Efficiency of Carbon-Based Electrodes on a Microbial Electrolysis System for the Treatment of Bilge Water
- 1Laboratory of Environmental Engineering, Department of Chemical Engineering, Cyprus University of Technology, Lemesos, Cyprus
- 2Nano/Micro Mechanics of Materials Laboratory and Research Unit for Nanostructured Materials Systems, Department of Mechanical Engineering and Materials Science and Engineering, Cyprus University of Technology, Limassol, Cyprus
A coupled Microbial Electrolysis Cell (MEC) – Anaerobic Granular Sludge (AGS) system was settled to investigate for the first time the ability of various carbon-based electrodes to enhance biodegradation of real bilge water (BW) and increase methane generation as an emerging technology for converting organic matter into value-added products. Results revealed that the performance of the three types of electrodes named carbon foam (CF), carbon cloth (CC) and three-dimensional graphene foam (3DG), was both time and organic load content dependent during the experimental cycles. Cumulative CH4 generation reached 235 mL in just 13 days after feeding the AGS with 50% of BW and application of 1.0 V at 3DG electrodes, followed by CC electrodes (148.3 mL). CF proved to be more resistant in higher BW concentration showing a sufficient performance of 1 month. However, in the third cycle, the performances of MECs containing 3DG and CC were higher compared to the CF and the control. Over the first cycle, the soluble Chemical Oxygen Demand (sCOD) removal was found to be around 70% to all MECs, and this value was around 10% higher than the control. Among the different Volatile Fatty Acids (VFAs), acetic acid was identified in the highest concentration in the first cycle, whereas propionic acid was detected in the second and third cycles. Microbial profile analysis showed that Methanobacterium and Desulfovibrio had substantially higher abundances in the cathodes than in the suspended anaerobic sludge. An X-ray diffraction (XRD) investigation of the used electrodes pointed out the formation of various crystalline compounds on their surface, which were different for the anode and cathode.
Introduction
Bilge water is the wastewater accumulated in a ship’s bilge. It consists of seawater and several types of wastes such as cleaning solvents, detergents, grease, oil additives, heavy metals, hydrocarbons, etc. (Vyrides et al., 2018) and is considered a recalcitrant wastewater (Mazioti A. A. et al., 2021). Its chemical composition differs from ship to ship and among days within the same ship (Tiselius and Magnusson, 2017). The characteristics of BW are also affected by ship’s operations, equipment performance and repairs (McLaughlin et al., 2014). Among other things, high values of COD (up to 15 g L−1), salinity (up to 35 g L−1) or oil content (up 2953 mg L−1) have been reported in the literature (Vyrides et al., 2018). These properties make BW treatment very difficult or unsatisfactory to meet international regulations regarding BW discharges by applying conventional treatment methods. Therefore, finding alternative economic and environment-friendly methods to enhance treatment effectiveness is needed.
So far, an alternative method for bilge water treatment against physicochemical and biological processes is the bioelectrochemical process. Hwang et al. (2019) used single chamber microbial fuel cells (MFCs) with Pseudomonas putida ATCC 49128 for synthetic bilge water biodegradation with simultaneous electricity generation. The COD removal was 71% in 20 days. Moreover, they investigated the effect of surfactant addition on the biodegradation of bilge water using an MFC. Their results showed that the addition of surfactant substantially improved the biodegradation of bilge water. Similarly, in another study, Hwang et al. (2021) used single and double-chamber microalgal fuel cells (SMAFC and DMAFC) capable of generating energy with a novel microalgal strain (Chlorella sorokiniana) that was isolated from oily wastewater to treat synthetic bilge water. Both SMAFC and DMAFC removed 67.2–77.4% of soluble COD from an initial 5576.5 ± 166.5 mg COD. Mei et al. (2019) assessed an integrated MFC with an electrocoagulation process to treat synthetic bilge water (2029 ± 61 mg L−1). In that study, three MFCs were connected in series using as a power source an electrocoagulation cell (ECC) for an onboard system for bilge water treatment. The results showed that acetate-fed or municipal waste MFC stacks can effectively provide enough energy to power ECC operation for bilge water treatment. The MFCs-ECC system removed successfully 93% of oil content; during a 4-h treatment, the COD of bilge was reduced to 347 ± 15 mg L−1 (83% removal) when powered by MFC series stack. Recently, Gatidou et al. (2022) examined real bilge water treatment using a microbial electrolysis cell (MEC) containing iron rod electrodes. After gradual biomass acclimatization, sCOD removal up to 70% was identified in this study. Furthermore, MEC operation at 1V resulted in better performance than the control or the operation at 2 V (Gatidou et al., 2022).
In MECs, electroactive microorganisms using an electrode surface as an external electron acceptor oxidize organic compounds producing protons, CO2 and electrons. Electrons generated by this electrogenic biofilm are transferred via extracellular electron transport (EET) to the anode and subsequently to the cathode through an externally applied electrical circuit (Zeng et al., 2016). The conversion of CO2 to CH4 in the cathode is achieved through direct or indirect (H2 mediator) pathways (Choi and Sang, 2016).
One of the key parameters of a MEC system is the selection of the electrode material because it determines the electrons transfer between exoelectrogens and electrodes which will take place (Mier et al., 2021). High conductivity, specific surface area and biocompatibility are considered core characteristics for achieving high rates of methanogenesis and organic load removal (Mier et al., 2021). Up to now, to the best of our knowledge, there is only one study regarding the application of the MEC-AD process for the treatment of real BW using commercial iron electrodes (Gatidou et al., 2022). However, colonization of metal surfaces such as iron by microbes may speed metallic corrosion in an aqueous environment (Krishnamurthy et al., 2013), leading to reduced performance of the MEC system. Thus, carbon-based materials like carbon cloth, carbon felt, graphite particles and others are often used instead due to their satisfactory stability in the microbial inoculum, great electrical conductivity and low toxicity (Mier et al., 2021). Unfortunately, simple carbon materials present poor biocompatibility and poor electrocatalytic activity and, for this reason often are modified to improve their performance (Mansoorian et al., 2020). Up to now, there are no data available regarding the application of carbon-based electrodes same or similar to those used in the study on the treatment of real BW by MEC coupled with AGS. Therefore, the current work, aimed to examine the efficiency of these types of electrodes on the biodegradation of real bilge water during batch experiments and using anaerobic granular sludge as an inoculum. More precisely electrodes of carbon foam (CF), carbon cloth (CC) and three-dimensional graphene foam (3DG) were produced and examined. The application of 1.0 V of external potential was investigated in order to enhance CH4 production and dCOD elimination for three consecutive batch cycles having total duration of almost 2 months (52d).
Materials and Methods
Semi-Batch Experiment
Anaerobic granular sludge used as inoculum, was provided from a full-scale upflow anaerobic sludge blanket (UASB) treating dairy wastewater (Charalambides Christis Ltd., Cyprus). Before its use, the sludge was sieved (1 mm), washed with distilled water and transferred into a duran glass bottle (1 L). Then, it was flushed with CO2 (99.99%) for 5 min to achieve anaerobic conditions and placed in a shaking incubator for a week at 34°C in order to activate the biomass. Real BW was collected from Ecofuel Ltd. Company (Zygi, Cyprus). The characteristics of pre-settled BW were as follows: pH: 7.5, COD: 2.9 g COD L−1, total Solids (TS): 9557 mg L−1, volatile solids (VS): 3053 mg L−1, conductivity: 16.3 mScm−1, salinity as g L−1 NaCl: 9.9 g L−1.
