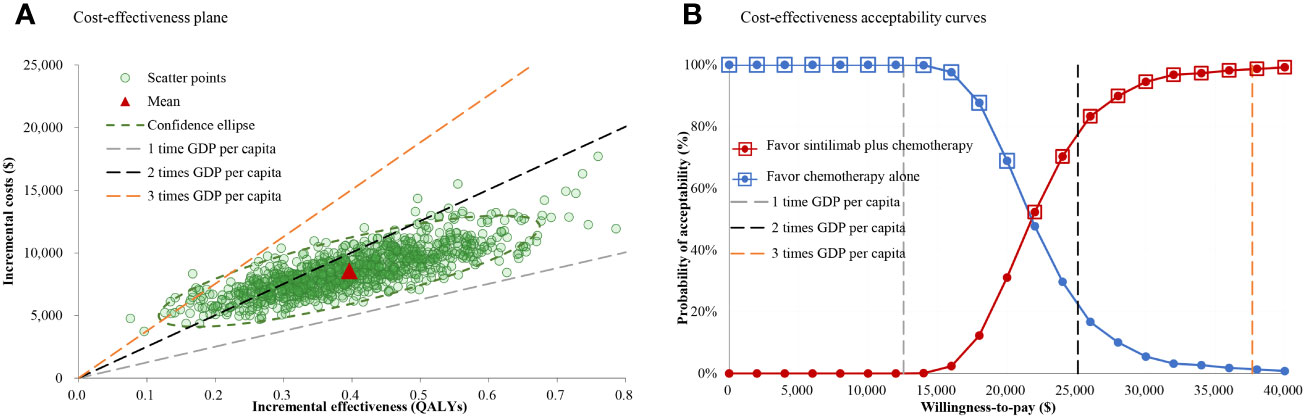
Figure 3 Cost-effectiveness plane and acceptability curves for sintilimab plus chemotherapy vs chemotherapy alone. (A) Cost-effectiveness plane. (B) Cost-effectiveness acceptability curves.
Subgroup analyses
The subgroup analyses, which were conducted by varying the HRs for OS and PFS, revealed that sintilimab plus chemotherapy was associated with primarily positive INHBs in the most subgroups at the threshold of 3-times GDP per capita per QALY (Figure 4). Only among patients with CPS<1, the point estimate of INHBs was negative, and in fewer subgroups (women, local advanced, cisplatin plus 5-fluorouracil, CPS<1), the lower bound of INHBs by varying the HRs for OS were negative.
