- 1College of Medical Informatics, Chongqing Medical University, Chongqing, China
- 2Department of General Practice, The Second Affiliated Hospital, Chongqing Medical University, Chongqing, China
- 3Department of Infectious Diseases, Key Laboratory of Molecular Biology for Infectious Diseases (Ministry of Education), Institute for Viral Hepatitis, the Second Affiliated Hospital, Chongqing Medical University, Chongqing, China
- 4Department of Cardiology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 5Graduate School, Guangxi University of Chinese Medicine, Nanning, China
- 6College of Osteopathic Medicine, California Health Sciences University, Clovis, CA, United States
- 7College of Osteopathic Medicine, Kansas City University, Kansas, MO, United States
- 8Department of Medical Quality Control, The First People’s Hospital of Zigong City, Zigong, China
Background: Liver resection (LR) and local tumor destruction (LTD) are effective treatments, but not commonly recommended for patients with intermediate/advanced hepatocellular carcinoma (HCC). This study aimed to explore whether LR/LTD could improve overall survival (OS) of these patients, and to identify the patients who will most likely benefit from LR/LTD.
Methods: Data of patients with intermediate/advanced HCC between 2001 and 2018 were extracted from Surveillance, Epidemiology, and End Results database. OS was compared between HCC patients who received LR/LTD and those who did not. A nomogram was constructed for predicting OS, and it was then validated.
Results: A total of 535 eligible patients were included, among which 128 received LR/LTD while 407 did not. Significantly higher OS in patients who received LR/LTD was observed (P<0.001). Based on independent prognostic factors obtained from univariate and multivariate analyses, a nomogram was constructed. The C-indices of nomogram were higher than those of the TNM staging system (training cohort: 0.74 vs. 0.59; validation cohort: 0.78 vs. 0.61). Similarly, areas under receiver operating characteristic curves and calibration curves indicated good accuracy of the nomogram. Decision curve analysis curves revealed good clinical practicability of the nomogram. Furthermore, low-risk patients (nomogram score: 0-221.9) had higher OS compared with high-risk patients (nomogram score: higher than 221.9) (P<0.001).
Conclusion: LR/LTD significantly improves OS in patients with intermediate/advanced HCC. The nomogram developed in the present study shows high predicating value for OS in patients with intermediate/advanced HCC, which might be useful in selecting patients who are most suitable for LR/LTD.
Introduction
With approximately 906,000 new cases and 830,000 deaths globally, primary liver cancer is the sixth most commonly diagnosed cancer and the third leading cause of cancer death in 2020 (1). Hepatocellular carcinoma (HCC) is the dominant type of liver cancer, leading to around 75% of all liver cancer cases (2). Due to the insidious onset, many patients were diagnosed with HCC at the intermediate/advanced stage. Considerable progresses have been made in the HCC treatment over the past years, and the current guidelines of the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of Liver (EASL) recommend the transarterial chemoembolization (TACE), transarterial radioembolization (TARE), liver transplantation and systemic treatment [tyrosine kinase inhibitors (TKIs), PD-1 inhibitors] for intermediate/advanced HCC (3–5). However, the outcomes of patients with intermediate/advanced HCC were still unsatisfactory. Thus, better treatment strategies are greatly needed for outcome improvement.
Liver resection (LR) and local tumor destruction [LTD, including heat-radio-frequency ablation (RFA), etc.] were usually recommended as first-line treatment modality only for early-stage HCC, but not for intermediate/advanced HCC (3–5). As the effective improvement of survival in patients with early HCC after LR/LTD (surgery) have been reported (6, 7), it would also be of clinical significance to explore whether LR/LTD could improve the prognosis of patients with intermediate/advanced HCC, and to identify which intermediate/advanced HCC patients are the best candidates for LR/LTD from the prognostic standpoint.
In this study, eligible patients with intermediate/advanced HCC [primary tumor (T) 3/4] were included from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute (8). The overall survival (OS) of the patients who received LR/LTD was compared with that of the patients who did not. A nomogram was constructed and validated to predict the OS of the patients to help predict which patients would be most suitable for LR/LTD.
