Application of commercial seaweed extract-based biostimulants to enhance adventitious root formation in ornamental cutting propagation protocols: a review
- Department of Soil, Plant and Food Sciences (Di.S.S.P.A.), University of Bari, “Aldo Moro”, Bari, Italy
Despite significant advancements in stem-cutting propagation, insufficient rooting efficiency remains an economic burden for the ornamental nursery industry. IBA and NAA play a critical role in generating adventitious roots (AR) when applied exogenously. In sustainable agriculture, the substitution of chemical inputs, with alternative natural eco-friendly products presents a key challenge. Biostimulants can form part of a solution to mitigate such risks deriving from the use of agrochemicals, they are generally considered to be non-toxic, non-polluting, biodegradable, and non-hazardous. The current knowledge of the use of commercial seaweed extract (SE) products applied to ornamental cutting propagation has not been summarized until now. Therefore, we conducted a systematic review, and we hypothesized that SE-based biostimulant application to ornamental stem cuttings improves AR formation in terms of rooting percentage, root number, and architecture. Moreover, they increase the overall quality of a rooted cutting as dry biomass and organic compound content. The authors chose SE-based biostimulants because they have been proven to have an extremely low carbon footprint; moreover, they are expected to account for more than 33% of the global market for biostimulants and reached a value of 894 million Euros by 2022. This review focuses on (i) SE-based biostimulants, in particular, brown algae; (ii) technical information on five commercial products: Goteo®, Kelpak®, AlgaminoPlant, Bio Rhizotonic, Actiwawe and others, less known, also used as phytoregulators substitutes; (iii) applied protocols, describing dose, application method, number of treatments, cutting type; (iv) effects of applied protocols on rooting rate, root architecture and overall rooted cutting quality. Outcomes show that findings vary based on crops, cuttings, location, raw materials, composition, dose, application number and procedures, and growth environment.
1 Introduction to ornamental stem cutting issues
Plant propagation is a vital part of both the agricultural and horticultural industries. Ornamentals such as rose, glossy abelia, wild sage, red tip photinia, ninebark, Pennisetum and others can be propagated using either vegetative multiplication, which produces genetically identical clones, or sexual reproduction (Hartmann and Kester, 2002; Winkelmann, 2013). For the industrial ornamental nursery, seed germination can be unreliable due to dormancy (Maghdouri et al., 2021) and seeds often have low viability (Cafourek et al., 2021). Vegetative propagation is insisted by farmers due to its lower cost, ease, and speed compared to sexual reproduction. Moreover, the resulting plants are clones that maintain the same morpho-physiological and genetic characteristics as the parent plants, ensuring uniformity and early production (Kentelky et al., 2021; Tsaktsira et al., 2021). Various techniques can be used for the ornamentals vegetative propagation: while layering is a simple technique, it is costly and only produces a limited number of clones (Braun and Wyse, 2019), grafting allows for adaptation to unfavorable environmental conditions and resistance to soil-borne pathogens and parasites but is labor-intensive and can lead to compatibility issues between the rootstock and graft (Baron et al., 2019; Rasool et al., 2020; Lesmes-Vesga et al., 2021). ‘In vitro’ clonal propagation can produce many plants in a short time (Gianguzzi et al., 2020; Park et al., 2021) but requires specialized laboratory facilities and skilled labor (Read, 2011).
Propagation by stem cutting, less expensive and easier than the ‘in vitro’ technique, is the method most widely used to multiplicate clones of landscape woody (Kaviani and Negahdar, 2017; Druege et al., 2019; Ross et al., 2021) and herbaceous ornamental. Adventitious roots (AR) “arise from non-root parts of plants, in response to stress and wounding” (Gogna et al., 2022) and are a key step in stem-cutting propagation (Assis et al., 2004; Oinam et al., 2011) of economically important landscaping ornamentals (Geiss et al., 2009).
The evaluation of the rooting quality of propagated cuttings is highly relevant from an economical viewpoint. AR quality is characterized by a high both percentage of rooted cuttings and the number and length of very fine roots, which are essential for continuous access to water and nutrients (Atkinson, 2000) and can help the plant withstand transplant shock, increasing survival and plant growth (Franco et al., 2011; Khan et al., 2016; Koevoets et al., 2016) and by an adequate number of higher caliber roots with mechanical support function. Indeed, inadequate environmental, nutritional and hormonal conditions of stem cutting can lead to the production of insufficient AR, with consequent loss of quality. The overall cutting quality is represented too by dry biomass of above-ground and ground parts and by some organic compound (chlorophyll and carbohydrate) content.
During the last few decades, intense interest has emerged in the role of other potential rooting co-factors, such as carbohydrates, phenolics, polyamines, and other plant growth regulators, as well as signal molecules, such as reactive oxygen and nitrogen species (Druege, 2023; Roussos, 2023).
The technical innovations for propagation by cuttings (automatic mist propagation unit, basal heating) provide optimal environmental conditions to improve rooting efficiency. Regarding stock plants nutritional conditions, it is widely recognized that, having adequate carbohydrate reserves, specifically soluble sugars, is crucial for survival and AR formation, particularly under unfavorable environmental conditions for photosynthesis (Hartmann and Kester, 2002). This is particularly relevant to producing young ornamental plants in Central Europe, where cuttings are rooted at relatively low irradiance levels, while in East and Central Africa and Latin America they are rooted under high irradiance conditions. This is because cuttings are unable to reach their light compensation point under low light conditions, especially during winter (Ahkami et al., 2009; Druege, 2009; Lohr et al., 2017; Tahir et al., 2022). A significant portion of sucrose is converted into starch, which is believed to be the primary carbon source for the growth of AR (Lohr et al., 2017).
Auxin is one of the major endogenous hormones involved in the process of adventitious rooting and the physiological stages of rooting are correlated with changes in endogenous auxin concentrations (Justamante et al., 2019). Indole-3-acetic acid (IAA) is the most abundant member of the auxin family of phytohormones, and its biosynthesis in plants and bacteria proceeds through tryptophan-dependent and independent pathways; it plays an important role in cell division and root initiation (Luziatelli et al., 2020).
Generally, on farms, the application of a specific synthetic plant phytoregulator (i.e. auxin) can enhance the rooting percentage, number and quality of AR and shortage of rooting time in some species but with little effect on others (Díaz-Sala, 2021; Justamante et al., 2019).
Compounds such as Indole-3-butyric acid (IBA) and 1-Naphthaleneacetic acid (NAA) play a critical role in generating AR when applied exogenously to cuttings (Hac-Wydro and Flasinski, 2015; Gonin et al., 2019). The IBA-containing preparation of Rhizopon and the water IBA solution positively affected the degree of rooting and the percentage of rooted cuttings in deciduous leafy shrubs (Pacholczak et al., 2016a). Loconsole et al. (2022a) experimented to improve the cutting propagation protocol and rooting quality of wild sage (Lantana camara) and glossy abelia (Abelia x grandiflora) treated with 0, 1,250, 2,500, and 5,000 mg L−1. Results showed that the species had remarkable sensitivity to IBA dosage, mostly for AR quality, reaching the best results with 5,000 mg L−1 IBA in L. camara ‘Yellow’ (increased root number, total length, surface area and the number of forks and crossings), and 1,250 mg L−1 A. x grandiflora ‘Edouard Goucher’.
More than 30 commercial products containing IBA have been registered by the United States Environmental Protection Agency for utilization on fruit, vegetable, and ornamental crops, which are categorized as biochemical pesticides (United States Environmental Protection Agency, 2022). IBA hazardous information (Globally Harmonized System of Classification and Labelling of Chemicals GHS Classification, US), directed to operators in the nursery sector, is represented by acute toxicity (Category 3), skin corrosion (Category 2), eye irritation (Category 2A), acute aquatic toxicity (Category 3), chronic aquatic toxicity (Category 3).
2 Biostimulants in sustainable agriculture
In sustainable agriculture, the substitution of chemical inputs, such as growth regulators too, with alternative natural eco-friendly products presents a key challenge (Bulgari et al., 2020; Malik et al., 2021; Carillo et al., 2022). Biostimulants could form part of a solution to mitigate such risks deriving from the use of agrochemicals (Lucini et al., 2020; Loconsole et al., 2022b). Several concepts have been proposed to define plant biostimulants, referred to as eco-friendly because they are generally considered to be non-toxic, non-polluting, biodegradable, and non-hazardous (Yakhin et al., 2017). Biostimulants are either organic, inorganic, or microbial substances, that can enhance in small amounts, the growth, yield and quality of crops (Rouphael and Colla, 2018; Sible et al., 2021; Kisvarga et al., 2022; Wei et al., 2022; Ganugi et al., 2023; Kaushal et al., 2023; Rouphael et al., 2023). They also provide anti-stress effects (Ambrosini et al., 2021; González-Morales et al., 2021; Franzoni et al., 2022; Jacomassi et al., 2022; Ma et al., 2022; El-Nakhel et al., 2023; Zhang et al., 2023). Li et al. (2022) reported a comprehensive meta-analysis on non-microbial biostimulants of over one thousand pairs of open-field data in a total of 180 qualified studies worldwide. They are frequently used in horticulture (Baltazar et al., 2021) but rarely in plant propagation. In 2014, Calvo defined biostimulants as any substances or microorganisms that benefit the plant (Calvo et al., 2014), while Du Jardin et al. stated, in 2015, that the definition is based on what they are not, rather than what they are; for instance, fertilizers and pesticides increase plant yields but do not fall into the biostimulants category. Most biostimulants are complex mixtures and on this basis (Povero et al., 2016), two definitions have been given and both share the requirement that the mode of action must be unknown but differ in the fundamental assumption that the function of the biostimulant is a consequence of the discrete components (Carletti et al., 2021; Pandey et al., 2022) or a consequence of the ‘emergent’ properties of the biostimulant. Therefore, Yakhin et al. (2017) have developed a third concept that integrates the two previous concepts ‘a formulated product of biological origin that improves plant productivity as a consequence of the novel, or emergent properties of the complex of constituents, and not as a sole consequence of the presence of known essential plant nutrients, plant growth regulators, or plant protective compound’.
In this review, the authors classified biostimulants into eight groups: (1) PGPR beneficial bacteria, (2) AMF beneficial fungi, (3) SE Seaweed extracts; (4) Ple Plant extracts, (5) PH protein hydrolysates and other nitrogen-containing compounds, (6) HFA humic acid and fulvic acid, (7) Ch chitosan and other biopolymers, and (8) Si silicon, having as references papers of du Jardin (2015) and Yakhin et al. (2017).
