- 1Taicang Affiliated Hospital of Soochow University, The First People’s Hospital of TaiCang, Soochow University, Suzhou, China
- 2Jiangsu Key Laboratory of Preventive and Translational Medicine for Geriatric Diseases, School of Public Health, Medical College of Soochow University, Suzhou, China
- 3Suzhou Center for Disease Prevention and Control, Suzhou, China
- 4Department of Sample Application and Management, Institute of Suzhou Biobank, Suzhou, China
- 5Department of Endocrinology and Metabolism, Institute of Post-Graduate Medical Education and Research and Seth Sukhlal Karnani Memorial Hospital (IPGME & R and SSKM Hospital), Kolkata, India
Objective: Gestational diabetes mellitus (GDM) is a common glucose metabolism disease occurs in pregnancy that affects both maternal and neonatal health. Recently, increasing studies have attached importance to the relationship between growth differentiation factor 15 (GDF-15) and GDM, but the results were inconclusive. Therefore, we conducted a meta-analysis to examine the association between GDF-15 and GDM.
Materials and methods: A systematical search was performed in Gene Expression Omnibus (GEO), PubMed and Google Scholar till Oct 27, 2022. We first calculated the mean and standard deviation of GDF-15 expression levels from the included eligible datasets and articles. Then, a meta-analysis was conducted to depict the difference in GDF-15 mRNA or GDF-15 protein expression between case and control groups by using conservative random effect model. Moreover, the potential publication bias was checked with the aid of Begg’s test and Egger’s test. Finally, sensitivity analyses were performed by changing the inclusion criteria.
Results: In summary, 12 GEO datasets and 5 articles were enrolled in our study, including 789 GDM patients and 1202 non-GDM pregnant women. It was found that the expression levels of GDF-15 mRNA and GDF-15 protein in late pregnancy were significantly higher in GDM patients compared with non-GDM pregnant women, with the standard mean difference (SMD) and 95% confidence interval (95% CI) of 0.48 (0.14, 0.83) and 0.82 (0.32-1.33), respectively. Meanwhile, a slightly weakened association between GDF-15 protein levels and GDM was also observed in the middle pregnancy, with SMD (95% CI) of 0.53 (0.04-1.02).
Conclusion: In all, our results suggested that the expression levels of GDF-15 were significantly higher in GDM patients compared with non-GDM pregnant women, especially in the late pregnancy.
Introduction
Gestational diabetes mellitus (GDM) is defined as diabetes or impaired glucose intolerance occurring for the first-time during pregnancy. It was estimated that the incidence of GDM ranged from 6.1% to 15.2% in the whole world (1). In China, the incidence of GDM was up to 14.8% (2). Without proper diagnosis and treatment, GDM can increase the risk of maternal complications (including gestational hypertension, urinary tract infection, and polyhydramnios) (3), as well as infant morbidities (such as macrosomia, erythrocytosis and hypoglycemia) (4). What’s more, GDM mothers and their offspring are more likely to develop obesity, type 2 diabetes mellitus (T2DM), and cardiovascular diseases (CVD) in later life (5–7). Therefore, it is of great clinical and health significance to explore the pathogenesis of GDM, and therefore to help in early screening, early diagnosis and thus early intervention of GDM to ensure maternal and fetal health.
Growth differentiation factor 15 (GDF-15) is a divergent member of the transforming growth factor-β superfamily (8). GDF-15 was reported to be an inflammation-induced central mediator of tissue tolerance (9). Levels of GDF-15 are markedly elevated in inflammatory disease states (10). GDF-15 was also found to suppress the intake of high-fat diets in animal models (11–13). Pharmacological treatment of recombinant GDF-15 proteins could reduce body weight and improve glucose tolerance in obese rodents and primates (12, 14). Moreover, metformin, the most commonly prescribed medication for T2DM, was revealed to achieve weight loss and glycemic control by stimulating the secretion of GDF-15 (14). Meanwhile, epidemic studies suggested that GDF-15 were associated with glucometabolic diseases (15), including T2DM (16).
GDM shares many features with T2DM in pathogenesis, for instance, glucose metabolism and insulin resistance (17, 18). Moreover, obesity, which could cause low-grade activation of inflammation and dysregulation of adipokines, is a major risk factor for these two diseases (19, 20). Along with observed associations of GDF-15 with T2DM, there was also an assumption that GDF-15 had a role in the development of GDM. However, the sample size of studies shed light in this field was relatively small and the results were far from conclusive. For example, two Norway studies reported that the GDF-15 levels were comparable between GDM patients and non-GDM pregnant women (21, 22), while Banerjee et al. found that GDF-15 concentrations at 24-32 weeks of gestation were significantly higher in GDM versus age-matched pregnant controls (23). Besides, Li et al. revealed a positive correlation between levels of GDF-15 in middle pregnancy and GDM (24), while Tang et al. reported that serum GDF-15 levels in the late pregnancy instead of the middle pregnancy were positively correlated with glucose metabolism (25).
Based on these inconsistent results in the current literature, we aimed to conduct a meta-analysis through search of GEO datasets and relevant literatures to figure out the association between GDF-15 and GDM.
