- 1Precision Medical Center, Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University of Science & Technology, Wuhan, China
- 2Department of Cardiology, Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University of Science & Technology, Wuhan, China
- 3Department of Clinical Laboratory, Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University of Science & Technology, Wuhan, China
- 4Rheumatology and Immunology Department, Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University of Science & Technology, Wuhan, China
Germline gain-of-function (GOF) mutations in the CARD11 gene lead to a rare primary immunodeficiency disease known as B cell expansion with NF-κB and T cell anergy (BENTA). Affected patients present with a polyclonal expansion of B cells, lymphadenopathy, and splenomegaly. Herein, we report a novel germline in-frame three base-pair deletion (c.1030_1032del, p.K344del) in the CARD11 gene in a patient with atypical BENTA, presenting with a recurrent fever and B cell lymphocytosis. This mutation was inherited from his mother, who is clinically asymptomatic and had a recurrent respiratory tract infection in her childhood. In vitro functional analysis demonstrated that this variant decreased the expression level of the CARD11 protein and activated the NF-κB signal pathway, leading to a higher expression of several NF-κB target gene transcripts in HCT116 cells transfected with mutant CARD11 (K344del-CARD11) as revealed by RNA sequencing analysis. To our knowledge, only 23 BENTA patients have been identified and carried seven distinct GOF mutations in CARD11. The clinical manifestations of patients are highly heterogeneous and there was no significant correlation between genotype and phenotype. In summary, we identified a novel in-frame three base-pair deletion that may be responsible for the pathogenesis of atypical BENTA in a Chinese family. Our study expands the mutational spectrum of the CARD11 gene and may be helpful in the understanding of diseases caused by CARD11 mutations and the clinical management of BENTA.
Introduction
B cell expansion with NF-κB and T cell anergy (BENTA) (OMIM 616452) was first reported in 2012 and is a rare primary immunodeficiency disease. Patients present with persistent B cell lymphocytosis in early childhood, usually accompanied by lymphadenopathy and splenomegaly (1, 2). BENTA is caused by heterozygous germline gain of function (GOF) mutations in the CARD11 gene, which encodes a caspase recruitment domain-containing protein (CARD11, also known as CARMA1) (3).
The CARD11 is a lymphocyte-specific scaffolding protein and acts as a critical signal transducer from the cell surface antigen receptor (AgR) in B or T cells to the cytoplasmic IκB kinase (IKK). This in turn activates the canonical NF-κB pathway (4). An overactive NF-κB pathway is associated with B cell malignancy (5). Somatic mutations in CARD11 have been reported in different cancers, especially in diffuse larger B cell lymphoma (DLBCL) (6). Germline CARD11 mutations have been associated with several primary immune disorders, including immunodeficiency 11A (OMIM 615206) (7), immunodeficiency with atopic dermatitis (OMIM 617638) (8), and BENTA caused by heterozygous GOF mutations (3). These immune disorders were caused by bi-allelic loss-of-function (LOF) mutations, heterozygous dominant negative, and GOF mutations, respectively. To date, 23 patients with BENTA have been identified to carry seven distinct GOF mutations, including C49Y, G123S, G123D, G126D, E134G, H234Ldel235-8, and K215del (3, 9–19). These mutations are located in the CARD, LATCH, or CC domains of CARD11.
In this study, a germline heterozygous mutation (c.1030_1032del, p.K344del) has been identified in the CARD11 gene in a Chinese boy with recurrent fever and B cell lymphocytosis. This mutation was inherited from his mother who had a recurrent respiratory tract infection in childhood and is clinically asymptomatic. Our functional study suggests that this mutation resulted in an overactive NF-κB in vitro. In addition, we review the literature and summarize the clinical phenotypes and genotypes of all BENTA patients.
Material and methods
Patient and samples
This work has been approved by the ethics committee of Wuhan Children’s Hospital, Tongji Medical College, and Huazhong University of Science & Technology, and informed consent was obtained from the parents of the patient. The patient was recruited in this study because of recurrent fever. After 3ml of peripheral blood was collected from both this patient and his parents, genomic DNA and RNA were extracted by using the MicroElute Genomic DNA Kit (OMEGA Bio-tek) and Trizol reagent (Invitrogen), respectively.
Whole exome sequencing
Trio WES was conducted with the help of the third-party medical testing laboratory (Chigene (Beijing) Translational Medical Research Center. China), and subsequent bioinformatic analysis was done as described previously in our laboratory (20).
The candidate gene variants were validated by Sanger sequencing in this patient and his parents. The primers sequence are as follows: CARD11-F: CAA CAG TCA GAT AGT CGG TTCC; CARD11-R: GAC AAA ACA CTC TGA AGG AGCC. The conservation analysis of the protein amino acid was conducted using MEGA software.
CARD11 gene plasmid construction and cell transfection
Site-directed mutagenesis was performed to generate K344del-CARD11 using specific primers for linear amplification, followed by DpnI digestion of methylated DNA. Positive colonies were subjected to sequencing analysis to verify the successful deletion of the guide sequence. The wildtype CARD11 plasmid (WT-CARD11) and positive control (C49Y-CARD11) were included as described previously (16).
After being cultured in DMEM supplemented with 10% fetal bovine serum (Gibco, Thermo Fisher Scientific) for 24 hours, HCT116 cells were transfected with 2 μg plasmids (pcDNA3.1, WT, C49Y, WT+K344del, and K344del using Lipofectamine 3000 (Invitrogen) according to the manufacturer’s instructions.).
