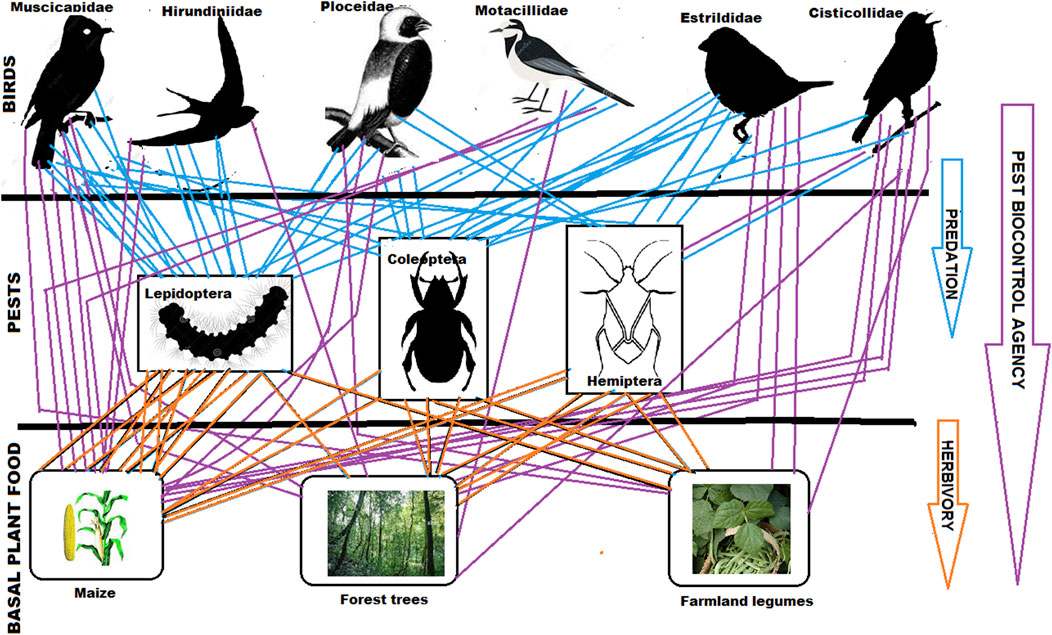
Tropical insectivorous birds’ predation patterns that promote forest–farmland trophic connectivity for integrated top–down pest biocontrol
- 1Zoology Department, National Museums of Kenya, Nairobi, Kenya
- 2Kakamega Environmental Education Project, Shinyalu, Kenya
Although conversion of natural forest to agriculture can negatively impact biodiversity in many ways, some affected species may respond by dispersing across the forest–farmland eco-zone, thereby facilitating functional connections through food-web interactions beneficial to crop production and forestry. This study examined patterns of Lepidoptera (butterflies and moths), Hemiptera (bugs), and Coleoptera (beetles) herbivory, and insectivorous bird predation within forest-adjacent farms in western Kenya, and how these processes trophically connect the two ecosystems to promote pest biocontrol. Through δ13C and δ15N stable isotope analyses, proportions of maize, farmland legumes and forest trees in pest diets, and pest-prey in bird’s diets were estimated. Birds’ habitat associations and diet specializations’ influence on pest consumption and basal plant carbon levels in birds’ tissues were determined to evaluate birds’ pest-biocontrol potential. Maize was the mostly consumed plant especially by Lepidoptera, but forest trees were peimarily consumed by Coleoptera and Hemiptera. In turn, Lepidoptera were mainly consumed by forest-associated birds, whereas Hemiptera and Coleoptera were mostly consumed by farmland-associated birds. Thus, birds showed cross-habitat pest consumption tendencies, though diet-specialization was unimportant in predicting those tendencies. Muscicapidae (flycatchers and allies); Hirundinidae (swifts and swallows); Motacillidae (pipits and wagtails); and Ploceidae (weavers) birds showed the highest contributory potential for pest biocontrol of Lepidoptera pests, but Estrildidae (manikins and waxbills), Muscicapidae, and Malaconotidae (boubous and gonoleks) birds showed the best potential against Hemiptera and Coleoptera. Furthermore, more maize basal carbon was assimilated by forest-associated compared to farmland-associated birds, whereas most basal carbon from farmland legumes and forest trees were assimilated by farmland birds, suggesting that unlike pest-prey choice, basal plant carbon pathways to avian insectivorous consumers did not strongly mirror birds’ habitat associations. Lepidoptera and Hemiptera were potentially the most significant interhabitat trophic connector arthropods, and for birds, Muscicapidae, Ploceidae, and Estrildidae. These findings show that such functional connectivity may be enhanced through increasing structural cover elements that promote insectivorous birds’ dispersal between farmland and adjacent forests to boost their pest-regulation ecosystem service contribution. The results serve to inform effective management practices by agronomists, foresters, and land-use planners toward promoting landscape-scale-integrated pest management for sustainable agriculture and biodiversity conservation.
1 Introduction
The rapid loss of forest cover to agricultural expansion is a major driver of ecological pressure on forest biodiversity due to habitat deterioration and diminishing foraging opportunities in the remnant forest patches (Bélanger and Grenier, 2002; Newmark and Stanley, 2011; Peter et al., 2015; Gonzalez et al., 2017). Furthermore, centuries of intensification of commercial agriculture, along with most farmers’ heavy dependency on chemical inputs for crop production, have done little to guarantee global food security or eliminate impacts of arthropod pests on crop yields. Instead, such agronomic practices have continued to adversely affect wildlife and human health, in addition to fostering resistance by some target arthropod crop herbivores (Gavrilescu, 2005; Tiryaki and Temur, 2010; Deguine et al., 2021). In addition, forest loss and fragmentation have rendered many forest-associated species more dependent on adjacent farmlands for survival (Berg and Pärt, 1994; Newmark and Stanley, 2011; Garbach et al., 2012). Likewise, many farm-dwelling organisms are adversely impacted not only by pollution from agrochemical inputs but also through anthropogenic disturbance and unpredictable fluxes in resource availability occasioned by agronomic and structural habitat management practices (Tscharntke et al., 2008; Rincon-Parra et al., 2022).
As a consequence of these factors, only species adapting to such perturbations can persist in forest–farm ecotones and have to disperse across habitat to obtain resources necessary for their survival (Bos et al., 2007; Gonzalez et al.;., 2017; Alvarez-Alvarez et al., 2022). For instance, bees and wasps normally establish colonies in forests but often forage for nectar on neighboring farmlands (Hill and Webster, 1995) and many butterfly species oviposit and breed on croplands but roost and hibernate in woodlands (Sosa-Aranda et al., 2018). Similarly, several avian insectivores like flycatchers and granivores like grosbeaks nest in wooded areas but forage on croplands (Douglas et al., 2014), whereas warblers, sparrows, stocks, cranes and many raptors, which feed predominantly within the farmland space, frequently utilize adjacent forests for roosting and nesting (Berg and Part, 1994). Under circumstances of profound anthropogenic disturbance such as loss or deterioration in habitat quality, such cross-boundary dispersal and functional tendencies become significantly enhanced.
There is a wide range of negative consequences of such enhanced dispersal by species. These include arthropod pest outbreaks on croplands following clear-cutting of the adjacent forest stands, crop damage by forest-associated pest birds (Peter et al., 2015), or livestock depredation by carnivorous forest mammals and birds of prey (Goswami et al., 2015). Dispersing individuals may also transport pollutants and other harmful chemical material into forests (Fujita and Koike, 2007). On the other hand, positive consequences and benefits of species’ dispersal within the forest–farm matrix may occur through enhanced ecological connections between the two adjacent ecosystems. This can support broader scale functional networks of fragmented habitat patches within the matrix (Pejchar et al., 2018; Grass et al., 2019) and may take the form of expanded food-webs arising from increased trophic interactions (Dias et al., 2019) or genetic linkages through breeding opportunities (Klinga et al., 2019). In turn, such processes can enhance biodiversity’s provisioning of important ecological services for both habitat zones, including natural pest biocontrol, crop pollination, seed dispersal, nutrient transport, and overall interhabitat energy subsidies or exchange (Decocq et al., 2016; Nyffeler et al., 2018; Grass et al., 2019). Based on such ecological processes and opportunities, biodiversity conservation practitioners now recognize that landscape management decisions for promoting sustainability of such benefits are likely to yield more long-term rewards for agricultural and silvicultural enterprises because they promote landscape resilience while also conserving biodiversity. Therefore, integrated pest management systems and field practices to conserve farm–forest landscape matrix connectivity can significantly boost cross-boundary food-web networks, and this can increase the potential for sustaining net benefits for both agriculture and forest management (Landis, 2017; Pejchar et al., 2018; Nelson et al., 2022).
Although many frugivorous and granivorous bird species mediate significant economic losses to farmers through crop damage (Firake et al., 2016; Lindell et al., 2016; Ernst et al., 2019; Kross et al., 2019), many insectivorous and omnivorous counterparts provide vital services by removing arthropod pests from many varieties of row crops (Girard et al., 2012; Garcia et al., 2018; Garfinkel et al., 2020). Depending on their foraging and ranging patterns, flocking behavior, diet specializations, or daily time budgets, some of these avian insectivores may provide pest removal services across many crop types and spatial scales (Shakelford and Corner, 1997). Nyffeler et al. (2018) estimated the annual global value of insects consumed by insectivorous birds on croplands to be in the region of 30 million metric tons. Insectivorous bird species inhabiting transitional habitat regions can be especially important in contributing to pest regulation from multiple plant hosts, whether agricultural or wild-growing, or across habitat boundaries such as farmland–prairie, farmland–wetland, or farmland–forest ecotones (Exnerová, et al., 2003; Massemin et al., 2006). Through this, such bird guilds can make significant contributions to reducing losses associated with crop damage, forest-tree defoliation, or pasture-land degradation (Exnerová, et al., 2003).
Majority of past studies on impacts of arthropod pests have focused on single crops types, single arthropod herbivore species, taxa, functional guild, or trophic relationships to a single specific suite of natural enemies or avian predator (Bendell et al., 1981; Tremblay et al., 2001; Amudavi et al., 2008; Hartman et al., 2016; Koch et al., 2016; Milligan et al., 2016; Ferger et al., 2012; Garcia et al., 2018). Few studies have extensively examined arthropod herbivory or insectivorous birds’ predation patterns within non-agricultural habitats or herbivorous arthropods’ impacts on specific non-crop host plants. Yet, in many countries in both temperate and tropical regions, substantial losses are incurred from arthropod pest damage in forestry and timber production systems, which have economic significance comparable to that of agriculture (Wylie and Speight, 2012; FAO, 2018; Li et al., 2019; Forest research, 2021). Furthermore, past studies on roles of birds in contributing to arthropod crop pest regulation in East Africa are still much fewer (Milligan et al., 2016; Otieno et al., 2019b) than those conducted in other regions (Tremblay et al., 2001; Karp et al., 2013; Garfinkel et al., 2020). Similarly, very few studies have applied stable isotopic techniques to assess the role of birds as contributors to arthropod pest regulation (Girard et al., 2012; Pagani-Núñez et al., 2017), even though a number of researchers have used them to assess diets of passerine birds in general (Hobson and Clark, 1992; Robbins et al., 2005; MacLellan, 2012; Ruhl et al., 2020). The advantage of stable isotope analyses (SIAs) over exclusion-cage, stomach-content analysis of, or other molecular techniques such as DNA barcoding, is that SIA facilitates explicit deciphering of consumers’ diet options as well as the estimation of proportional contribution of each food option to consumers’ diets, when combined with Bayesian mixing model procedures (Phillips et al., 2005; Parnell et al., 2013; Stock et al., 2018).
This study’s overall aim was to apply δ13C and δ15N stable isotope analyses in assessing patterns and extent to which trophic interactions of common passerine insect-eating birds with three herbivorous arthropod taxa mediate the interflow of diet carbon subsidies between forest and adjacent farmland habitats, with implication for natural pest biocontrol in both habitats. The specific objectives were as follows:
1) To estimate dietary proportions of maize, farmland legume plants, and forest-trees assimilated in tissues of Lepidoptera, Hemiptera, and Coleoptera pests
2) To assess dietary proportions of the three pest-food sources in the common passerine birds of the farmland–forest ecotone
3) To determine whether birds’ habitat associations and diet specializations relate to choice and dietary proportions of arthropod pests they consume
4) To evaluate birds for their relative significance in contributing to top–down pest regulation based on their dietary pest-food proportions and levels of basal plant carbon (diet carbon derived from the bottom of the food chain)
To achieve those objectives, we interrogated the following two working hypotheses: 1) farmland- and forest-associated birds predominantly consume pest-prey primarily associated with the respective habitat zones and 2) proportions of plant basal carbon assimilated into tissues of bird families directly reflect the bird’s dietary proportion of the pest-prey predominantly consuming that plant food resource.
In the first hypothesis, we assumed that forest and farmland habitats are functionally distinct with little or no trophic exchange, while the second one assumed that birds’ arthropod pest-prey were essentially monophagous with significant trophic fidelity to specific plant food sources or types.
