- 1National Center for International Research on Animal Genetics, Breeding and Reproduction (NCIRAGBR), College of Animal Science and Technology, Huazhong Agricultural University, Wuhan, China
- 2Faculty of Veterinary and Animal Sciences, Muhammad Nawaz Shareef University of Agriculture, Multan, Pakistan
- 3College of Veterinary Medicine, Northwest Agricultural and Forestry University, Yangling, China
- 4Hubei Engineering Research Center in Buffalo Breeding and Products, Wuhan, China
Introduction: Inhibin DNA vaccine has already been proven to improve the fertility of animals. This study aimed to investigate the effects of a novel Anti-Müllerian hormone (AMH)-Inhibin (INH)-RF-amide-related peptides (RFRP) DNA vaccine on immune response and reproductive performance in buffalo.
Methods: A total of 84 buffaloes were randomly divided into four groups and nasally immunized twice a day with 10 ml of either AMH-INH-RFRP DNA vaccines (3 × 1010 CFU/ml in group T1, 3 × 109 CFU/ml in group T2, and 3 × 108 CFU/ml in group T3) or PBS (as a control) for 3 days, respectively. All animals received a booster dose at an interval of 14 days.
Results: ELISA assay revealed that primary and booster immunization significantly increased the anti-AMH, anti-INH, and anti-RFRP antibody titers in the T2 group compared with that in the T3 group. After the primary immunization, the antibody positive rate was significantly higher in the T2 group than that in the T3 group. In addition, ELISA results indicated that concentrations of E2, IFN-γ, and IL-4 were significantly higher in the antibody-positive (P) group compared to the antibody-negative (N) group. In contrast, there was no significant difference in the concentrations of P4 between the P and N groups. Ultrasonography results revealed a highly significant increase of 2.02 mm in the diameter of ovulatory follicles in the P group compared to the N group. In parallel, growth speed of dominant follicles was significantly higher in the P group than that in the N group (1.33 ± 1.30 vs 1.13 ± 0.12). Furthermore, compared to N group, the rates of oestrus, ovulation, and conception were also significantly higher in the P group.
Conclusion: The novel AMH-INH-RFRP DNA vaccine improves the proportion of oestrus, ovulation, and conception in buffalo by promoting the production of E2 and the growth of follicles.
1 Introduction
Buffalo has important economic and biological significance for tropical and subtropical regions due to characteristics of good adaptability and stress resistance (1). Compared with cattle, buffaloes have many known reproductive disorders including poor sign of estrus, longer postpartum quiescence, delayed puberty, and seasonal breeder, resulting in the reduction of buffalo fertility and the buffalo industry’s developmental limits (2–4). Biotechnologies such as estrus synchronization and fixed-timed artificial insemination (FTAI) have been well known for improving the fertility of buffalo (5). Nevertheless, some researchers reported that the conception rate was approximately 30% of buffaloes using Ovsynch-TAI protocol (6, 7), suggesting that the reproductive effect induced by Ovsynch-TAI protocol is not always satisfactory. The main reason might be due to differences in the age and diameter of the preovulatory follicles during synchronized ovulation with the second GnRH treatment, resulting in a different level of maturation of the ovulatory follicles (8).
Inhibin (INH) has long been thought to be a hormone that inhibits follicle-stimulating hormone (FSH) secretion and regulates ovarian function through pituitary-gonadal negative feedback (9, 10). Notably, the recombinant inhibin DNA vaccine delivered by Salmonella choleraesuis has already been proven to improve the fertility of mice, rats, and sheep (11–13). Meanwhile, Liu Q et al. reported that the proportion of estrous and ovulation could be improved by immunization with recombinant inhibin DNA vaccine, eventually leading to an increase in the conception rate of buffaloes (14, 15). A novel hypothalamic neuropeptide called gonadotropin-inhibitory hormone (GnIH) is known to inhibit FSH and luteinizing hormone (LH) from the anterior pituitary since its detection in quail. In mammals, it is also called RF-amide-related peptides (RFRP), which have molecules similar to GnIH (16, 17). In addition, the applying a recombinant DNA vaccine harboring the INH and RFRP genes has revealed better immunogenicity and more litter size of mice (18). Anti-Müllerian hormone (AMH), a member of the transforming growth factor-beta (TGF-β) super-family, is responsible for the regression of the Müllerian duct during sexual differentiation in males (19). In females, AMH has been shown to suppress the primordial follicle initiation, and inhibit FSH-dependent increase in aromatase activity, LH receptor expression, and COC in vitro maturation (20–22). Moreover, it inhibited the growth of mouse secondary follicles (23). Yu X et al. reported that co-immunization with AMH and INH plasmids could increase the litter size of mice (24).
Based on the above studies, it can be concluded that INH, INH-RFRP, and INH-AMH DNA vaccines exhibit promising effects on enhancing animals’ reproductive performance. Here, the current study aimed to investigate the immune response of a novel AMH-INH-RFRP DNA vaccine harboring AMH, INH, and RFRP encoding genes, and evaluate the reproductive effect of this novel vaccine on oestrus, ovulation, and conception in buffalo.
2 Materials and methods
2.1 Preparation of AMH-INH-RFRP DNA vaccine
AMH-INH-RFRP DNA vaccine has previously been constructed and preserved in our laboratory. Briefly, the recombinant plasmid AMH-INH-RFRP contained three fused fragments including S/AMH, S/INH, and S/RFRP, which were linked with 2A peptide (Figure 1). Subsequently, AMH-INH-RFRP plasmid was transformed into attenuated salmonella choleraesuis (C500) with asd and crp double deletion, producing AMH-INH-RFRP DNA vaccine delivered by C500 strain.
AMH-INH-RFRP DNA vaccine was added (1: 100) to LB liquid medium (200 ml) and cultured in 37°C shaker at 220 rpm/min for 16 h. After harvesting through 4°C centrifugation at 6000 rpm for 10 min, the vaccine was re-suspended by phosphate-buffered saline (PBS). Before immunization, LB agar plates were used to place the bacteria in triplicate to determine the number of colony-forming units and acquire adjusted roughly 3 × 1010 CFU/ml by PBS.
2.2 Animals and immunization protocol
Experimental buffaloes (n = 84) of 3-6 years old with first to third lactations were from local buffalo farms (Hubei JinNiu Co., Ltd. Shayang, China) and enrolled in this study, which has the body condition score (BCS) between 2.5 to 3 (1-5 score) and are free from reproductive problems. Experimental animals were fed a total mixed ration (TMR) containing forages and concentrates in routine. Forages were comprised of corn silage, rice straw, peanut, and concentrates included corn (38.0%), soybean meal (16.0%), linen (6.0%), cottonseed cake (6.0%), cornmeal (17.5%), vinasse (10.0%), baking soda (0.5%) and premixed material (6.0%). The experimental animals had free access to fresh and clean water during the experimental period. A guideline followed for using animals and experimental procedures was from the Animal Care and Use Committee of Huazhong Agricultural University (HZAUBU-2019-001).