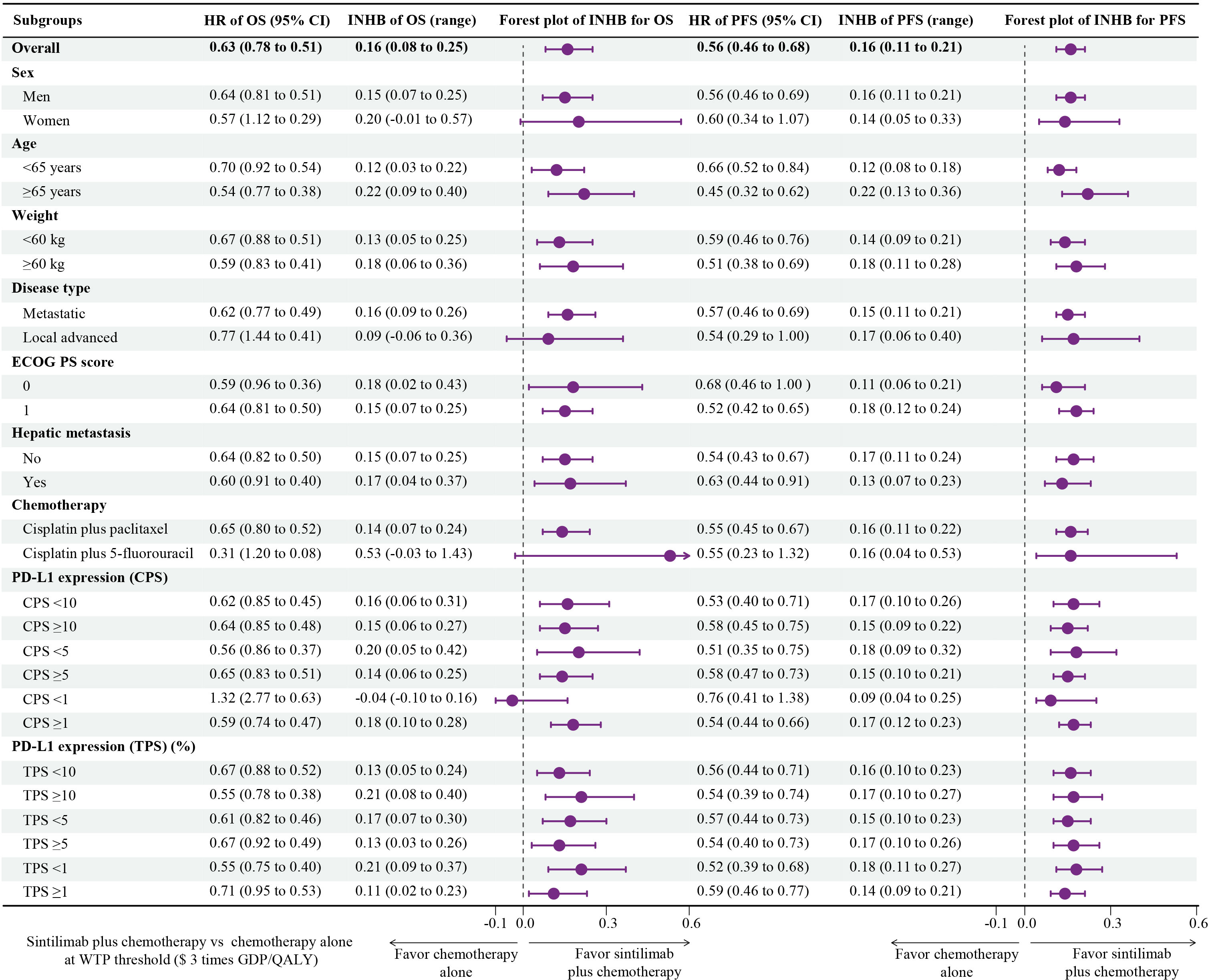
Figure 4 Subgroup Analysis Results of Incremental Net Health Benefits (INHBs) Obtained by Varying the Hazard Ratios (HRs) for Overall Survival and Progression-Free Survival.
Scenario analyses
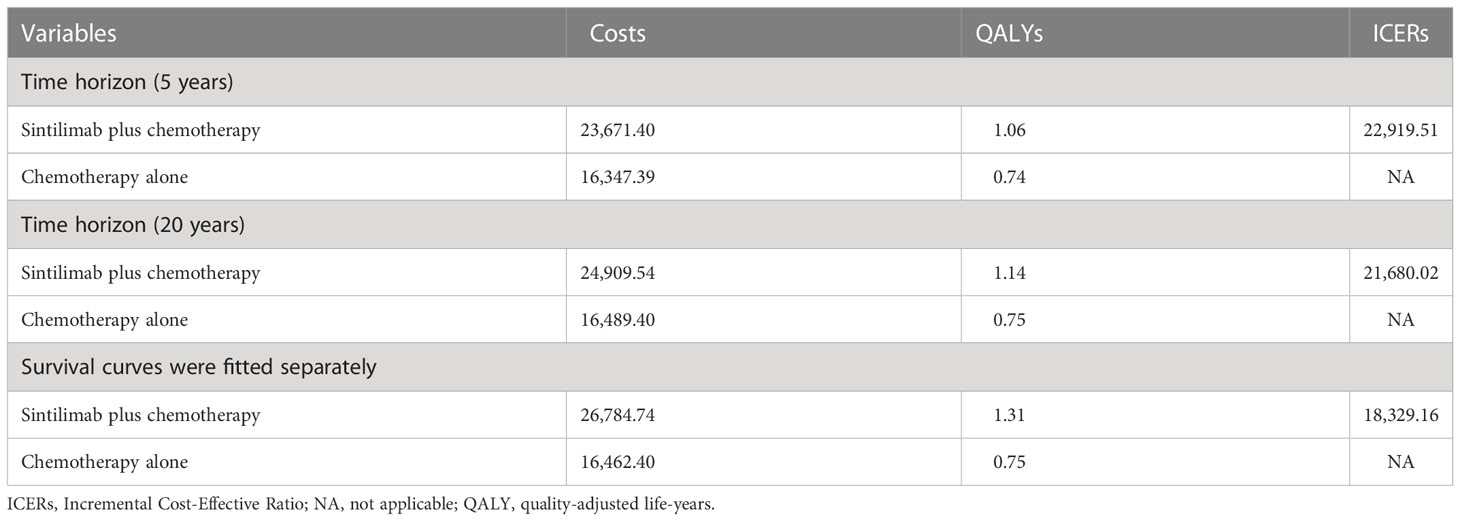
When the time horizon was 5 or 20 years, the overall cost of sintilimab plus chemotherapy was $23,671.40 or $24,909.54, resulting in an ICER of sintilimab plus chemotherapy vs chemotherapy alone of $ 22,919.51 or $21,680.02 per QALY. When four survival curves (OS and PFS curves for sintilimab plus chemotherapy vs chemotherapy alone) were fitted separately, the overall cost of sintilimab plus chemotherapy was $26,784.74, resulting in an ICER of $18,329.16/QALY (Table 3).
