- 1Department of Endocrinology, The Second Affiliated Hospital of Fujian Medical University, Quanzhou, China
- 2Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Fujian Medical University, Quanzhou, China
Background: Diabetic peripheral neuropathy (DPN) is the most common diabetes-associated complication and imposes a significant burden to healthcare systems. Thus, early diagnosis of DPN is extremely critical for management and outcome of diabetic patients. Supersonic Shear Wave Imaging (SSI) enables the noninvasive measurement of nerve stiffness. However, previous studies on SSI in the diagnosis of DPN were limited in sample sizes and reported various results. In this meta-analysis, we aimed to obtain comprehensive evidence on the value of tibial nerve stiffness measurement by SSI in the diagnosis of DPN.
Methods: A comprehensive literature search in English and Chinese electronic database was conducted for studies (published until January 25, 2022) that investigated the diagnostic performance of tibial nerve stiffness measurement by SSI for detecting DPN. Summary receiver operating characteristics (SROC) modelling was constructed to conduct the meta-analysis of diagnostic accuracy of SSI for detecting DPN.
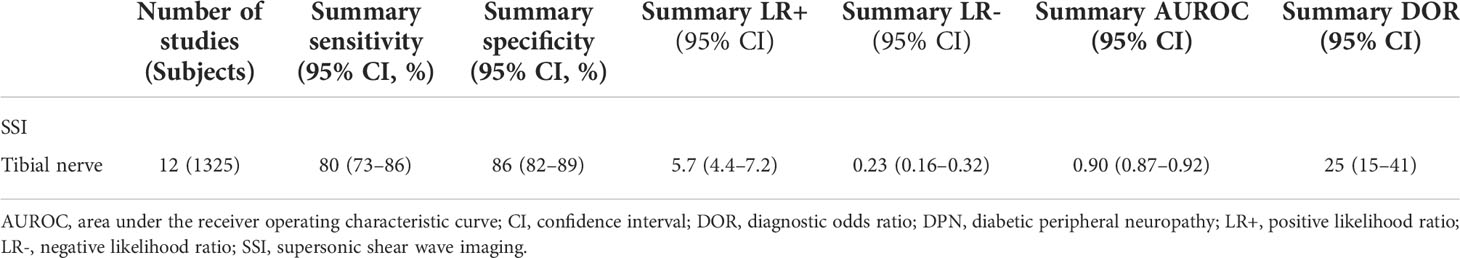
Results: Finally, a total of 12 eligible studies with 1325 subjects were included for evaluation, and a meta-analysis was conducted to evaluate the diagnostic performance of tibial nerve stiffness measurement by SSI for detecting DPN. For tibial nerve stiffness measurement by SSI, the summary sensitivity and specificity for the diagnosis of DPN were 80% (95% confidence interval [CI]: 73%–86%) and 86% (95% CI: 82%–89%), respectively. The summary area under the ROC curve (AUROC) value of the SROC was 0.90 (95% CI: 0.87–0.92), for diagnosing DPN. A subgroup analysis of 11 SSI studies from China revealed similar diagnostic performance, with a summary sensitivity of 79% (95% CI: 72%–85%), specificity of 86% (95% CI: 82%–89%) and summary AUROC value of the SROC of 0.90 (95% CI: 0.87–0.92) for diagnosing DPN.
Conclusions: Our meta-analysis suggests that a tibial nerve stiffness measurement by SSI shows good performance in diagnosing DPN and has considerable potential as a noninvasive tool for detecting DPN.
Introduction
Diabetes mellitus (DM) is a chronic progressive metabolic disease, which is characterized by systemic hyperglycemia (1). The incidence and prevalence of both type 1 and type 2 DM is increasing rapidly throughout the world and becoming a major public health problem (2, 3). Over the past 20 years, the number of adults living with diabetes has more than tripled (4). By 2045, the number of people with diabetes worldwide is expected to rise to 700 million (5). According to International Diabetes Federation (IDF) Atlas (9th edition), in 2019 alone, approximately 4.2 million deaths worldwide were attributable to diabetes and its complications (5).
The final outcome of diabetes is a variety of chronic complications (1). Among these, diabetic peripheral neuropathy (DPN) is the most common diabetes-associated complication (6). Notably, there is a high incidence of DPN in the diabetic population. The previous study has reported that approximately 50% of people with diabetes will develop DPN (7). If it is not timely diagnosed and treated, it may cause further serious complications, such as foot ulcers, gangrene, and amputation (8, 9). Importantly, this condition continues to have a great impact on the quality of life of people with diabetes and also imposes a significant burden to healthcare systems (10, 11). In fact, DPN is known as the strongest initiating risk factor for diabetic foot ulceration (12, 13). Thus, early diagnosis of DPN is extremely critical for management and outcome of diabetic patients.
Currently, there are several methods, including quantitative sensory testing (e.g., monofilament examination), clinical neurological function scoring system (e.g., Toronto clinical scoring system), high-frequency ultrasound and nerve conduction studies (NCS), are commonly used to detect DPN (10, 14). Among these, NCS is considered to be one of the gold standard methods for the diagnosis of DPN (15). However, this technique also has disadvantages; for instance, it is expensive, time-consuming, and requires professional doctors to operate, making it difficult to implement in many primary care settings (10, 14). As a consequence, there is a strong need for a simple and effective tool for detecting DPN.