Methods
Patient inclusion
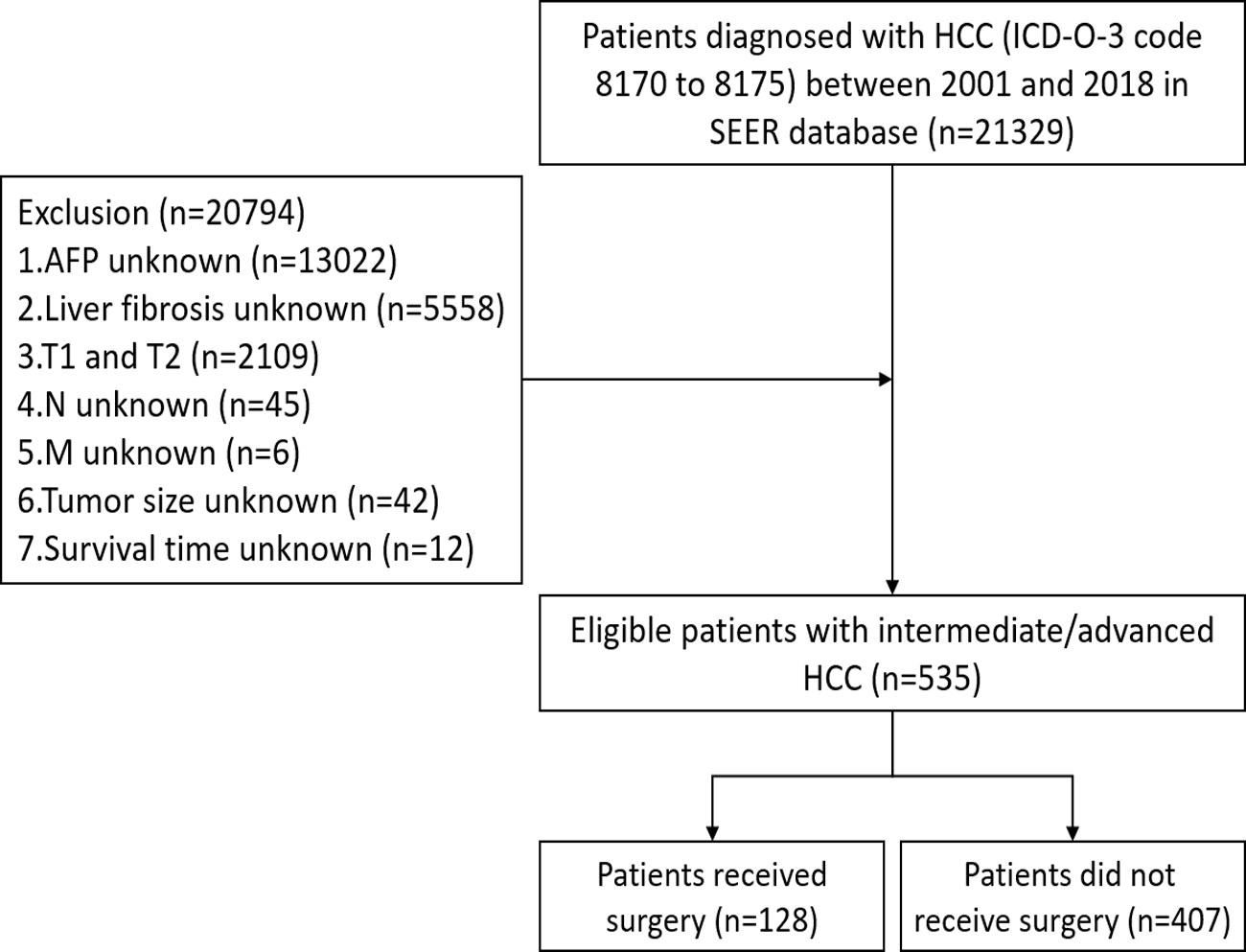
Data of eligible patients from 2001 to 2018 were extracted from SEER database using SEER∗Stat software (version 8.3.8). The inclusion criteria were as follows: 1) International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) code 8170 to 8175; and 2) T3/T4. The exclusion criteria were as follows: 1) race unknown; 2) histological grade unknown; 3) tumor size unknown; 4) regional lymph nodes (N) unknown; 5) distant metastasis (M) unknown; 6) alpha fetoprotein (AFP) unknown; 7) liver fibrosis unknown; and 8) survival time unknown. After screening, a total of 535 eligible patients with intermediate/advanced HCC were eventually included in this study (Figure 1). The institutional review board approval and written patient consent were not required for this study, as the data for all patients are deidentified in the publicly available SEER database.
Statistical analysis
Propensity score matching (PSM) was performed with 1:1 nearest neighbor method to balance the characteristics of the patients who received LR/LTD (surgery) and those who did not. Chi-square test and Mann-Whitney U test were used to compare the difference of characteristics between the two groups. Kaplan–Meier curves and log-rank tests were applied for survival comparison.
Univariate and multivariate Cox proportional hazards regression analyses were used to obtain independent factors that significantly affected OS. The obtained independent factors were used for the nomogram construction. The concordance index (C-index) and receiver operating characteristic (ROC) curve were applied to reflect the discrimination and predictive accuracy of the nomogram, and their values range from 0 and 1.0. 1.0 represents perfect predicting accuracy (9). Calibration curves using a bootstrap approach with 1,000 resamples were used to compare the predicted survival with the observed survival (10). Decision curve analysis (DCA) was used to assess the clinical practicability of the nomogram (11). The cutoff value was obtained based on the log-rank test results, and then based on cutoff value, patients were divided into low-risk and high-risk groups.
R software (4.1.2, R Core Team) and relevant packages (“Matching,” “nonrandom,” “tableone,” “plyr,” “rms,” “DynNom,” “nomogramFormula,” “survival,” “foreign,” “survivalROC,” “ggDCA,” “survminer,” “glmnet,” “riskRegression”) were used for statistical analyses and plotting. The cutoff value was obtained using log-rank test. A two-sided P-value < 0.05 was considered statistically significant.