Materials and methods
Data acquisition and search strategy
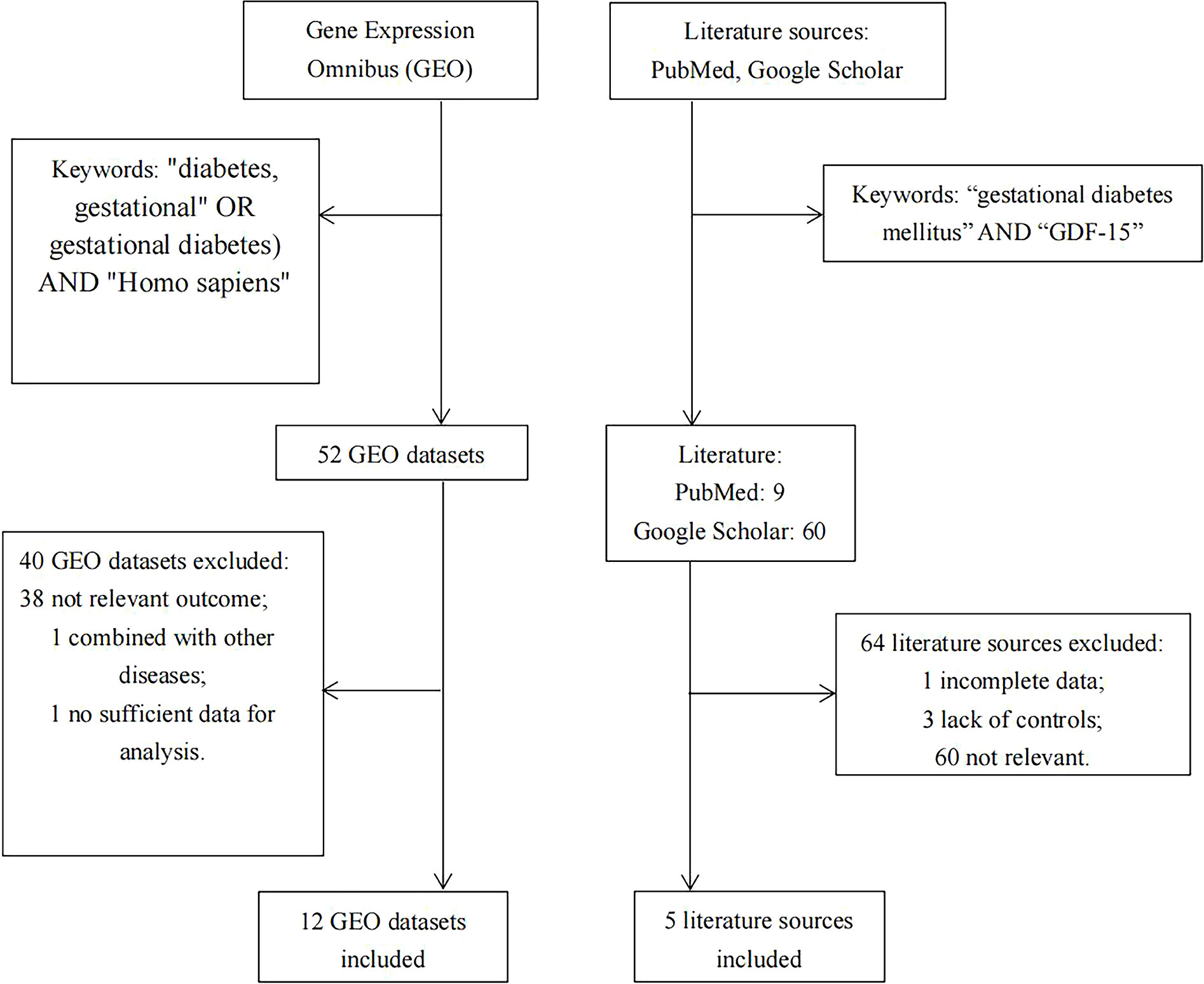
Relevant databases were searched in the Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) for the meta-analysis up to Oct 27, 2022, using the following subject terms (“diabetes, gestational” OR “gestational diabetes”) AND “Homo sapiens”. Then, suitable literatures were manually searched in PubMed and Google Scholar, using the keywords of “gestational diabetes mellitus” AND “GDF-15”. The detailed search strategy was illustrated in Figure 1.
Inclusion and exclusion criteria
Studies were considered eligible if they met the following criteria (1): the expression levels of GDF-15 were compared between GDM patients and non-GDM pregnant women; (2) the mean and standard deviation (SD) of GDF-15 should be extracted or calculated; (3) only human samples could be included; (4) because little evidence was reported for early pregnancy, thus only data from the middle and late pregnancy were included if GDF-15 was evaluated at different gestational weeks in one study. Data collected after delivery was categorized into the late pregnancy group. Exclusion criteria were as follows: (1) patients enrolled in the study have developed diabetes before pregnancy; (2) studies were performed in animals or cell lines; (3) samples have overlapped with other studies. One article was excluded because of insufficient data for analysis (26).
Quality control and data extraction
Two authors (Yicheng Lu and Yushan Zhang) independently assessed the eligible datasets according to the inclusive and exclusive criteria, and any divergence related to study inclusion was settled within the team. The following information of each study was extracted: the first author, country of origin, publication year, study subjects (disease status and sample type), the exclusion criteria of case/controls, as well as expression values, means, and SD of GDF-15.
In GSE65737, 30 pairs of GDM macrosomia and normal controls were divided into three subgroups randomly, and the umbilical cord vein blood from each subgroup was mixed and hybridized to a microarray. Therefore, the mean and SD were calculated based on the data from the pooled subgroups. In GSE203346, the dataset simultaneously collected samples of both placenta from pregnant women and umbilical cord blood, our study only included data from maternal placenta.
Statistical analysis
We first calculated the mean and SD of GDF-15 expression levels from the included eligible datasets and articles. If the included literature reported median and interquartile range, we used the compiled formula online calculator provided by Wan et al. (27) and Luo et al. (28) (https://www.math.hkbu.edu.hk/~tongt/papers/median2mean.html) to transform these values into mean and SD. Then, a meta-analysis was conducted to depict the difference in GDF-15 expression between case and control groups. Forest plots were used to express the pooled standard mean difference (SMD) and 95% confidence interval (95% CI). Considering the included studies used various tissues and platforms for mRNA quantification, we used conservative random effect model for data combination. Moreover, the potential publication bias was checked with the aid of Begg’s test and Egger’s test. Finally, sensitivity analyses were performed by excluding one study in turn to assess possible biases caused by a single study, or by adopting different inclusion criteria. All analyses were conducted with the assistance of SAS (SAS Institute Inc., NC, USA) and STATA (STATA Corporation, College Station, TX, USA).
Results
Basic characteristics of included studies
As illustrated in Figure 1, there were 5 articles (22–25, 29) and 12 GEO datasets finally met the inclusion criteria. For the 5 articles measured serum GDF-15 protein levels, 640 GDM patients and 880 controls were recruited during their middle or late pregnancy in China, India, and Turkey. Among the 12 GEO datasets including 149 GDM patients and 322 controls detecting GDF-15 mRNA, the vast majority were conducted at delivery; in contrast, there was only one relevant dataset (GSE154377) with 7 GDM patients and 9 controls who were recruited in their middle pregnancy. Meanwhile, the diagnostic criteria for GDM varied in studies, 3 datasets (including GSE65737, GSE103552 and GSE203346) and 1 article (23) used criteria from the International Association of Diabetes and Pregnancy Study Groups, 1 dataset (GSE154377) and 1 article (29) followed the American College of Obstetricians and Gynecologists, 3 articles (22, 24, 25) referred to the World Health Organization, while the remained studies (including GSE49524, GSE70493, GSE87295, GSE51546, GSE128381, GSE150621, GSE154414 and GSE194119) did not mention the diagnostic criteria. What’s more, 5 GEO datasets (including GSE70493, GSE65737, GSE128381, GSE154377 and GSE203346) and 4 articles (22, 24, 25, 29) excluded preeclampsia or other diseases that may influence GDF-15 expression during sample selection. The detailed information on exclusion criteria of each study was shown in Supplementary Table 1.