Recently, measurement of nerve stiffness by ultrasound elastography becomes a noninvasive alternative method for the diagnosis of DPN. Ultrasound elastography is a promising imaging technology, which measures the speed of a shear wave, providing a quantitative estimate of tissue stiffness (16). Three types of ultrasound elastography, including transient elastography, point shear wave elastography and two-dimensional shear wave elastography, have now been commercialized (17). In contrast, two-dimensional shear wave elastography represents a novel quantitative ultrasound elastography technique, which uses an acoustic radiation force impulse to generate shear waves (18). This technique has several strengths; for example, it enables a simultaneous display of a color-coded elastographic map on a conventional B-mode ultrasound image in time, and to visualize the stiffness of the examined areas (19, 20). Notably, among different ultrasound equipments installed with this technique, the estimates of the speed of a shear wave in the same nerve are widely heterogeneous. Supersonic Shear Wave Imaging (SSI; Aix-en-Provence, France) is one of the most widely used equipments of two-dimensional shear wave elastography for the evaluation of neuromuscular pathologies, including DPN, to the best of our knowledge.
Some studies have reported on the value of SSI for diagnosing DPN. However, previous studies on SSI in the diagnosis of DPN were limited in sample sizes and reported various results. Therefore, in this meta-analysis, we aimed to obtain comprehensive evidence on the value of tibial nerve stiffness measurement by SSI in the diagnosis of DPN. Additionally, we also conducted a subgroup analysis to summarize the diagnostic performance of SSI studies from China. In the present study, only relevant data from the SSI was collected for evaluation and meta-analysis.
Methods
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for performing and reporting our current meta-analysis were followed (21).
Search strategy
A computerized search was conducted using the four English electronic databases (PubMed, EMBASE, Web of Science, and Cochrane Library) and four Chinese electronic databases (China National Knowledge Infrastructure, Chinese Biomedical Database, Chinese VIP Information Database, and Wanfang Med Database) to identify studies (published until January 25, 2022) that investigated the diagnostic performance of tibial nerve stiffness measurement by SSI for detecting DPN. The following search terms were used: ((diabetes) OR (diabetic peripheral neuropathy) OR (diabetic complications)) AND ((shear wave elastography) OR (supersonic shear wave imaging)). Moreover, references of the identified articles were also examined for other relevant publications. We used EndNote X9 software (Clarivate Analytics, Philadelphia, PA, United States) to import and manage retrieved records.
Inclusion and exclusion criteria
Overall, the diagnostic performance of studies that investigated the accuracy of tibial nerve stiffness measurement by SSI for detecting DPN was considered eligible for our meta-analysis. Specifically, studies were included in the present meta-analysis according to the following criteria: (1) the study examined the accuracy of the tibial nerve stiffness measurement by SSI for diagnosing DPN; (2) the study enrolled more than 30 patients; and (3) the study provided sufficient data and therefore allowed us to construct at least one 2 × 2 table for test performance. The exclusion criteria were as follows: (1) studies were not relevant to the topic; (2) studies were performed on patients without DM; (3) studies had incomplete data sets; (4) duplicate publications (we excluded the studies with the smaller population); and (5) non-original research articles, including guidelines, conference abstracts, patents, reviews, protocols and commentary. Of note, only studies that used the SSI were included in this analysis, we therefore excluded other types of ultrasound elastography techniques, such as transient elastography and point shear wave elastography, and other ultrasound equipments for two-dimensional shear wave elastography technique, such as Siemens Virtual Touch tissue imaging and quantification (VTIQ) and Toshiba shear wave elastography.
Data extraction and quality assessment
In this meta-analysis, we extracted the following data from the included studies: (1) study characteristics, including author, year of publication, region, number of study population, etc.; (2) patients’ data, including age, patient gender, body mass index (BMI), etc.; (3) SSI characteristics, including probe, number of measurements for each subject, number of readers, representative value of elasticity (median or mean), blinding to the reference standard, and interval time between the reference standard and SSI; and (4) the reference standard used for diagnosing DPN.
The revised Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool (22) was used to assess the quality of the studies included in this meta-analysis. Two reviewers (Y.P. Chen and H.H. Duan) of our research independently evaluated the study eligibility and quality and extracted the outcome data, with a third reviewer (H.B. Huang) adjudicating on disagreements.
Data synthesis and analysis
Statistical analysis was performed by Stata version 15 (STATA Corp., College Station, TX, USA) and Meta-Disc software version 1.4. In this meta-analysis, our main purpose was to investigate the performance of tibial nerve stiffness measurement by SSI for the diagnosis of DPN. The data was extracted from the included studies and the 2 × 2 table for test performance was constructed, which was then used to calculate the sensitivity, specificity, positive and negative likelihood ratio and diagnostic odds ratio (DOR). Moreover, the summary receiver operating characteristic (SROC) curve was also constructed. We then examined the summary area under the ROC curve (AUROC) and the corresponding 95% confidence intervals (CIs) to further assess the accuracy of tibial nerve stiffness measurement by SSI for the diagnosis of DPN. Furthermore, a subgroup analysis was also conducted to evaluate the diagnostic performance of SSI studies from China.