The present study is therefore significant in presenting an integrated examination of the arthropod pest management question from a perspective of multiple consumer taxa, trophic levels and host plants within two different land use types. It is also unique in being the first in the region to use SIA to address the objectives outlined.
2 Materials and methods
2.1 Study area
The study was conducted in western Kenya’s County of Kakamega, on the farmland to the eastern side of Kakamega Forest (00o 11′ 09″N-00o 26′ 08″N and 34o 44′ 30″E−34o 51′ 26″E), an area of mid-level elevation c. 1,600 m asl with crop-field matrices, human settlements, small towns, and water courses, with the main crop grown being non-Bt maize (Farwig et al., 2008; Laube et al., 2008). The average temperature in this area is 21.4°C, and rainfall is approximately 1,800 mm per annum, with longer-period seasonal rains peaking between March and May and shorter-period rains peaking around November (The Kenya Meteorological Department, 2021). Kakamega Forest is the only true tropical rainforest in Kenya, which has undergone substantial cover loss form anthropogenic encroachment and conversion to agriculture and settlement over the past decades, leaving only 85 km2 of closed-canopy primary forest from 300 km2 nearly a century ago (Farwig et al.). As a relic of a previously contiguous continental Guineo-Congolean rainforest belt, it retains considerably high endemism of original flora and fauna, particularly birds, including two globally endangered and 15 regionally threatened species (BirdLife International, 2012; IUCN, 2023). Partly as a result of such significance, the forest is listed among Kenya’s network of Important Bird Areas and as a UNESCO World Heritage Centre (Bird Life International, 2012). Except from the last two and a half decades when official forest protection enforcement began to improve, the trends in forest’s destruction, degradation, and fragmentation had traditionally been tied to the fact that it is surrounded by farmland and a dense human population practicing mainly subsistence agriculture and small-scale livestock husbandry but with considerable dependence on the forest for wood, fiber, and other products, though this is now regulated by forest management authorities (Farwig et al., 2008). The main crop on the eastern side of the forest includes mainly non-Bt maize, which is commonly intercropped with legume crops (Otieno et al., 2019a). Farmers also typically maintain some stand of woodlots and fruit orchards, and also plant trees along property boundaries, and occasionally establish fast-growing agroforestry trees alongside row-crops (Diwani et al., 2013).
Field sampling was carried out during two maize cropping seasons (long-rain and short-rain) in 2007 at three cropping stages each between October and the following July. The cropping stages were early crop (from germination to first weeding), midcrop (from second weeding through flowering to ear formation), and mature crop (from cob hardening to harvesting). This was aimed at minimizing potential sampling bias on the temporal scale (Lancaster et al., 2006; Meadows et al., 2012).
2.2 Study farms selection and characterization
Farm selection was based primarily on the cropping system and proximity to forest such that a total of 15 farms were selected, which had fields of maize and at least some legume plants either intercropped with maize, or planted in separate field plots, or growing wild within fallow field patches. The forest’s proximity to the crop fields had to be at most 1 km to ensure there was reasonable chance of trophic interactivity between birds and arthropods across the two habitats through dispersal (Ding et al., 2011). A 2-km minimum interfarm distance was maintained to ensure sampling independence with regards to the more mobile bird and arthropod groups (Lancaster et al., 2006). Furthermore, only farms in which maize or maize with other legume crops had been grown for at least two consecutive seasons were selected, in order to reduce significant short-term probabilistic fluctuations in arthropod and bird assemblage patterns.
2.3 Arthropod sampling
Plant and arthropod samples were taken during the three crop-growing stages outlined previously. Arthropods were collected using both standard sweep nets (using the standardized 100 sweeps along transects in each farm) and pitfall traps, which comprised the standard 70-mm diameter and 120-mm high plastic cups inserted upright and flush with the ground surface (Sekamatte et al., 2003). The pitfall traps were filled to one-third volume with 25% sodium chloride solution for preservation and to maintain samples’ isotopic integrity, with and conical plastic rain shields propped above each trap. Three replicates were randomly placed along a diagonal line running across each maize field, at distance intervals which depended on the farm size. The traps were collected after 3 days. The samples were discarded, and traps were reset if flooding occurred from rainfall. Arthropods were additionally sampled on selected individual plants using a search-and-pick procedure on the leaves, stems, and flowers using forceps. At trap collection, the samples were transferred to airtight bags and frozen. Using illustrated field guides and identification keys tools, the arthropod samples were later identified to family, with only primary consumers (herbivores) retained. Subsequently, in order to achieve a sufficiently clear analytical resolution of isotopic signatures, the arthropod samples were pooled into three taxonomic groups (Orders), namely, Coleopteran (leaf beetles, flower beetles, sap beetles, and weevils), Hemiptera (leaf bugs, true bugs, and stink bugs), and Lepidoptera (moths and butterflies) (Hyodo, 2015).
2.4 Bird sampling
Bird samples were collected by the use mist nets. Bird mist-netting was conducted once on each farm, at the onset of the long rains in early April, and in each case, two 12-m mist nets were set up running perpendicular to the forest edge, one starting at the forest edge itself and a second one 25 m into the forest interior, ensuring that the two net lines were not directly aligned to each other (Newmark and Stanley, 2011; Ralph et al., 2014). The nets were set up and left out for 2 hours from 0700–0900 at each study farm, with inspections conducted every 15 min to extract captured individuals and to avoid any stressful impacts or injuries to the birds. At net inspection, the birds were identified to species, and subsequently a small portion was clipped off the tip of a primary wing feather (Hobson and Clark, 1992). Birds awaiting processing were kept in dark seclusion inside well-aerated wide-fitting cotton-fabric bags. To further prevent birds from being stressed out, it was ensured that the sampled birds were subsequently released back at the exact place of capture, within a maximum of 10 min after capture (Ralph et al., 2014). Clipped feather samples were immediately sealed in paper envelopes to minimize contamination, labelled, and sent to the laboratory.
2.5 Plant sampling
From each study farm, 12 plant-leaf samples were collected randomly (four each from maize, legume plants, and forest trees). On croplands, systematic randomization was achieved by sampling from the fifth plant of every 10th row, whereas in the forest, samples were collected from the central plant within each of 2-m radius quadrats established from forest edge toward forest interior at 10-m intervals, hence ∼100 m into forest (Ilić et al., 2018). The plants were divided into three categories for purposes of SIA, so as to establish three isotopically distinct basal food-source origins for consumers (Ostrom et al., 1997; Layman et al., 2012). The three groups were: 1) maize, a crop plant which follows a C4 photosynthetic pathway, 2) farmland legumes, field crops or farmland plants which follow a C3 photosynthetic pathway, and 3) forest trees, which follow the C3 photosynthetic pathway. Immediately after collection, the samples were sealed in paper envelopes to reduce moisture loss or air contamination, labelled, and sent to the laboratory.
2.6 Pre-test processing of samples
In the laboratory, bird feathers were rinsed in 4% solution of formaldehyde to remove excessive oil and impurities before further handling (Paritte and Kelly, 2009; Haché et al., 2012). All bird-feather, arthropod, and plant-leaf samples were then oven-dried at 60°C to constant mass before being ground to fine powder. Arthropods from each of the taxonomic group were ground whole. For each farm, two 5-mg subsample replicate portions were then prepared per sample of each arthropod and plant group. The subsamples were subsequently transferred into tinfoil capsules for subsequent isotopic analyses.
2.7 SIA for consumer diet compositions
To identify the array of consumers’ food sources, the processed samples were analyzed at the Environmental Isotope Laboratory of the iThemba Laboratory for the Accelerator Based Sciences (LABS) in Johannesburg, South Africa. These tests were conducted to determine signatures of δ13C and δ15N isotopes in the consumer and food source tissues. This was achieved using Flash HT Plus elemental analyzer coupled to a Delta V Advantage isotope ratio mass spectrometer by a ConFloIV interface combusting at 1 020°C (ThermoFisher, Bremen, Germany). These models are founded on the isotopic mass balance equation: δX = [(Rsample/Rstandard-1)] * 1 000, where X refers to 13C or 15N and R is the corresponding ratio between 13C/12C and 15N/14N, respectively, and the standards based on the International Atomic Energy Agency standard sample stock in Vienna (Phillips et al., 2005).
2.8 Data analyses
Analyses of data for arthropod consumers (pests) were conducted at three taxonomic group (order) levels (Coleoptera, Hemiptera, and Lepidoptera). Each of the bird species was assigned to one of the two diet specialization guilds and a taxonomic family. The two guilds of diet specialization were obligate insectivore (predominantly consumes arthropods) and omnivore (consumes both arthropods and plant food material). The birds were further classified into two habitat association categories, that is, those primarily associated with the forest habitat and those primarily associated with the farmland. This categorization combines the classification by Bennun et al. (2009) for forest birds and that of the field guide to the birds of Kenya and Northern Tanzania (Zimmerman et al. (1999). Bird families represented by individual species that were encountered only once were excluded from the analyses. Thus, of the total 79 species encountered representing 24 families, 70 species from 16 families were subjected to analyses (Supplementary Table S1, listing according BirdLife International and NatureServe, 2011).
2.8.1 Food source contributions to consumers’ diets
From the stable isotopic signatures incorporated in their tissues, estimates were made of relative contributions of the plant food sources to arthropod pest diets and arthropod pest-food sources to birds’ diets. This was modeled using the mixing model procedure with the MixSIAR tool in R v.4.0 (R Core Team, 2021). In addition to identifying the range of various food source options to consumer’s diets, MixSIAR incorporates appropriate fractionation discrimination values (or trophic enrichment factor (TEF)) to calculate the relative contribution of each food source to the consumer diets (Caut et al., 2009; Layman et al., 2012; Parnell et al., 2013; Stock and Semmens, 2016). TEFs serve to correct for any significant errors attributable to a consumer-specific metabolic process (Hobson and Clark, 1992; Layman et al., 2012; Parnell et al., 2013). The TEF values applied here were averages relevant to herbivorous arthropod consumers from a review by Spence and Rosenheim (2005), while for avian consumers, the values applied were those specific to avian feathers as applied by Ferger et al. (2012). In addition, evaluation was made of proportions of the plant basal carbon assimilated into birds’ tissues via the arthropod pests to track the broader pathway for the farmland–forest trophic carbon subsidy and exchange (Decocq et al., 2016). For this purpose, the TEFs of the two trophic steps (plants to pests and pests to birds) were each combined by summing up into one final value. The MixSIAR model platform further facilitates testing of the role of environmental or other habitat explanatory factors that underpin the proportional food source contributions to consumer diets (Stock and Semmens, 2016). Accordingly, in this study, the role of bird habitat association in driving dietary proportions of the various pest-food sources into birds’ diets was analyzed.
The models were run separately for pest-to-plant (herbivory) and bird-to-pest (predation) trophic linkages. In running each model, mixture data (consumer file) were input, followed by source files (food sources data) subsequently incorporating respective TEFs (Stock and Semmens, 2016). Further analyses involved selecting and running uninformative priors, designing Jags models with specific error structures (residual and process, in the present case) (Stock and Semmens, 2016) followed by running a series of Jags models starting with a test Markov Chain Monte Carlo (MCMC) chain (1,000 length, 500 burn-in iterations) and building up chain lengths in subsequent runs through “very short,” “normal,” or “long” (chain length and burn-in iterations of 1,000, 5,000; 50,000, 25,000; and 100,000, 50,000, respectively) until convergences on true posterior distributions of estimated food source proportion contribution to consumer diets were achieved from the Jags outputs. This was confirmed using the Gelman–Rubin diagnostic procedure (Stock et al., 2018). The final outputs of summary statistics and posterior density plots of consumers’ dietary proportions of food sources were then evaluated for median and mean quantile distributions and 95% credible intervals of such estimates.
Assessment was also made of the relationship between observed dietary proportions of birds’ pest-food sources, to bird family identity, arthropod taxa identity, birds’ habitat association, and birds’ diet specialization. This was achieved using permutational analysis of variance, PERRMANOVA in Primer v.7 with PERMANOVA + add-on (Anderson, 2017; PRIMER-E, 2019). Here, the models were run for main tests with 9,999 permutations. The results of the analysis were plotted using principal coordinate ordination (PCO). Finally, canonical analyses of principal ordinates, CAP, was used to evaluate and plot relative importance of bird’s habitat association and pest taxa identity, in relation to pests’ contribution to birds’ diets. All tests in PRIMER were performed on log (X+1) transformed Euclidean distance resemblance matrices derived from respective dissimilarity matrices of consumers’ dietary proportions data.
3 Results
A total of 248 individual birds were captured and of the 70 bird species identified, 11 families were obligate insectivores (six farmland-associated and five forest-associated) and five families were omnivores (three farmland-associated and two forest-associated) as outlined in Supplementary Table S1.
