Nesting success and potential nest predators of the red Junglefowl (Gallus gallus jabouillei) based on camera traps and artificial nest experiments
- 1College of Forestry, Hainan University, Haikou, China
- 2Intelligent Forestry Key Laboratory of Haikou City, College of Forestry, Hainan University, Haikou, China
- 3College of Ecology and Environment, Hainan University, Haikou, China
- 4Hainan Tropical Rain Forest National Park Service Wuzhishan Branch, Wuzhishan, China
- 5Hainan Datian National Nature Reserve Administration, Dongfang, China
- 6Hainan Bangxi Provincial Nature Reserve Management Station, Bangxi, China
Breeding success is an important factor determining fecundity with nest predation being the main factor limiting avian breeding success. Understanding of nest predation and its influencing factors are highly significant to explore the dynamics of bird populations and developing appropriate conservation strategies. In two breeding seasons of the year 2020 and 2021, natural nests of the red junglefowl (Gallus gallus jabouillei) were systematically searched and monitored using infrared camera, in two nature reserves (Datian and Bangxi) of tropical Hainan island, China. Results showed that breeding season of the red junglefowl is mainly from March to July, with April being the breeding peak. The clutch size was 5.15 ± 1.28 (n = 13), and nesting success of natural nests was 31.2%, with nest predation accounting for 45.4% of nest failure. Artificial nest experiments showed that predation rates of artificial nests were 25% (Datian, 2020), 6.67% (Datian, 2021), and 0% (Bangxi, 2020). Rodents, reptiles, and coucals are the main nest predators of red junglefowls, while activities of Hainan Eld’s deers (Panolia siamensis) may interfere with the reproduction of red junglefowls. We suggest that the conservation management policies should consider the impacts on junglefowls’ breeding success when reconstructing the suitable habitat of the Hainan Eld’s deer.
Introduction
The study of reproductive biology and the estimation of nest survival rates are critical to understanding avian life history (Martin, 2004; Stutchbury and Morton, 2008, 2023). Life history traits related to reproduction, such as clutch size, parental care, chick development, and survival rate, can provide insights for solving problems related to the assessment of population status and conservation (Martin, 2002, 2008; Martin et al., 2017). Most recent studies on reproductive biology have focused on northern temperate zone birds (Martin, 2004; Lloyd et al., 2014; Xiao et al., 2017), while birds in other regions, especially tropical birds, have been relatively less studied (Xiao et al., 2017). The main reason may be that tropical birds are relatively difficult to monitor (Martin, 1996; Jiang et al., 2017; McCullough and Londoño, 2017). Studies have shown that the reproductive strategies of birds in different regions are different mainly due to the differences in the breeding habitats of birds and their nest predation rates (Martin, 1996). Compared with temperate birds, tropical birds are characterized by a longer breeding season, smaller clutch size, longer brooding, and hatching periods (Martin, 1996; Lloyd et al., 2014). Therefore, expanding the understanding of the life history of tropical birds can help us better understand the global trend of life history strategies (Ricklefs and Wikelski, 2002; Slevin et al., 2020).
Nest predation is a major factor limiting the success of bird reproduction and affects the life history and population dynamics of birds (Reidy and Thompson, 2012; Thompson and Ribic, 2012; Ibáñez-Álamo et al., 2015; Chen et al., 2020). Especially for ground-nesting birds, nest predation has become one of the most critical factors affecting their reproductive performance and population growth (Sanders and Maloney, 2002; MacDonald and Bolton, 2008; Pedersen et al., 2011; Melville et al., 2014). Based on this, understanding predation on birds and its influencing factors is of great significance for studying bird population dynamics and proposing conservation strategies (Martin, 1993; Chalfoun et al., 2002; Seibold et al., 2013; Chen et al., 2020).