Results
Patient characteristics
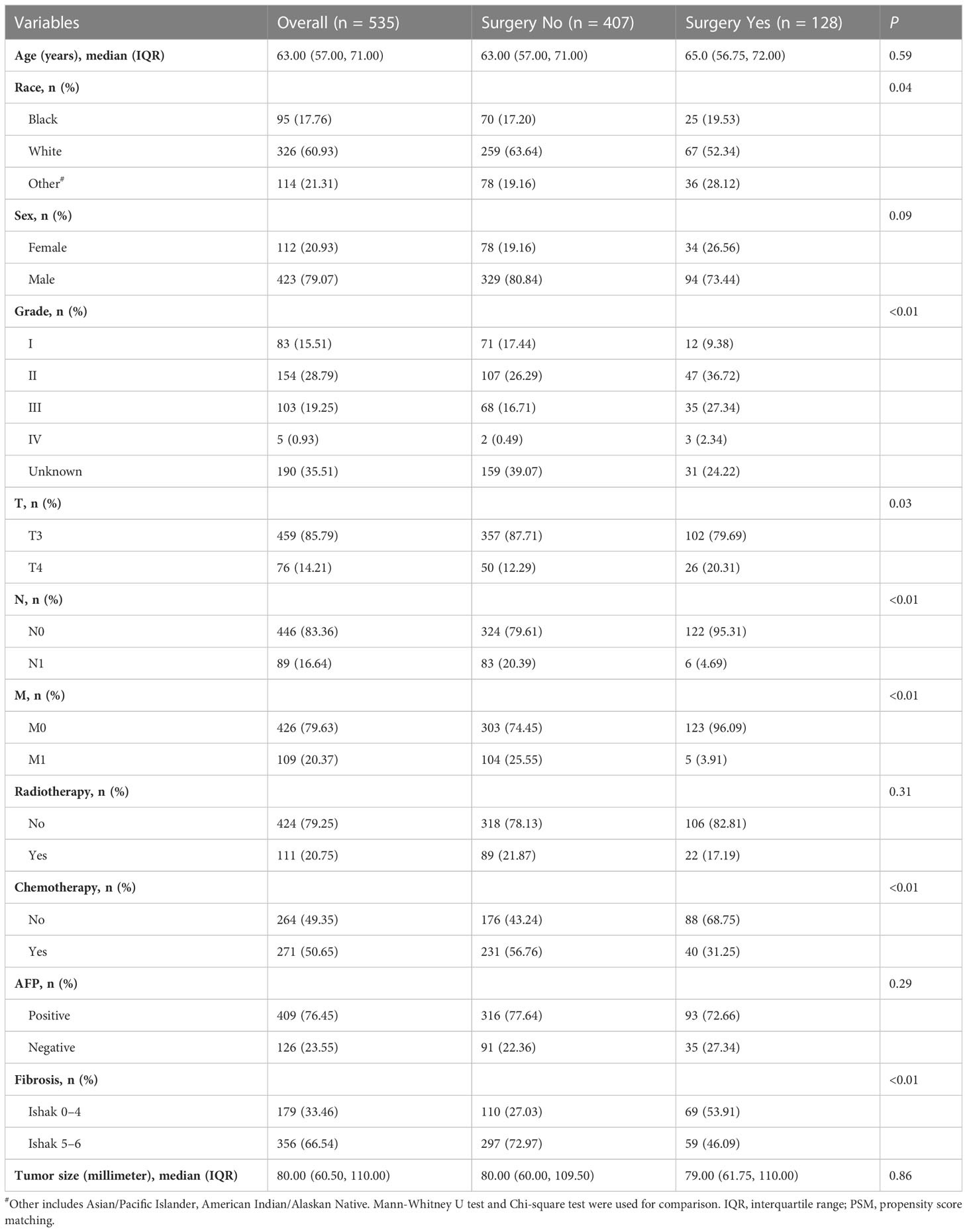
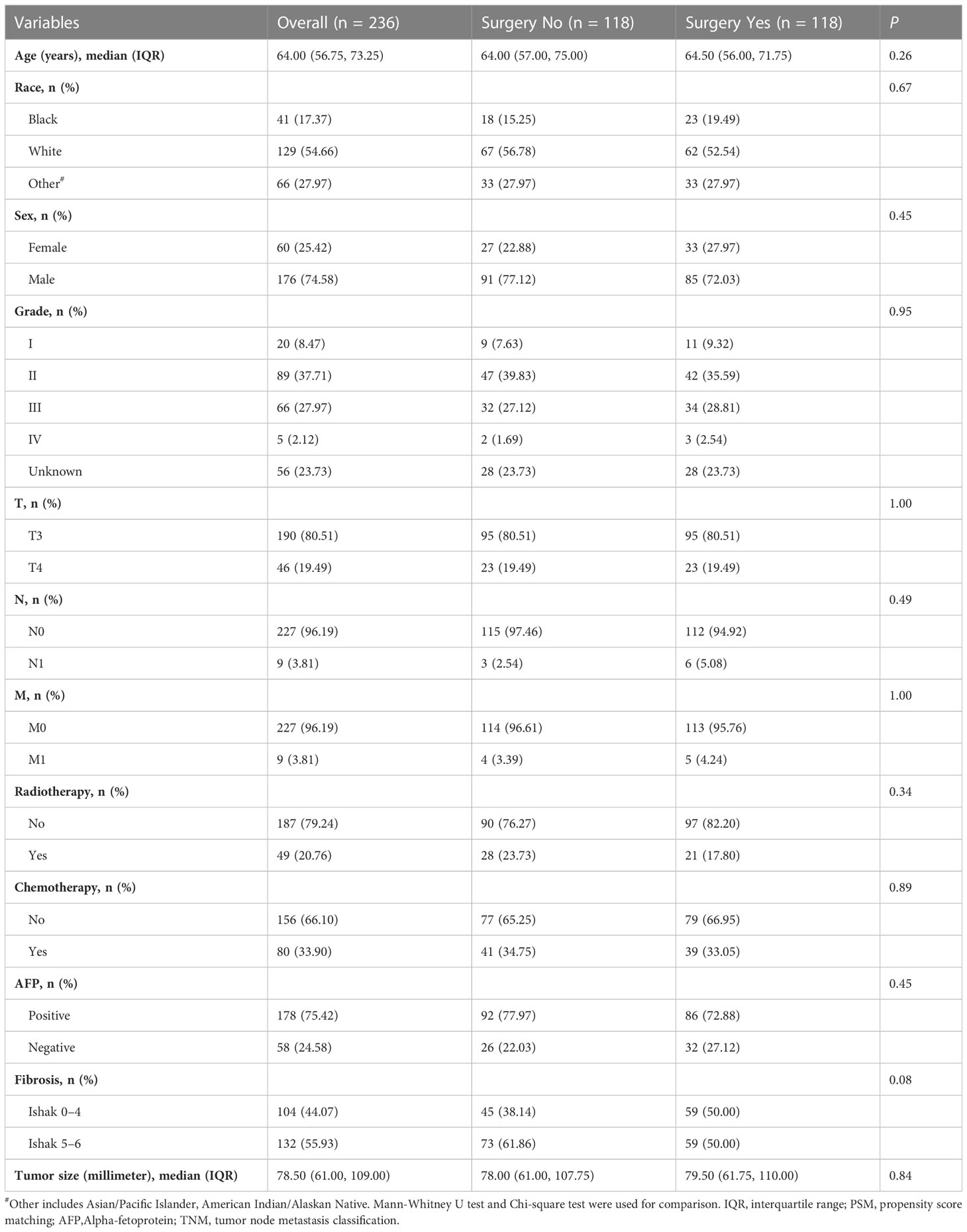
The characteristics of the 535 eligible patients with intermediate/advanced HCC were summarized in Table 1. Of these, 128 (23.9%) patients received LR/LTD (surgery), while 407 (76.1%) patients did not. Most (85.8%) patients were at T3 stage, while 14.21% of them were at T4. 83.36% of patients were at N0, and 79.63% of them were at M0. Then, PSM was performed to balance the characteristics between the patients who received surgery and the patients who did not. Finally, 118 patients who received surgery and 118 patients who did not were enrolled for further analysis (Table 2).
Surgery improved the survival of patients with intermediate/advanced HCC
The OS were compared between the patients with intermediate/advanced HCC who received surgery and those who did not. Before PSM, the OS was significantly