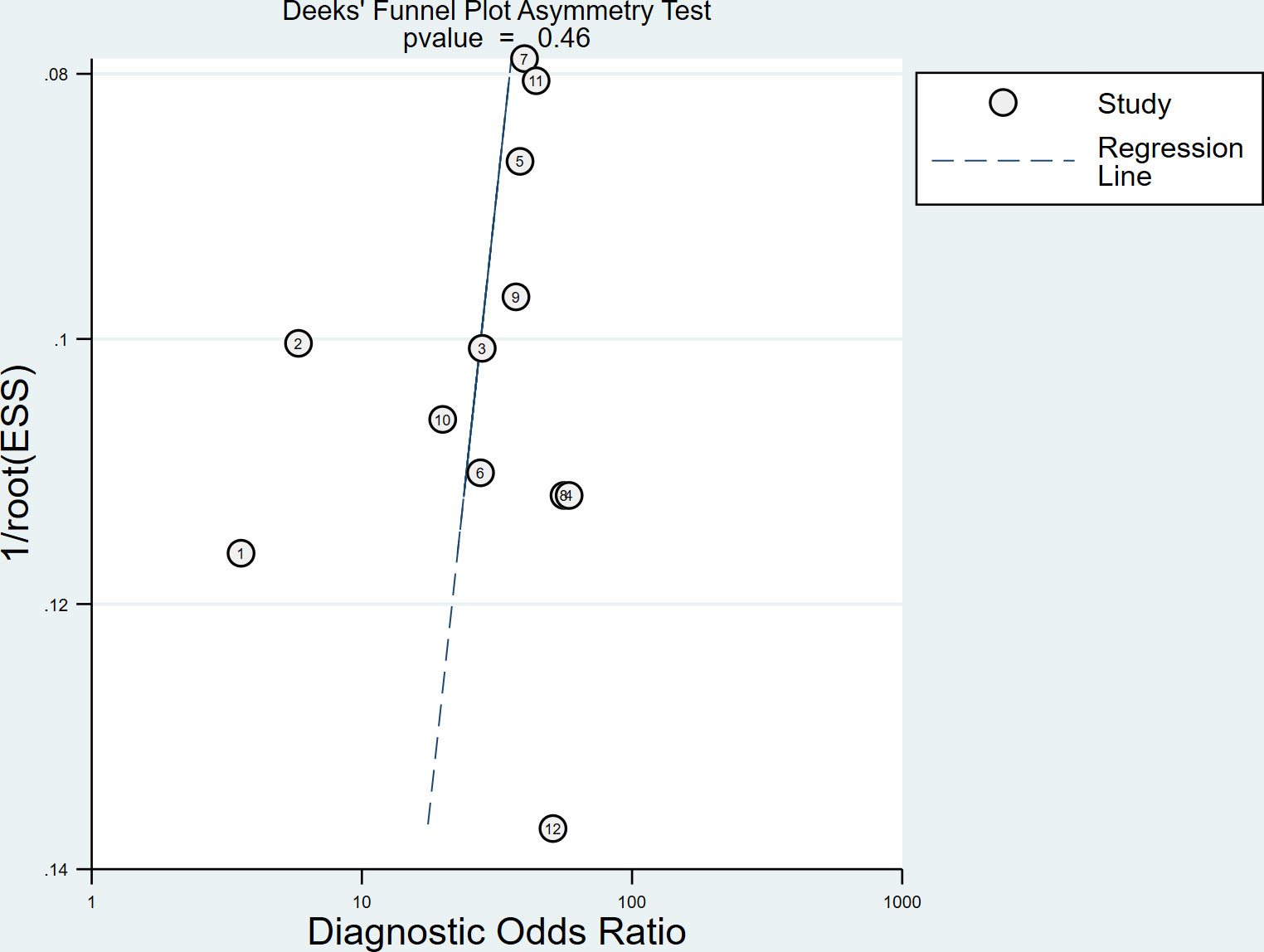
Cochran’s Q-test (P < 0.1 indicated significant heterogeneity) and inconsistency index (I2) was used to assess the non-threshold heterogeneity of the results between SSI studies in this meta-analysis (23). The inconsistency index I2 was calculated in our analysis and then used to qualify the amount of non-threshold heterogeneity. It may be considered to represent moderate, substantial or considerable heterogeneity if the inconsistency index I2 value ≥ 30%, ≥ 50% or ≥ 75%. In addition, the Spearman correlation coefficient was also calculated in order to evaluate the threshold effect of the included studies. A P value < 0.05 was considered to indicate the existence of a threshold effect. Publication bias was assessed using the Deeks’ funnel plots (24). When P value < 0.05, the results were suggested that significant publication bias existed.