3.1 Plant food sources in arthropod consumer diets
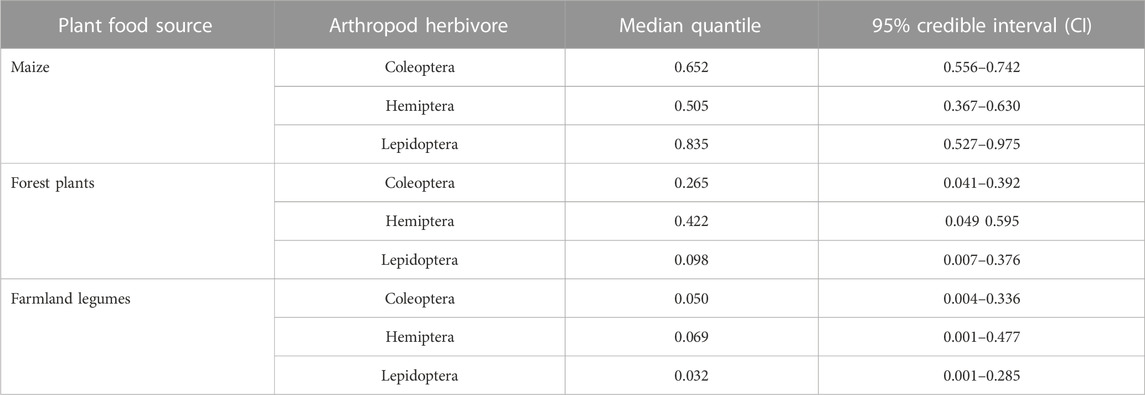
Each of the three arthropod pest taxa derived diet carbon from all the three plant food sources (Table 1). Nonetheless, in general, the bulk of pest consumer’s plant food carbon originated from the farmland rather than from the forest, though maize was more targeted by the pests than were farmland legume plants, with each of the three pest groups deriving more than half of its plant carbon from maize (Table 1). Maize was a particularly important food source for Lepidoptera and Coleoptera pests (Figure 1A), while forest trees and farmland legumes were consumed mainly by Hemiptera pests (Table 1; Figure 1B). Although Lepidoptera pests incorporated the highest proportions of plant food in general, with a major trophic focal bias toward maize, Coleoptera and Hemiptera pests showed a more widespread trophic linkage to all three plant food options (Figures 1A–C).
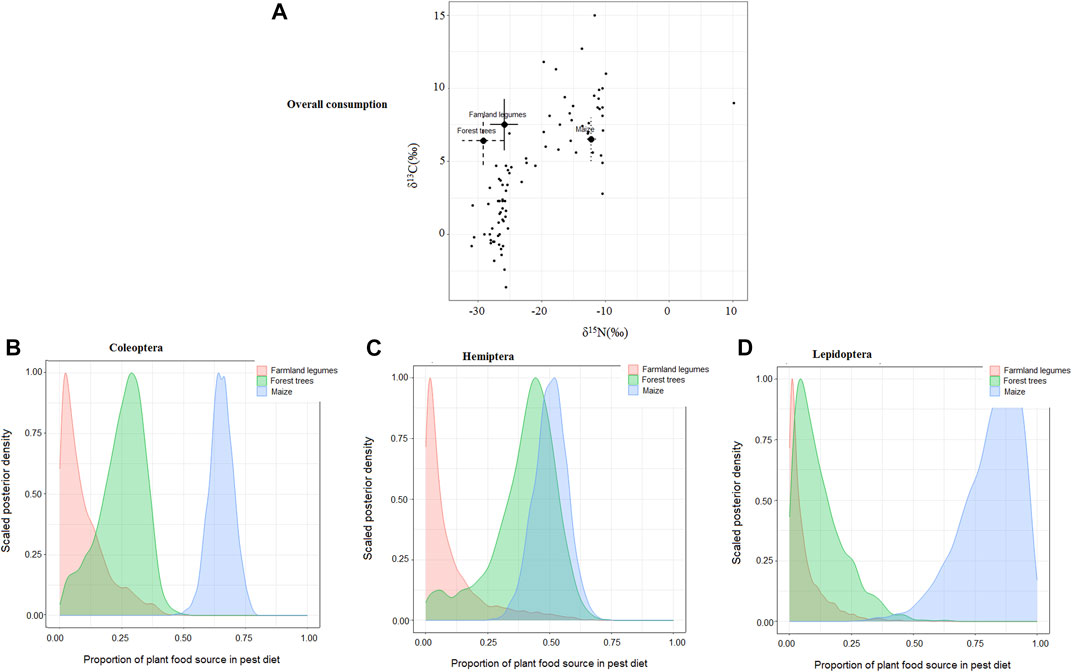
FIGURE 1. (A) Iso-space biplot showing the overall clustering pattern of consumption of plant food choices by the three arthropod pest taxa and (B–D) posterior density plots showing the estimated proportions of plant food source contributions to diets of the three arthropod pest taxa.
3.2 Arthropod pest food in birds’ diets
3.2.1 Food source proportions overall
Lepidoptera was disproportionately the most important pest-prey for birds overall (median = 0.777, 95% CI = 0.656–0.913), followed by Hemiptera pests (median = 0.136, 95% CI = 0.011-0.289), while Coleoptera was the least preferred food source (median = 0.062, 95% CI = 0.003-0.248), making Lepidoptera almost six times more preferred by birds as compared to the other two options combined (Figure 2A).
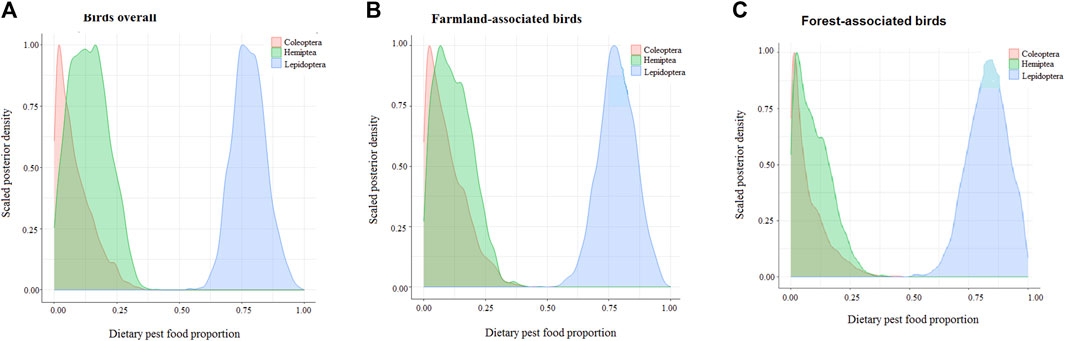
FIGURE 2. Posterior density plot comparing estimated overall proportions of the arthropod pest food sources incorporated in tissues of (A) birds overall, (B) birds associated with farmland, and (C) birds associated with forest.
3.2.2 Arthropod food proportions at bird families and habitat association scales
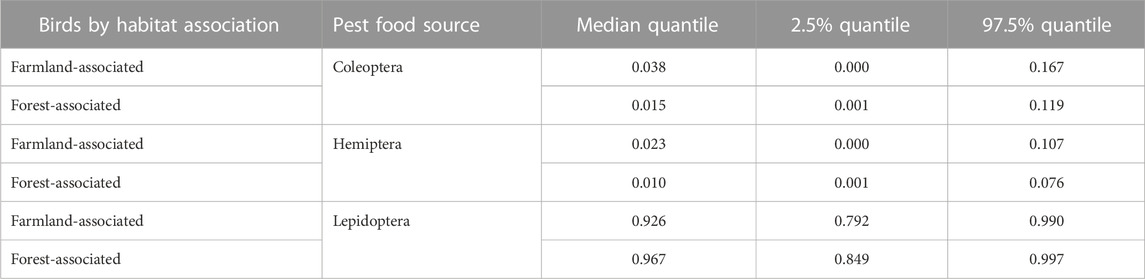
At the family level, although every bird family consumed Lepidoptera pests proportionately more than they did the other pest items (Table 2; Figure 2A), the four most significant consumers of Lepidoptera were muscicapids (flycatchers), hirundinids (swallows, martins, and saw-wings), ploceids (weavers), and motacillids (pipits and wagtails), each of these consuming a medial value of at least 85% of this pest-prey item. The four most important consumers of Coleoptera pests were estrildids (manikins, bluebills, and waxbills), muscicapids, ploceids, and malaconotids (boubous, gonoleks, and tchagras) (Table 2).
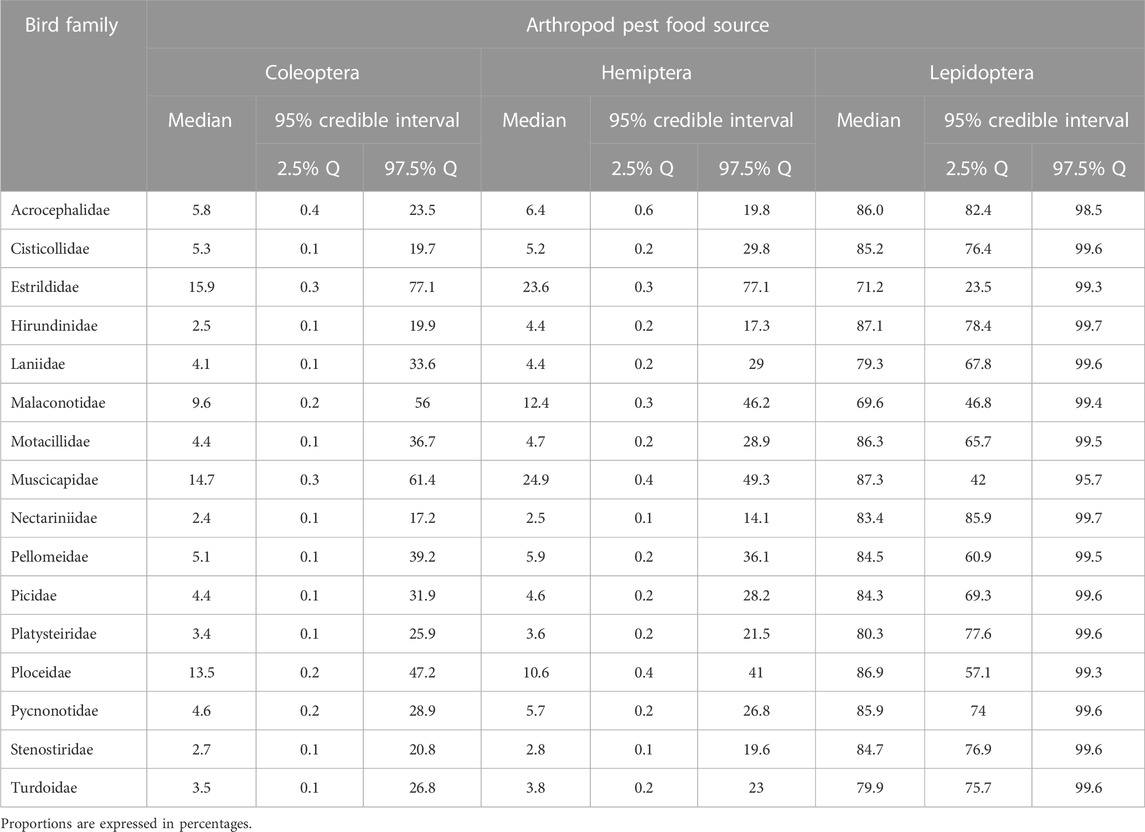
TABLE 2. Estimated dietary proportions of arthropod pest food sources assimilated by various bird families.
From the PERMANOVA results, these patterns were also revealed through the ordination analysis, with a 49% Eigenvalue in the first axis of PCO alone (Figure 3A). Nonetheless, bird’s family identities in general (F = 0.101, p = 0.676, and df = 15), their habitat associations (F = 0.432, p = 0.711, and df = 1), and diet specializations (F = 0.178, p = 0.928, and df = 1) were not as significant in explaining variations in dietary pest food proportions as were identities of pest-prey items (F = 147.7, p < 0.001, and df = 2) (Figure 3B). Furthermore, ordination plots from CAP analyses showed that birds’ pest-food choice had greater significance than habitat association in accounting for variations in birds’ dietary pest food proportions (Figure 3B), with birds targeting prey items largely regardless of their own habitat associations.
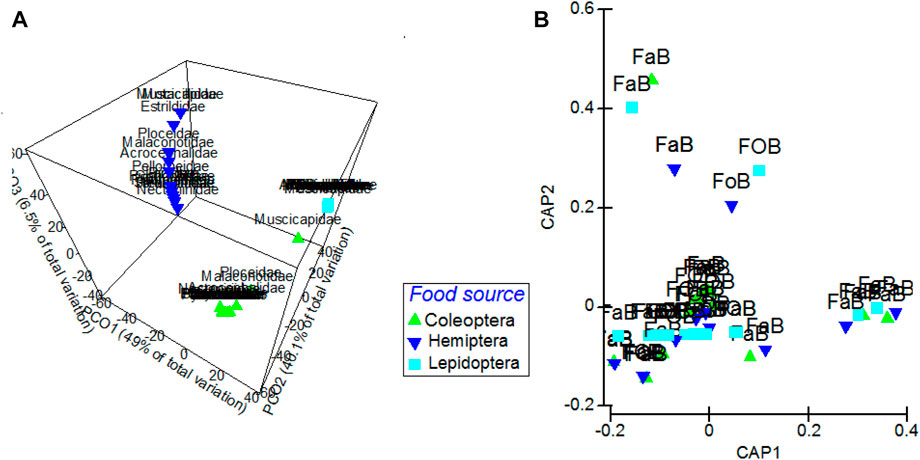
FIGURE 3. (A) Principal coordinate ordination (PCO) plots of dietary proportion distributions showing associational clustering of the various bird families to the three arthropod food source options, and (B) canonical analysis of principal ordinates (CAP) plot of the relationship between birds’ habitat association and pest food proportions in birds’ diets. FaB = Farmland-associated birds and FOB = Forest-associated birds. Ordination is based on log (X+normalized resemblance matrix of the Euclidean distance of the dietary proportions data.