higher in patients with intermediate/advanced HCC who received surgery than in patients who did not (median months: 21.0 vs. 5.0, P<0.001) (Figure 2A). The 1-, 3-, and 5-year OS rates were all higher in patients who received surgery than in patients who did not (1-year: 73.9% vs. 27.5%, 3-year: 34.3% vs. 10.2%, 5-year: 13.0% vs. 5.9%, respectively). Similarly, after PSM, the OS (median months: 21.0 vs. 4.0, P<0.001) (Figure 2B), and the 1-, 3-, and 5-year OS rates were all higher in patients who received surgery than in patients who did not.
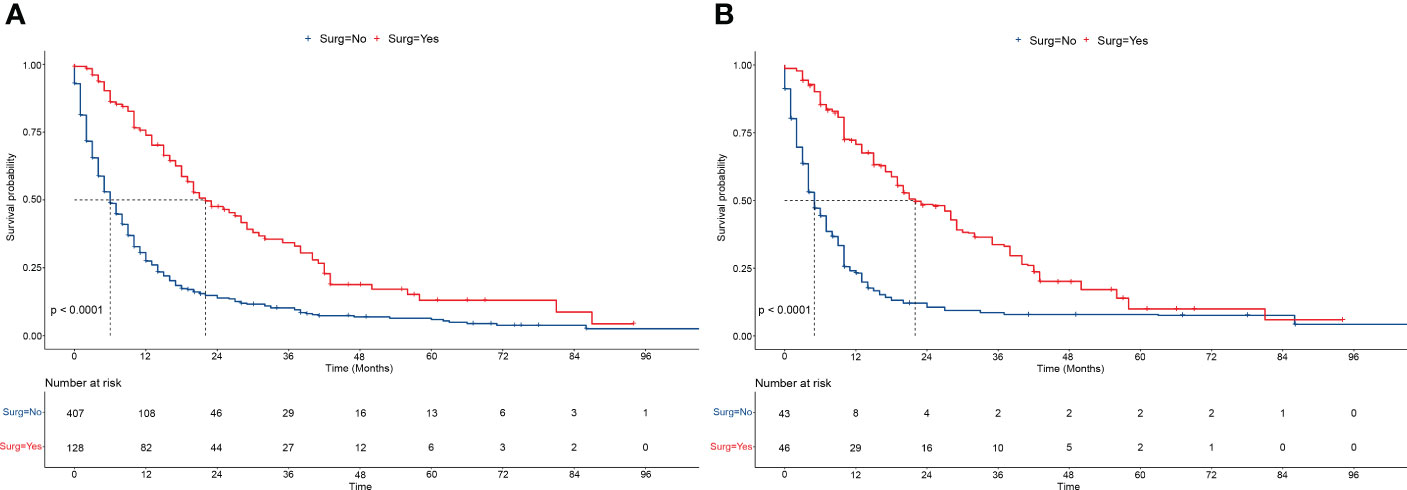
Figure 2 Overall survivals of patients receiving/not receiving surgery. (A, B) The overall survivals of patients receiving/not receiving surgery (LR/LTD) before PSM (A), and after PSM (B). Log-rank tests were used for survival comparison. LR, liver resection; LTD, local tumor destruction; PSM, propensity score matching.