A total of 84 buffaloes was randomly divided into four groups: groups T1 (3 × 1010 CFU/ml, n = 21), T2 (3 × 109 CFU/ml, n = 21), and T3 (3 × 108 CFU/ml, n = 21) were nasally immunized twice a day with 10 ml of AMH-INH-RFRP DNA vaccine for 3 days, respectively. The control group (n = 21) was nasally immunized twice daily with 10 ml PBS for 3 days. At 2 weeks after primary immunization, all animals were boosted with the same procedures as primary immunization. After a booster immunization, buffaloes were subjected to the classic Ovsynch-TAI protocol (GPG). During the experiment, a total of 10 buffaloes did not participate in the complete GPG procedure due to buffalo injuries and management negligence. The details of the treatment procedure are presented in Figure 2. During the experiment, blood samples from the jugular vein of buffaloes were collected on days 14 and 28. The blood was then centrifuged at 3000 rpm for 10 min to separate the serum. The serum samples were stored at -80°C for further analysis.
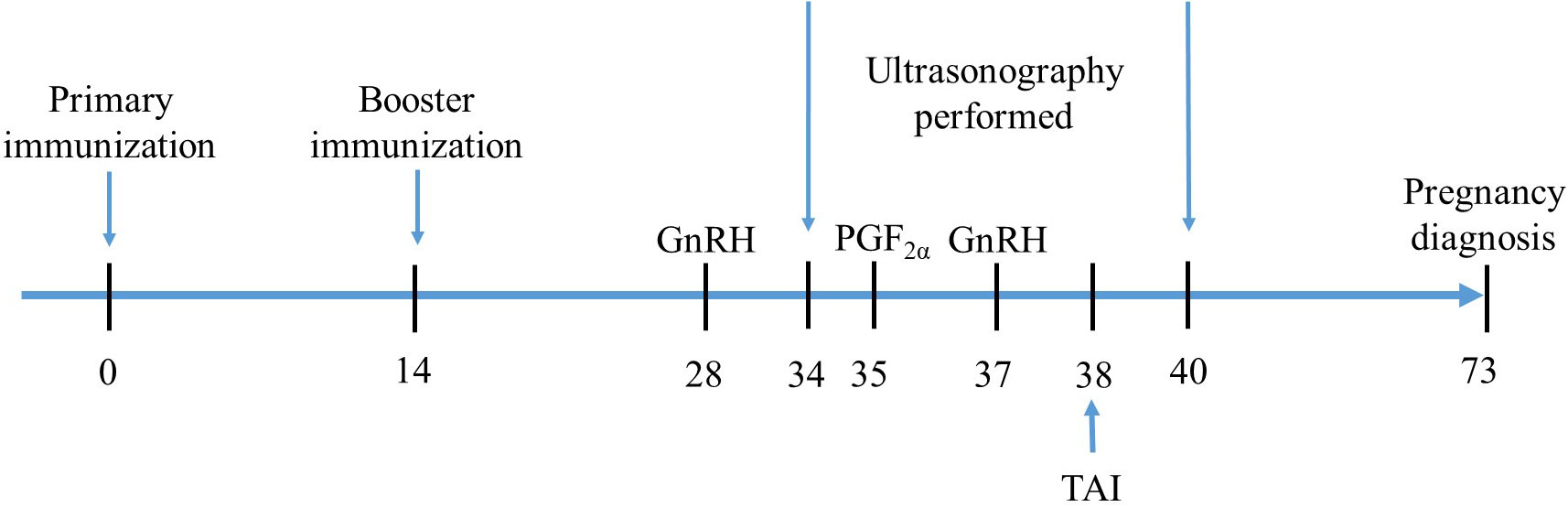
Figure 2 An outline of experimental design. Experimental buffaloes were immunized with AMH-INH-RFRP DNA vaccine on days 0 and 14, and control group were administered with PBS. All buffaloes underwent the Ovsynch-TAI program after 2 weeks of booster immunization. Ultrasonography was conducted from day 34 to 40 at 12 h interval to evaluate the folliculogenesis and ovulation. At day 73, pregnancy diagnosis was performed for all buffaloes.
2.3 Ultrasonography detection
From day 34 (a day earlier than PGF2α treatment) to day 40 (72 h post 2nd GnRH injection), all buffaloes were scanned using B-type ultrasound (Shenzhen Well.D Medical Electronics Co., Ltd. China) to evaluate follicles and ovulation at 12 h intervals. Oestrus was observed and confirmed by obvious vaginal mucous discharge twice a day (8:00 and 16:00). Ovulation was confirmed at the subsequent ultrasonographic examination by the abrupt disappearance of the previously recorded dominant follicle. At 24 h intervals, the prominent follicles were compared to determine their growth rate (mm/day). A pregnancy diagnosis was conducted on the 35th day following artificial insemination (AI) to assess the rate of conception.
2.4 Oestrous synchronization procedure
The Ovsynch-TAI program was used in this study. Briefly, all buffaloes were injected with 200 μg of GnRH analog (Sansheng Pharmaceutical Co., Ltd., Ningbo, China) on day 28. Further, after an intramuscular injection of 0.4 mg PGF2α (Sansheng Pharmaceutical Co., Ltd., Ningbo, China), the buffaloes received another shot of 200 μg GnRH on day 37. From 18 to 24 h after the second GnRH injection, TAI was performed for all buffaloes.
2.5 Detection of antibody titer
Anti-AMH, INH, and RFRP antibodies in serum were measured by indirect ELISA. Briefly, each 96-well microtiter plate was coated 100 ng/100 μl AMH, INH, and RFRP antigens, which were synthesized by Sangon-Peptide Biotech CO., Ltd. (Shanghai, China), and then incubated overnight at 4°C. After discarding the reaction reagent, 300 μl of PBS with Tween 20 (Tween 20, 1247ML100, Bioroxx, Germany) (PBST) was added into each well and washed for 5 times. Next, 200 μl of 1% bovine serum albumin (BSA, 4240GR100, Bioroxx, Germany) was added into each well for blocking and then incubated at 37°C for 90 min. After washing with PBST, serum samples (100 μl) diluted with PBS (1:25, 1:50, 1:100, 1:200, 1:400, 1:800, 1:1600, and 1:3200) were added into each well and incubated at 37°C for 1 h. Following PBST wash, goat anti-bovine IgG-HRP (1:5000; 6030-05, Southern Biotechnology Associates, Inc. USA) was added (100 μl/well) and incubated at 37°C for 1 h. After washing with PBST, 150 μl of tetramethylbenzidine (TMB, ATO00001, AtaGenix, Wuhan, China) were added into each well and incubated at 37°C for 15 min. Then 50 μl of stop solution (C1058, Solarbio Science & Technology Co., Ltd. China) were added to stop the reaction. Finally, the absorbance at 450 nm was measured by a Microplate Reader (PerkinElmer, USA). Under the same dilution factor, if the OD450 value of the experimental group was greater than the mean OD450 value in the control group + 2SD (standard deviation), it was judged as a positive value (18, 25).