Meta-analysis of GDF-15 mRNA expression in GDM patients and controls
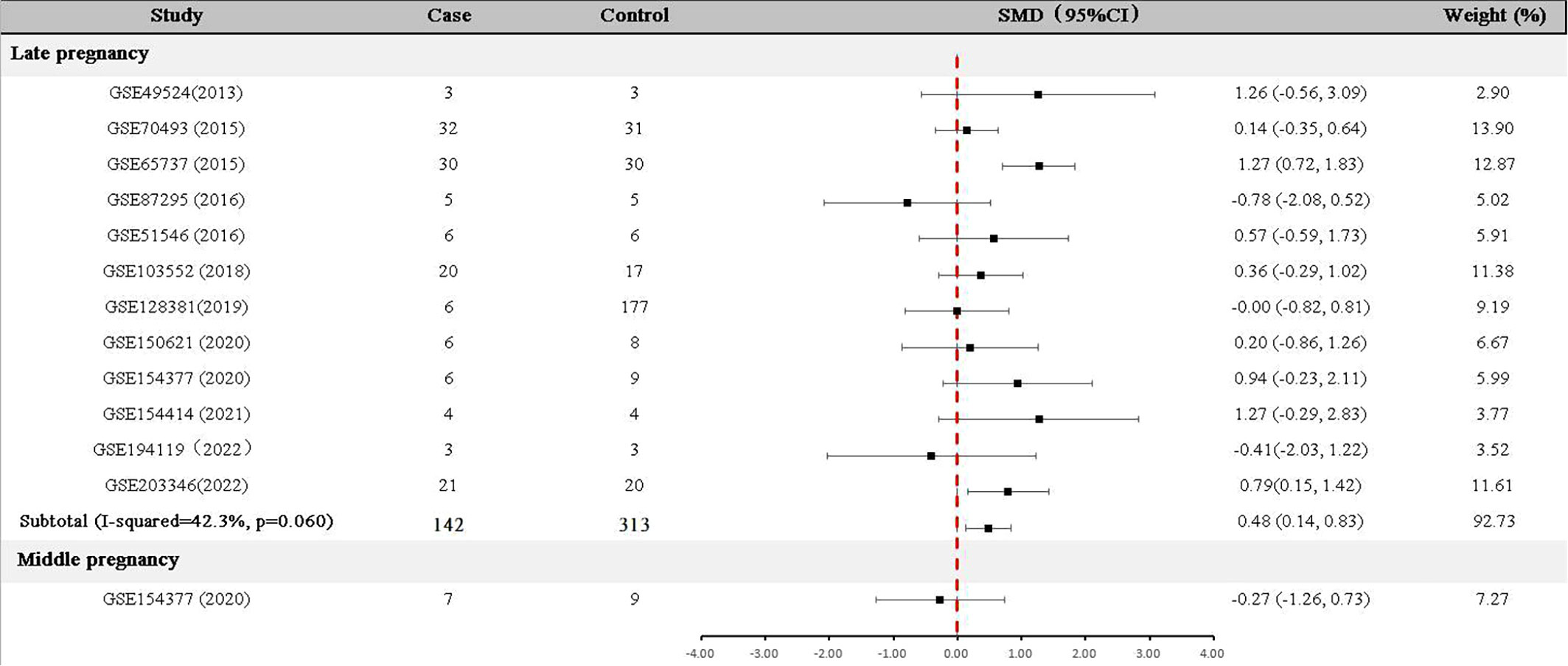
For data collected in the late pregnancy, 12 relevant datasets (GSE49524, GSE70493, GSE65737, GSE87295, GSE51546, GSE103552, GSE128381, GSE150621 GSE154377, GSE154414, GSE194119 and GSE203346) with 142 GDM patients and 313 controls were available (Table 1). As shown in Figure 2, the expression of GDF-15 mRNA in the late pregnancy was significantly higher in GDM patients compared with that in controls (SMD=0.48, 95% CI=0.14-0.83). There was only one relevant dataset (GSE154377) that was conducted in the middle pregnancy, reporting that GDF-15 mRNA was not statistically different between controls and GDM (SMD=-0.27, 95% CI=-1.26-0.73).
