Self-assembly synthesis of petal-like MoS2/Co9S8/carbon nanohybrids for enhanced lithium storage performance
- 1MOE Key Laboratory of Resources and Environmental Systems Optimization, College of Environment and Chemical Engineering, North China Electric Power University, Beijing, China
- 2Guangdong Provincial Key Laboratory of Petrochemical Pollution Process and Control, School of Environmental Science and Engineering, Guangdong University of Petrochemical Technology, Maoming, China
Transition metal sulfides are favored as anode materials for the next generation of lithium-ion batteries because of their high theoretical capacities and abundant natural resources. However, serious volume changes during charging and discharging pose great challenges to their stability. In this work, petal-like MoS2/Co9S8/C nanohybrids were synthesized via the immobilization of molybdyl acetoacetonate MoO2(acac)2 in ZIF-67 and subsequent combined vulcanization and thermolysis process. Benefiting from the homogeneous bimetallic sulfide and highly conductive carbon layer, the as-obtained MoS2/Co9S8/C nanohybrids exhibited a high initial discharge capacity of 988.3 mAh g−1 at 200 mA g−1 and a capacity retention > 99.9% after 50 cycles. Even at a high current density of 1000 mA g−1, the reversible capacity of MoS2/Co9S8/C is still as high as 754.6 mAh g−1, revealing extraordinary rate ability. This work can provide a general approach to design and synthesize other advanced bimetallic chalcogenides for boosting lithium-ion batteries storage performance.
Introduction
With the rapid increase in fossil energy consumption and ensuing environmental concerns, the development of clean and efficient energy storage is of great significance to fulfill the demands of higher specific energy, higher power density, and longer lifespan (Liu et al., 2012; Li et al., 2017; Chen et al., 2018a). Rechargeable lithium-ion batteries (LIBs) are a very appealing portable electronic device of energy conversion for large-scale applications (Zhou et al., 2015a; Wang et al., 2021; Miao et al., 2022). Nevertheless, the practical application of commercial graphite-based anodes have been frustrated due to their low theoretical capacity of 372 mAh g−1 (Zhou et al., 2014; Wang et al., 2017; Zhang et al., 2018). In the context of developing highly efficient energy storage electrode materials, extensive research has been conducted on various advanced anode materials, including metal oxides, metal sulfides, and their composites (Ren et al., 2019; Tao et al., 2021). Recently, layer-structured transition metal chalcogenides (TMCs), such as MoS2 (Jiang et al., 2015), Co9S8 (Liu et al., 2016), SnS (Ru et al., 2020), and FeS (Wang et al., 2018a), have become the limelight of research due to their high theoretical capacity, abundant natural resources, and environmental friendliness (Zhou et al., 2015b; Kim et al., 2020; Ke et al., 2021; Li et al., 2021).
Among those TMCs, molybdenum disulfide (MoS2), in which S–Mo–S layers are held together by weak van der Waals forces, has received intensive research attention in lubrication, drug delivery, and energy storage due to its extraordinary activity and perfect two-dimensional structure (Maksoud et al., 2021). Particularly, MoS2 is emerging as a potential candidate for lithium ion storage because of its excellent electrical, chemical, mechanical, and thermal properties (Hu et al., 2018). Excitingly, MoS2 can uptake four Li ions during the lithiation process, corresponding to 669 mAh g−1 specific capacity, which is significantly higher than that of a commercial graphite anode (372 mAh g−1) (Wang et al., 2016; Zhang et al., 2017). Unfortunately, the rapid capacity decay and poor rate performance of MoS2 electrodes have greatly affected the commercialization of MoS2 as an anode material (Wang et al., 2014; Yu et al., 2015). Numerous studies have been devoted to improve the intrinsic/ionic conductivity of MoS2. In addition, the large volume excursion of MoS2 brings about capacity fading during charge/discharge cycles (Chen et al., 2018b), and the higher surface energy and interlayer van der Waals forces of 2D materials leads to MoS2 stacking, which further restricts its practical application (Zhang et al., 2015). In order to solve these issues, incorporation of other conductive materials with MoS2 is considered as an effective way to achieve outstanding performance. For example, Fang et al. (2016) designed a vertically stacked ultrathin two-dimensional ordered mesoporous carbon/MoS2–layered heterogeneous material with high reversible discharge capacity (1140 mAh g−1) at a current density of 100 mA g−1. Wu et al. (2019) synthesized a low-crystalline MoS2 nanosheet encapsulated on nitrogen-doped carbon nanotubes (MoS2/N-CNT), and the results showed that the material could reach 1003 mAh g−1 after 800 cycles at 2 A g−1, demonstrating its high specific capacity and ultra-long cycle stability. In order to seek alternative anode materials, the combination of MoS2 with other highly conductive sulfides, such as Co3S4 (Lei et al., 2018), SnS (Ru et al., 2020), and MnS (Chen et al., 2021), to form heterogeneous structures is also an important way to improve its electrochemical performance. Related studies have shown that the composites containing the MoS2 component inherit the advantages of each component. In other words, MoS2-based composites synergistically improve the electrochemical kinetics and structural stability, thus offering superior electrochemical performance and commercialization prospects (Yang et al., 2020). Taking the aforementioned issues into account, the rational design of MoS2, carbon, and other metal sulfides could synergistically promote the electronic conductivity of nanohybrids and reduce the bulk effect during cycling.
Metal organic frameworks (MOFs), an emerging class of functional nanomaterials, possess outstanding tunable nanostructures, high porosity, and large specific surface area, making them ideal carbon matrixes for electrode materials (Aslam et al., 2018; Huang et al., 2021). Furthermore, such assembly of metal ions/clusters with organic linkers offers prospective application in catalysis, energy storage, and other fields (Fu et al., 2021; Tu et al., 2021). Encouragingly, serving as sacrificial templates, the reactive metal components embedded in MOFs could be transformed in situ into metal sulfides after vulcanization reaction, while the organic ligands are converted to amorphous carbon during the annealing treatment. The metal sulfides/carbon composite–derived MOFs would be favored for their excellent electrochemical properties due to their porous nanostructures. Recently, Guo et al. (2020) reported a strategy using zeolitic imidazolate framework 67 (ZIF-67) as a precursors to prepare the Co9S8/C anode material, delivering a specific capacity of 700 mAh g−1 at a current density of 500 mA g−1 after 150 cycles. Note that Co9S8 owns high theoretical capacity (545 mAh g−1), low cost, outstanding electrical conductivity, and superior thermal stability (Huang et al., 2020). However, to date, there have been few studies on coupling with layered MoS2 and MOF-derived other metal sulfides/carbon ingredients, which are capable in enhancing the electrochemical performances in LIBs.