The red junglefowl (Gallus gallus) belong to the genus Gallus of the pheasant family (Phasianidae) in the order Galliformes and is a ground-active bird (Zheng, 2022). According to the differences in their distribution range and morphological characteristics, the world’s red junglefowl can be divided into five subspecies, all of which are distributed in southern Asia (Johnsgard, 1999; del Hoyo et al., 2001). There are two subspecies in China, the southern Yunnan subspecies (G. g. spadiceus) and the Hainan subspecies (Gallus gallus jabouillei; Zheng, 2022).
In this study, we searched and monitored the natural nests of red junglefowls, the Hainan subspecies, in Datian and Bangxi Nature Reserves, tropical Hainan Island, China, using infrared cameras. Considering that the number of natural nests of Galliformes birds is limited and nest sites are relatively difficult to find (Luo et al., 2017), the use of artificial nests for simulation studies can not only reduce human interference on hatching in the wild but also has the advantage of ease of use, and can provide sufficient samples for research (Martin, 1987; Major and Kendal, 1996; Wilson et al., 1998; Zanette and Jenkins, 2000; McDonald et al., 2009; Melville et al., 2014; Luo et al., 2017), we carried out artificial nest experiments in the study area to compare the survival rates and potential predators of natural nests and artificial nests.
Materials and methods
Study area
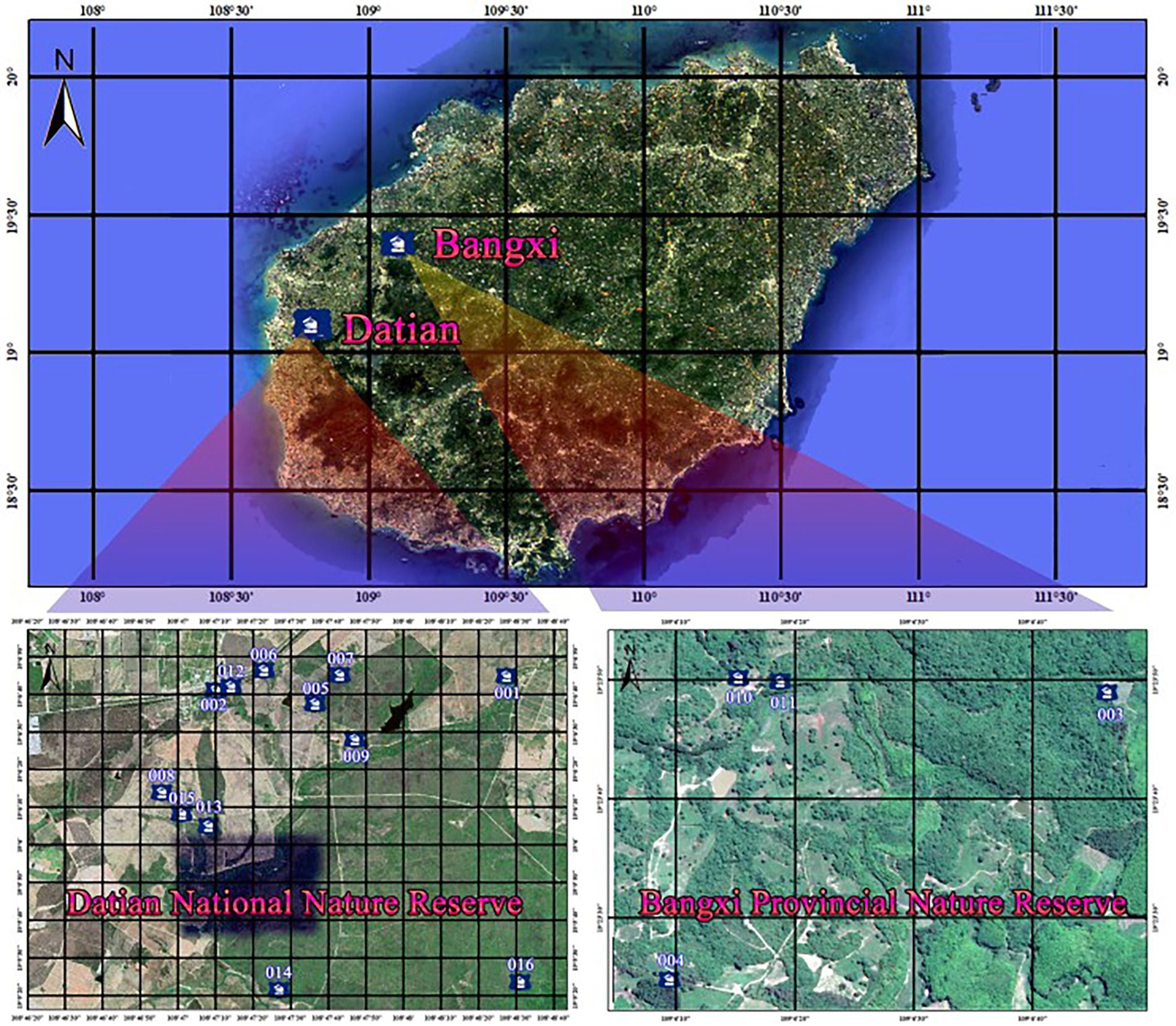
This study was conducted in Datian and Bangxi Nature Reserves. The Hainan Datian National Nature Reserve (19°05′-17′ N, 108°47′-49′ E, with 30–80 m in elevation and 1,310 ha in total area) is located in Dongfang city, Hainan Province, with the Hainan Eld’s deer and its habitat being the main target for protection. Bangxi Provincial Nature Reserve (19°22′-24′ N, 109°05′-06′ E, with an elevation of 17–80 m and a total area of 361.8 ha) is located in Bangxi town, northwest of Baisha County, Hainan Province, with the Hainan Eld’s deer and its habitat also being the main target for protection.
Monitoring of natural nests
In 2020 and 2021, we carried out a search for the natural nests of red junglefowls following Conkling et al. (2012). The search was divided between two groups. The first group consisted of forest rangers in the reserve, who searched for nests during daily routine patrols; the second group consisted of researchers who used the behavioral observation and systematic search technology of adult birds and monitored them at 06:00 ~ 09:00 and 16:00 ~ 19:00 every day. Once the nest was found, we used a GPS device (eTrex 32x, Garmin) to mark its location and recorded the status of the female (brooding or flew away) and of the nest (building, hatched, brooded, or abandoned). To avoid interference, we used a telescope to observe the nest every 3–5 days. If at least one egg in the nest successfully hatched, we considered the reproduction to be successful; if all the eggs were not hatched or were destroyed, we considered the reproduction to be a failure (Visco and Sherry, 2015).