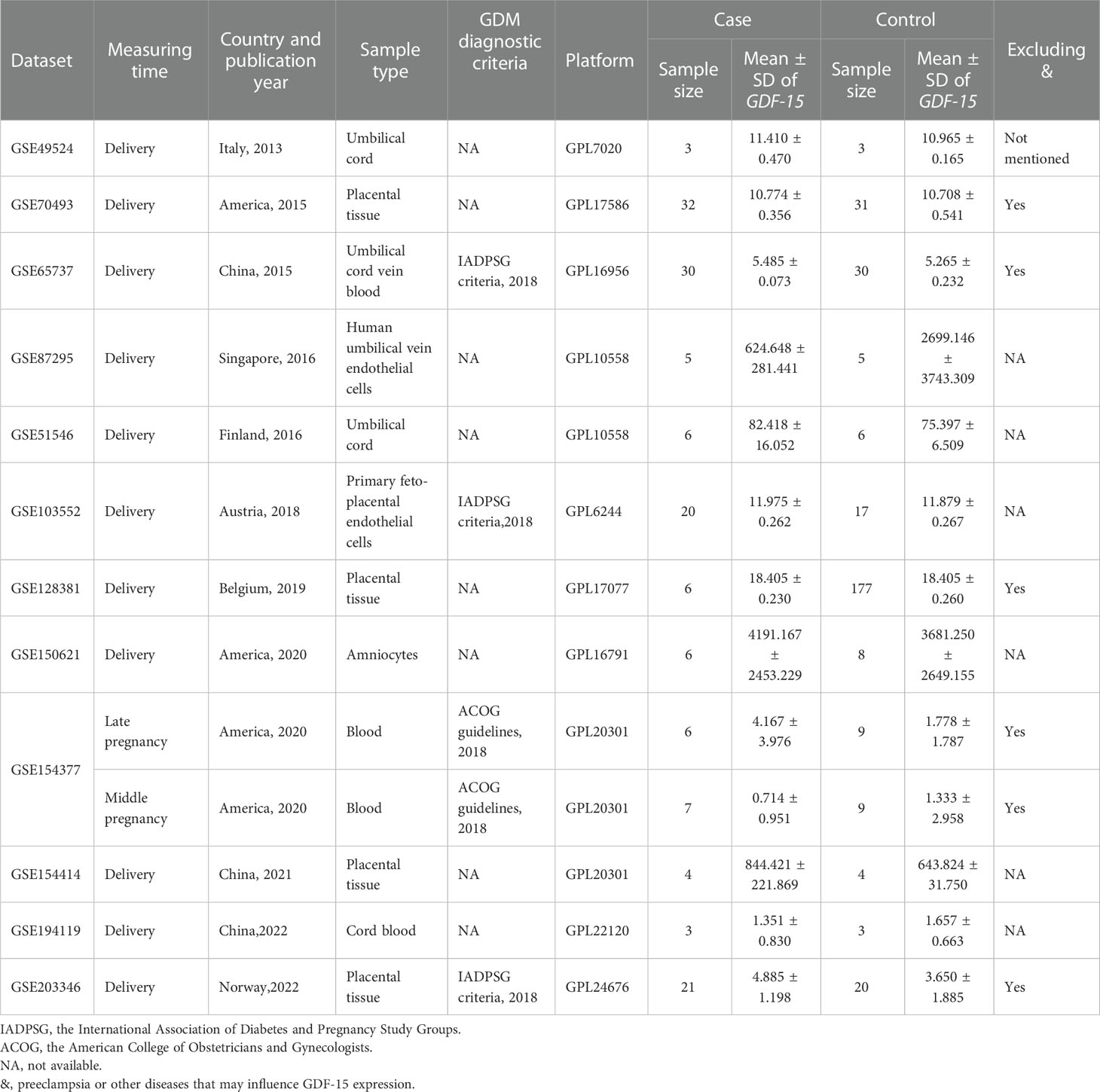
Table 1 Characteristics of GDF-15 mRNA expression profiling datasets included in the current meta-analysis between GDM and controls.
Meta-analysis of GDF-15 protein expression in GDM patients and controls
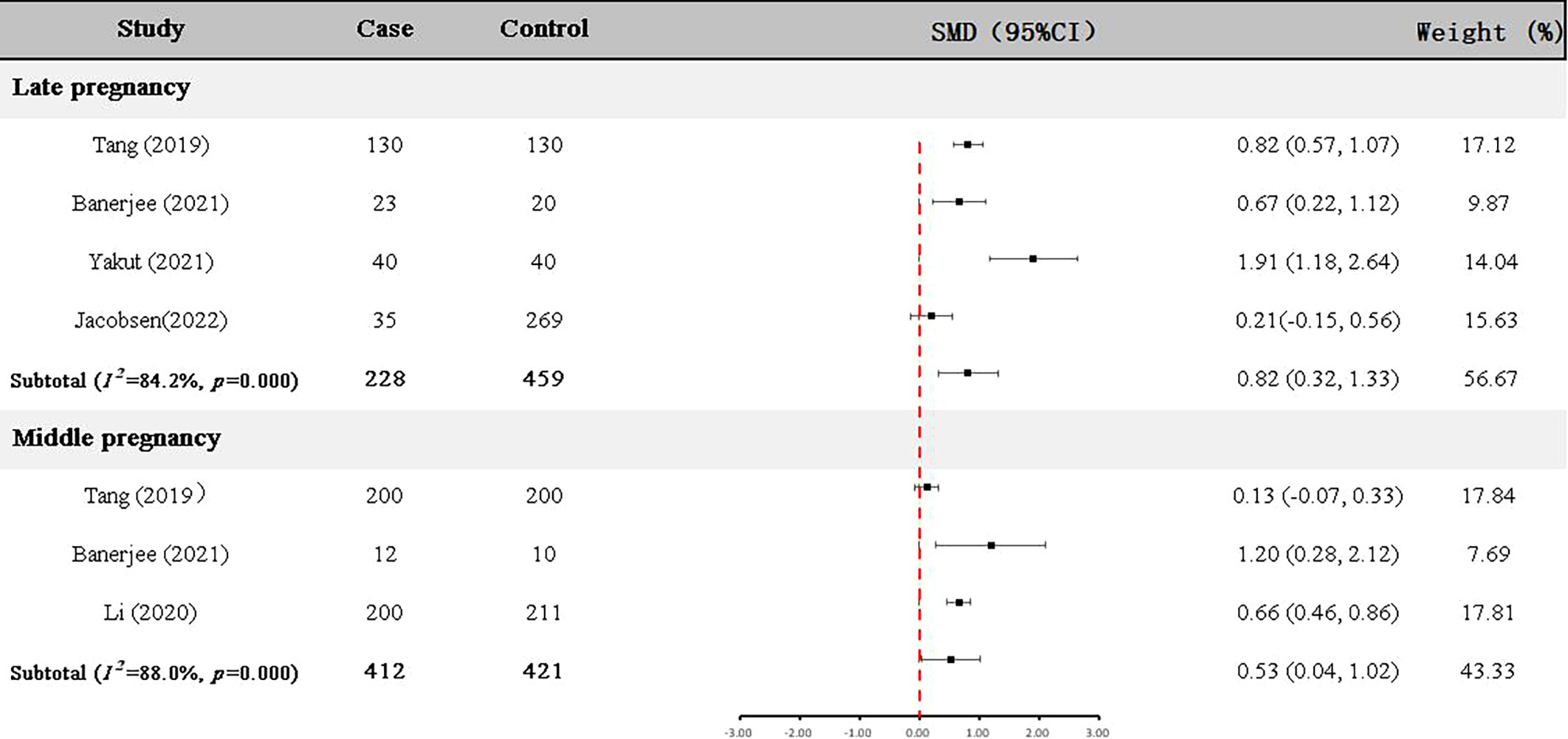
Four articles (22, 23, 25, 29) with 228 GDM patients and 459 controls examined GDF-15 protein in the late pregnancy (Table 2). As shown in Figure 3, the expression level of GDF-15 protein in GDM patients was statistically higher than that in controls (SMD=0.82, 95% CI=0.32-1.33).