The objectives of this study are 1) to synthesize MoS2/Co9S8/C nanohybrids via the coordination-driven self-assembly of molybdyl acetoacetonate [MoO2(acac)2] into ZIF-67, followed by an in situ vulcanization strategy and finally a thermal annealing process; 2) to characterize the physicochemical properties of MoS2/Co9S8/C using various techniques; and 3) to estimate the lithium storage performance as anode materials in LIBs. In this approach, thanks to the advantage of ZIF-67’s unique hydrocarbon networks, bimetal sulfides could be simultaneously converted to MoS2/Co9S8 embedded in the carbon matrix (MoS2/Co9S8/C), which effectively prevented aggregation of the ZIF-67–derived sulfides. This unique structure is able to act as a buffer to mitigate severe volume changes during charging/discharging. In addition, the synergistic effect of MoS2, Co9S8, and carbon not only imparts excellent electrical conductivity and high structural stability but also promotes electron/ion transfer and reaction kinetics. As expected, in comparison with individual bulk MoS2 and Co9S8/C, the MOF-derived MoS2/Co9S8/C exhibited excellent lithium storage performance as anode materials in LIBs, maintaining a capacity of 1270.2 mAh g−1 at a current density of 100 mA g−1 and a remarkable rate capability of 754.6 mAh g−1 at 1000 mA g−1. Their synergy endows MOF-derived multiple metal sulfides favorable for the effective storage of lithium ions.
Experimental section
Chemicals
All chemicals used in the experiments were obtained from commercial sources and were not further purified before use. Molybdyl acetoacetonate [MoO2(acac)2, 97%], 2-methylimidazole (98%), and cobalt nitrate hexahydrate [Co(NO3)2·6H2O, 99%] were purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd. Ammonium molybdate tetrahydrate ((NH4)6Mo7O24·4H2O, 99%) and thioacetamide (TAA, 99%) were supplied by Shanghai Macklin Bio-Chem Technology Co., Ltd.
Materials synthesis
Synthesis of MoO2(acac)2/ZIF-67: 5 g of MoO2(acac)2 and 3.49 g of Co(NO3)2·6H2O were first dissolved in 100 ml of absolute methanol. Afterward, 3.94 g of 2-methylimidazole was dissolved in another 100 ml of absolute methanol to generate a clear solution. Subsequently, both the solutions were mixed together in a flask and then kept at 60°C for 24 h. The product was separated by centrifugation and washed with methanol several times. Finally, the obtained MoO2(acac)2/ZIF-67 powder was dried and stored in a vacuum oven at 60°C for 12 h.
Synthesis of MoS2/Co9S8/C: In a typical procedure, 180 mg of MoO2(acac)2/ZIF-67 and 360 mg of TAA were ultrasonically dispersed in 30 ml of ultrapure water, and then the solution was transferred to a teflon-lined stainless steel autoclave and kept at 220°C for 18 h. After cooling down, the black product was collected by centrifugation, washed five times with ultrapure water and then once with ethanol, and finally dried under vacuum at 60°C for overnight. The obtained MoCoS powders were directly annealed under N2 atmosphere at 800 C for 2 h with a ramping rate of 5°C min−1. The individual MoS2 and Co9S8/C samples were fabricated according to the typical vulcanization reaction of (NH4)6Mo7O24·4H2O or ZIF-67 with TAA under identical condition.
Characterization
The crystal structures of the materials were analyzed by X-ray diffraction (XRD, Rigaku SmartLab SE) using Cu Kα radiation (λ = 1.5406 Å) with a sweep rate of 10°/min. The morphological characterizations of the samples were performed by a scanning electron microscope (SEM, Thermo Fisher Scientific, Quattro). The transmission electron microscopy (TEM) and energy-dispersive X-ray spectroscopy (EDS) images were performed on a Hitachi H-7650 microscope. The X-ray photoelectron spectroscopy (XPS) measurements were performed using a PerkinElmer IR-843 spectrometer. According to the Barrett Joyner Halenda (BJH) model, the pore size distributions were tested by using the TriStar II 3020 analyzer. A thermogravimetric analysis (TGA) was investigated under air atmosphere from indoor temperature to 700°C on a NETZSCH STA 2500 thermal gravimetric analyzer.
Electrochemical measurement
The working electrodes were prepared by mixing active materials (MoS2, Co9S8/C, and MoS2/Co9S8/C), conductive agent carbon black, binder polyvinylidene difluoride in the ratio of 8:1:1 with appropriate amount of N-methyl-2-pyrrolidinone. Then the mixture was coated on the copper foil and dried at 80°C under vacuum for 12 h. Lithium metal disks worked as the counter/reference electrode, and the solution of 1.0 M LiPF6 in a 1:1:1 volume ratio of ethylene carbonate (EC), diethyl carbonate (DEC), and ethyl methyl carbonate (EMC) was employed as the electrolyte for the cells. We assembled CR2032 type coin cells in a glove box filled with argon and used them for various electrochemical tests. The galvanostatic charge/discharge behaviors of the batteries were tested at different current densities from 0.01–3.0 V with the LAND battery test system. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were tested using an electrochemical workstation (Metrohm AG, PGSTAT302N). CV curves were collected at a scan rate of 0.1 mV s−1 from 0.01 to 3.0 V, and EIS data were performed at the frequency range from 100 kHz to 10 mHz with an amplitude of 5 mV. In the calculated specific capacities, the composited electrodes were based on the total mass of MoS2, Co9S8/C, or MoS2/Co9S8/C nanohybrids.
Results and discussion
Morphologies and microstructures of the nanocomposites
The synthetic route of petal-like MoS2/Co9S8/C nanohybrids is depicted in Figure 1. First, we prepared ZIF-67–wrapped MoO2(acac)2 as a precursor by introducing Mo source during ZIF-67 generation. Then a bimetallic sulfide was synthesized by hydrothermal vulcanization of the as-obtained MoO2(acac)2/ZIF-67 particles in the presence of TAA. Finally, in order to improve the electrical conductivity and crystallinity of the sulfide, the composites were heated under N2 atmosphere at 800oC for 2 h. During the process of thermal carbonization, amorphous CoS was converted to Co9S8, accompanying with the transformation in situ of organic moiety into a more conductive carbon layer. As a result, MoS2/Co9S8/C nanohybrids can be eventually achieved.
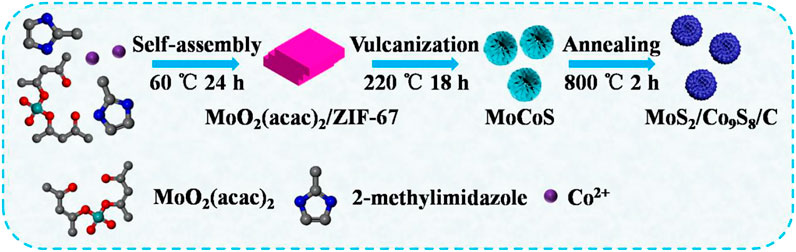
FIGURE 1. Schematic illustration of the formation process of the petal-like MoS2/Co9S8/C nanohybrids.