In addition, an infrared camera was also aimed at each breeding nest to accurately identify predators and assess the fate of the nests. To avoid abandonment by parent birds due to human interference, the infrared cameras were installed in the incubation period (DeGregorio et al., 2016). We usually adopted the method of using local materials to fix the camera to the tree trunk at 1–1.5 m away from the nest; for situations in which we were unable to fix the camera to a tree trunk, we used the method of piling objects to set up the camera and disguise it appropriately. The infrared camera was set to record continuously throughout the day, and the shooting mode was set to “photograph + video.” Each time the camera was triggered, it shot three photos and 15 s of video, and the trigger sensitivity was set to “medium” (Xiao et al., 2014). After the nest was preyed upon, we reviewed the camera records to identify the type of predator and recorded the time of predation. For predation events in which the predators were not recorded due to camera failure or other reasons, we speculated on the identity of the predators based on the status of the nests after the predation event occurred because some predators will leave evidence behind. For example, rodents usually leave behind eggshell fragments, while some reptiles prey on nests without leaving any traces (Klug et al., 2010).
After breeding, we recorded and measured the following nest site parameters: (1) nest size (long and short diameters); (2) nest depth; (3) nest materials; (4) distance from nest to the nearest water source; and (5) shortest distance from the nest to the road. For the natural nests in which reproduction failed, the following parameters were recorded and measured: (1) clutch size; (2) egg size; and (3) weight of the eggs. We used a tape measure which ranging from 0 to 500 cm to measure the nest size, nest depth, distance from the nest to the nearest water source, and shortest distance from the nest to the road. For indicators that exceeded the measuring range of the tape measure, we used the method of walking estimation. Eggs were measured using a digital Vernier caliper; the egg size measurement was accurate to 0.01 mm. The egg weight measurement was accurate to 0.01 g.
Artificial nest experiments
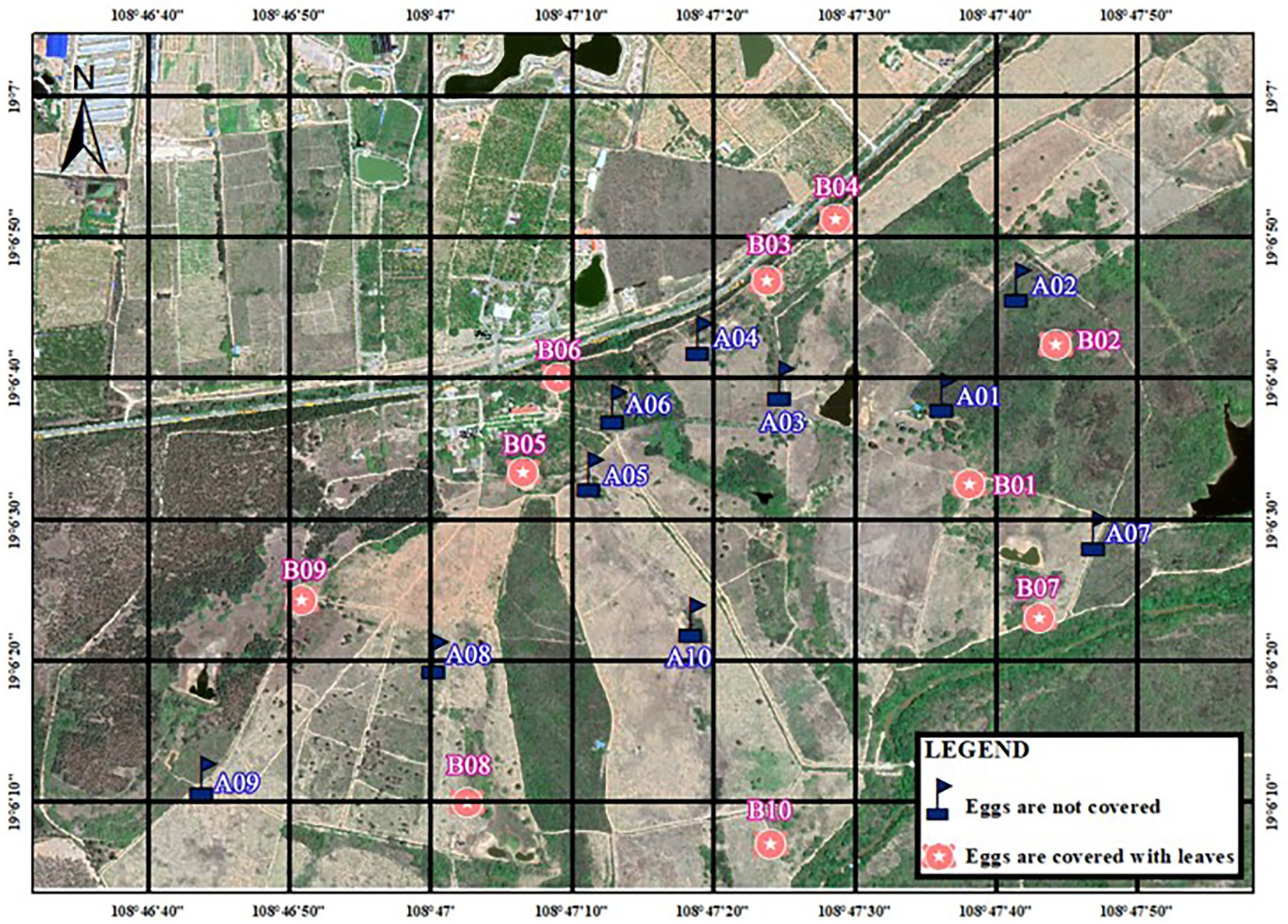
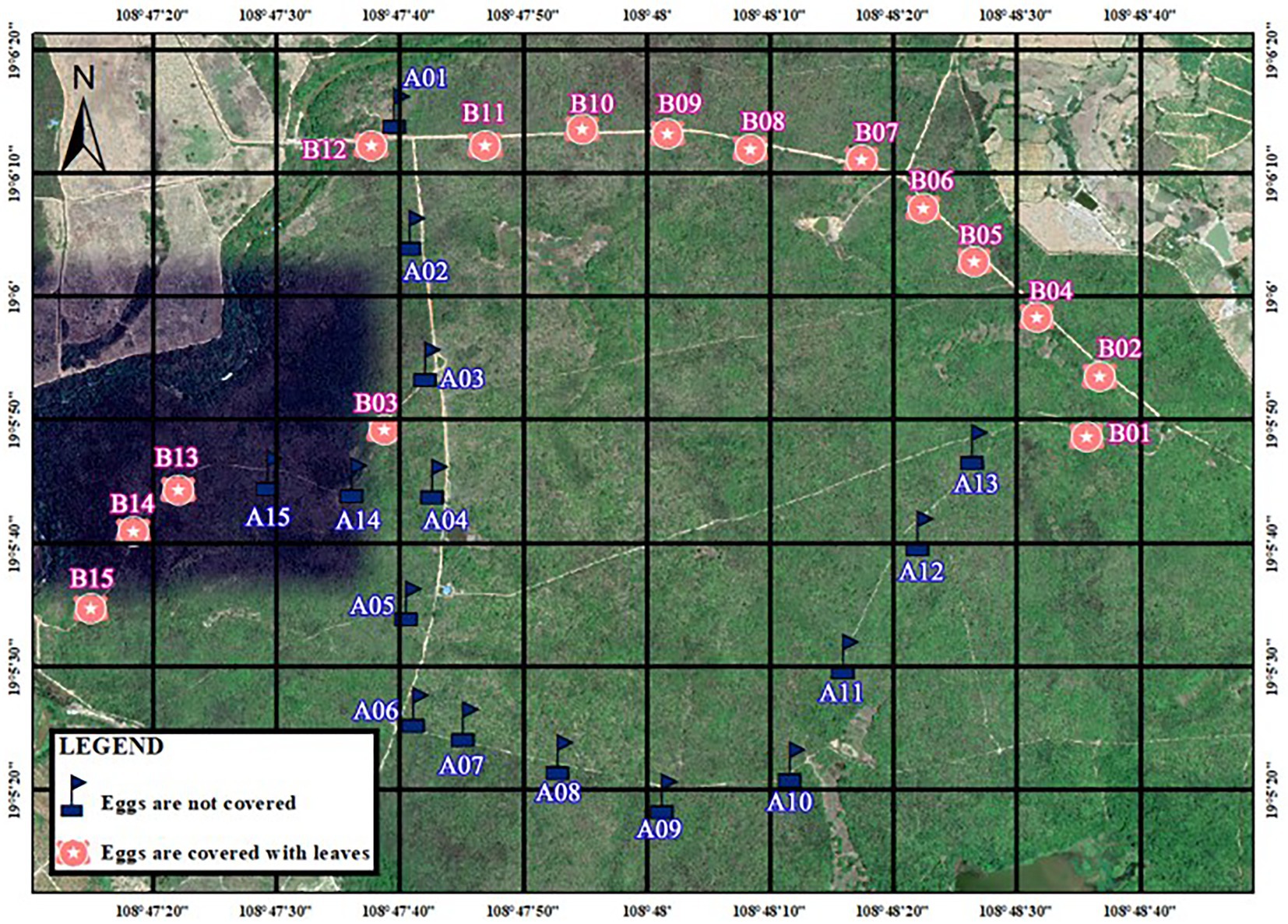
During the breeding season in 2020, we conducted artificial nest experiments in Datian (6–25 May 2020) and Bangxi (7–25 May 2020). The location for setting up the artificial nests was selected in the same area where natural nests of red junglefowl were found in a previous study (Yuan, 2009) or was determined according to the characteristics of the site of the natural nests (Yuan et al., 2009a). Artificial nests were constructed by imitating the structure of natural nests, which are shallow pits, and using dry leaves, twigs, and feathers as nest materials. Two eggs of domesticated chickens that were similar in size and color to those of wild red junglefowl were placed in each artificial nest. Two groups of experiments were set up at each study site (the distribution of artificial nests is shown in Figures 1, 2). The first group consisted of 10 nests for the non-covered group (infrared camera), and the second group consisted of 10 nests, with dry leaves used to cover the experimental eggs. The artificial nest incubation period was set as 19 days, which similar to its natural incubation period (Yuan, 2009). The artificial nests were inspected every 6 days for a total of three times, on the 7th day, 13th day, and 19th day after nest set up (Dinsmore et al., 2002). In each inspection, the artificial nest was photographed, and the nest number was recorded. If there were two eggs or one egg in the artificial nest, the nest was inspected again the next time (fully covered nests were covered with leaves again after the inspection); if the two eggs were preyed on or disappeared, the results were recorded immediately after taking pictures, and this signified the end of the experiment. The inspection results were divided into: (1) two eggs intact; (2) one egg intact, one egg preyed on (with eggshell or predation traces); (3) one egg intact, one egg missing (no traces); (4) two eggs preyed on (with eggshell or predation traces); (5) two eggs missing; and (6) experimental eggs were moved by unknown animals.