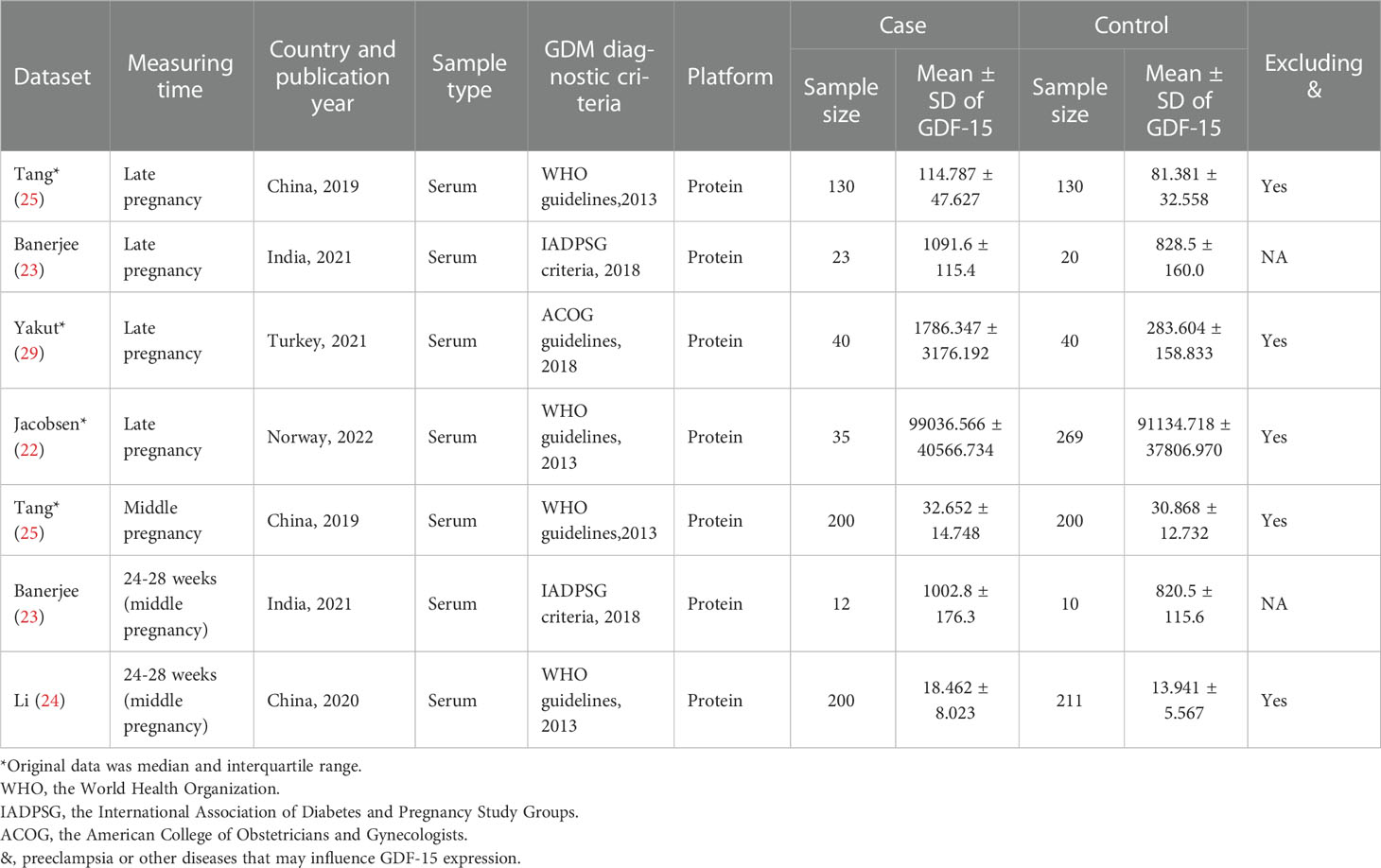
Table 2 Characteristics of GDF-15 protein expression profiling datasets included in the current meta-analysis between GDM and controls.
In addition, GDF-15 protein of 412 GDM patients and 421 controls were assessed in the middle pregnancy in three articles (23–25) (Table 2). As shown in Figure 3, the expression level of GDF-15 protein was slightly elevated in GDM patients compared with controls (SMD=0.53, 95% CI=0.04-1.02).
Meanwhile, we tried to calculated the reference range by using random effect model (inverse variance method) (30). Representing as the 95%CI of the averaged level among controls, the reference range of circulating GDF-15 in late pregnancy was 1913.73-3396.96 pg/ml, corresponding reference range in middle pregnancy was 75.64-126.54 pg/ml.
Publication bias and sensitivity analyses
Overall, Begg’s test and Egger’s test consistently indicated that there was no publication bias for the mentioned combinations above (all P values were above 0.05, shown in Supplementary Figures S1A, S1B).
A sensitivity analysis was performed by excluding one study in turn to assess possible biases caused by a single study, the results were basically stable (Supplementary Figures S2A, S2B).
We then explored whether the results were changed if we restricted to studies that excluded preeclampsia or other diseases that may influence GDF-15 expression during their sample selection. In the 5 GEO datasets (including GSE70493, GSE65737, GSE128381, GSE154377 and GSE203346), it was found that the expression level of GDF-15 mRNA in late pregnancy was significantly higher in 95 GDM patients compared with 267 non-GDM pregnant women, with SMD (95% CI) of 0.61(0.09,1.13) (Supplementary Figure S3). In three articles enrolled 205 GDM patients and 439 controls in late pregnancy (22, 25, 29), the expression level of GDF-15 protein was significantly elevated in GDM patients compared with controls (SMD=0.57, 95%CI=0.18-0.96) (Supplementary Figure S4). However, in the two articles containing 400 GDM patients and 411 controls (24, 25), it was found that the difference of the expression levels of GDF-15 protein in the middle pregancy was not significant between GDM patients and non-GDM pregnant women, with SMD and 95%CI of 0.39 (-0.12, 0.91) (Supplementary Figure S4).
