Status, mechanism, suitable distribution areas and protection countermeasure of invasive species in the karst areas of Southwest China
- 1State Key Laboratory of Environmental Criteria and Risk Assessment, Chinese Research Academy of Environmental Sciences, Beijing, China
- 2College of Water Sciences, Beijing Normal University, Beijing, China
- 3Institute of Botany, Chinese Academy of Sciences, Beijing, China
- 4College of Ecology, Lanzhou University, Lanzhou, China
Biological invasion is one of the major threats to global biodiversity attracting a primary focus of scientific attention. During the past decades, due to the diversity and peculiarity of species, coupled with the vulnerable ecosystem, karst areas have received more and more attention. Numerous investigations and studies have confirmed that the karst areas in Southwest China are suffering from biological invasions under the intensified human activities and the climate change they caused. Despite some fundamental research on invasive species that has been conducted to understand the species and distribution in the karst areas, the mechanism of biological invasions and the response of karst ecosystem are still lack sufficient knowledge. In this paper, we summarized the habitat characteristics and invasion status of karst areas to biological invasions. This paper comprehensively analyzed the research results on biological invasions in karst areas to understand the status and development trends of biological invasions in the karst of China, so as to promote the relevant research on biological invasions in the karst areas. We found that the biological invasions in the karst areas were increasing with years. We also revealed the possible mechanism including competition, mutualism, allelopathy and phenotypic plasticity of biological invasion in karst by summarizing the relevant research results of in the karst areas. Moreover, the response of karst to biological invasion was described from the aspects of ecosystem, community, species and genetic levels, etc. By comparing the characteristics of invasive species that have been found in karst area, we analyzed the common characteristics including strong fecundity and rapid growth rate, strong environmental adaptability, strong phenotypic plasticity and high genetic diversity of the existing invasive species, we simulated and predicted the habitat of invasive species. Overall, we found three areas with high habitat suitability covering Chinese southwest Karst ecosystem, which include the southern Yunnan-Guizhou Plateau, foothill area on the Min-Yue-Gui and foothill area of southern Yunnan. It is also worth noting that the Sichuan Basin has a higher invasive risk compared to its surrounding Karst ecosystem, mainly because of the high habitat suitability of some invasive species. Therefore, we suggest that a general survey of alien invasive species in the karst areas of Southwest China should be carried out as soon as possible, focusing on the survey of the suitable areas of alien species for early warning. In addition, to establish a database of invasive alien species in the karst areas of southwest China, strengthen the monitoring of alien species, and evaluate the impact of invasive species in key areas on the biodiversity and ecosystem in the karst areas of Southwest China, so as to maintain the stability of cave biodiversity and the fragile ecosystem.
Introduction
Biological invasion has been recognized as one of the main factors driving biodiversity declines, economic losses, and zoonotic disease emergences in the world (Pysek et al., 2020; Diagne et al., 2021; Zhang et al., 2022). By 2018, 667 invasive species had been recorded in China (Xu, 2018). Same as the “fully invasive species” in unified framework for biological invasions (Blackburn et al., 2011), these species with individuals reproducing, surviving and spreading at multiple sites have a serious impact. Biological invasion is a complex process that is influenced by various factors (Lake and Leishman, 2004; Geng et al., 2007). The invasiveness of alien species and the invasibility of the ecosystem being invaded are key factors for the successful invasion of alien species (Wang et al., 2015).
Southwest China’s karst areas are the largest in the world and one of the hotspots of global biodiversity (Lu et al., 2013). The unique geological structure of karst areas has formed their unique habitats and provides rich ecological niches for the species in them (Xu, 2011). The characteristics of the karst ecosystem, such as shallow, calcium-rich soil and extensive caves, have led to the emergence of many unique and endemic species of plants and animals (Lu et al., 2013). Karst habitats in Southwest China are rich in rare, endangered and protected wildlife (Jiang, 2014; Shui, 2017). The specific habitat of karst not only promotes biodiversity but also determine the vulnerability of the ecosystem (Guo et al., 2011). For instance, the barren and easily erodible soil in the habitat is one of the major factors leading to the vulnerability of karst vegetation, which causes biodiversity decline and instability of the dynamic balance of the karst ecosystem (Jiang, 2014; Shui., 2017). Habitat disturbances in the karst area result from serious ecological disasters such as rocky desertification (Wang et al., 2004). Human activities also disturb habitat and will lead to biodiversity declines and biological invasions (Dukes and Mooney, 1999). For instance, land-use modifications significant affected biological invasion (Ficetola et al., 2010). Habitat loss facilitated the biological invasion of the American bullfrog (Rana catesbeiana) at Liuji Town, Jiangsu Province, China (Wang et al., 2022). At present, there are no relevant reports on the status and diffusion trend of biological invasion in the karst areas of Southwest China. The impact of biological invasion on the biodiversity of karst landforms has not attracted international attention. In this work, we collected, sorted and analyzed invasive species data in the karst areas of Southwest China, clarified the current status of biological invasion in those karst areas, and summarized the mechanism of biological invasion. We also put forward corresponding countermeasures and suggestions for the prevention and control of biological invasion in the karst areas of Southwest China.
Distribution and ecosystem characteristics of karst areas in southwest China
Distribution of karst areas in southwest China
The global extent of karst areas is approximately 2.2 million km2 (Groves and Meiman, 2011), which accounts for approximately 12% of the world’s total land area and provides drinking water to nearly 25% of the world’s population. Karst ecosystems are an important part of the Earth’s surface ecosystem (Goldscheider et al., 2020). Karst landforms in China are widely distributed and extensive. In particular, karst landforms in Southwest China are characterized by large area, rich biodiversity and a fragile ecosystem. (Lu et al., 2013). With the Yunnan-Guizhou Plateau as the core area, karst landforms in Southwest China are distributed in Guizhou, Yunnan, Sichuan and Chongqing (Jiang, 2014; Xu and Zhang, 2014). The Southwest karst region is the most well-known karst in the world, and its grassland ecosystem is vulnerable (Lu, 2012).
Ecosystem characteristics of karst areas in southwest China
The ecosystem of the karst areas in Southwest China are unique. The karst areas in Southwest China have a subtropical or tropical humid monsoon climate (Shui, 2017) and are warm in winter and hot in summer, with abundant annual rainfall but uneven seasonal distribution (Lu, 2012). In terms of habitat, the soil of the karst uplands is shallow and discontinuous, with a thick soil layer at the foot of the mountains and barren soil at the top, alkaline and calcium rich (Zhu, 2003). The different ecological niches such as stone crevices, caves, stone surfaces and soil layers have formed diversified small landforms in the karst areas in Southwest China (Jiang, 2014; Shui, 2017). For example, sinkholes provide refuge for many plants and caves combine with underground rivers to form a complex underground water system (Xiong, 2006). These isolated habitats provide rich ecological niches for organisms (Xu, 2011). In terms of plant species, the karst vegetation has evolved unique adaptive characteristics, including calcium and drought tolerance and strong roots, some of which can cling to rocks (Shui, 2017). These plant characteristics are due to strong water permeability and poor retention capacity of the shallow, calcium-rich, alkaline soil; fewer available water resources; and proclivity to drought of the karst landforms (Xiong, 2006; Shui, 2017). There are many unique cave animals and rock-dwelling animals in the karst areas due to the cave-rich ecosystems and special habitats, such as karst caves and underground water networks (Jiang, 2014). The uniqueness of karst plants also promotes the emergence of many unique and endemic species of animals in the ecosystem (Xu, 2011). Maolan Nature Reserve, for example, has more than 200 endemic species, including Gekko liboensis, Nemacheilus liboensis, Sinocyclocheilus macrolepis etc. (Jiang, 2014). Karst areas in Southwest China are rich in rare, endangered and protected wildlife. The unique karst habitats promote biodiversity and determine the vulnerability of their ecosystems. Once the karst landform is disturbed by human activities, serious ecological disasters such as rocky desertification occur easily (Wang et al., 2004; Guo et al., 2011; Xu and Zhang, 2014). Soil is one of the major factors among many leading to the vulnerability of karst ecosystems (Guo et al., 2011). For example, karst landforms in Southwest China have obvious seasonal droughts and weakly alkaline, high-calcium soil; the soil layer in many habitats is barren and easily erodible (Lu, 2012). These characteristics cause vulnerability of vegetation, which leads to the instability of the dynamic balance of the karst ecosystem.