The morphologies of the aforementioned materials at different stages were determined by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). From the SEM image of the precursor MoO2(acac)2/ZIF-67 (Figure 2A), one can see that MoO2(acac)2/ZIF-67 consisted of irregular nanoplatelets, which are clearly different from the typical polyhedral structure of ZIF-67 (Supplementary Figure S1A). This phenomenon was mainly attributed that the presence of MoO2(acac)2 in the reaction interferes the pairing of 2-methylimidazole with cobalt ions, thus preventing the formation of the well-defined polyhedron structure. When undergoing hydrothermal vulcanization, in which the MoO2(acac)2/ZIF-67 nanosheets self-assembled into nanospheres, the intermediate MoCoS exhibits a uniform petal-like nanostructure with a diameter of about 300 nm (as shown in Figure 2B). From Figure 2C, we can observe that after thermal annealing treatment, MoS2/Co9S8/C nanohybrids maintained the original petal-like structure without any damage in morphology. Also, for comparison, bulk MoS2 and Co9S8/C were further synthesized, as shown in Supplementary Figure S1A, S2. Apparently, Co9S8/C derived from ZIF-67 retained the former polyhedral structure. However, the as-synthesized Co9S8/C underwent some agglomeration, while the individual MoS2 exhibited a classical blocky structure. Moreover, the SEM images of both MoS2 and Co9S8/C showed several microns in size. Compared with MoS2 and Co9S8/C, MoS2/Co9S8/C nanohybrids assembled by thin layers greatly increase the contact area between electrode and electrolyte and shorten the lithium ion transport path (Yang et al., 2020). In addition, such a special structure of nanospherical shape would play a positive role in alleviating the severe volume change during the charging/discharging process (Hu et al., 2018). These results suggested that synthesized MoS2/Co9S8/C nanohybrids would be capable of implementing a good Li+ storage performance when used as the anode of LIBs. As observed from Figure 2D, the TEM image further demonstrated that MoS2/Co9S8/C nanohybrids were assembled from a series of nanosheets into petal-like nanospheres. As shown in Figure 2E, energy-dispersive X-ray spectroscopy (EDS) mappings of MoS2/Co9S8/C revealed the homogeneous distribution of Mo, Co, S, C, and N throughout the whole nanohybrids, indicating that MoO2(acac)2 species was uniformly encapsulated in ZIF-67 when the cobalt ion was paired with 2-methylimidazole to form ZIF-67.

FIGURE 2. (A–C) SEM images of MoO2(acac)2/ZIF-67, MoCoS, and MoS2/Co9S8/C composites. (D) TEM image of MoS2/Co9S8/C. (E) TEM image of MoS2/Co9S8/C and the corresponding elemental mapping images of Mo, Co, S, C, and N.
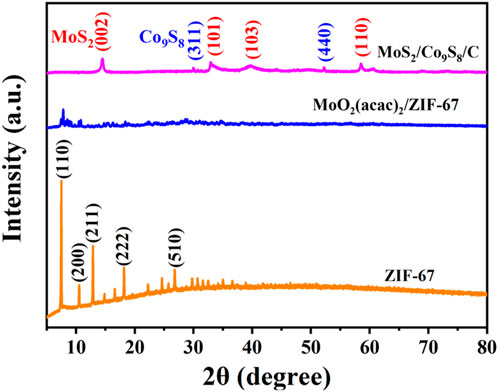
X-ray diffraction (XRD) was used to identify the crystallographic structure of these samples derived at different stages. As shown in Figure 3, the diffraction peaks of the synthesized ZIF-67 could be readily indexed to the reported patterns of the ZIF-67 crystal (Yang et al., 2018). The XRD pattern of MoO2(acac)2/ZIF-67 were similar to that of pristine ZIF-67, and the presence of MoO2(acac)2 dramatically interfered the formation of well-defined nucleation and growth of ZIF-67 crystals, resulting in the relative low intensity in the XRD pattern of MoO2(acac)2/ZIF-67. After vulcanization and calcination processes (Figure 3), the characteristic peaks located at 14.4°, 33.4°, 39.5°, 44.2°, 58.2°, and 60.3° were related to the (002), (101), (103), (104), (110), and 112) reflections of a typical hexagonally structural MoS2 (JCPDS No. 37–1492) (Geng et al., 2017). While the diffraction peaks at 29.9° and 52.0° correspond to (311) and (440) planes of face centered cubic Co9S8 (JCPDS No. 65–6801) (Geng et al., 2017). These results implied that the metal chalcogenide hybrids are the mixture of MoS2 and Co9S8 without other phases, revealing the high purity of the product.
The amount of carbon component in MoS2/Co9S8/C nanohybrids was determined by a thermogravimetric analysis (TGA). From Figure 4A, we can clearly see that there were three weight loss processes in the TGA analysis of MoS2/Co9S8/C. The first weight loss was started from room temperature to 180°C, which can be attributed to the evaporation or desorption of physically adsorbed water. The second weight loss of the sample occurred at 180–482°C, which is due to the oxidation of the metal sulfides by O2 in the air. Ultimately, as the temperature continues to rise, the decomposition of carbon in MoS2/Co9S8/C nanohybrids is accompanied by releasing of CO2 (Ren et al., 2019). The result of TGA curve showed that the carbon mass percentage in MoS2/Co9S8/C was about 6.1%. The pore distribution of MoS2/Co9S8/C nanohybrids was investigated by N2 adsorption and desorption analysis. The corresponding pore size distribution calculated by the Barrett Joyner Halenda (BJH) method displayed a narrow distribution centered at 3.3 nm in diameter.
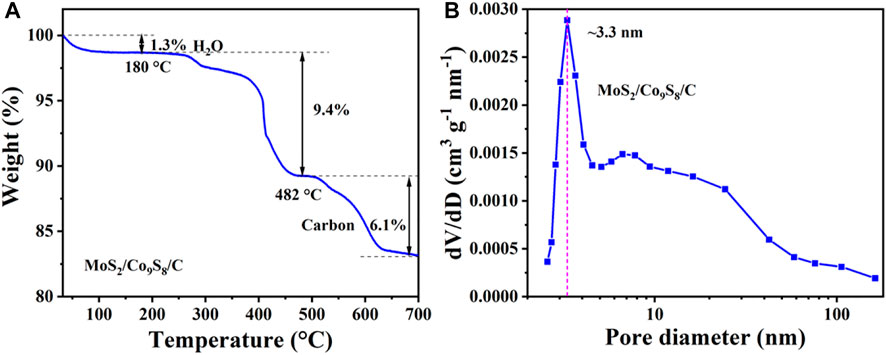
FIGURE 4. (A) Thermogravimetric data of MoS2/Co9S8/C powder sample in air atmosphere. (B) Pore size distributions of MoS2/Co9S8/C.
The chemical constituents and valance information of MoS2/Co9S8/C nanohybrids were further analyzed by X-ray photoelectron spectroscopy (XPS). As shown in Figure 5A, the survey spectrum confirmed that Mo 3d, Co 2p, S 2p, C 1s, N 1s, and O 1s peaks exist in the sample of MoS2/Co9S8/C nanohybrids. From the high-resolution XPS spectrum of Mo 3d (Figure 5B), the binding energies located at 229.8, 232.9, and 226.9 eV originated from Mo 3d5/2, Mo 3d3/2, and S 2s, respectively (Wang et al., 2018b), implying the presence of Mo4+ species and the formation of MoS2 in the composites. Meanwhile, a broad peak at 235.7 eV of Mo6+ was indicative of the existence of oxidized phases (i.e., Mo6+) (Hou et al., 2018). As observed from Figure 5C, two pairs of peaks were the features of Co2+ (Co 2p1/2 at 799.5 eV and Co 2p3/2 at 782.8 eV) and Co3+ (Co 2p1/2 at 788 eV and Co 2p3/2 at 779.6 eV) (Ren, et al., 2019). Moreover, two peaks located at 804.4 and 784.8 eV were the shakeup satellites (Hou et al., 2018). Combined with the core level of the S 2p spectrum (Figure 5D), the characteristic peaks of S 2p3/2 at 162.6 eV and S 2p1/2 at 163.8 eV were attributed to S2− of both Co-S and Mo-S bonds, and the shakeup satellite peak in 169.6 eV might be ascribed to the partial surface oxidization (Geng et al., 2017). The region of C 1s spectrum could be grouped into three components originating from centered at C-C (284.7 eV), C-N (285.7 eV), and C=O bonds (287.4 eV), respectively, as displayed in Figure 5E, while the N 1s XPS spectrum of MoS2/Co9S8/C could be fitted into four peaks, which are associated with the Mo 3p (395.6 eV), pyridinic N (398.5 eV), pyrrolic N (400 eV), and graphitic N (402.1 eV) (Zhao et al., 2018). The result of XPS spectra survey demonstrated the formation of heterostructures between Co9S8 and MoS2.
