- 1Division of Endocrinology and Metabolism, Department of Obstetrics, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing Maternal and Child Health Care Hospital, Beijing, China
- 2National Research Institute for Family Planning, Beijing, China
- 3National Human Genetic Resources Center, Beijing, China
Objective: There is a lack of risk factors that can effectively identify gestational diabetes mellitus (GDM) in early pregnancy. It is unclear whether serum taurine in the first trimester and dynamic changes have different characteristics in GDM women. Whether these features are associated with the occurrence of GDM has not yet been elucidated. The main objective of this study was to observe the dynamic changes of serum taurine during pregnancy and investigate the relationship between serum taurine levels and GDM in the first and second trimesters.
Methods: This was a nested case-control study in 47 women with GDM and 47 age-matched normoglycemic women. We examined serum taurine at 8-12 weeks’ gestation and 24-28 weeks’ gestation. The serum taurine of the two groups was compared. Multivariable logistic regression analysis was performed to investigate how serum taurine was associated with GDM.
Results: The serum taurine concentration of GDM women was significantly lower than that of normoglycemic women in the first trimester(2.29 vs 3.94 μmol/L, P<0.001). As the pregnancy progressed, serum taurine concentration in normoglycaemic women decreased significantly(3.94 vs 2.47 μmol/L, P<0.001), but not in the GDM group(2.29 vs 2.37 μmol/L, P=0.249), resulting in the disappearance of differences between the two groups(2.47 vs 2.37 μmol/L, P=0.160). After adjustment for pre-pregnancy body mass index(BMI), fasting plasma glucose(FPG), and lipid profiles in the first trimester, the serum taurine concentration in the first trimester was negatively correlated with the risk of GDM(OR=0.017, 95% CI=0.003-0.107, P<0.001). Furthermore, dynamic change of serum taurine showed a significantly positive correlation with the risk of GDM(OR=9.909, 95% CI=3.556-27.610, P<0.001).
Conclusion: Low serum taurine concentration in the first trimester was significantly associated with the development of GDM. As the pregnancy progressed, the association between serum taurine and GDM disappeared in the second trimester, which might be related to the inhibition of taurine transporter(TauT) activity by high glucose.
Introduction
Gestational diabetes mellitus (GDM) is the most common metabolic disease in pregnancy, with an incidence of 9%-25% globally according to the International Diabetes Federation (IDF) (1). Women with GDM are at an increased risk of gestational hypertension, pre-eclampsia, and cesarean section, as well as long-term risk of type 2 diabetes (T2DM) and cardiovascular disease (2). Maternal hyperglycemia will increase the risk of large for gestational age(LGA), shoulder dystocia or birth injury, and neonatal hypoglycemia (3). The offspring of GDM women are at increased long-term risk of obesity, abnormal glucose metabolism, and cardiovascular disease (4). With the continuous progress in knowledge of GDM, the oral glucose tolerance test (OGTT) at 24-28 gestational weeks was the diagnostic criteria for GDM (2). Recent studies evaluating maternal glycemia in relation to fetal growth trajectory have confirmed the early impact of maternal glycemia on fetal overgrowth and obesity prior to the diagnosis of standard GDM (5, 6). Lifestyle interventions such as dietary counseling or physical activity in the first trimester were demonstrated to effectively reduce the incidence of GDM and its associated adverse pregnancy outcomes (7, 8). As a result, it is of great clinical value to identify risk factors for GDM, especially in the first trimester.
Taurine which is the most abundant free amino acid in the human body and the key component of bile acid has many biological effects such as antioxidant, anti-inflammatory, improvement of insulin resistance(IR), neuroprotection, and anti-neurotoxicity (9, 10). Taurine can be made endogenous from cysteine or methionine, provided extrinsic from the diet, or affected by gut microbiota (11, 12). There was a significant negative correlation between taurine and non-gestational blood glucose, and taurine supplementation was effective in improving diabetes and other chronic metabolic diseases and preventing related complications (10). A recent study suggested a lower plasma taurine level in the first trimester seemed to be a fair marker of inadequate insulin secretion and to be more closely associated with a higher risk of GDM development in multiparas (13). However, the dynamic changes in serum taurine from the first to second trimester were unknown.
The main objective of this study was to observe the dynamic changes of serum taurine during pregnancy and investigate the relationship between serum taurine levels and GDM in the first and second trimesters.