Status quo of biological invasions in the karst areas of southwest China
Damage caused by biological invasion
In recent years, biological invasion caused by human activities have seriously affected the ecosystem in the karst areas of Southwest China. The species richness and diversity indexes of these areas are high. However, in recent years, these karst areas in Southwest China have suffered from serious biological invasion, which has decreased the biodiversity (Wang et al., 2004). Invasive species not only threaten the biodiversity of karst areas, but also may carry pathogens that threaten wildlife and human health (Zhang et al., 2022). Large numbers of invasive species were found in the karst areas of Guizhou, and malignant invasive species were found in more than 60% of survey sites (Yang et al., 2022). In the karst areas of Guizhou, the occurrence areas of invasive species Ageratina adenophora, Alternanthera philoxeroides and Mikania micrantha were 2,238, 79, and 1,467 km2 respectively; and Chromolaena odorata has flooded the Nanpan River, Beipan River and Red River basins (Yang, 2020). After Ageratina adenophora and other invasive plants invaded the karst areas of Guizhou, the local biodiversity and the total number of soil animal groups decreased significantly, including a 41.3% decrease and 43.25% in grassland (Yang, 2020). As plant diversity decreases, herbivores lack food, and the food chain breaks down; thus, many karst endemic species have become endangered (Yang, 2020). In Caohai, Guizhou province, China, Rana catesbiana has occupied a niche and is proliferating (Liu and Li, 2009; Lv et al., 2020). The invasion of Rana catesbiana led to the extinction of Cynops wolterstorffi in Dianchi Lake, Yunnan Province (Li and Xie, 2004). Invasive species, such as Pomacea canaliculata and Oreochromis nilotica, are increasingly endangering the ecosystem in the karst areas of Guizhou, and in some areas invasive species are out of control (Qiu et al., 2019). The invasive species Achating fulica has endangered more than 200 species in Yunnan Province, posing a serious threat to local biodiversity (Li and Li, 2008). The impact of biological invasion on biodiversity in karst areas has not attracted broad international attention, but there are some case studies. For example, biological invasions have resulted in a significant reduction of biodiversity and total numbers of animal and plant groups and even endangered species (Jiang, 2014; Shui, 2017). The invasive plant Aster subulatus is one of the most harmful plants in nurseries and gardens in Southwest China, but the research on it is very limited (Pan et al., 2010). Therefore, biological invasions in the karst areas in Southwest China should receive more attention and research to prevent ecosystem degradation.
Invasive species
In recent years, the karst areas in Southwest China are suffering from serious biological invasion. To understand the status quo of biological invasion in these areas, we summarized the invasive species according to published literatures (Supplementary Table S1). A total of 172 invasive species were recorded, including 21 animal species (excluding insects) and 151 plants species. The 21 invasive animal species belonged to 16 families and six groups, of which fishes were the most abundant, with 12 species accounting for 57.14% of all species; mammals were the second abundant, with four species accounting for 19.05% of all species. Other groups had one or two species. The 151 invasive plant species belonged to 36 families, of which Compositae was the most abundant, with 34 species accounting for 22.52% of all species; the second abundant was Leguminosae, with 19 species accounting for 12.58%. In addition, there are 14 species of Amaranthaceae accounting for 9.27% of all species and 13 species of Gramineae, accounting for 8.61% of all invasive species. Among the invasive plants, 32 species were malignant, 48 species were severe, 21 species were local, and 24 species were general. The malignant and severe invasive plants accounting for 52.98% of the total invasive plant species. Supplementary Table S1 showed that among the typical invasive animals in the karst areas of Southwest China, except for muroid, the other animal species were aquatic or amphibian. Among the invasive plants, Ageratina Adenophora, Lantana camara, Alternanthera philoxeroides and Eichhornia crassipes were hygrophytic species. These descriptions demonstrate that karst areas are being threatened by invasive species, especially some aquatic alien species, which have seriously affected the biodiversity of water ecosystems and the ecological safety in the karst areas. Effective measures are urgently needed to prevent and control invasive species.
Distribution of invasive species
We summarized the distribution of 151 invasive plant species and 21 invasive animal species with county-level resolution. For each animal species, we obtained information about their distribution from literatures. Because the data of most animal species’ occurrence are only at the provincial level and some animal species, such as Pomacea canaliculata, Pterygoplichthys pardalis and Rattus norvegicus are widely distributed species, we believe that they are located in all counties of the recorded province. For plants, distribution data were extracted from the Invasive Plants module of the National Specimen Information Infrastructure (NSII) database. The Asteraceae family is widely distributed in all provinces including the karst areas, among which the most widely distributed species are Bidens pilosa, Conyza canadensis, Crassocephalum crepidioides, Erigeron annuus and Galinsoga parviflora. From Figure 1, it can be seen that there were more invasive species in the foothills area of the Min-Yue-Gui, Hengduan Mountain Area and Guizhou Plateau. The invasive species richness was low in Sichuan Basin and northwest Sichuan Province. We found that most species were not only distributed in the karst areas shown in Figure 1, but also distributed in other areas to varying degrees. For example, the adjacent Guangxi Province had a high invasive species richness, which might be related to the large karst area in this province.
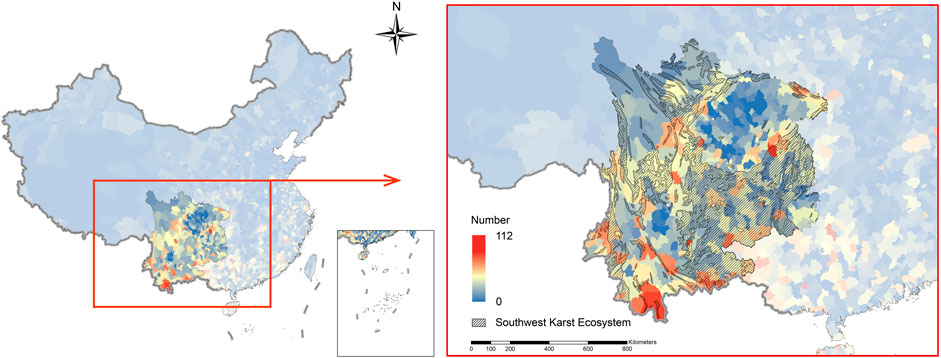
FIGURE 1. Richness of the typical invasive alien species among karst systems in southwest China. Blue represents counties with low richness, red represents counties with high richness. Crosshatch regions mean karst systems in southwest China.
Biological characteristics and mechanisms of invasive species in the karst areas of Southwest China
Biological invasion is a complex process and there are many factors affecting the spread of invasive species. The invasiveness of invasive species and the resistance to the invasion of the ecosystem are the key factors that determine the success of invasive species (Wang et al., 2015). Studies have shown that the invasiveness of invasive species is significantly related to its competitiveness, spatial growth ability, fecundity, resource utilization capacity and allelochemicals release etc. (Pyek and Richardson, 2007). The resistance to the invasion of the ecosystem is related to the species diversity and available resources of the local ecosystem (Lonsdale, 1999). During the process of biological invasion, alien species ensure their dominance in interspecific competition with local species especially for resources through the plasticity of their morphological characteristics, physiological characteristics and reproductive characteristics to enhance their invasiveness (Sodhi et al., 2019). In addition, alien species can increase their invasiveness by releasing allelochemicals that inhibit the growth of native species (Callaway and Ridenour, 2004). Generally, the biological invasion of alien species is the result of the combined action of different invasion methods.
Typical biological characteristics of invasive species
According to some studies, successful invasive species have several significant characteristics including high fecundity, strong environmental adaptability and high phenotypic plasticity (Mack et al., 2003). Among the 21 invasive animal species (insects excepted) in the karst areas of Southwest China, the successful invasive species had several significant characteristics in common. The first characteristic was omnivory (Table 1). Omnivorous alien species can find food and adapt to local environments rapidly. The American bullfrog (Rana catesbeiana) that invaded Yunnan, China, for instance, fed on more than 30 species from 10 taxonomic classes, including frogs native to Yunnan (Liu et al., 2015). Procambarus clarkii, which invaded Caohai, China, fed on plants, plankton, aquatic invertebrates, insects, etc. Its extensive diet contributed to maintaining and expanding the population (Tao, 2020). The second characteristic was strong fecundity (Table 1). Invasive animals showed strong fecundity. For example, each female American bullfrog laid 10,000 eggs at a time, with a survival rate of approximately 10% (Li and Xie, 2004). Gambusia affinis showed strong fecundity and high seasonal characteristics, which posed a serious threat to the survival of local fish in the areas invaded (Gao et al., 2019). The third characteristic was strong environmental tolerance with wide habitat adaptability (Cruz and Rebelo, 2007) (Table 1). Procambarus clarkii was highly adaptable to the hydrologic and temperature conditions of the new habitat (Gutiérrez-Yurrita et al., 1998), and it can reproduce in most water, even in the extreme environment of polluted water or the high-salinity water (Barbaresi and Gherardi, 2000). The last characteristic was high phenotypic plasticity (Table 1), which enables invasive animals to improve their adaptability to the environments. For example, Gambusia affinis had strong phenotypic plasticity, and environmental factors such as temperature, precipitation, altitude, salinity, electrical conductivity and dissolved oxygen all affected their life-history traits (Jourdan et al., 2016; Cheng et al., 2018). In addition, higher genetic diversity or genetic variation is beneficial to the adaptive evolution of invasive animals (Wang, 2015).