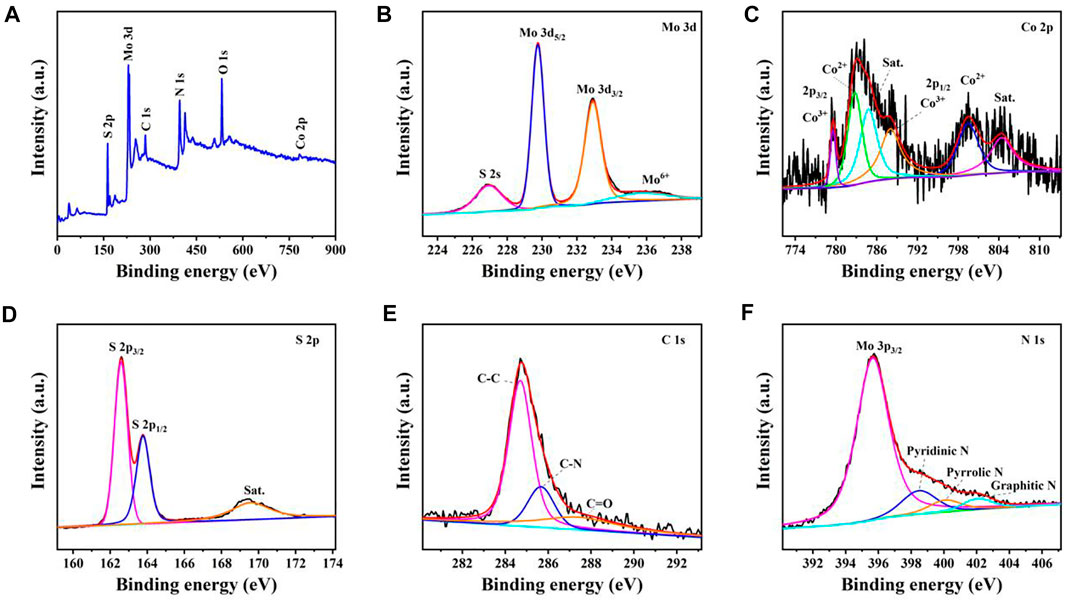
FIGURE 5. XPS spectra of MoS2/Co9S8/C nanohybrids: (A) survey spectrum, (B) Mo 3d, (C) Co 2p, (D) S 2p, (E) C 1s, and (F) N 1s.
Electrochemical performances
The anodic electrochemical performance of MoS2/Co9S8/C nanohybrids in LIBs was systematically evaluated by assembling a series of half-cells. Figures 6A–C showed the cyclic voltammetry (CV) curves of MoS2/Co9S8/C, MoS2, and Co9S8/C electrodes for the first three cycles at a scanning rate of 0.1 mV s−1 and a voltage range of 0.01–3.0 V. As shown in Figure 6A, in the first cathode scan, we can observe that MoS2/Co9S8/C has four reduction peaks at 0.47, 1.08, 1.18, and 1.33 V, respectively, revealing a multistep electrochemical reaction. The two peaks located at 1.18 and 1.33 V can be ascribed to the reduction of Co9S8 to metallic Co (Zhao et al., 2018; Ren et al., 2019). Also, the strong peak at 1.08 V is the result of the intercalation of lithium ions into the MoS2 layer, while the pronounced reduction peak at 0.47 V in the first cycle was identified to the reduction of LixMoS2 and the formation of a solid electrolyte interphase (SEI) layer (Ren et al., 2019). During the reverse anodic sweep of the MoS2/Co9S8/C, two intensive peaks at 2.05 and 2.29 V could be attributed to the oxidation of metallic Co and Mo, respectively (Geng et al., 2017). In the following CV cycle, the lithium ion storage mechanism of MoS2 turns into a reversible redox reaction of sulfur (Yang et al., 2020). The overlapped of the second and third cycles also demonstrated that Li+ ions could be reversibly inserted into and extracted from MoS2/Co9S8/C nanohybrids. The CV curves of individual MoS2 and Co9S8/C electrodes were also investigated for comparison. As shown in Figure 6B, two reduction peaks at 0.5 and 1.1 V can be attributed to the embedding of lithium ions and the reduction of LixMoS2, respectively, while the three reduction peaks located at 0.71, 1.09, and 1.22 V in Figure 6C were the result of the reduction of Co9S8 to metallic Co. The oxidation peaks of MoS2 at 2.31 V and Co9S8 at 2.09 V were assigned to the oxidation peaks of MoS2 and Co9S8. These results indicated that MoS2 and Co9S8 exhibited synergistic effects in MoS2/Co9S8/C nanohybrids, which would contribute to good reversibility and cyclic stability together with boosting LIBs storage.
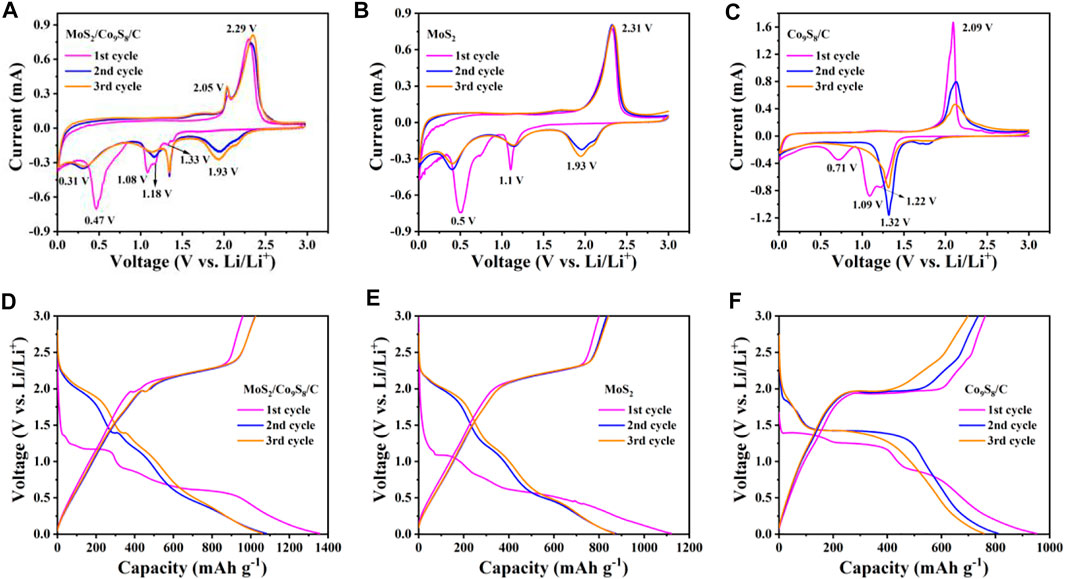
FIGURE 6. (A–C) CV curves of MoS2/Co9S8/C, MoS2, and Co9S8/C electrodes at a scanning rate of 0.1 mV s−1 during the initial three cycles. (D–F) Galvanostatic discharge/charge profiles of MoS2/Co9S8/C, MoS2, and Co9S8/C electrodes at 100 mA g−1.