Results
Literature search
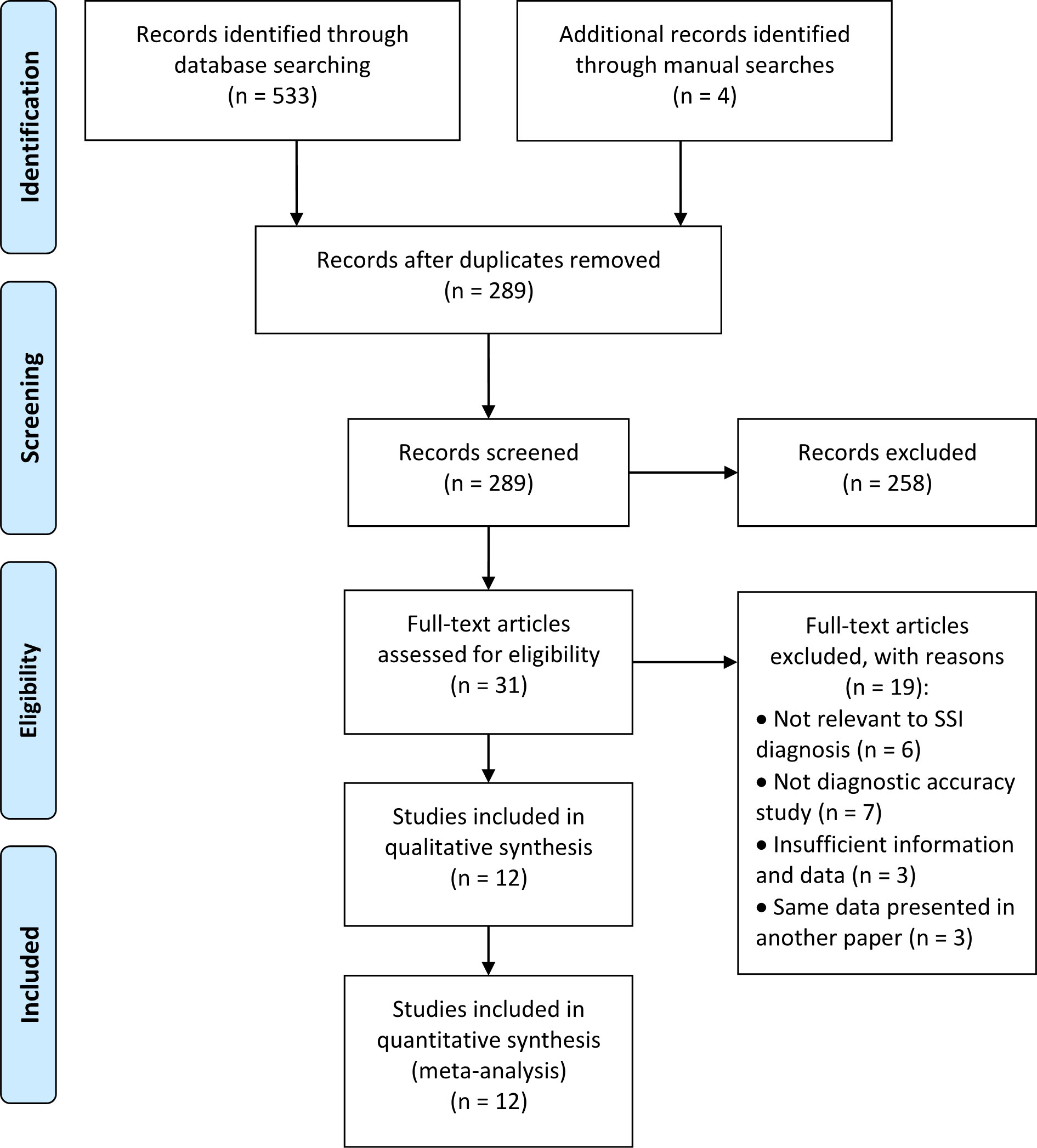
Figure 1 shows a flow diagram for the literature selection process. Using the above-mentioned search strategy, a total of 537 records were retrieved. After removal of 248 duplicates, 289 records were retained. However, 277 studies were excluded for some reasons, such as conference abstracts, patents, reviews, protocols, studies were not relevant to the topic, etc. Finally, 12 eligible studies (25–36) were ultimately reserved for evaluation and meta-analysis. Specifically, five of these studies were retrieved from the English database and 7 from Chinese database.
Study and technical characteristics
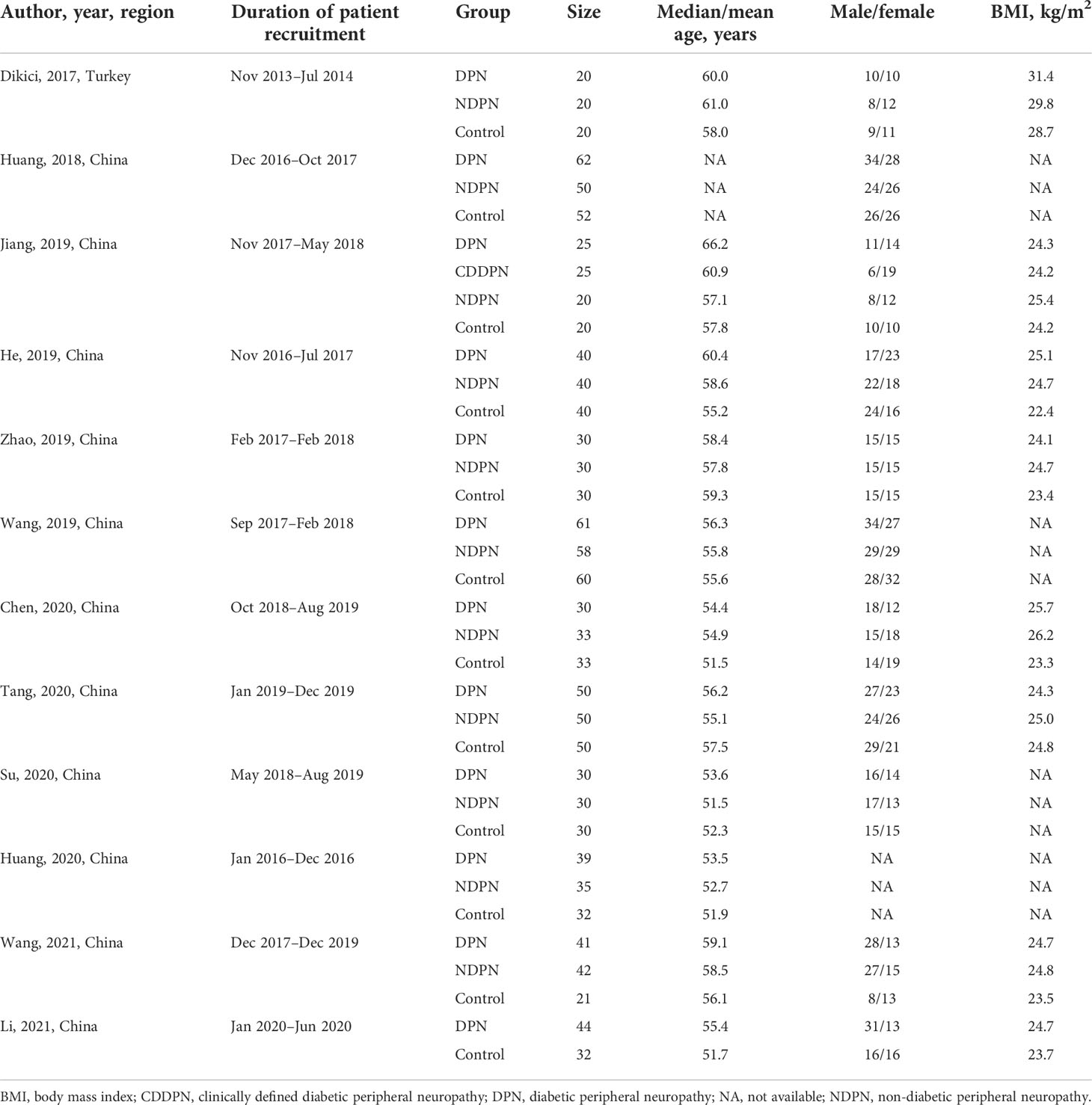
Table 1 demonstrates the basic characteristics of the studies included in this meta-analysis. Among the included studies, 10 (83.3%) studies were published between 2019 and 2021. With the exception of one study from the Turkey, the vast majority of published studies were from China. In total, 1325 subjects were included in this meta-analysis for evaluation. Specifically, they were 472 DPN patients, 25 clinically defined