Materials and methods
Patient cohorts
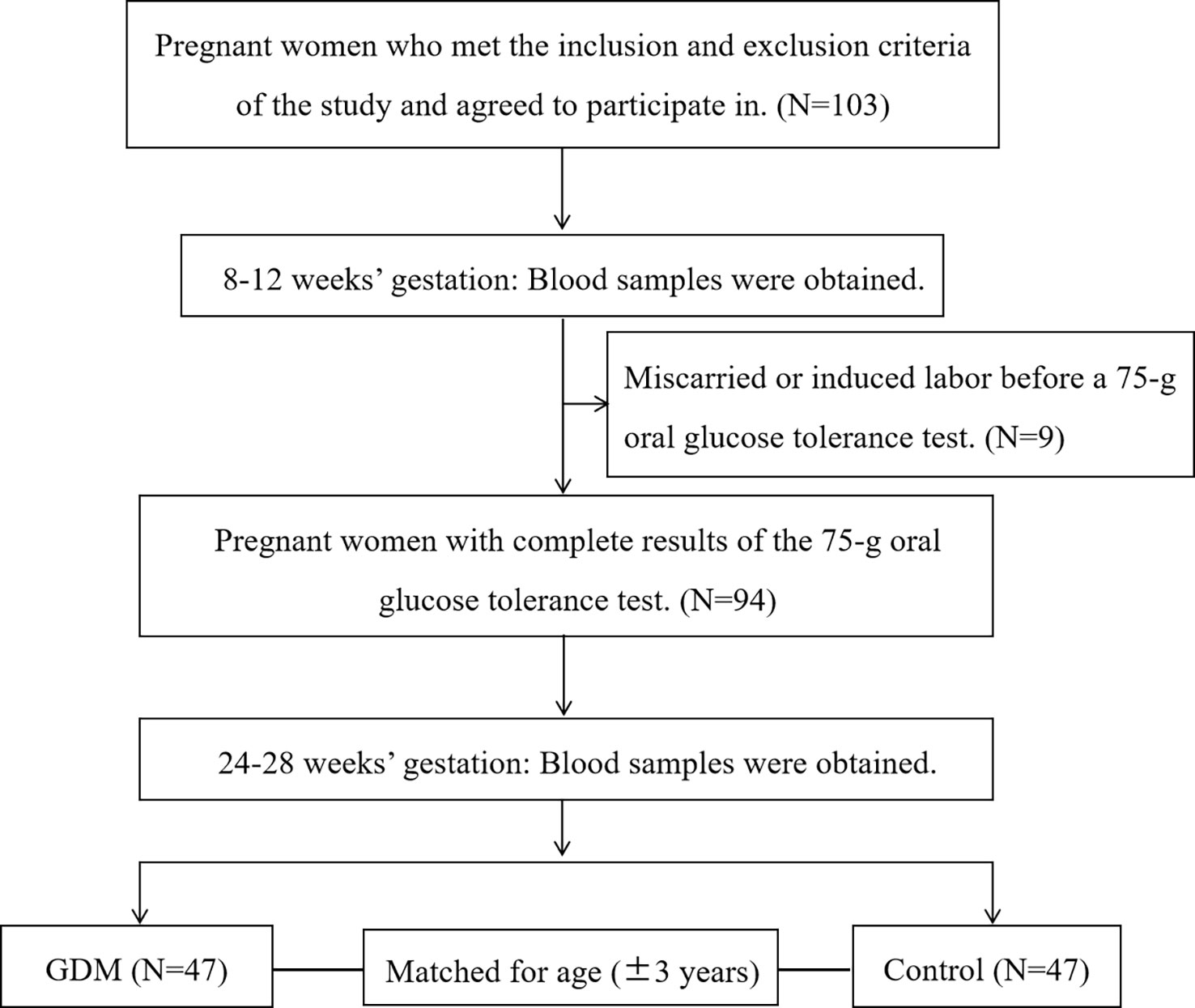
The participants in this nested case-control study were from a prospective cohort study in the Beijing Obstetrics and Gynecology Hospital, Capital Medical University. All pregnant women who intended to give birth in this hospital were enrolled in the cohort study at 8-12 gestational weeks and followed up until delivery. To evaluate the relationship between serum taurine and GDM, we selected eligible subjects from the recruited pregnant women above. Singleton pregnant women aged 18 to 44 years were recruited and only participants with complete clinical information were included in the analysis. Women with hypertension, diabetes, hyperlipidemia, liver or kidney dysfunction, and infectious diseases (hepatitis, pulmonary tuberculosis, etc.) before pregnancy were excluded. A 75-g OGTT was carried out at 24-28 gestational weeks. The diagnosis of GDM was made when any one of the following values was met or exceeded in the 75-g OGTT: 0 h (fasting), 5.1 mmol/L; 1 h, 10.0 mmol/L; and 2 h, 8.5 mmol/L according to ADA criteria (14). Normoglycaemic women were matched for age ( ± 3 years) to each case of GDM women in the same cohort (Figure 1).
Clinical measurements and covariates
Anthropometric measurements of participants were completed by trained medical staff at recruitment using a standardized protocol. Clinical data were collected by medical record review. Pre-pregnancy body weight was self-reported. A family history of diabetes was defined as a first-degree relative with T2DM. The fasting plasma glucose(FPG) and lipid profiles, including cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL), were determined as described in a previous study (15).
Taurine examination
Blood samples were collected from participants following an overnight fast at 8-12 weeks and 24-28 weeks, and serum specimens were isolated and stored at -80°C for further examination. The serum taurine levels were examined by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS, Thermo Scientific, USA). First, 100 μL of human serum was briefly added to a 0.5 mL glass centrifuge tube. After centrifugation at 14000 r/min for 5 min, the serum sample was dried under nitrogen at 50°C. Then, 60 μL of N-butyl alcohol and 12 mol/L HCI (95:5, v/v) were added and vortexed for 30 seconds in a seal. After incubation at 65°C for 15 min for derivatization, the derivatized solution was centrifuged, and dried under nitrogen at 50°C again. The residue was reconstituted by adding 100 μL of acetonitrile and water (4:1, v/v), vortexed for 30 seconds, centrifuged at 14000 r/min for 5 min, and injected at 20 μL for LC-MS/MS analysis.