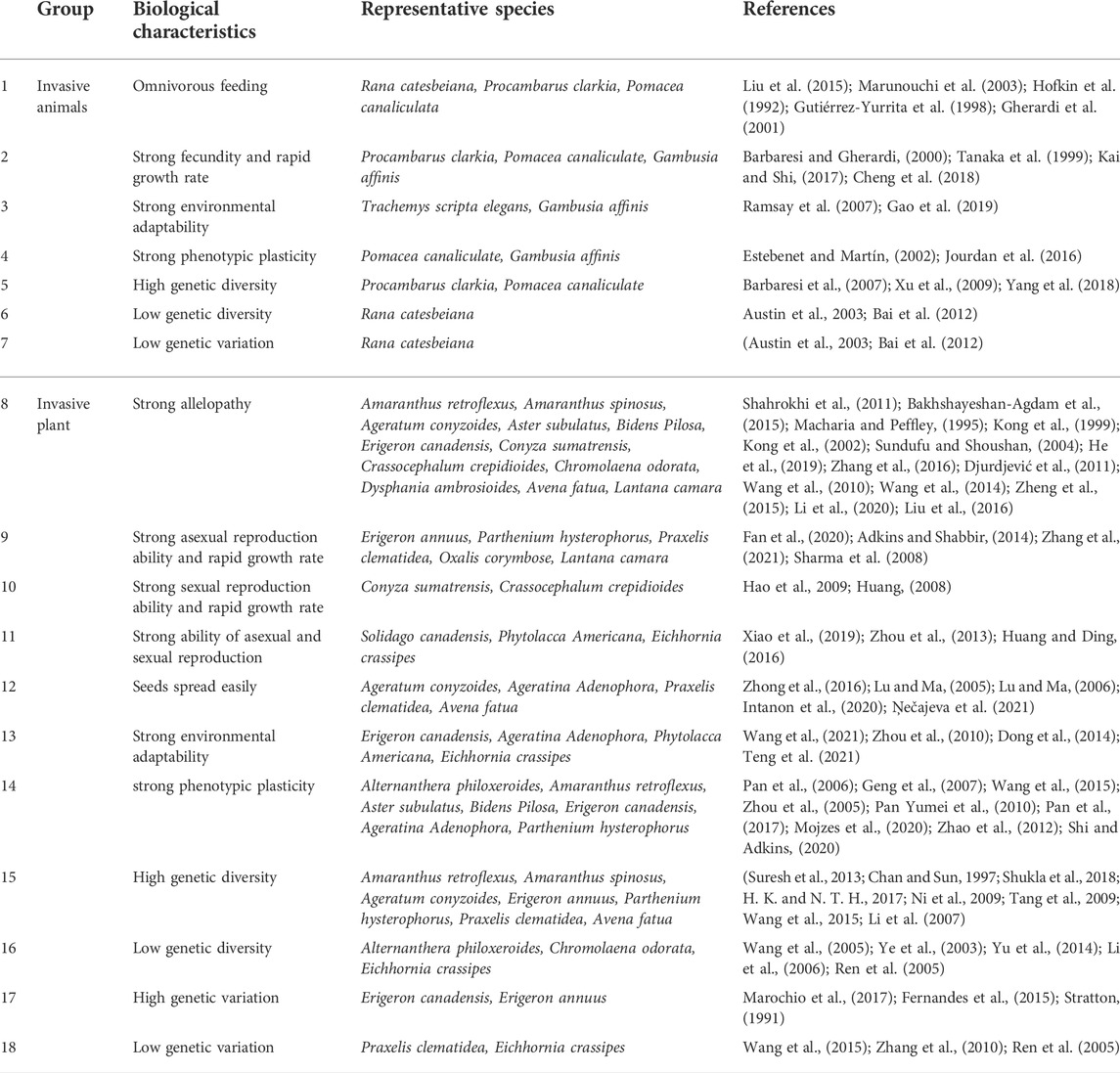
TABLE 1. Main biological characteristics of invasive alien species in Karst areas of Southwest China.
Among the 151 invasive, mostly herbaceous, plant species in the karst areas of Southwest China, the successful invasive species had several significant, common characteristics. The first characteristic was strong allelopathy (Table 1). For example, Ageratum conyzoides, Parthenium hysterophorus and Lantana camara have strong allelopathy (Kong et al., 2002). Studies have shown that Lantana camara and its extracts had allelopathic effects on seed germination and seedling growth of Capsicum annuum, Brassica rapa and Brassica campestris. The second characteristic was strong fecundity (Table 1). Table 1 shows that the invasive plants in the karst areas of Southwest China had strong fecundity, including vegetative propagation and sexual propagation. Some species had both propagation modes. For example, Ageratum conyzoides reproduced sexually in suitable conditions to ensure the variability of the population, and reproduced asexually under harsh conditions to ensure the fecundity of the species (Zhang et al., 2020). Amaranthus spinosus is a hermaphrodite angiosperms, with bisexual flowers and self-pollination. It can produce up to 10,000 seeds per plant and has strong fecundity (Li, 2016). The third characteristic was easy seed dispersal (Table 1). The reproductive strategy of annual invasive plants generally relies on the production of a large number of individuals to maintain the populations. This was usually manifested as a large number of seeds, while small seeds that spread easily by wind or river (Lu and Ma, 2005, 2006). For example, the seeds of Bidens pilosa and Praxelis clematidea were small, with long pappi and strong diffusibility in the wind (Zhong et al., 2016). The fourth characteristic was high phenotypic plasticity (Table 1). Phenotypic plasticity in plants plays an important role in the survival and maintenance of species under new environments and pressures by obtaining maximum fitness (Hendry, 2016). For example, Alternanthera philoxeroides showed high phenotypic plasticity in a variable environment (Geng et al., 2007). The last characteristic was higher genetic diversity or genetic variation. Most invasive plants in the karst areas of Southwest China have high genetic variation or diversity, such as Praxelis clematidea, Amaranthus retroflexus, Amaranthus spinosus, etc. (Chan and Sun, 1997; Li et al., 2007; Tang et al., 2009; Wang et al., 2015).
Ecosystem resistance to the invasive species
The ecosystem resistance to invasive species is related to available resources, species diversity, and human disturbance, etc. (Lonsdale, 1999). Biological invasions in the karst areas of Southwest China are related to available resources. If there are unused resources suitable for invasive species in the karst ecosystem, this is conducive to the early establishment of invasive species and to decreasing the ecosystem’s resistance to invasion (Wan et al., 2015). For instance, in degraded karst sinkholes, invasive plants had fewer resources to use, which effectively reduced the invasion of Ageratina adenophora, resulting in different degrees of invasion in different karst habitats (Jiang et al., 2019). There were abundant aquatic plants and mosquitoes in the shallow lakes and wetlands in Caohai, Guizhou province, and the suitable conditions and available resources were conducive to the invasibility of the American bullfrog in this area (Lv et al., 2020). The species diversity of the karst ecosystem is also a factor affecting the degree of alien species invasion. For example, in western Panzhihua, Sichuan province, the higher species diversity of the shrub layer and the composition of the herb layer inhibited the invasion of Ageratina adenophora; the higher the species diversity of shrub layer, the lower the invasive degree of Ageratina adenophora, which was consistent with the diversity resistance hypothesis proposed by Albertson (1960).
Ecosystem biodiversity is positively correlated with invasive species resistance. However, different conclusions were drawn about the impact of species diversity on ecosystem resistance to invasive species at different spatial scales. For example, at the scale of 25 m2, the invasive degree of Ageratina adenophora was negatively correlated with local species diversity; at the scale of 400 m2, there were both positive and negative correlations; and at the provincial scale, the invasive degree was positively correlated with the local biodiversity (Lu et al., 2008). In addition to the previously mentioned factors, human disturbance also affects the invasiveness of the karst ecosystem. With the intensification of human activities and global climate change, many natural ecosystems have been disturbed at various degrees. Excessive disturbance will lead to ecological imbalance and degradation, which will decrease the ecosystem resistance to invasion (Wan et al., 2015).
The mechanisms of alien species invasions
The main mechanisms of alien species invasion include competition, symbiosis, allelopathy and phenotypic plasticity. Compared with native species, invasive species have advantages in life-history traits, genetics and evolution (Callaway and Ridenour, 2004; Wan et al., 2015). The inherent superiority hypothesis holds that the successful invasion of some alien species is due to their morphological, physiological, ecological and other specific traits, which make them superior to other species in resource competition and environmental adaptation (Sax and Brown, 2000; Hufbauer and Torchin, 2008). Phenotypic plasticity in environmental adaptation is also an important strategy and mechanism for alien species invasion.
Species in the same ecological niche or trophic level compete for resources. Competition is an important way for native species to prevent biological invasion (Wan et al., 2015). For example, Rana catesbeianuscan compete with native species in the same ecological niche of Caohai lake in Guizhou province for food and space resources because of their omnivory and large food intake (Liu and Li, 2009; Lv et al., 2020). Studies on the life-history traits of invasive plants showed that they had higher spatial growth capacity, resource utilization capacity, photosynthetic rate (Shen et al., 2011) as well as stronger fecundity (Qi et al., 2014). The height, specific leaf area, concentration of representative nutrients, and photosynthetic rate of invasive plants were significantly higher than those of native plants (Sodhi et al., 2019). These functional traits of invasive plants represent their efficient use of resources and contribute to their competitive ability and successful invasion (Lake and Leishman, 2004). Studies have shown that symbiosis is also a factor in plant invasion in the karst areas (Wan et al., 2015). The karst landforms have shallow and discontinuous soils that are calcium rich and nutrient poor, which determines their obvious spatial heterogeneity (Lu, 2012). These characteristics will significantly affect the development and distribution of plant populations (Qi et al., 2013). Most plants are symbiotic with arbuscular mycorrhizal (AM) fungi, which is highly adaptable in the heterogeneous habitat of karst landforms. Some research found that AM fungi can significantly improve the growth and nutrient use of the invasive plant Bidens pilosa in the karst areas and significantly improve its adaptability to the karst heterogeneous habitats (Xu, 2020). Some studies also found that mycorrhizal fungi can reduce the competitiveness of the native species Kummerowia striata and promote the invasion of Solidago Canadensis (Yang et al., 2014). Researchers have regarded invasive plants and AM fungi as a symbiotic relationship that facilitates invasion (Cui and He, 2009).