Western blot
The detailed experimental protocol is similar to our previously published article (16). Cells were lysed in NP-40 lysis buffer on ice, and then centrifugated at 12000rpm for 10 minutes at 4°C. Cell nucleoprotein was extracted using NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific). The extracted protein was separated by SDS-PAGE and WB was used to detect the expression level of related proteins with the following antibodies: anti-CARD11(Proteintech, 21741-1-AP), anti-NF-κB p65(Cell Signaling Technology, #8242), lamin B (Santa cruz, sc-377000), and GAPDH(Proteintech, 60004-1-Ig).
Immunofluorescence
Cells cultured in a confocal dish were fixed for 15 minutes with 3% paraformaldehyde and then permeabilized for 15 minutes in 0.1% Triton X-100/PBS. After blocking in a 3% BSA buffer, cells were incubated with the CARD11 antibody (Proteintech, 21741-1-AP) for 90 minutes. Cells were washed in PBS and then incubated with a secondary antibody conjugated to Alexa Fluor 488 (Invitrogen) at normal temperature. After washing three times, DAPI was added to the cell culture dish. Fluorescent images were obtained on a confocal microscope (Leica Stellaris 5).
IgH rearrangement analysis
DNA was extracted from peripheral blood using MicroElute Genomic DNA Kit (OMEGA Bio-tek). PCR was performed using fluorescent-labeled primers to the VH framework region (FR II) and joining region(JH) of the IgH gene. The amplification products were separated by capillary electrophoresis and analyzed on Genetic Analyzer (3500DX, ABI) using GeneMapper software version 5.0 (Applied Biosystems).
Luciferase reporter assays
HCT116 cells were plated in 12-well culture plates (3 × 105 cells/well) for 12h. The cells were then co-transfected with either 500 ng of pcDNA3.1+, CARD11-WT, and -C49Y- -K344del, 300 ng of pNFκB-luc (Bayotime, China) containing NF-κB binding motifs, or 100 ng of pRL-TK vector as a control (Promega, Madison, WI). After 36h, cells are lysed in lysate buffer and then analyzed for luciferase activity using the Dual-Luciferase reporter Assay System (Promega, Madison, WI). Three independent experiments were performed to assess luciferase activity.
RNA-seq analysis
The experimental protocol was carried out as described previously (16). RNA was isolated from HCT116 cells transfected with CARD11-K344del or CARD11-WT plasmids, respectively. After constructing cDNA libraries, sequencing analysis was carried out on an Illumina sequencer instrument. Gene expression was calculated as transcripts per million (TPM) mapped reads by using the TopHat alignment program with redundant reads removed, and the expression values were normalized by using . The raw data was submitted to the SRA database (SUB11737083).
Quantitative PCR analysis
After the cDNA was generated from the total RNA isolated from HCT116 cells transfected with wild-type and mutant CARD11 gene, respectively, a quantitative PCR experiment was performed on ABI 7500 instrument (Applied Biosystems, USA) using SYBR green preMix Taq Kit (TAKARA, Dalian). Single amplicons of predicted size were confirmed by agarose gel electrophoresis analysis. Raw data was calculated as an average of three independent experiments. ACTIN was included as an internal control.
Statistics methods