Batch lab-scale experiments were performed using four 1 L glass reactors as single chamber MEC (working volume of 0.7 L). The four reactors were set up and operated simultaneously as follows: R0 without electrodes inside which served as the control (R0-C), R1 containing carbon foam CF electrodes (R1-CF), R2 containing carbon cloth CC electrodes (R2-CC) and R3 containing 3DG graphene foam electrodes (R3-3DG). Anode and cathode in the different MECs constituted by the same material. The performance of the MECs was studied by applying a constant potential of 1.0 V using a DC power supply (GW-Instek, Taiwan). Each reactor was fed with 50 g of AGS and BW at different concentrations; cycle A: 50%, cycle B: 75% and cycle C: 75% BW. The remaining volume was filled with anaerobic medium (Angelidaki et al., 2009) and the pH was adjusted to 7.5. Finally, CO2 was flushed inside (5 min) the bioreactors only at the beginning of each cycle, for achieving anaerobic conditions and the reactors were placed at 34 °C. In total, three consecutive batch cycles were run for almost 2 months (52d) (cycle A: 13d, cycle B: 19d and cycle C: 21d). Throughout the experiments the output voltage in MEC-AD was recorded every 5 min by a data acquisition system (Keithley 2700) connected to a computer.
Carbon-Based Electrodes
Carbon based electrodes used in this study were produced with commercially available materials that were used as received (CC) or after processing/modification (CF and 3DG). Carbon foam CF electrodes (CF) were produced through a pyrolysis process of a commercially available melamine-based foam (
The synthesis of three-dimensional graphene foam (3DG) was performed using a commercial nickel foam (
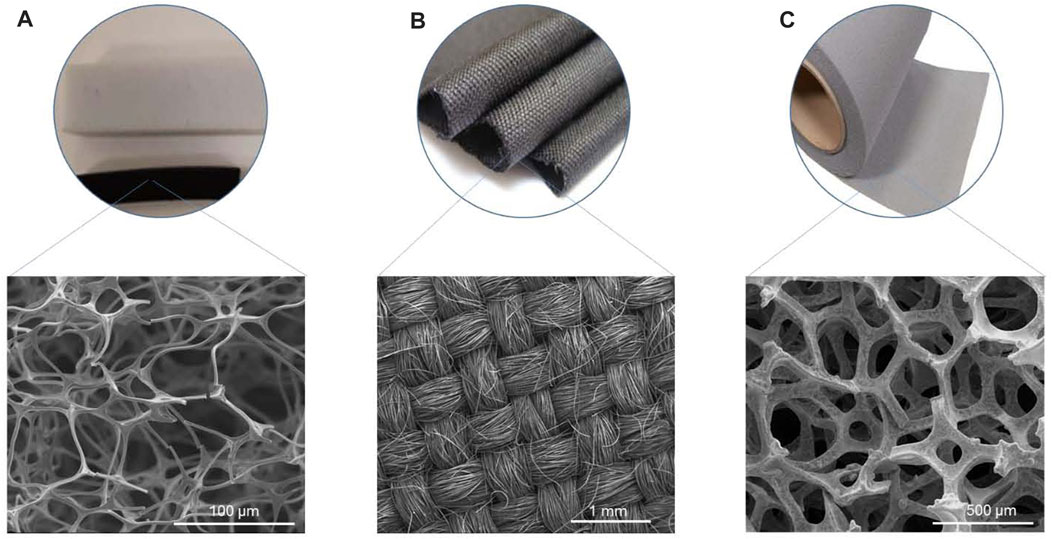
FIGURE 1. Materials for the production of electrodes used in this study: (A) Carbon foam (CF), (B) Commercial carbon cloth (CC), and (C) three-dimensional graphene foam (3DG).
Analysis of Electrodes
At the end of cycle C used electrodes were dried and prepared for characterization d. Used cathodes and anodes were studied by XRD measurements in a Rigaku Ultima IV diffractometer equipped with a copper tube and operated at 40 kV and 40 mA. The system was configured in parallel beam mode by a multilayer mirror and patterns were recorded in θ–2θ (Bragg-Brentano) scans using Cu Kα radiation (λ = 0.15419 nm). A fixed graphite monochromator was also used at receiving optics to suppress fluorescence.
Due to the nature (size and shape) of the used electrodes, it was very difficult to achieve proper forms for the XRD scans, which resulted in low signal to noise ratio. Furthermore, the unknown composition of the bilge water made the peak identification a rather challenging process. Thus, the XRD patterns are considered in a qualitative manner and treated as indications for the involved processes between the electrodes and the bilge water.
Microbial Profile Analysis
AGS samples (∼250 mg) were collected from bioreactors and electrodes similarly to Gatidou et al. (2022) at the end of cycle C. DNeasy PowerSoil Pro kit (Quiagen, Germany) was used for the total genomic DNA extraction following manufacturers’ instructions and the final extracts were analyzed by DNASense Apps Company (Denmark). Analytical description of the used protocol is given in the Supplementary Material (Section A. Analytical Methods).
Analytical Methods
Total solids (TS), volatile solids (VS) and sCOD were performed based on Standard Methods (APHA 2005). Composition of produced biogas analysis was achieved by an Agilent Technologies Gas Chromatographer 7820A connected to a Thermal Conductivity Detector (Wilmington, DE, United States) according to Vyrides et al. (2018). Separation of the several gases (H2, O2, N2, CH4, and CO2) was performed with a ShinCarbon ST packed column (Restek Corporation, Bellefonte, PA, United States) using argon (1 mL min−1) as a carrier gas and the following column temperature program: 2 min at 60°C, then increase to 160°C with a rate of 20°C min−1 and hold for 1 min. The injector and detector were maintained at 125 and 250°C, respectively. The produced volumes of biogas were measured using a 50 mL glass syringe and reported at atmospheric pressure.
Concentrations of VFAs (acetic, propionic, iso-butyric, butyric and valeric acid) were determined by injecting 1 μl of the sample to High-Performance Liquid Chromatographer- UV detector (LC-20AD, Shimadzu, Japan) according to Vyrides and Stuckey (2009). Elution of the compounds was performed isocratically with a flow rate 0.7 mL min−1 on a Rezex™ ROA-Organic Acid H+ (8%) (150 × 7.8 mm, Phenomenex) with 5 mM H2SO4 at 50°C. The UV detector was set at 210 nm during the analysis.
Calculations
The cumulative methane yield was calculated according to (Charalambous and Vyrides, 2021). The removal efficiency of sCOD was calculated according to Eq. 1:
Where, COD0 and CODt are the major pollutants’ concentrations at the start and at the end of the experiment.
First order kinetic equation was fitted to COD concentration profiles for each reactor and the removal rate constants (k), the half-life values (t1/2) and the correlation coefficients (R2) were calculated as follows (Eq. 2):
Where Ct and C0 are the COD concentration at time t and t = 0, respectively, (mg L−1), k is the removal coefficient (d−1) and t is the time at the end of each experimental cycle.