In addition to competition and symbiosis, invasive plants interact with native species by allelopathy. The allelopathy of invasive plants is mainly related to their rapid evolution and lack of coevolution with native plants. The new weapon hypothesis holds that an important reason for the success of alien plants is that they can release allelochemicals to inhibit the growth of native species (Callaway and Ridenour, 2004). A typical invasive weed in the Sichuan Basin, Erigeron canadensis, releases phenolic acid that can inhibit seed germination and seedling growth of species such as Trifolium repens (Djurdjević et al., 2011). The invasive weed Parthenium hysterophorus releases phenolic substances that inhibit the growth of Brassica campestris, Brassica rapa and Brassica oleracea seedlings (Singh et al., 2005). A tissue extract of the invasive weed Amaranthus retroflexus had allelopathic effects on the seed germination and growth of four important crops: cucumber (Cucumis sativus), alfalfa (Medicago sativa), kidney bean (Phaseulus vulgaris) and wheat (Triticum aestivum) (Bakhshayeshan-Agdam et al., 2015).
Phenotypic plasticity enables alien species to maintain high fitness in a variety of habitats, especially when the environment undergoes unsuitable changes. For example, the phenotypic plasticity of Procambarus clarkii is reflected in its feeding habits. An optimal feeding strategy can be achieved by adjusting feeding strategies at different times (Smart et al., 2002). Research found that in three different karst habitats, Alternanthera philoxeroides increased its adaptability to different habitats by changing a series of morphological characteristics and adjusting reproductive strategies (Zhang et al., 2017). The invasive species Erigeron annuus in Chongqing had strong phenotypic plasticity. In low-altitude areas, it adjusted a series of phenotypic traits to enhance ecological adaptability (Li, 2014). The most seriously invaded areas of Ageratina adenophora in China are in the Yunnan-Guizhou Plateau. Due to the large environmental differences between the invasion and origin, and the low genetic variation between and within populations in the invasion site, this species has great ecological adaptability (Sang et al., 2010), revealing the high phenotypic plasticity of its functional traits. That is the main strategy for its invasion of China (Zhao et al., 2012). This overview indicates that the main mechanisms of biological invasion in the karst ecosystems of Southwest China are competition, symbiosis, allelopathy and phenotypic plasticity.
Suitable habitats of invasive species in the karst areas of Southwest China
We collected global presence records for nine terrestrial invasive animal species and 80 malignant and severe invasive plant species (Supplementary Table S1) from the Global Biodiversity Information Facility (GBIF) and the citizen science database iNaturalist. Elevation data and 19 bioclimatic variables such as annual mean temperature are obtained from the WorldClim Climate Database (www.worldclim.org). The global potential habitat suitabilities for 89 invasive species were calculated by the maximum entropy niche model, using the software MaxEnt 3.4.4 and combined with these 20 environmental variables. For models of all species, the average training area under the curve (AUC) ranged from 0.656 to 0.99 (mean: 0.869; standard deviation: 0.076). Because of the need to use different model variables, aquatic invasive animals are not considered in this part. Habitat suitability in each grids were calculated as the accumulative distribution probabilities of these invasive species. A higher habitat suitability value for a given grid cell indicates a higher relative probability of invasion.
Except for two human-associated species, Mus musculus and Rattus norvegicus, the other seven invasive animal species had low habitat suitability in the karst area (Figure 2A.). For invasive plants, we found three areas with high habitat suitability, including the southern Yunnan-Guizhou Plateau, the Min-Yue-Gui foothills and the foothill of southern Yunnan. It can be observed from Figure 2 that, consistent with the current distribution pattern shown in Figure 1, the habitat suitability of 80 typical invasive species is highest in Guangxi Province adjacent to the study area and in Guangdong Province, which is at the same latitude. Amaranthus lividus, Amaranthus viridis, Amaranthus spinosus, Conyza bonariensis and Pharbitis nil have the largest suitable habitats areas in eastern and central China. In addition, the Sichuan Basin has a higher invasive risk compared to the surrounding karst areas, mainly because of the high habitat suitability of Amaranthus lividus, Conyza bonariensis, Pharbitis nil, Pharbitis purpurea and Solanum khasianum.
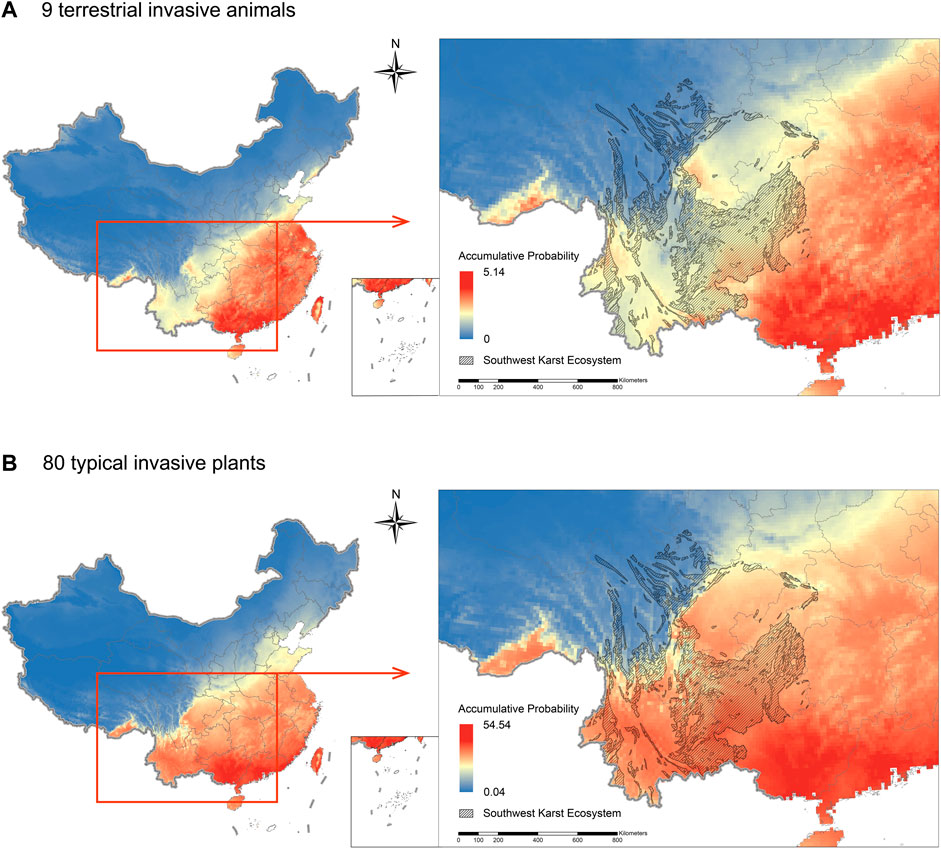
FIGURE 2. Accumulative potential distribution probability of typical invasive species in China predicted by the MaxEnt niche model. Blue represents grids with low probability which means low habitat suitability, red represents grids with high probability which means high habitat suitability. Crosshatch regions mean karst systems in southwest China.
The Maxent model was used to predict the suitable distribution areas of Alternanthera philoxeroides in China, which were mainly distributed in tropical, subtropical and southeast warm temperate regions of China. In addition, high-suitability areas were mainly located in Guangxi, Shanghai, Jiangsu, Sichuan provinces and in Chongqing, where invasive species exhibit continuous expansion (Yan et al., 2020).
Countermeasures and suggestions
The plant communities in the karst areas of southwest China show complex and diverse species compositions, high species richness, and high diversity indexes and occupy important position in the global karst ecosystem. However, biological invasion caused by human activities has seriously affected the ecosystem of the karst areas in Southwest China in recent years. To effectively prevent and control biological invasion in this area, some countermeasures and suggestions are put forward as follows. It is necessary to conduct a survey of invasive species in the karst areas of Southwest China. A systematic survey of invasive species has not been carried out although there are many invasive species in the karst areas. We suggest that a survey of invasive species in the karst areas of Southwest China be conducted as soon as possible, especially in the important wetlands, plateaus, basins, and other suitable distribution areas. We must obtain the information of invasive species types, quantities, and key areas threatened to facilitate the development of an early warming and protection strategy. Moreover, for our models’ results, the ROC analyses show fairly good performance by MaxEnt for training AUC values. But the high AUC values may result from the arbitrary selection of pseudo-absence data (Phillips et al., 2009; VanDerWal et al., 2009; Liu et al., 2011). The accurate presence-absence data is the key to further modeling.
It is necessary to establish a database of invasive species and a monitoring and early warning system for invasive species in the karst areas of Southwest China. Timely supplement and improvement of the database should be part of the follow-up investigation and research, to provide a basis for the establishment of the monitoring and early warning system. We also suggest increased monitoring of invasive species in the karst areas of Southwest China and evaluation of the impact of invasive species on local biodiversity and ecosystem in key areas. Concurrently, the key invasive species’ habitat suitability should be simulated, so that species with a serious expansion trend can be monitored, warned against and controlled.