Discussion
By combining expression data of mRNA and protein, we found that GDF-15 was upregulated in GDM patients in different tissues compared with non-GDM pregnant women, indicating that GDF-15 may be used as a biomarker of GDM. It should be noted that one study, which was excluded due to incomplete data for meta-analysis, reported consistent results with ours (26). Similarly, GDF-15 was found to be associated with another common gestation complication [preeclampsia (31)].
We hypothesized that GDF-15 may be compensatively upregulated in GDM, similar to the results reported for other disorders of glucose metabolism (16, 32–34). On one hand, as we mentioned before, GDF-15 appears to maintain systemic energy homeostasis. In pregnant women, GDF-15 was positively related with nausea and vomiting (35), a common gestational condition that may cause low gestational weight gain. Consistently, studies observed that GDF-15 was inversely correlated with maternal BMI and gestational weight gain during pregnancy (36, 37), which are important risk factors of GDM (38). On the other hand, there was evidence that GDF-15 exerted anti-inflammatory role through inhibiting the activation of macrophages (39), while the role of immune activation and inflammation in the pathogenesis of GDM has widely accepted (40). Therefore, GDF-15 may be induced in response to the altered energy metabolism and increased inflammation of GDM.
GDF-15 exerted protective effect in the disorders of glucose metabolism in preclinical studies but possessed paradoxically positive association with such disease in epidemiological surveys. Similar paradoxical results of GDF-15 were also observed in other disease settings. For example, epidemiological studies have demonstrated that higher GDF-15 levels are unfavorably associated with CVD progression and prognosis (41). However, animal studies suggested that GDF-15 was probably a cardioprotective factor (42). For another example, GDF-15 levels are found to be correlated with an increased risk of chronic kidney disease progression (43) or albuminuria in patients with T2DM (44), while animal study suggested that GDF-15 could be reno-protective (45). It also should be mentioned that mendelian randomization studies revealed that there was a null association between GDF-15 and the risk of CVD (46) and T2DM (47), suggesting that GDF-15 was only a biomarker for related diseases instead of a causal factor. Therefore, our results support that GDF-15 could serve as a biomarker of GDM. Nevertheless, whether GDF-15 plays a causal role in the pathogenesis of GDM or is just a bystander, requires further investigation.
Several limitations in our study should be noted. Firstly, it is reported that the level of serum GDF-15 gradually increased during the progression of gestation (48), and the association between GDF-15 and GDM may differ depending on the trimester of pregnancy (25). However, only three articles (23–25) and one study (GSE154377) with a total of 419 GDM patients and 430 controls assessed GDF-15 in the middle pregnancy. Therefore, further studies aimed for the early and middle pregnancy should be encouraged. Secondly, the combined analyses of the current study were based on mean and SD. But three original literatures (22, 25, 29) only reported median, interquartile range or range, the conversion of which to mean and SD may cause some bias. Thirdly, the included studies used different diagnostic criteria for GDM and collected different maternal or fetal tissues. Therefore, we used a conservative random effect model for the combination. Fourthly, tissues collected at delivery was categorized into the late pregnancy group, which may cause some bias. Lastly, as a feature of meta-analysis, our study could not explore the mechanism of GDF-15 in GDM. Further studies are warranted to figure this out.
Conclusions
Our results suggested that the expression levels of GDF-15 were significantly higher in GDM patients than in non-GDM pregnant women, especially in the late pregnancy, indicating that GDF-15 may act as a biomarker for GDM.
Author contributions
Y-CL and S-LL searched data and draft the manuscript; JY-Y and QP-M designed the study and revised the manuscript; all other authors contributed in the data extraction or manuscript revision.
Funding
Q-PM is supported by the Healthcare Applied Basic Research Project of Science and Technology Bureau of Taicang (grant number: TC2021JCYL03), the 28th batch of Science and Technology Development plan (Medical and Health Technology Innovation) of Suzhou (grant number:SKYD2022061), and 2020 Science and the Technology Development Plan of Suzhou (Livelihood Science and Technology - Basic Research on Medical and Health Application, grant number:SYSD2020024). J-YY is currently receiving grant from the National Natural Science Fund of China (grant number: 82273635) and is supported by a Project Funded by Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). For the remaining authors none were declared.
Acknowledgments
We are grateful to Cheng Hu and Sudipta Banerjee for providing original data of GDF-15 in the middle pregnancy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1084896/full#supplementary-material
Supplementary Figure 1 | Begg’s funnel plot for the assessment of potential publication bias in the samples (A: mRNA; B: protein).
Supplementary Figure 2 | Sensitivity test of GDF-15 expression between GDM patients and non-GDM pregnant women (A: mRNA; B: protein).
Supplementary Figure 3 | Difference of GDF-15 mRNA expression between GDM patients and non-GDM pregnant women (excluding preeclampsia or other diseases that may influence GDF-15 expression).
Supplementary Figure 4 | Difference of GDF-15 protein expression between GDM patients and non-GDM pregnant women (excluding preeclampsia or other diseases that may influence GDF-15 expression).
Abbreviations
GDM, gestational diabetes mellitus; GDF-15, growth differentiation factor 15; SMD, standard mean difference; 95% CI, 95% confidence interval; BMI, body mass index;LGA, large for gestational age; T2DM, type 2 diabetes mellitus; MIC-1, macrophage inhibiting cytokine-1; TGF-β, transforming growth factor-β SD, standard deviation; GEO, Gene Expression Omnibus; WHO, the World Health Organization; IADPSG, the International Association of Diabetes and Pregnancy Study Groups; ACOG, the American College of Obstetricians and Gynecologists.