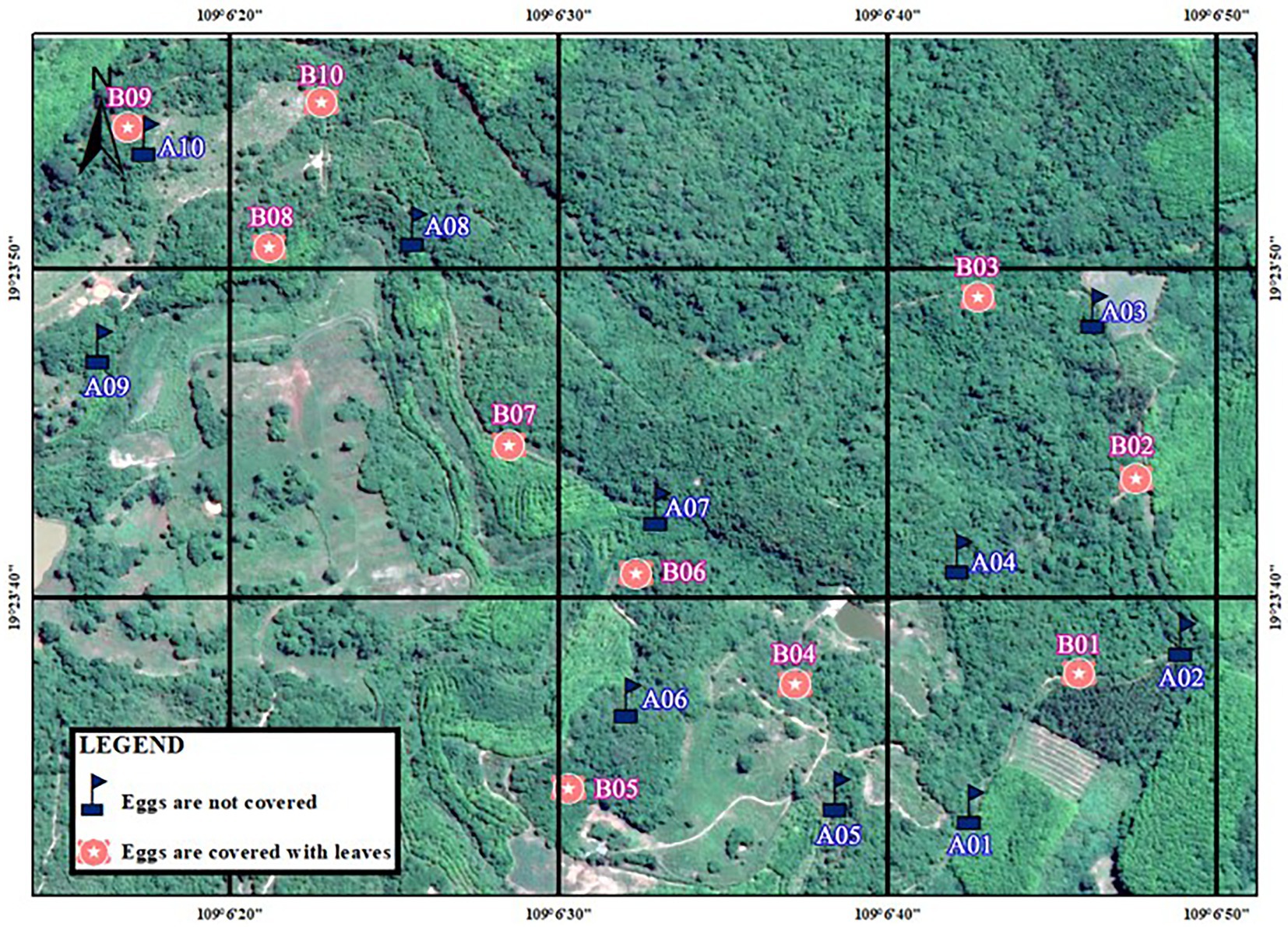
During the breeding season in 2021, we conducted artificial nest experiments in Datian (11–29 March 2021). Similarly, we set up two sets of experiments, with 15 nests in each group (the distribution of artificial nests is shown in Figure 3). In the non-covered group, every nest was deployed together with an infrared camera, and only some of the nests (three totals) in the fully covered group were deployed together with a camera. The specific operations and methods were the same as described above.
To reduce the influence of researchers on the experiment, human interference should be minimized during artificial nest inspection, for example, to leave as little footprint as possible around the artificial nest and to avoid the behavior that may affect the nest, such as touching the experimental eggs (Driscoll et al., 2005). In the wild, if the nest is disturbed or the eggs in the nest are preyed upon, the female chickens will abandon the nest (Yuan, 2009). Therefore, if the eggs in the artificial nest are damaged, removed or disappeared, we will not replace the new experimental eggs (Nour et al., 1993) and define the nest as a reproductive failure (Noske et al., 2008). Otherwise, the nest is considered a successful breeding nest.
Species identification was performed based on photos or videos taken by the infrared cameras. When identifying species, we mainly referred to the “Chinese Wildlife Manual of Mammals” (Smith and Xie, 2009), “A Checklist on the Classification and Distribution of the Birds of China (third Edition)” (Zheng, 2022) and the “The CNG field guide to the birds of China” (Liu and Chen, 2021). To calculate the number of animals captured by the cameras, when an animal was photographed by the same camera more than 30-min apart, this was considered an independent capture and an individual animal (O’Brien et al., 2003; Rovero et al., 2014).
Results
Natural nests
In the breeding season of 2020 and 2021, we found 16 natural nests of red junglefowl at the two study sites (Table 1; Figure 4). There were 12 nests in Datian and four nests in Bangxi (Figures 5A–D). Among the 16 nests found, infrared cameras were installed in 13 nests for monitoring (Figure 6). In total, five out of the 16 nests reproduced successfully (Figures 7A–C), and the reproductive success rate was 31.25%. Among them, five nests were preyed upon and nest predation accounted for 45.45% of nest failures. Nests 008 and 013 were both visited by the Hainan Eld’s deer during the hatching period (Supplementary Videos S1, S2; Figure 8); the female bird in nest 008 abandoned it (one chick hatched successfully), and the eggs were preyed upon after the female bird abandoned nest 013.

Table 1. Active nests of red junglefowls (Gallus gallus jabouillei) in Datian and Bangxi Nature Reserves in 2020 and 2021.

Figure 5. (A,B) Natural nests of red junglefowl (Gallus gallus jabouillei) found in the study sites (females brooding), (C,D) Natural nests of red junglefowl (Gallus gallus jabouillei) found in the study sites (female birds flew away).

Figure 6. Infrared camera monitoring of a natural nest of red junglefowl (Gallus gallus jabouillei).

Figure 7. (A,B) Natural nests with successful reproduction; (C) Natural nests that failed to reproduce.
The clutch size of red junglefowls was 4.50 ± 1.83 (n = 16). The egg parameters were showed as follows: weight: 24.26 ± 0.60 g, long diameter: 43.88 ± 1.18 mm, and short diameter: 33.45 ± 0.52 mm, n = 9. The nest parameters were showed as follows: long diameter: 15.51 ± 4.21 cm, short diameter: 13.00 ± 4.23 cm, and depth: 3.55 ± 0.80 cm, n = 8. The distance between the natural nest and the nearest water source was 56.18 ± 57.58 m (n = 11), and the closest distance between the natural nest and the road was 12.65 ± 27.40 m (n = 13). The number of nests found was the highest in April, with 11 nests, accounting for 68.75% of the total number of nests, followed by May, with two nests, accounting for 15.38%. Only one nest was found in July.