DPN patients (i.e., patients with clinical signs or symptoms of DPN but normal NCS), 408 non-diabetic peripheral neuropathy (NDPN) patients, and 420 control participants. In the DPN and NDPN groups, the study populations were confirmation of type 2 DM patients.
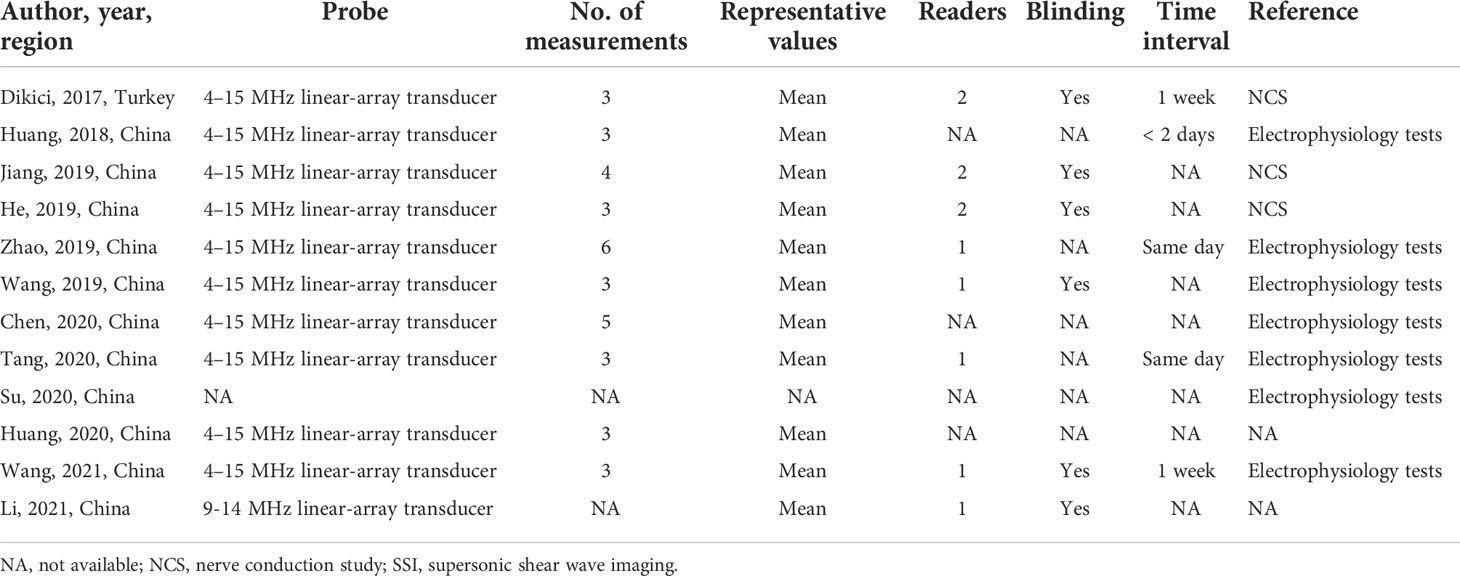
The technical characteristics of the SSI technique used in the included studies are summarized in Table 2. Most of the included studies were used a 4–15 MHz linear array transducer (10, 83.3%), when performing examinations with SSI technique. In addition to one study, 11 studies used the mean value to determine the representative value of elasticity. As the measure of tibial nerve stiffness by SSI, 10 studies used elasticity (kilopascals), and two studies used the shear wave speed (meters per second).
Diagnostic performance of SSI for the detection of DPN
In total, 12 studies (with 472 DPN patients, 25 clinically defined DPN patients, 408 NDPN patients, and 420 control participants) were included for the quantitative analysis to investigate the accuracy of tibial nerve stiffness measurement by SSI for the diagnosis of DPN.