Actually, “Fertilising Products Regulation (2019/1009)” represents a huge step forward for plant biostimulants (European Commission, 2019): for the first time, EU law recognizes biostimulants, and there is a common definition across all the Member States. The agricultural sector has been challenged to increase productivity to feed the growing global population while reducing adverse impacts on ecosystems and human health (Rouphael and Colla, 2020). One solution to reduce chemical use, without compromising crop quality and production (Li et al., 2022), could be by using biostimulants (Zulfiqar et al., 2020), even if their effects and mechanisms of action are still not fully understood (Toscano et al., 2018). The recent Green Deal Europe guidelines will probably increase the research and innovation in the biostimulants sector (Corsi et al., 2022).
Despite significant advancements in stem-cutting propagation, insufficient rooting efficiency remains an economic burden for the horticultural nursery industry (Ahkami et al., 2009). The current knowledge of the use of commercial SE products applied to ornamental cutting propagation has not been summarized until now.
Therefore, we conducted a systematic review to update evidence presented in scientific articles from 2011 to 2023: we hypothesize that commercial SE-based biostimulant products application to ornamental stem cuttings improves AR formation in terms of rooting percentage, root number, and overall quality of rooted cutting.
The authors chose SE-based biostimulants because they have been proven to have an extremely low carbon footprint (Ghosh et al., 2015); moreover, they are expected to account for more than 33% of the global market for biostimulants and reached a value of 894 million Euros by 2022 (El Boukhari et al., 2020).
The treated areas in this review could fill the gaps in knowledge on the SE applications in clonal propagation by cutting. It is undoubtedly thanks to the reviews of Kisvarga et al. (2022) on the use of biostimulants in ornamental greenhouse production (i.e. micro propagated orchids, endangered Brazilian, gladioli and seed-sown species) and Parađiković et al. (2019) on pot wild roses, marigold, and zinnia that ornamental crops have the place they deserve in the general landscape of biostimulants in horticulture.
3 Methodology
This review focuses on ornamental commercially important crops (Table 1). Demand for ornamentals has been increasing lately, due to the dynamic development of urban infrastructure (Francini et al., 2022). Green areas have become an inseparable element of modern settings. In response to this trend, ornamental nursery production has been increasing to the point of becoming the most profitable branch of ornamental horticulture.
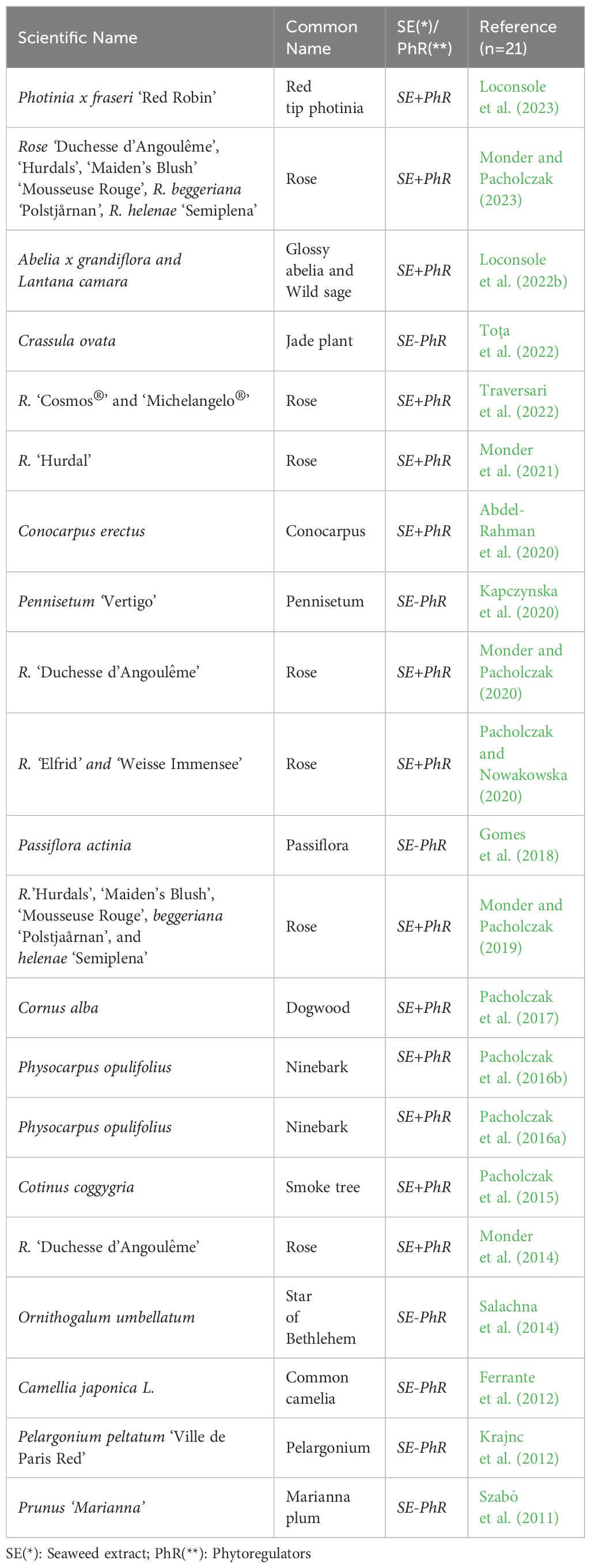
Table 1 List of references regarding ornamental commercially important crops propagated by cutting using SE-based biostimulants with (+) or without (-) phytoregulators (PhR). (n=21).
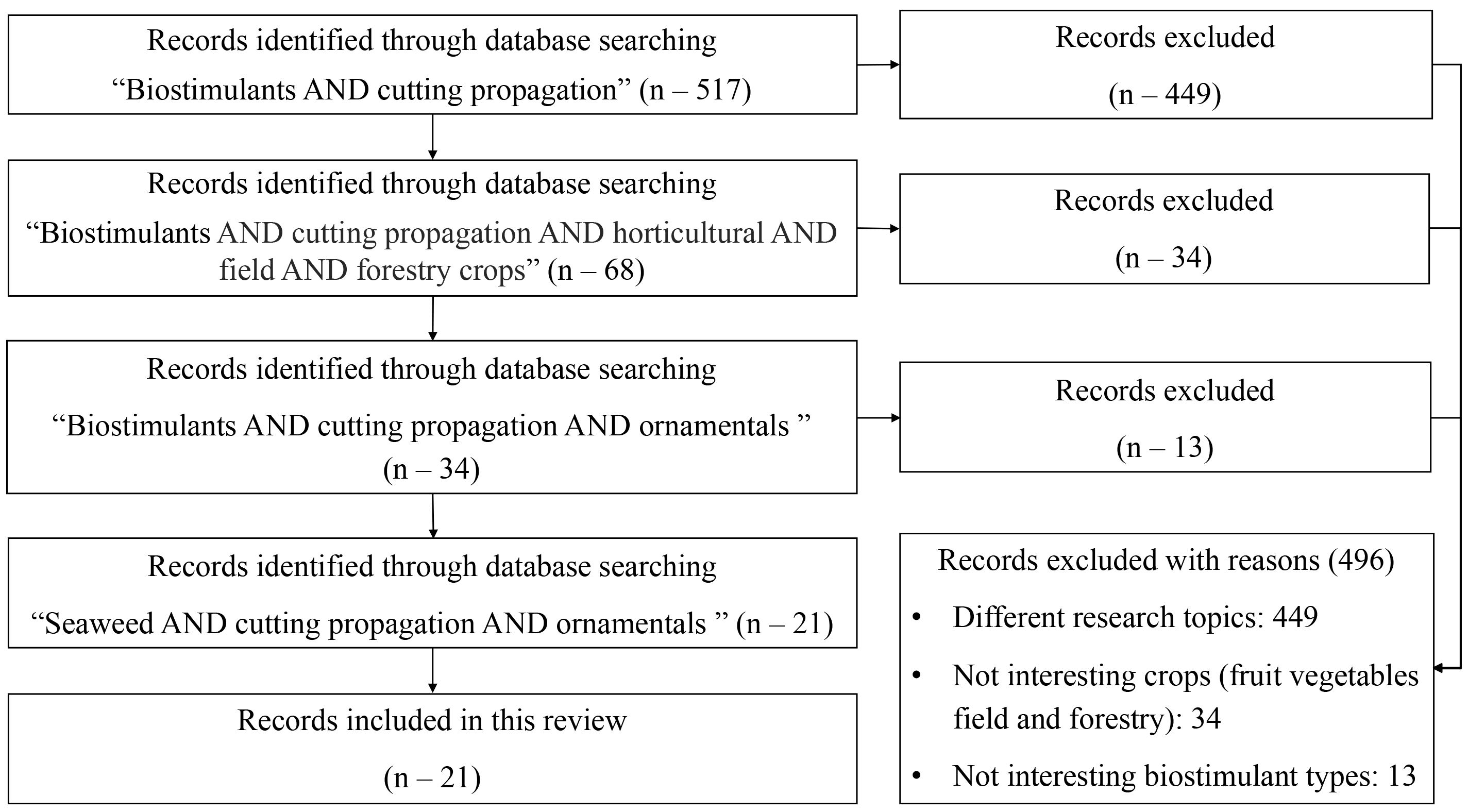
The applied methodology, shown in Figure 1, was divided into five successive phases:
1. Formulation of the problem: Despite significant advancements in stem-cutting propagation, insufficient rooting efficiency remains an economic burden for the floricultural nursery industry.
2. Objectives: to describe: (i) SE-based biostimulants; (ii) technical information on five commercial SE-based products: Goteo®, Kelpak®, AlgaminoPlant, Bio Rhizotonic, Actiwawe and some others less known also used as phytoregulators substitutes; (iii) applied protocols: crop, cutting type, commercial SE product, dose, application method, number of treatments; (iv) effects of applied protocols on rooting rate, root architecture and overall quality.
3. Keyword identification: Thomson Reuters’ Web of Science, Elsevier’s Scopus, and Google Scholar were queried until November 2023 for published scientific publications, written in English and identified using the following keywords: in the first step, “biostimulants AND cutting propagation AND horticulture AND field AND forestry crops” (n=68); in the second: “biostimulants AND cutting propagation AND ornamentals” (n=34), in the third: “Seaweed extract biostimulants AND cutting propagation AND ornamentals” (n=21) through the process of identification of bibliographical references and discarding those irrelevant.