Discussion
At present, ICIs alone or in combination with other regimens has becoming a prominent option for the treatment of advanced oesophageal cancer. However, there are few studies on the pharmacoeconomic evaluation of immunotherapies for OSCC. Based on our model, sintilimab plus chemotherapy cost $21,782.24 per additional QALY gained compared with chemotherapy alone, suggesting sintilimab plus chemotherapy would be considered cost-effective when assuming a WTP threshold at 2- or 3-times GDP per capita per QALY. Results were generally robust, as evidenced by the results of one-way sensitivity analysis and probabilistic sensitivity analysis. At a WTP threshold of 3 times GDP per capita per QALY, all subgroups other than CPS<1 subgroup favored sintilimab plus chemotherapy treatment due to its association with positive INHBs by varying the HRs for OS and PFS compared with chemotherapy alone. Economic evaluations of the 26 subgroups may be useful in tailoring treatment decisions by physicians, patients and policy makers.
Interestingly, different PD-1 inhibitors showed completely distinct cost-effectiveness in the first-line treatment of OSCC. Contrary to our previous study, camrelizumab plus chemotherapy was not a cost-effective option as the first-line treatment for advanced or metastatic OSCC because the ICER of camrelizumab plus chemotherapy versus chemotherapy was $46,671.10 per QALY gained, which was higher than the WTP threshold of China (23). Another two recent studies reported by Wu et al. (29) and Zheng et al. (30) investigated the cost-effectiveness of pembrolizumab plus chemotherapy vs placebo plus chemotherapy in the first-line therapy of oesophageal cancer patients in China, and also obtained results contrary to the current study with ICERs of $115,391.84/QALY and $41,805.12/QALY, respectively, exceeding the threshold of WTP. These opposite results to ours maybe owing to different trial data and survival curve simulation techniques and different costs of PD-1 inhibitors, leading to various incremental costs and QALYs. In these settings, clinicians and decision-makers can leverage knowledge of economics to advance policy discussions about health care costs and to take action on accessibility, affordability, and value for use of these novel immunotherapies. Although there is no cost-effectiveness analysis study of sintilimab in the treatment of OSCC, some studies have reported that sintilimab plus chemotherapy is cost-effective in the first-line therapy of locally advanced or metastatic non-small cell lung cancer (31, 32). Moreover, the combination of sintilimab plus bevacizumab is also likely to be a cost-effective option compared with sorafenib as the first-line treatment of patients with unresectable hepatocellular carcinoma in China (33–35).