Birds associated with farmland consumed higher proportions of Coleoptera and Hemiptera pests, which they derived mostly from forests (Table 3; Figure 2B). Forest-associated birds, on the other hand, incorporated higher proportions of Lepidopteran pest-prey items, mainly associated with the farmland habitat (Figure 2C).
3.3 Plant diet carbon in bird tissues
Of the three basal plant food options, maize showed the highest proportion in tissues of birds in general (median = 0.889 and CI = 0.744–0.979) followed by forest trees (median = 0.086 and CI = 0.006–0.240) and farmland legumes (median = 0.015 and CI = 0.001–0.079).
3.3.1 Proportions of basal plant diet carbon in birds by habitat association
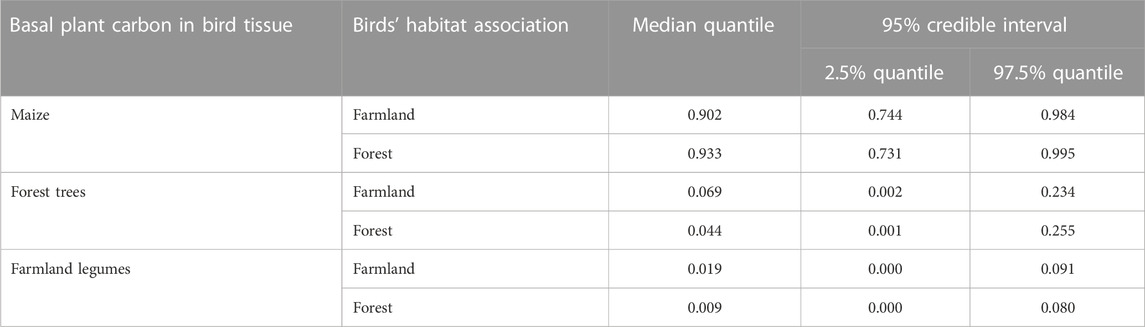
Counterintuitively, higher proportions of maize diet carbon were detected in tissues of forest-associated birds compared to those detected in farmland birds (Table 4; Figures 4B, C). Conversely, higher proportions of forest-tree carbon were evident in farmland-associated birds, even though the farmland-associated birds also assimilated higher proportions of legume plants (Table 4; Figure 4B).
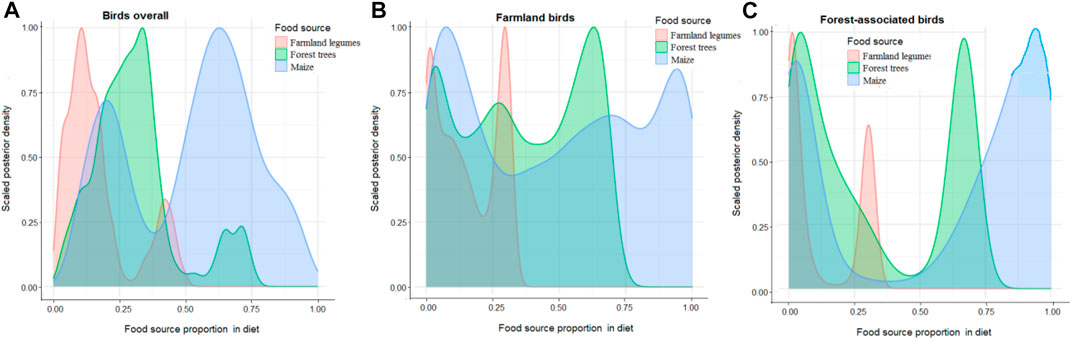
FIGURE 4. Posterior density plots of the estimated proportions of basal plant diet carbon incorporated in tissues of (A) birds overall; (B) farmland-associated birds; and (C) forest-associated birds.
3.3.2 Proportions of basal plant diet carbon in birds at family scale
Whereas proportions of maize diet carbon was the highest in muscicapid, cisticollid (warblers and allies), ploceids, and stenosterid (blue flycatchers) tissues, diet carbon from forest trees occurred in highest proportions in tissues of muscicapids, estrildids, malaconotids, and acrocephalids (yellow warblers), while the leading assimilators of farmland legume carbon were estrildids, muscicapids, ploceids, and malaconotids (Table 5). Just as was seen for the overall case, of the three basal plant food sources, the highest proportion of dietary carbon across all bird species was contributed by maize.
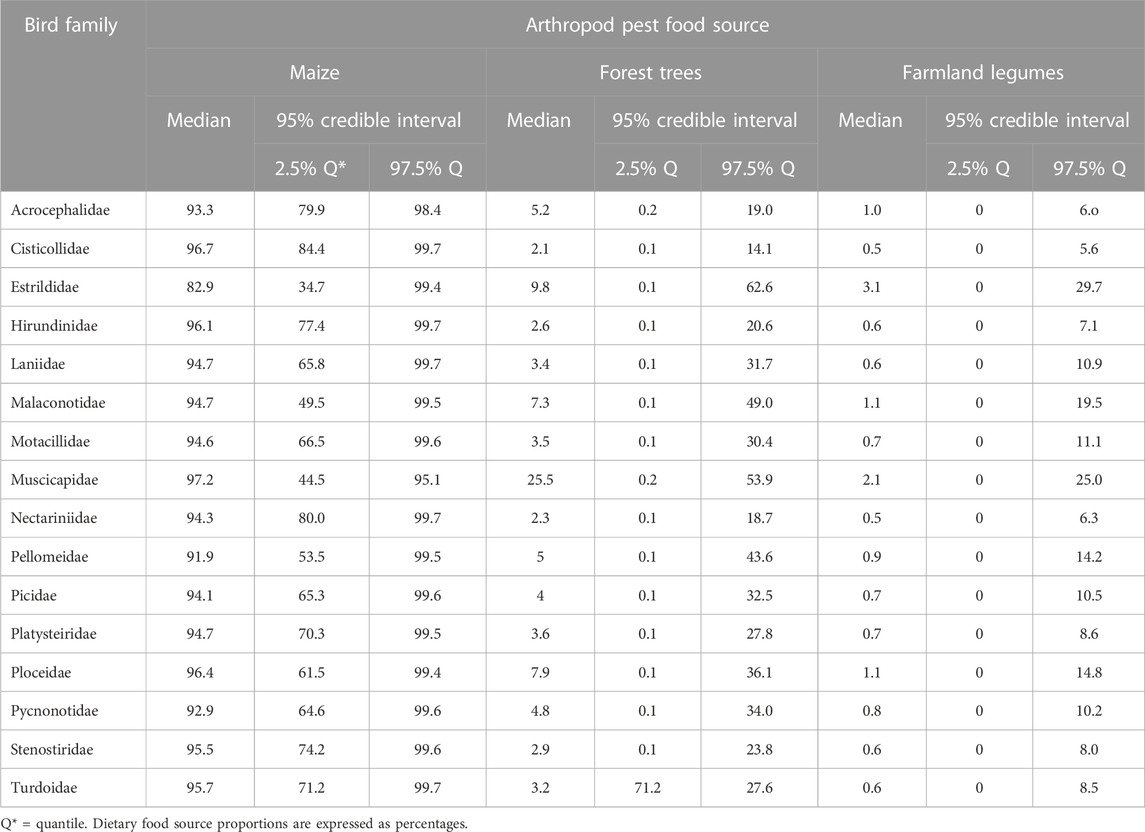
TABLE 5. Estimated median dietary proportions of basal plant diet carbon incorporated in tissues of various bird families.
These patterns were also depicted by PCO ordination results (Figure 5B), with PERMANOVA test outcomes further showing that variations in proportions of basal plant diet carbon in bird’s tissues may be significantly explained by birds’ diet specialization (F = 7.520, p = 0.002, and df = 1) and plant food choices (F = 171.4, p < 0.01, and df = 2), but not by birds’ family identity (F = 0.881, p = 0.628, and df = 15) or habitat associations (F = 0.812, p = 0.415, and df = 1). The PCO ordination here showed a total of 47% of these similarity patterns explained by the Eigenvalues of the first two axes (Figure 5B).
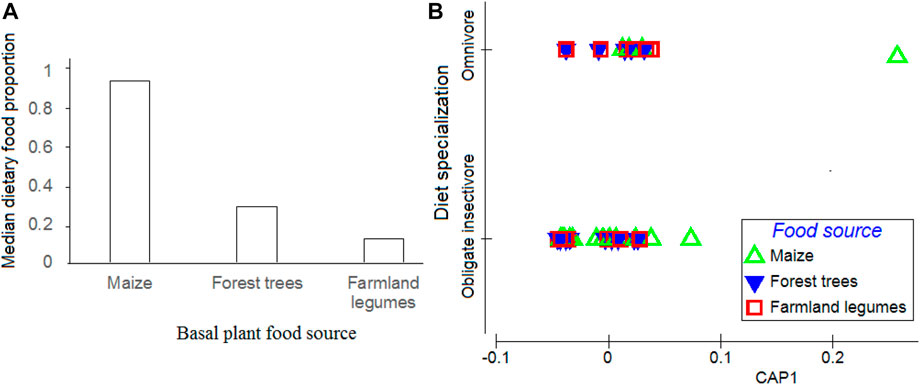
FIGURE 5. (A) Estimated median dietary proportion of basal plant food sources to diets of birds in general. Error bars represent standard deviations at 95% confidence intervals and (B) canonical analysis of principal ordinates (CAP) plot showing associational clustering of various birds to the three basal plant food sources based on diet specialization. Ordination is based on log (X+normalized resemblance matrix of the Euclidean distance of the dietary proportions data.
4 Discussion
Even though the location of agricultural land in forest neighborhoods may not always compensate for the loss of native forest cover (Douglas et al., 2014; Kross et al., 2020), this study found nearly equitable representation of bird families between the two adjacent ecosystems. This is significant as it indicates that this farm–forest ecotone bears correspondingly equal importance to the respective avian communities (Deikumah et al., 2017). Within the prevailing land use systems in the study area, much of the original old-growth forest has been significantly reduced and fragmented over the decades to give way for agriculture and human settlement (Farwig et al., 2008). This study, therefore, has revealed that the surrounding farmland is important as a non-permanent but dynamic buffer providing dispersal and foraging opportunities to many affected non-specialist but forest-dependent insectivorous birds (Laube et al., 2008; Newmark and Stanley, 2011). Şekercioğlu (2002) demonstrated that, in fragmented forests, opportunity for insectivorous birds to utilize adjacent non-forest habitat or to disperse to the nearest forest patch is critical for long-term persistence. This is due to the necessity for such birds to compensate for declines in abundance and variety of prey resources occasioned by fragmentation-driven declines in sizes of foraging habitat and ranges. Conversely, the forest habitat may be of strategic significance to farmland insectivores for roosting, nesting, and as refuge from predation risk (Berg and Pärt, 1994). These complementary values of the two adjacent habitat types may thus promote the conservation of birds and other taxa, thereby contributing to overall landscape-scale functional diversity through expanded ecological networks underpinned by trophic interactions (Grass et al., 2019; Kross et al., 2020). This can, in turn, potentially promote provisioning of the ecological service of crop pest regulation through cross-boundary spillover effects of vertebrate and invertebrate predators associated with either habitat zone (Blitzer et al., 2012; Lucey and Hill., 2012; Gonzalez et al., 2017). However, the observed dominance of obligate insectivores within the assemblage of birds assessed in this ecotone primarily confirms that many species of this guild actually disperse widely within the forest–farmland mosaic to track the variety of arthropod prey or other proximate resources across habitat boundaries (Jones et al., 2005a). The predominance of obligate insectivores may further be attributed to the general dominance of avian insectivory in most ecosystems when compared to other avian diet guilds (Nyffeler et al., 2018). The significance of this finding is that insectivorous birds of both habitat zones bear a greater potential for facilitating the functional connectivity of the two habitat types despite the inherent anthropogenic perturbations in both zones.