References
1. Szmuilowicz ED, Josefson JL, Metzger BE. Gestational diabetes mellitus. Endocrinol Metab Clin North Am (2019) 48(3):479–93. doi: 10.1016/j.ecl.2019.05.001
2. Gao C, Sun X, Lu L, Liu F, Yuan J. Prevalence of gestational diabetes mellitus in mainland China: A systematic review and meta-analysis. J Diabetes Investig (2019) 10(1):154–62. doi: 10.1111/jdi.12854
3. Johns EC, Denison FC, Norman JE, Reynolds RM. Gestational diabetes mellitus: Mechanisms, treatment, and complications. Trends Endocrinol Metab (2018) 29(11):743–54. doi: 10.1016/j.tem.2018.09.004
4. Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: The consequences of not treating. Am J Obstet Gynecol (2005) 192(4):989–97. doi: 10.1016/j.ajog.2004.11.039
5. Lowe WL Jr., Scholtens DM, Lowe LP, Kuang A, Nodzenski M, Talbot O, et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA (2018) 320(10):1005–16. doi: 10.1001/jama.2018.11628
6. Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol (2012) 8(11):639–49. doi: 10.1038/nrendo.2012.96
7. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia (2019) 62(6):905–14. doi: 10.1007/s00125-019-4840-2
8. Breit SN, Johnen H, Cook AD, Tsai VW, Mohammad MG, Kuffner T, et al. The TGF-beta superfamily cytokine, MIC-1/GDF15: A pleotrophic cytokine with roles in inflammation, cancer and metabolism. Growth Factors (2011) 29(5):187–95. doi: 10.3109/08977194.2011.607137
9. Luan HH, Wang A, Hilliard BK, Carvalho F, Rosen CE, Ahasic AM, et al. GDF15 is an inflammation-induced central mediator of tissue tolerance. Cell (2019) 178(5):1231–44. doi: 10.1016/j.cell.2019.07.033
10. Desmedt S, Desmedt V, De Vos L, Delanghe JR, Speeckaert R, Speeckaert MM. Growth differentiation factor 15: A novel biomarker with high clinical potential. Crit Rev Clin Lab Sci (2019) 56(5):333–50. doi: 10.1080/10408363.2019.1615034
11. Borner T, Shaulson ED, Ghidewon MY, Barnett AB, Horn CC, Doyle RP, et al. GDF15 induces anorexia through nausea and emesis. Cell Metab (2020) 31(2):351–62.e5. doi: 10.1016/j.cmet.2019.12.004
12. Chrysovergis K, Wang X, Kosak J, Lee SH, Kim JS, Foley JF, et al. NAG-1/GDF-15 prevents obesity by increasing thermogenesis, lipolysis and oxidative metabolism. Int J Obes (Lond) (2014) 38(12):1555–64. doi: 10.1038/ijo.2014.27
13. Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med (2017) 23(10):1215–9. doi: 10.1038/nm.4393
14. Coll AP, Chen M, Taskar P, Rimmington D, Patel S, Tadross JA, et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature (2020) 578(7795):444–8. doi: 10.1038/s41586-019-1911-y
15. Wang D, Day EA, Townsend LK, Djordjevic D, Jørgensen SB, Steinberg GR. GDF15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat Rev Endocrinol (2021) 17(10):592–607. doi: 10.1038/s41574-021-00529-7
16. Lu J, Zhang Y, Dong X, Lu J, Zhang C, Liu J, et al. Association between MIC-1 and type 2 diabetes: A combined analysis. Dis Markers (2019) 2019:7284691. doi: 10.1155/2019/7284691
17. Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia (2003) 46(1):3–19. doi: 10.1007/s00125-002-1009-0
18. Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci (2018) 19(11):3342. doi: 10.3390/ijms19113342
19. Vrachnis N, Belitsos P, Sifakis S, Dafopoulos K, Siristatidis C, Pappa KI, et al. Role of adipokines and other inflammatory mediators in gestational diabetes mellitus and previous gestational diabetes mellitus. Int J Endocrinol (2012) 2012:549748. doi: 10.1155/2012/549748
20. Halim M, Halim A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab Syndr (2019) 13(2):1165–72. doi: 10.1016/j.dsx.2019.01.040
21. Sugulle M, Dechend R, Herse F, Weedon-Fekjaer MS, Johnsen GM, Brosnihan KB, et al. Circulating and placental growth-differentiation factor 15 in preeclampsia and in pregnancy complicated by diabetes mellitus. Hypertension (2009) 54(1):106–12. doi: 10.1161/HYPERTENSIONAHA.109.130583
22. Jacobsen DP, Roysland R, Strand H, Moe K, Sugulle M, Omland T, et al. Cardiovascular biomarkers in pregnancy with diabetes and associations to glucose control. Acta diabetologica (2022) 59(9):1229–36. doi: 10.1007/s00592-022-01916-w
23. Banerjee S, Bhattacharjee R, Sur A, Adhikary P, Chowdhury S. A study of serum growth differentiation factor 15 in Indian women with and without gestational diabetes mellitus in the third trimester of pregnancy and its association with pro-inflammatory markers and glucose metabolism. Diabetol Int (2021) 12(3):254–9. doi: 10.1007/s13340-020-00478-y
24. Li E, Chen P, Lu J, Dai J, Yi J, Zhang S, et al. Serum growth differentiation factor 15 is closely associated with metabolic abnormalities in Chinese pregnant women. J Diabetes Investig (2020) 12(08):1501–7. doi: 10.1111/jdi.13488
25. Tang M, Luo M, Lu W, Wang S, Zhang R, Liang W, et al. Serum growth differentiation factor 15 is associated with glucose metabolism in the third trimester in Chinese pregnant women. Diabetes Res Clin Pract (2019) 156:107823. doi: 10.1016/j.diabres.2019.107823
26. Yarsilikal Guleroglu F, Selvi E, Turan Bakirci I, Bafali O, Argun Atalmis H, Yasti Dayan M, et al. Clinical value of serum BMP-4, BMP-2, GDF-15, MMP-9, GP39 levels in pregnant women with obesity and the related comorbidities diabetes mellitus and gestational hypertension. Z Geburtshilfe Neonatol (2022). doi: 10.1055/a-1937-1155
27. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol (2014) 14:135. doi: 10.1186/1471-2288-14-135
28. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res (2018) 27(6):1785–805. doi: 10.1177/0962280216669183