2.6 Assay of reproductive hormone and cytokine concentrations
Bovine ELISA Kits (Ruixin Biological Technology Co., Ltd.; Quanzhou, China) were used to measure concentrations of IL-4 (RX1600854B), IFN-γ (RX1600863B), E2 (RXJ1600845B), and P4 (RXJ1600748B) concentrations, according to the manufacturer’s instructions. The intra- and inter-assay coefficients of variation were less than 10.0% and 15.0% for IL-4, 15.0% and 15.0% for IFN-γ, 15.0% and 15.0% for E2, and 15.0% and 15.0% for P4, respectively.
2.7 Statistical analyses
The data were analysed using the MIXED procedure of SAS 9.4 software (SAS Institute, Inc., Cary, NC, USA). The mean values were compared using Tukey’ multiple range test with a significance level of p < 0.05. Nonparametric data were analysed using the chisquared test. Variability in the data was expressed as the standard error of means (SEM), and p < 0.05 was considered statistically significant.
3 Results
3.1 Immune response of AMH-INH-RFRP DNA vaccine against buffalo
As shown in Figure 3, buffaloes immunized with AMH-INH-RFRP DNA vaccine could induce anti-AMH, anti-INH, and anti-RFRP antibodies on day 14 after primary immunization (PI) and booster immunization (BI). After primary immunization, T2 group showed remarkably higher anti-AMH and anti-INH antibody levels than T3 group (P < 0.01), and T1 and T2 groups exhibited significantly higher anti-RFRP antibody titer than T3 group (P < 0.05). After booster immunization, there was an increasing trend in anti-AMH, anti-INH, and anti-RFRP antibody titers. Anti-AMH and anti-INH antibody titers in T2 group were significantly greater than that in T3 group (P < 0.05), and anti-RFRP antibody titers were significantly greater in T1 and T2 groups compared to that in T3 group (P < 0.01).
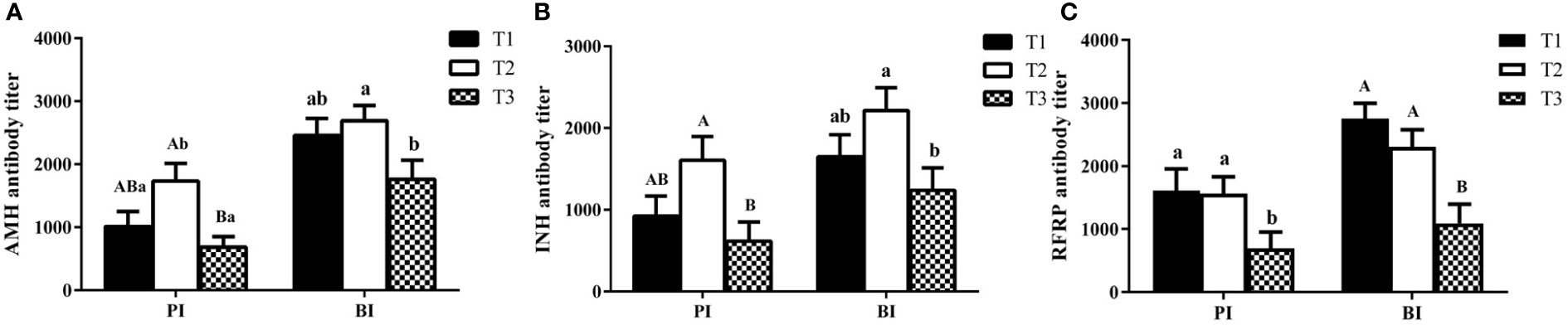
Figure 3 The titers of IgG antibody in buffaloes after immunization with various concentration of AMH-INH-RFRP DNA vaccine. Anti-AMH (A), anti-INH (B), anti-RFRP (C) were detected by ELISA. Data are presented as mean ± SEM. The bars with different letters indicate significant differences (a,bP < 0.05, A,BP < 0.01).
On the other hand, we also calculated the antibody positive rate in different treatment groups (Table 1). After primary immunization, the anti-AMH antibody positive rate in T1 and T2 groups was significantly higher than that in T3 group (85.71% and 85.71% vs 47.62%, P < 0.01). In addition, the antibody positive rates for anti-INH, and anti-RFRP in T2 group were significantly increased by 38.09% compared to T3 group (P < 0.05). After booster immunization, the antibody positive rates increased compared to primary immunization, even though there were no significant changes among different groups.
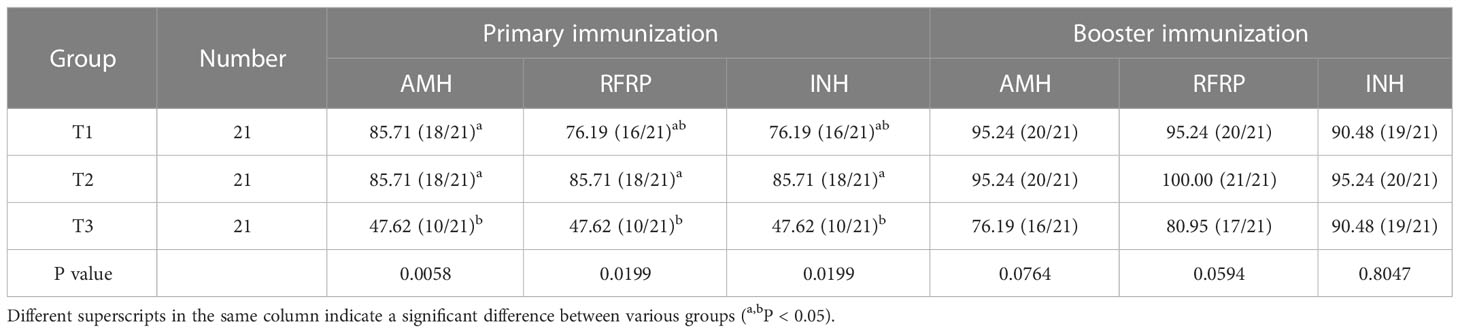
Table 1 Percentage (and proportion) of buffalo with positive antibody against AMH, RFRP and INH at various intervals after immunization of DNA vaccine.