Artificial nest experiments and potential nest predators
Among the 20 nests in Datian, five nests were preyed upon, and the predation rate was 25%. In one of the nests, the experimental eggs were moved (Figure 9A). However, no predation occurred in the Bangxi. Vertebrates were the main cause of nest predation in the experiments, including two bird incidents (coucals) and two rodent incidents (Figure 9B). There were similarities in the types and numbers of animals that visited the artificial nests at the two study sites. Among them, Hainan Eld’s deers (Figure 10A) and several small rodents (Rattus spp.; Figure 10B) were photographed at the two experimental sites. Wild boars (Sus scrofa; Figure 11A), greater coucals (Centropus sinensis; Figure 11B), and lesser coucals (Centropus bengalensis; Figure 11C) were only photographed in Datian, while red-bellied squirrels (Callosciurus erythraeus; Figure 11D) and domestic chicken (Gallus gallus domesticus; Figure 12) were only photographed in Bangxi.

Figure 9. (A) Experimental eggs were moved by unknown animals; (B) Rodent predation of experimental eggs.

Figure 10. (A) Hainan Eld’s deer near the artificial nest; (B) Small rodent near the artificial nest.

Figure 11. (A) Wild boar near the artificial nest, (B) Greater coucal near the artificial nest, (C) Lesser coucal near the artificial nest, and (D) Red-bellied squirrel near the artificial nest.
Among 30 artificial nests set up in Datian in March 2021, two nests that were preyed on (Figure 9B), and experimental eggs in two nests were moved by unknown animals. However, they were not preyed upon. The predation event was not captured by the infrared camera, but animals that appeared near the artificial nests were recorded. These animals were the red junglefowl (Figure 13), black-throated laughingthrush (Garrulax chinensis monachus), wild boar, Hainan muntjac (Muntiacus nigripes; Figure 14), leopard (Prionailurus bengalensis alleni; Figure 15), and several small rodents.
Discussion
In the two breeding seasons of 2020 and 2021, we found a total of 16 natural nests and nesting success of the red junglefowl was 31.25%, which was higher than the result observed by Yuan (2009) in the same area (18.2%). However, the rate of nest abandonment was as high as 68.75%, higher than the results of previous studies (55.6%; Yuan, 2009).
In a study of the Indian subspecies, it was found that after the first reproductive failure, red junglefowls would choose to reproduce a second time (Collias and Collias, 1967). Similarly, the reproduction record of the southern Yunnan subspecies also mentions that it may reproduce twice a year (Zheng et al., 1978). It is likely that the Hainan subspecies may reproduce multiple times to improve the reproductive success, which needs further confirmation in the wild. The clutch size in our study areas was similar to that of the Indian subspecies (Anwar et al., 2016) but was significantly lower than that of the southern Yunnan subspecies (Zheng et al., 1978). In southern Yunnan, female and male birds brood and hatch eggs together (Zheng et al., 1978). Yuan (2009) observed that in the field, both male and female birds brood nestlings, while we found that only female birds were responsible for brooding in the wild.
We found that the Hainan subspecies was breeding from March to July, and that the number of broods was highest in April. It is thus speculated that the breeding peak in the Hainan subspecies is April. This is consistent with the results of Yuan (2009), but there are differences between the Indian subspecies from different areas and the southern Yunnan subspecies. For example, in India, broiler flocks were found to breed in January–October, and the breeding peak period was March–May (Ali and Ripley, 1987; Anwar et al., 2016); in the Malay Peninsula region, the breeding time is from December to May of the following year, and the breeding peak is from January to February (del Hoyo et al., 2001). In areas with sufficient food, red junglefowl can even breed year-round (Arshad and Zakaria, 1999). In southern Yunnan, the breeding time is from February to October (Zheng et al., 1978). Tropical birds have different life history traits (Stutchbury and Morton, 2023). Anyway, we only carried out the breeding monitoring of the red junglefowl for 2 years, which may be the reason that the breeding season in Hainan subspecies differs from other subspecies. There should be a long-term monitoring to the subspecies in the wild.
Studies have found that red junglefowl tend to choose areas with well-developed herbs and patches for nesting (Collias and Collias, 1967). However, some studies have suggested that deciduous trees and bamboo forests with scattered patches are the favorite nesting habitats of red junglefowls during the breeding season (Johnson, 1963). In this study, it was observed that female birds mostly nested on patrol roads or in open areas near the edge of forests with fewer deciduous leaves. This reflects the geographical differences in the selection of nesting sites among different subspecies. The edge effect hypothesis indicates that because the edge area of the habitat has more abundant vegetation resources and a more complex environment, the predation pressure is usually greater than that of the central area (Ewers and Didham, 2007). However, studies on the Reeves’s pheasant (Syrmaticus reevesii) do not support this hypothesis, as this species tends to nest in marginal areas with more human interference (Luo et al., 2017). Our results are consistent with that study, as the female birds of the Hainan subspecies chose to nest on patrol roads or at the edge of the forest. It is possible that human activities in these areas are more frequent, and some predators are forced to enter the central area where there are fewer disturbances, thereby reducing predation by natural enemies. Nest predation is the main factor in the failure of pheasant reproduction (Lu and Zheng, 2001; Sherry et al., 2015). In the predation events we observed, eggshells were either left in the nest or no trace of the predator was left after the eggs were preyed upon. Therefore, we speculated that rodents and reptiles may be the main nest predators. This is because rodents usually leave eggshells or debris behind after predation, and some reptiles leave no traces when preying on nests (Klug et al., 2010). In addition, in the study area, small carnivorous mammals, such as leopards, falcons, and raptors are the main natural enemies of the subspecies (Zheng et al., 1978; Evans et al., 1993), and snakes are also the main predators of red junglefowls (Anwar et al., 2016).
In our study, although artificial nests were set up in the same study area, their predation rate was lower than that of natural nests, and the types of predators were also different. This result is consistent with the results of a study of the reed parrotbill (Paradoxornis heudei) (Chen et al., 2020). This is different from the results of other studies. For example, snakes were found to be the main predators of natural nests, while mammals were found to be the main predators of artificial nests (Thompson and Burhans, 2003; Chen et al., 2020).
The use of infrared camera technology to monitor artificial nests is ideal for the determination of nest predator species (Anthony et al., 2006; Kross et al., 2013). In this study, the infrared cameras captured a number of wild animals, such as greater coucals, lesser coucals, and Hainan Eld’s deers. Among them, the greater coucal was the main predator of the experimental eggs, and the feeding activity of Hainan Eld’s deers caused female birds to abandon nests. Studies have shown that wild boars can change the terrain by overturn large areas of soil, thereby changing the vegetation structure and composition of their habitats (Singer et al., 1984; Lacki and Lancia, 1986), which is particularly harmful to ground-nesting birds (Barrios-Garcia and Ballari, 2012; Sanders et al., 2020). Therefore, the wild boar may be an opportunistic predator of nests. A recent study has shown that gray squirrels (Sciurus carolinensis) are widely considered to be important predators of bird eggs and chicks (Broughton, 2020). We photographed red-bellied squirrels in the vicinity of artificial nests many times and speculate that the red-bellied squirrel is a potential nest predator. In addition, studies have found that bats are the main nest predators of tropical forest birds (Perrella et al., 2019). In our study, we did not find predation of eggs by bats. In follow-up work, we intend to carry out effective and continuous monitoring of breeding nests and clarify the antipredation strategies of red junglefowl.