GraphPad Prism 5 for Windows software was used for data evaluation.
Current (I) was calculated using the Ohm’s law (Eq. 3):
Where V is the applied voltage (V) and R is the resistor connected in the MECs (10 Ω).
The energy efficiency of microbial electrolysis cell was calculated according to Xing et al. (2021) following the Eq. 4:
Where μE is the energy efficiency of microbial electrolysis cell, Ii is the current (A) of external circuit for microbial electrolysis cell, E is the voltage (V) of MEC, Δt is the digestion time in each cycle (s), V is the methane volume (L) of microbial electrolysis cell, Vc is the methane volume (L) of bioreactor without extranet voltage, ΔH is the upper heating value of methane (890.31 × 103 J mol−1), Vm is molar volume of gas at ambient temperature and pressure (22.4 L mol−1).
Results and Discussion
Methane Production
Cumulative methane production during each running cycle for all reactors is presented in Figure 2. According to the results throughout the experiments, higher methane generation was always detected in MECs than in the control anaerobic bioreactor. After the first operation cycle with diluted 50% real bilge water (cycle A), the cumulative methane was found to be 1.5, 2.6, and 4.1 times higher than the control for R1-CF, R2-CC and R3-3DG, respectively confirming the advantage of using MEC systems for the treatment of recalcitrant wastewater. The 3DG electrodes had the highest performance among the three different electrodes. At the end of cycle A, a total of 235.3 mL methane was produced in the R3-3DG reactor. This volume was about 58 and 179% higher than the measured gas in R2-CC and R1-CF, respectively. Higher methane production in R3-3DG reaction was probably connected with its preeminent properties like high electrical conductivity and/or large accessible surface (Mao et al., 2015) that favor the colonization of this material by microorganisms (Yu et al., 2016). This is also in line with the higher energy efficiency found in R3-3DG compared to other reactors (please see section regarding energy efficiency) Furthermore, the possible release of nickel ions from the electrode into the liquid phase could also be a possible reason for the higher methane content in the R3-3DG reactor. Nickel (the base of 3DG electrodes) is an essential trace element next to others and plays a critical role in methanogenesis. It is involved in the function of enzymes such as NiFe hydrogenases, carbon monoxide dehydrogenase/acetyl-CoA synthase, hydrogenases, and F420-reducing hydrogenases (Khan et al., 2021). Additionally, the nickel template may be also oxidized, contributing to hydrogen production and, therefore, enhanced hydrogenotrophic methanogenesis.
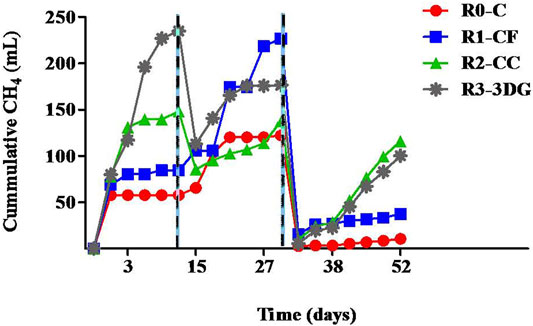
FIGURE 2. Cumulative methane (mL) produced over time in control (R0-C) and in MECs containing carbon foam (R1-CF), carbon cloth (R2-CC) and 3-dimensional graphene (R3-3DG). Dot lines represent the beginning of the next experimental cycle.
Estimating the cycle, A, a new feeding was performed on day 13, applying higher organic load content in the reactors. The results indicated that when the concentration of bilge water was increased to 75% (cycle B), methane generation was enhanced in R1-CF reactor and reached 226.7 mL (Figure 2) at day 31 (end of cycle B). On the contrary, in R2-CC methane production, although presented an increase, at the end of cycle B, its value fluctuated at the same levels as cycle A. Regarding the R3-3DG reactor, feeding with a higher organic load resulted in about 33% less gas production (176.3 mL) on 31d than cycle A, indicating that this material loses its effectiveness soon enough, probably due to the oxidation of nickel. Nevertheless, the overall methane generation in MECs was still higher than in control. Specifically, it was found to be 12.3, 44.2, and 85.3% increased for R2-CC, R3-3DG and R1-CF, respectively.
Replacement of liquor on day 31 with fresh BW (start of cycle C) resulted in even lower methane generation in all MEC reactors than in previous cycles (Figure 2), while in the control bioreactor, the production was negligible. At the end of the experiment (52d), the cumulative methane was 37.5, 115.8 and 100.3 mL for R1-CF, R2-CC and R3-3DG, respectively. Nevertheless, methane production increased R2-CC and R3-3DG, whereas R1-CF reached a steady-state 3 days after the feeding, similar to cycle A. This lower methane production during the last cycle could be due to the biomass’s possible weakening and inability to cope with the toxic and high salinity environment for a long time, given that no previous adaptation in saline conditions took place before the beginning of the experiment. As reported in the literature, the existence of high osmotic pressure for an extended period (use of 75% BW from day 13 until 52) may force biomass to consume the organic load for their survival instead of methanogenesis (Vyrides and Stuckey, 2009). Furthermore, the presence of specific pollutants like organic solvents or hydrocarbons often detected in BW may also contribute to reduced methane generation. According to the literature, several authors have reported the existence of different compounds. For instance Vyrides et al. (2018) identified methyl isobutyl ketone and heptane in untreated BW. Similarly, McLaughlin et al. (2014) detected methyl ethyl ketone, while Cazoir et al. (2012).
In a recent study, Gatidou et al. (2022) evaluated the performance of commercial iron electrodes in MEC reactors for the treatment of real BW. According to their results, during the first 15 days of the experiment, the cumulative methane in the MEC reactor working at 1 V was negligible although only 25% of BW was used. On the contrary, the results of the current study show that carbon-based electrodes are more effective for the start-up of a MEC system than the commercial iron electrodes.
COD Removal Efficiency
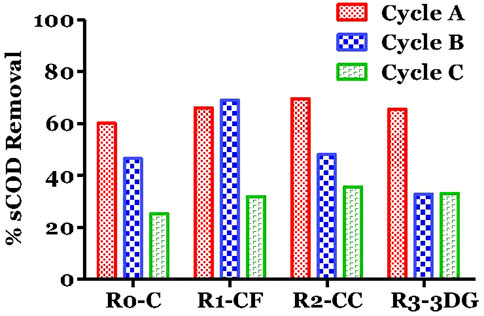
The sCOD removals at the end of each cycle are shown in Figure 3. At the end of cycle A the sCOD removal in all MECs found to be similar to all reactors and up to 69.6%. This value was higher than Gatidou et al. (2022) during their cycle A of experiment, considering the double content of BW that was used in the present study (50%). However, the measured values were only about 5–10% higher than the control, indicating a slight improvement in organic load removal due to MEC application. Moreover, the CH4, as was shown in Figure 2, was higher for MEC systems than the control, and this is not in line with the COD results (Figure 3). At the end of cycle A, the produced gas corresponded to 26.7, 46,4 and 77.1% of the initial COD for R1-CF, R2-CC and R3-3DG, respectively. This could be due to hydrogen production from water electrolysis and the subsequent utilization by hydrogenotrophic methanogens.