4.1 Plant food sources in arthropod pest diets
Although a number of individual arthropod pest species are known to be monophagous and thus exhibit trophic fidelity to specific crop or non-crop host plants (Gates, 1980), it was evident from this study that at the taxa level, each of the three pest groups obtained food from both crops and non-crop plants. From the perspective of spatial and temporal fluxes in resource abundance such as the cycles of crop planting seasons followed by after-harvest transitions, such taxon-level food resource diversification and flexibility are important for the herbivores’ survival and persistence (Driscoll et al., 2013). However, the herbivores ‘apparent overall preference of maize over the other two plant food sources likely owed to the position of maize as the predominant and most abundant subsistence row-crop, as well as being the most common across these particular farming landscapes (Laube et al., 2008; Otieno et al., 2019a) For this reason. therefore, of the three plant food options assessed here, maize was the most readily available to most herbivorous species of the three arthropod taxa. An additional plausible explanation is that maize was more frequently encountered than forest trees or farmland legumes within the ecotone region, especially since legumes are typically planted primary as intercrops in between maize rows. In comparison to the other two plant food source options, maize is also more likely to be selected by arthropod herbivore consumers due to relatively higher interaction likelihood. This is because in addition to its value as a food source, maize’s structural attributes also offer a wide variety of niches and microhabitats for various herbivorous arthropods that can utilize these for oviposition, aestivation, or refuge from predation risk (Broad et al., 2008; Suckling et al., 2017). For these reasons, the leading role of Lepidoptera pests as maize consumers shows that Lepidoptera constitutes the most significant arthropod pests of maize in this region. This pattern is well documented form studies in many maize growing regions of the world (Amudavi et al., 2008; Suckling et al., 2017; Khan et al., 2022).
On the other hand, the results showed Hemiptera bugs to be potentially the most significant pests of forest trees and farmland legumes. For farmland legume plants, such hemipteran herbivores would mainly include leaf bugs such as stink bugs (Penatomidae) and plant bugs (Miridae) (Hartman et al., 2016; Garfinkel et al., 2020) and sap-sucking bugs such as aphids (Aphididae) (Koch et al., 2016), while for forest trees, these include mainly the sap-sucking bugs of the scale insect variety (Coccomorpha). As insect pests, scale insects are among the most economically important in timber production systems worldwide (MacLean, 2016; Ülgentürk and Dokuyucu, 2019; Marini et al., 2022), although some Lepidopteran pest species in the genus Hypsipyla, Condylorrhiza, and Doratifera are also recognized for causing serious damage in tropical forestry through tree defoliation (Wylie and Speight, 2012).
It was noteworthy that despite being a widespread taxa across many habitats especially croplands, and although they essentially derived their diet carbon from all the three plant food sources, Lepidoptera showed the strongest trophic connection bias toward maize with only marginal dependence on farmland legumes and forest plants (Broad et al., 2008). Contrastingly, despite consuming lower proportions of maize when compared to the case for Lepidoptera, Hemiptera, and Coleoptera pests exhibited nearly proportionate trophic linkages across the range of three plant food resources. This further underscores the relatively higher threat that Lepidoptera herbivores pose to maize as compared to most coleopteran and hemipteran pests (Khan et al., 2022). It also indicates the relatively lower herbivory risk that Lepidoptera pose to farmland legumes and forest plants in this region. However, Wylie and Speight (2012) showed the risk of certain Lepidoptera pest species to specific timber varieties across the world. On the other hand, the finding also implies that by not being trophically biased in their plant food selection, Coleoptera and Hemiptera pests are likely more resilient herbivores and more capable than Lepidoptera, of thriving in diversified landscape matrices bearing row-crops and patches of natural habitat. For instance, Ülgentürk and Dokuyucu showed that some Hemiptera species within the scale insect family (Pseudococcidae and Hemiptera) have a wide range of polyphagy covering up to 300 host plant species which they target for herbivory. The authors observed that the host plants spanned a wide spectral range of land uses and husbandry types from agriculture, forestry to greenhouse horticulture, and even wild-growing species.
4.2 Arthropod pest food sources in bird diets
The dominance of Lepidoptera in bird’s overall diets highlights this pest taxa’s abundance, landscape spread, and availability to insectivorous bird consumers. This pattern has been reported from many past studies in many types of croplands (Janzen, 1988; Sosa-Aranda et al., 2018). As seen previously, this pest taxon derived its diet carbon from all of the three plant food sources across both the forest and farmland habitats. This observation should be particularly relevant in association with passerine bird’s breeding periods during which the fledgling young are fed exclusively on easily available invertebrate prey such as butterfly and moth larvae (McKinnon et al., 2012; Sánchez-Fernández et al., 2020). In addition, of all arthropod herbivores in farmland and other ecosystems, Lepidoptera have one of the highest taxonomic diversities with a wide range of host food plants most of which are also used for habitation, oviposition, and aestivation (Goldstein, 2017; Suckling et al., 2017). However, the considerably low level of birds’ consumption of Hemiptera and Coleoptera pests is not easy to explain, given that most species of these taxa also breed during the same periods as do Lepidoptera and should therefore be abundant and widely available to insectivorous birds (Johansson et al., 2020), albeit at somewhat lower scales compared to Lepidoptera. In fact, a number of studies have cited Coleoptera pests as being nearly as preferred as Lepidoptera to many insectivorous birds (Nyffeler et al., 2018).
In terms of pest regulation roles, by being the most avid predators on Lepidoptera pests, muscicapids, hirundinids, ploceids, and motacillids seemed to be the most important contributors to the removal of these pest taxa from the cropland. Similarly, muscicapids, estrildids, malaconotids, and ploceids seemed to be the most significant contributors to removal of Hemiptera and Coleoptera pests’ from farm legumes and forest plants. Indeed, by consuming approximately more than double the proportions of Hemiptera and Coleoptera compared to any other bird species, muscicapids, estrildids, and ploceids would be the most efficient avian contributors for the regulation of these pest taxa in the forest–farmland zone as a whole. These findings are supported by some related past studies on avian–arthropod feeding relations in farmland habitats. For instance, Ferger et al. (2012) showed that muscicapids flycatchers, ploceid weavers, and blue flycatchers (Stenosteridae) were the leading overall arthropod pest consumers on the farmlands of Kakamega County’s sugarcane-dominated farming landscape, which is located to the western side of Kakamega Forest opposite to the area of the present study. A study within an east European forest–farmland mosaic that encompassed row-crops, pasture, and hedgerows also reported the importance of Hemiptera herbivores in diets of several muscicapid insectivorous bird species (Exnerová et al., 2003). However, so far, few quantitative studies have been conducted on the roles of hirundinids or motacillid birds in the removal of insect pests from agricultural crops. Similarly, no published reports have highlighted the significance of estrildid waxbills and manikins, or weavers in regulating Hemiptera or Coleoptera pests of forest plants, the latter two being more commonly associated with damage to cereal crops.
With regards to removal of specific arthropod pest taxa, a review by Jones et al. (2005b) showed that most caterpillars (Lepidoptera larvae) constituted substantial proportions of the diet of most insectivorous farmland birds across many parts of the United Sates. Garfinkel et al. (2020), however, demonstrated that the maize arthropod pests targeted most by insectivorous farmland birds were corn rootworms (Diabrotica barberi, Chrysomelidae: Coleoptera) and tarnished plant bugs (Lygus lineolarus, Miridae: Hemiptera) rather than the Lepidoptera group. Tremblay et al. (2001) further indicated that the levels at which lepidopteran cutworm pest (Agotis sp) populations were regulated in some American cornfield by insectivorous birds were not very different from those levels observed for corn leaf aphids (Rhopalosiphum maidis, Aphididae: Hemiptera), weevils (Sphenophorus sp., Curculionidae: Coleoptera), or corn rootworms. The authors did not, however, provide the pests’ trophic linkages to any of the bird species.
Such variations in study findings, though partly latitudinal, also suggest that although Lepidoptera arthropods may be the most widely targeted pest group, insectivorous birds’ dietary choices and trophic fidelity to the range of arthropod food is largely flexible and may be subjected to a wider array of opportunistic local factors. These may include the landscape structural context, regional climate, and finer-scale anthropogenic impacts such as farmers’ selective and discretionary application of chemical inputs in crop production or weed control (Kross et al., 2019; Kross et al., 2020). Such diet flexibility is particularly important for the survival of forest-associated generalist insectivores, in the face of loss, fragmentation, or changes in quality of their forest habitat resulting from anthropogenic impacts (Şekercioğlu, 2002). Furthermore, certain cases are considered in which some insectivorous birds opportunistically predate on beneficial arthropod pest’ natural enemies (Grass et al., 2017; Pejchar et al., 2018) resulting in trophic cascades that may undermine overall pest suppression, especially where the dominant arthropod pests occur at outbreak abundance scales. In such cases, for instance, farmland avian predators may consume more Hemiptera than Lepidoptera prey during a leaf aphid outbreak period (Gonzalez et al., 2017; Riedl et al., 2018). Similarly, they may predate on more Coleoptera prey on croplands next to a recently clear-cut forest stands due to local upsurges in such prey items dispersing from the disturbed habitat (Yard et al., 2002; Exnerová et al., 2003; Bakx et al., 2020).
Despite these individual trends and patterns in insectivorous bird’ predation, family-level bird identity, habitat association, or their diet-guild specialization did not seem to be important predictors of bird communities’ overall dietary pest-food proportions. In other words, whether Muscicapidae or Laniidae, Cisticollidae or Ploceidae, farmland- or forest-associated, did not matter in generally explaining how much pest food from the various options was likely to be consumed by birds overall. This means, in particular, that there was no remarkable dominance of any one bird family in the rate of consumption of arthropod pests which is, however, is contrary to findings by Maas et al. (2015) in Indonesian cacao agroforestry systems. Likewise, it did not seem to matter whether birds were obligate insectivores or omnivorous; hence each family had similar contributory potential to the total proportions of food incorporated from across the range of options into diets of birds overall. However, identities of pest-prey items were important in predicting overall pest-food proportions in birds’ diets. Therefore, just as was as seen earlier on, muscicapid, hirundinid or ploceid birds would more likely consume Lepidoptera pests whereas, estrildids, malaconotid and ploceid birds consume Coleoptera and Hemiptera pest food resources without regard to whether these were closely linked to farmland or forest. On the one hand, this implies that birds of either zone are likely to disperse across the boundaries in order to secure preferred pest-prey types in the right proportions. Furthermore, birds of particular habitat zones are unlikely to discriminate against prey associated with the opposite habitat, when they opportunistically encounter these, as long as such prey are of a suitable taxa. The implication here is that pest-prey items were generally available at abundance scales that justified choice discretion among most bird families. This could be presumably based on prey suitability or quality, such that Lepidoptera prey were the most preferred overall. This presumption is founded on the tendency of predatory passerine birds to exhibit diet selectivity when prey resources are non-limiting (Jones et al., 2005b). Furthermore, in their study, Razeng and Watson (2014) showed that such selectivity can be significantly driven by birds’ perception of the relative nutritional value of prey items. This fact might partly explain the global decline in populations of insectivorous birds in small forest fragments whose sizes can no longer support many such consumers due to reducing biomass and variety of high quality insect prey (Şekercioğlu, 2002; Peter et al., 2015).
4.3 Plant basal carbon in bird tissues
Contrary to expectation, forest-associated birds exhibited higher proportions of tissue-assimilated maize-derived basal diet carbon when compared to the case for farmland-associated birds. This means that substantial quantities of arthropods consumed by forest-associated birds, in turn, derived their basal plant food from maize. This is confirmed by the aforementioned results that Lepidoptera had the strongest trophic connection to maize, which is a farmland crop plant. It is also supported by the fact that Lepidoptera were the most highly consumed pest by birds in general, including by forest birds. In contrast, the bulk of the forest-tree diet carbon was detected in farmland-associated birds, showing that these birds derive considerable basal plant carbon through arthropod herbivores of forest trees, especially Coleoptera and Hemiptera, most of which are also known to be abundant in forests and wooded habitats (Bos et al., 2007; Bellamy et al., 2018). These patterns suggest that the forest-associated birds observed in this study do play more significant roles than do their farmland counterparts in contributing to the removal of arthropod pests within the open farmland. This may be accounted for in two possible ways. First, Lepidoptera pest-prey, which was mainly consumed by forest birds, may be so significant in the diets of most forest birds that such birds are prepared to disperse into the farmland to track them down. The second possibility is that some Lepidoptera herbivores within the farmland also disperse into the forest for various purposes, including breeding, refuge, or roosting, during which they are consumed by many forest birds.
Similarly, forest-tree basal carbon occurring in the highest proportions in farmland-associated birds points to the possibility that many farmland birds also cross habitat boundaries to forage on forest-tree arthropod herbivores. This may particularly owe to the importance of forest and other wooded habitats adjacent to farms as roosting, nesting, breeding, or refuge habitats for such birds (Berg and Part, 1994). Indeed just as in the case for forest birds, while in the forest, such farmland birds may also predate on arthropod pest-prey that primarily consume forest trees and thus acquire forest-based plant carbon, and in the process, contribute to the removal of forest-tree pests. Such dynamic dispersal opportunities and trophic interaction options may be pivotal in facilitating avian and arthropod functional roles in promoting ecological connectivity and energy exchange between the agricultural and forest ecosystems (Alvarez-Alvarez et al., 2022). Furthermore, maize’s highest proportion in birds’ tissues at both the overall and the family levels underscores its importance over legume plants in the overall sustenance of all insectivorous birds across the farm–forest edge zone in this broader agrarian landscape.