The last suggestion is to protect and improve the biodiversity of native species in the karst areas of Southwest China. We suggest strengthening the relevant research on the ecological characteristics of native species and restoring suitable ecosystem structures and functions to establish a benign successive ecological community and provide a basis for scientific replacement control of invasive species. In the prevention and control of alien plants, replacement control is a method that uses plants with ecological and economic value to replace invasive plants according to the succession rule of a plant community. We suggest strengthening the research on the ecological characteristics of native species, and giving full consideration to plant replacement restoration technologies in the prevention and control of alien species.
Author contributions
YL and JC designed the article; YL, TS, YL and LF collected the data and information; TS, YL and YH drew the figures; YL and JC wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fundamental Research Funds for the Central Public-interest Scientific Institution (2020YSKY-008), the Third Xinjiang Scientific Expedition Program (Grant No.2021xjkk0600) and the Open Foundation of Key Laboratory of Forest Plant Ecology, Ministry of Education, Northeast Forestry University (K2020B01).
Acknowledgments
We thank Jiang Zhou and Lina Zhao for their valuable suggestions. Additionally, we would like to thank two anonymous reviewers who gave constructive comments on the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.957216/full#supplementary-material
References
Adkins, S., and Shabbir, A. (2014). Biology, ecology and management of the invasive parthenium weed (Parthenium hysterophorus L.). Pest Manag. Sci. 70, 1023–1029. doi:10.1002/ps.3708
Austin, J. D., Dávila, J. A., Lougheed, S. C., and Boag, P. T. (2003). Genetic evidence for female-biased dispersal in the bullfrog, Rana catesbeiana (Ranidae). Mol. Ecol. 12, 3165–3172. doi:10.1046/j.1365-294x.2003.01948.x
Bai, C., Ke, Z., Consuegra, S., Liu, X., and Li, Y. (2012). The role of founder effects on the genetic structure of the invasive bullfrog (Lithobates catesbeianaus) in China. Biol. Invasions 14, 1785–1796. doi:10.1007/s10530-012-0189-x
Bakhshayeshan-Agdam, H., Salehi-Lisar, S. Y., Motafakkerazad, R., Talespour, A., and Farsad, N. (2015). Allelopathic effects of redroot pigweed (Amaranthus retroflexus L.) on germination & growth of cucumber, alfalfa, common bean and bread wheat. Acta Agric. Slov. 105, 193–202. doi:10.14720/aas.2015.105.2.02
Barbaresi, S., Gherardi, F., Mengoni, A., and Souty-Grosset, C. (2007). “Genetics and invasion biology in fresh waters: A pilot study of Procambarus clarkii in europe,” in Biological invaders in inland waters: Profiles, distribution, and threats. Editor F. Gherardi (Berlin: Springer), 381–400.
Barbaresi, S., and Gherardi, F. (2000). The invasion of the alien crayfish Procambarus clarkii in europe, with particular reference to Italy. Biol. invasions 2, 259–264. doi:10.1023/a:1010009701606
Blackburn, T. M., Pyšek, P., Bacher, S., Carlton, J. T., Duncan, R. P., Jarošík, V., et al. (2011). A proposed unified framework for biological invasions. Trends Ecol. Evol. 26, 333–339. doi:10.1016/j.tree.2011.03.023
Callaway, R. M., and Ridenour, W. M. (2004). Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2, 436–443. doi:10.1890/1540-9295(2004)002[0436:nwisat]2.0.co;2
Chan, K. F., and Sun, M. (1997). Genetic diversity and relationships detected by isozyme and RAPD analysis of crop and wild species of Amaranthus. Theor. Appl. Genet. 95, 865–873. doi:10.1007/s001220050637
Cheng, Y., Xiong, W., Tao, J., He, D., Chen, K., and Chen, Y. (2018). Life-history traits of the invasive mosquitofish (Gambusia affinis baird and girard, 1853) in the central yangtze river, China. Bioinvasions Rec. 7, 309–318. doi:10.3391/bir.2018.7.3.13
Cruz, M. J., and Rebelo, R. (2007). Colonization of freshwater habitats by an introduced crayfish, Procambarus clarkii, in Southwest Iberian Peninsula. Hydrobiologia 575, 191–201. doi:10.1007/s10750-006-0376-9
Cui, Q., and He, W. (2009). Soil biota, but not soil nutrients, facilitate the invasion of Bidens pilosa relative to a native species Saussurea deltoidea. Weed Res. 49, 201–206. doi:10.1111/j.1365-3180.2008.00679.x
Diagne, C., Leroy, B., Vaissière, A.-C., Gozlan, R. E., Roiz, D., Jarić, I., et al. (2021). High and rising economic costs of biological invasions worldwide. Nature 592, 571–576. doi:10.1038/s41586-021-03405-6
Djurdjević, L., Mitrović, M., Gajić, G., Jarić, S., Kostić, O., Oberan, L., et al. (2011). An allelopathic investigation of the domination of the introduced invasive Conyza canadensis L. Flora - Morphol. Distribution Funct. Ecol. Plants 206, 921–927. doi:10.1016/j.flora.2011.06.001
Dong, Z. Y., Bai, X. F., Zhang, J. Z., Hou, Y. P., and Bu, Q. M. (2014). Adaptability of an invasive plant Phytolacca americana to varied light environment. Chin. J. Ecol. 33, 316–320. doi:10.13292/j.1000-4890.2014.0008 [Chinese with abstract in English]
Dukes, J. S., and Mooney, H. A. (1999). Does global change increase the success of biological invaders? Trends Ecol. Evol. 14, 135–139. doi:10.1016/s0169-5347(98)01554-7
Estebenet, A. L., and Martín, P. R. (2002). Pomacea canaliculata (gastropoda: Ampullariidae): Life-history traits and their plasticity. Biocell. 26, 83–89.
Fan, J. J., Yi, Y. M., and Zhu, X. Z. (2020). Advances on the invasive weed, Erigeron annuus. J. Weed Sci. 38, 1–8. doi:10.19588/j.issn.1003-935X.2020.02.001[Chinese with abstract in English]
Fernandes, S., Torre, F. A. M. D., Denis, B., Aparecida, M. C., Aparecida, D. O. C. S., Oliveira, J. R. S., et al. (2015). Evidence of high gene flow between samples of horseweed (Conyza canadensis) and hairy fleabane (Conyza bonariensis) as revealed by isozyme polymorphisms. Weed Sci. 63, 604–612. doi:10.1614/ws-d-14-00044.1
Ficetola, G. F., Maiorano, L., Falcucci, A., Dendoncker, N., Boitani, L., Padoa-Schioppa, E., et al. (2010). Knowing the past to predict the future: Land-use change and the distribution of invasive bullfrogs. Glob. Change Biol. 16, 528–537. doi:10.1111/j.1365-2486.2009.01957.x
Gao, J., Santi, F., Zhou, L., Wang, X., and Plath, M. (2019). Geographical and temporal variation of multiple paternity in invasive mosquitofish (Gambusia holbrooki, Gambusia affinis). Mol. Ecol. 28, 5315–5329. doi:10.1111/mec.15294
Gao, J., Santi, F., Zhou, L., Wang, X., Riesch, R., and Plath, M. (2019). Geographical and temporal variation of multiple paternity in invasive mosquitofish (Gambusia holbrooki , Gambusia affinis). Mol. Ecol. 28, 5315–5329. doi:10.1111/mec.15294
Geng, Y., Pan, X., Xu, C., Zhang, W., Li, B., Chen, J., et al. (2007). Phenotypic plasticity rather than locally adapted ecotypes allows the invasive alligator weed to colonize a wide range of habitats. Biol. Invasions 9, 245–256. doi:10.1007/s10530-006-9029-1
Gherardi, F., Renai, B., and Corti, C. (2001). Crayfish predation on tadpoles: A comparison between A native (Austropotamobius pallipes) and an alien species (Procambarus clarkii). Bull. Français de Pêche de Piscic. 361, 659–668. Knowledge & Management of Aquatic Ecosystems. doi:10.1051/kmae:2001011
Goldscheider, N., Chen, Z., Auler, A. S., Bakalowicz, M., Broda, S., Drew, D., et al. (2020). Global distribution of carbonate rocks and karst water resources. Hydrogeol. J. 28, 1661–1677. doi:10.1007/s10040-020-02139-5
Groves, C., and Meiman, J. (2011). Weathering, geomorphic work, and karst landscape evolution in the Cave City groundwater basin, Mammoth Cave, Kentucky. Geomorphology 67, 115–126. doi:10.1016/j.geomorph.2004.07.008
Guo, K., Liu, C. C., and Dong, M. (2011). Ecological adaptation of plants and control of rocky-desertification on karst region of Southwest China. Chin. J. Plant Ecol. 35, 991–999. doi:10.3724/sp.j.1258.2011.00991
Gutiérrez-Yurrita, P. J., Sancho, G., Bravo, M. Á., Baltanás, Á., Montes, C., Gutierrez-Yurrita, P. J., et al. (1998). Diet of the red swamp crayfish Procambarus clarkii in natural ecosystems of the doñana national park temporary fresh-water marsh (Spain). J. Crustacean Biol. 18, 120–127. doi:10.2307/1549526
Hao, J., Qiang, S., Liu, Q., and Cao, F. (2009). Reproductive traits associated with invasiveness in Conyza sumatrensis. J. Syst. Evol. 47, 245–254. doi:10.1111/j.1759-6831.2009.00019.x
He, P., Deng, Y. J., Hu, X. Y., Pan, H. M., and Deng, H. P. (2019). Potential allelopathic effect of Aster subulayus on Triticum aestivum and Brassica chinensis. Acta Prataculturae Sin. 28, 101–109. [Chinese with abstract in English]
Hendry, A. P. (2016). Key questions on the role of phenotypic plasticity in eco-evolutionary dynamics. J. Hered. 107, 25–41. doi:10.1093/jhered/esv060
Hofkin, B. V., Hofinger, D. M., Koech, D. K., and Loker, E. S. (1992). Predation of Biomphalaria and non-target molluscs by the crayfish Procambarus clarkii: Implications for the biological control of schistosomiasis. Ann. Trop. Med. Parasitol. 86, 663–670. doi:10.1080/00034983.1992.11812723
Huang, Q. S. (2008). “Studies on invasive biology and integrated management of the exotic plant Crassocephalum crepidioides,” ([Beijing: Zhejiang Normal University). [dissertation/master’s thesis].