During the next cycle, the increase of BW content (75%) seemed to affect the performance of some reactors. According to the results, the sCOD removal was significantly lower in R3-3DG and R2-CC than cycle A, with values as high as 32.9 and 48%, respectively on day 32 (end of cycle B), revealing a possible shock of microorganisms due to the higher salinity of the new feeding. As reported in the literature (Ma et al., 2020; Ahmadi et al., 2017), the lack of acclimation of non-halophilic biomass to high saline environments can result in gradual cell dehydration and enzyme deactivation to the osmotic difference, which can even lead to lower removal of organic loads. On the contrary, the COD removal in R1-CF remained high (69%), possibly due to higher porosity of carbon foam material (100 pores cm−1) compared to carbon cloth and 3D-graphene (39 porescm−1), which led to more significant adsorption of the organic load from the liquid phase. However, the high % of COD removal in R1-CF was not balance by a high percentage of methane since the produced methane at that time (end of cycle B) found to be only 36% of the initial COD. Renewal of the organic load on 31d (start of cycle C) with the same BW content further decreased the removal of dCOD, indicating that the performance of the three materials is reduced over time (Supplementary Figures S1–S3). Indeed, at the end of the experiment, the range found to be between 31.9 (R1-CF) and 35.6% (R2-CC) demonstrated ineffective microbial activity.
A first-order kinetic equation was fitted to COD concentration profiles at each experimental cycle, and the removal rate constants (k), the half-life values (t1/2) and the correlation coefficients (R2) were calculated using the software GraphPad Prism 5 (Table 1). According to the results, the half-life values ranged between 6.2d (R2-CF, cycle A) and 29.1d (R1-CC, cycle C), indicating that as time passes, the performance of the reactors reduces. The F-test in the Graphpad prism showed that the rate constants and the half-lives were similar for all the reactors for a given cycle. No statistically significant differences existed despite the use of different electrodes. p values were 0.9220, 0.0733 and 0.0783 for cycles A, B and C, respectively.
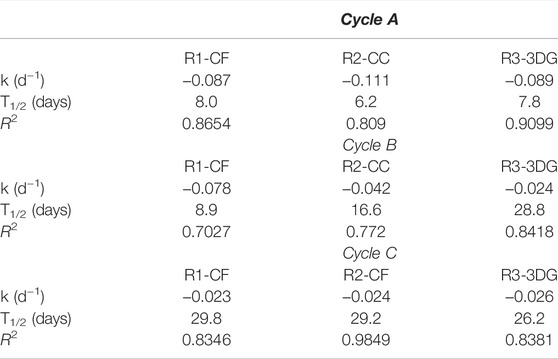
TABLE 1. First order kinetics (k), half-life (t1/2) and correlation coefficients (R2) values calculated for sCOD concentrations in batch experiments during different feeding cycles.
Regarding the methane produced, this accounts for 27.6, 38.8, 51.5, and 62.5% of the total COD for R0, R1, R2 and R3, respectively. Part of the higher CH4 produced in MEC reactors could be due to the hydrogen produced in the cathode and the utilization by hydrogenotrophic methanogens.
Soluble microbial products (SMPs) are widely classified as biomass-associated products (BAP-due to cell lysis) and utilization-associated products (UAP-which are produced during catabolism/anabolism) (Soh et al., 2021). The SMPs at the end of cycle 1 were higher for the control bioreactor than for the MEC-AC (Figure 4). However, the SMPs are built up and increased in all bioreactors at the end of the following cycles. The SMPs in cycles 2 and 3 were higher than in the first cycle; SMPs might be increased due to the higher bilge concentration in the second and third cycles. The SMPs remained higher in control bioreactor than in the other three MEC-ACs. The SMPs that could be recalcitrant organic compounds could have inhibited the anaerobic microorganisms, which is likely the reason for the lower performance in the third cycle compared to the first cycle.
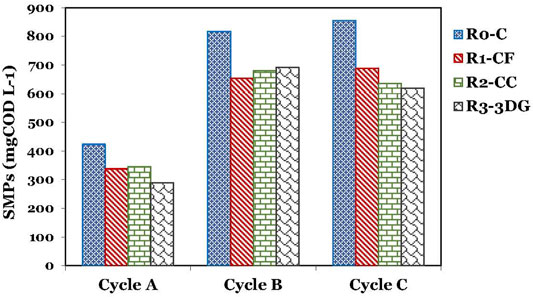
FIGURE 4. Production of Soluble Microbial Products (SMPs) at the end of each experimental cycle A, B and C.
VFAs Analysis
Chromatographic analysis of liquid samples for VFAs detection is presented in Figure 5. Regarding the different types of analyzed VFAs, acetic, propionic and valeric acid were only detected. During experimental cycle A, all three acids were present at concentrations between 26 and 573 mg L−1. While the percentage of BW was increased from 50 to 75% moving from cycle A to B, both propionic and acetic acid were present. Their values were found to be up to 228 (R0-C) and 163 (R1-CF) mg L−1 for acetic and propionic acid, respectively. However, after the first two to 4 days of cycle B no acetic acid was detected, indicating its utilization by acetoclastic methanogens (Yu et al., 2016). Propionic acid was the only detectable compound until the end of cycle C (52d), and its concentrations over time varied from 26 (R1-CF) to 279 mg L-1 (R0-C) (Supplementary Figure S4).
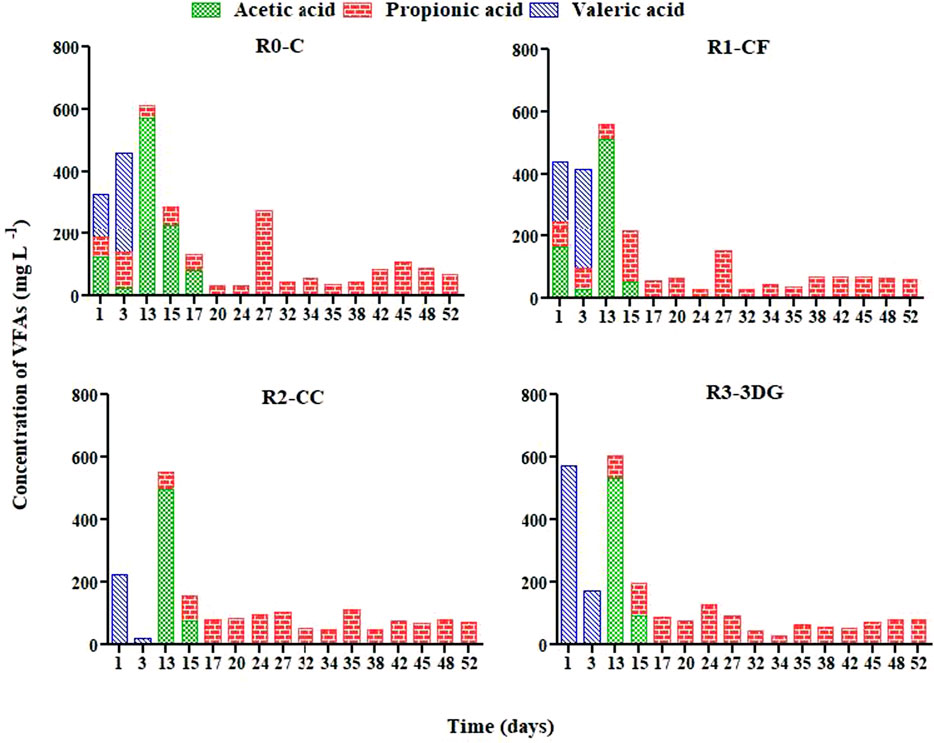
FIGURE 5. Variation in VFAs composition (mg L−1) over time in the different reactors during the three consecutive cycles.