4. Evaluation by title/keywords/abstract: n=21 (Supplementary Table 1).
5. Critical reading and text analysis of papers
The processing of bibliographic data shows that ornamental crops constitute as much as 45% of all those propagated by stem cuttings applying biostimulants (Figure 2). Figure 3 focuses on the comparison between the different types of biostimulants mentioned in the cutting propagation protocols; SE has higher relative frequency values both in horticultural (field, forest, fruit, vegetable and ornamental crops) and ornamental crops.
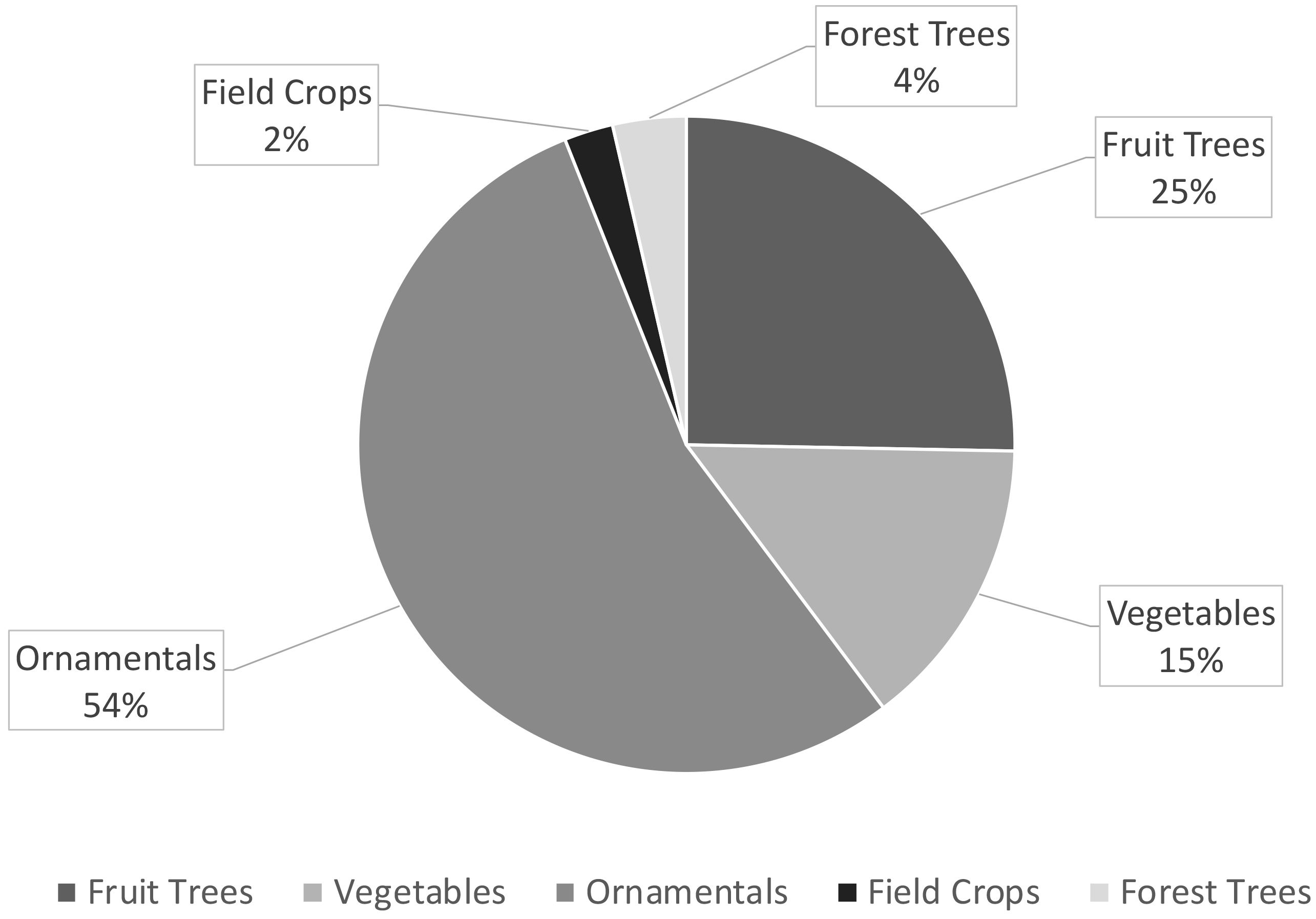
Figure 2 Relative frequency (%) of different crop categories treated with biostimulants in the cutting propagation protocols.
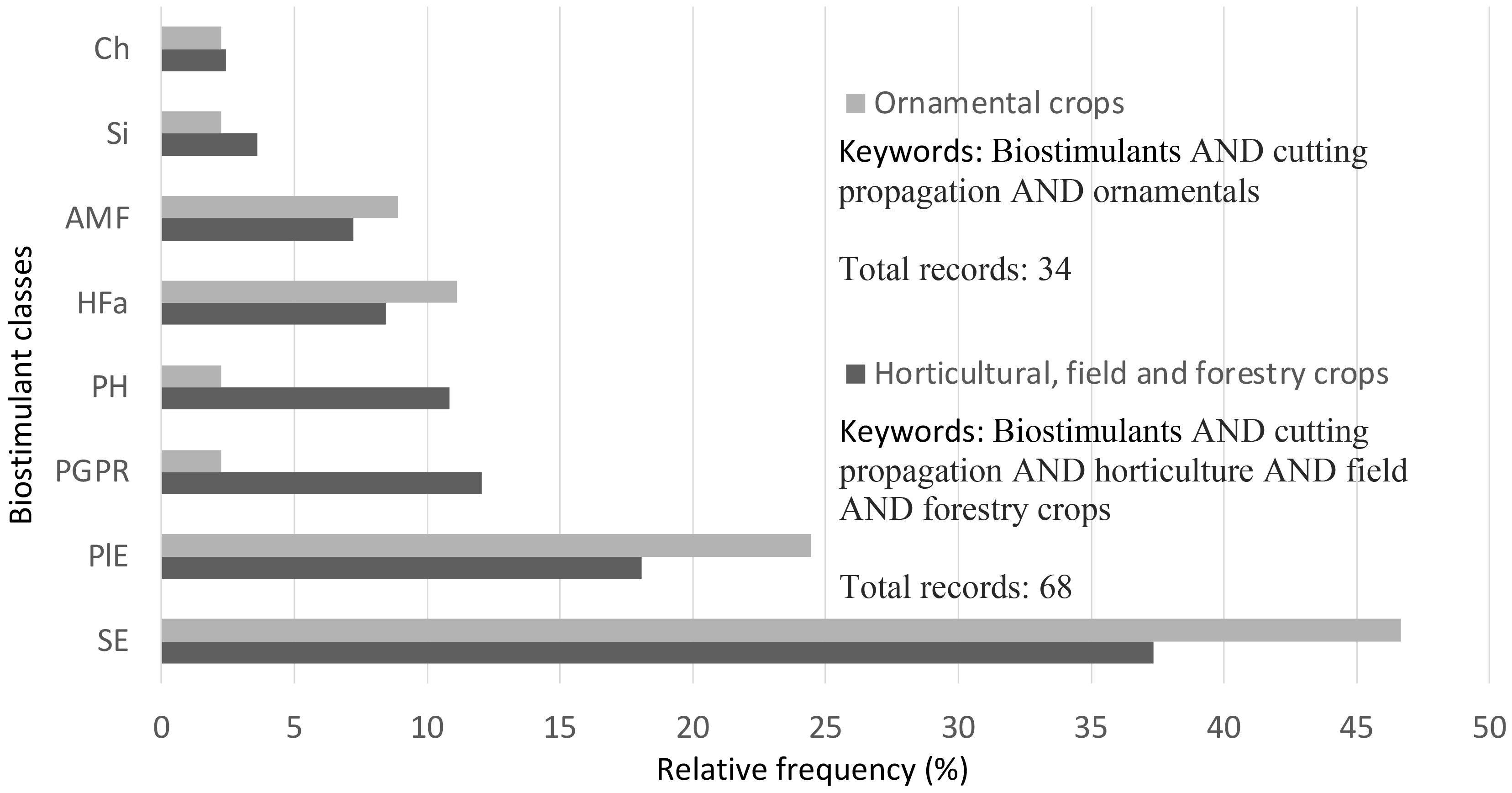
Figure 3 Relative frequency (%) of biostimulant classes used in the cutting propagation protocols: comparison between ornamental and horticultural crops.
4 SE-based biostimulants
Seaweeds are a diverse group of organisms, with approximately 9,000 species of red, brown, and green seaweeds identified. Among these groups, brown seaweeds (Phaeophyta) are commonly used for commercial extract manufacturing in agriculture (Atzmon and van Staden, 1994) and horticulture (Khan et al., 2009), due to their high content of macro- and micronutrients, amino acids, polyunsaturated fatty acids, vitamins, and polysaccharides such as alginates, fucoidans, laminarians, lichenan-like glucans, and fucose containing glucans (Khan et al., 2009; Battacharyya et al., 2015; Shukla et al., 2016). The biostimulant function of SE is commonly associated with the content of phytohormone, such as cytokinins, auxin, gibberellin and other hormone like compounds (Petropoulos, 2020; Stirk et al., 2020; Ali et al., 2021; Cardarelli et al., 2024). These compounds can act directly (i.e. exogenous apport) or indirectly [i.e. stimulation of genes expression and promoting the endogenous biosynthesis of auxin, cytokinins and gibberellins (Ali et al., 2021)], stimulating plant growth and development (Jannin et al., 2013; Stirk and Van Staden, 2014; Elansary et al., 2017) and improving nutrient uptake (González et al., 2013). All these components have a synergistic effect in the crops, providing a strong root system, promoting plant growth, and improving leaf development and flowering (Mukherjee and Patel, 2019; Valverde et al., 2022).
It is crucial to emphasize that biostimulants derived from SEs do not constitute a homogeneous category of products. The characteristics of SE can vary significantly based on factors such as the family and species of seaweed utilized in the manufacturing process (e.g., brown, green, or red seaweed), the source of the seaweed raw material, and the specific extraction methods employed (Fletcher et al., 2017). Commercial brown SEs are a variable mixture of Ascophyllum nodosum, Ecklonia maxima, Fucus, Laminaria, Sargassum, and Turbinaria spp. (Craigie, 2011; Sharma et al., 2012; Górka et al., 2018). Biostimulants derived from A. nodosum extract have demonstrated various positive effects on fruit and vegetable crops: plant vigor, increased root development, enhanced chlorophyll synthesis, promotion of earlier flowering, enhancement of fruit set and uniformity, delayed senescence, and increased tolerance to abiotic stress (Shukla et al., 2019). SE application can introduce microbial communities at the point of inoculation; on the other hand, foliar application to vegetative growth stages is aimed at stimulating signal tracts to reduce abiotic stresses (Adedayo and Babalola, 2023; Krawczuk et al., 2023). These beneficial outcomes highlight the potential of A. nodosum extract biostimulants in contributing to overall plant health and productivity (Łangowski et al., 2019).