In this study, the predation rates of artificial nests varied in Datian. The main reason is that the artificial nest experiments were done at the buffer zone in 2020, while at the core zone in 2021. The habitats in the two regions are significantly different, and so are the predation rates. The predation rate was 0% in Bangxi. There are two possible reasons. Firstly, our sample size is not large enough, secondly, there is a relative lack of potential predators for red junglefowl eggs. Although the survival rates of the artificial nests in the two study sites were higher than those of the natural nests. The possible reason is that the experiment was conducted in May 2020, while in March 2021, the birds had just started breeding, and most of them were in the hatching period in May. Most previous studies used the quadrat survey method (Johnson, 1963; Kalsi, 1992; Arshad and Zakaria, 2009; Yuan et al., 2009a,b) or radio tracking technology (Arshad and Zakaria, 2011) to investigate habitat selection and habitat utilization in red junglefowls. Satellite tracking technology is a tool to effectively track the small-scale movement of highly mobile and inaccessible species (Cagnacci et al., 2010; Coxen et al., 2017). The application of this technology to the study of ground-dwelling pheasants such as red junglefowl is very important for understanding how these birds use their habitat, the motivation for their movements, and the process of space utilization, to ultimately improve pheasant protection.
Conclusion
Red junglefowl are ground-nesting pheasants that are highly alert, which makes field research and tracking difficult (Arshad and Zakaria, 2011; Anwar et al., 2016). Human interference or direct observation of brooding parent birds may lead to abandonment of the nest and reproduction failure (Yuan, 2009). Therefore, there is limited information on the survival rate of red junglefowl nests. Protecting the habitat and reducing human disturbance will play a positive role in the growth of the red junglefowl population in our study areas. Datian and Bangxi are both protected areas with the main goal of protecting Hainan Eld’s deer. Burning grassland vegetation, thinning, plowing, and manual removal of waste can alleviate the degradation of Hainan Eld’s deer habitat quality (Fu et al., 2016, 2018). We suggest that the conservation area should take into account the impact on the reproduction of ground-dwelling pheasants when rebuilding or reconstructing the suitable habitat of Hainan Eld’s deer. In addition, domestic chickens were photographed in the Bangxi Nature Reserve, indicating the possibility of hybridization between wild red junglefowl and domestic chickens. It is recommended that free-range domestic chickens be banned within protected areas to avoid the problem of genetic contamination of wild red junglefowls.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the animal study because we have obtained the administrative licensing decision of Hainan Forestry Bureau on the study of Red Junglefowl [Hainan Forestry Bureau approval (2019) 083].
Author contributions
XR designed the study. XR, JL, BH, HW, GW, and TT carried out field experiments. XR and QL performed cartography and statistical analyses. XR wrote the draft manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Joint Fund of the Natural Science Foundation of Hainan Province (no. 320RC506 to XR) and the National Natural Science Foundation of China (no. 31800320 to XR). XR was funded by the project supported by the Scientific Research start-up Fund of Hainan University [no. KYQD (ZR) 20057] and the Teaching Research of Hainan University (no. Hdsz20-9).
Acknowledgments
We are grateful to Datian and Bangxi Nature Reserves for their help and co-operation. We thank Biaoyi Tang for assistance with field work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1127139/full#supplementary-material
References
Ali, S., and Ripley, S. D. (1987). The Compact Handbook of the Birds of India and Pakistan. Bombay: Oxford University Press
Anthony, R. M., Grand, J. B., Fondell, T. F., and Millar, D. A. (2006). Techniques for identifying predators of goose nests. Wildl. Biol. 12, 249–256. doi: 10.2981/0909-6396(2006)12[249:TFIPOG]2.0.CO;2
Anwar, M., Ali, S., Rais, M., and Mahmood, T. (2016). Breeding ecology of red jungle fowl (Gallus gallus) in Deva Vatala National Park, Azad Jammu and Kashmir, Pakistan. J. Appl. Agric. Biotechnol. 1, 59–65.
Arshad, M. I., and Zakaria, M. (1999). Breeding ecology of red junglefowl (Gallus gallus spadiceus) in Malaysia. J. Malayan Nat. 53, 355–365.
Arshad, M. I., and Zakaria, M. (2009). Roosting habits of red Junglefowl in orchard area. Pak. J. Life Soc. Sci. 7, 86–89.
Arshad, M. I., and Zakaria, M. (2011). Variation in home range size exhibited by red Junglefowl (Gallus gallus spadiceus) in oil palm plantation habitat, Malaysia. Pak. J. Zool. 43, 33–840.
Barrios-Garcia, M. N., and Ballari, S. A. (2012). Impact of wild boar (Sus scrofa) in its introduced and native range: a review. Biol. Invasions 14, 2283–2300. doi: 10.1007/s10530-012-0229-6
Broughton, R. K. (2020). Current and future impacts of nest predation and nest-site competition by invasive eastern grey squirrels Sciurus carolinensis on European birds. Mammal Rev. 50, 38–51. doi: 10.1111/mam.12174
Cagnacci, F., Boitani, L., Powell, R. A., and Boyce, M. S. (2010). Animal ecology meets GPS-based radiotelemetry: a perfect storm of opportunities and challenges. Philos. Trans. Roy. Soc. B. Biol. Sci. 365, 2157–2162. doi: 10.1098/rstb.2010.0107
Chalfoun, A. D., Thompson, F., and Ratnaswamy, M. J. (2002). Nest predators and fragmentation: a review and meta-analysis. Conserv. Biol. 16, 306–318. doi: 10.1046/j.1523-1739.2002.00308.x
Chen, P., Chen, T., Liu, B., Zhang, M., Lu, C., and Chen, Y. (2020). Snakes are the principal nest predators of the threatened reed parrotbill in a coastal wetland of eastern China. Glob. Ecol. Conserv. 23:e01055. doi: 10.1016/j.gecco.2020.e01055
Collias, N. E., and Collias, E. C. (1967). A field study of the red junglefowl in north-Central India. Condor 69, 360–386. doi: 10.2307/1366199
Conkling, T. J., Pope, T. L., Smith, K. N., Mathewson, H. A., Morrison, M. L., Wilkins, R. N., et al. (2012). Black-capped vireo nest predator assemblage and predictors for nest predation. J. Wildl. Manag. 76, 1401–1411. doi: 10.1002/jwmg.388
Coxen, C. L., Frey, J. K., Carleton, S. A., and Collins, D. P. (2017). Species distribution models for a migratory bird based on citizen science and satellite tracking data. Glob. Ecol. Conserv. 11, 298–311. doi: 10.1016/j.gecco.2017.08.001
Degregorio, B. A., Weatherhead, P. J., Ward, M. P., and Sperry, J. H. (2016). Do seasonal patterns of rat snake (Pantherophis obsoletus) and black racer (Coluber constrictor) activity predict avian nest predation? Ecol. Evol. 6, 2034–2043. doi: 10.1002/ece3.1992
del Hoyo, J., Elliott, A., and Sargatal, J. (2001). Hand Book of Bird of the World. 2: New World vultures to Guinea fowl. Barecelona: Lynx Editions
Dinsmore, S. J., White, G. C., and Knopf, F. L. (2002). Advanced techniques for modeling avian nest survival. Ecology 83, 3476–3488. doi: 10.1890/0012-9658(2002)083[3476:ATFMAN]2.0.CO;2
Driscoll, M. J. L., Donovan, T., Mickey, R., Howard, A., and Fleming, K. K. (2005). Determinants of wood thrush nest success: a multi-scale, model selection approach. J. Wildl. Manag. 69, 699–709. doi: 10.2193/0022-541X(2005)069[0699:DOWTNS]2.0.CO;2
Evans, C. S., Macedonia, J. M., and Marter, P. (1993). Effects of apparent size and speed on the response of chickens (Gallus gallus) to computer-generator stimulations of aerial predators. Anim. Behav. 46, 1–11. doi: 10.1006/anbe.1993.1156
Ewers, R. M., and Didham, R. K. (2007). The effect of fragment shape and species' sensitivity to habitat edges on animal population size. Conserv. Biol. 21, 926–936. doi: 10.1111/j.1523-1739.2007.00720.x
Fu, M., Bai, Z., Jia, K., Wen, X., Feng, Y., Zhang, E., et al. (2018). Habitat selection of Hainan Eld's deer after Forest thinning in a deciduous monsoon Forest. Chin. J. Wildl. 39, 502–506.