Graphpad Prism 5 (Graphpad software, USA) was used for statistical analyses. The Student’s t-test was used to compare two groups, a one-way analysis of variance (ANOVA) was used to compare one variable among three or more groups, and statistical significance is indicated by a P-value (*P<0.05, **P<0.01, ***P<0.001). All experiments were performed in duplicates and repeated three times, and data are described as the mean ± SD.
Results
Case presentation
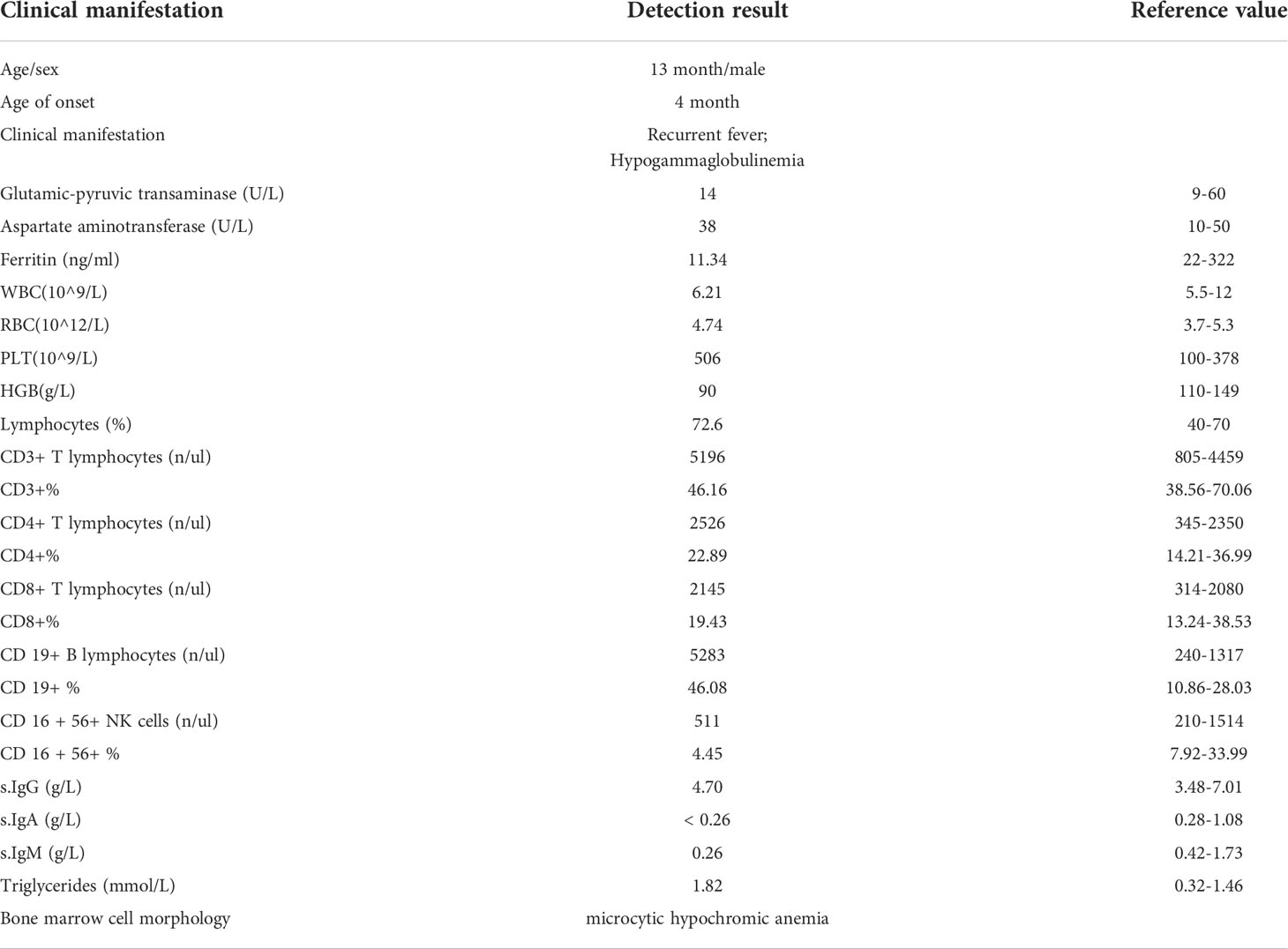
The patient was a 13-month-old male of Chinese descent who was referred for genetic evaluation because of unexplained recurrent fever. He is the only child born to healthy non-consanguineous parents without familial history of genetic diseases. There were no complications during pregnancy and no obvious abnormality was found at birth. When he was 7 months old, the patient presented with a recurrent fever (8 times within two months), with a maximum temperature of 39.2°C, and a persistent upper respiratory tract infection. He had a small rash on his face after the fever. The rest of his physical examination was unremarkable and he had no palpable lymph nodes. Clinical laboratory examination showed the proportion of lymphocytes (84.8%, normal range 40-70%) increased significantly with normal morphological characteristics and normal total white blood cell counts (9.67*109/L). The CD19+ B cell population was expanded at presentation (3,432 cells/μL, normal range 240-1,317cells/μL), and the proportion of NK cells was decreased (3.03%). The patient did not receive any special treatment, other than immunization and other symptomatic treatments. Over the next six months, the patient still had periodic fever episodes occurring every 8 days and lasting for 2-3 days. When the patient was 13 months old, he was admitted to our hospital again because of recurrent fever. The summary of routine blood tests and immunological investigations is provided in Table 1. Initial blood analyses showed the proportion of lymphocyte cells and CD19+ B-cells were increased, and the hemoglobin level was decreased (90g/L, normal range 110-149g/L). A bone marrow biopsy showed hyperplastic anemia (Figure 1A). An IgH rearrangement experiment indicated that the patient’s B cells were polyclonal (Figure 1B). An abdominal CT scan showed multiple mesenteric lymphadenopathies and an ultrasound examination revealed a normal spleen size and an accessory spleen at the hilum.
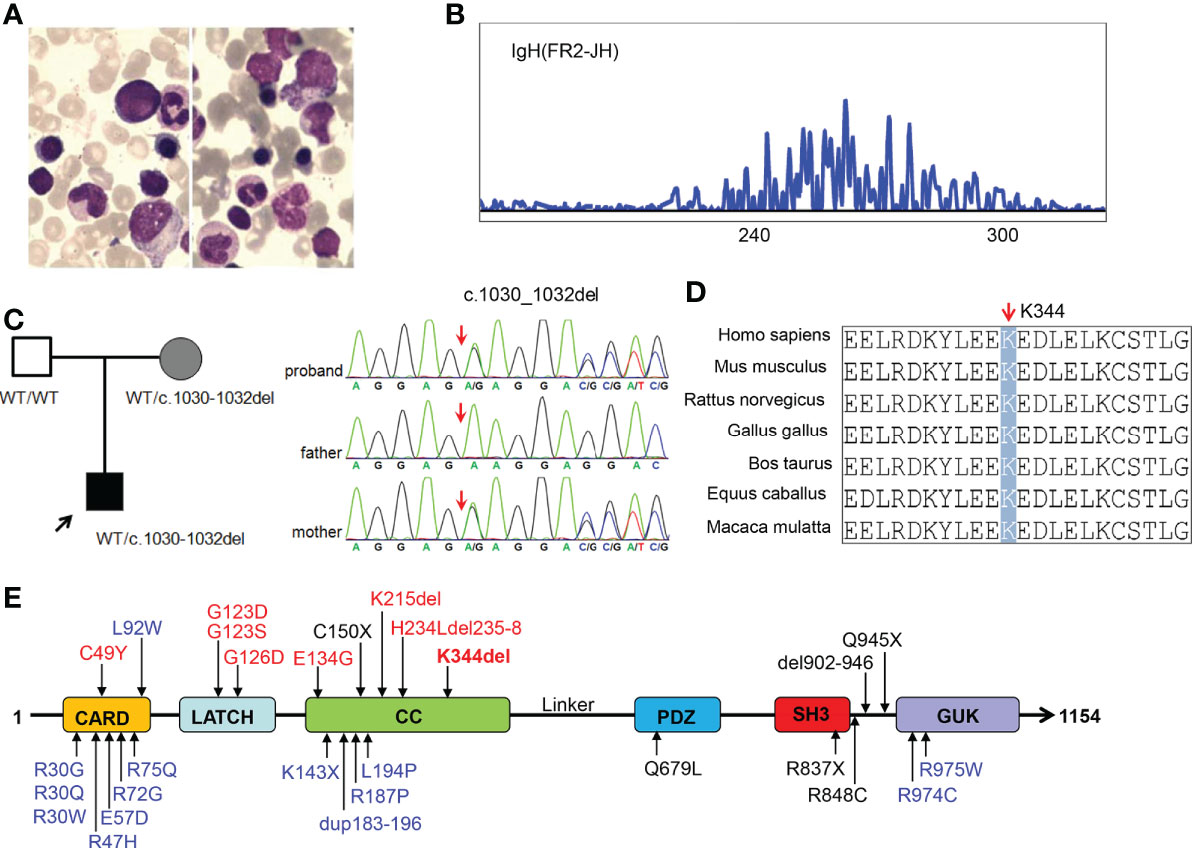
Figure 1 Characterization of our patient with CARD11 gene mutation and conserved features of the CARD11 protein. (A) Bone marrow smear from the patient showing microcytic hypochromic anemia; (B) Result of IgH rearrangement of our patient; (C) Pedigrees of this family and sanger sequencing of CARD11 mutation in this family; (D) Aligned amino acid sequence at this mutation among different species. The position at residue 344 is noted by a gray bar and highly conserved throughout all indicated species; (E) Scheme of the distribution of the CARD11 gain-of-function mutations, and the mutation noted in red was reported in this study. Gain of function mutations are highlighted in red, dominant negative mutations are in blue, and loss of function mutations are in black.
Identification of germline CARD11 mutation