At the end of the first cycle, the diluted bilge was biodegraded to acetic acid by acidogenic and acetogens microorganisms. The rate-limiting step was the acetoclastic methanogens. However, at cycles B and C, high propionic acid in an anaerobic bioreactor indicated low performance, and its biodegradation required low hydrogen partial pressure (Yu et al., 2016). The higher percentage of bilge at these cycles inhibited hydrogen utilizing microorganisms such as hydrogenotrophic methanogens and homoacetogens, and therefore, the propionic acid utilization was not thermodynamically favorable (Charalambous and Vyrides 2021).
Energy Efficiency
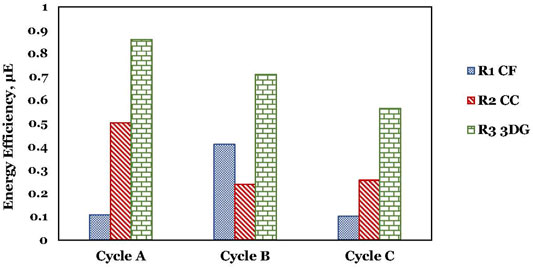
The energy efficiency for the three MEC bioreactors is shown in Figure 6. Over the three cycles, R3 showed a higher efficiency compared to R1 and R2. However, the highest efficiency for R3 was only 0.86; this means that the incremental methane yield produced in the MEC cannot cover the electrical energy consumed in MEC. Even so, MEC-AD performance was better than the control, but the recalcitrant nature of bilge water did not allow the system to balance the consumed energy. From cycle 1 to cycle 3, as the SMPs increased, the energy efficiency decreased, probably due to the toxic compounds in bilge and of SMPs towards anaerobic microorganisms. In a similar system using MEC with commercial iron rods electrodes (Gatidou et al., 2022), an efficiency of 0.23 was found. Therefore, using carbon electrodes in MEC pointed out higher performance than the use of iron electrodes. The highest energy efficiency was for R3-3DG.
Microbial Profile
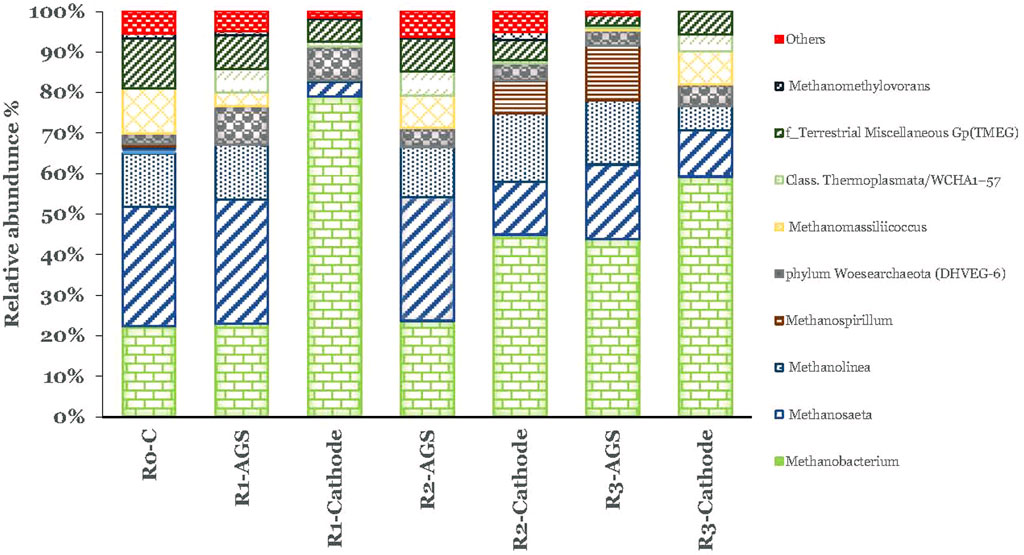
Regarding archaeal communities, the diversity of Euryarchaeota was the most abundant phylum in all samples (Figure 7). Methanobacterium, a typical hydrogen-utilizing methanogen that uses H2 as an electron donor and CO2 as an acceptor, was significantly higher in the cathodes than in the suspended AGS. At the carbon foam cathode, the relative abundance of Methanobacterium found to be 79.2%, whereas at the suspended AGS, this was around 23.9%. On the contrary, the relative abundance of Methanosaeta found to be only 3.6% and was significantly higher in the suspended sludge (30.8%). The same trend was observed in R2-CC and R3-3DG systems but the difference was not so pronounced (Figure 7). The results follow Siegert et al. (2015), who reported cathodes’ attachment mainly by the genus Methanobacterium while studying methane production in acetate-fed MECs systems. In addition, hydrogenotrophic Methanolinea was found in similar relative abundance to all samples; it is exclusively found in microbial communities enriched with syntrophic substrates and is likely to play a role in propionic biodegradation.
Methanosaeta, an acetoclastic methanogen, was more abundant in the suspended AGS (Figure 7) and has been suggested as one of the prominent microbial groups responsible for methanogenic granule formation (Andronikou et al., 2022). Previous work (Gatidou et al., 2022) with MECs using iron rode electrodes also revealed high abundance of Methanosaeta in suspended sludge compared to the electrode surface.
Regarding the bacteria diversity, Desulfovibrio was identified at higher percentage in the cathodes compared to the suspended AGS. According to Carmona-Martínez et al. (2015) several microorganisms within the deltaproteobacteria class have been proved to possess electroactivity. This genus was also identified on iron rod electrodes during MEC treating bilge water (Gatidou et al., 2022). Apart from Desulfovibrio no other clear trend was detected among the different cathodes and the suspended AGS (Figure 8). However, the genus Mesotoga was found in all samples (Figure 8). This is in line with the findings of Mazioti A. A. et al. (2021) and Gatidou et al. (2022) that also exposed AGS to bilge water. Nesbø et al. (2019) reported that Mesotoga are frequently identified in oil-polluted marine mesophilic anaerobic environments. Mesotoga, although they belong to the thermophilic phylum of Thermotogae, are the only strictly mesophilic genus (Pollo et al., 2015) and may take part in syntrophic acetate limitation (Nobu et al., 2015).