The high proportions of maize carbon assimilated by muscicapid flycatchers, cisticollid warblers, ploceid weavers, and hirundinid birds primarily confirms that these four families mainly consumed maize arthropod pests, and this has a number of explanations. For instance, majority of these bird families frequently occur in the farmland or open habitat and therefore have a higher likelihood of trophic interaction with maize arthropod pests. Additionally, ploceid weavers are omnivorous feeders and thus may obtain a part of their maize diet carbon through direct granivory in addition to preying on maize arthropod pests such as Lepidoptera. By comparison, in addition to muscicapids and ploceids, the highest proportions of basal plant carbon from forest trees and farmland legumes appeared in estrildid and malaconotid bird families, which indicate that besides their dietary intake of Lepidoptera, these bird families significantly depend on pests of legumes and forest trees. Therefore, estrildid and malconitid birds could be uniquely suited as important contributory agents of arthropod pest biocontrol in legume crops and in forest trees. Within the context of birds’ roles in interhabitat connectivity, these patterns clearly demonstrate the pathway for the diet carbon exchange between the farmland and the forest habitats by trophic interactions. Thus muscicapid flycatchers and malaconotids are the forest-associated bird families that are most likely responsible for the high levels of farmland diet carbon in the overall forest birds. On the other hand, estrildids, ploceids, and hirundinids may be responsible for the high forest diet carbon in farmland birds’ tissues. Therefore, in functional terms, muscicapids, ploceids, estrildids, and hirundinids, either by being the leading consumers of the most important pest-prey or by virtue of their well-known wide mobility within the farm–forest ecotone, are potentially the best avian agents in contributing to arthropod pest biocontrol across the two habitats. They may also be efficient ecological connectors of fragmented forest patches within the forest–farmland habitat mosaic (Garbach et al., 2012; Alvarez-Alvarez, et al., 2022). Ploceids and estrildids should be particularly instrumental for this role given their typically gregarious, highly-itinerant, and sporadic-dispersal foraging patterns (Yard et al., 2002). Kross et al. (2019) suggested that such connectivity may be enhanced even further thorough establishment of such seminatural features as hedgerows, fallow-field patches, and other forms of woody vegetation within the farmland, which Lourenço et al. (2021) also showed to be important in supporting birds’ provisioning of arthropod pest biocontrol services within vineyard orchards. Such features can additionally help in boosting the recovery of populations of beneficial parasitoid wasps following stochastic population crashes (Schindler et al., 2022).
Considering the dietary proportions of pest-prey against proportions of basal plant carbon assimilated in bird’s tissues, some variations were evident. For instance, cisticollid warblers were not among the leading consumers of Lepidoptera pests which were the principal herbivores of maize, or of Hemiptera, the main pest of forest trees and legume plants. Yet, these warblers were among the groups which incorporated maize diet carbon in the highest proportions. This implies that cisticollid warblers derived much of their maize diet carbon through arthropod pest-prey belonging to taxa beyond the scope of the three that were examined here, but which also constituted maize pests. As such, despite not being as trophically dependent on Lepidoptera pest-prey, these warblers do not necessary play a role subliminal to that of flycatchers, swallows, or weavers, in contributing to maize pest biocontrol. Similarly, although motacillids evidently exhibited considerable Lepidoptera carbon in their tissues, they showed relatively insignificant proportions of assimilated maize carbon, implying that these birds derived Lepidoptera pest-prey from plant sources extending beyond maize. However, these discrepancies may also arise from the intraguild predation processes (Sanders et al., 2011), which were, however, not focal to the present study. For instance, cisticollid warblers could also derive maize carbon through consuming arthropod natural enemies of maize pests such as ants and spiders (Sanders et al., 2011; Otieno et al., 2019a).
From the ordination analysis results, the more significant role of arthropod prey taxa and diet specialization over habitat association or family identity in accounting for birds’ basal plant food signatures demonstrates that birds consumed pest food based on significant fidelity to diet guild orientation. It also shows therefore, that bird’s associations to each habitat zone did not necessarily translate to them incorporating significantly more basal plant food from that zone. Further, the finding points to availability of prey resources being in such sufficient abundances as to allow birds of both guild classes to feed on a broad spectrum of prey options in accordance to their specific diet orientation without omnivores species having to switch to herbivory at any time, for instance. This is unsurprising, considering the theory of relatively higher variety of prey items within most tropical ecosystems as compared to those in temperate latitudes (Willig et al., 2003; Andrew and Hughes, 2005). As is widely known, in colder temperate regions, the arthropod prey resource supplies undergo significantly higher seasonal fluctuations, with significant declines in distribution and abundances during winters. This imposes dietary plasticity in avian omnivores in such regions to rely less on arthropods but more on plant-based food during this time (McKinnon et al., 2012; Garfinkel et al., 2020; Kross et al., 2020).
5 Conclusion
The overall high proportion of maize in diets of all three pest taxa reflects how important the crop is in the diets of these arthropod herbivores across the study area, but especially for Lepidoptera, while Hemiptera and Coleoptera pests were more liberal in their diet choices. Similarly, Lepidoptera pests were the favorite prey source for most insectivorous birds regardless of bird’s diet specialization but especially forest-associated birds, while Hemiptera and Coleoptera pests were consumed nearly proportionately by both farmland- and forest-associated birds. Thus, with regards to predation, prey taxa identity and habitat association were highly important in explaining the overall distribution of pest food proportions in birds’ diets. Furthermore, through the comparative tests of arthropod–bird and plant–bird trophic connections, the study showed that birds incorporated significantly more basal plant diet carbon from maize than from other plant sources, and that as the key drivers of these patterns, five avian families, namely, muscicapids, estrildids, ploceids, malaconotids, and hirundinids, are the most important agents of trophic connectivity of the two habitat zones. Thus, integrated pest management practices for maximizing birds’ functional roles as contributors to top–down arthropod pest suppression should include measure to conserve such keystone interhabitat connector species. Accordingly, assessing the proportions of basal plant carbon assimilated in predators’ tissues to complement results from direct-predation assessments offers a more explicit underpinning of the predators’ role in driving the herbivory component of the prevailing food-web linkages. In particular, such a procedure is necessary for evaluating predators’ relative importance in biocontrolling pest-prey that target the respective plants for herbivory. In small-scale farming ventures, such as the one predominant in much of East Africa, collaborating with forest-edge farmers to embrace agronomic practices that enhance landscape structural complexity such as through agroforestry and maintaining hedgerows or uncultivated margins would be especially important (Maas et al., 2015; Lindell and Dayer, 2022). For example, native trees on farms have been shown to be attractive to insedtivirous birds at high densities and variety since they serve as feeding habitats as well as stepping-stone elements facilitating birds’ dispersal across the landscape (Douglas et al., 2014), thus boosting the trophic component of the broader functional ecological linkage across the farm-forest mosaic (Bos et al., 2007; Stewart et al., 2019; Kross et al., 2020; Alvarez-Alvarez et al., 2022; Otieno and Pryke, 2022).
6 Limitations of the study and further research
This study examined arthropod pests at the Order taxa levels to assess their trophic linkages to plant food sources and to their avian predators from a stable isotopic perspective. In our opinion, this approach offered the most optimal analytical resolution for a spatio-temporal mapping of the pest-prey trophic interaction in the landscape. However, through this strategy, the study did not capture roles that individual pest species or genera might play in contributing to the overall dietary proportions assimilated. Second, because of the single-event effort in mist-netting, a number of bird species might have been missed out within the study sites, and therefore for a more complete picture of the trophic linkages, future studies in the study area should consider increasing effort in the bird-capture exercise. Third, a more complete assessment of bird’s relative importance in contributing to the removal of arthropod pest in each of the three plant groups examined here could be achieved by assessing a broader spectrum of herbivore taxa than the three that are treated here. Finally, more in-depth studies incorporating both stable isotope analyses and a sampling protocol for exclusion of either birds or pests, and plant damage rate assessment, would facilitate a precise dollar-value quantification of the pest control services provided by the birds.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by National Museums of Kenya and Kenya Wildlife Service.
Author contributions
NO: methodology, formal analysis, investigation, data curation, data analyses, writing—original draft, and project administration. JM: field investigation, bird systematics and identification, data collation, and draft manuscript approval. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the German Catholic Academic Exchange Service (KAAD) and Ornithological component of the Biodiversity Transect Analysis in East Africa (BIOTA-East 11).
Acknowledgments
The authors would like to thank all of the landowners in the entire eastern region of Kakamega Forest where the study was carried out for permitting access to their property on multiple occasions for purposes of the research. The authors also thank the local leadership for the administrative facilitation of our interaction with the farmers and Benson Munyasia for field assistantship help. The National Museums of Kenya provided additional administrative logistical support for the project. The field work complied with the research regulatory framework of the laws of Kenya regarding handling of wild animals, and approval from the wildlife authorities at the time of the study.
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2023.1194267/full#supplementary-material
References
Alvarez-Alvarez, E., Almazán-Núñez, R., Corcuera, P., González-García, F., Brito-Millán, M., and Alvarado-Castro, V. (2022). Land use cover changes the bird distribution and functional groups at the local and landscape level in a Mexican shaded-coffee agroforestry system. Agric. Ecosys. Environ. 330, 107882. doi:10.1016/j.agee.2022.107882
Amudavi, D., Khan, Z., Pickett, J., Lynam, J., and Pittchar, J. (2008). “Push-pull technology and determinants influencing expansion among smallholder producers in Western Kenya,” in Proc 24th Ann Meet. AIAEE 33-50.URL.
Anderson, M. J. (2017). Permutational multivariate analysis of variance (PERMANOVA). Wiley StatsRef: Statistics Reference Online. Available at: https://doi.org/10.1002/9781118445112.stat07841 (Accessed January 14, 2023).
Andrew, N. R., and Hughes, L. (2005). Arthropod community structure along a latitudinal gradient: Implications for future impacts of climate change. Austr. Ecol. 30, 281–297. doi:10.1111/j.1442-9993.2005.01464.x
Bakx, T. R., Lindström, A., Ram, D., Pettersson, L. B., Smith, H. G., van Loon, E. E., et al. (2020). Farmland birds occupying forest clear-cuts respond to both local and landscape features. For. Ecol. Manag. 478, 118519. doi:10.1016/j.foreco.2020.118519
Bélanger, L., and Grenier, M. (2002). Agriculture intensification and forest fragmentation in the St. Lawrence valley, Québec, Canada. Landsc. Ecol. 17, 495–507. doi:10.1023/a:1021443929548
Bellamy, A. S., Svenssson, O., van den Brink, P., Gunnarsson, J., and Tedengren, M. (2018). Insect community composition and functional roles along a tropical agricultural production gradient. Environ. Sci. Poll. Res. Intern. 25 (14), 13426–13438. doi:10.1007/s11356-018-1818-4
Bendell, B. E., Weatherhead, P. J., and Steward, R. K. (1981). The impact of predation by red-winged blackbirds on European corn borer populations. Can. J. Zoo. 59 (8), 1535–1538. doi:10.1139/z81-208
Bennun, L. A., Dranzoa, C., and Pomeroy, D. (2009). The forest birds of Kenya and Uganda. J. E. Af. Nat. Hist. 85, 23–48. doi:10.2982/0012-8317(1996)85[23:TFBOKA]2.0.CO;2
Berg, A., and Pärt, T. (1994). Abundance of breeding farmland birds on arable and set-aside fields at forest edges. Ecogr 17 (2), 147–152. doi:10.1111/j.1600-0587.1994.tb00087.x
BirdLife International and NatureServe (2011). Bird species distribution maps of the world. Arlington, USA: BirdLife International.
BirdLife International (2012). Important bird areas factsheets: Kakamega forest. Available at: http://www.birdlife.org/datazone/sitefactsheet.