3.2 The effect of AMH-INH-RFRP DNA vaccine on steroid hormones and cytokines in buffalo
The levels of steroid hormones (P4 and E2) and cytokines (IL-4 and IFN-γ) were detected by ELISA kits (Figure 4). Regarding serum P4 concentration, no significant difference was observed between the different groups (P > 0.05, Figure 4A). Compared with the control (Ctrl) group, the E2 concentration in T1 group significantly increased by 432.65 pg/mL (P < 0.05, Figure 4B). In addition, we observed that the levels of IL-4 and IFN-γ were significantly greater in T1 and T2 groups than that in Ctrl group (P < 0.05, Figures 4C, D). Furthermore, we compared the differences in steroid hormones and cytokines between the antibody-positive (P) and antibody-negative (N) buffaloes. The results showed that the concentrations of IL-4, IFN-γ and E2 in antibody-positive buffaloes were significantly increased compared to those in antibody-negative buffaloes (P < 0.01), while there was no significant difference in the concentration of P4 (P > 0.05, Figure 4E).
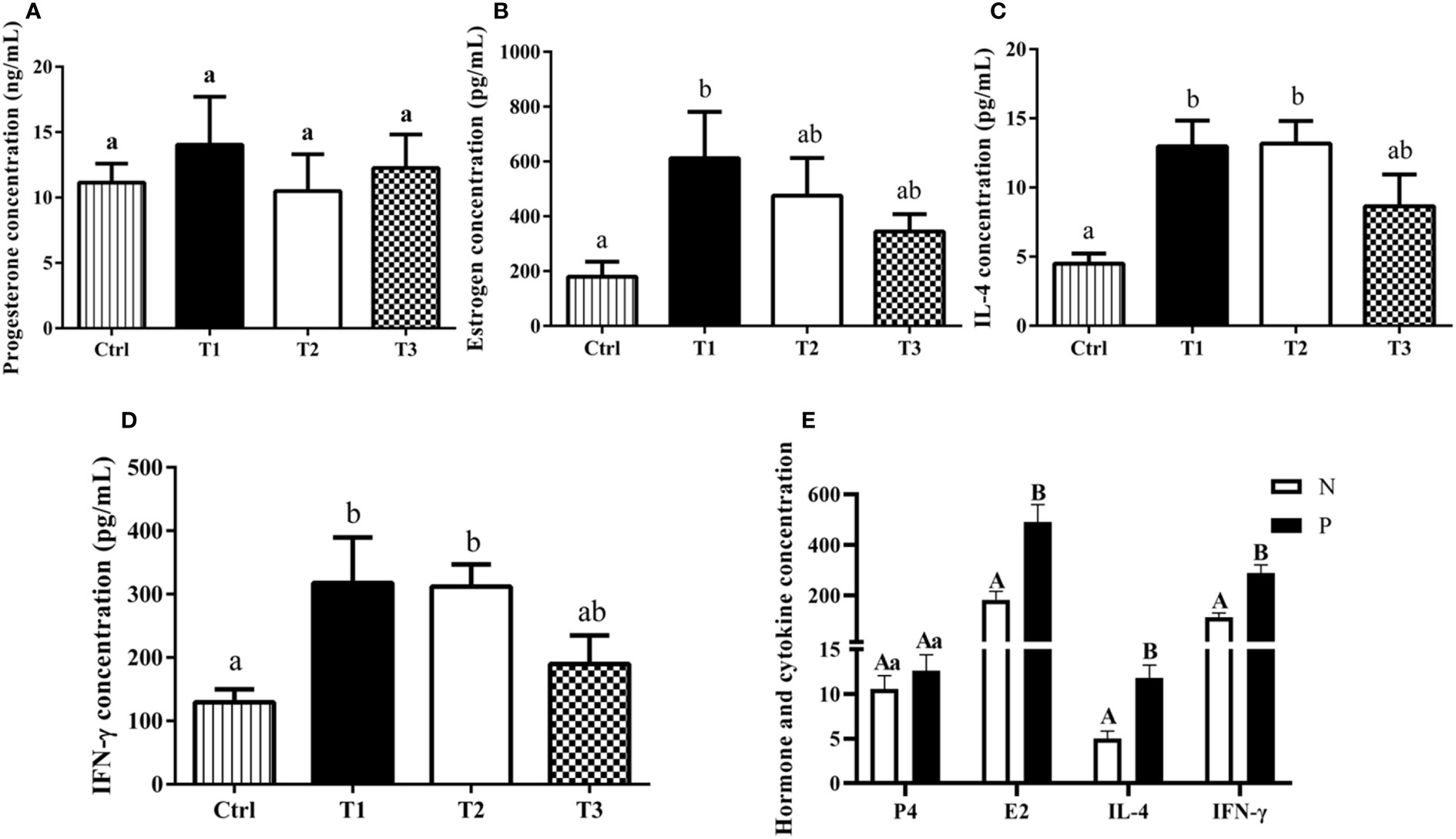
Figure 4 The effects of AMH-INH-RFRP DNA vaccine on steroid hormones and cytokines in buffalo after nasal immunization. (A-D) represent the effect on P4, E2, IFN-γ, and IL-4, respectively. (E) The effect of antibody-positive and antibody-negative buffaloes on P4, E2, IL-4 and IFN-γ. Data are presented as mean ± SEM. The bars with difference letters indicated significant differences (a,bP < 0.05; A,BP < 0.01).
3.3 The effect of AMH-INH-RFRP DNA vaccine on folliculogenesis in buffalo
All buffaloes were monitored by ultrasonography to record the diameter of follicle and the growth rate of the dominant follicle (mm/day). As shown in Table 2, the buffaloes in T1 (12.98 ± 0.46), T2 (13.55 ± 0.44) and T3 (12.56 ± 0.46) groups had a significantly greater diameter of ovulatory follicle compared to the Ctrl group (10.74 ± 0.46, P < 0.05). In addition, there was a significant increase in the growth speed of dominant follicle in T1 (1.34 ± 0.07) and T2 (1.32 ± 0.08) groups compared to Ctrl group (1.10 ± 0.08, P < 0.05). However, there were no significant differences in diameter of ovulatory follicles and the growth speed of dominant follicle between DNA vaccine immunization groups (P > 0.05).