In order to determine the cause of the patient’s recurrent fever and rule out the genetic disorder, a trio WES was conducted. Bioinformatic analysis was performed to identify candidate variants according to filtering strategy and the pathogenicity of identified variants was assessed as described previously (20). After analysis, a heterozygous in-frame three base-pair deletion mutation (c.1030_1032del, p.K344del) was identified in the CARD11 gene (NM_032415), and a Sanger sequencing analysis showed this mutation was inherited from the patient’s mother (Figure 1C). The mutated site is conserved among different species (Figure 1D). Interestingly, similar to most other BENTA-associated variants, the K344del variant was located in CC domain (Figure 1E). The germline variant has not been listed in the Clinvar, ExAC, gnoMAD, or dbSNP databases, while the somatic variant was included in the Catalogue of Somatic Mutations in Cancer (COSMIC) in a patient with diffuse large B cell lymphomas (DLBCL). We submitted this variant to the Clinvar database (https://submit.ncbi.nlm.nih.gov/subs/clinvar_wizard/SUB11735856/overview).
Given that the K344del variant was inherited from the mother, we also made a detailed inquiry into whether the mother had similar symptoms. The mother, who is clinically asymptomatic, told us that she had a recurrent respiratory tract infection in childhood. The clinical laboratory examinations, including the total white blood cell counts, the proportion of lymphocytes, CD19+ B cell population, and the proportion of NK cells were in normal ranges.
K344del spontaneously aggregates and constitutively activates NF-κB
To examine the effect of K344del, K344del-CARD11, positive control C49Y-CARD11, WT-CARD11, and empty plasmids were individually transfected into HCT116 cells. Our results showed that K344del significantly decreased the expressions of both gene and protein of CARD11 (Figures 2A, B). Similar to the positive control (C49Y), K344del resulted in CARD11 protein aggregation into large complexes, whereas WT-CARD11 dispersed throughout the cytoplasm (Figure 2C). In addition, p65 protein was significantly increased in mutant CARD11 compared to wild-type CARD11(Figure 3A). NF-κB activation was also examined by measuring pNF-kB-luc reporter gene expression in HCT116 cells transfected with pcDNA3.1(+), CARD11-WT, -K344del, or -C49Y plasmids. As shown in (Figure 3B), the luciferase activity in cells with mutated K344del-CARD11 was significantly higher than that in cells with CARD11-WT. Taken together, overexpression of K344del drives NF-κB activation. To mimic the proband’s state, K344del CARD11 was co-transfected at a 1:1 ratio with wild-type CARD11, and a similar change in the NF-κB signaling pathway was also detected in a heterozygous state by both western blot and luciferase reporter gene experiments (Figures 3A, B). Given that the cells only transfected with wild-type CARD11 had a higher level of CARD11 compared to those with only K344del-CARD11, a more appropriate ratio of WT-CARD11 to K344del-CARD11 used for transfection was determined to rule out the effect of wild-type CARD11. We found that the expression levels of wild-type and mutant CARD11 were comparable when the ratio of wild-type to mutant plasmid was 1 to 4 (Figure 3C). In the heterozygous state, similar to the proband, the activity of NF-κB luciferase was elevated significantly in the cells carrying the K344del plasmids in vitro (Figure 3D), suggesting that the mutation does not have a dominant negative effect. In addition, we also noted that several NF-κB target gene transcripts had higher expressions in HCT116 cells transfected with mutated CARD11 compared to that of wild-type CARD11 detected by RNA-seq (Figure 3E).