In terms of biological activity on plants, the main known elicitors from seaweeds are cell wall polysaccharides that also contain a wide range of organic and inorganic molecules that are known to contribute to their biostimulant activity (Magnabosco et al., 2023). Their application helps in promoting physiological actions like photosynthesis, nutrient metabolism, enzymatic activities, chlorophyll, and carbohydrate content. And consequently, improve the rooting process. It is known that during rhizogenesis, carbohydrates are an important source of energy for plant tissues. Furthermore, a significant portion of sucrose is converted into starch, which is believed to be the primary carbon source for the growth of AR (Lohr et al., 2017).
5 Commercial SE-based products as an example
Analysis of the 21 references (Supplementary Table 1) showed that there were 10 commercial SE-based products used in the trials.
Supplementary Figure 1 shows that, in our reference list (n=21), the most widely used commercial SE-based products in the propagation of ornamental plants turn out to be Goteo (23%), AlgaminoPlant (19%), Bio Rhizotonic (19%), Kelpak (15%) and others (4%). Supplementary Figure 2 illustrates the distribution of other commercial biostimulant categories, non-SE-based: Bio Roots and RootJuice (Ple) are the those with the highest percentage of use. Among phytoregulators, IBA is the most widely used in cutting propagation (60%), followed by NAA (25%) (Supplementary Figure 3). The dose indicated on the label of the following commercial SE-based products is missing for the ornamental crops: Goteo®, Kelpak®, AlgaminoPlant, BioRhizotonic.
Goteo® (Goteo – Goactiv, UPL, Cesena, Italy) is a commercial liquid seaweed-based biostimulant, containing GA142, a filtrate from the seaweed A. nodosum, a source of auxins and cytokinins, polysaccharides and vitamins (Stępowska, 2008; Francke et al., 2022). In the preparation, GA142 is added with organo-mineral fertilizers (w/v): 13% P2O5, 5% K2O, and 1.3–2.4% organic substances (Loconsole et al., 2022b).
Kelpak® (Kelp Products Pty Ltd, Simon’s Town, South Africa) (Table 2) is a liquid concentrate derived from the stipes and laminae of the brown kelp, E. maxima (Osbeck) Papenfuss (Crouch and Van Staden, 1991). It is commercially used as a plant growth stimulator reducing nursery periods before out-planting and increasing the yield and quality of a variety of terrestrial crops (Crouch and Van Staden, 1991; Al-Hawezy, 2015; Kocira et al., 2018). It is also known to aid in producing stronger and healthier crops by enhancing root formation and reducing transplant shock resistance.
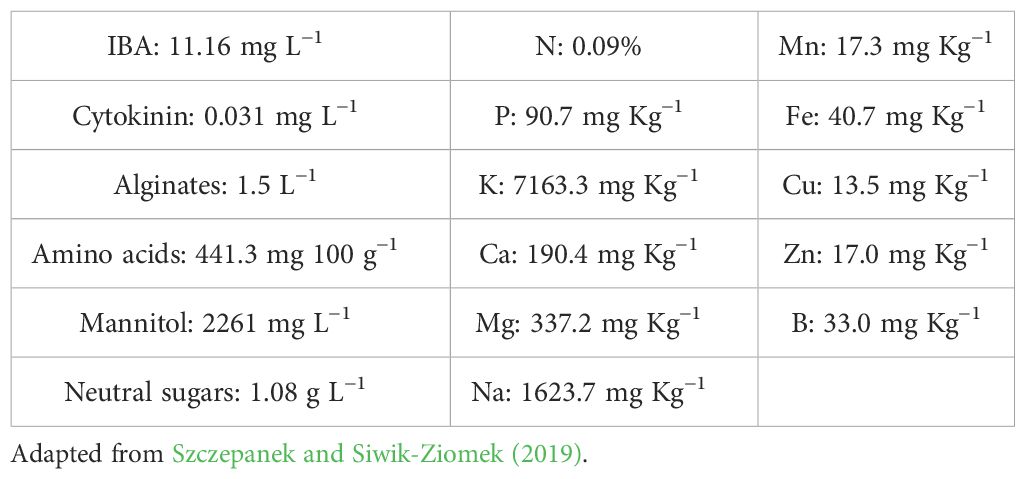
AlgaminoPlant (Varichem, Poland), is a liquid preparation composed of SE derived from Sargassum, Laminaria, Ascophyllum, and Fucus at 18% concentration. It incorporates phytohormones with gibberellin-like activity equivalent to 0.005% gibberellic acid (GA3), cytokinin activity equivalent to 0.0005% benzyl adenine (BA), and auxin-like activity corresponding to 0.003% IAA. Additionally, it is enriched with potassium salts of amino acids at a concentration of 10% (Matysiak et al., 2010).
Bio Rhizotonic (Canna Continental, Los Angeles, USA), identified as an algae-based rooting liquid stimulator, includes vitamins such as B1, B2, and N-P-K at a ratio of 0.6–0.2-0.6 (Monder and Pacholczak, 2020).
Actiwave® (Valagro SpA., Atessa, Italy) is a SE product derived from A. nodosum and its three major components are kahydrin, alginic acid, and betaine. Its composition includes organic carbon (12%), organic N (1%), Total N (3%), N ureic (2%), K2O (7%) at 6.4 pH. The dose indicated on the label is 300–500 mL 100 L−1 for the ornamental crops.
6 Applied protocols with commercial SE-based products
‘The ability to demonstrate that a product is indeed a bona fide biostimulant will depend on a demonstration of its effect’ (Ricci et al., 2019).
6.1 Goteo®
Goteo® has been observed to stimulate the greenhouse development of the Ornitogalum bulb’s twin scales, excised from the outer and inner of the parent bulb and soaked for 30 minutes in 0.2% solution of Goteo® (Salachna et al., 2014). The study revealed that exciting twin scales from the outer layer of the parent bulb led to a greater formation of adventitious bulbs that exhibited higher fresh weight and circumference, as well as a greater number and length of roots.
Although not in a greenhouse protocol comparing SE-based biostimulants with IBA, Kapczynska et al. (2020) found that in Pennisetum ‘Vertigo’ cuttings Goteo®, at a dose of 0.1%, stimulated AR development, having a significant impact on root elongation, regardless of whether peat or perlite was used as the rooting medium. Watering the cuttings with Goteo® 5 times every 5 days proved to be highly effective, achieving a 100% success rate, compared to soaking the 2 cm cuttings base for 20 min and applying foliar Goteo spraying 5 times every 5 days. For each Goteo treatment, a control was set up in which the cuttings were treated as follows: soaking with water, watering with water and spraying with water. Furthermore, Goteo® watering stimulated the generation of new shoots during greenhouse cultivation. The application of Goteo® resulted in an increased phosphorus and potassium content. Khan et al. (2009) observed a beneficial effect of plant P nutrition because of the application of SE-based biostimulants.
Loconsole et al. (2022b) investigated the effects of Goteo® compared to IBA application, in the process of rhizogenesis of two ornamental shrubs, Lantana camara and Abelia x grandiflora, in a propagation greenhouse. The treatments applied to semi-hardwood stem cuttings were as follows: control (distilled water as spray); IBA (Sigma, St. Louis, MO, USA) solution at concentration 1,250 mg L−1; Goteo® concentrations at 1, 2, and 3 mL L−1 as spray application at two-week intervals, totaling three applications. Regarding the concentration, the company recommends 0.1% solution (1 mL L−1) on vegetable crops and does not provide a dosage for ornamentals. On the other hand, Gajc-Wolska et al. (2012) recommended the dosage for hastening the rooting system regeneration as being equal to three to four treatments with a 0.2% solution (2 mL L−1) every 2 weeks. SE biostimulant was found to be effective in enhancing rooting percentage as well as in root architecture.
Findings revealed that Goteo® at a dose of 3 mL L−1 in wild sage and 1 mL L−1 in glossy abelia, Goteo® produced similar results to those obtained with IBA regarding rooting percentage (in lantana: IBA= 91%, Goteo® 3 mL L−1 = 87%; in abelia: IBA= 93%, 1 mL L−1 = 90%). Moreover, the stimulator was successful in promoting the growth of many roots and enhancing architecture parameters when compared to IBA. Better results were found in wild sage at a dose of 3 mL L−1 compared to IBA treatment, regarding root length (+27%), surface area (+47%), diameter (+22%), number of tips (+41%), forks (+61%), and crossings (+31%). In A. × grandiflora, on the contrary, the applications of IBA and Goteo® 1–2 mL L−1 determined the greatest root length (391, 396 and 336 mm, respectively) and root surface area values (67, 70 and 61 mm2, respectively).
In addition to observing that SE-based biostimulant had a beneficial effect on AR and shoot quality of cuttings, Pacholczak and Nowakowska (2020) also noted, in two ground cover roses ‘Elfrid’ and ‘Weisse Immensee’ under a plastic tunnel, those on both chlorophyll contents (chlorophyll a+b) and soluble sugars (Supplementary Figures 4, 5). The goal of their experiment was to compare the effects of Goteo® and AlgaminoPlant, applied at a concentration of 0.2% for foliage spraying, to IBA (Rhizopon 1% or water solution 200 mg L−1). AlgaminoPlant and Goteo® treated cuttings, compared to the control, led to an increase in chlorophyll content by 23% and 28% in Elfrid and by 19% in Weisse Immensee, respectively. Compared to untreated cuttings, those treated with Goteo® (0.2%) had the highest values of total soluble sugars and chlorophyll compared to those treated with Rhizopon (1% IBA) or water solution.
Monder et al. (2014) and Monder and Pacholczak (2018) in rose cuttings showed the positive impacts of IBA and SE-based biostimulants on sugar levels, vital for effective rhizogenesis since the effectiveness of photosynthesis in cuttings is low. AR formation is a highly energetic process; the capability of young cutting to produce carbohydrates both as storage starch and ready-to-use simple sugar forms is fundamental.
6.2 Kelpak®
Leafy shoot cuttings are exposed to several environmental stresses during rooting. Szabó et al. (2011) tested, on stockplants of Prunus ‘Marianna 8-1’ raised in the open field, two biostimulants: Kelpak® (0.2%) and Wuxal Ascofol® (0.2%, containing A. nodosum extract) a PGPR-based biostimulant, Pentakeep®V (0.03%), containing 5-amino-levulic-acid, and untreated control. applied three times before cutting. Kelpak® and Wuxal Ascofol® increased the number and the weight of shoots, the weight of cuttings and the leaf chlorophyll content. Therefore, shoots of treated stock plants reached earlier the optimum size for cutting propagation. Application of Kelpak® resulted in the highest rooting rate and increased the fresh weight of cuttings during rooting surpassing both control and cuttings treated with the other two preparations tested.