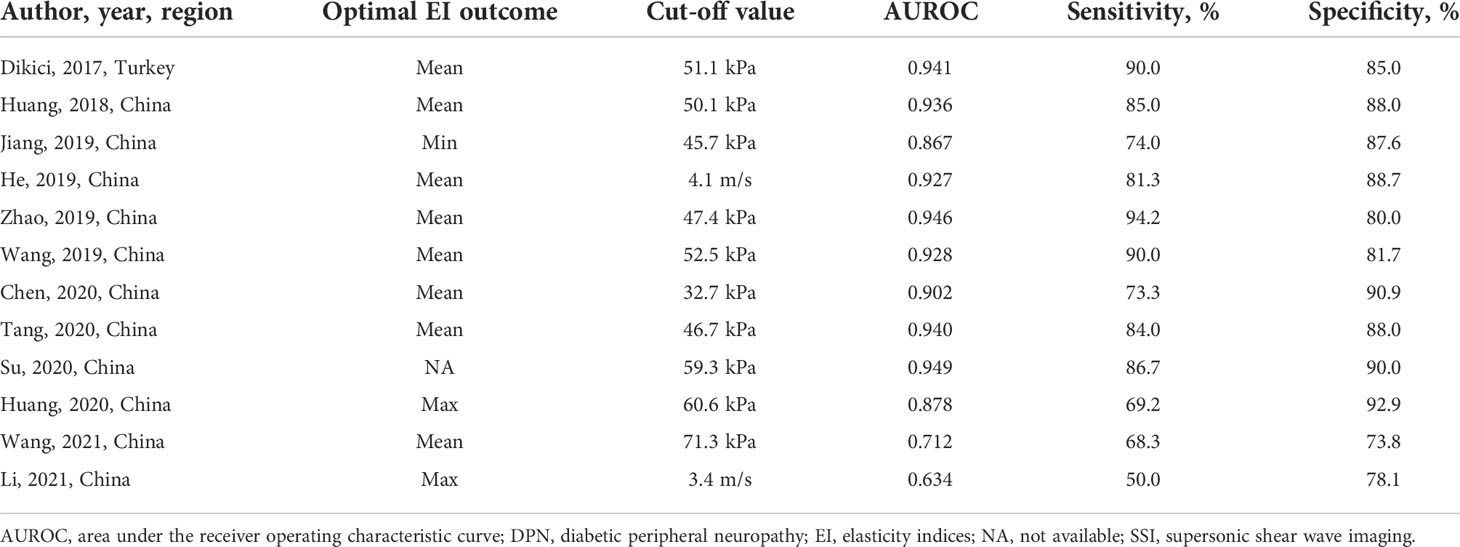
The sensitivity and specificity of the included studies ranged from 50.0% to 94.2% and 73.8% to 92.9%, respectively, as shown in Table 3. Figure 2 demonstrates that, for tibial nerve stiffness measurement by SSI, the summary sensitivity and specificity for the diagnosis of DPN were 80% (95% CI: 73%–86%) and 86% (95% CI: 82%–89%), respectively. The mean AUROC value of tibial nerve stiffness measurement by SSI for diagnosing DPN was 0.880 (range, 0.634–0.949). When the results were combined, the summary AUROC value of the SROC was 0.90 (95% CI: 0.87–0.92), as shown in Figure 3. Moreover, we found that the summary DOR of tibial nerve stiffness measurement by SSI was 25 (95% CI: 15–41; Table 4), for diagnosing DPN.
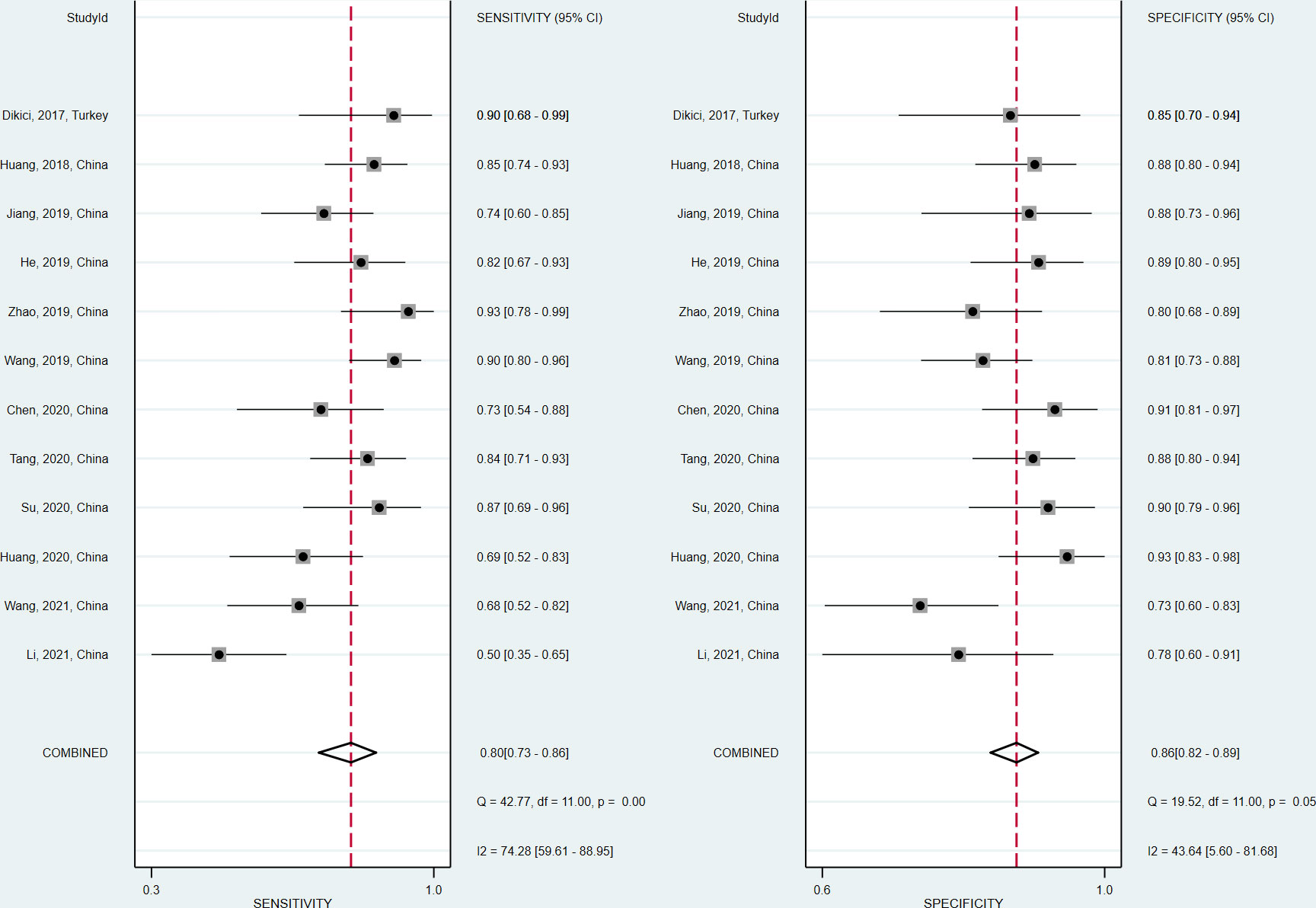
Figure 2 Coupled forest plots of the sensitivity and specificity of tibial nerve stiffness measurement by Supersonic Shear Wave Imaging (SSI) for diagnosing diabetic peripheral neuropathy (DPN).