Fu, Y., He, B., Wang, J., Wang, L., and Fu, X. (2016). Discussion and research on habitat reform and reconstruction of Hainan Eld's deer protection area in Datian town. Trop. For. 44, 26–30. doi: 10.3969/j.issn.1672-0938.2016.04.008
Ibáñez-Álamo, J. D., Magrath, R. D., Oteyza, J. C., Chalfoun, A. D., Haff, T. M., Schmidt, K. A., et al. (2015). Nest predation research: recent findings and future perspectives. J. Ornithol. 156, 247–262. doi: 10.1007/s10336-015-1207-4
Jiang, A., Jiang, D., Zhou, F., and Goodale, E. (2017). Nest site selection and breeding ecology of streaked wren-babbler (Napothera brevicaudata) in a tropical limestone forest of southern China. Avian Res. 8, 1–8. doi: 10.1186/s40657-017-0086-1
Johnsgard, P. A. (1999). The Pheasants of the World: Biology and Natural History 2nd Edn. Washington D.C.: Smithsonian Press
Johnson, R. A. (1963). Habitat preference and behavior of breeding jungle fowl in central western Thailand. Wilson Bull 75, 270–272.
Kalsi, R. (1992). Habitat selection of red junglefowl at Kalesar reserve Forest, Haryana, India Pheasants in Asia.
Klug, P. E., Jackrel, S. L., and With, K. A. (2010). Linking snake habitat use to nest predation risk in grassland birds: the dangers of shrub cover. Oecologia 162, 803–813. doi: 10.1007/s00442-009-1549-9
Kross, S. M., Mcdonald, P. G., and Nelson, X. J. (2013). New Zealand falcon nests suffer lower predation in agricultural habitat than in natural habitat. Bird Conserv. Int. 23, 512–519. doi: 10.1017/S0959270913000130
Lacki, M. J., and Lancia, R. A. (1986). Effects of wild pigs on beech growth in Great Smoky Mountains National Park. J. Wildl. Manag. 50, 655–659. doi: 10.2307/3800976
Liu, Y., and Chen, S. (2021). The CNG Field Guide to the Birds of China. Changsha: Hunan Science and Technology Press
Lloyd, P., Abadi, F., Altwegg, R., and Martin, T. E. (2014). South temperate birds have higher apparent adult survival than tropical birds in Africa. J. Avian Biol. 45, 493–500. doi: 10.1111/jav.00454
Lu, X., and Zheng, G. M. (2001). Habitat selection and use by hybrid White/Tibetan eared-pheasants in eastern Tibet during post-incubation. Can. J. Zool. 79, 319–324. doi: 10.1139/z00-203
Luo, X., Zhao, Y., Ma, J., Li, J., and Xu, J. (2017). Nest survival rate of Reeves’s pheasant (Syrmaticus reevesii) based on artificial nest experiments. Zool. Res. 38, 49–54. doi: 10.13918/j.issn.2095-8137.2017.008
MacDonald, M. A., and Bolton, M. (2008). Predation on wader nests in Europe. Ibis 150, 54–73. doi: 10.1111/j.1474-919X.2008.00869.x
Major, R. E., and Kendal, C. E. (1996). The contribution of artificial nest experiments to understanding avian reproductive success: a review of methods and conclusions. Ibis 138, 298–307. doi: 10.1111/j.1474-919x.1996.tb04342.x
Martin, T. E. (1987). Artificial Nest experiments: effects of nest appearance and type of predator. Condor 89, 925–928. doi: 10.2307/1368547
Martin, T. E. (1993). Nest predation among vegetation layers and habitat types: revising the dogmas. Am. Nat. 141, 897–913. doi: 10.1086/285515
Martin, T. E. (1996). Life history evolution in tropical and south temperate birds: what do we really know? J. Avian Biol. 27, 263–272. doi: 10.2307/3677257
Martin, T. E. (2002). A new view of avian life-history evolution tested on an incubation paradox. Proc. R. Soc. London, Ser. B 269, 309–316. doi: 10.1098/rspb.2001.1879
Martin, T. E. (2004). Avian life-history evolution has an eminent past: does it have a bright future? Auk 121, 289–301. doi: 10.1642/0004-8038(2004)121[0289:ALEHAE]2.0.CO;2
Martin, T. E. (2008). Egg size variation among tropical and temperate songbirds: an embryonic temperature hypothesis. PNAS 105, 9268–9271. doi: 10.1073/pnas.0709366105
Martin, T. E., Riordan, M. M., Repin, R., Mouton, J. C., and Blake, W. M. (2017). Apparent annual survival estimates of tropical songbirds better reflect life history variation when based on intensive field methods. Glob. Ecol. Biogeogr. 26, 1386–1397. doi: 10.1111/geb.12661
McCullough, J. M., and Londoño, G. A. (2017). Nesting biology of the black-throated Tody-tyrant (Hemitriccus granadensis) with notes on mating displays. Wilson J. Ornithol. 129, 820–826. doi: 10.1676/16-122.1
McDonald, P. G., Wilson, D. R., and Evans, C. S. (2009). Nestling begging increases predation risk, regardless of spectral characteristics or avian mobbing. Behav. Ecol. 20, 821–829. doi: 10.1093/beheco/arp066
Melville, H. I. A. S., Conway, W. C., Morrison, M. L., Comer, C. E., and Hardin, J. B. (2014). Artificial nests identify possible nest predators of eastern wild turkeys. Southeast. Nat. 13, 80–91. doi: 10.1656/058.013.0106
Noske, R. A., Fischer, S., and Brook, B. W. (2008). Artificial nest predation rates vary among habitats in the Australian monsoon tropics. Ecol. Res. 23, 519–527. doi: 10.1007/s11284-007-0403-y
Nour, N., Matthysen, E., and Dhondt, A. A. (1993). Artificial nest predation and habitat fragmentation: different trends in bird and mammal predators. Ecography 16, 111–116. doi: 10.1111/j.1600-0587.1993.tb00063.x
O’Brien, T. G., Kinnaird, M. F., and Wibisono, H. T. (2003). Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical frost landscape. Anim. Conserv. 6, 131–139. doi: 10.1017/S1367943003003172
Pedersen, Å. Ø., Asmyhr, L., Pedersen, H. C., and Eide, N. E. (2011). Nest⁃predator prevalence along a mountain birch⁃alpine tundra ecotone. Wildl. Res. 38, 525–536. doi: 10.1071/WR11031