Sample size calculation
The sample size was calculated using the mean and standard deviation of serum taurine in two groups. The test level (α) was 0.05, and the power (1-β) was 0.8. Serum taurine concentrations are 0.6 ± 0.1 mmol/L in diabetic patients and 0.8 ± 0.2 mmol/L in healthy adults (16). The minimum sample size was 48, and the sample size of this study was 94, which was sufficient according to the sample size calculation.
Statistics
Data were analyzed using the SPSS 26.0 software. Data with normal distributions were shown as the mean ± standard deviation, and nonnormal distributed data were shown as the median (interquartile range), respectively. T-tests and Wilcoxon tests were used to analyze the differences in continuous variables between the GDM group and the control group. Serum taurine concentrations were also compared by t-test. Categorical variables, including serum taurine levels (categorized into quartiles), were evaluated using the Cochran-Armitage. As pre-pregnancy body mass index(BMI) remained higher in the GDM group, we adjusted for pre-pregnancy BMI when comparing serum taurine levels in the two groups. Binary logistic regression for the association between GDM and serum taurine was carried out with adjustment for potentially confounding variables. The results are represented by the odds ratio (OR) and 95% confidence interval (CI). The differences were considered statistically significant when P<0.05.
Results
Clinical and laboratory characteristics
The study included 47 GDM women and 47 normoglycemic women. There was no history of GDM, macrosomia, or low birth weight delivery in both two groups. Pre-pregnancy BMI was significantly higher in the GDM women(22.32 vs 20.67, p=0.001), and other clinical indicators were similar, including gravidity, primipara, and history of polycystic ovary syndrome. However, FPG and lipid profiles including TC, TG, and LDL, were significantly higher among GDM women in the first trimester(FPG: 4.86 vs 4.64mmol, P=0.017; TC: 4.46 vs 4.12, P=0.021; TG: 1.26 vs 1.02, P=0.023; LDL: 2.33 vs 2.08, P=0.025)(Table 1). At OGTT, the blood glucose value of the GDM group was significantly higher, but there was no difference in lipid profiles between the two groups in the second trimester (Table S1 in the supplemental material).
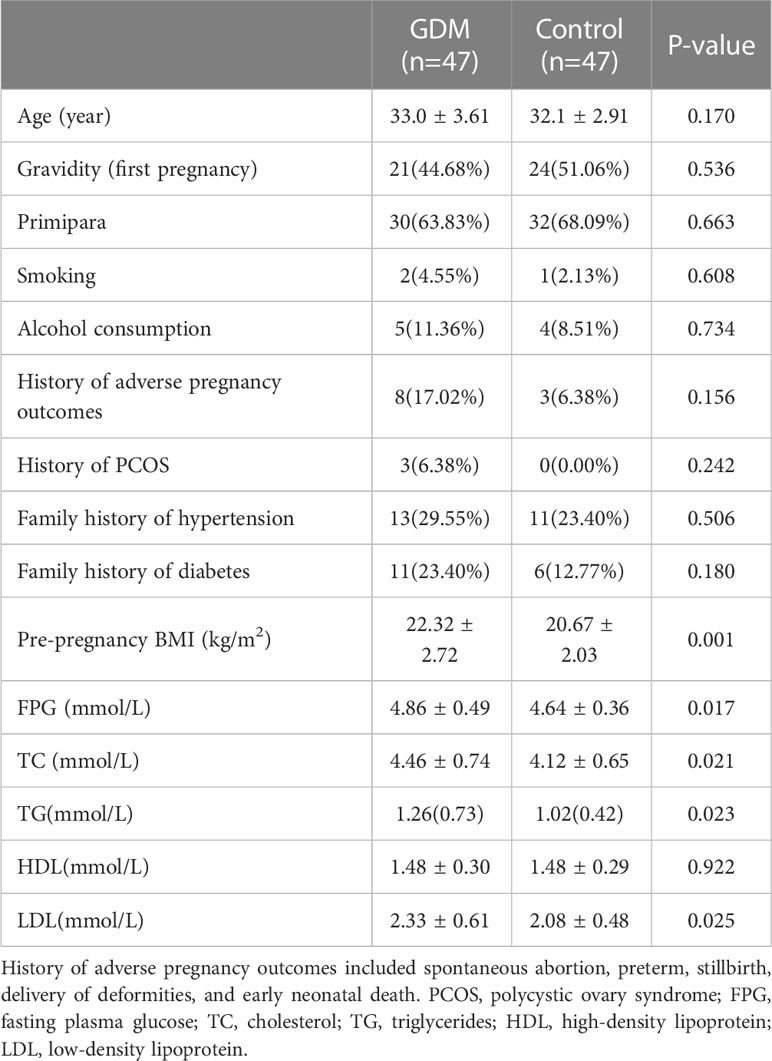
Table 1 Baseline characteristics and glycolipids metabolism in the first trimester between two groups.