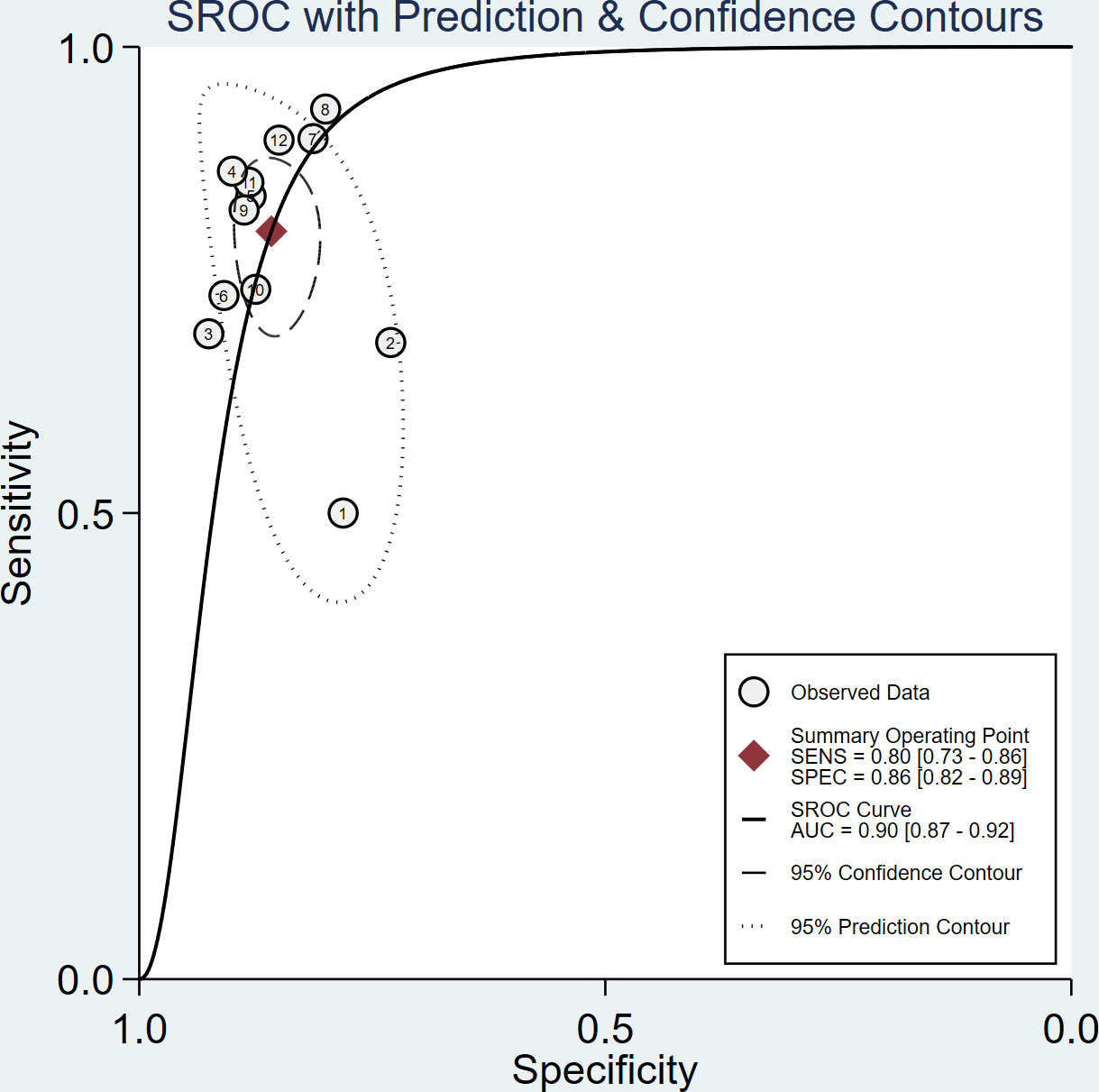
Figure 3 Summary receiver operating characteristic (SROC) curve of tibial nerve stiffness measurement by Supersonic Shear Wave Imaging (SSI) for diagnosing diabetic peripheral neuropathy (DPN).