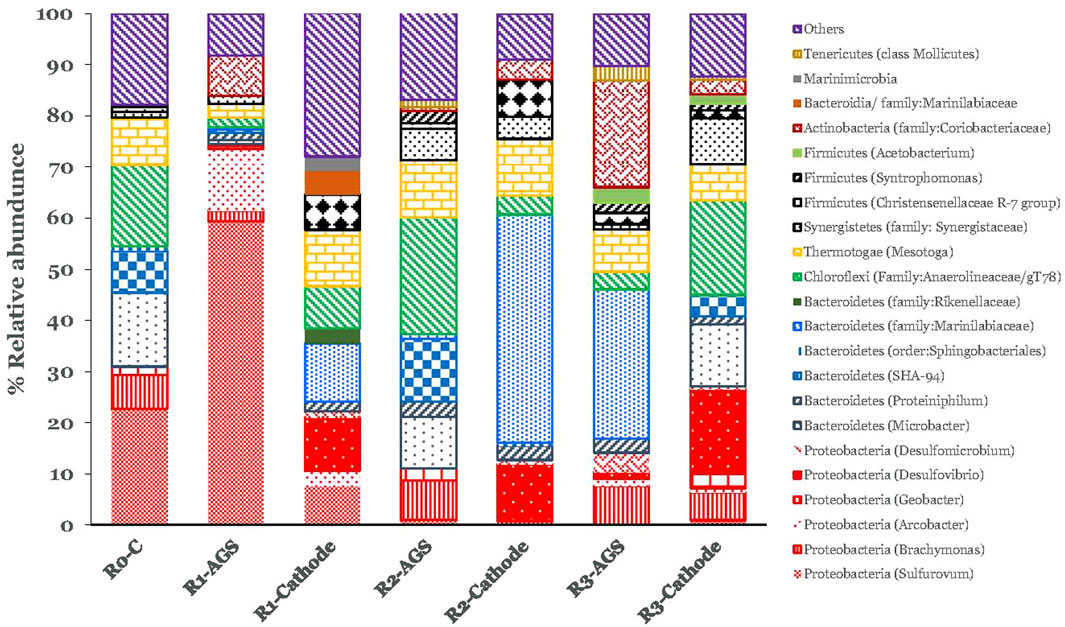
FIGURE 8. Identified relative abundance at the bacterial community at the phylum and genus level in the bioreactors in the suspended sludge and on the cathodes.
Anaerolineaceae (T78) was also found in all samples and the highest relative abundance was observed in the suspended AGS of R2-CC (Figure 8). Anaerolineales family can maintain the physical integrity of the anaerobic granular structure (Zhu et al., 2017). Furthemore, this specific species found to be the most dominant group of bacteria in a semi-continuous anaerobic digester treated lipid wastewater (Nakasaki et al., 2020). Marinilabiaceae was identified only at high relative abundance in R1-CF and R2-CC cathodes (Figure 8) but not in R3-3DG. The family Marinilabiaceae are an anaerobic mesophilic bacterium that can ferment various substrates and produce propionate, acetate, and succinate (Poirier et al., 2017).
Electrodes Analysis
Despite the difficulties that occurred during electrodes XRD analysis, some initial conclusions were still possible to draw. As shown in Figure 9, XRD pattern of R1-CF cathode (carbon foam material) revealed the characteristic peaks of calcite (CaCO3) and halite (NaCl). Formation of CaCO3 was probably due to biomineralization by the bacterial metabolic activity which resulted in mineral precipitation and growth (Keren-Paz and Kolodkin-Gal, 2020). Mazioti A. A. et al. (2021) also observed the formation of CaCO3 in biocarrier during BW treatment. However, it is also possible that the formation of CaCO3 was caused due to the slight increase of pH (7.72 at the end of cycle C). According to the literature (Zeppilli et al., 2021; Ceballos-Escalera et al., 2022), increase of pH near cathode enhances the precipitation of cations like Ca+2 leading to overlap of biofilm and electrode surface. This fact negatively affects the operation of the MEC system decreasing the effectiveness of the treatment.
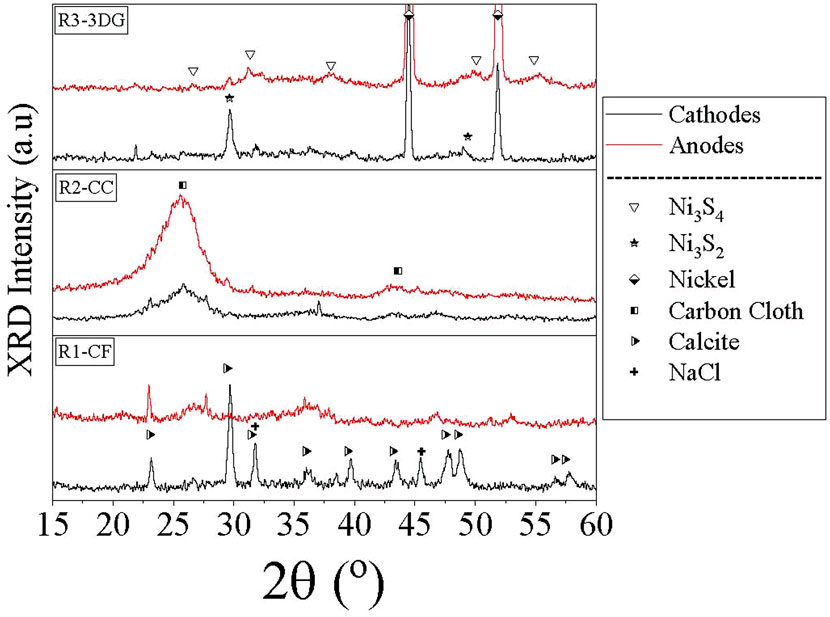
FIGURE 9. XRD patterns of anodes (red lines) and cathodes (black lines) for the three types of electrodes (R1: carbon foam, R2: carbon Cloth, R3: 3-dimensional graphene).
NaCl obviously came from seawater content in BW. The presence of calcite on the surface of the electrode may have reduced the available surface area of the electrode contributing to its lower performance. On the contrary, the XRD pattern of the anode consisted of a messy set of low intensity and broad peaks which was unable to assign to a specific crystalline phase.
Regarding CC material, both electrodes of R2-CC reactor exhibited only the characteristic broad peaks of carbon cloth at 25.8° and 43.6° 2θ.
As for R3-3DG, the XRD patterns revealed the presence of Ni3S4 and Ni3S2 in the anode and cathode, respectively. The formation of nickel sulfides could be attributed to the oxidation of 3DG and especially the nickel foam template by HS− released from sulfur compounds that were contained in the BW. Moreover, both electrodes exhibited pure metallic Ni peaks due to the nickel foam used for their synthesis, while in the cathode some minor unidentified peaks were also observed. The presence of sulfides is in line with the microbial profile findings and the high relative abundance of Desulfovibrio in the cathode of R3-3DG.