Maintaining green, functionally active leaves during root induction is crucial since root formation depends on adequate carbohydrate supply from the cutting’s source leaves to the region of root regeneration.
Traversari et al. (2022), aimed to test the replacement of synthetic phytoregulators, such as auxins and brassinosteroids, with a commercial SE, for AR formation, analyzed in greenhouse, ‘Michelangelo®’ and ‘Cosmos®’ rose cuttings, tested with five different treatments: distilled water (control), 10% Kelpak® and 10% Phylgreen in distilled water, 4000 ppm IBA and NAA in distilled water; 5 ppm of 22(S),23(S)-homobrassinolide in distilled water. Results showed that Kelpak® improved rooting percentage and root biometric parameters in both cultivars. Moreover, root fresh weight was significantly higher under both auxin and Kelpak® treatments, with increases of +99% and +62%, respectively. Additionally, root length demonstrated notable enhancements under auxin and Kelpak® treatments in the ‘Michelangelo®’, with increases of +103% and +75%, respectively, compared to the control.
Loconsole et al. (2023) observed that, in Photinia x fraseri ‘Red Robin’ cuttings, Kelpak® and Goteo® at the doses of 2 and 3 mL L−1 applied as foliar spray four times in greenhouse propagation, stimulated the production of callus in over 80% of cuttings treated, whereas IBA produced the highest rooting percentage. Moreover, it appears that the abundant callus tissue production hinders the rooting, creating a structural barrier to the development of AR. These results are consistent with those of Monder and Pacholczak (2017) and Monder et al. (2021), who found that in the rhizogenesis of the ‘Hurdal’ rose, an increase in rooting percentage was only observed when the percentage of cuttings with callus decreased.
6.3 AlgaminoPlant and Bio Rhizotonic
Pacholczak et al. (2017) reported a comparable effect on the rooting potential of cuttings of Cornus alba ‘Aurea’ and ‘Elegantissima’, treated with AlgaminoPlant at 0.2% water solution with cuttings treated with Rhizopon AA (1% IBA) and IBA 200 mg L−1.
Pacholczak et al. (2016b) reported a beneficial impact of AlgaminoPlant (0.2%), on the rooting of ninebark cuttings (Physocarpus opulifolius), sprayed once, twice or three times during the rooting period, compared to Rhizopon AA (2% IBA) and a water solution of 200 mg dm−3 IBA. The control cuttings were sprayed with distilled water. Two nodal stem cuttings were rooted in styrofoam boxes with a mixture of peat, perlite and sand in a greenhouse propagation. The trial was repeated in two years (2012 and 2013). A triple treatment with AlgaminoPlant was observed to increase the percentage of rooted cuttings by 36% (2012) and 26% (2013) compared to the control and showed comparable or slightly weaker effects to the treatments with synthetic IBA.
Pacholczak and Nowakowska (2020) compared the effects of AlgaminoPlant, Goteo® and Rhizopon (1% IBA) on cuttings in two ground cover roses Elfrid’ and ‘Weisse Immensee’ growth under a plastic tunnel. SEs were applied by foliar spraying at a concentration of 0.2%. The experiment’s findings revealed that AlgaminoPlant exerted a positive influence on rhizogenesis in both cultivars, comparable to or only slightly less potent than those induced by the IBA. Furthermore, the application of Goteo® led to an increase in the content of chlorophyll and total soluble sugars in cuttings. On the other hand, the levels of free amino acids and polyphenolic acids were reduced under the influence of Goteo®.
The research carried out by Monder et al. (2021) focused on the response of stem cuttings of Rosa ‘Hurdal’ treating the cuttings with Bio Rhizotonic and showed that the anatomical structure of cuttings was influenced by plant-derived biostimulants.
Historical roses are especially difficult to propagate, with the process of rhizogenesis lasting for a minimum of 12 weeks and connected with low quality of rooted cuttings. Monder and Pacholczak (2023) pointed out that, on six aged, once-flowering rose cultivars (‘Duchesse d’Angoulême,’ ‘Hurdals,’ ‘Maiden’s Blush,’ ‘Mousseuse Rouge,’ Rosa beggeriana ‘Polstjårnan,’ R. helenae ‘Semiplena’) treated with Bio Rhizotonic, the polyphenolic acid content decreased during the rhizogenesis period.
6.4 Other SE-based products
Ferrante et al. (2012), pointed out an experiment on cuttings of Camellia japonica L. using Actiwave® (Valagro Spa) at the doses of 0.015 mL and 0.03 mL per application, for a total of eight applications per month, directly on the substrate. The effect of the biostimulant on the rooting of cuttings was evaluated by comparing it with applications of gibberellic acid (GA3) at doses of 1.25 mg and 2.5 mg. These doses were administered at identical intervals of Actiwave®, with nebulization on both the leaves and the substrate of the cutting, resulting in a cumulative application of 10 and 20 mg. After 127 days, the rooting percentage for cuttings treated with 0.12 ml/L Actiwave® was 82%, contrasting with the control group, which exhibited a rooting percentage of 18%. By the end of the experiment (161 days), the rooting value for the Actiwave®-treated cuttings further increased to 88%, while the control group showed a rooting percentage of 55%.
Toța et al. (2022) carried out a study on Crassula ovata clonal propagation, comparing several biostimulants, including Raiza, a liquid product containing bioactive substances extracted from A. nodosum, 20 free amino acids including proline and serine, phytohormones including cytokinins, auxins and gibberellins. The experimental protocol lacks in SE doses and number of applications.
Abdel-Rahman et al. (2020) assessed the impact of a commercial SE-based biostimulant (Tera at 3 mL L−1 or 10 mL/pot), IBA, both independently and in combination with PGPR (Agrobacterium rhizogenes) at 108 CFU mL−1, and Ple biostimulants (coconut water), on the root quality of cuttings in Conocarpus erectus L. The treatments were administered as follows: for IBA, at 100 ppm, the basal ending of cuttings was soaked for 20 hours at the start of the trial; PGPR were inoculated in the rooting substrate before sticking cuttings; Tera was sprayed (3 mL L−1) or drenched (10 mL pot−1) once every 5 days for three times in total. Finally, for coconut water, the basal ending of the cuttings was soaked for either 3 min or 1 h before planting.
The findings revealed that all treatments involving IBA and/or biostimulants significantly increased the rooting percentage, as well as the root and vegetative characteristics of the rooted cuttings when compared to untreated cuttings. Notably, the individual applications of seaweed extract and coconut water demonstrated greater efficacy than IBA or A. rhizogenes alone. Additionally, treatments involving seaweed extract as a drench, with or without IBA, outperformed those of seaweed extract treatments as a spray.
Gomes et al. (2018) tested a commercial SE product (name not given) manufactured by Acadian Seaplants® in the greenhouse, to assess its impact when applied to the bases of P. actinia stem cuttings. Five concentrations of the extract in distilled water were tested: 0, 10, 20, 30, and 40%. Stem cuttings were dipped for 2 min in solution before planting. On average, a rooting percentage of 51% was achieved. The rooting percentage exhibited a linear increase in correlation with the concentrations of the brown seaweed extract. In comparison to the control treatment, a notable 10% increase in rooting was observed with the 40% seaweed extract treatment.
7 Conclusions
The ornamental’s worldwide production has increased in recent years due to their great use in urban parks and green areas, ensuring ecosystem services. Despite significant advancements in stem-cutting propagation, a complicated process that starts from high-quality rooted cuttings, insufficient rooting efficiency remains an economic burden for the ornamental nursery industry. Synthetic phytoregulator as IBA, with a high carbon footprint, when applied exogenously to cutting, however, enhances AR formation.
In sustainable agriculture, the substitution of chemical inputs, with alternative natural eco-friendly products presents a key challenge. Biostimulants, renewable sources, can form part of a solution to mitigate such risks deriving from the use of agrochemicals.
This review shows the positive effects of the main commercial SE-based products on AR formation and quality of rooted cutting, highlighting their effectiveness on rooting percentage, root number and architecture. Moreover, they increase the overall quality of a rooted cutting as dry biomass and organic compound content.
The findings show that the effects, unfortunately, are species-specific and product-specific, depending on: (i) the type of seaweed resource; (ii) the quality and composition of the extract; (iii) the method, concentration, and frequency of application.
Especially, when the row material comes from macroalgae, it turns out to be extremely complex, to identify the numerous components exerting a role in the biostimulation mechanism. Despite the intricate nature of this product, identification of its bioactive ingredients is critical to building trust and credibility in its commercialization.
To conclude, it is possible to state that:
- The same dose can prove effective if administered with one SE commercial product and ineffective with another (i.e. Kelpak vs Phylgreen);
- The same dose should be optimized with the species and cultivar (i.e. Goteo® at a dose of 3 mL L−1 in wild sage and 1 mL L−1 in glossy abelia);
- The optimal dose depends on the growing season too: as occurred in ‘GiSelA 5’ softwood cuttings;
- SEs as a drench with or without IBA surpassed those of SE treatments as a spray (i.e. in C. erectus cuttings);
- Where no relationships exist between the type of cutting (herbaceous or woody, top or branch) and the most suitable biostimulants, need to operate empirically.
This review also shows that the application of SE-based biostimulants in replacement of IBA can ensure results of comparable quality in ornamental cutting propagation.
Therefore, it will be necessary to create newly standardized protocols to validate and give credibility to the effects of SE-based biostimulants applied to clonal multiplication.
The specific application of other biostimulants, such as AMF and PGPR, can be useful in the sector of ornamentals to pursue research.