These corresponding plateaus were further observed in the galvanostatic charge/discharge profiles of the MoS2/Co9S8/C nanohybrids, MoS2 and Co9S8/C at a current density of 100 mA g−1 within the voltage window of 0.01–3.0 V (vs. Li/Li+) (Figures 6D–F). For the MoS2/Co9S8/C electrode, the initial discharge/charge capacities achieved 1356.5 and 958.9 mA h g−1, respectively, higher than those of MoS2 (1121.2 and 800 mAh g−1) and Co9S8/C (951.1 and 762.2 mAh g−1). The corresponding initial Coulombic efficiencies (CEs) of MoS2/Co9S8/C, MoS2 and Co9S8/C reached up to ∼70.69%, ∼71.35 and ∼80.14%, respectively. After the initial adjustment cycle, petal-like nanospheres–structured MoS2/Co9S8/C delivered the highest discharge/charge capacities and stability compared to those of MoS2 and Co9S8/C in the 2nd and 3rd cycles. More importantly, by comparing the charge/discharge curves and CV curves of MoS2/Co9S8/C, it was found that MoS2/Co9S8/C brought two charging and multiple discharge plateaus, which were consistent with the result in the CV profile of MoS2/Co9S8/C.
The long-term cycling performance and coulombic efficiencies (CEs) of the MoS2/Co9S8/C, MoS2 and Co9S8/C were subsequently explored at a current density of 100 mA g−1 between 0.01 and 3.0 V. From Figure 7A, one can see that the discharge capacities of MoS2/Co9S8/C, MoS2 and Co9S8/C after 50 charge/discharge cycles were 1270.2, 668.5, and 455.3 mAh g−1, presenting 93.64%, 59.62% and 47.87% retention of 50th discharge capacity. Furthermore, Figure 7B further displayed the compared cycling performance of MoS2/Co9S8/C, MoS2 and Co9S8/C electrodes at a higher current density of 200 mA g−1. It was found that the initial discharge capacities of MoS2/Co9S8/C, MoS2 and Co9S8/C were 988.3, 776.3, and 770.6 mAh g−1, respectively. After 50 cycles, the discharge capacities of MoS2 and Co9S8/C faded to 438.6 and 236.7 mAh g−1, respectively. Surprisingly, MoS2/Co9S8/C possessed a remarkable discharge capacity of 987.7 mAh g−1 after 50 cycles, which was almost 2.25 times of that of the bare MoS2 and 4.17 times of that of the Co9S8/C. Notably, the CE of MoS2/Co9S8/C approached nearly 100% throughout the overall cycle. These results fully proved that the MoS2/Co9S8/C nanohybrids’ capacity and cyclability were significantly higher than the MoS2 and Co9S8/C. Furthermore, the high specific capacity (1270.2 mAh g−1) at 100 mA g−1 was achieved for MoS2/Co9S8/C nanohybrids by comparison with most reported MoS2-based anode materials. Surprisingly, the ternary MoS2/Co9S8/C nanohybrids present better lithium storage performances than previous bare MoS2 and MoS2/carbon composites, indicating that integrating MoS2/Co9S8/C nanohybrids with hierarchical structure could offer a scalable approach to develop promising anode materials for LIBs.
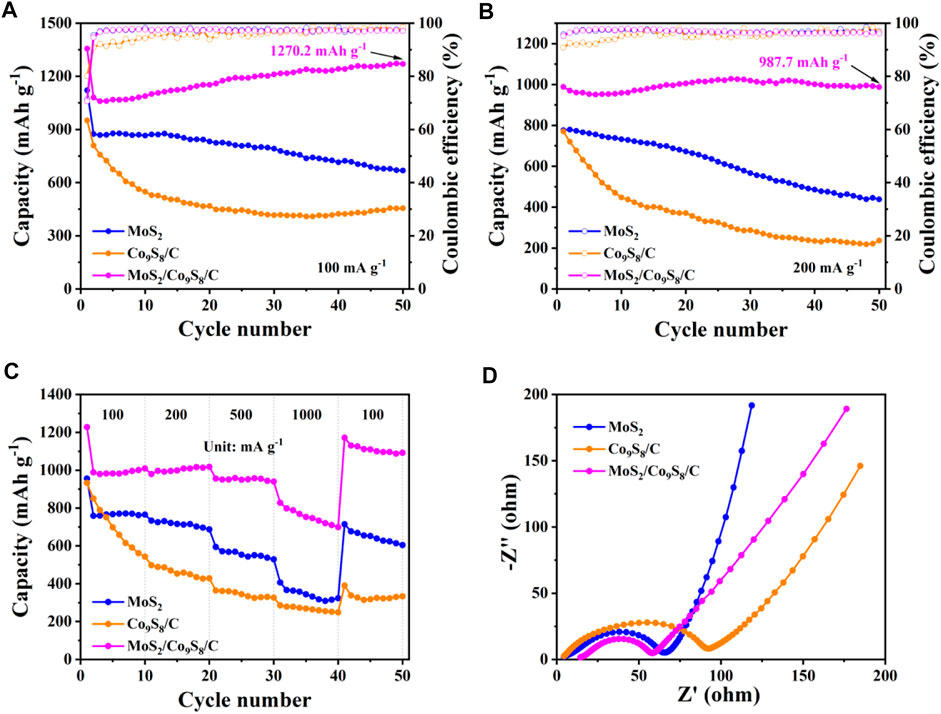
FIGURE 7. Cycling performance together with Coulombic efficiency of MoS2/Co9S8/C, MoS2, and Co9S8/C at a current density of (A) 100 mA g−1 and (B) 200 mA g−1, respectively. (C) Rate capability performance of MoS2/Co9S8/C, MoS2, and Co9S8/C. (D) Nyquist plots of MoS2/Co9S8/C, MoS2, and Co9S8/C in the frequency range between 100 kHz and 10 mHz.