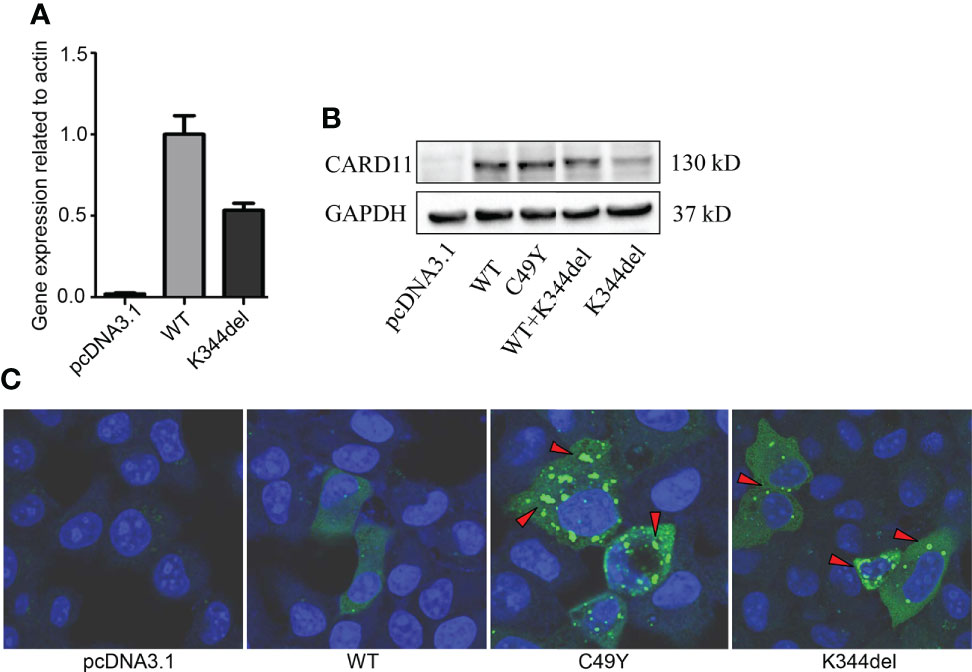
Figure 2 Expressions and Distribution of CARD11-WT and K344del in HCT116 cells. Expressions of RNA and protein of mutant CARD11 and its controls (A, B, respectively); (C) Distribution of CARD11-WT, -C49Y, and –K344del in HCT116 cells investigated by immunofluorescence.
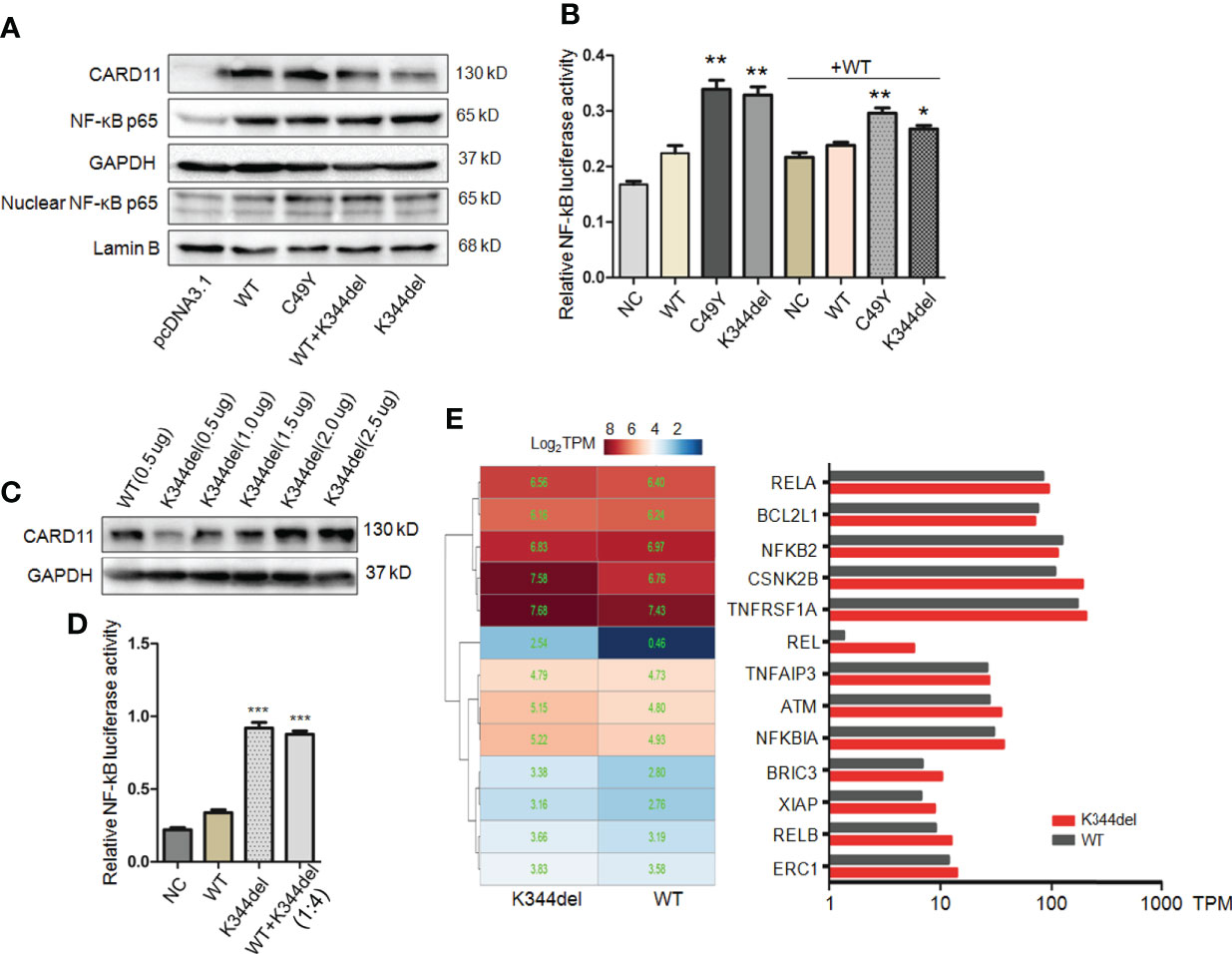
Figure 3 Functional analysis of K344del mutation in HCT116 cells. (A) The expression levels of CARD11, NF-κB p65 in HCT116 cells transfected with wildtype or mutant CARD11 in whole cell lysates and nuclear lysates, GAPDH and Lamin B serve as a loading control; (B) The activity of NF-κB-dependent luciferase of cell extracts from each sample was measured and recorded as a fold increase compared to control cells with WT-CARD11 plasmid. (C) The expression levels of CARD11 in different ratios of plasmid transfection. (D) The luciferase activity of cells transfected with mutants was increased in the case of consistent expression levels. The results from three independent experiments are described as the mean + standard deviation (*P < 0.05; **P < 0.01, ***P < 0.001). (E) Heat map of differentially expressed genes involved in NF-κB signal pathway between WT and K344del by RNA-seq analysis in HCT116 cells, the color scales of heatmap refer to Log2TPM.