Blitzer, E. J., Dormann, C. F., Holzschuh, A., Klein, A. M., Rand, T. A., and Tscharntke, T. (2012). Spillover of functionally important organisms between managed and natural habitats. Agric. Ecosys. Environ. 146, 34–43. doi:10.1016/j.agee.2011.09.005
Bos, M., Höhn, P., Saleh, S., Büche, B., Buchori, D., Steffan-Dewenter, I., et al. (2007). “Insect diversity responses to forest conversion and agroforestry management,” in Stability of tropical rainforest margins. Environmental science and engineering. Editors T. Tscharntke, C. Leuschner, M. Zeller, E. Guhardja, and A. Bidin (Berlin, Heidelberg: Springer), 277–294. doi:10.1007/978-3-540-30290-2_14
Broad, S. T., Schellhorn, N. A., Lisson, S. N., and Mendham, N. J. (2008). Host location and oviposition of lepidopteran herbivores in diversified broccoli cropping systems. Agric. For. Entomol. 10 (2), 157–165. doi:10.1111/j.1461-9563.2008.00374.x
Caut, S., Angulo, E., and Courchamp, F. (2009). Variation in discrimination factors (δ15N and δ13C), effect of diet isotopic values and applications for diet reconstruction. J. Appl. Ecol. 46, 443–453. doi:10.1111/j.1365-2664.2009.01620.x
Decocq, G., Andrieu, E., Brunet, J., Chabrerie, O., De Frenne, P., De Smedt, P., et al. (2016). Ecosystem services from small forest patches in agricultural landscapes. Curr. For. Rep. 2, 30–44. doi:10.1007/s40725-016-0028-x
Deguine, J. P., Aubertot, J. N., Flor, R. J., Lescourret, F., Wyckhuys, K. A., and Ratnadass, A. (2021). Integrated pest management: Good intentions, hard realities. A review. Agron. Sust. Dev. 41, 38. doi:10.1007/s13593-021-00689-w
Deikumah, J. P., Kwafo, R., and Konadu, V. A. (2017). Land use types influenced avian assemblage structure in a forest-agriculture landscape in Ghana. Ecol. Evol. 7, 8685–8697. doi:10.1002/ece3.3355
Dias, B. S., Frisk, M. G., and Jordaan, A. (2019). Opening the tap: Increased riverine connectivity strengthens marine food web pathways. PLoSONE 14 (5), e0217008. doi:10.1371/journal.pone.0217008
Ding, L. Y., Quie, L., Sodhi, M., Koh, L. P., Peh, K., Lee, T. M., et al. (2011). Do insectivorous bird communities decline on land-bridge forest islands in Peninsular Malaysia? J. Trop. Ecol. 27, 1–14. doi:10.1017/S0266467410000520
Diwani, T., Asch, F., Becker, M., and Mussgnug, F. (2013). Characterizing farming systems around Kakamega Forest, Western Kenya, for targeting soil fertility–enhancing technologies. J. Plant. Nutr. Soil Sci. 176, 585–594. doi:10.1002/jpln.201200036
Douglas, D. J., Nalwanga, D., Katebaka, R., Atkinson, P., Pomeroy, D., Nkuutu, D., et al. (2014). The importance of native trees for forest bird conservation in tropical farmland. Anim. Cons. 17 (3), 256–264. doi:10.1111/acv.12087
Driscoll, D. A., Banks, S. C., Barton, P. S., Lindenmayer, D. B., and Smith, A. L. (2013). Conceptual domain of the matrix in fragmented landscapes. Trend. Ecol. Evol. 28, 605–613. doi:10.1016/j.tree.2013.06.010
Ernst, K., Elser, J., Linz, G., Kandel, H., Holderieath, J., DeGroot, S., et al. (2019). The economic impacts of blackbird (Icteridae) damage to sunflower in the USA. Pest Manag. Sci. 75, 2910–2915. doi:10.1002/ps.5486
Exnerová, A., Štys, P., Krištín, A., and Volf, O. (2003). Birds as predators of true bugs (Heteroptera) in different habitats. Biol. Bratislav. 58 (2), 253–264. Available at: https://www.researchgate.net/publication/235447321_Birds_as_predators_of_true_bugs_Heteroptera_in_different_habitats (Accessed January 22, 2023).
FAO (2018). Global forest products facts and figures 2018. Rome: FAO. Available at: https://www.fao.org/3/ca7415en/ca7415en.pdf (Accessed February 12, 2023).
Farwig, N., Sajita, N., and Bo¨hning-Gaese, K. (2008). Conservation value of forest plantations for bird communities in Western Kenya. For. Ecol. Manag. 255, 3885–3892. doi:10.1016/j.foreco.2008.03.042
Ferger, S. W., Böhning-Gaese, K., Wilcke, W., Oelmann, E., and Schleuning, M. (2013). Distinct carbon sources indicate strong differentiation between tropical forest and farmland bird communities. Oecolog. 171, 473–486.
Firake, D., Behere, G., and Chandra, S. (2016). An environmentally benign and cost-effective technique for reducing bird damage to sprouting soybean seeds. Field crop. Res. 188, 74–81. doi:10.1016/j.fcr.2016.01.008
Forest Research (2021). Forestry statistics 2021: International forestry. Edinburgh: The Research Agency of the National Forestry Commission. Available at: https://cdn.forestresearch.gov.uk/2022/02/ch9_international_fs2021.pdf (Accessed March 5, 2023).
Fujita, M., and Koike, F. (2007). Birds transport nutrients to fragmented forests in an urban landscape. Ecol. Appl. 17 (3), 648–654. https//doi.org/10.1890/06-0118.
Garbach, K., Estrada-Carmona, N., Martínez-Salinas, A., and Declerck, F. (2012). Connectivity by design: Enhancing functional connectivity for forest-dependent birds in tropical agroecosystems. The 97th Annual Meeting of the Ecological Society of America. Portland, Oregon. Available at: https://www.esa.org/history/2013/03/97th-annual-meeting/(Accessed August 5-10, 2012).
Garcia, D., Miñarro, M., and Martínez Sastre, R. (2018). Birds as suppliers of pest control in cider apple orchards: Avian biodiversity drivers and insectivory effect. Agric. Ecosys. Environ. 254, 233–243. doi:10.1016/j.agee.2017.11.034
Garfinkel, M. B., Monor, E. S., and Wheelan, C. J. (2020). Birds suppress pests in corn but release them in soybean crops within a mixed prairie/agriculture system. Condor 122 (2), duaa009. doi:10.1093/condor/duaa009
Gates, R. G. (1980). Feeding patterns of monophagous, oligophagous, and polyphagous insect herbivores: The effect of resource abundance and plant chemistry. Oecolog 49 (1), 22–31. doi:10.1007/BF00346961
Gavrilescu, M. (2005). Fate of pesticides in the environment and its bioremediation. Eng. Life Sci. 5 (6), 497–526. doi:10.1002/elsc.200520098
Girard, J., Baril, A., Mineau, P., and Fahrig, L. (2012). Foraging habitat and diet of song sparrows (Melospiza melodia) nesting in farmland: A stable isotope analysis. Can. J. Zoo. 90 (11), 1339–1350. doi:10.1139/z2012-103
Goldstein, P. Z. (2017). “Diversity and significance of Lepidoptera: A phylogenetic perspective,” in Insect biodiversity: Science and society. Editors R G Foottit,, and P H Adler. 2nd ed. (New York: John Wiley and Sons), 463–495.
Gonzalez, E., Salvo, A., and Valladares, G. (2017). Natural vegetation cover in the landscape and edge effects: Differential responses of insect orders in a fragmented forest. Ins. Sci. 24 (5), 891–901. doi:10.1111/1744-7917.12377
Goswami, V. R., Medhi, K., Nichols, J. D., and Oli, M. K. (2015). Mechanistic understanding of human-wildlife conflict through a novel application of dynamic occupancy models. Cons. Biol. 29 (4), 1100–1110. doi:10.1111/cobi.12475
Grass, I., Lehmann, K., Thies, C., and Tscharntke, T. (2017). Insectivorous birds disrupt biological control of cereal aphids. Ecol 98 (6), 1583–1590. doi:10.1002/ecy.1814
Grass, I., Loos, J., Baensch, S., Batáry, P., Librán-Embid, F., Ficiciyan, A., et al. (2019). Land-sharing/-sparing connectivity landscapes for ecosystem services and biodiversity conservation. Peop. Nat. 1, pan3.21–272. doi:10.1002/pan3.21
Haché, S., Hobson, K. A., Villard, M. A., and Bayne, M. A. (2012). Assigning birds to geographic origin using feather hydrogen isotope ratios (δ2H): importance of year, age, and habitat. Can. J. Zoo. 90, 722–728. doi:10.1139/z2012-039
Hartman, G. L., Pawlowski, M. L., Herman, T. K., and Eastburn, D. (2016). Organically grown soybean production in the USA: Constraints and management of pathogens and insect pests. Agron 6, 16. doi:10.3390/agronomy6010016
Hill, D. B., and Webster, T. C. (1995). Apiculture and forestry (bees and trees). Agrofor. Sys. 29, 313–320. doi:10.1007/BF00704877
Hobson, K. A., and Clark, R. G. (1992). Assessing avian diets using stable isotopes II: Factors influencing diet-tissue fractionation influencing diet-tissue fractionation. Condor 94, 189–197. doi:10.2307/1368808
Hyodo, F. (2015). Use of stable carbon and nitrogen isotopes in insect trophic ecology: Use of isotope in insect trophic ecology. Sci. 18 (3), 295–312. doi:10.1111/ens.12128
Ilić, M., Igić, R., Ćuk, M., and Vukov, D. (2018). Field sampling methods for investigating forest-floor bryophytes: Microcoenose vs. random sampling. Arch. Biol.Sci. 70, 589–598. doi:10.2298/ABS180422020I
IUCN (2023). The IUCN red list of threatened species. Version 2022-2. Available at: https://www.iucnredlist.org (Accessed January 18, 2023).
Janzen, D. H. (1988). Ecological characterization of a Costa Rican dry forest caterpillar fauna. Biotr 20, 120–135. doi:10.2307/2388184
Johansson, F., Orizaola, G., and Nilsson-Örtman, V. (2020). Temperate insects with narrow seasonal activity periods can be as vulnerable to climate change as tropical insect species. Sci. Rep. 10 (1), 8822. doi:10.1038/s41598-020-65608-7
Jones, G. A., Sieving, K. E., Avery, M. L., and Meagher, R. L. (2005b). Parasitized and non-parasitized prey selectivity by an insectivorous bird. Crop Prot. 24, 185–189. doi:10.1016/j.cropro.2004.07.002
Jones, G. A., Sieving, K. E., and Jacobson, J. S. (2005a). Avian diversity and functional insectivory on north-central Florida farmlands. Conserv. Biol. Rev. 19, 1234–1245. doi:10.1111/j.1523-1739.2005.00211.x
Karp, D. S., Mendenhall, C. D., Sandí, R. F., Chaumont, N., Ehrlich, P. R., Hadly, E. A., et al. (2013). Forest bolsters bird abundance, pest control and coffee yield. Ecol. Lett. 16, 1339–1347. doi:10.1111/ele.12173
Khan, Z., Sharawi, S. E., Khan, M. S., Xing, L. X., Ali, S., Ahmed, N., et al. (2022). Prevalence of insect pests on maize crop in district mansehra, khyber pakhtunkhwa, Pakistan insect pests on maize crop in district mansehra, khyber pakhtunkhwa, Pakistan. Braz. J. Biol. 84, e259217. doi:10.1590/1519-6984.259217
Klinga, P., Mikoláš, M., Smolko, P., Tejkal, M., Höglund, J., and Paule, L. (2019). Considering landscape connectivity and gene flow in the Anthropocene using complementary landscape genetics and habitat modelling approaches. Landsc. Ecol. 34, 521–536. doi:10.1007/s10980-019-00789-9
Koch, R. L., Potter, B. D., Glogoza, P. A., Hogson, E. W., Krupke, C., Tooker, J., et al. (2016). Biology and economics of recommendations for insecticide-based management of soybean aphid. Pl. Heal. Progr. 17 (4), 265–269. doi:10.1094/PHP-RV-16-0061
Kross, S. M., Martinico, B. L., Bourbour, R. P., Townsend, J. M., McColl, C., and Kelsey, T. R. (2019). Net effects of field and landscape scale habitat on insect and bird damage to sunflowers. bioRxiv. doi:10.1101/804328
Kross, S. M., Martinico, B. L., Bourbpur, R. P., Townsend, J. M., McColl, C., and Kesley, T. R. (2020). Effects of field and landscape scale habitat on insect and bird damage to sunflowers. Front.Sust. Food Sys. 4 (40), 1–11. doi:10.3389/fsufs.2020.00040
Lancaster, G. A., Green, M., and Lane, S. (2006). Reducing bias in ecological studies: An evaluation of different methodologies. J. Roy. Stat. Soc. Ser. A. Stat. Soc. 69 (4), 681–700. doi:10.1111/j.1467-985x.2006.00418.x
Landis, D. A. (2017). Designing agricultural landscapes for biodiversity based ecosystem services. Bas. Appl. Ecol. 18, 1–12. doi:10.1016/j.baae.2016.07.005
Laube, I., Breitbach, N., and Bo¨hning-Gaese, K. (2008). Avian diversity in a Kenyan agroecosystem: Effects of habitat structure and proximity to forest. J. Ornith 149, 181–191. doi:10.1007/s10336-007-0258-6
Layman, C. A., Araujo, M. S., Boucek, R., Harrison, E., Jud, Z. R., Matich, P., et al. (2012). Applying stable isotopes to examine food-web structure: An overview of analytical tools. Biol. Rev. 87, 545–562. doi:10.1111/j.1469-185X.2011.00208.x
Li, Y., Mei, B., and Juvenal, T. L. (2019). The economic contribution of the world's forest sector. For. Pol. Econ 100, 236–253. doi:10.1016/j.forpol.2019.01.004
Lindell, C., and Dayer, A. (2022). Six principles for working effectively with landowners to advance bird conservation. Ornithol. Appl. 124 (4), 108. doi:10.1093/ornithapp/duac035
Lindell, C., Steensma, K., Curtis, P., B., Carroll, J., Burrows, C., Lusch, D., et al. (2016). Proportions of bird damage in tree fruits are higher in low-fruit-abundance contexts. Crop Prot. 90, 40–48. doi:10.1016/j.cropro.2016.08.011
Lourenço, R., Pereira, P. F., Oliveira, A., Ribeiro-Silva, J., Figueiredo, D., Rabaça, J. E., et al. (2021). Effect of vineyard characteristics on the functional diversity of insectivorous birds as indicator of potential biocontrol services. Ecol. Indic. 122, 107251. doi:10.1016/j.ecolind.2020.107251
Lucey, J. M. M., and Hill, J. K. (2012). Spillover of insects from rain forest into adjacent oil palm plantations. Biotr 44 (3), 368–377. doi:10.1111/j.1744-7429.2011.00824.x
Maas, B., Tscharntke, T., Saleh, S., Putra, D. D., and Clough, Y. (2015). Avian species identity drives predation success in tropical cacao agroforestry. J. Appl. Ecol. 53 (3), 735–743.