Serum taurine levels between or within GDM and normoglycemic women
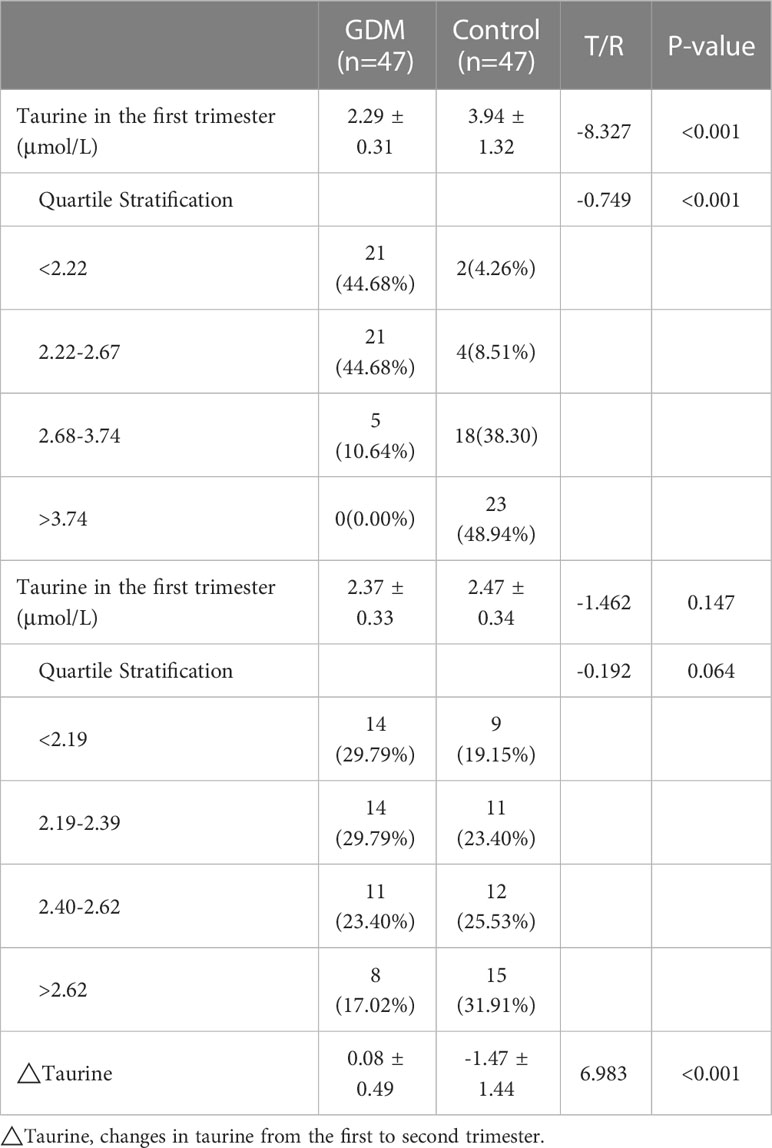
We compared serum taurine concentrations of GDM women and normoglycemic women at different stages of pregnancy, as well as the dynamic changes of serum taurine in the two groups(Table 2). The serum taurine concentration of GDM women was significantly lower than that of normoglycemic women in the first trimester(2.29 vs 3.94 μmol/L, P<0.001). When stratified by quartile, there were 23 controls and no GDM women with a taurine concentration less than 2.22 and there were 2 controls and 21 GDM women with a taurine concentration greater than 3.74(P<0.001). The serum taurine concentration was similar between the two groups in the second trimester(2.37 vs 2.47 μmol/L, P=0.147), and there was no significant difference in quartile stratification(P=0.064). With the progress of pregnancy, serum taurine concentration decreased significantly in the control group(3.94 vs 2.47 μmol/L, P<0.001), but not in the GDM group(2.29 vs 2.37 μmol/L, P=0.249) (Figure 2).