Results of the one-way sensitivity analysis confirmed that the model was most sensitive to the utility of PFS and cost of sintilimab. When the cost of sintilimab was reduced, sintilimab plus chemotherapy treatment would become more favorable because its ICER was lower than 1 times GDP per capita per QALY. After that, HR for PFS and OS had the greatest effect on the model results, demonstrating sintilimab plus chemotherapy was more cost-effective for patients with a favorable prognosis, such as patients with age ≥ 65 years and weight ≥ 60 kg. Notably, as these parameters varied within the specified range, ICERs dramatically increased or decreased, which induced ICERs still below the WTP threshold of 3 times GDP per capita per QALY, making sintilimab plus chemotherapy consistently cost-effective, which was further supported by the cost-effectiveness acceptability curve that the probability of sintilimab plus chemotherapy being cost-effective was 76.80% and 98.60% at 2 or 3 times GDP per capita per QALY threshold, respectively. Furthermore, the scenario analyses also affirmed the stability of the model results. Although altering the time horizon of the model or fitting survival curves separately, the ICERs remained below 2 times GDP per capita per QALY ($25,108.84/QALY).
Strengths and limitations
The strengths of this study are worth highlighting. To the best of our knowledge, this study is the first to evaluate the economic outcomes of sintilimab plus chemotherapy in the first-line treatment for advanced or metastatic OSCC by integrating the latest evidence through a pharmacoeconomic modeling technology. Besides, the partitioned survival model is employed in this economic analysis for the advantages that survival curve can be directly used to obtain the proportion of patients with different health states, and complex risk functions can be directly reconstructed by extrapolation method, so the calculation is relatively simple. Furthermore, there is no need to make additional assumptions on the model to calculate the probability of transition between states, so it is more suitable for the actual survival of patients.
There are also several limitations in our study. First, we only evaluated the cost-effectiveness of sintilimab plus chemotherapy vs chemotherapy alone for patients with advanced OSCC. In fact, the cost-effectiveness of sintilimab vs similar drugs (other ICIs) should also be assessed. However, due to the lack of robust head-to-head trial data, we could not compare the economics of other immunotherapies with that of sintilimab directly, such as pembrolizumab, camrelizumab, and nivolumab, which had also shown favorable health benefits for patients with advanced OSCC. Therefore, future research will perform network meta-analyses to obtain HRs for OS and PFS between different ICIs regimens, subsequently achieving cost-effectiveness comparisons. Second, though ORIENT-15 is a large and well-designed trial, any biases such as rigorous eligible patients, high medication adherence and racial difference, can affect its validity and generalizability. Thus, there’s a difference in generalizing our cost-effectiveness analysis results outside the Chinese setting. Third, clinical benefits beyond the observation time of the ORIENT-15 trial were assumed through fitting of parametric distributions to the reported Kaplan-Meier PFS and OS data, which may lead to uncertainty in the model outcomes, although flexible parametric models were applied and the modeled and observed data were validated. However, we have conducted a series of sensitivity, subgroup and scenario analyses to evaluate the uncertainty, and they showed the model results were robust. Fourth, the costs of grade 1 and 2 AEs were excluded, which might result in the overestimation of the economic results associated with sintilimab plus chemotherapy. Lastly, the disutility value for AEs extracted from a study outside Chinese setting, may also cause some bias. These limitations may not be major factors, as showed in the one-way sensitivity analysis indicating that the influence of the costs/disutility values of AEs were minor.
Conclusion
This economic evaluation showed that sintilimab plus chemotherapy was associated with improved QALYs and an additional cost but was estimated to be cost-effective compared with chemotherapy as a first-line treatment for patients with advanced or metastatic OSCC at the commonly adopted WTP threshold of 3 times GDP per capita per QALY in China. The findings may help clinicians and oncologists determine the preferred economic treatment strategy of advanced OSCC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
YS and QZ conceived and administered the study. LL, QZ, and YS conducted the analysis, drafted the manuscript and interpreted the results. LW and YD substantively revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82104476).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1092385/full#supplementary-material
References
1. Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology (2018) 154(2):360–73. doi: 10.1053/j.gastro.2017.08.023
2. Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. (2020) 69(9):1564–71. doi: 10.1136/gutjnl-2020-321600
3. International Agency for Research on Cancer. Global cancer observatory: cancer today . Available at: https://gco.iarc.fr/today/data/factsheets/populations/160-china-fact-sheets.pdf (Accessed March, 2021).
4. Muro K, Lordick F, Tsushima T, Pentheroudakis G, Baba E, Lu Z, et al. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with metastatic oesophageal cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol (2019) 30(1):34–43. doi: 10.1093/annonc/mdy498
5. National Comprehensive Cancer Network. NCCN guidelines in esophageal and esophagogastric junction cancers. version 4 (2022). Available at: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1433 (Accessed Feb 6, 2022).
6. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20(11):1506–17. doi: 10.1016/s1470-2045(19)30626-6
7. Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: The ESCORT-1st randomized clinical trial. Jama (2021) 326(10):916–25. doi: 10.1001/jama.2021.12836
8. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet (London England) (2021) 398(10294):27–40. doi: 10.1016/s0140-6736(21)00797-2
9. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet (London England) (2021) 398(10302):759–71. doi: 10.1016/s0140-6736(21)01234-4
10. Hoy SM. Sintilimab: First global approval. Drugs (2019) 79(3):341–6. doi: 10.1007/s40265-019-1066-z
11. Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Efficacy and safety of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous NSCLC: A randomized, double-blind, phase 3 study (Oncology pRogram by InnovENT anti-PD-1-11). J Thorac Oncol (2020) 15(10):1636–46. doi: 10.1016/j.jtho.2020.07.014
12. Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. Bmj (2022) 377:e068714. doi: 10.1136/bmj-2021-068714
13. Chinese Society of Clinical Oncology. CSCO guidelines in diagnosis and treatment of esophageal cancer (2022). Available at: http://www.csco.org.cn/cn/index.aspx (Accessed April 11, 2022).
14. Williams C, Lewsey JD, Mackay DF, Briggs AH. Estimation of survival probabilities for use in cost-effectiveness analyses: A comparison of a multi-state modeling survival analysis approach with partitioned survival and Markov decision-analytic modeling. Med Decis Making (2017) 37(4):427–39. doi: 10.1177/0272989x16670617
15. Su D, Wu B, Shi L. Cost-effectiveness of atezolizumab plus bevacizumab vs sorafenib as first-line treatment of unresectable hepatocellular carcinoma. JAMA Netw Open (2021) 4(2):e210037. doi: 10.1001/jamanetworkopen.2021.0037
16. Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: Updated reporting guidance for health economic evaluations. Value Health (2022) 25(1):3–9. doi: 10.1016/j.jval.2021.11.1351
17. Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol (2012) 12:9. doi: 10.1186/1471-2288-12-9
18. Latimer NR. Survival analysis for economic evaluations alongside clinical trials–extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Making (2013) 33(6):743–54. doi: 10.1177/0272989x12472398
19. Felizzi F, Paracha N, Pöhlmann J, Ray J. Mixture cure models in oncology: A tutorial and practical guidance. Pharmacoecon Open (2021) 5(2):143–55. doi: 10.1007/s41669-021-00260-z
20. Martinez EZ, Achcar JA, Jácome AA, Santos JS. Mixture and non-mixture cure fraction models based on the generalized modified Weibull distribution with an application to gastric cancer data. Comput Methods Programs BioMed (2013) 112(3):343–55. doi: 10.1016/j.cmpb.2013.07.021
21. Chinese drug. Chinese drug price of drug centralized bid procurement (2021). Available at: https://db.yaozh.com/yaopinzhongbiao (Accessed Sep 30, 2022).
22. Lin YT, Liu TX, Chen J, Wang C, Chen Y. Cost-effectiveness of nivolumab immunotherapy vs. paclitaxel or docetaxel chemotherapy as second-line therapy in advanced esophageal squamous cell carcinoma in China. Front Public Health (2022) 10:923619. doi: 10.3389/fpubh.2022.923619
23. Zhang Q, Wu P, He X, Ding Y, Shu Y. Cost-effectiveness analysis of camrelizumab vs. placebo added to chemotherapy as first-line therapy for advanced or metastatic esophageal squamous cell carcinoma in China. Front Oncol (2021) 11:790373. doi: 10.3389/fonc.2021.790373
24. Amdahl J, Diaz J, Park J, Nakhaipour HR, Delea TE. Cost-effectiveness of pazopanib compared with sunitinib in metastatic renal cell carcinoma in Canada. Curr Oncol (2016) 23(4):e340–54. doi: 10.3747/co.23.2244
25. Yue X, Li Y, Wu J, Guo JJ. Current development and practice of pharmacoeconomic evaluation guidelines for universal health coverage in China. Value Health Reg Issues (2021) 24:1–5. doi: 10.1016/j.vhri.2020.07.580
26. National Bureau of Statistics of China. National bureau of statistics of China (2021). Available at: http://data.stats.gov.cn/english/index.htm (Accessed Sep 30, 2022).