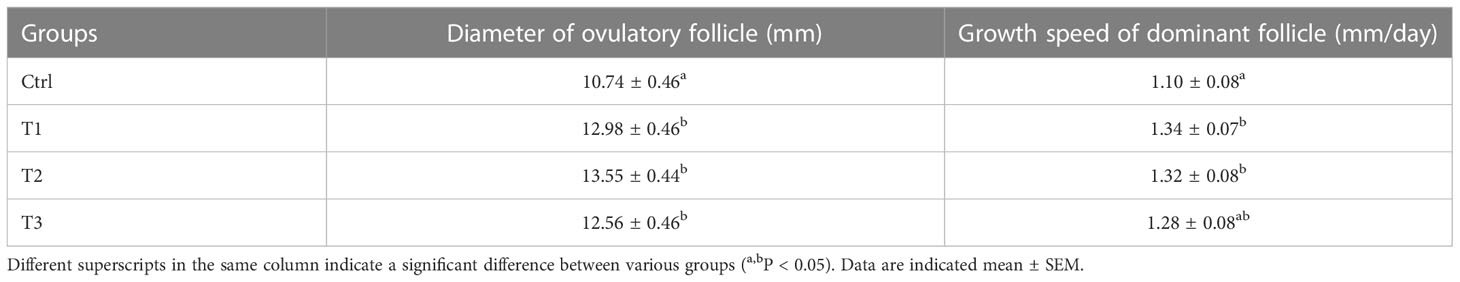
Table 2 The diameter of ovulatory follicles and dominant follicles’ growth speed in buffaloes after immunization with different vaccine concentrations.
Moreover, we further compared the changes in diameter of ovulatory follicles and the growth speed of dominant follicle between antibody-positive and antibody-negative buffaloes. As shown in Table 3, the diameter of ovulatory follicles in the P group was highly significantly increased by 2.02 mm compared to the N group (P < 0.01), and growth speed of dominant follicle in the P group was much faster than that in the N group (1.33 ± 0.04 vs 1.13 ± 0.06, P < 0.05).
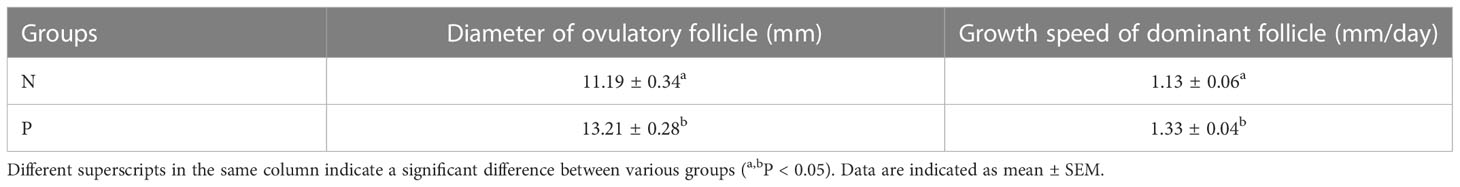
Table 3 Diameter of ovulatory follicles and growth speed of dominant follicle between antibody-positive and antibody-negative buffaloes.
3.4 The effects of AMH-INH-RFRP DNA vaccine on oestrus, ovulation, and conception rates
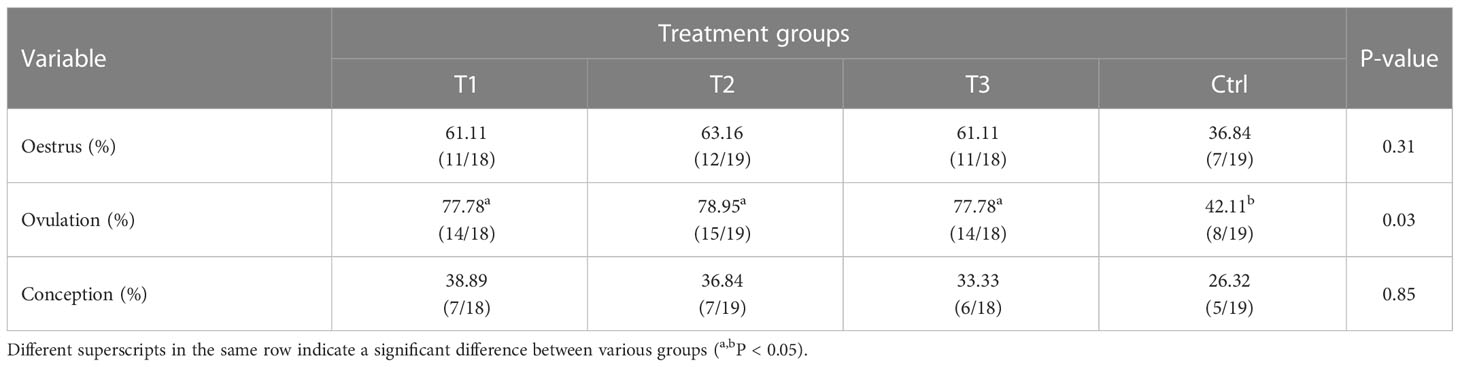
After immunization with AMH-INH-RFRP DNA vaccine, the oestrus, ovulation, and conception rates of buffaloes were observed and recorded. As shown in Table 4, oestrus and conception rates of buffaloes in groups T1, T2, and T3 tended to be higher than the Ctrl group, although the difference didn’t reach significance (P > 0.05). Furthermore, the proportion of ovulation in T1, T2, and T3 groups was significantly superior to that of the Ctrl group by 35.67%, 36.84%, and 35.67%, respectively (P < 0.05).
Notably, there were extremely significant differences in oestrus and ovulation rates between antibody-positive and antibody-negative buffaloes in Table 5 (P < 0.05). Likewise, the conception rate in P group was also significantly higher by 24.03% than that in N group (P < 0.05).