Univariate and multivariate analyses for independent prognostic factors
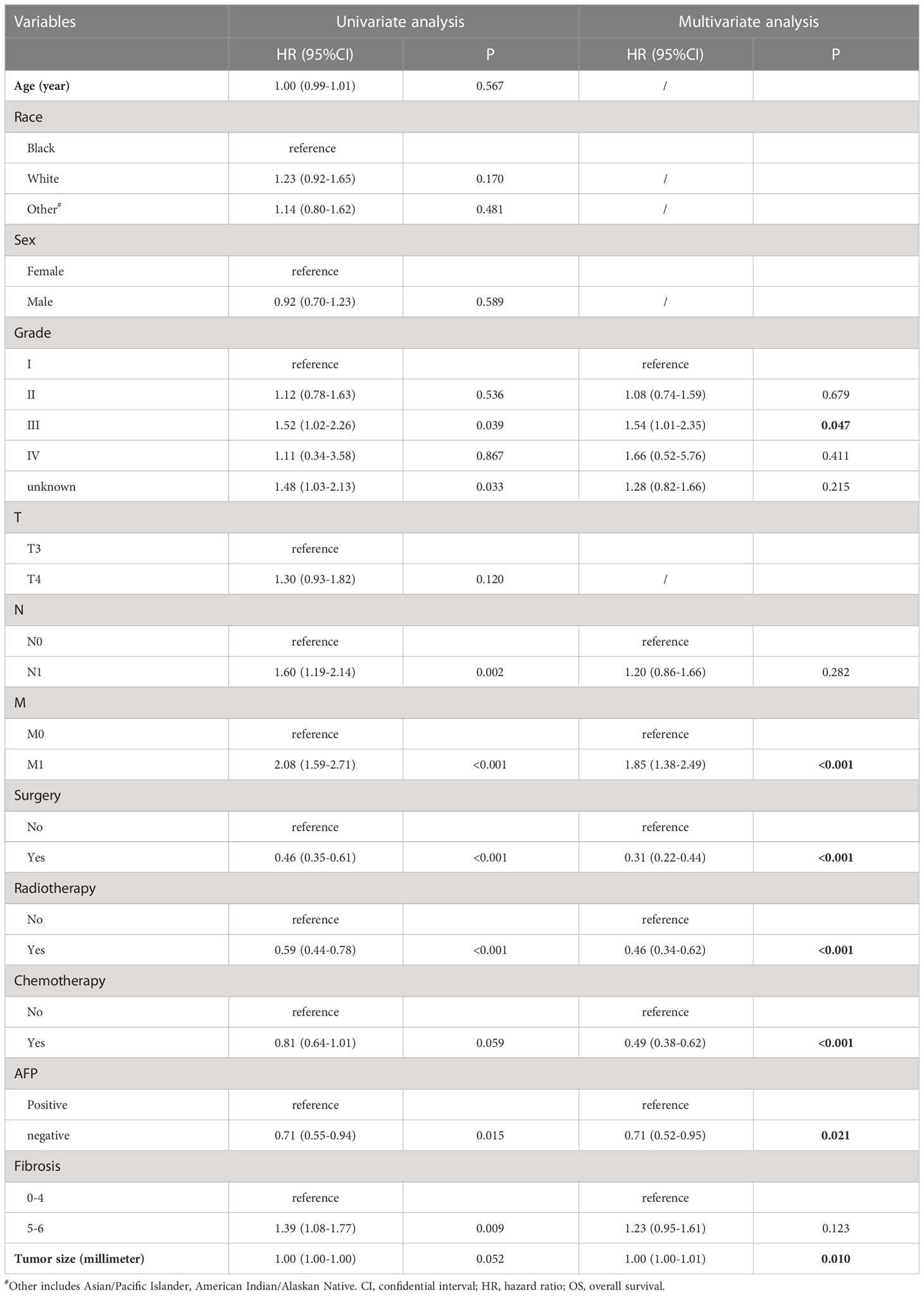
Univariate and multivariate analyses were performed to obtain the factors that significantly affected the OS. The variable was included in the multivariate analyses when its P-value was < 0.01. The results showed that grade, M, surgery (LR/LTD), radiation, chemotherapy, AFP, and tumor size significantly affected the OS of patients with intermediate/advanced HCC (Table 3).
Construction and validation of the nomogram
The independent prognostic factors above were included in the nomogram for predicting the 1-, 3-, and 5-year OS of patients with intermediate/advanced HCC (Figure 3A). To construct and validate the nomogram, 70% (n = 380) and 30% (n = 155) of all included patients were randomly assigned to the training and validation cohorts, respectively (Supplementary Tables 1, 2). The C-indices of the nomogram were higher than those of the American Joint Committee on Cancer (AJCC) TNM staging system (training cohort: 0.74 vs. 0.59; validation cohort: 0.78 vs. 0.61). The 1-, 3-, and 5-year areas under the ROC curves (AUCs) of the nomogram were 0.77(95%CI:0.72-0.82), 0.73(95%CI:0.66-0.80), and 0.72(95%CI:0.61-0.83), respectively, in the training cohort (Figure 3B). The 1-, 3-, and 5-year AUCs in the validation cohort were 0.87(95%CI:0.81-0.92), 0.81(95%CI:0.71-0.91), and 0.64(95%CI:0.50-0.78), respectively (Figure 3C). Similarly, the calibration curves showed moderate-to-high consistency between the nomogram predictions and the actual survivals both in training and validation cohorts (Figures 3D, E). Furthermore, the DCA curves demonstrated that the nomogram tended to have better positive net benefits than the TNM staging system, suggesting good clinical practicability (Supplementary Figure 1).