27. Craig BA, Black MA. Incremental cost-effectiveness ratio and incremental net-health benefit: two sides of the same coin. Expert Rev Pharmacoecon Outcomes Res (2001) 1(1):37–46. doi: 10.1586/14737167.1.1.37
28. Stinnett AA, Mullahy J. Net health benefits: A new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making (1998) 18(2 Suppl):S68–80. doi: 10.1177/0272989x98018002s09
29. Wu M, Qin S, Wang L, Tan C, Peng Y, Zeng X, et al. Cost-effectiveness of pembrolizumab plus chemotherapy as first-line therapy for advanced oesophageal cancer. Front Pharmacol (2022) 13:881787. doi: 10.3389/fphar.2022.881787
30. Zheng Z, Lin J, Zhu H, Cai H. Cost-effectiveness analysis of pembrolizumab plus chemotherapy vs. chemotherapy alone as first-line treatment in patients with esophageal squamous cell carcinoma and PD-L1 CPS of 10 or more. Front Public Health (2022) 10:893387. doi: 10.3389/fpubh.2022.893387
31. Rui M, Fei Z, Wang Y, Zhang X, Ma A, Sun H, et al. Cost-effectiveness analysis of sintilimab + chemotherapy versus camrelizumab + chemotherapy for the treatment of first-line locally advanced or metastatic nonsquamous NSCLC in China. J Med Econ (2022) 25(1):618–29. doi: 10.1080/13696998.2022.2071066
32. Chen P, Wang X, Zhu S, Li H, Rui M, Wang Y, et al. Economic evaluation of sintilimab plus chemotherapy vs. pembrolizumab plus chemotherapy for the treatment of first-line advanced or metastatic squamous NSCLC. Front Public Health (2022) 10:956792. doi: 10.3389/fpubh.2022.956792
33. Peng Y, Zeng X, Peng L, Liu Q, Yi L, Luo X, et al. Sintilimab plus bevacizumab biosimilar versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma: A cost-effectiveness analysis. Front Pharmacol (2022) 13:778505. doi: 10.3389/fphar.2022.778505
34. Zhou T, Cao Y, Wang X, Yang L, Wang Z, Ma A, et al. Economic evaluation of sintilimab plus bevacizumab versus sorafenib as a first-line treatment for unresectable hepatocellular carcinoma. Adv Ther (2022) 39(5):2165–77. doi: 10.1007/s12325-022-02079-4
35. Li L, Yang S, Chen Y, Tian L, He Y, Wu B, et al. Immune checkpoint inhibitors plus an anti-VEGF antibody as the first-line treatment for unresectable hepatocellular carcinoma: A network meta-analysis and cost-effectiveness analysis. Front Pharmacol (2022) 13:891008. doi: 10.3389/fphar.2022.891008
Keywords: cost-effectiveness, immunotherapy, oesophageal squamous cell carcinoma, quality-adjusted life years, sintilimab
Citation: Liu L, Wang L, Chen L, Ding Y, Zhang Q and Shu Y (2023) Cost-effectiveness of sintilimab plus chemotherapy versus chemotherapy alone as first-line treatment of locally advanced or metastatic oesophageal squamous cell carcinoma. Front. Immunol. 14:1092385. doi: 10.3389/fimmu.2023.1092385
Received: 08 November 2022; Accepted: 09 January 2023;
Published: 23 January 2023.
Edited by:
Domenico Criscuolo, Italian Society of Pharmaceutical Medicine, ItalyReviewed by:
Bálint Tamaskovics, Heinrich Heine University of Düsseldorf, GermanyChangfa Xia, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2023 Liu, Wang, Chen, Ding, Zhang and Shu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yamin Shu, shuyamin1990hust@163.com; Qilin Zhang, qilinzhang88@163.com
 Lulu Liu
Lulu Liu Lei Wang
Lei Wang Li Chen
Li Chen Yiling Ding4
Yiling Ding4 Qilin Zhang
Qilin Zhang Yamin Shu
Yamin Shu