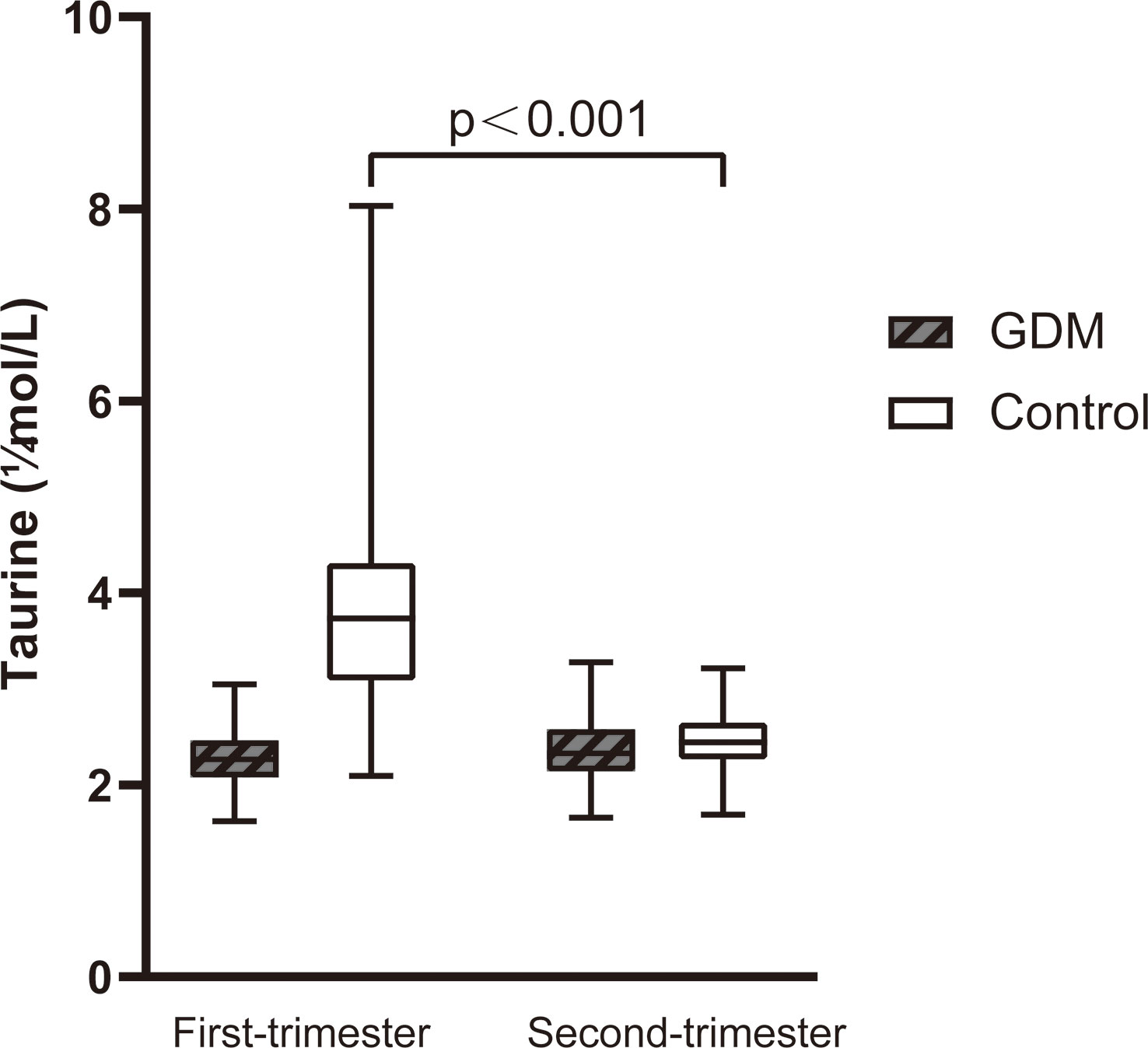
Figure 2 The dynamic changes of serum taurine between the first and second trimester of women with GDM and controls. Serum taurine concentration in control decreased significantly (P<0.001).
The association between serum taurine and GDM
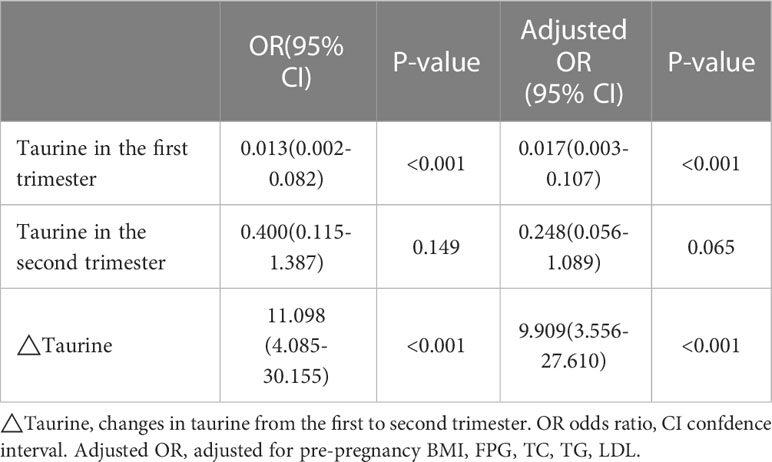
Univariate logistic regression analysis showed that there was a significant negative correlation between serum taurine concentration in the first trimester and the risk of GDM(OR=0.013, 95% CI=0.002-0.082, P<0.001, Table 3). Furthermore, dynamic change of serum taurine showed a significantly positive correlation with GDM(OR=11.098, 95% CI=4.085-30.155, P<0.001, P<0.001, Table 3). Results did not change after adjustment for pre-pregnancy BMI, FPG, and lipid profiles in the first trimester(Taurine in the first trimester: OR=0.017, 95% CI=0.003-0.107, P<0.001; ΔTaurine: OR=9.909, 95% CI=3.556-27.610, P<0.001; Table 3). However, serum taurine concentration in the second trimester was not correlated with GDM in any case.
Discussion
Our study showed that serum taurine concentration in the first trimester was significantly lower in women who were later diagnosed with GDM. As the pregnancy progressed, serum taurine concentration in normoglycaemic women decreased significantly, resulting in the disappearance of differences between the two groups. Low serum taurine concentration in the the first trimester was significantly associated with the occurrence of GDM, and this correlation also no longer existed in the second trimester.