Huang, W., and Ding, J. (2016). Effects of generalist herbivory on resistance and resource allocation by the invasive plant, Phytolacca americana. Insect Sci. 23, 191–199. doi:10.1111/1744-7917.12244
Hufbauer, R. A., and Torchin, M. E. (2008). Integrating ecological and evolutionary theory of biological invasions. Berlin Heidelberg: Springer, 193, 79–96. doi:10.1007/978-3-540-36920-2_6
Intanon, S., Wiengmoon, B., and Mallory-Smith, C. A. (2020). Seed morphology and allelopathy of invasive Praxelis clematidea. Not. Bot. Horti Agrobot. Cluj. Napoca. 48, 261–272. doi:10.15835/nbha48111831
Jiang, C., Shui, W., Jian, X. M., Guo, P. P., and Chen, Y. P. (2019). Soil microbial community characteristics in degraded karst tiankeng invaded by Eupatorium adenophorum. Chin. J. Appl. Ecol. 30, 2002–2010. doi:10.13287/j.1001-9332.201906.032
Jiang, Z. G. (2014). Survey and study on the important wild animals in the karst regions of southwest China. Beijing: China Environment Publishing Group.[In Chinese]
Jourdan, J., Krause, S. T., Lazar, V. M., Zimmer, C., Sommer-Trembo, C., Arias-Rodriguez, L., et al. (2016). Shared and unique patterns of phenotypic diversification along a stream gradient in two congeneric species. Sci. Rep. 6, 38971. doi:10.1038/srep38971
Kai, M., and Shi, H. (2017). “Red-Eared slider Trachemys scripta elegans (Wied-Neuwied),” in Biological invasions and its management in China. Editors F. H. Wan, M. X. Jiang, and A. B. Zhan (Berlin: Springer), 49–76. doi:10.1007/978-981-10-3427-5_4
Kong, C., Hu, F., Xu, T., and Lu, Y. (1999). Allelopathic potential and chemical constituents of volatile oil from Ageratum conyzoides. J. Chem. Ecol. 25, 2347–2356. doi:10.1023/a:1020882109682
Kong, C., Hu, F., and Xu, X. (2002). Allelopathic potential and chemical constituents of volatiles from Ageratum conyzoides under stress. J. Chem. Ecol. 28, 1173–1182. doi:10.1023/a:1016229616845
Lake, J. C., and Leishman, M. R. (2004). Invasion success of exotic plants in natural ecosystems: The role of disturbance, plant attributes and freedom from herbivores. Biol. Conserv. 117, 215–226. doi:10.1016/s0006-3207(03)00294-5
Li, C., and Xie, F. (2004). Invasion of bullforg (Rana catesbeinan Show) in China and its management strategies. Chin. J. Appl. Environ. Biol. 10, 95–98.[Chinese with abstract in English]
Li, J., Yang, X., Yu, J., Li, Z., Deng, Q., Cao, Y., et al. (2020). Chemical composition of the volatile oil of Chenopodium ambrosioides L. from Mianyang in Sichuan Province of China and its sub-chronic toxicity in mice. Trop. J. Pharm. Res. 19, 1985–1991. doi:10.4314/tjpr.v19i9.26
Li, P. C. (2016). “Effects of potential allelochemicals in Amaranthus spinosus on crops growth and its physiobiochemical mechanism,” ([Hangzhou]: China Jiliang University). [dissertation/master’s thesis].[In Chinese]
Li, P., and Li, Y. (2008). Research the African snails occurs and control measures in Yunnan. China: Journal of Yunnan University, 203–205.[In Chinese]
Li, R., Wang, S., Duan, L., Li, Z., Christoffers, M. J., and Mengistu, L. W. (2007). Genetic diversity of wild oat (avena fatua) populations from China and the United States. Weed Sci. 55, 95–101. doi:10.1614/ws-06-108.1
Li, W., Wang, B., and Wang, J. (2006). Lack of genetic variation of an invasive clonal plant Eichhornia crassipes in China revealed by RAPD and ISSR markers. Aquat. Bot. 84, 176–180. doi:10.1016/j.aquabot.2005.09.008
Li, Z. (2014). “Adaptation in traits of growth and reproduction of Erigeron annuus in different regions of China,” ([Wuhan]: Huazhong Agricultural University). [dissertation/master’s thesis].[In Chinese]
Liu, X., Guo, Z., Ke, Z., Wang, S., and Li, Y. (2011). Increasing potential risk of a global aquatic invader in Europe in contrast to other continents under future climate change. PloS one 6, e18429. doi:10.1371/journal.pone.0018429
Liu, X., and Li, Y. M. (2009). Aquaculture enclosures relate to the establishment of feral populations of introduced species. PLoS ONE 4, e6199–7. doi:10.1371/journal.pone.0006199
Liu, X., Luo, Y., Chen, J., Guo, Y., Bai, C., and Li, Y. (2015). Diet and prey selection of the Invasive American bullfrog (Lithobates catesbeianus) in southwestern China. Asian herpetological Res. 6, 34–44. doi:10.16373/j.cnki.ahr.140044
Liu, X., Tian, F., Tian, Y., Wu, Y., Dong, F., Xu, J., et al. (2016). Isolation and identification of potential allelochemicals from aerial parts of avena fatua L. And their allelopathic effect on wheat. J. Agric. Food Chem. 64, 3492–3500. doi:10.1021/acs.jafc.5b05498
Lonsdale, W. M. (1999). Global patterns of plant invasions and the concept of invasibility. Ecology 80, 1522–1536. doi:10.1890/0012-9658(1999)080[1522:gpopia]2.0.co;2
Lu, P., Sang, W., and Ma, K. (2008). Differential responses of the activities of antioxidant enzymes to thermal stresses between two invasive eupatorium species in China. J. Integr. Plant Biol. 50, 393–401. doi:10.1111/j.1744-7909.2007.00583.x
Lu, Y., Liu, Q., and Zhang, F. E. (2013). Environmental characteristics of karst in China and their effect on engineering. Carbonates Evaporites 28, 251–258. doi:10.1007/s13146-013-0158-1
Lu, Z., and Ma, K. (2005). Scale dependent relationships between native plant diversity and the invasion of croftonweed (Eupatorium adenophorum) in southwest China. Weed Sci. 53, 600–604. doi:10.1614/ws-04-188r2.1
Lu, Z., and Ma, K. (2006). Spread of the exotic croftonweed (Eupatorium adenophorum) across southwest China along roads and streams. Weed Sci. 54, 1068–1072. doi:10.1614/ws-06-040r1.1
Lv, J. C., Li, Y. L., Dai, L. L., Li, Z. J., Wei, G., Wang, C. Q., et al. (2020). Distribution and preferences of Rana catesbeiana in Caohai wetland of Guizhou province. J. Hydroecology 41, 133–140.[Chinese with abstract in English]
Macharia, C., and Peffley, E. B. (1995). Suppression of Amaranthus spinosus and kochia scoparia: Evidence of competition or allelopathy in allium fistulosum. Crop Prot. 14, 155–158. doi:10.1016/0261-2194(95)92870-S
Marochio, C. A., Bevilaqua, M., Takano, H. K., Mangolim, C. A., Junior, R. O., and Machado, M. (2017). <b>Genetic admixture in species of Conyza (Asteraceae) as revealed by microsatellite markers. Acta Sci. Agron. 39, 437–445. doi:10.4025/actasciagron.v39i4.32947
Marunouchi, J., Tsuruda, K., and Noguchi, T. (2003). Cynops pyrrhogaster (Japanese newt): Predation by introduced Rana catesbeiana (bullfrog). Herpetol. Bull., 83, 31–32.