Figure 7C showed the rate performance comparison of these three electrodes at different current densities ranging from 100 to 1000 mA g−1. Upon comparison of the MoS2 and Co9S8/C electrodes, the MoS2/Co9S8/C nanohybrids delivered superior capacities and improved rate performance, with average discharge capacities of 1013.8, 1002.9, 951.4, and 754.6 mAh g−1 at current densities of 100, 200, 500, and 1000 mA g−1, respectively. Note that the discharge capacity of MoS2/Co9S8/C could recover to 1112.2 mAh g−1 as the current density returned to 100 mA g−1, which was higher than the capacity after 10 cycles at 100 mA g−1 initially. Meanwhile, the discharge capacities of MoS2/Co9S8/C were much higher than those of MoS2 and Co9S8/C at any current densities, and the bare MoS2 and Co9S8/C electrodes only maintained discharge capacities of 323.6 and 249 mAh g−1 at high rate of 1000 mA g−1. Such enhanced rate performance of high power output was mainly attributed to the integrating features of MoS2/Co9S8/C nanohybrids: 1) the unique petal-like structure promoted the fast electronic transportation in the bulk electrode, 2) the carbon layer preserved the reactive surface and high conductivity, 3) the MoS2 and Co9S8 active materials contributed to the high capacity.
To understand the enhanced rate capability, resistance of three electrodes was further tested by electrochemical impedance spectroscopy (EIS). All the assembled batteries were cycled once at a current density of 100 mA g−1 prior to EIS testing. The Nyquist plots of the cells assembled with the three materials were shown in Figure 7D, and each curve was composed of a semicircle and a straight line, where the size of the semicircle reflected the magnitude of the charge transfer impedance (Rct) between the electrolyte and the electrode (Ke et al., 2021). Clearly, the Rct value of MoS2/Co9S8/C is the smallest relative to that of Co9S8/C and MoS2, indicating that the reaction speed of MoS2/Co9S8/C is faster in the process of lithium intercalation/delamination (Zhao et al., 2020). This also confirmed that in the MoS2/Co9S8/C nanohybrids, there is a synergistic effect between MoS2 and Co9S8/C, resulting in enhanced electrochemical cycling and rate properties (Han et al., 2020).
Conclusion
In conclusion, we have rationally designed and successfully prepared petal-like MoS2/Co9S8/C nanohybrids by a simple self-assembly approach. This unique structure can not only buffer the huge volume change of the electrode during the charging/discharging process of LIBs but also greatly shorten the electron and ion transfer pathway and promote the kinetics of the reaction. In addition, the synergistic effect between MoS2 and Co9S8 embedded in the carbon matrix significantly imparts to MoS2/Co9S8/C outstanding lithium storage capacity, conductivity, and stability. Excitingly, when serving as the anode of LIBs, MoS2/Co9S8/C delivers a high reversible specific capacity of 1270.2 mAh g−1 at 100 mA g−1 after 50 cycles, which is a significant improvement compared to those of MoS2 electrode (668.5 mAh g−1) and Co9S8/C electrode (455 mAh g−1). Moreover, the nanohybrids display superior average discharge capacities of 1013.8, 1002.9, 951.4, and 754.6 mAh g−1 at current densities of 100, 200, 500, and 1000 mA g−1, respectively. These integrating features demonstrate that the synthesized petal-like MoS2/Co9S8/C nanohybrids would be capable in boosting the lithium storage performance. In the future research directions, we will devote to investigating the intelligent synthetic process and exploring the in situ galvanostatic discharge/charge process using advanced spectroscopic techniques (Table 1).
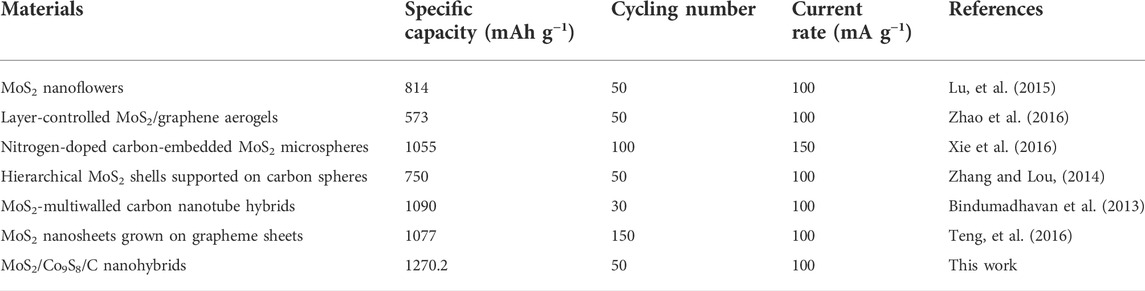
TABLE 1. Lithium storage performances of the present MoS2/Co9S8/C with the reported MoS2-based anode materials.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
BW performed the most experiments and wrote the manuscript. RM and XL performed partial experiments. YZ and SG performed data analysis. YY plotted the figures. MS designed the experiments. SW edited the manuscript. TW proposed the project and revised the manuscript.
Funding
This work was financially supported by the National Key Research and Development Program of China (2018YFC1900105), Science Challenge Project (TZ2016004), and Beijing Outstanding Young Scientist Program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenrg.2022.918494/full#supplementary-material
References
Aslam, M. K., Shah, S. S. A., Li, S., and Chen, C. (2018). Kinetically controlled synthesis of MOF nanostructures: Single-holed hollow core-shell ZnCoS@Co9S8/NC for ultra-high performance lithium-ion batteries. J. Mat. Chem. A 6, 14083–14090. doi:10.1039/c8ta04676j
Bindumadhavan, K., Srivastava, S. K., and Mahanty, S. (2013). MoS2−MWCNT hybrids as a superior anode in lithium-ion batteries. Chem. Commun. 49 (18), 1823–1825. doi:10.1039/c3cc38598a
Chen, B., Lu, H., Zhou, J., Ye, C., Shi, C., Zhao, N., et al. (2018a). Porous MoS2/carbon spheres anchored on 3D interconnected multiwall carbon nanotube networks forUltrafast Na storage. Adv. Energy Mat. 8, 1702909. doi:10.1002/aenm.201702909
Chen, C., Xie, X., Anasori, B., Sarycheva, A., Makaryan, T., Zhao, M., et al. (2018b). MoS2-on-MXene heterostructures as highly reversible anode materials for lithium-ion batteries. Angew. Chem. Int. Ed. 57, 1846–1850. doi:10.1002/anie.201710616
Chen, F., Shi, D., Yang, M., Jiang, H., Shao, Y., Wang, S., et al. (2021). Novel designed MnS-MoS2 heterostructure for fast and stable Li/Na storage:insights into the advanced mechanism attributed to phase engineering. Adv. Funct. Mat. 31, 2007132. doi:10.1002/adfm.202007132
Fang, Y., Lv, Y., Gong, F., Elzatahry, A. A., Zheng, G., and Zhao, D. (2016). Synthesis of 2D-mesoporous-carbon/MoS2 heterostructures with well-defined interfaces for high-performance lithium-ion batteries. Adv. Mat. 28, 9385–9390. doi:10.1002/adma.201602210