Author contributions
DL: Conceptualization, Writing – original draft, Writing – review & editing. ES: Writing – original draft. AS: Writing – original draft. GC: Writing – original draft, Writing – review & editing. BD: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The authors declare that this study received funding from Apulia Region (Italy). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fhort.2024.1371090/full#supplementary-material
References
Abdel-Rahman S., Abdul-Hafeez E., Saleh A. M. (2020). Improving rooting and growth of conocarpus erectus stem cuttings using indole-3-butyric acid (IBA) and some biostimulants. S.J.F.O.P. 7, 109–129. doi: 10.21608/sjfop.2020.96213
Adedayo A. A., Babalola O. O. (2023). The potential of biostimulants on soil microbial community: a review. Front. Ind. Microbiol. 1. doi: 10.3389/finmi.2023.1308641
Ahkami A. H., Lischewski S., Haensch K. T., Porfirova S., Hofmann J., Rolletschek H., et al. (2009). Molecular physiology of adventitious root formation in petunia hybrida cuttings: involvement of wound response and primary metabolism. New Phytol. 181, 613–625. doi: 10.1111/j.1469-8137.2008.02704.x
Al-Hawezy S. M. N. (2015). The use of Kelpak to improve the vegetative growth of loquat (Eriobotya Jappanica L.) seedling. Zanco J. pure Appl. Sci. 27, 1–4. doi: 10.21271/zjpas.v27i6.334
Ali O., Ramsubhag A., Jayaraman J. (2021). Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 10, 531. doi: 10.3390/plants10030531
Ambrosini S., Sega D., Santi C., Zamboni A., Varanini Z., Pandolfini T. (2021). Evaluation of the potential use of a collagen-basedProtein hydrolysate as a plantMulti-stress protectant. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.600623
Assis T. F., Fett-Neto A. G., Alfenas A. C. (2004). ““ Current techniques and prospects for the clonal propagation of hardwoods with emphasis on eucalyptus,” in Plantation forest biotechnology for the 21st century. Eds. Walter C., Carson M. (Research Signpost, Thiruvananthapuram, India), 1109–1119.
Atkinson D. (2000). “Root characteristics: why and what to measure,” in Root methods. Eds. Smit A. L., Bengough A. G., Engels C., van Noordwijk M., Pellerin S., van de Geijn S. C. (Springer, Berlin, Heidelberg), 1–32. doi: 10.1007/978-3-662-04188-8_1
Atzmon N., van Staden J. (1994). The effect of seaweed concentrate on the growth of Pinus pinea seedlings. New For. 8, 279–288. doi: 10.1007/BF00025373
Baltazar M., Correia S., Guinan K. J., Sujeeth N., Bragança R., Gonçalves B. (2021). Recent advances in the molecular effects of biostimulants in plants: An overview. Biomolecules 11, 1096. doi: 10.3390/biom11081096
Baron D., Amaro A. C. E., Pina A., Ferreira G. (2019). An overview of grafting re-establishment in woody fruit species. Sci. Hortic. 243, 84–91. doi: 10.1016/j.scienta.2018.08.012
Battacharyya D., Babgohari M. Z., Rathor P., Prithiviraj B. (2015). Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 196, 39–48. doi: 10.1016/j.scienta.2015.09.012
Braun L., Wyse D. (2019). Optimizing IBA concentration and stem and segment size for rooting of hybrid hazelnuts from hardwood stem cuttings. J. Environ. Hortic. 37, 1–8. doi: 10.24266/0738-2898-37.1.1
Bulgari R., Cocetta G., Trivellini A., Ferrante A. (2020). Borage extracts affect wild rocket quality and influence nitrate and carbon metabolism. Physiol. Mol. Biol. Plants 26, 649–660. doi: 10.1007/s12298-020-00783-5
Cafourek J., Maděra P., Střítecký J., Adolt R., Smola M. (2021). Experimental Examination of Vegetative Propagation Methods of Nothofagus Antarctica (G. Forst.) Oerst. for Restoration of Fire-Damaged Forest iIn Torres del Paine National Park, Chile. Forests 12, 1238. doi: 10.3390/f12091238
Calvo P., Nelson L., Kloepper J. W. (2014). Agricultural uses of plant biostimulants. Plant Soil 383, 3–41. doi: 10.1007/s11104-014-2131-8
Cardarelli M., El Chami A., Rouphael Y., Ciriello M., Bonini P., Erice G., et al. (2024). Plant biostimulants as natural alternatives to synthetic auxins instrawberry production: physiologicaland metabolic insights. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1337926
Carillo P., Pannico A., Cirillo C., Ciriello M., Colla G., Cardarelli M., et al. (2022). Protein hydrolysates from animal or vegetal sources affect morpho-physiological traits, ornamental quality, mineral composition, and shelf-life of chrysanthemum in a distinctive manner. Plants 11, 2321. doi: 10.3390/plants11172321
Carletti P., García A. C., Silva C. A., Merchant A. (2021). Towards a functional characterization of plant biostimulants. Front. Plant Sci. 12. doi: 10.3389/978-2-88966-794-9
Corsi S., Ruggeri G., Zamboni A., Bhakti P., Espen L., Ferrante A., et al. (2022). A bibliometric analysis of the scientific literature on biostimulants. Agronomy 12, 1257. doi: 10.3390/agronomy12061257
Craigie J. S. (2011). Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 23, 371–393. doi: 10.1007/s10811-010-9560-4
Crouch I. J., Van Staden J. (1991). Evidence for rooting factors in a seaweed concentrate prepared from Ecklonia maxima. J. @ P. Phy. 137, 319–322. doi: 10.1016/S0176-1617(11)80138-0
Díaz-Sala C. (2021). Adventitious root formation in tree species. Plants 10, 486. doi: 10.3390/plants10030486
Druege U. (2009). “Involvement of carbohydrates in survival and adventitious root formation of cuttings within the scope of global horticulture,” in Adventitious root formation of forest trees and horticultural plants – from genes to applications. Eds. Niemi K., Scagel C. (Research Signpost, Kerala), 187–208.
Druege U. (2023). Facing the dynamic environment: a systemic perspective on the physiology of leafy cuttings. Acta Hortic. 1368, 93–102. doi: 10.17660/ActaHortic.2023.1368.13
Druege U., Hilo A., Pérez-Pérez J. M., Klopotek Y., Acosta M., Shahinnia F., et al. (2019). Molecular and physiological control of adventitious rooting in cuttings: phytohormone action meets resource allocation. Ann. Bot. 123, 929–949. doi: 10.1093/aob/mcy234
du Jardin P. (2015). Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 196, 3–14. doi: 10.1016/j.scienta.2015.09.021
Elansary H. O., Yessoufou K., Abdel-Hamid A. M. E., El-Esawi M. A., Ali H. M., Elshikh M. S. (2017). Seaweed extracts enhance salam turfgrass performance during prolonged irrigation intervals and saline shock. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00830
El Boukhari M. E. M., Barakate M., Bouhia Y., Lyamlouli K. (2020). Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants 9, 359. doi: 10.3390/plants9030359
El-Nakhel C., Cristofano F., Colla G., Pii Y., Lucini L., Rouphael Y., et al. (2023). and its fractions provide differential growth and modulate qualitative traits of lettuce grown under non-saline and mild salinity conditions. Sci. Hortic. 319, 112130. doi: 10.1016/j.scienta.2023.112130
European Commission (2019) Regulation of the european parliament and of the council laying down rules on the making available on the market of EU fertilising products and amending regulations (EC) no 1069/2009 and (EC) no 1107/2009 and repealing regulation (EC) no 2003/2003. Available online at: https://data.consilium.europa.eu/doc/document/PE-76-2018-INIT/en/pdf (Accessed December 19, 2023).
Ferrante A., Trivellini A., Vernieri P., Piaggesi A. (2012). Application of Actiwave® for improving the rooting of Camellia cuttings. Acta Hortic. 1009, 213–218. doi: 10.17660/ActaHortic.2013.1009.25
Fletcher H. R., Biller P., Ross A. B., Adams J. M. M. (2017). The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res. 22, 79–86. doi: 10.1016/j.algal.2016.10.015
Francini A., Romano D., Toscano S., Ferrante A. (2022). The contribution of ornamental plants to urban ecosystem services. Earth 3, 1258–1274. doi: 10.3390/earth3040071
Francke A., Majkowska-Gadomska J., Kaliniewicz Z., Jadwisieńczak K. (2022). No effect of biostimulants on the growth, yield and nutritional value of shallots grown for bunch harvest. Agronomy 12, 1156. doi: 10.3390/agronomy12051156
Franco J. A., Bañón S., Vicente M. J., Miralles J., Martínez-Sánchez J. J. (2011). Root development in horticultural plants grown under abiotic stress conditions -a review. J. Hortic. Sci. Biotechnol. 86, 543–556. doi: 10.1080/14620316.2011.11512802
Franzoni G., Cocetta G., Prinsi B., Ferrante A., Espen L. (2022). Biostimulants on crops: their impact under abiotic stress conditions. Horticulturae 8, 189. doi: 10.3390/horticulturae8030189
Gajc-Wolska J., Kowalczyk K., Nowecka M., Mazur K., Metera A. (2012). Effect of organic-mineral fertilizers on the yield and quality of endive (Cichorium endivia L.). Acta Sci. Pol. 11, 189–200.
Ganugi P., Caffi T., Gabrielli M., Secomandi E., Fiorini A., Zhang L., et al. (2023). A 3-year application of different mycorrhiza-basedplant biostimulants distinctively modulatesphotosynthetic performance, leafmetabolism, and fruit quality ingrapes (Vitis vinifera L.). Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1236199
Geiss G., Gutierrez L., Bellini C. (2009). Adventitious root formation: new insights and perspectives (Hoboken, NJ, USA: Wiley-Blackwell). doi: 10.1002/9781444310023.ch5
Ghosh A., Anand K. G. V., Anand Seth A. (2015). Life cycle impact assessment of seaweed based biostimulant production from onshore cultivated Kappaphycus alvarezii (Doty) Doty ex Silva—Is it environmentally sustainable? Algal Res. 12, 513–521. doi: 10.1016/j.algal.2015.10.015
Gianguzzi V., Barone E., Sottile F. (2020). In vitro rooting of Capparis spinosa L. as affected by genotype and by the proliferation method adopted during the multiplication phase. Plants 9, 398. doi: 10.3390/plants9030398
Gogna M., Kumar R., Tiwari L. D., Tailor A., Kumari A., Mehta S. (2022). Chapter 15 - Strigolactones: A new player in regulating adventitious root formation. Editor(s): Husen A. In Plant Biology, Sustainability and Climate Change, Environmental, Physiological and Chemical Controls of Adventitious Rooting in Cuttings (Academic Press), Academic Press, 343–366. doi: 10.1016/B978-0-323-90636-4.00004-0
Gomes E. N., Vieira L. M., Tomasi J. D. C., Tomazzoli M. M., Grunennvaldt R. L., Fagundes C. D. M., et al. (2018). Brown seaweed extract enhances rooting and roots growth on Passiflora actinia Hook stem cuttings. Ornam. Hortic. 24, 269–276. doi: 10.14295/oh.v24i3.1221
Gonin M., Bergougnoux V., Nguyen T. D., Gantet P., Champion A. (2019). What makes adventitious roots? Plants 8, 240. doi: 10.3390/plants8070240
González A., Castro J., Vera J., Moenne A. (2013). Seaweed oligosaccharides stimulate plant growth by enhancing carbon and nitrogen assimilation, basal metabolism, and cell division. J. Plant Growth. Regul. 32, 443–448. doi: 10.1007/s00344-012-9309-1
González-Morales S., Solís-Gaona S., Valdés-Caballero M. V., Juárez-Maldonado A., Loredo-Treviño A., Benavides-Mendoza A. (2021). Transcriptomics of biostimulation of plants under abiotic stress. Front. Gen. 12. doi: 10.3389/fgene.2021.583888
Górka B., Korzeniowska K., Lipok J., Wieczorek P. P. (2018). “The biomass of algae and algal extracts in agricultural production,” in Algae biomass: characteristics and applications, vol. 8 . Eds. Chojnacka K., Wiezorek P., Schroeder G., Michalak I. (Springer International Publishing, Cham, Switzerland), 103–114. doi: 10.1007/s10725-021-00794-6
Hac-Wydro K., Flasinski M. (2015). The studies on the toxicity mechanism of environmentally hazardous natural (IAA) and synthetic (NAA) auxin—The experiments on model Arabidopsis thaliana and rat liver plasma membranes. Colloids Surf. B Bio. 130, 53–60. doi: 10.1016/j.colsurfb.2015.03.064
Hartmann H. T., Kester D. E. (2002). Plant propagation: principles and practices (New Jersey, NJ: Prentice-Hall).