Literature review of patients with BENTA
A literature review of BENTA cases was conducted by searching for all cases published from 2012 (BENTA was first reported in 2012) to 2021 with the keywords “CARD11 gene” and “B cell expansion with NF-κB and T cell anergy “. The database included Pubmed, Medline, and Clinvar et al. We reviewed 12 articles which included 23 cases with BENTA, and the clinical features of these patients were summarized in Table 2.
Among the 24 BENTA patients (17 families), there were 9 male patients and 15 female patients, aged from 2 months to 80 years. The main clinical manifestations in the patients were lymphocytosis, splenomegaly, lymphadenopathy, recurrent infection, and recurrent fever.
The clinical presentation and course are highly variable, from severe lymphocytosis and dying at a very young age (Tab, P1, P3, P4), to repeated infection in childhood and clinically asymptomatic in adulthood (P22). A total of 7 germline GOF mutations (C49Y, G123S, G123D, G126D, E134G, K215del, and H234Ldel235-8) have been reported (8–13), and these mutations are located in the CARD, LATCH, or CC domains of CARD11 (Figure 1E). The K344del reported in this study is located in the CC domain. There was no significant correlation between genotype and phenotype. For instance, Buchbinder reported that 3 patients carrying C49Y mutation presented with milder clinical symptoms, while patients (P3 and P4) with this mutation died at a young age (13). All these variants, except E134G and H234Ldel235-8, were included in the COSMIC database and are identified as somatic mutations in patients with DLBCL, chronic lymphocytic leukemia, or lymphoma. This information indirectly indicates that BENTA patients with GOF mutation are at a high risk of developing lymphoma or leukemia, and some BENTA patients had developed lymphoma or B-ALL in adulthood (3, 11).
Discussion
In this work, we have reported on a 13-month-old patient with B cell lymphocytosis, persistent recurrent fever, and upper respiratory tract infection but without lymphadenopathy or splenomegaly. The WES revealed a novel germline in-frame three base-pair deletion, c.1030_1032del(p.K344del) in the CARD11 gene, which was associated with BENTA, severe combined immune deficiency, and severe atopic disease. According to the patient’s clinical manifestations, laboratory results of B cell lymphocytosis, and genetic findings, a diagnosis of atypical BENTA was considered.
The NF-κB family of transcription factors plays a crucial role in inflammation, immunity, and cell proliferation (5, 21). CARD11 is expressed mainly in lymphocytes and is a large scaffold protein bridging the AgR of B or T cells with several signaling pathways, including NF-κB (22). GOF mutants in the CARD11 gene result in the spontaneous assembly of the CARD11-BCL10-MALT1 complex, which drives constitutive activation of NF-κB (23). Somatic K344del mutation was reported in one patient with diffuse large B-cell lymphoma (24), and to our knowledge, this variant has not been reported to be inherited (germline). To investigate the effects of K344del, we expressed the mutant and wild-type constructs along with a positive control (C49Y) in HCT116. Our findings showed that the K344del variant resulted in the aggregation of CARD11 and increased activation of NF-κB, consistent with previous studies on GOF mutations in the CARD11 gene (16). Besides the GOF effect, CARD11 could lead to a dominant negative effect, involving immunodeficiency with atopic dermatitis. We noted that affected members in a four-generation family with a novel heterozygous germline mutation (c.701-713delinsT, p.H234Ldel235-8) in CARD11 exhibited “mixed” clinical manifestations of BENTA (milder to moderate B cell lymphocytosis) with atopic dermatitis, which is consistent with both the GOF and dominant negative signaling effects (11). To investigate if the K344del CARD11 has a dominant negative effect, indicated by decreased activity in the heterozygous state compared with wild-type, plasmids with K344del CARD11 were co-transfected at a 1:1 ratio (or 4:1 ratio) with wild-type CARD11. We found that the NF-κB signaling pathway was also activated in the heterozygous state and K344del did not have a dominant negative effect.