A significant negative association between taurine and T2DM has been demonstrated (16). Previous RCT studies have shown that taurine supplementation could effectively improve metabolic indicators of T2DM, including glycemic indexes, lipid profiles, and inflammatory biomarkers, and prevent related complications (17–19). The T2DM patients in these studies were all detected with improvement in clinical metabolic markers after supplementing with 3000mg/day of taurine for 8 weeks. In addition, animal experiments showed that taurine had a protective effect on liver damage in GDM offspring (20). A study conducted the dietary survey at 24-28 gestational weeks and found that taurine intakes were lower in GDM than non-GDM in normal-weight women (21). However, there were few studies establishing a link between serum taurine levels and the risk of GDM. A recent study suggested a lower plasma taurine level in the first trimester seemed to be a fair marker of inadequate insulin secretion and to be more closely associated with a higher risk of GDM development in multiparas (13). This was consistent with our findings regarding the relationship between low serum taurine concentration in the first trimester and GDM.
Our study further compared the serum taurine concentrations in the second trimester and analyzed its dynamic changes. We found that as the pregnancy progressed, serum taurine concentration decreased significantly in normoglycaemic women but not in GDM women, resulting in the disappearance of differences between the two groups. The taurine decline trend from the first to second trimester was significantly negatively associated with the occurrence of GDM. Taurine is an amino acid that links the mother with the offspring during pregnancy, and fetuses depend on the taurine supplied by mothers via the placenta (22). The concentration of taurine in the placental tissue is 100-150 times higher than that of the fetus and mother (23). The placental tissues concentrate taurine efficiently and transfer taurine to fetal circulation based on the taurine transporter(TauT) activity (22). Animal studies have demonstrated that taurine concentration correlated with the peak of neurogenesis (24), which explained the decrease in serum taurine concentration in normoglycaemic women as the pregnancy progressed. However, high glucose levels could acutely inhibit taurine’s transport by TauT (25), which might be the reason why there was no difference in serum taurine concentration between the first and second trimester of GDM women in our study. The offspring of GDM have a long-term risk of neurodevelopmental disorder (26), and the role of taurine transport inhibition is worth further study.
The beneficial effects of taurine on T2DM and its related complications have been widely reviewed in human clinical practice (27). Taurine played a hypoglycemic role by improving insulin sensitivity, stimulating insulin secretion, and reducing inflammation and oxidative stress (27). Previous studies have reported the role of taurine in maintaining glucose homeostasis involving several possible mechanisms, such as modulating several pancreatic cells (28) and inhibiting inflammatory factor and nuclear factor kappa-B(NF-κB) activity to reduce inflammatory-mediated destruction of pancreatic β cells (29). It is not clear whether the pathogenesis of GDM induced by taurine deficiency in the first trimester is identical to that in non-pregnant women. In our former study, we reported gut microbiota changes in the first trimester were potentially associated with the development of GDM (30). The gut microbiota could trigger inflammatory processes by increasing gut permeability by exposing tight gap junction proteins to bacterial lipopolysaccharides (31, 32). Taurine is a microbiota-related metabolite derived from bile acids by certain microorganisms (33), and animal studies have shown that taurine has a protective effect on intestinal barrier function (34). Taurine deficiency might play a critical role in the pathogenesis of GDM, resulting in the loss of intestinal barrier protection and chronic inflammation. Although a direct causal relationship between taurine and its pathological state has not been established, it might be a potential marker for GDM. We hope to develop a sensitive and reliable GDM prediction model with serum taurine in the first trimester to help identify high-risk individuals at an early stage. In addition, the clinical intervention can be stratified according to the high-risk degree to avoid the waste of medical resources.
Strengths and limitations
This was the first study to compare the dynamic changes of serum taurine concentrations from the first to second trimester. Our results demonstrated that small molecule metabolites varied during pregnancy and should be combined with dynamic changes to analyze their relationship with disease. Unfortunately, we were unable to collect umbilical cord blood to test their serum taurine levels to verify the relationship between taurine transport and the dynamic change of serum taurine concentration during pregnancy. In the future, the serum taurine levels of mothers and newborns could be detected simultaneously to reveal this correlation and its role in offspring nervous system development. In addition, this study was a single-center study, limited by the sample size and limited geographical area.
Conclusion
Our study revealed that GDM women had a reduced serum taurine level in the first trimester. Elevated serum taurine concentration from the first to second trimester was significantly associated with the development of GDM. The relationship between taurine deficiency and GDM may be related to increased intestinal permeability and systemic inflammation, and the specific mechanism needs to be further explored.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Beijing Obstetrics and Gynecology Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All the authors contributed significantly to the manuscript. GL and XM contributed to the study design and interpretation of the data. JW and YW contributed to the drafting and revision of the manuscript. JW and WZ coordinated and executed the statistical analysis. CL, XY, and YZ contributed to the collection of data. WS, XW, and SL contributed to the enrollment and follow-up in clinic. All authors reviewed and approved the final submitted version.