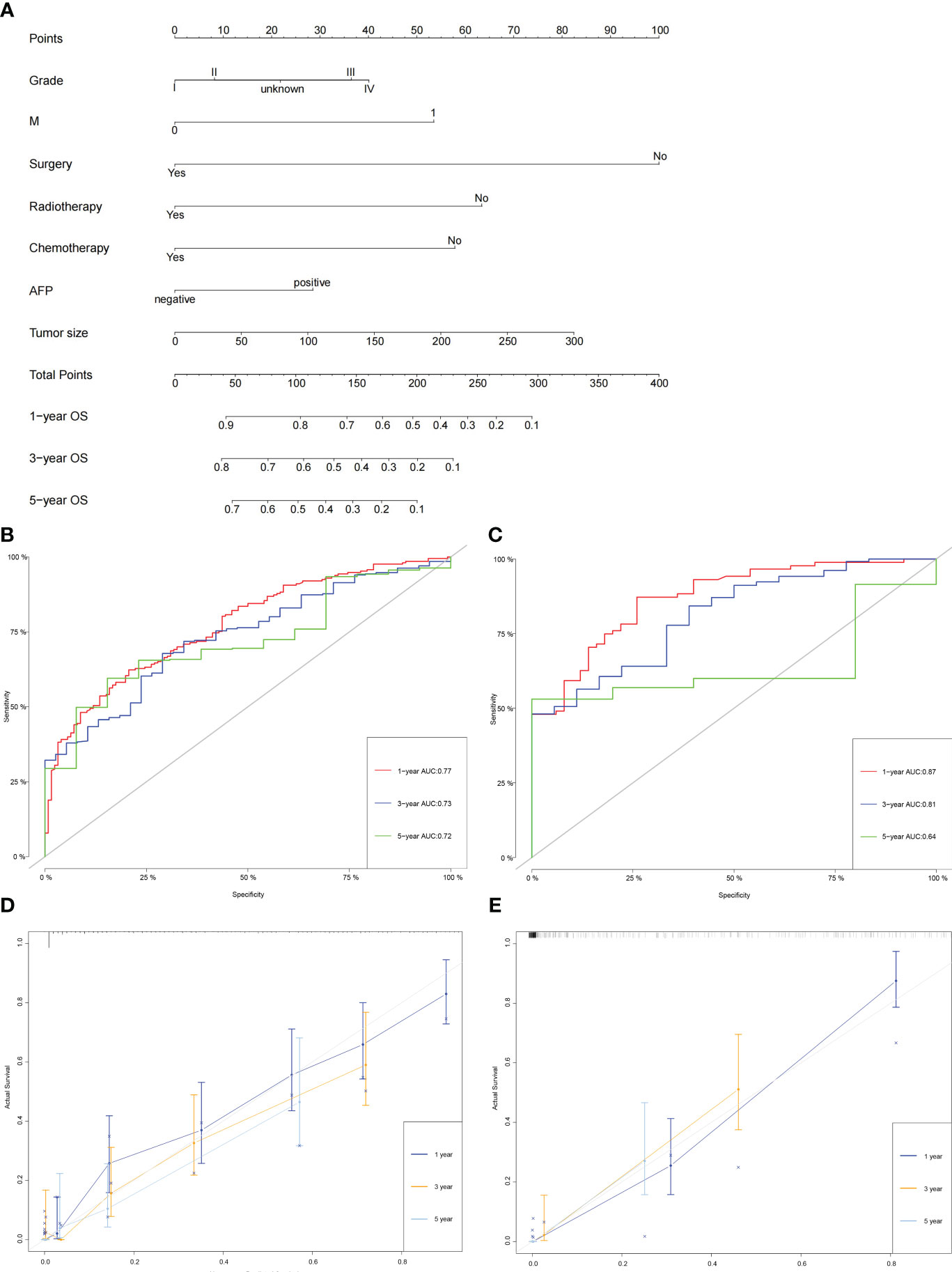
Figure 3 Construction and validation of the nomogram. (A) The nomogram for predicting the 1-, 3-, and 5-year OS of patients with intermediate/advanced HCC. (B, C) The 1-, 3-, and 5-year AUCs of the nomogram in the training cohort (B) and validation cohort (C). (D, E) Calibration curves of the nomogram for 1-, 3-, and 5-year OS in the training cohort (D) and validation cohort (E).
Overall survival in low-risk and high-risk patient groups
According to the log-rank test results of OS, patients were divided into a low-risk group (nomogram scores ranged from 0 to 221.9) and a high-risk group (nomogram scores higher than 221.9). Kaplan–Meier curves revealed that the OS were significantly higher in the low-risk group than in the high-risk group (P <0.001) (Figures 4A–C). And the 1-, 3-, and 5-year OS rates were all higher in the low-risk group than in the high-risk group in all cohort, training cohort, and validation cohort (Supplementary Table 3). The results indicated good discrimination and clinical practicability of our nomogram.