Conclusion
Single-chamber MEC-AGS reactors used to treat real bilge water revealed higher methane production than the control (AGS reactor) in all experimental cycles. The performance of the three types of electrodes investigated in the present study for the first time was significantly affected by time and organic load content. 3DG was more effective at lower BW concentrations and generated higher CH4 production among the different materials examined. On the contrary, carbon foam demonstrated better resistance at higher BW content during the second cycle. As for CC performance, results indicated the existence of a ceiling on methane production regardless of the BW concentration or operation time. Considerable COD removal was observed in all MECs, confirming the benefit of using them. However, as the organic load content was increased the biomass seemed to be distressed mainly in the R2-CC and R3-3DG reactors (third cycle). VFAs analysis demonstrated the presence of acetic, propionic and valeric acid, but as the percentage of BW was increased, propionic acid was dominated. The energy efficiency was higher for the system with 3DG electrode than the other systems; however, the methane energy produced in the MECs cannot cover the electrical energy consumed in MECs. Results regarding DNA sequencing analysis indicated the higher predominance of Methanobacterium and Desulfovibrio in the cathodes compared to suspended AGS. Additionally, the XRD analysis of used electrodes confirmed the formation of calcite (CaCO3) and halite (NaCl) on the R1-CF cathode. At R3-3DG, the presence of Ni3S4 and Ni3S2 in the anode and cathode were identified.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
GG: Investigation, Methodology, Validation, Visualization, Writing- Original draft preparation. MC: Investigation, Validation. LK: Investigation. GC: Conceptualization, Methodology, Visualization, Project administration, Supervision, Writing - Review and Editing. IV: Conceptualization, Methodology, Visualization, Project administration, Supervision, Writing - Review and Editing. All authors contributed to the article and approved the submitted version.
Funding
The work has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 841797. Synthesis and characterization of carbon-based electrodes was conducted using support from the project POST-DOC/0718/0213 which is co-funded by the European Regional Development Fund and the Republic of Cyprus through the Research and Innovation Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to clarify that Ecofuel Ltd. (Cyprus) had no role in study funding, design, data collection and analysis, decision to publish or preparation of the current manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.894240/full#supplementary-material
References
Ahmadi, M., Jorfi, S., Kujlu, R., Ghafari, S., Darvishi, R., Soltani, Ch., et al. (2017). A Novel Salt-Tolerant Bacterial Consortium for Biodegradation of Saline and Recalcitrant Petrochemical Wastewater. J. Environ. Manage. 191, 198–208. doi:10.1016/j.jenvman.2017.01.010
Andronikou, M., Adamou, V., Koutsokeras, L., Constantinides, G., and Vyrides, I. (2022). Magnesium Ribbon and Anaerobic Granular Sludge for Conversion of CO2 to CH4 or Biogas Upgrading. Chem. Eng. J. 435, 134888. doi:10.1016/j.cej.2022.134888
Angelidaki, I., Alves, M., Bolzonella, D., Borzacconi, L., Campos, J. L., Guwy, A. J., et al. (2009). Defining the Biomethane Potential (BMP) of Solid Organic Wastes and Energy Crops: a Proposed Protocol for Batch Assays. Water Sci. Technol. 59 (5), 927–934. doi:10.2166/wst.2009.040
Carmona-Martínez, A. A., Trably, E., Milferstedt, K., Lacroix, R., Etcheverry, L., and Bernet, N. (2015). Long-term Continuous Production of H2 in a Microbial Electrolysis Cell (MEC) Treating Saline Wastewater. Water Res. 81, 149–156. doi:10.1016/j.watres.2015.05.041
Cazoir, D., Fine, L., Ferronato, C., and Chovelon, J.-M. (2012). Hydrocarbon Removal from Bilgewater by a Combination of Air-Stripping and Photocatalysis. J. Hazard. Mater. 235-236, 159–168. doi:10.1016/j.jhazmat.2012.07.037
Ceballos-Escalera, A., Pous, N., Balaguer, M. D., and Puig, S. (2022). Electrochemical Water Softening as Pretreatment for Nitrate Electro Bioremediation. Sci. Total Environ. 806, 150433. doi:10.1016/j.scitotenv.2021.150433
Charalambous, P., and Vyrides, I. (2021). In Situ biogas Upgrading and Enhancement of Anaerobic Digestion of Cheese Whey by Addition of Scrap or Powder Zero-Valent Iron (ZVI). J. Environ. Manag. 280, 111651. doi:10.1016/j.jenvman.2020.111651
Choi, O., and Sang, B. I. (2016). Extracellular Electron Transfer from Cathode to Microbes: Application for Biofuel Production. Biotechnol. Biofuels 9 (1), 11–14. doi:10.1186/s13068-016-0426-0
Gatidou, G., Samanides, C. G., Fountoulakis, M. S., and Vyrides, I. (2022). Microbial Electrolysis Cell Coupled with Anaerobic Granular Sludge: A Novel Technology for Real Bilge Water Treatment. Chemosphere 296, 133988. doi:10.1016/j.chemosphere.2022.133988
Hwang, J.-H., Kim, K.-Y., Resurreccion, E. P., and Lee, W. H. (2019). Surfactant Addition to Enhance Bioavailability of Bilge Water in Single Chamber Microbial Fuel Cells (MFCs). J. Hazard. Mater. 368, 732–738. doi:10.1016/j.jhazmat.2019.02.007
Hwang, J.-H., Ryu, H., Rodriguez, K. L., Fahad, S., Domingo, J. S., Kushima, A., et al. (2021). A Strategy for Power Generation from Bilgewater Using a Photosynthetic Microalgal Fuel Cell (MAFC). J. Power Sources 484, 229222. doi:10.1016/j.jpowsour.2020.229222
Keren-Paz, A., and Kolodkin-Gal, I. (2020). A Brick in the Wall: Discovering a Novel Mineral Component of the Biofilm Extracellular Matrix. New Biotechnol. 56, 9–15. doi:10.1016/j.nbt.2019.11.002
Khan, M. S., Lu, F., Kashif, M., and Shen, P. (2021). Multiple Effects of Different Nickel Concentrations on the Stability of Anaerobic Digestion of Molasses. Sustainability 13 (9), 4971. doi:10.3390/su13094971
Krishnamurthy, A., Gadhamshetty, V., Mukherjee, R., Chen, Z., Ren, W., Cheng, H.-M., et al. (2013). Passivation of Microbial Corrosion Using a Graphene Coating. Carbon 56, 45–49. doi:10.1016/j.carbon.2012.12.060
Ma, X.-Ch., Li, X.-K., Wang, X.-W., Liu, G.G., Zuo, J. L., Wang, S.-T., et al. (2020). Impact of Salinity on Anaerobic Microbial Community sStructure in High Organic Loading Purified Terephthalic Acid Wastewater Treatment System. J. Hazard. Mater. 383, 121132. doi:10.1016/j.jhazmat.2019.121132