By reviewing the literature, a total of 23 patients were identified, and their clinical manifestations were highly heterogeneous. Previous studies reported that patients with G123D, G123S, or G126D mutation had more severe symptoms, including B cell lymphocytosis, immunodeficiency, or death at a young age (13, 15, 16). Patients possessing the C49Y variant could present with recurrent fever and mild infection, and/or fatal hemophagocytic lymphohistiocytosis, and have a high risk for mortality at a young age (13, 19). It is clear that GOF mutation within CARD and LATCH domains is associated with severe phenotypes but more studies are needed for further confirmation. The heterogeneity of clinical phenotypes could be due to the potency of the GOF effect, additional genomic variants or modifications, and environmental factors. In addition, recurrent infection also affects CARD11 activity and disease severity. Lastly, the structural biology of the mutant CARD11 within the CARD11-BCL10-MALT1(CBM) signalosome complex may be another factor to mediate the CARD11 activity. This in turn, affects clinical symptoms and needs further study. For the patients with BENTA that survive to adulthood, as B cell lymphocytosis gets milder with aging (14), it is likely that patients are clinically asymptomatic, like the mother of our patient, and could be missed.
Polyclonal expansion of B cells is a hallmark of BENTA and polyclonal expansion of B cells increases the risk of B cell malignancy later in life. In addition, BENTA patients also have recurrent infections, including Epstein–Barr virus (EBV) infections. Impaired control of EBV infection has emerged as a recurring problem in BENTA disease (18, 25). A previous study demonstrated that the control of viral infection was inadequate, especially of chronic, low-grade EBV viremia, and the presence of EBV viremia may also increase the risk of B cell lymphomagenesis (26). Therefore, BENTA patients should be regularly monitored for B cell clonal expansion and infection, even EBV viral load.
There are some limitations in our study. Due to the difficulty in obtaining specimens from the patient and his mother, an experiment to examine the NF-κB activity and cellular distribution of CARD11 was not done. In addition, the clinical laboratory examination regarding the presence or absence of B cell expansion was missing. Finally, in vitro functional study on the mutant CARD11 was performed in HCT116 cells but not in lymphocyte cell lines, even though the expression level of CARD11 was low in HCT116 cells (Figures 2A, B), which can reduce background interference.
In conclusion, we reported a germline heterozygous GOF variant in the CARD11 gene from a patient with B cell lymphocytosis and constitutive NF-κB activation. Our study provides functional evidence of the pathogenicity of this mutation. The literature review indicates that the clinical manifestations of BENTA patients are highly heterogeneous. Additional basic research focusing on the CARD11 signal and the balance of CARD11 activity will help determine the factors that influence BENTA pathogenesis.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: SRA PRJNA855490, ClinVar SCV002564141.1.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional review board of Wuhan Children’s Hospital, Tongji Medical College, Huazhong University of Science & Technology. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
Study concepts: XH, YD. Study design: PZ, DS, QM, YD, XH. Literature reserch: QM, PZ, YH. Clinical information collection: DS, QM, YH. Data acquisition: QM, XZ, LZ, YZ, YH. Data analysis/interpretation: LZ, QM, XZ, LT. Manuscript preparation: XH, PZ. Manuscript editing: XH. Manuscript final version approval: YD. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the Wuhan Municipal Health Commission (NO.WX19C19) and the Youth Program of National Natural Science Foundation of China (NO.81700302).
Acknowledgments
We thank the patient for participating in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Turvey SE, Durandy A, Fischer A, Fung SY, Geha RS, Gewies A, et al. The CARD11-BCL10-MALT1 (CBM) signalosome complex: Stepping into the limelight of human primary immunodeficiency. J Allergy Clin Immunol (2014) 134(2):276–84. doi: 10.1016/j.jaci.2014.06.015
2. Stinson JR, Dorjbal B, McDaniel DP, David L, Wu H, Snow AL. Gain-of-function mutations in CARD11 promote enhanced aggregation and idiosyncratic signalosome assembly. Cell Immunol (2020) 353:104129. doi: 10.1016/j.cellimm.2020.104129
3. Snow AL, Xiao W, Stinson JR, Lu W, Chaigne-Delalande B, Zheng L, et al. Congenital b cell lymphocytosis explained by novel germline CARD11 mutations. J Exp Med (2012) 209(12):2247–61. doi: 10.1084/jem.20120831
4. Thome M, Charton JE, Pelzer C, Hailfinger S. Antigen receptor signaling to NF-kappaB via CARMA1, BCL10, and MALT1. Cold Spring Harb Perspect Biol (2010) 2(9):a003004. doi: 10.1101/cshperspect.a003004
5. DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev (2012) 246(1):379–400. doi: 10.1111/j.1600-065X.2012.01099.x
6. Reimann M, Schrezenmeier J, Richter-Pechanska P, Dolnik A, Hick TP, Schleich K, et al. Adaptive T-cell immunity controls senescence-prone MyD88- or CARD11-mutant b-cell lymphomas. Blood (2021) 137(20):2785–99. doi: 10.1182/blood.2020005244