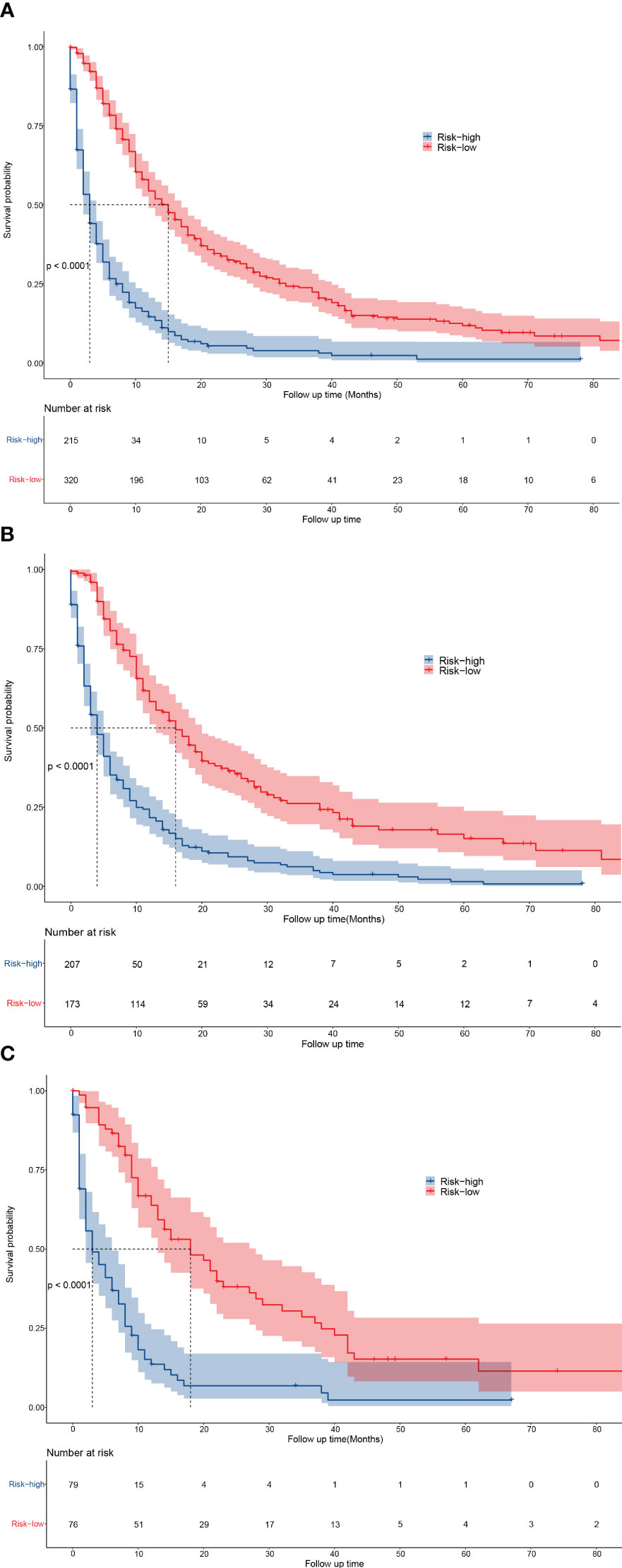
Figure 4 Kaplan-Meier curves for predicting the OS of low- and high-risk patients. (A–C) The OS of low- and high-risk patients in all cohort (A), training cohort (B), and validation cohort (C).
Web application for OS prediction
Based on the Nomogram prediction model constructed in this study, we have developed and deployed a web-based application for predicting the overall survival of patients with intermediate/advanced liver cancer. The application can be accessed at https://advancedlivercancer.shinyapps.io/DynNomapp/. By entering the clinicopathological characteristics of the patient, the application immediately provides the predicted survival probability for the current patient. It is designed to be simple to operate and easy to understand.
Discussion
This study mainly explored whether surgery (LR/LTD) could significantly improve the OS of patients with intermediate/advanced HCC. Furthermore, a nomogram was constructed and validated to predict the OS of the patients, which could help to identify the patients who would most likely benefit from LR/LTD.
Previous studies have shown that both LR and LTD improved the OS and disease-free survival of patients with HCC, especially those with early HCC (6, 7). LTD is usually an alternative to LR. To date, the guidelines of AASLD and EASL still recommend LR and LTD as first-line treatment for patients with early-stage HCC, but not for patients with intermediate/advanced HCC (3–5). However, the guidelines of the Asian Pacific Association for the Study of the Liver (APASL) (12) and China (13) both point out that LR could be performed in some eligible patients with intermediate/advanced HCC. Moreover, some studies have shown that LR improved the survival in selected patients with advanced HCC (14, 15). Similarly, in this study, LR/LTD was proved to significantly improve the OS of patients with intermediate/advanced HCC, indicating good clinical benefits. Furthermore, surgery (LR/LTD) was identified to be the most important prognostic factor in our nomogram, which also suggested that receiving surgery could significantly improve the OS of patients with intermediate/advanced HCC. Though considerable progresses have been made in the HCC treatment in recent years, patients with intermediate/advanced HCC still have poor prognosis. Hence, LR/LTD might be used in certain patients to improve their OS.
A nomogram is a reliable predictive tool to calculate and predict individual survival by integrating cancer-related prognostic factors (10, 11). The results of C-indices, AUCs, calibration curves in training and validation cohorts all indicated good predictive accuracy of the nomogram developed in this study, which is better than that of the TNM staging system. Similarly, the DCA curves suggested that this nomogram tended to have better clinical practicability than the TNM staging system. Therefore, the nomogram constructed in the present study could provide great clinical value in prognostic evaluation, and assist clinicians and patients in the therapeutic strategy selection.
To further explore the values of the nomogram, the low-risk patients and high-risk patients were categorized based on their nomogram scores. It revealed that the OS was significantly higher in low-risk patients than in high-risk patients. Our results also suggested that patients with certain characteristics (low grade, M0, radiotherapy received, chemotherapy received, negative AFP, and small tumor size), namely the patients with low risk, may have good survival when receiving surgery. Therefore, surgery (LR/LTD) might be an appropriate alternative treatment in the eligible patients with intermediate/advanced HCC.