Mansoorian, H. J., Mahvi, A., Nabizadeh, R., Alimohammadi, M., Nazmara, S., and Yaghmaeian, K. (2020). Evaluating the Performance of Coupled MFC-MEC with Graphite felt/MWCNTs Polyscale Electrode in Landfill Leachate Treatment, and Bioelectricity and Biogas Production. J. Environ. Health Sci. Eng. 18 (2), 1067–1082. doi:10.1007/s40201-020-00528-2
Mao, S., Lu, G., and Chen, J. (2015). Three-Dimensional Graphene-Based Composites for Energy Applications. Nanoscale 7, 6924–6943. doi:10.1039/C4NR06609J
Mazioti, A. A., Koutsokeras, L. E., Constantinides, G., and Vyrides, I. (2021b). Untapped Potential of Moving Bed Biofilm Reactors with Different Biocarrier Types for Bilge Water Treatment: A Laboratory-Scale Study. Water 13, 13131810. doi:10.3390/w13131810
Mazioti, A. A., Vasquez, M. I., and Vyrides, I. (2021a). Comparison of Different Cultures and Culturing Conditions for the Biological Deterioration of Organic Load from Real Saline Bilge Wastewater: Microbial Diversity Insights and Ecotoxicity Assessment. Environ. Sci. Pollut. Res. 28 (27), 36506–36522. doi:10.1007/s11356-021-13153-9
McLaughlin, C., Falatko, D., Danesi, R., and Albert, R. (2014). Characterizing Shipboard Bilgewater Effluent before and after Treatment. Environ. Sci. Pollut. Res. 21, 5637–5652. doi:10.1007/s11356-013-2443-x
Mei, X., Wang, H., Hou, D., Lobo, F. L., Xing, D., and Ren, Z. J. (2019). Shipboard Bilge Water Treatment by Electrocoagulation Powered by Microbial Fuel Cells. Front. Environ. Sci. Eng. 13 (4), 1–7. doi:10.1007/s11783-019-1134-3
Mier, A. A., Olvera-Vargas, H., Mejía-López, M., Longoria, A., Verea, L., Sebastian, P. J., et al. (2021). A Review of Recent Advances in Electrode Materials for Emerging Bioelectrochemical Systems: From Biofilm-Bearing Anodes to Specialized Cathodes. Chemosphere 283, 131138. doi:10.1016/j.chemosphere.2021.131138
Nakasaki, K., Nguyen, K. K., Ballesteros, F. C., Maekawa, T., and Koyama, M. (2020). Characterizing the Microbial Community Involved in Anaerobic Digestion of Lipid-Rich Wastewater to Produce Methane Gas. Anaerobe 61, 102082. doi:10.1016/j.anaerobe.2019.102082
Nesbø, C. L., Charchuk, R., Pollo, S. M. J., Budwill, K., Kublanov, I. V., Haverkamp, T. H. A., et al. (2019). Genomic Analysis of the Mesophilic Thermotogae Genus Mesotoga Reveals Phylogeographic Structure and Genomic Determinants of its Distinct Metabolism. Environ. Microbiol. 21 (1), 456–470. doi:10.1111/1462-2920.14477
Nobu, M. K., Narihiro, T., Rinke, C., Kamagata, Y., Tringe, S. G., Woyke, T., et al. (2015). Microbial Dark Matter Ecogenomics Reveals Complex Synergistic Networks in a Methanogenic Bioreactor. Isme J. 9 (8), 1710–1722. doi:10.1038/ismej.2014.256
Poirier, S., Madigou, C., Bouchez, T., and Chapleur, O. (2017). Improving Anaerobic Digestion with Support Media: Mitigation of Ammonia Inhibition and Effect on Microbial Communities. Bioresour. Technol. 235, 229–239. doi:10.1016/j.biortech.2017.03.099
Pollo, S. M. J., Zhaxybayeva, O., and Nesbø, C. L. (2015). Insights into Thermoadaptation and the Evolution of Mesophily from the Bacterial Phylum Thermotogae. Can. J. Microbiol. 61 (9), 655–670. doi:10.1139/cjm-2015-0073
Siegert, M., Li, X.-F., Yates, M. D., and Logan, B. E. (2015). The Presence of Hydrogenotrophic Methanogens in the Inoculum Improves Methane Gas Production in Microbial Electrolysis Cells. Front. Microbiol. 5, 778. doi:10.3389/fmicb.2014.00778
Soh, Y. N. A., Kunacheva, C., Menon, S., Webster, R. D., and Stuckey, D. C. (2021). Comparison of Soluble Microbial Product (SMP) Production in Full-Scale Anaerobic/aerobic Industrial Wastewater Treatment and a Laboratory Based Synthetic Feed Anaerobic Membrane System. Sci. Total Environ. 754, 142173. doi:10.1016/j.scitotenv.2020.142173
Tiselius, P., and Magnusson, K. (2017). Toxicity of Treated Bilge Water: The Need for Revised Regulatory Control. Mar. Pollut. Bull. 114 (2), 860–866. doi:10.1016/j.marpolbul.2016.11.010
Vyrides, I., Drakou, E.-M., Ioannou, S., Michael, F., Gatidou, G., and Stasinakis, A. S. (2018). Biodegradation of Bilge Water: Batch Test under Anaerobic and Aerobic Conditions and Performance of Three Pilot Aerobic Moving Bed Biofilm Reactors (MBBRs) at Different FIlling Fractionsfilling Fractions. J. Environ. Manag. 217, 356–362. doi:10.1016/j.jenvman.2018.03.086
Vyrides, I., and Stuckey, D. C. (2009). Effect of Fluctuations in Salinity on Anaerobic Biomass and Production of Soluble Microbial Products (SMPs). Biodegradation 20 (2), 165–175. doi:10.1007/s10532-008-9210-6
Xing, T., Yun, S., Li, B., Wang, K., Chen, J., Jia, B., et al. (2021). Coconut-shell-derived Bio-Based Carbon Enhanced Microbial Electrolysis Cells for Upgrading Anaerobic Co-digestion of Cow Manure and Aloe Peel Waste. Bioresour. Technol. 338, 125520. doi:10.1016/j.biortech.2021.125520
Yu, F., Wang, C., and Ma, J. (2016). Applications of Graphene-Modified Electrodes in Microbial Fuel Cells. Materials 9 (10), 807. doi:10.3390/ma9100807
Zeng, X., Borole, A. P., and Pavlostathis, S. G. (2016). Inhibitory Effect of Furanic and Phenolic Compounds on Exoelectrogenesis in a Microbial Electrolysis Cell Bioanode. Environ. Sci. Technol. 50 (20), 11357–11365. doi:10.1021/acs.est.6b01505
Zeppilli, M., Paiano, P., Torres, C., and Pant, D. (2021). A Critical Evaluation of the pH Split and Associated Effects in Bioelectrochemical Processes. Chem. Eng. J. 422, 130155. doi:10.1016/j.cej.2021.130155
Keywords: bilge water, carbon foam, carbon cloth, 3D graphene foam, microbial electrolysis cell, anaerobic treatment
Citation: Gatidou G, Constantinou M, Koutsokeras L, Vyrides I and Constantinides G (2022) Efficiency of Carbon-Based Electrodes on a Microbial Electrolysis System for the Treatment of Bilge Water. Front. Environ. Sci. 10:894240. doi: 10.3389/fenvs.2022.894240
Received: 11 March 2022; Accepted: 20 April 2022;
Published: 11 May 2022.
Edited by:
Narcis Pous, University of Girona, SpainReviewed by:
Marco Zeppilli, Sapienza University of Rome, ItalyZhao Yuxiao, Shandong Academy of Sciences, China
Copyright © 2022 Gatidou, Constantinou, Koutsokeras, Vyrides and Constantinides. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Georgia Gatidou, ggatid@env.aegean.gr; Ioannis Vyrides, ioannis.vyrides@cut.ac.cy
 Georgia Gatidou
Georgia Gatidou Marios Constantinou
Marios Constantinou Loukas Koutsokeras
Loukas Koutsokeras Ioannis Vyrides
Ioannis Vyrides Georgios Constantinides
Georgios Constantinides