Perrella, D. F., Zima, P. V. Q., Ribeiro-Silva, L., Biagolini, C. H., Carmignotto, A. P., Galetti, P. M., et al. (2019). Bats as predators at the nests of tropical forest birds. J. Avian Biol. 51:e02277. doi: 10.1111/jav.02277
Reidy, J. L., and Thompson, F. (2012). Predatory identity can explain nest predation patterns. Stud. Avian Biol. 43, 135–148. doi: 10.1525/california/9780520273139.003.0011
Ricklefs, R. E., and Wikelski, M. (2002). The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468. doi: 10.1016/S0169-5347(02)02578-8
Rovero, F., Martin, E., Rosa, M., Ahumada, J. A., and Spitale, D. (2014). Estimating species richness and modeling habitat preferences of tropical forest mammals from camera trap data. PLoS One 9:e103300. doi: 10.1371/journal.pone.0103300
Sanders, H. N., Hewitt, D. J., Vercauteren, K. C., and Snow, N. P. (2020). Opportunistic predation of wild Turkey nests by wild pigs. J. Wildl. Manag. 84, 293–300. doi: 10.1002/jwmg.21797
Sanders, M. D., and Maloney, R. F. (2002). Causes of mortality at nests of ground-nesting birds in the upper Waitaki Basin, South Island, New Zealand: a 5-year video study. Biol. Conserv. 106, 225–236. doi: 10.1016/s0006-3207(01)00248-8
Seibold, S., Hempel, A., Piehl, S., Bässler, C., Brandl, R., Rösner, S., et al. (2013). Forest vegetation structure has more influence on predation rate of artificial ground nests than human activities. Basic Appl. Ecol. 14, 687–693. doi: 10.1016/j.baae.2013.09.003
Sherry, T. W., Wilson, S., Hunter, S., and Holmes, R. T. (2015). Impacts of nest predators and weather on reproductive success and population limitation in a long-distance migratory songbird. J. Avian Biol. 46, 559–569. doi: 10.1111/jav.00536
Singer, F. J., Swank, W. T., and Clebsch, E. E. (1984). Effects of wild pig rooting in a deciduous forest. J. Wildl. Manag. 48, 464–473. doi: 10.2307/3801179
Slevin, M. C., Bin Soudi, E. E., and Martin, T. E. (2020). Breeding biology of the mountain wren-babbler (Gypsophila crassus). Wilson J. Ornithol. 132, 124–133. doi: 10.1676/1559-4491-132.1.124
Smith, A. T, and Xie, Y. (2009). A Guide to the Mammals of China. Changsha: Hunan Education Publishing House
Stutchbury, B. J. M., and Morton, E. S. (2008). Recent advances in the behavioural ecology of tropical birds. Wilson J. Ornithol. 120, 26–37. doi: 10.1676/07-018.1
Stutchbury, B. J. M., and Morton, E. S. (2023). Behavioral Ecology of Tropical Birds. San Diego (CA): Academic Press
Thompson, F., and Burhans, D. E. (2003). Predation of songbird nests differs by predator and between field and forest habitats. J. Wildl. Manag. 67, 408–416. doi: 10.2307/3802781
Thompson, F., and Ribic, C. A. (2012). “Conservation implications when the nest predators are known” in Video Surveillance of Nesting Birds. Studies in Avian Biology (no.43). eds. C. A. Ribic, F. R. Thompson, and P. J. Pietz (Berkeley, CA: University of California Press), 23–34.
Visco, D. M., and Sherry, T. W. (2015). Increased abundance, but reduced nest predation in the chestnut-backed antbird in costa rican rainforest fragments: surprising impacts of a pervasive snake species. Biol. Conserv. 188, 22–31. doi: 10.1016/j.biocon.2015.01.015
Wilson, G. R., Brittingham, M. C., and Goodrich, L. J. (1998). How well do artificial nests estimate success of real nests? Condor 100, 357–364. doi: 10.2307/1370277
Xiao, H., Hu, Y., Lang, Z., Fang, B., Guo, W., Zhang, Q., et al. (2017). How much do we know about the breeding biology of bird species in the world? J. Avian Biol. 48, 513–518. doi: 10.1111/jav.00934
Xiao, Z., Li, X., and Jiang, G. (2014). Applications of camera trapping to wildlife surveys in China. Biodivers. Sci. 22, 683–684. doi: 10.3724/SP.J.1003.2014.14244
Yuan, L. (2009). Field Studies on Red Junglefowl (Gallus gallus jabouillei) in Hainan, China. Beijing: University of the Chinese Academy of Sciences
Yuan, L., Zhang, C., Zhang, H., Fu, H., Lin, X., Fu, Y., et al. (2009a). Nest-site characteristics of red jungle fowl, Gallus gallus jabouillei. Zool. Res. 30, 457–462. doi: 10.3724/SP.J.1141.2009.04457
Yuan, L., Zhang, C., Zhang, H., Zhang, C., Fu, Y., Lin, X., et al. (2009b). Roosting site selection of red jungle fowl during the breeding season in Hainan. Sichuan J. Zool. 28, 652–657.
Zanette, L., and Jenkins, B. (2000). Nesting success and nest predators in forest fragments: a study using real and artificial nests. Auk 117, 445–454. doi: 10.1093/auk/117.2.445
Zheng, G. (2022). Checklist on the Classification and Distribution of the Birds of China. Beijing: Science Press
Keywords: artificial nest, breeding success, Gallus gallus jabouillei, infrared camera, nest predation
Citation: Rao X, Li J, He B, Wang H, Wu G, Teng T and Ling Q (2023) Nesting success and potential nest predators of the red Junglefowl (Gallus gallus jabouillei) based on camera traps and artificial nest experiments. Front. Ecol. Evol. 11:1127139. doi: 10.3389/fevo.2023.1127139
Edited by:
Xiang Liu, Lanzhou University, ChinaReviewed by:
Haitao Wang, Northeast Normal University, ChinaLongwu Wang, Guizhou Normal University, China
Copyright © 2023 Rao, Li, He, Wang, Wu, Teng and Ling. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaodong Rao, 993676@hainanu.edu.cn
 Xiaodong Rao
Xiaodong Rao Jialing Li3,4
Jialing Li3,4