This study had several advantages. First, this is the first study to explore the effect of LR/LTD in the OS of patients with intermediate/advanced HCC in a relatively large cohort. Second, a nomogram with good accuracy and clinical practicality was constructed to predict the OS of patients with intermediate/advanced HCC. And patients with low risk (based on the nomogram) were demonstrated to be eligible for LR/LTD, which may be helpful for clinical practice. However, limitations also existed. First, data from the SEER database did not include some clinical variables, such as liver function parameters. Second, although LR/LTD were proved to improve the OS of patients with intermediate/advanced HCC, the specific treatment strategies for different patients still needed further investigation.
In summary, this study revealed that surgery (LR/LTD) could significantly improve the OS of patients with intermediate/advanced HCC, especially the low-risk patients. Our nomogram with good accuracy and clinical practicality may be used to predict the OS of patients with intermediate/advanced HCC, and to help identify the patients who would most likely benefit from LR/LTD.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://seer.Cancer.gov.
Author contributions
YaZ, YiZ, TH, DK, XL, JC contributed to the idea and design. GL, MD and YaZ collected and analyzed the data. XL, JH, CH, SL, and YaZ drew the figures and tables. YiZ: Writing - original draft. DK: Writing - review & editing. JC: Writing - review & editing; investigating. All authors contributed to manuscript writing and revision. All authors have reviewed the final version of the manuscript and approved its submission.
Acknowledgments
We thank the open access to SEER database.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1191822/full#supplementary-material
Abbreviations
AASLD, American Association for the Study of Liver Diseases; AFP, alpha fetoprotein; APASL, Asian Pacific Association for the Study of the Liver; AUCs, areas under the ROC curves; C-index, concordance index; DCA, decision curve analysis; EASL, European Association for the Study of Liver; HCC, hepatocellular carcinoma; LR, liver resection; LTD, local tumor destruction; OS, overall survival; PSM, propensity score matching; RFA, heat-radio-frequency ablation; ROC, receiver operating characteristic; SEER, Surveillance, Epidemiology, and End Results; TACE, transarterial chemoembolization; TARE, transarterial radioembolization; TKIs, tyrosine kinase inhibitors.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology (2021) 73(Suppl 1):4–13. doi: 10.1002/hep.31288
3. Bruix J, Sherman M. American Association for the study of liver d. management of hepatocellular carcinoma: an update. Hepatology (2011) 53(3):1020–2. doi: 10.1002/hep.24199
4. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology (2018) 67(1):358–80. doi: 10.1002/hep.29086
5. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol (2018) 69(1):182–236. doi: 10.1016/j.jhep.2018.03.019
6. Hasegawa K, Makuuchi M, Takayama T, Kokudo N, Arii S, Okazaki M, et al. Surgical resection vs. percutaneous ablation for hepatocellular carcinoma: a preliminary report of the Japanese nationwide survey. J Hepatol (2008) 49(4):589–94. doi: 10.1016/j.jhep.2008.05.018
7. Yamakado K, Nakatsuka A, Takaki H, Yokoi H, Usui M, Sakurai H, et al. Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology (2008) 247(1):260–6. doi: 10.1148/radiol.2471070818
8. NC I. Surveillance, epidemiology, and end results (SEER) program (2022). Available at: https://seer.cancer.gov/.
9. Royston P, Moons KG, Altman DG, Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ (2009) 338:b604. doi: 10.1136/bmj.b604
10. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol (2008) 26(8):1364–70. doi: 10.1200/JCO.2007.12.9791
11. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol (2015) 16(4):e173–e80. doi: 10.1016/S1470-2045(14)71116-7
12. Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int (2017) 11(4):317–70. doi: 10.1007/s12072-017-9799-9
13. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer (2020) 9(6):682–720. doi: 10.1159/000509424
14. Shindoh J, Kawamura Y, Kobayashi Y, Kobayashi M, Akuta N, Okubo S, et al. Prognostic impact of surgical intervention after lenvatinib treatment for advanced hepatocellular carcinoma. Ann Surg Oncol (2021) 28(12):7663–72. doi: 10.1245/s10434-021-09974-0
Keywords: liver resection, local tumor destruction, hepatocellular carcinoma, survival, nomogram
Citation: Zhang Y, Zhang Y, He T, Liu G, Duan M, Huang J, Huang C, Lowe S, Ke D, Liu X and Cao J (2023) Local tumor destruction and liver resection increase overall survival in intermediate/advanced hepatocellular carcinoma patients: evidence from a population-based study. Front. Endocrinol. 14:1191822. doi: 10.3389/fendo.2023.1191822
Received: 22 March 2023; Accepted: 03 July 2023;
Published: 28 July 2023.
Edited by:
Houjuan Zhu, Institute of Materials Research and Engineering (A*STAR), SingaporeReviewed by:
Yanlong Shi, Nanjing Medical University, ChinaGouling Zhan, Central South University, China
Nan Ding, Maastricht University, Netherlands
Copyright © 2023 Zhang, Zhang, He, Liu, Duan, Huang, Huang, Lowe, Ke, Liu and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dazhi Ke, kedazhi@hospital.cqmu.edu.cn; Xiaozhu Liu, xiaozhuliu@hospital.cqmu.edu.cn; Junyi Cao, junyi_cao@yeah.net
†These authors have contributed equally to this work and share first authorship
 Yang Zhang
Yang Zhang Yi Zhang2†
Yi Zhang2† Taiyu He
Taiyu He Minjie Duan
Minjie Duan Jian Huang
Jian Huang Scott Lowe
Scott Lowe Xiaozhu Liu
Xiaozhu Liu