29. Yakut K, Öcal DF, Öztürk FH, Öztürk M, Oğuz Y, Sınacı S, et al. Is GDF-15 level associated with gestational diabetes mellitus and adverse perinatal outcomes? Taiwan J Obstet Gynecol (2021) 60(2):221–4. doi: 10.1016/j.tjog.2020.12.004
30. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synthesis Methods (2010) 1(2):97–111. doi: 10.1002/jrsm.12
31. Wang L, Yang Q. Circulating growth differentiation factor 15 and preeclampsia: A meta-analysis. Horm Metab Res (2022). doi: 10.1055/a-1956-2961
32. Carstensen M, Herder C, Brunner EJ, Strassburger K, Tabak AG, Roden M, et al. Macrophage inhibitory cytokine-1 is increased in individuals before type 2 diabetes diagnosis but is not an independent predictor of type 2 diabetes: The Whitehall II study. Eur J Endocrinol (2010) 162(5):913–7. doi: 10.1530/EJE-09-1066
33. Hong JH, Chung HK, Park HY, Joung KH, Lee JH, Jung JG, et al. GDF15 is a novel biomarker for impaired fasting glucose. Diabetes Metab J (2014) 38(6):472–9. doi: 10.4093/dmj.2014.38.6.472
34. Shin MY, Kim JM, Kang YE, Kim MK, Joung KH, Lee JH, et al. Association between growth differentiation factor 15 (GDF15) and cardiovascular risk in patients with newly diagnosed type 2 diabetes mellitus. J Korean Med Sci (2016) 31(9):1413–8. doi: 10.3346/jkms.2016.31.9.1413
35. Fejzo MS, Trovik J, Grooten IJ, Sridharan K, Roseboom TJ, Vikanes A, et al. Nausea and vomiting of pregnancy and hyperemesis gravidarum. Nat Rev Dis Primers (2019) 5(1):62. doi: 10.1038/s41572-019-0110-3
36. Wang P, Ma W, Zhou Y, Zhao Y, Shi H, Yang Q, et al. Circulating metal concentrations, inflammatory cytokines and gestational weight gain: Shanghai MCPC cohort. Ecotoxicol Environ Saf (2020) 199:110697. doi: 10.1016/j.ecoenv.2020.110697
37. Andersson-Hall U, Joelsson L, Svedin P, Mallard C, Holmang A. Growth-differentiation-factor 15 levels in obese and healthy pregnancies: Relation to insulin resistance and insulin secretory function. Clin Endocrinol (Oxf) (2021) 95(1):92–100. doi: 10.1111/cen.14433
38. Li N, Liu E, Guo J, Pan L, Li B, Wang P, et al. Maternal prepregnancy body mass index and gestational weight gain on pregnancy outcomes. PLoS One (2013) 8(12):e82310. doi: 10.1371/journal.pone.0082310
39. Breit SN, Johnen H, Cook AD, Tsai VW, Mohammad MG, Kuffner T, et al. The TGF-β superfamily cytokine, MIC-1/GDF15: A pleotrophic cytokine with roles in inflammation, cancer and metabolism. Growth Factor (2011) 29(5):187–95. doi: 10.3109/08977194.2011.607137
40. Lekva T, Norwitz ER, Aukrust P, Ueland T. Impact of systemic inflammation on the progression of gestational diabetes mellitus. Curr Diabetes Rep (2016) 16(4):26. doi: 10.1007/s11892-016-0715-9
41. Eddy AC, Trask AJ. Growth differentiation factor-15 and its role in diabetes and cardiovascular disease. Cytokine Growth Factor Rev (2021) 57:11–8. doi: 10.1016/j.cytogfr.2020.11.002
42. Emmerson PJ, Duffin KL, Chintharlapalli S, Wu X. GDF15 and growth control. Front Physiol (2018) 9:1712. doi: 10.3389/fphys.2018.01712
43. Nair V, Robinson-Cohen C, Smith MR, Bellovich KA, Bhat ZY, Bobadilla M, et al. Growth differentiation factor-15 and risk of CKD progression. J Am Soc Nephrol (2017) 28(7):2233–40. doi: 10.1681/ASN.2016080919
44. Hellemons ME, Mazagova M, Gansevoort RT, Henning RH, de Zeeuw D, Bakker SJ, et al. Growth-differentiation factor 15 predicts worsening of albuminuria in patients with type 2 diabetes. Diabetes Care (2012) 35(11):2340–6. doi: 10.2337/dc12-0180
45. Kim YI, Shin HW, Chun YS, Park JW. CST3 and GDF15 ameliorate renal fibrosis by inhibiting fibroblast growth and activation. Biochem Biophys Res Commun (2018) 500(2):288–95. doi: 10.1016/j.bbrc.2018.04.061
46. Cheung CL, Tan KCB, Au PCM, Li GHY, Cheung BMY. Evaluation of GDF15 as a therapeutic target of cardiometabolic diseases in human: A mendelian randomization study. EBioMedicine (2019) 41:85–90. doi: 10.1016/j.ebiom.2019.02.021
47. Au Yeung SL, Luo S, Schooling CM. The impact of GDF-15, a biomarker for metformin, on the risk of coronary artery disease, breast and colorectal cancer, and type 2 diabetes and metabolic traits: a mendelian randomisation study. Diabetologia (2019) 62(9):1638–46. doi: 10.1007/s00125-019-4913-2
48. Moore AG, Brown DA, Fairlie WD, Bauskin AR, Brown PK, Munier ML, et al. The transforming growth factor-ss superfamily cytokine macrophage inhibitory cytokine-1 is present in high concentrations in the serum of pregnant women. J Clin Endocrinol Metab (2000) 85(12):4781–8. doi: 10.1210/jcem.85.12.7007
Keywords: gestational diabetes mellitus, growth differentiation factor 15, macrophage inhibiting cytokine-1, meta-analysis, diabetes
Citation: Lu Y-C, Liu S-L, Zhang Y-S, Liang F, Zhu X-Y, Xiao Y, Wang J, Ding C, Banerjee S, Yin J-Y and Ma Q-P (2023) Association between growth differentiation factor 15 levels and gestational diabetes mellitus: A combined analysis. Front. Endocrinol. 14:1084896. doi: 10.3389/fendo.2023.1084896
Received: 31 October 2022; Accepted: 03 January 2023;
Published: 20 January 2023.
Edited by:
Sandeep Mathur, Sawai ManSingh Medical College, IndiaReviewed by:
Amitabh Sur, Peerless Hospital, IndiaFangchao Liu, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2023 Lu, Liu, Zhang, Liang, Zhu, Xiao, Wang, Ding, Banerjee, Yin and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie-Yun Yin, jyyin@suda.edu.cn; Qiu-Ping Ma, 343587729@qq.com
†These authors have contributed equally to this work
 Yi-Cheng Lu
Yi-Cheng Lu Song-Liang Liu1†
Song-Liang Liu1† Xiao-Yan Zhu
Xiao-Yan Zhu Yue Xiao
Yue Xiao Sudipta Banerjee
Sudipta Banerjee Jie-Yun Yin
Jie-Yun Yin