7. Greil J, Rausch T, Giese T, Bandapalli OR, Daniel V, Bekeredjian-Ding I, et al. Whole-exome sequencing links caspase recruitment domain 11 (CARD11) inactivation to severe combined immunodeficiency. J Allergy Clin Immunol (2013) 131(5):1376–83.e3. doi: 10.1016/j.jaci.2013.02.012
8. Dadi H, Jones TA, Merico D, Sharfe N, Ovadia A, Schejter Y, et al. Combined immunodeficiency and atopy caused by a dominant negative mutation in caspase activation and recruitment domain family member 11 (CARD11). J Allergy Clin Immunol (2018) 141(5):1818–30.e2. doi: 10.1016/j.jaci.2017.06.047
9. Buchbinder D. Sassoon A. a case of bad carma! Blood (2017) 129(12):1737. doi: 10.1182/blood-2016-12-756007
10. Shields AM, Bauman BM, Hargreaves CE, Pollard AJ, Snow AL, Patel SY. A Novel, Heterozygous three base-pair deletion in CARD11 results in b cell expansion with NF-κB and T cell anergy disease. J Clin Immunol (2020) 40(2):406–11. doi: 10.1007/s10875-019-00729-x
11. Desjardins M, Arjunaraja S, Stinson JR, Dorjbal B, Sundaresan J, Niemela J, et al. A unique heterozygous CARD11 mutation combines pathogenic features of both gain- and loss-of-Function patients in a four-generation family. Front Immunol (2018) 9:2944. doi: 10.3389/fimmu.2018.02944
12. Ludgate JL, Weeks RJ, Morison IM. Aberrant immunoglobulin kappa locus rearrangement in a patient with CARD11-related b cell lymphocytosis. J Clin Immunol (2021) 41(8):1943–5. doi: 10.1007/s10875-021-01114-3
13. Gupta M, Aluri J, Desai M, Lokeshwar M, Taur P, Lenardo M, et al. Clinical, immunological, and molecular findings in four cases of b cell expansion with NF-κB and T cell anergy disease for the first time from India. Front Immunol (2018) 9:1049. doi: 10.3389/fimmu.2018.01049
14. Outinen T, Syrjänen J, Rounioja S, Saarela J, Kaustio M, Helminen M. Constant b cell lymphocytosis since early age in a patient with CARD11 mutation: A 20-year follow-up. Clin Immunol (2016) 165:19–20. doi: 10.1016/j.clim.2016.02.002
15. Brohl AS, Stinson JR, Su HC, Badgett T, Jennings CD, Sukumar G, et al. Germline CARD11 mutation in a patient with severe congenital b cell lymphocytosis. J Clin Immunol (2015) 35(1):32–46. doi: 10.1007/s10875-014-0106-4
16. Zhao P, Meng Q, Huang Y, Zhang L, Luo S, Zhang X, et al. Identification and characterization of a germline mutation in CARD11 from a Chinese case of b cell expansion with NF-κB and T cell anergy. Front Immunol (2021) 12:676386. doi: 10.3389/fimmu.2021.676386
17. Arjunaraja S, Angelus P, Su HC, Snow AL. Impaired control of Epstein-Barr virus infection in b-cell expansion with NF-κB and T-cell anergy disease. Front Immunol (2018) 9:198. doi: 10.3389/fimmu.2018.00198
18. Buchbinder D, Stinson JR, Nugent DJ, Heurtier L, Suarez F, Sukumar G, et al. Mild b-cell lymphocytosis in patients with a CARD11 C49Y mutation. J Allergy Clin Immunol (2015) 136(3):819–21.e1. doi: 10.1016/j.jaci.2015.03.008
19. Neishabury M, Azarkeivan A, Mehri M, Najmabadi H, Cheraghi T. The first case of BENTA disease (B cell expansion with NF-κB and T cell anergy) from Iran. J Clin Immunol (2021) 41(4):811–3. doi: 10.1007/s10875-021-00965-0
20. Luo S, Chen L, Wei W, Tan L, Zhang M, Duan Z, et al. Prenatal genetic diagnosis in three fetuses with left heart hypoplasia (LHH) from three unrelated families. Front Cardiovasc Med (2021) 8:631374. doi: 10.3389/fcvm.2021.631374
21. Zinatizadeh MR, Schock B, Chalbatani GM, Zarandi PK, Jalali SA, Miri SR. The nuclear factor kappa b (NF-kB) signaling in cancer development and immune diseases. Genes Dis (2020) 8(3):287–97. doi: 10.1016/j.gendis.2020.06.005
22. Rawlings DJ, Sommer K, Moreno-Garcia ME. The CARMA1 signalosome links the signalling machinery of adaptive and innate immunity in lymphocytes. Nat Rev Immunol (2006) 6(11):799–812. doi: 10.1038/nri1944
23. Meininger I, Krappmann D. Lymphocyte signaling and activation by the CARMA1-BCL10-MALT1 signalosome. Biol Chem (2016) 397(12):1315–33. doi: 10.1515/hsz-2016-0216
24. Hattori K, Sakata-Yanagimoto M, Okoshi Y, Goshima Y, Yanagimoto S, Nakamoto-Matsubara R, et al. MYD88 (L265P) mutation is associated with an unfavourable outcome of primary central nervous system lymphoma. Br J Haematol (2017) 177(3):492–4. doi: 10.1111/bjh.14080
25. Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol (2007) 25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553
Keywords: BENTA, CARD11, gain-of-function, lymphocytosis, NF-κB
Citation: Zhao P, Hu Y, Sun D, Meng Q, Zhang L, Zhang X, Tan L, Zhang Y, Ding Y and He X (2022) A novel CARD11 germline mutation in a Chinese patient of B cell expansion with NF-κB and T cell anergy (BENTA) and literature review. Front. Immunol. 13:943027. doi: 10.3389/fimmu.2022.943027
Received: 13 May 2022; Accepted: 18 August 2022;
Published: 20 September 2022.
Edited by:
Andrew L. Snow, Uniformed Services University of the Health Sciences, United StatesReviewed by:
Saul Oswaldo Lugo Reyes, National Institute of Pediatrics, MexicoAnne Helene Durandy, Institut National de la Santé et de la Recherche Médicale (INSERM), France
Copyright © 2022 Zhao, Hu, Sun, Meng, Zhang, Zhang, Tan, Zhang, Ding and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuelian He, hexuelian2013@hotmail.com; Yan Ding, dingy129@sina.com; Yong Zhang, 1539210298@qq.com
†These authors have contributed equally to this work
 Peiwei Zhao1†
Peiwei Zhao1† Lei Zhang
Lei Zhang Yong Zhang
Yong Zhang Yan Ding
Yan Ding Xuelian He
Xuelian He