Funding
This work was supported by National Natural Science Foundation of China (82171671), Beijing Hospitals Authority’ Ascent Plan(DFL20191402), Scientific Research Common Program of Beijing Municipal Commission of Education (KM202110025007), Beijing Natural Science Foundation (No. 7214231).
Acknowledgments
The authors thank the participants for participating in the study and the medical staff for their work on information collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1116044/full#supplementary-material
References
1. Yuen L, Saeedi P, Riaz M, Karuranga S, Divakar H, Levitt N, et al. Projections of the prevalence of hyperglycaemia in pregnancy in 2019 and beyond: Results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract (2019) 157:107841. doi: 10.1016/j.diabres.2019.107841
2. Sweeting A, Wong J, Murphy HR, Ross GP. A clinical update on gestational diabetes mellitus. Endocr Rev (2022) 43(5):763–93. doi: 10.1210/endrev/bnac003
3. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med (2008) 358(19):1991–2002. doi: 10.1056/NEJMoa0707943
4. Scholtens DM, Kuang A, Lowe LP, Hamilton J, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): Maternal glycemia and childhood glucose metabolism. Diabetes Care (2019) 42(3):381–92. doi: 10.2337/dc18-2021
5. Li M, Hinkle SN, Grantz KL, Kim S, Grewal J, Grobman W, et al. Glycaemic status during pregnancy and longitudinal measures of fetal growth in a multi-racial US population: a prospective cohort study. Lancet Diabetes Endocrinol (2020) 8(4):292–300. doi: 10.1016/S2213-8587(20)30024-3
6. Venkataraman H, Ram U, Craik S, Arungunasekaran A, Seshadri S, Saravanan P. Increased fetal adiposity prior to diagnosis of gestational diabetes in south asians: More evidence for the 'thin-fat' baby. Diabetologia (2017) 60(3):399–405. doi: 10.1007/s00125-016-4166-2
7. Koivusalo SB, Rönö K, Klemetti MM, Roine RP, Lindström J, Erkkola M, et al. Gestational diabetes mellitus can be prevented by lifestyle intervention: The Finnish gestational diabetes prevention study (RADIEL): A randomized controlled trial. Diabetes Care (2016) 39(1):24–30. doi: 10.2337/dc15-0511
8. Coomar D, Hazlehurst JM, Austin F, Foster C, Hitman GA, Heslehurst N, et al. Diet and physical activity in pregnancy to prevent gestational diabetes: A protocol for an individual participant data (IPD) meta-analysis on the differential effects of interventions with economic evaluation. BMJ Open (2021) 11(6):e048119. doi: 10.1136/bmjopen-2020-048119
9. Tochitani S. Functions of maternally-derived taurine in fetal and neonatal brain development. Adv Exp Med Biol (2017) 975 Pt 1:17–25. doi: 10.1007/978-94-024-1079-2_2
10. Inam-U-Llah PF, Aadil RM, Suleman R, Li K, Zhang M, et al. Ameliorative effects of taurine against diabetes: A review. Amino Acids (2018) 50(5):487–502. doi: 10.1007/s00726-018-2544-4
11. Zhou Y, Holmseth S, Guo C, Hassel B, Höfner G, Huitfeldt HS, et al. Deletion of the γ-aminobutyric acid transporter 2 (GAT2 and SLC6A13) gene in mice leads to changes in liver and brain taurine contents. J Biol Chem (2012) 287(42):35733–46. doi: 10.1074/jbc.M112.368175
12. Levy M, Thaiss CA, Zeevi D, Dohnalová L, Zilberman-Schapira G, Mahdi JA, et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell (2015) 163(6):1428–43. doi: 10.1016/j.cell.2015.10.048
13. Liu PJ, Liu Y, Ma L, Liu L, Hu T, An Z, et al. The relationship between plasma taurine levels in early pregnancy and later gestational diabetes mellitus risk in Chinese pregnant women. Sci Rep (2021) 11(1):7993. doi: 10.1038/s41598-021-87178-y
14. American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2018. Diabetes Care (2018) 41(Suppl 1):S13–27. doi: 10.2337/dc18-S002
15. Zheng W, Huang W, Zhang L, Tian Z, Yan Q, Wang T, et al. Early pregnancy metabolic factors associated with gestational diabetes mellitus in normal-weight women with polycystic ovary syndrome: A two-phase cohort study. Diabetol Metab Syndr (2019) 11:71. doi: 10.1186/s13098-019-0462-6
16. Sak D, Erdenen F, Müderrisoglu C, Altunoglu E, Sozer V, Gungel H, et al. The relationship between plasma taurine levels and diabetic complications in patients with type 2 diabetes mellitus. Biomolecules (2019) 9(3):96. doi: 10.3390/biom9030096
17. Maleki V, Alizadeh M, Esmaeili F, Mahdavi R. The effects of taurine supplementation on glycemic control and serum lipid profile in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Amino Acids (2020) 52(6-7):905–14. doi: 10.1007/s00726-020-02859-8