Fu, L., Kang, C., Xiong, W., Tian, P., Cao, S., Wan, S., et al. (2021). WS2 nanosheets@ZIF-67-derived N-doped carbon composite as sodium ion battery anode with superior rate capability. J. Colloid Interface Sci. 595, 59–68. doi:10.1016/j.jcis.2021.03.127
Geng, H., Yang, J., Dai, Z., Zhang, Y., Zheng, Y., Yu, H., et al. (2017). Co9S8/MoS2 yolk-shell spheres for advanced Li/Na storage. Small 13, 201603490. doi:10.1002/smll.201603490
Guo, S., Zhang, P., Feng, Y., Wang, Z., Li, X., and Yao, J. (2020). Rational design of interlaced Co9S8/carbon composites from ZIF-67/cellulose nanofibers for enhanced lithium storage. J. Alloys Compd. 818, 152911. doi:10.1016/j.jallcom.2019.152911
Han, Y., Li, W., Zhou, K., Wu, X., Wu, H., Wu, X., et al. (2020). Bimetallic sulfide Co9S8/N-C@MoS2 dodecahedral heterogeneous nanocages for boosted Li/K storage. Chemnanomat 6, 132–138. doi:10.1002/cnma.201900601
Hou, X., Zhang, Y., Dong, Q., Hong, Y., Liu, Y., Wang, W., et al. (2018). Metal organic framework derived core-shell structured Co9S8@N-C@MoS2 nanocubes for supercapacitor. ACS Appl. Energy Mat. 1, 3513–3520. doi:10.1021/acsaem.8b00773
Hu, X., Li, Y., Zeng, G., Jia, J., Zhan, H., and Wen, Z. (2018). Three-dimensional network architecture with hybrid nanocarbon composites supporting few-layer MoS2 for lithium and sodium storage. Acs Nano 12, 1592–1602. doi:10.1021/acsnano.7b08161
Huang, Y., Hu, X., Li, J., Zhang, J., Cai, D., Sa, B., et al. (2020). Rational construction of heterostructured core-shell Bi2S3@Co9S8 complex hollow particles toward high-performance Li- and Na-ion storage. Energy Storage Mat. 29, 121–130. doi:10.1016/j.ensm.2020.04.004
Huang, S., Jin, Z., Ding, Y., Ning, P., Chen, Q., Fu, J., et al. (2021). Encapsulating Fe2O3 nanotubes into carbon-coated Co9S8 nanocages derived from a MOFs-directed strategy for efficient oxygen evolution reactions and Li-ions storage. Small 17, 2103178. doi:10.1002/smll.202103178
Jiang, H., Ren, D., Wang, H., Hu, Y., Guo, S., Yuan, H., et al. (2015). 2D monolayer MoS2-carbon interoverlapped superstructure: Engineering ideal atomic interface for lithium ion storage. Adv. Mat. 27, 3687–3695. doi:10.1002/adma.201501059
Ke, G., Chen, H., He, J., Wu, X., Gao, Y., Li, Y., et al. (2021). Ultrathin MoS2 anchored on 3D carbon skeleton containing SnS quantum dots as a high-performance anode for advanced lithium ion batteries. Chem. Eng. J. 403, 126251. doi:10.1016/j.cej.2020.126251
Kim, M., Anjum, M. A. R., Choi, M., Jeong, H. Y., Choi, S. H., Park, N., et al. (2020). Covalent 0D-2D heterostructuring of Co9S8-MoS2 for enhanced hydrogen evolution in all pH electrolytes. Adv. Funct. Mat. 30, 2002536. doi:10.1002/adfm.202002536
Lei, X., Yu, K., Qi, R., and Zhu, Z. (2018). Fabrication and theoretical investigation of MoS2-Co3S4 hybrid hollow structure as electrode material for lithium-ion batteries and supercapacitors. Chem. Eng. J. 347, 607–617. doi:10.1016/j.cej.2018.04.154
Li, H., Qian, X., Xu, C., Huang, S., Zhu, C., Jiang, X., et al. (2017). Hierarchical porous Co9S8/nitrogen-doped carbon@MoS2 polyhedrons as pH universal electrocatalysts for highly efficient hydrogen evolution reaction. ACS Appl. Mat. Interfaces 9, 28394–28405. doi:10.1021/acsami.7b06384
Li, J., Han, L., Zhang, X., Sun, H., Liu, X., Lu, T., et al. (2021). Multi-role TiO2 layer coated carbon@few-layered MoS2 nanotubes for durable lithium storage. Chem. Eng. J. 406, 126873. doi:10.1016/j.cej.2020.126873
Liu, H., Su, D., Zhou, R., Sun, B., Wang, G., and Qiao, S. Z. (2012). Highly ordered mesoporous MoS2 with expanded spacing of the (002) crystal plane for ultrafast lithium ion storage. Adv. Energy Mat. 2, 970–975. doi:10.1002/aenm.201200087
Liu, J., Wu, C., Xiao, D., Kopold, P., Gu, L., van Aken, P. A., et al. (2016). MOF-derived hollow Co9S8 nanoparticles embedded in graphitic carbon nanocages with superior Li-ion storage. Small 12, 2354–2364. doi:10.1002/smll.201503821
Lu, Y., Yao, X., Yin, J., Peng, G., Cui, P., and Xu, X. (2015). MoS2 nanoflowers consisting of nanosheets with a controllable interlayer distance as high-performance lithium ion battery anodes. RSC Adv. 5 (11), 7938–7943. doi:10.1039/c4ra14026E
Maksoud, M. I. A. A., Bedir, A. G., Bekhit, M., Abouelela, M. M., Fahim, R. A., Awed, A. S., et al. (2021). MoS2-based nanocomposites: Synthesis, structure, and applications in water remediation and energy storage: A review. Environ. Chem. Lett. 19, 3645–3681. doi:10.1007/s10311-021-01268-x
Miao, Y. J., Zheng, Y. F., Tao, F., Chen, Z. J., Xiong, Y., Ren, F. Z., et al. (2022). Synthesis and application of single-atom catalysts in sulfur cathode for high-performance lithium–sulfur batteries. Chin. Chem. Lett. doi:10.1016/j.cclet.2022.01.014
Ren, H., Gu, C., Zhao, J., Joo, S. W., and Huang, J. (2019). Co9S8@MoS2 core-shell nanostructure anchored on reduced graphene oxide with improved electrochemical performance for lithium-ion batteries. Appl. Surf. Sci. 473, 918–927. doi:10.1016/j.apsusc.2018.12.257
Ru, J., He, T., Chen, B., Feng, Y., Zu, L., Wang, Z., et al. (2020). Covalent assembly of MoS2 nanosheets with SnS nanodots as linkages for lithium/sodium-ion batteries. Angew. Chem. Int. Ed. 59, 14621–14627. doi:10.1002/anie.202005840
Tao, F., Liu, Y., Ren, X. Y., Jiang, A. J., Wei, H. J., Zhai, X. L., et al. (2021). Carbon nanotube-based nanomaterials for high-performance sodium-ion batteries: Recent advances and perspectives. J. Alloys Compd. 873, 159742. doi:10.1016/j.jallcom.2021.159742
Teng, Y., Zhao, H., Zhang, Z., Li, Z., Xia, Q., Zhang, Y., et al. (2016). MoS2 nanosheets vertically grown on graphene sheets for lithium-ion battery anodes. ACS Nano 10 (9), 8526–8535. doi:10.1021/acsnano.6b03683