MacLean, D. (2016). Impacts of insect outbreaks on tree mortality, productivity, and stand development. Can. Entomol. 148 (S1), 138–159. doi:10.4039/tce.2015.24
MacLellan, C. R. (2012). Role of woodpeckers in control of the codling moth in nova scotia. Can. Entomol. 90 (1), 18–22. doi:10.4039/Ent9018-1
Marini, L., Ayres, M. P., and Jactel, H. (2022). Impact of stand and landscape management on forest pest damage. Ann. Rev. Entomol. 67, 181–199. doi:10.1146/annurev-ento-062321-065511
Massemin, S., Gendner, J., Samtmann, S., Pichegru, L., W., and Le Maho, Y. (2006). The effect of migration strategy and food availability on White Stork Ciconia ciconia breeding success. Ibis 148, 503–508. doi:10.1111/j.1474-919X.2006.00550.x
McKinnon, L., Picotin, M., Bolduc, E., Juillet, C., and Bêty, J. (2012). Timing of breeding, peak food availability, and effects of mismatch on chick growth in birds nesting in the High Arctic. Can. J. Zoo. 90 (8), 961–971. doi:10.1139/z2012-064
Meadows, S., Moller, H., and Weller, F. (2012). Reduction of bias when estimating bird abundance within small habitat fragments Advances in tools for bird population monitoring in New Zealand. New zeal. J. Ecol. 36 (3), 1–7.
Milligan, M., Johnson, M., Garfinkel, M., Smith, C., and Njoroge, P. (2016). Quantifying pest control services by birds and ants in Kenyan coffee farms. Biol. Conserv. 194, 58–65. doi:10.1016/j.biocon.2015.11.028
Nelson, K., Patalee, B., and Yao, B. (2022). Higher landscape diversity associated with improved crop production resilience in Kansas-USA. Environ. Res. Lett. 17 (8), 084011. doi:10.1088/1748-9326/ac7e5f
Newmark, W. D., and Stanley, T. R. (2011). Habitat fragmentation reduces nest survival in an Afrotropical bird community in a biodiversity hotspot. Proc. Nat. Acad. Sci. U. S. A. 108 (28), 11488–11493. doi:10.1073/pnas.1104955108
Nyffeler, M., Şekercioğlu, Ç. H., and Whelan, C. J. (2018). Insectivorous birds consume an estimated 400–500 million tons of prey annually. Sci. Nat. 105, 47. doi:10.1007/s00114-018-1571-z
Ostrom, P. H., Colunga-Garcia, M., and Gaze, S. H. (1997). Establishing pathways of energy flow for insect predators using stable isotope ratios: Field and laboratory evidence. Oecolog 109, 108–113. doi:10.1007/s004420050064
Otieno, N. E., Jacobs, S. M., and Pryke, J. S. (2019b). Maize-field complexity and farming system influence insectivorous birds’ contribution to arthropod herbivore regulation. Biotrop 51 (6), 851–861. doi:10.1111/btp.12701
Otieno, N. E., Pryke, J. S., Butler, M., and Jacobs, S. M. (2019a). Top-down suppression of arthropod herbivory in intercropped maize and organic farms evidenced from δ13C and δ15N stable isotope analyses. Agron. Sust. Dev. 39, 39. doi:10.1007/s13593-019-0585-z
Otieno, N. E., and Pryke, J. S. (2022). Tree and hedgerow configurations on maize farms are key drivers of granivorous passerine bird assemblage patterns. Agric. Ecosys. Environ. 338 (1), 108104. doi:10.1016/j.agee.2022.108104
Pagani-Núñez, E., Renom, M., Mateos-Gonzalez, F., Cotín, J., and Senar, J. C. (2017). The diet of great tit nestlings: Comparing observation records and stable isotope analyses. Bas. Appl. Ecol. 18, 57–66. doi:10.1016/j.baae.2016.11.004
Paritte, J. M., and Kelly, J. F. (2009). Effect of cleaning regime on stable isotope ratios of feathers in Japanese quail (Coturnix japonica). Auk 126 (1), 165–174. doi:10.1525/auk.2009.07187
Parnell, A., Phillips, D. L., Bearhop, S., Semmens, B. X., Ward, E. J., Moore, W. J., et al. (2013). Bayesian stable isotope mixing models. Environ 24, 387–399. doi:10.1002/env.2221
Pejchar, L., Clough, Y., Ekroos, J., Nicholas, K. A., Olsson, O., Ram, D., et al. (2018). Net effects of birds in agroecosystems. BioSci 68 (11), 896–904. doi:10.1093/biosci/biy104
Peter, F., Berens, D., Grieves, G. R., and Farwig, N. (2015). Forest fragmentation drives the loss of insectivorous birds and an associated increase in herbivory. Biotrop 47 (5), 626–635. doi:10.1111/btp.12239
Phillips, D. L., Newsome, S. D., and Greg, J. W. (2005). Combining sources in stable isotope mixing models: Alternative methods. Oecolog 144, 520–527. doi:10.1007/s00442-004-1816-8
R Core Team (2021). R: A language and environment for statistical computing R foundation for statistical computing Vienna Austria. Available at: https://wwwr-projectorg/.
Ralph, C. J., Dunn, E. H., Peach, W. J., and Handei, C. M. (2014). Recommendations for the use of mist nets for inventory and monitoring of bird populations. Stud. Av. Biol. 29, 187–196. Available at: https://www.fs.usda.gov/research/treesearch/31580 (Accessed April 03, 2023).
Razeng, R., and Watson, D. (2014). Nutritional composition of the preferred prey of insectivorous birds: Popularity reflects quality. J. Av. Biol. 46. doi:10.1111/jav.00475
Riedl, H. L., Stinson, L., Pejchar, L., and Clements, W. H. (2018). An introduced plant affects aquatic-derived carbon in the diets of riparian birds. PLoSONE 13 (11), e0207389. doi:10.1371/journal.pone.0207389
Rincon-Parra, V. J., Echeverry-Galvis, M. A., and Alvarez, S. J. (2022). Functional responses of bird assemblages to land-use change in the Colombian Llanos Region. Front. Environm. Sci. 9, 689745. doi:10.3389/fenvs.2021.689745
Robbins, C. T., Felicetti, L. A., and Sponheimer, M. (2005). The effect of dietary protein quality on nitrogen isotope discrimination in mammals and birds. Oecolog 144, 534–540. doi:10.1007/s00442-005-0021-8
Ruhl, P. J., Flaherty, E. A., and Dunning, J. B. (2020). Using stable isotopes of plasma, red blood cells, feces, and feathers to assess mature-forest bird diet during the post-fledging period. Can. J. Zoo. 98 (1), 39–46. doi:10.1139/cjz-2019-0109
Sánchez-Fernández, J., Vílchez-VivancoNavarro, A. J. B. F., Castro-Rodríguez, J., and Castro-RodrÍguez, J. (2020). Farming system and soil management affect butterfly diversity in sloping olive groves. Ins. Cons. Divers. 13, 456–469. doi:10.1111/icad.12435
Sanders, D., Schaefer, M., Platner, C., and Griffiths, G. J. (2011). Intra-guild interactions among generalist predator functional groups drive impact on herbivore and decomposer prey. Oikos 120 (3), 418–426. doi:10.1111/j.1600-0706.2010.18924.x
Schindler, B., Gavish-Regev, E., and Keasar, T. (2022). Parasitoid wasp community dynamics in vineyards following insecticide application. Front. Environ. Sci. 9, 785669. doi:10.3389/fenvs.2021.785669
Sekamatte, B. M., Ogenga-Latigo, M., and Russell-Smith, A. (2003). Effects of maize-legume intercrops on termite damage to maize, activity of predatory ants and maize yields in Uganda. Crop Prot. 22 (1), 87–93. doi:10.1016/S0261-2194(02)00115-1
Şekercioğlu, C. H. (2002). Forest fragmentation hits insectivorous birds hard. Dir. Sci. 1, 62–64. doi:10.1100/tsw.2002.190
Shakelford, C. E., and Corner, R. N. (1997). Woodpecker abundance and habitat use in three forest types in eastern Texas. Wilson Bull. 109 (4), 614–629. https://www.srs.fs.usda.gov/pubs/ja/ja_shakelford001.pdf.
Sosa-Aranda, I., del-Val, E., Hernández-Martínez, G., Arroyo-Lambaer, D., Uscanga, A., and Boege, K. (2018). Response of lepidopteran herbivore communities to crop management in coffee plantations. Agric. Ecosys. Environ. 265, 37–44. doi:10.1016/j.agee.2018.05.018
Spence, K., and Rosenheim, J. (2005). Isotopic enrichment in herbivorous insects: a comparative field-based study of variation. Oecolog. 146, 89–97. doi:10.1007/s00442-005-0170-9
Stewart, F. E. C., Darlington, S., Volpe, J. P., McAdie, M., and Fisher, J. T. (2019). Corridors best facilitate functional connectivity across a protected area network. Sci. Rep. 9 (1), 10852. doi:10.1038/s41598-019-47067-x
Stock, B. C., Jackson, A. L., Ward, E. J., Parnell, A. C., Phillips, D. L., and Semmens, B. X. (2018). Analyzing mixing systems using a new generation of Bayesian tracer mixing models. PeerJ 6, e5096. doi:10.7717/peerj.5096
Stock, B. C., and Semmens, B. X. (2016). MixSIAR GUI user manual. Version 3.1. Available at: https://github.com/brianstock/MixSIAR/.
Suckling, D. M., Conlong, D. E., Carpenter, J. E., Bloem, K. A., Vireysen, M. J., and Vreysen, M. J. B. (2017). Global range expansion of pest Lepidoptera requires socially acceptable solutions. Biol. Inv. 19, 1107–1119. doi:10.1007/s10530-016-1325-9
The Kenya Meteorological Department (2021). State of the climate Kenya 2020. Nairobi: Kenya Ministry of Environment and Forestry.
Tiryaki, O., and Temur, C. (2010). The fate of pesticide in the environment. J. Biol. Envrion. Sci. 4 (10), 29–38. https://uludag.edu.tr/dosyalar/jbes/10/mak05.pdf.
Tremblay, A., Mineau, P., and Stewart, R. K. (2001). Effects of bird predation on some pest insect populations in corn. Agric. Ecosys. Environ. 83 (1-2), 143–152. doi:10.1016/S0167-8809(00)00247-4
Tscharntke, T., Şekercioğlu, C. H., Dietsch, T. V., Sodhi, N. S., Hoehn, P., and Tylianakis, J. M. (2008). Landscape constraints on functional diversity of birds and insects in tropical agroecosystems. Ecol 89 (4), 944–951. doi:10.1890/07-0455.1
Ülgentürk, S., and Dokuyucu, Ö. (2019). Pest species of coccoidea (Hemiptera; coccomorpha) in forest of Turkey. Turk. J. For. 20 (4), 482–491. doi:10.18182/tjf.616353
Willig, M. R., Kauffman, D., and Stevens, R. (2003). Latitudinal gradients of biodiversity: Pattern, process, scale, and synthesis. Ann. Rev. Ecol. Evol. Sys. 34, 273–309. doi:10.1146/annurev.ecolsys.34.012103.144032
Wylie, F. R., and Speight, M. R. (Editors) (2012). Insect pests in tropical forestry. 2nd ed. (Wallingford, UK: CAB International).
Yard, H., van Riper, C., Li, R., Brown, B., and Kearsley, M. (2002). Diets of insectivorous birds along the Colorado river in grand canyon, Arizona. Condor 106 (1), 106–115. doi:10.1093/condor/106.1.106
Keywords: ecotone, trophic linkage, biocontrol, ecological networks, connectivity, insectivorous birds, stable isotopes
Citation: Otieno NE and Mukasi J (2023) Tropical insectivorous birds’ predation patterns that promote forest–farmland trophic connectivity for integrated top–down pest biocontrol. Front. Environ. Sci. 11:1194267. doi: 10.3389/fenvs.2023.1194267
Received: 26 March 2023; Accepted: 23 June 2023;
Published: 27 July 2023.
Edited by:
Tien Ming Lee, Sun Yat-sen University, ChinaReviewed by:
Cheng Huang, Shenzhen University, ChinaLuis M. Bautista-Sopelana, National Museum of Natural Sciences (MNCN-CSIC), Spain
Copyright © 2023 Otieno and Mukasi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nickson Erick Otieno, otieno_nickson@yahoo.com
 Nickson Erick Otieno
Nickson Erick Otieno Jonathan Mukasi1
Jonathan Mukasi1