18. Maleki V, Mahdavi R, Hajizadeh-Sharafabad F, Alizadeh M. The effects of taurine supplementation on oxidative stress indices and inflammation biomarkers in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Diabetol Metab Syndr (2020) 12:9. doi: 10.1186/s13098-020-0518-7
19. Esmaeili F, Maleki V, Kheirouri S, Alizadeh M. The effects of taurine supplementation on metabolic profiles, pentosidine, soluble receptor of advanced glycation end products and methylglyoxal in adults with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Can J Diabetes (2021) 45(1):39–46. doi: 10.1016/j.jcjd.2020.05.004
20. Luo Y, Tian Y, Zhao C. Taurine attenuates liver autophagy and injury of offspring in gestational diabetic mellitus rats. Life Sci (2020) 257:117889. doi: 10.1016/j.lfs.2020.117889
21. Park S, Kim MY, Baik SH, Woo JT, Kwon YJ, Daily JW, et al. Gestational diabetes is associated with high energy and saturated fat intakes and with low plasma visfatin and adiponectin levels independent of prepregnancy BMI. Eur J Clin Nutr (2013) 67(2):196–201. doi: 10.1038/ejcn.2012.207
22. Ramamoorthy S, Leibach FH, Mahesh VB, Han H, Yang-Feng T, Blakely RD, et al. Functional characterization and chromosomal localization of a cloned taurine transporter from human placenta. Biochem J (1994) 300(Pt 3):893–900. doi: 10.1042/bj3000893
23. Kulanthaivel P, Cool DR, Ramamoorthy S, Mahesh VB, Leibach FH, Ganapathy V. Transport of taurine and its regulation by protein kinase c in the JAR human placental choriocarcinoma cell line. Biochem J (1991) 277(Pt 1):53–8. doi: 10.1042/bj2770053
24. Tochitani S, Furukawa T, Bando R, Kondo S, Ito T, Matsushima Y, et al. GABAA receptors and maternally derived taurine regulate the temporal specification of progenitors of excitatory glutamatergic neurons in the mouse developing cortex. Cereb Cortex (2021) 31(10):4554–75. doi: 10.1093/cercor/bhab106
25. Tochitani S. Taurine: A maternally derived nutrient linking mother and offspring. Metabolites (2022) 12(3):228. doi: 10.3390/metabo12030228
26. Farahvar S, Walfisch A, Sheiner E. Gestational diabetes risk factors and long-term consequences for both mother and offspring: A literature review. Expert Rev Endocrinol Metab (2019) 14(1):63–74. doi: 10.1080/17446651.2018.1476135
27. Sirdah MM. Protective and therapeutic effectiveness of taurine in diabetes mellitus: A rationale for antioxidant supplementation. Diabetes Metab Syndr (2015) 9(1):55–64. doi: 10.1016/j.dsx.2014.05.001
28. Santos-Silva JC, Ribeiro RA, Vettorazzi JF, Irles E, Rickli S, Borck PC, et al. Taurine supplementation ameliorates glucose homeostasis, prevents insulin and glucagon hypersecretion, and controls β, α, and δ-cell masses in genetic obese mice. Amino Acids (2015) 47:1533–48. doi: 10.1007/s00726-015-1988-z
29. Imae M, Asano T, Murakami S. Potential role of taurine in the prevention of diabetes and metabolic syndrome. Amino Acids (2014) 46(1):81–8. doi: 10.1007/s00726-012-1434-4
30. Zheng W, Xu Q, Huang W, Yan Q, Chen Y, Zhang L, et al. Gestational diabetes mellitus is associated with reduced dynamics of gut microbiota during the first half of pregnancy. mSystems (2020) 5(2):e00109–20. doi: 10.1128/mSystems.00109-20
31. Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med (2013) 34(1):39–58. doi: 10.1016/j.mam.2012.11.001
32. Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia (2007) 50(11):2374–83. doi: 10.1007/s00125-007-0791-0
33. Stacy A, Andrade-Oliveira V, McCulloch JA, Hild B, Oh JH, Perez-Chaparro PJ, et al. Infection trains the host for microbiota-enhanced resistance to pathogens. Cell (2021) 184(3):615–627.e17. doi: 10.1016/j.cell.2020.12.011
Keywords: biomarker, gestational diabetes mellitus, taurine, taurine transporter, dynamic change
Citation: Wang J, Wang Y, Zheng W, Yuan X, Liu C, Zhang Y, Song W, Wang X, Liang S, Ma X and Li G (2023) Dynamic changes of serum taurine and the association with gestational diabetes mellitus: A nested case-control study. Front. Endocrinol. 14:1116044. doi: 10.3389/fendo.2023.1116044
Received: 05 December 2022; Accepted: 13 March 2023;
Published: 23 March 2023.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenCopyright © 2023 Wang, Wang, Zheng, Yuan, Liu, Zhang, Song, Wang, Liang, Ma and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guanghui Li, liguanghui@ccmu.edu.cn; Xu Ma, nfpcc_ma@163.com
†These authors have contributed equally to this work and share first authorship
 Jia Wang1†
Jia Wang1† Xianxian Yuan
Xianxian Yuan Cheng Liu
Cheng Liu Wei Song
Wei Song Xiaoxin Wang
Xiaoxin Wang Xu Ma
Xu Ma Guanghui Li
Guanghui Li