Mojzes, A., Ónodi, G., Lhotsky, B., Kalapos, T., and Kröel-Dulay, G. (2020). Experimental drought indirectly enhances the individual performance and the abundance of an invasive annual weed. Oecologia 193, 571–581. doi:10.1007/s00442-020-04711-y
Ņečajeva, J., Bleidere, M., Jansone, Z., Gailīte, A., and Ruņģis, D. (2021). Variability of seed germination and dormancy characteristics and genetic analysis of Latvian avena fatua populations. Plants (Basel). 10, 235. doi:10.3390/plants10020235
Ni, F. M., Li, J. M., Wu, J. J., Wang, D. X., and Li, J. H. (2009). Genetic diversity and differentiation of Erigeron annuns in different salinity gradients. Jour Fujian For. Sci Tech 36, 44–47+92.[Chinese with abstract in English]
Pan, X., Geng, Y., Zhang, W., Li, B., and Chen, J. (2006). The influence of abiotic stress and phenotypic plasticity on the distribution of invasive Alternanthera philoxeroides along a riparian zone. Acta Oecol. 30, 333–341. doi:10.1016/j.actao.2006.03.003
Pan, Y. M., Tang, S. C., Cen, Y. X., Pu, G. Z., Wei, C. Q., and Chen, Q. X. (2010). Biomass allocation on the modules of aster subulatusMichx population at flowering stage. J. Trop. Subtropical Bot. 18, 176–181. doi:10.3724/SP.J.1238.2010.00516[Chinese with abstract in English]
Pan, Y. M., Tang, S. C., Wei, C. Q., and Li, X. Q. (2017). Comparison of growth, photosynthesis and phenotypic plasticity between invasive and native Bidens species under different light and water conditions. Biodivers. Sci. 25, 1257–1266. doi:10.17520/biods.2016366
Phillips, S. J., Dudík, M., Elith, J., Graham, C. H., Lehmann, A., Leathwick, J., et al. (2009). Sample selection bias and presence-only distribution models: Implications for background and pseudo-absence data. Ecol. Appl. 19, 181–197. doi:10.1890/07-2153.1
Pysek, P., and Richardson, D. M. (2007). Traits associated with invasiveness in alien plants: Where do we stand? Biol. Invasions 193, 97–125. doi:10.1007/978-3-540-36920-2_7
Pysek, P., Hulme, P. E., Simberloff, D., Bacher, S., Blackburn, T. M., Carlton, J. T., et al. (2020). Scientists' warning on invasive alien species. Biol. Rev. 95, 1511–1534. doi:10.1111/brv.12627
Qi, S., Dai, Z., Miao, S., Zhai, D., Si, C., Huang, P., et al. (2014). Light limitation and litter of an invasive clonal plant, Wedelia trilobata, inhibit its seedling recruitment. Ann. Bot. 114, 425–433. doi:10.1093/aob/mcu075
Qi, X., Wang, K., and Zhang, C. (2013). Effectiveness of ecological restoration projects in a karst region of southwest China assessed using vegetation succession mapping. Ecol. Eng. 54, 245–253. doi:10.1016/j.ecoleng.2013.01.002
Qiu, J. S., Zhang, N. N., Tian, M. J., Xu, Z. Z., and Ban, Q. M. (2019). Influence of invasive alien species on people's livelihood and ecology in north & south Pan River and hongshui river valley. Guizhou For. Sci. Technol. 47, 18–22. [In Chinese]
Ramsay, N. F., Ng, P., O'Riordan, R. M., and Chou, L. M. (2007). The red-eared slider (Trachemys scripta elegans) in asia: A review. Biol. invaders inland waters Profiles, distribution, threats 8, 161–174. doi:10.1007/978-1-4020-6029-8_8
Ren, M., Zhang, Q., and Zhang, D. (2005). Random amplified polymorphic DNA markers reveal low genetic variation and a single dominant genotype in Eichhornia crassipes populations throughout China. Weed Res. 45, 236–244. doi:10.1111/j.1365-3180.2005.00445.x
Sang, W., Zhu, L., and Axmacher, J. C. (2010). Invasion pattern of Eupatorium adenophorum Spreng in southern China. Biol. Invasions 12, 1721–1730. doi:10.1007/s10530-009-9584-3
Sax, D. F., and Brown, J. H. (2000). The paradox of invasion. Glob. Ecol. Biogeogr. 9, 363–371. doi:10.1046/j.1365-2699.2000.00217.x
Shahrokhi, S., Hejazi, S. N., Khodabandeh, H., Farboodi, M., and Faramarzi, A. (2011). Allelopathic effect of aqueous extracts of pigweed, Amaranthus retroflexus L. organs on germination and growth of five barley cultivars. 3rd Int. Conf. Chem. Biol. Environ. Eng. Singap. 20, 80–84.
Sharma, O. P., Sharma, S., Pattabhi, V., Mahato, S. B., and Sharma, P. D. (2008). A review of the hepatotoxic plant Lantana camara. Crit. Rev. Toxicol. 37, 313–352. doi:10.1080/10408440601177863
Shen, X., Peng, S., Chen, B., Pang, J., Chen, L., Xu, H., et al. (2011). Do higher resource capture ability and utilization efficiency facilitate the successful invasion of native plants? Biol. Invasions 13, 869–881. doi:10.1007/s10530-010-9875-8
Shi, B., and Adkins, S. (2020). The phytotoxic activity of Parthenium hysterophorus L. seedlings on a range of pasture species. Crop Prot. 137, 105211. doi:10.1016/j.cropro.2020.105211
Shui, Y. M. (2017). Checklist of seed plants in the karst regions in China. Beijing: Science Press. [In Chinese]
Shukla, A., Srivastava, N., Suneja, P., Yadav, S. K., Hussain, Z., Rana, J. C., et al. (2018). Untapped amaranth (Amaranthus spp.) genetic diversity with potential for nutritional enhancement. Genet. Resour. Crop Evol. 65, 243–253. doi:10.1007/s10722-017-0526-0
Singh, H. P., Batish, D. R., Pandher, J. K., and Kohli, R. K. (2005). Phytotoxic effects of Parthenium hysterophorus residues on three Brassica species. Weed Biol. Manag. 5, 105–109. doi:10.1111/j.1445-6664.2005.00172.x
Smart, A. C., Harper, D. M., Malaisse, F., Schmitz, S., Coley, S., and Beauregard, A. C. G. D. (2002). Feeding of the exotic Louisiana red swamp crayfish, Procambarus clarkii (Crustacea, Decapoda), in an African tropical lake: Lake Naivasha, Kenya. Hydrobiologia 488, 129–142. doi:10.1023/A:1023326530914
Sodhi, D. S., Livingstone, S. W., Carboni, M., and Cadotte, M. W. (2019). Plant invasion alters trait composition and diversity across habitats. Ecol. Evol. 9, 6199–6210. doi:10.1002/ece3.5130
Stratton, D. A. (1991). Life history variation within populations of an asexual plant, Erigeron annuus (asteraceae). Am. J. Bot. 78, 723–728. doi:10.1002/j.1537-2197.1991.tb12596.x
Sundufu, A. J., and Shoushan, H. (2004). Chemical composition of the essential oils of Ageratum conyzoides L. occurring in south China. Flavour Fragr. J. 19, 6–8. doi:10.1002/ffj.1198
Suresh, S., Chung, J. W., Cho, G. T., Sung, J. S., Park, J. H., Gwag, J. G., et al. (2013). Analysis of molecular genetic diversity and population structure in Amaranthus germplasm using SSR markers. Plant Biosyst. - Int. J. Deal. all Aspects Plant Biol. 148, 635–644. doi:10.1080/11263504.2013.788095
Tanaka, K., Watanabe, T., Higuchi, H., Miyamoto, K., Yusa, Y., Kiyonaga, T., et al. (1999). Density-dependent growth and reproduction of the apple snail, Pomacea canaliculata: A density manipulation experiment in a paddy field. Popul. Ecol. 41, 253–262. doi:10.1007/s101440050029
Tang, S. Q., Wei, F., Zeng, L. Y., Li, X. K., Tang, S. C., Zhong, Y., et al. (2009). Multiple introductions are responsible for the disjunct distributions of invasive parthenium hysterophorus in China: Evidence from nuclear and chloroplast DNA. Weed Res. 49, 373–380. doi:10.1111/j.1365-3180.2009.00714.x
Tao, H. M. (2020). “Study on the population distribution characteristics and control Technology of Procambarus clarkii in Caohai,” (Guizhou: Guizhou Normal University). [dissertation/master’s thesis]. [Guiyang].[In Chinese]
Teng, Q. M., Sun, Y. J., Shen, Y. Y., Zhang, D. N., Xu, G. P., Zhou, L. W., et al. (2021). Growth and phenotypic plasticity variability of Eichhornia crassipes in response to different eutrophic water in karst wetland. J. Lake Sci. 33, 123–137. doi:10.18307/2021.0115
VanDerWal, J., Shoo, L. P., Graham, C., and Williams, S. E. (2009). Selecting pseudo-absence data for presence-only distribution modeling: How far should you stray from what you know? Ecol. Model. 220, 589–594. doi:10.1016/j.ecolmodel.2008.11.010
Wang, B., Li, W., and Wang, J. (2005). Genetic diversity of Alternanthera philoxeroides in China. Aquat. Bot. 81, 277–283. doi:10.1016/j.aquabot.2005.01.004
Wang, C., Cheng, H., Wu, B., Jiang, K., Wang, S., Wei, M., et al. (2021). The functional diversity of native ecosystems increases during the major invasion by the invasive alien species, Conyza canadensis. Ecol. Eng. 159, 106093. doi:10.1016/j.ecoleng.2020.106093
Wang, C., Dang, H. S., Tan, S. D., and Zhang, Q. F. (2010). Study on allelopathy and invasiveness of Conyza sumatrensis in the three gorges reservoir of the yangtze river. Plant Sci. J. 28, 90–98. doi:10.3724/sp.j.1142.2010.00090
Wang, M. H., Gao, X., and Guan, Y. L. (2020). Genetic diversity analysis of parthenium hysterophor L. Based on SRAP molecular markers. Mol. Plant Breed. 18, 193–199. [Chinese with abstract in English]
Wang, Q., Huang, M., Downie, S. R., Chen, Z., and Chen, Y. (2015). Genetic diversity and structure of the noxious alien grass Praxelis clematidea in southern China. Biochem. Syst. Ecol. 59, 183–189. doi:10.1016/j.bse.2015.01.021
Wang, R., Zheng, Z., Wang, G., and Kong, X. (2014). Allelopathic potential and antifeeding activity of Crassocephalum crepidioides against native plants and Spodoptera litura. Allelopathy J. 33, 245.