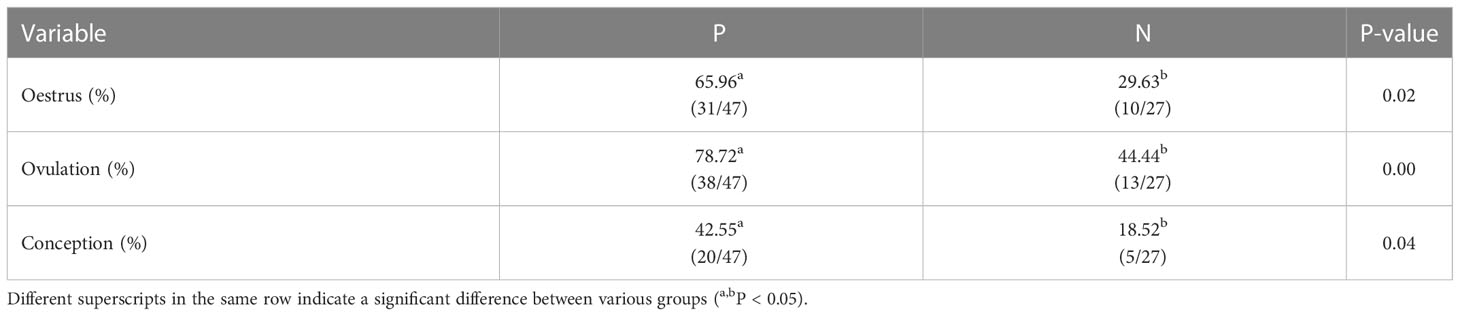
Table 5 Oestrus, ovulation and conception rates between antibody-positive and antibody-negative buffaloes.
4 Discussion
It is well known that DNA vaccine has several obvious advantages over conventional vaccines, including easy to design and manufacture, low cost, convenient transportation, unsafe sources of infection are not involved, and encoding multiple immunogenic epitopes (26). Unfortunately, inadequate immunogenicity is still the biggest challenge for application of practical DNA vaccines (27). In the present study, to improve the immune response of DNA vaccine, the hepatitis B surface antigen gene (HBsAg-S) was used to fuse with AMH, INH, and RFRP genes, and 2A peptide was employed to ligate the fragments of S/AMH, S/INH, and S/RFRP (28, 29). DNA vaccines can induce both humoral and cellular immune responses (30). Our results showed that antibody titers were higher in T1 and T2 groups than that in T3 group after both primary and booster immunizations. There was no significant difference between T1 and T2 groups, suggesting that the antibody levels evoked by AMH-INH-RFRP DNA vaccines are not in a dose-dependent manner. Notably, all experimental groups showed a high rate of antibody positivity after 14 days of booster immunization, ensuring booster efficacy. On the other hand, we further measured expression levels of IL-4 and IFN-γ, and observed that IL-4 and IFN-γ were significantly higher in the P group than that in the N group. T helper (Th) cells play a key role in regulating the immune response by synthesizing and releasing cytokines into the surrounding microenvironment. The Th1 cells produce IL-2, IFN-γ, tumor necrosis factor (TNF), and lymphotoxin and are associated with cell-mediated immune functions. The Th2 cells produce IL-4, IL-5, IL-6, and IL-10, to assist the humoral immune response (31, 32). Inagawa et al. found that IFN-γ and IL-4 significantly enhanced specific Th1 and Th2 cell immune responses, respectively (33). All the above evidence suggests that AMH-INH-RFRP DNA vaccine can induce both humoral and cellular immune response through nasal immunization in buffalo, which is consistent with the previous studies on GPV-VP1 DNA vaccine (34).
It was previously reported that the concentrations of E2 were significantly higher in large follicles compared to those in medium and small follicles, but the progesterone concentrations were not affected in buffaloes (35). In addition, E2 levels in plasma correlated with estrus performance in buffalo (36). Estrogens can promote granulosa cell proliferation and follicle growth and inhibit atresia of small luminal follicles or preluminal follicles, and also promote maturation of large follicles and ovulation (37). Importantly, cumulative studies showed that INH showed an additive inhibitory effect on FSH-induced estradiol production (10, 24). Similarly, AMH and RFRP also can negatively regulate the synthesis and secretion of steroid hormones by downregulation the aromatase (38, 39). Here, we found that antibody-positive buffaloes had higher serum estrogen levels than antibody-negative buffaloes, the possible reason may be due to the decrease of endogenous AMH, INH, and RRPP hormones, which were immune neutralized by anti-AMH, anti-INH, and anti-RFRP antibodies (13, 15). However, the immunized and control groups did not show any change in P4 concentration, presumably since mature follicles did not ovulate. Corpus luteum (CL) formation didn’t occur (40). In addition, the increase of estrogen in antibody-positive buffaloes significantly accelerates the growth speed of the dominant follicle, and increase the diameter of the ovulatory follicle, results in high proportions of estrus and ovulation. Agree with Wang et al. studies in rats, showing that plasma concentration of FSH and E2 was significantly increased following pcISI DNA vaccination in rats and stimulated follicular development (41).
A previous study demonstrated that estrus synchronization in buffalo with Ovsynch resulted in the estrus rate of 96.1%, ovulation of 84.6%, and pregnancy rate of 30.8% (5), which are higher than our control result. The possible reason may be attributed to the season of experiment which was between May and July in this study, which is the low breeding season for buffalo (6). Evidence showed that buffaloes had a greater calving incidence and oestrous expression from August to November in most parts of India (42, 43). Moreover, pregnancy rates were poor during the low breeding season showing that 26% in cyclic buffalo and 7% in acyclic buffalo synchronized with Ovsynch (7, 44). On the other hand, sexed semen for TAI in this study might be another important reason for the low conception rate. Though sexed semen is attributed to improving the genetic merit of future cows, however, the conception rate with sexed semen is low (45). In a previous study, compared to the conventional semen group (55%), cattle in the sexed semen group (44%) had an 11% reduction in conception rate (46). Although conception rates in N group were low due to seasonal and sexed semen factors, by a combination of AMH-INH-RFRP DNA vaccine immunization and Ovsynch-TAI protocol, we observed that the conception rate in T1, T2, T3 groups increased to 38.89%, 36.84%, and 33.33% as compared to Ctrl group (26.32%), and the conception rate in the P group was significantly increased by 24.03% compared to the N group (42.55% vs 18.52%). This data indicated that AMH-INH-RFRP DNA vaccine could overcome the problem of poor effects resulting from Ovsynch-TAI protocol in non-breeding seasons and the use of sexed semen.
5 Conclusions
In summary, nasal immunization against buffalo with the AMH-INH-RFRP DNA vaccine induced the cellular and humoral immune responses, and increased the estrogen concentration. Meanwhile, AMH-INH-RFRP DNA vaccine increased the diameter of ovulatory follicle and accelerated the growth speed of dominant follicle, thus improving the behavioral oestrus and ovulation, ultimately leading to an increase in the conception rate of buffaloes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Animal Care and Use Committee of Huazhong Agricultural University (HZAUBU-2019-001). Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
CC performed the whole experiment and wrote the manuscript. CC and MA conceived and designed the experiment data curation. ZA, PN, XXZ, KN, JT, and XHZ contributed animals’ arrangement and sample collection. AL and LY supervised the study and revised the manuscript. All the authors have reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Natural Science Foundation of China (32072729) and the earmarked fund for CARS36.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hegde NG. Buffalo husbandry for sustainable development of small farmers in India and other developing countries. Asian J Res Anim Vet Sci (2019) 3(1):1–20.