Jacomassi L. M., Viveiros J. D. O., Oliveira M. P., Momesso L., de Siqueira G. F., Crusciol C. A. C. (2022). A seaweed extract-based biostimulant mitigates drought stress in sugarcane. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.865291
Jannin L., Arkoun M., Etienne P., Laîné P., Goux D., Garnica M., et al. (2013). Brassica napus growth is promoted by Ascophyllum nodosum (L.) Le Jol. seaweed extract: microarray analysis and physiological characterization of N, C, and S metabolisms. J. Plant Growth Regul. 32, 31–52. doi: 10.1007/s00344-012-9273-9
Justamante M. S., Acosta-Motos J. R., Cano A., Villanova J., Birlanga V., Albacete A., et al. (2019). Integration of phenotype and hormone data during adventitious rooting in carnation (Dianthus caryophyllus L.) stem cuttings. Plants 8, 226. doi: 10.3390/plants8070226
Kapczynska A., Kowalska I., Prokopiuk B., Pawłowska B. (2020). Rooting Media and Biostimulator Goteo Treatment effect the adventitious root formation of Pennisetum ‘Vertigo’cuttings and the Quality of the Final Product. Agriculture 10, 570. doi: 10.3390/agriculture10110570
Kaushal P., Ali N., Saini S., Pati P. K., Pati A. M. (2023). Physiological and molecular insight of microbial biostimulants for sustainable agriculture. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1041413
Kaviani B., Negahdar N. (2017). Propagation, micropropagation and cryopreservation of Buxus hyrcana Pojark., an endangered ornamental shrub. S. Afr. J. Bot. 111, 326–335. doi: 10.1016/j.sajb.2017.04.004
Kentelky E., Jucan D., Cantor M., Szekely-Varga Z. (2021). Efficacy of different concentrations of NAA on selected ornamental woody shrubs cuttings. Horticulturae 7, 464. doi: 10.3390/horticulturae7110464
Khan M. A., Gemenet D. C., Villordon A. (2016). Root system architecture and abiotic stress tolerance: current knowledge in root and tuber crops. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01584
Khan W., Rayirath U. P., Subramanian S., Jithesh M. N., Rayorath P., Hodges D. M. (2009). Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 28, 386–399. doi: 10.1007/s00344-009-9103-x
Kisvarga S., Farkas D., Boronkay G., Neményi A., Orlóci L. (2022). Effects of biostimulants in horticulture, with emphasis on ornamental plant production. Agronomy 12, 1043. doi: 10.3390/agronomy12051043
Kocira A., Świeca M., Kocira S., Złotek U., Jakubczyk A. (2018). Enhancement of yield, nutritional and nutraceutical properties of two common bean cultivars following the application of seaweed extract (Ecklonia maxima). Saudi J. Biol. Sci. 25, 563–571. doi: 10.1016/j.sjbs.2016.01.039
Koevoets I. T., Venema J. H., Elzenga J. T. M., Testerink C. (2016). Roots withstanding their environment: exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01335
Krajnc A. U., Ivanus A., Kristl J., Susek A. (2012). Seaweed extract elicits the metabolic responses in leaves and enhances growth of Pelargonium cuttings. Eur. J. Hortic. Sci. 77, 170–181. doi: 10.3389/fpls.2016.01335
Krawczuk A., Huyghebaert B., Rabier F., Parafiniuk S., Przywara A., Koszel M., et al. (2023). The technical parameters of seaweed biostimulant spray application as a factor in the economic viability of soybean production. Appl. Sci. 13, 1051. doi: 10.3390/app13021051
Łangowski Ł., Goñi O., Quille P., Stephenson P., Carmody N., Feeney E. (2019). A plant biostimulant from the seaweed Ascophyllum nodosum (Sealicit) reduces podshatter and yield loss in oilseed rape through modulation of IND expression. Sci. Rep. 9, 16644. doi: 10.1038/s41598-019-52958-0
Lesmes-Vesga R. A., Chaparro J. X., Sarkhosh A., Ritenour M. A., Cano L. M., Rossi L. (2021). Effect of propagation systems and indole-3-butyric acid potassium salt (K-IBA) concentrations on the propagation of peach rootstocks by stem cuttings. Plants 10, 1151. doi: 10.3390/plants10061151
Li J., Van Gerrewey T., Geelen D. (2022). A meta-analysis of biostimulant yield effectiveness in field trials. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.836702
Loconsole D., Cristiano G., De Lucia B. (2022a). Image analysis of adventitious root quality in wild sage and glossy abelia cuttings after application of different indole-3-butyric acid concentrations. Plants 11, 290. doi: 10.3390/plants11030290
Loconsole D., Cristiano G., De Lucia B. (2022b). Improving aerial and root quality traits of two landscaping shrubs stem cuttings by applying a commercial brown seaweed extract. Horticulturae 8, 806. doi: 10.3390/horticulturae8090806
Loconsole D., Sdao A. E., Cristiano G., De Lucia B. (2023). Different Responses to Adventitious Rhizogenesis under Indole-3-Butyric Acid and Seaweed Extracts in Ornamental’s Cuttings: First Results in Photinia x fraseri ‘Red Robin’. Agriculture 13, 513. doi: 10.3390/agriculture13030513
Lohr D., Tillmann P., Druege U., Zerche S., Rath T., Meinken E. (2017). Non-destructive determination of carbohydrate reserves in leaves of ornamental cuttings by near-infrared spectroscopy (NIRS) as a key indicator for quality assessments. Biosyst. Engin. 158, 51–63. doi: 10.1016/j.biosystemseng.2017.03.005
Lucini L., Miras-Moreno B., Rouphael Y., Cardarelli M., Colla G. (2020). Combining molecular weight fractionation and metabolomics to elucidate the bioactivity of vegetal protein hydrolysates in tomato plants. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00976
Luziatelli F., Ficca A. G., Bonini P., Muleo R., Gatti L., Meneghini M., et al. (2020). A genetic and metabolomic perspective on the production of indole-3-acetic acid by Pantoea agglomerans and use of their metabolites as biostimulants in plant nurseries. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.01475
Ma Y., Freitas H., Dias M. C. (2022). Strategies and prospects for biostimulants to alleviate abiotic stress in plants. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1024243
Maghdouri M., Ghasemnezhad M., Rabiei B., Golmohammadi M., Atak A. (2021). Optimizing seed germination and seedling growth in different kiwifruit genotypes. Horticulturae 7, 314. doi: 10.3390/horticulturae7090314
Magnabosco P., Masi A., Shukla R., Bansal V., Carletti P. (2023). Advancing the impact of plant biostimulants to sustainable agriculture through nanotechnologies. Chem. Biol. Technol. Agric. 10, 117. doi: 10.1186/s40538-023-00491-8
Malik A., Mor V. S., Tokas J., Punia H., Malik S., Malik K., et al. (2021). Biostimulant-treated seedlings under sustainable agriculture: A global perspective facing climate change. Agronomy. 11, 14. doi: 10.3390/agronomy11010014
Matysiak K., Kaczmarek S., Kierzek R., Kardasz P. (2010). Effect of seaweeds extracts and humic and fulvic acids on the germination and early growth of winter oilseed rape (Brassica napus L.). J. Res. Appl. Agric. Eng. 55, 28.
Monder M. J., Kozakiewicz P., Jankowska A. (2021). The role of plant origin preparations and phenological stage in anatomy structure changes in the rhizogenesis of rosa “Hurdal”. Front. Plant Sci. 7. doi: 10.3389/fpls.2021.696998
Monder M. J., Niedzielski M., Wolinski K. (2014). Effect of rooting preparations on protein, chlorophyll and carotenoid content in leaves of Rosa gallica ‘Duchesse d’Angouleme’cuttings. Dendrobiology 72, 29-39. doi: 10.12657/denbio.072.002
Monder M. J., Pacholczak A. (2018). Preparations of plant origin enhance carbohydrate content in plant tissues of rooted cuttings of rambler roses: Rosa beggeriana ‘Polstjärnan’ and Rosa helenae ‘Semiplena’. Acta Agric. Scand. B Soil Plant Sci. 68, 189–198. doi: 10.1080/09064710.2017.1378365
Monder M. J., Pacholczak A. (2019). Rhizogenesis and contents of polyphenolic acids in cuttings of old rose cultivars treated with rooting stimulants. Acta Hortic. 1232. 99–104. doi: 10.17660/ActaHortic.2019.1232.16
Monder M. J., Pacholczak A. (2020). Rhizogenesis and concentration of carbohydrates in cuttings harvested at different phenological stages of once-blooming rose shrubs and treated with rooting stimulants. Biol. Agric. Horticult. 36, 53–70. doi: 10.1080/01448765.2019.1685407
Monder M. J., Pacholczak A. (2023). Polyphenolic acid changes in stem cuttings of rosa cultivars in relation to phenological stage and rooting enhancers. Agronomy 13, 1405. doi: 10.3390/agronomy13051405
Mukherjee A., Patel J. S. (2019). Seaweed extract: bioestimulator of plant defense and plant productivity. IJEST 17, 553–558. doi: 10.1007/s13762-019-02442-z
Oinam G., Yeung E., Kurepin L., Haslam T., Lopez-Villalobos A. (2011). Adventitious root formation in ornamental plants: I. General overview and recent successes. Propag. Ornam. Plants 11, 78–90.