Wang, S. J., Liu, Q. M., and Zhang, D. F. (2004). Karst rocky desertification in southwestern China: Geomorphology, landuse, impact and rehabilitation. Land Degrad. Dev. 15, 115–121. doi:10.1002/ldr.592
Wang, X., Yi, T., Li, W., Xu, C., Wang, S., Wang, Y., et al. (2022). Anthropogenic habitat loss accelerates the range expansion of a global invader. Divers. Distributions 00, 1610–1619. doi:10.1111/ddi.13359
Wang, Y., Li, W. H., Li, D., and Zhang, Z. (2015). Research progress on invasion mechanism and prevention strategy of Alternanthera philoxeroides. J. Zhejiang A F Univ. 32, 625–634. doi:10.11833/j.issn.2095-0756.2015.04.020 [Chinese with abstract in English]
Xiao, L., Hervé, M. R., Carrillo, J., Ding, J., and Huang, W. (2019). Latitudinal trends in growth, reproduction and defense of an invasive plant. Biol. Invasions 21, 189–201. doi:10.1007/s10530-018-1816-y
Xiong, K. N. (2006). South China karst cone karst ecological processes and biodiversity of libo. Guiyang: Guizhou People's Publishing House.[In Chinese]
Xu, E. Q., and Zhang, H. Q. (2014). Characterization and interaction of driving factors in karst rocky desertification: A case study from changshun, China. Solid earth. 5, 1329–1340. doi:10.5194/se-5-1329-2014
Xu, H. G. (2011). Biodiversity in the karst area of southwest Guangxi. Beijing: Encyclopedia of China Publishing House.[In Chinese]
Xu, J. R., Han, X. L., Li, N., Yu, J. F., Qian, C. H., and Bao, Z. M. (2009). Analysis of genetic diversity of three geographic populations of Pomacea canaliculata by AFLP. Acta Ecol. Sin. 29, 4119–4126.[Chinese with abstract in English]
Xu, X. Y. (2020). “Effects of karst soil heterogeneity and AM fungi on growth and nutrients utilization of Bidens pilosa,” ([Guiyang]: Guizhou University). [dissertation/master’s thesis].[In Chinese]
Yan, H., Feng, L., Zhao, Y., Feng, L., Wu, D., and Zhu, C. (2020). Prediction of the spatial distribution of Alternanthera philoxeroides in China based on ArcGIS and MaxEnt. Glob. Ecol. Conservation 21, e00856. doi:10.1016/j.gecco.2019.e00856
Yang, C. (2020). Species, harm, Prevention and control Status and future development trend of Alien Species invasion in Guizhou. J. Agric. Catastropholgy 10, 144–148+150. doi:10.19383/j.cnki.nyzhyj.2020.04.059[In Chinese]
Yang, Q., Jin, B., Zhao, X., Chen, C., Cheng, H., Wang, H., et al. (2022). Composition, distribution, and factors affecting invasive plants in grasslands of Guizhou province of southwest China. Diversity 14, 167. doi:10.3390/d14030167
Yang, Q., Liu, S., He, C., and Yu, X. (2018). Distribution and the origin of invasive apple snails, Pomacea canaliculata and P. maculata (Gastropoda: Ampullariidae) in China. Sci. Rep. 8, 1185. doi:10.1038/s41598-017-19000-7
Yang, R., Zhou, G., Zan, S., Guo, F., Su, N., and Li, J. (2014). Arbuscular mycorrhizal fungi facilitate the invasion of Solidago canadensis L. in southeastern China. Acta Oecol. 61, 71–77. doi:10.1016/j.actao.2014.10.008
Ye, W. H., Li, J., Cao, H. L., and Ge, X. J. (2003). Genetic uniformity of Alternanthera philoxeroides in South China. Weed Res. 43, 297–302. doi:10.1046/j.1365-3180.2003.00346.x
Yu, X., He, T., Zhao, J., and Li, Q. (2014). Invasion genetics of Chromolaena odorata (asteraceae): Extremely low diversity across asia. Biol. Invasions 16, 2351–2366. doi:10.1007/s10530-014-0669-2
Zhang, B. C., Peng, Y., Zang, L. F., Qin, K. N., Li, X. B., and Sui, C. L. (2017). Plasticity of Alternanthera philoxeroides in response to three karst habitats. Guihaia 37, 702–706+733.[Chinese with abstract in English]
Zhang, H., Goncalves, P., Copeland, E., Qi, S., Dai, Z., Li, G., et al. (2020). Invasion by the weed Conyza canadensis alters soil nutrient supply and shifts microbiota structure. Soil Biol. Biochem. 143, 143107739. doi:10.1016/j.soilbio.2020.107739
Zhang, K., Shen, Y., Fang, Y., and Liu, Y. (2016). Changes in gametophyte physiology of Pteris multifida induced by the leaf leachate treatment of the invasive Bidens pilosa. Environ. Sci. Pollut. Res. 23, 3578–3585. doi:10.1007/s11356-015-5589-x
Zhang, L., Rohr, J., Cui, R., Xin, Y., Han, L., Yang, X., et al. (2022). Biological invasions facilitate zoonotic disease emergences. Nat. Commun. 13, 1762. doi:10.1038/s41467-022-29378-2
Zhang, Y., Wu, H., Hrandl, E., Franca, R., and Hao, J. (2021). Autonomous apomixis in Praxelis clematidea (Asteraceae: Eupatorieae), an invasive alien plant. AoB PLANTS 13, plab007. doi:10.1093/aobpla/plab007
Zhang, Y., Zhang, D., and Barrett, S. C. H. (2010). Genetic uniformity characterizes the invasive spread of water hyacinth (Eichhornia crassipes), a clonal aquatic plant. Mol. Ecol. 19, 1774–1786. doi:10.1111/j.1365-294x.2010.04609.x
Zhao, Y., Yang, X., Xi, X., Gao, X., and Sun, S. (2012). Phenotypic plasticity in the invasion of crofton weed (Eupatorium adenophorum) in China. Weed Sci. 60, 431–439. doi:10.1614/ws-d-11-00198.1
Zheng, Y. L., Feng, Y. L., Zhang, L. K., Callaway, R. M., Valiente-Banuet, A., Luo, D. Q., et al. (2015). Integrating novel chemical weapons and evolutionarily increased competitive ability in success of a tropical invader. New Phytol. 205, 1350–1359. doi:10.1111/nph.13135
Zhong, J. D., Zhou, H. B., Liu, K. D., Yuan, C. C., Li, X. K., and Liu, w. g. (2016). Comparative study on seed biological characteristics of three invasive Compositae species. J. Weed Sci. 34, 7–11. doi:10.19588/j.issn.1003-935x.2016.02.002[Chinese with abstract in English]
Zhou, B., Yan, X. H., Xiao, Y. A., Zhang, Z. G., Li, X. H., and Yang, J. Q. (2013). Traits of reproductive biology associated with invasiveness in alien invasive plant Phytolacca americana. Ecol. Environ. Sci. 22, 567–574. doi:10.16258/j.cnki.1674-5906.2013.04.010[Chinese with abstract in English]
Zhou, D., Wang, T., and Valentine, I. (2005). Phenotypic plasticity of life-history characters in response to different germination timing in two annual weeds. Can. J. Bot. 83, 28–36. doi:10.1139/b04-148
Zhou, Z., Jiang, H., Yang, C., Yang, M., and Zhang, H. (2010). Microbial community on healthy and diseased leaves of an invasive plant Eupatorium adenophorum in Southwest China. J. Microbiol. 48, 139–145. doi:10.1007/s12275-010-9185-y
Keywords: karst areas, invasive species, status quo, mechanism of biological invasions, potential distributions, countermeasures and proposals
Citation: Li Y, Song T, Lai Y, Huang Y, Fang L and Chang J (2022) Status, mechanism, suitable distribution areas and protection countermeasure of invasive species in the karst areas of Southwest China. Front. Environ. Sci. 10:957216. doi: 10.3389/fenvs.2022.957216
Received: 30 May 2022; Accepted: 25 July 2022;
Published: 31 August 2022.
Edited by:
Tian Zhao, Chengdu Institute of Biology (CAS), ChinaReviewed by:
Xuan Liu, Institute of Zoology (CAS), ChinaZuofu Xiang, Central South University Forestry and Technology, China
Copyright © 2022 Li, Song, Lai, Huang, Fang and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiang Chang, conservation1@126.com
 Yonghua Li
Yonghua Li Tianjian Song
Tianjian Song Yangjun Lai3
Yangjun Lai3