Tu, C., Peng, A., Zhang, Z., Qi, X., Zhang, D., Wang, M., et al. (2021). Surface-seeding secondary growth for CoO@Co9S8 P-N heterojunction hollow nanocube encapsulated into graphene as superior anode toward lithium ion storage. Chem. Eng. J. 425, 130648. doi:10.1016/j.cej.2021.130648
Wang, J., Liu, J., Chao, D., Yan, J., Lin, J., and Shen, Z. X. (2014). Self-assembly of honeycomb-like MoS2 nanoarchitectures anchored into graphene foam for enhanced lithium-ion storage. Adv. Mat. 26, 7162–7169. doi:10.1002/adma.201402728
Wang, Y., Yu, L., and Lou, X. W. (2016). Synthesis of highly uniform molybdenum-glycerate spheres and their conversion into hierarchical MoS2 hollow nanospheres for lithium-ion batteries. Angew. Chem. Int. Ed. 55, 7423–7426. doi:10.1002/anie.201601673
Wang, S., Guan, B. Y., Yu, L., and Lou, X. W. (2017). Rational design of three-layered TiO2@Carbon@MoS2 hierarchical nanotubes for enhanced lithium storage. Adv. Mat. 29, 1702724. doi:10.1002/adma.201702724
Wang, J., He, H., Wu, Z., Liang, J., Han, L., Xin, H. L., et al. (2018a). Controllable construction of flower-like FeS/Fe2O3 composite for lithium storage. J. Power Sources 392, 193–199. doi:10.1016/j.jpowsour.2018.04.107
Wang, Y., Kang, W., Cao, D., Zhang, M., Kang, Z., Xiao, Z., et al. (2018b). A yolk-shelled Co9S8/MoS2-CN nanocomposite derived from a metal-organic framework as a high performance anode for sodium ion batteries. J. Mat. Chem. A 6, 4776–4782. doi:10.1039/c8ta00493e
Wang, F., Liu, Y., Wei, H.-J., Li, T.-F., Xiong, X.-H., Wei, S. Z., et al. (2021). Recent advances and perspective in metal coordination materialsbased electrode materials for potassium-ion batteries. Rare Met. 40 (2), 448–470. doi:10.1007/s12598-020-01649-1
Wu, T., Jing, M., Liu, Y., and Ji, X. (2019). Binding low crystalline MoS2 nanoflakes on nitrogen-doped carbon nanotube: Towards high-rate lithium and sodium storage. J. Mat. Chem. A 7, 6439–6449. doi:10.1039/c9ta00123a
Xie, D., Xia, X., Wang, Y., Wang, D., Zhong, Y., Tang, W., et al. (2016). Nitrogen-doped carbon embedded MoS2 microspheres as advanced anodes for lithium- and sodium-ion batteries. Chem. Eur. J. 22 (33), 11617–11623. doi:10.1002/chem.201601478
Yang, Q., Lu, R., Ren, S., Chen, C., Chen, Z., and Yang, X. (2018). Three dimensional reduced graphene oxide/ZIF-67 aerogel: Effective removal cationic and anionic dyes from water. Chem. Eng. J. 348, 202–211. doi:10.1016/j.cej.2018.04.176
Yang, K., Mei, T., Chen, Z., Xiong, M., Wang, X., Wang, J., et al. (2020). Chinese hydrangea lantern-like Co9S8@MoS2 composites with enhanced lithium-ion battery properties. Nanoscale 12, 3435–3442. doi:10.1039/c9nr09260a
Yu, X.-Y., Hu, H., Wang, Y., Chen, H., and Lou, X. W. (2015). Ultrathin MoS2 nanosheets supported on N-doped carbon nanoboxes with enhanced lithium storage and electrocatalytic properties. Angew. Chem. Int. Ed. 54, 7395–7398. doi:10.1002/anie.201502117
Zhang, L., and Lou, X. W. (2014). Hierarchical MoS2 shells supported on carbon spheres for highly reversible lithium storage. Chem. Eur. J. 20 (18), 5219–5223. doi:10.1002/chem.201400128
Zhang, S., Chowdari, B. V. R., Wen, Z., Jin, J., and Yang, J. (2015). Constructing highly oriented configuration by few-layer MoS2: Toward high-performance lithium-ion batteries and hydrogen evolution reactions. Acs Nano 9, 12464–12472. doi:10.1021/acsnano.5b05891
Zhang, X., Zhao, R., Wu, Q., Li, W., Shen, C., Ni, L., et al. (2017). Petal-like MoS2 nanosheets space-confined in hollow mesoporous carbon spheres for enhanced lithium storage performance. Acs Nano 11, 8429–8436. doi:10.1021/acsnano.7b04078
Zhang, Z., Zhao, H., Teng, Y., Chang, X., Xia, Q., Li, Z., et al. (2018). Carbon-sheathed MoS2 nanothorns epitaxially grown on CNTs: Electrochemical application for highly stable and ultrafast lithium storage. Adv. Energy Mat. 8, 1700174. doi:10.1002/aenm.201700174
Zhao, B., Wang, Z., Gao, Y., Chen, L., Lu, M., Jiao, Z., et al. (2016). Hydrothermal synthesis of layer-controlled MoS2/graphene composite aerogels for lithium-ion battery anode materials. Appl. Surf. Sci. 390, 209–215. doi:10.1016/j.apsusc.2016.08.078
Zhao, Y., Bi, M., Qian, F., Zeng, P., Chen, M., Wang, R., et al. (2018). Heterostructure CoS/NC@MoS2 hollow spheres for high-performance hydrogen evolution reactions and lithium-ION batteries. Chemelectrochem 5, 3953–3960. doi:10.1002/celc.201801166
Zhao, R., Han, Y., Li, W., Li, J., Chen, M., and Chen, L. (2020). Construction of nanocage-structured heterogeneous binary metal sulfides via step-by-step confined growth for boosted lithium storage properties. Chem. Commun. 56, 6798–6801. doi:10.1039/d0cc00962h
Zhou, F., Xin, S., Liang, H.-W., Song, L.-T., and Yu, S.-H. (2014). Carbon nanofibers decorated with molybdenum disulfide nanosheets: Synergistic lithium storage and enhanced electrochemical performance. Angew. Chem. Int. Ed. 53, 11552–11556. doi:10.1002/anie.201407103
Zhou, J., Qin, J., Zhang, X., Shi, C., Liu, E., Li, J., et al. (2015a). 2D space-confined synthesis of few-layer MoS2 anchored on carbon nanosheet for lithium-ion battery anode. Acs Nano 9, 3837–3848. doi:10.1021/nn506850e
Keywords: lithium-ion batteries, MoS2/Co9S8/C nanohybrids, electrochemical performances, synergistic effect, self-assembly
Citation: Wu B, Ma R, Liu X, Zheng Y, Guo S, Yi Y, Sun M, Wang S and Wen T (2022) Self-assembly synthesis of petal-like MoS2/Co9S8/carbon nanohybrids for enhanced lithium storage performance. Front. Energy Res. 10:918494. doi: 10.3389/fenrg.2022.918494
Received: 12 April 2022; Accepted: 18 July 2022;
Published: 19 August 2022.
Edited by:
Vivekanand Shukla, Chalmers University of Technology, SwedenReviewed by:
Ahmed I Osman, Queen’s University Belfast, United KingdomYong Liu, Henan University of Science and Technology, China
Copyright © 2022 Wu, Ma, Liu, Zheng, Guo, Yi, Sun, Wang and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Wen, twen@ncepu.edu.cn
 Bo Wu
Bo Wu Ran Ma1
Ran Ma1  Tao Wen
Tao Wen