Pacholczak A., Jędrzejuk A., Sobczak M. (2017). Shading and natural rooting biostimulator enhance potential for vegetative propagation of dogwood plants (Cornus alba L.) via stem cuttings. S. Afr. J. Bot. 109, 34–41. doi: 10.1016/j.sajb.2016.12.009
Pacholczak A., Nowakowska K. (2020). The effect of biostimulators and indole-3-butyric acid on rooting of stem cuttings of two ground cover roses. Acta Agrobot 73, 1-9. doi: 10.5586/aa.7314
Pacholczak A., Nowakowska K., Mika N., Borkowska M. (2016a). The effect of the biostimulator Goteo on the rooting of ninebark stem cuttings. Folia Hortic. 28, 109–116. doi: 10.1515/fhort-2016-0013
Pacholczak A., Nowakowska K., Pietkiewicz S. (2016b). The effects of synthetic auxin and a seaweed-based biostimulator on physiological aspects of rhizogenesis in ninebark stem cuttings. Not. Bot. Horti. Agrobot. Cluj Napoca 44, 85–91. doi: 10.15835/nbha44110061
Pacholczak A., Petelewicz P., Jagiełło-Kubiec K., Ilczuk A. (2015). The effect of two biopreparations on rhizogenesis in stem cuttings of Cotinus coggygria Scop. Eur. J. Hortic. Sci. 80, 183-189. doi: 10.17660/eJHS.2015/80.4.6
Pandey B., Bhardwaj V., Ramawat N. (2022). “The role of biostimulants in plant growth, development, and abiotic stress management: recent insights,” in Biostimulants: exploring sources and applications. Plant life and environment dynamics. Eds. Ramawat N., Bhardwaj V. (Springer, Singapore). doi: 10.1007/978-981-16-7080-0_9
Parađiković N., Teklić T., Zeljković S., Lisjak M., Špoljarević M. (2019). Biostimulants research in some horticultural plant species—A review. Food Energy Secur. 8, e00162. doi: 10.1002/fes3.162
Park H. Y., Kim K.-S., Ak G., Zengin G., Cziáky Z., Jekó J., et al. (2021). Establishment of a rapid micropropagation system for Kaempferia parviflora wall. Ex Baker: Phytochemical analysis of leaf extracts and evaluation of biological activities. Plants 10, 698. doi: 10.3390/plants10040698
Petropoulos S. A. (2020). Practical applications of plant biostimulants in greenhouse vegetable crop production. Agronomy 10, 1569. doi: 10.3390/agronomy10101569
Povero G., Mejia J. F., Di Tommaso D., Piaggesi A., Warrior P. (2016). A systematic approach to discover and characterize natural plant biostimulants. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00435
Rasool A., Mansoor S., Bhat K. M., Hassan G. I., Baba T. R., AlYemeni M. N. (2020). Mechanisms underlying graft union formation and rootstock scion interaction in horticultural plants. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.590847
Read P. E. (2011). “Tough nuts to crack: Advances in micropropagation of woody species,” in V international symposium on acclimatization and establishment of micropropagated plants (International Society for Horticultural Science, Leuven, Belgium), 115–122. doi: 10.17660/ActaHortic.2013.988.12
Ricci M., Tilbury L., Daridon B., Sukalac K. (2019). General principles to justify plant biostimulant claims. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00494
Ross S. G., Souza-Pérez M., Ávila N., Pietro F., González A. M. (2021). Stem-cutting anatomy and biochemical responses associated with competence for adventitious root differentiation in Acca sellowiana (Myrtaceae). Trees 35, 1221–1232. doi: 10.1007/s00468-021-02110-1
Rouphael Y., Carillo P., Ciriello M., Formisano L., El-Nakhel C., Ganugi P., et al. (2023). Copper boosts the biostimulant activity of a vegetal-derived protein hydrolysate in basil: morpho-physiological and metabolomics insights. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1235686
Rouphael Y., Colla G. (2018). Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.01655
Rouphael Y., Colla G. (2020). Editorial: biostimulants in agriculture. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00040
Roussos P. A. (2023). Adventitious root formation in plants: the implication of hydrogen peroxide and nitric oxide. Antioxidants 12, 862. doi: 10.3390/antiox12040862
Salachna P., Zawadzinska A., Piechocki R., Wilas J. (2014). Propagation of arabian star flower (Ornithogalum arabicum L.) by twin scales using seaweed extracts. Folia Pomeranae Univ. Technol. Stetin 310, 105–112. doi: 10.5555/20143318262
Sharma S., Lyons G., McRoberts C., McCall D., Carmichael E., Andrews F., et al. (2012). Biostimulant activity of brown seaweed species from Strangford Lough: compositional analyses of polysaccharides and bioassay of extracts using mung bean (Vigno mungo L.) and pak choi (Brassica rapa chinensis L.). J. Appl. Phycol. 24, 1081–1091. doi: 10.1007/s10811-011-9737-5
Shukla P. S., Borza T., Critchley A. T., Prithiviraj B. (2016). Carrageenans from red seaweeds as promoters of growth and elicitors of defense response in plants. Front. Mar. Sci. 3. doi: 10.3389/fmars.2016.00081
Shukla P. S., Mantin E. G., Adil M., Bajpai S., Critchley A. T., Prithiviraj B. (2019). Ascophyllum nodosum-based biostimulants: sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00655
Sible C. N., Seebauer J. R., Below F. E. (2021). Plant biostimulants: A categorical review, their implications for row crop production, and relation to soil health indicators. Agronomy 11, 1297. doi: 10.3390/agronomy11071297
Stępowska A. (2008). “Effect of GA 142 (Goëmar Goteo) and (Goëmar BM 86) extracts on sweet pepper yield in non-heated tunnels,” in Monographs series: biostimulators in modern agriculture: solanaceous crops. Ed. Dąbrowski Z. T. (Editorial House Wieś Jutra, Warsaw), 45–51.
Stirk W. A., Rengasamy K. R., Kulkarni M. G., van Staden J. (2020). “Plant biostimulants from seaweed: An overview, 31–55,” in The chemical biology of plant biostimulants. Eds. Geelen D., Xu L. (John Wiley & Sons Ltd). doi: 10.1002/9781119357254.ch2
Stirk W. A., Van Staden J. (2014). Plant growth regulators in seaweeds: occurrence, regulation and functions. Sea Plants 71, 125–159. doi: 10.1016/B978-0-12-408062-1.00005-6
Szabó V., Sárvári A., Hrotkó K. (2011). Treatment of stockplants with biostimulators and their effects on cutting propagation of Prunus marianna ‘GF 8-1’. Acta Hortic. 923, 277–281. doi: 10.17660/ActaHortic.2011.923.41
Szczepanek M., Siwik-Ziomek A. (2019). P and K accumulation by rapeseed as affected by biostimulant under different NPK and S fertilization doses. Agronomy 9, 477. doi: 10.3390/agronomy9090477
Tahir M. M., Mao J., Li S., Li K., Liu Y., Shao Y., et al. (2022). Insights into factors controlling adventitious root formation in apples. Horticulturae 8, 276. doi: 10.3390/horticulturae8040276
Toța C., Bala M., Sala F. (2022). Vegetative propagation in jade tree using rooting biostimulators of stem cuttings. Sci. Papers: Management Economic Eng. Agric. Rural Dev. 22, 743–750.
Toscano S., Romano D., Massa D., Bulgari R., Franzoni G., Ferrante A. (2018). Biostimulant applications in low input horticultural cultivation systems. Italus Hortus. 25, 27–36. doi: 10.26353/j.itahort/2018.1.2736
Traversari S., Cacini S., Nesi B. (2022). Seaweed extracts as substitutes of synthetic hormones for rooting promotion in rose cuttings. Horticulturae 8, 561. doi: 10.3390/horticulturae8070561
Tsaktsira M., Chavale E., Kostas S., Pipinis E., Tsoulpha P., Hatzilazarou S., et al. (2021). Vegetative propagation and ISSR-based genetic identification of genotypes of Ilex aquifolium ‘Agrifoglio Commune’. Sustainability 13, 10345. doi: 10.3390/su131810345
United States Environmental Protection Agency (2022) Biopesticides. Available online at: https://www.epa.gov/pesticide-registration/pesticide-data-submitters-list-pdsl (Accessed 10/01/2024).
Valverde S., Williams P. L., Mayans B., Lucena J. J., Hernández-Apaolaza L. (2022). Comparative study of the chemical composition and antifungal activity of commercial brown seaweed extracts. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1017925
Wei X., Zhang W., Zulfiqar F., Zhang C., Chen J. (2022). Ericoid mycorrhizal fungi as biostimulants for improving propagation and production of ericaceous plants. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1027390
Winkelmann T. (2013). Recent advances in propagation of woody plants. Acta Hortic. 990, 375–381. doi: 10.17660/ActaHortic.2013.990.47
Yakhin O. I., Lubyanov A. A., Yakhin I. A., Brown P. H. (2017). Biostimulants in plant science: A global perspective. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.02049
Zhang L., Freschi G., Rouphael Y., De Pascale S., Lucini L. (2023). The differential modulation of secondary metabolism induced by a protein hydrolysate and a seaweed extract in tomato plants under salinity. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1072782
Keywords: AlgaminoPlant, Bio Rhizotonic, Goteo®, Kelpak®, phytoregulator, root architecture, rooting percentage
Citation: Loconsole D, Scaltrito E, Sdao AE, Cristiano G and De Lucia B (2024) Application of commercial seaweed extract-based biostimulants to enhance adventitious root formation in ornamental cutting propagation protocols: a review. Front. Hortic. 3:1371090. doi: 10.3389/fhort.2024.1371090
Received: 15 January 2024; Accepted: 15 April 2024;
Published: 16 May 2024.
Edited by:
Daniela Romano, University of Catania, ItalyReviewed by:
Roberta Bulgari, University of Turin, ItalyStefania Toscano, University of Messina, Italy
Copyright © 2024 Loconsole, Scaltrito, Sdao, Cristiano and De Lucia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danilo Loconsole, danilo.loconsole@uniba.it
 Danilo Loconsole*
Danilo Loconsole*  Eugenio Scaltrito
Eugenio Scaltrito Anna Elisa Sdao
Anna Elisa Sdao Giuseppe Cristiano
Giuseppe Cristiano Barbara De Lucia
Barbara De Lucia