2. Rensis FD, López-Gatius F. Protocols for synchronizing estrus and ovulation in buffalo (Bubalus bubalis): A review. Theriogenology (2007) 67:209–16. doi: 10.1016/j.theriogenology.2006.09.039
3. Li J, Liu J, Campanile G, Plastow G, Yang L. Novel insights into the genetic basis of buffalo reproductive performance. BMC Genomics (2018) 19(1):814. doi: 10.1186/s12864-018-5208-6
4. Harun-Or-Rashid M, Sarkar AK, Hasan M, Hasan M, Juyena NS. Productive, reproductive, and estrus characteristics of different breeds of buffalo cows in Bangladesh. J Adv Vet Anim Res (2019) 6(4):553–60. doi: 10.5455/javar.2019.f382
5. Abulaiti A, El-Qaliouby HS, El Bahgy HEK, Naseer Z, Ahmed Z, Hua GH, et al. GPGMH, a new fixed timed-AI synchronization regimen for swamp and river crossbred buffaloes (Bubalus bubalis). Front Vet Sci (2021) 8. doi: 10.3389/fvets.2021.646247
6. Warriach HM, Channa AA, Ahmad N. Effect of oestrus synchronization methods on oestrus behaviour, timing of ovulation and pregnancy rate during the breeding and low breeding seasons in nili-ravi buffaloes. Anim Reprod Sci (2008) 107:62–7. doi: 10.1016/j.anireprosci.2007.06.007
7. Baruselli PS. Control of follicular development applied to reproduction biotechnologies in buffalo. In Proceedings of the Book of the Congress, Congresso Nazionale sull’allevamento del Bufalo. Rome, Italy. (2001) pp. 128–146.
8. Mussard ML, Burke CR, Behlke EJ, Gasser CL, Day ML. Influence of premature induction of a luteinizing hormone surge with gonadotropin-releasing hormone on ovulation, luteal function, and fertility in cattle. J Anim Sci (2007) 85:937–43. doi: 10.2527/jas.2006-592
9. Lu C, Wei Y, Min C, Tao L, Liu Y. Inhibin a inhibits follicle-stimulating hormone (FSH) action by suppressing its receptor expression in cultured rat granulosa cells. Mol Cell Endocrinol (2009) 298:48–56. doi: 10.1016/j.mce.2008.09.039
10. Burger HG. Evidence for a negative feedback role of inhibin in follicle stimulating hormone regulation in women. Hum Reprod (1993) 8:129–32. doi: 10.1093/humrep/8.suppl_2.129
11. Hui FM, Meng CL, Guo NN, Yang LG, Shi FX, Mao DG. Evaluation of attenuated salmonella choleraesuis-mediated inhibin recombinant DNA vaccine in rats. Genet Mol Res (2014) 13:6113–25. doi: 10.4238/2014.August.7.27
12. Han L, Zhen Y-H, Liang A-X, Zhang J, Riaz H, Xiong J-J, et al. Oral vaccination with inhibin DNA delivered using attenuated salmonella choleraesuis for improving reproductive traits in mice. J Basic Microbiol (2014) 54:962–8. doi: 10.1002/jobm.201300052
13. Dan X, Liu X, Han Y, Liu Q, Yang L. Effect of the novel DNA vaccine fusing inhibin α (1-32) and the RF-amide related peptide-3 genes on immune response, hormone levels and fertility in tan sheep. Anim Reprod Sci (2016) 164:105–10. doi: 10.1016/j.anireprosci.2015.11.018
14. Liu Q, Han L, Ur Rehman Z, Dan X, Liu X, Bhattarai D, et al. The efficacy of an inhibin DNA vaccine delivered by attenuated salmonella choleraesuis on follicular development and ovulation responses in crossbred buffaloes. Anim Reprod Sci (2016) 172:76–82. doi: 10.1016/j.anireprosci.2016.07.004
15. Liu Q, Rehman ZU, Liu JJ, Han L, Liu XR, Yang LG. Nasal immunization with inhibin DNA vaccine delivered by attenuated salmonella choleraesuis for improving ovarian responses and fertility in cross-bred buffaloes. Reprod Domest Anim (2017) 52:189–94. doi: 10.1111/rda.12876
16. Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, et al. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun (2000) 275:661–7. doi: 10.1006/bbrc.2000.3350
17. Ubuka T, Son YL, Tobari Y, Tsutsui K. Gonadotropin-inhibitory hormone action in the brain and pituitary. Front Endocrinol (2012) 3:148. doi: 10.3389/fendo.2012.00148
18. Dan X, Han L, Riaz H, Luo X, Liu X, Chong Z, et al. Construction and evaluation of the novel DNA vaccine harboring the inhibin α (1-32) and the RFamide related peptide-3 genes for improving fertility in mice. Exp Anim (2016) 65:17–25. doi: 10.1538/expanim.15-0044
19. Teixeira J, Maheswaran S, Donahoe PK. Müllerian inhibiting substance: an instructive developmental hormone with diagnostic and possible therapeutic applications. Endocrine Rev (2001) 22:657–74. doi: 10.1210/edrv.22.5.0445
20. Diclemente N, Goxe B, Remy JJ, Cate R, Josso N, Vigier B, et al. INHIBITORY EFFECT OF AMH UPON THE EXPRESSION OF AROMATASE AND LH RECEPTORS BY CULTURED GRANULOSA-CELLS OF RAT AND PORCINE IMMATURE OVARIES. Endocrine (1994) 2:553–8.
21. Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen A, Hovatta O. Anti-mullerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod (2006) 21(9):2223–7. doi: 10.1093/humrep/del165
22. Chang H-M, Klausen C, Leung PCK. Antimullerian hormone inhibits follicle-stimulating hormone-induced adenylyl cyclase activation, aromatase expression, and estradiol production in human granulosa-lutein cells. Fertil Steril (2013) 100:585–+. doi: 10.1016/j.fertnstert.2013.04.019
23. Durlinger ALL, Gruijters MJG, Piet K, Bas K, Rajendra KT, Matzuk MM, et al. Anti-müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology (2001) 142:4891–9. doi: 10.1210/endo.142.11.8486
24. Yu X, Qiao T, Hua L, Liu S, Zhao X, Lv C, et al. Synergistic regulatory effect of inhibin and anti-mullerian hormone on fertility of mice. Front Vet Sci (2021) 8. doi: 10.3389/fvets.2021.747619
25. Zhang F, Fang F, Chang H, Peng B, Wu J, Chen J, et al. Comparison of protection against H5N1 influenza virus in mouse offspring provided by maternal vaccination with HA DNA and inactivated vaccine. Arch Virol (2013) 158:1253–65. doi: 10.1007/s00705-013-1621-y
26. Schalk JAC, Mooi FR, Berbers GAM, van Aerts L, Ovelgonne H, Kimman TG. Preclinical and clinical safety studies on DNA vaccines. Hum Vaccines (2006) 2:45–53. doi: 10.4161/hv.2.2.2620
27. Li L, Petrovsky N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev Vaccines (2016) 15:313–29. doi: 10.1586/14760584.2016.1124762
28. Valenzuela P, Coit D, Medina-Selby MA, Kuo CH, Nest GV, Burke RL, et al. Antigen engineering in yeast: Synthesis and assembly of hybrid hepatitis b surface antigen-herpes simplex 1 gD particles. Nat Biotechnol (1985) 3:323–6. doi: 10.1038/nbt0485-323
29. Fang JM, Qian JJ, Yi SL, Harding TC, Tu GH, VanRoey M, et al. Stable antibody expression at therapeutic levels using the 2A peptide. Nat Biotechnol (2005) 23:584–90. doi: 10.1038/nbt1087
30. Kobiyama K, Jounai N, Aoshi T, Tozuka M, Takeshita F, Coban C, et al. Innate immune signaling by, and genetic adjuvants for DNA vaccination. Vaccines (2013) 1(3): 278–92. doi: 10.3390/vaccines1030278
31. Li-Weber M, Laur O, Davydov IV, Hu CG, Salgame P, Krammer PH. What controls tissue-specific expression of the IL-4 gene? Immunobiology (1997) 198:170–8. doi: 10.1016/S0171-2985(97)80038-1
32. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Pillars article: Two types of murine helper T cell clone. i. definition according to profiles of lymphokine activities and secreted proteins. J Immunol (2005) 175:5–14.
33. Inagawa H, Nishizawa T, Honda T, Nakamoto T, Takagi K, Soma G. Mechanisms by which chemotherapeutic agents augment the antitumor effects of tumor necrosis factor: Involvement of the pattern shift of cytokines from Th2 to Th1 in tumor lesions. Anticancer Res (1998) 18:3957–64.
34. Deng S-x, Cai M-s, Cui W, Huang J-l, Li M-l. Evaluation of the immune response in shitou geese (Anser anser domesticus) following immunization with GPV-VP1 DNA-based and live attenuated vaccines. Vet Q (2014) 34:180–4. doi: 10.1080/01652176.2014.966173
35. Palta P, Bansal N, Prakash BS, Manik RS, Madan ML. Endocrinological observation of atresia in individual buffalo ovarian follicles. Indian J Anim Sci (1998) 68:444–7.
36. Roy KS, Prakash BS. EFFICACY OF OVSYNCH TREATMENT FOR IMPROVEMENT OF CYCLICITY IN MURRAH BUFFALO HEIFERS DURING SUMMER STRESS. Indian Vet J (2008) 85:833–6.
37. Emmen JMA, Couse JF, Elmore SA, Yates MM, Korach KS. In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER){alpha} and ER{beta} null mice indicate a role for ER{beta} in follicular maturation. Endocrinology (2013) 146:2817–26. doi: 10.1210/en.2004-1108
38. Dewailly D, Robin G, Peigne M, Decanter C, Pigny P, Catteau-Jonard S. Interactions between androgens, FSH, anti-mullerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum Reprod Update (2016) 22:709–24. doi: 10.1093/humupd/dmw027
39. Smith JT. The role of kisspeptin and gonadotropin inhibitory hormone in the seasonal regulation of reproduction in sheep. Domest Anim Endocrinol (2012) 43:75–84. doi: 10.1016/j.domaniend.2011.11.003
40. Medan MS, Watanabe G, Sasaki K, Nagura Y, Taya K. Ovarian and hormonal response of female goats to active immunization against inhibin. J Endocrinol (2003) 177:287. doi: 10.1677/joe.0.1770287
41. Wang SL, Han L, Ahmad S, Cao SX, Xue LQ, Xing ZF, et al. Effect of a DNA vaccine harboring two copies of inhibin α (1-32) fragments on immune response, hormone concentrations and reproductive performance in rats. Theriogenology (2012) 78(2):393–401. doi: 10.1016/j.theriogenology.2012.02.019
42. Gunwant P, Pandey AK, Kumar A, Singh I, Magotra A. Polymorphism of melatonin receptor (MTNR1A) gene and its association with seasonal reproduction in water buffalo (Bubalus bubalis). Anim Reprod Sci (2018) 199:51–9. doi: 10.1016/j.anireprosci.2018.10.006
43. Reddy AO, Ramesha KP, Rao MK. Effect of climate on the incidence of oestrus, conception and cycle length in murrah buffaloes. Indian J Anim Sci (1999) 69:485–9.
44. Chohan KR. Estrus synchronization with lower dose of PGF2 alpha and subsequent fertility in subestrous buffalo. Theriogenology (1998) 50:1101–8. doi: 10.1016/S0093-691X(98)00211-8
45. Khalajzadeh S, Nejati-Javaremi A, Yeganeh HM. Effect of widespread and limited use of sexed semen on genetic progress and reproductive performance of dairy cows. Animal (2012) 6:1398–406. doi: 10.1017/S1751731112000651
Keywords: AMH, INH, RFRP, DNA vaccine, reproduction, buffalo
Citation: Chen C, Zhao X, An Z, Ahmad MJ, Niu K, Zhang X, Nie P, Tang J, Liang A and Yang L (2023) Nasal immunization with AMH-INH-RFRP DNA vaccine for improving follicle development and fertility in buffaloes. Front. Endocrinol. 14:1076404. doi: 10.3389/fendo.2023.1076404
Received: 21 October 2022; Accepted: 27 January 2023;
Published: 20 February 2023.
Edited by:
Feng Wang, Nanjing Agricultural University, ChinaReviewed by:
Shunfeng Cheng, Qingdao Agricultural University, ChinaChunjin Li, Jilin University, China
Copyright © 2023 Chen, Zhao, An, Ahmad, Niu, Zhang, Nie, Tang, Liang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liguo Yang, ylg@mail.hzau.edu.cn; Aixin Liang, lax.pipi@mail.hzau.edu.cn
 Chao Chen
Chao Chen Xuhong Zhao1
Xuhong Zhao1 Zhigao An
Zhigao An Muhammad Jamil Ahmad
Muhammad Jamil Ahmad Aixin Liang
Aixin Liang Liguo Yang
Liguo Yang