A subgroup analysis of 11 SSI studies from China revealed similar diagnostic performance. The results showed that the summary sensitivity and specificity of tibial nerve stiffness measurement by SSI for diagnosing DPN were 79% (95% CI: 72%–85%) and 86% (95% CI: 82%–89%). The summary AUROC value of the SROC was 0.90 (95% CI: 0.87–0.92), for diagnosing DPN. Detailed results are provided in Supplementary Materials.
Heterogeneity and publication bias
Spearman correlation coefficient was calculated to evaluate the threshold effect of the included studies. We found that there was no threshold effect among the SSI studies, as depicted by the Spearman correlation coefficient of 0.042 (P = 0.897). Cochran’s Q-test and I2 statistic showed substantial heterogeneity with regard to the summary sensitivity (I2 = 74.28%, P < 0.1) and moderate heterogeneity with regard to the summary specificity (I2 = 43.63%, P < 0.1). There was no evidence of publication bias in SSI studies in diagnosing DPN in this meta-analysis (P = 0.46), as shown in Figure 4.
Discussion
In the present study, we only collected relevant data from the SSI for evaluation and meta-analysis and excluded other types of ultrasound elastography, and therefore, this major source of heterogeneity may be avoided. Based on a systematically literature search in English and Chinese electronic database, we aimed to obtain comprehensive evidence on the value of tibial nerve stiffness measurement by SSI in the diagnosis of DPN.
In this meta-analysis, we identified 12 eligible studies to assess the performance of tibial nerve stiffness measurement by SSI for diagnosing DPN. Pooled analysis of 12 included studies showed that a tibial nerve stiffness measurement by SSI exhibited good diagnostic performance, with a summary sensitivity of 80% (95% CI: 73%–86%), specificity of 86% (95% CI: 82%–89%), and AUROC of 0.90 (95% CI: 0.87–0.92). A subgroup analysis of 11 SSI studies from China revealed similar diagnostic performance, it showed that the summary sensitivity and specificity were 79% (95% CI: 72%–85%) and 86% (95% CI: 82%–89%), respectively, and the summary AUROC was 0.90 (95% CI: 0.87–0.92). Our findings thus indicate that SSI, as a novel ultrasound elastography technique, can be used as a noninvasive tool for diagnosing DPN.
In recent years, ultrasound elastography has gained traction as a new imaging technique for obtaining tissue biomechanical characteristics (18, 37). SSI is a novel as well as one of the most widely used ultrasound elastography for the evaluation of neuromuscular pathologies, which allows real-time visualization of tissue viscoelastic properties beyond conventional B mode ultrasound, and shear wave velocity or Young’s modulus for region of interest can be obtained quantitatively and dynamically (38). SSI has some potential advantages over other types of ultrasound elastography techniques, such as transient elastography and point shear wave elastography (37, 38). Indeed, previous studies have revealed that in diabetic patients, the tibial nerve is stiffer when measured with SSI (25, 35). Even in diabetic patients without DPN, the tibial nerve stiffness was significantly higher than in control subjects (25, 35). This suggests that SSI-based stiffness measurements of the nerve can reflect the nerve changes in diabetic patients indirectly.
Electrophysiological examinations are still the cornerstone of neuropathy diagnosis, which can be provide detailed information about the dysfunction of affected nerves (39). Notably, this technique mainly evaluates the changes of the large nerve fibers, while in DPN patients, small nerve fibers are the first to be affected (10, 40). Compared to the controls, the tibial nerve is stiffer in NDPN groups when measured with SSI (25, 35). It may appear earlier than can be seen in abnormal electrophysiological examinations. Therefore, SSI has potential value as an effective tool in the early detection of subelectrophysiological DPN (35).
It is necessary to mentioned that heterogeneity was found across the studies included for the present meta-analysis. This may be due to several factors, such as characteristics of study population, methods of DPN diagnosis and SSI measurements, and sometimes a variable distribution of the severity of diabetes. In the study conducted by Wang et al. (35), authors concluded that no significant correlation between the tibial nerve stiffness and the DM duration was existed, regardless of the DPN history. However, other factors, such as age and height of patients (27, 41), may have potential influence on the measurement of tibial nerve stiffness by SSI. Moreover, ultrasound machine transducer and its orientation, and other technical considerations and artifacts may also influence the resulting values when performing SSI measurements (18). We did not conducted meta-regression and subgroup analyses to explore these confounding factors, however, by the small number of studies included in this meta-analysis. As a consequence, more research is needed in the future.
Some limitations of this meta-analysis are noteworthy. First, in addition to the tibial nerve, the most frequently involved site (25), DPN may affect many more peripheral nerves, such as the median nerve and sural nerve. DPN is a multiple peripheral nerve disease; therefore, future studies will be needed for the investigation of other nerves and multiple anatomic regions of the same nerve using SSI (28). Second, all but one of the studies included in this meta-analysis were based on Chinese patients, and the results may not be generalizable to other populations. A prospective, multicenter investigation with a large sample is thus warrented. Third, in this meta-analysis, we did not studied the relationship of the stiffness of the tibial nerve and the degree of diabetic neuropathy. Finally, the optimal cut-off value of the tibial nerve on SSI for diagnosing DPN was not established. In order to ensure its applicability to clinical use, further work is required to identify the optimal cut-off value for SSI.
In conclusion, our meta-analysis has suggested that a tibial nerve stiffness measurement by SSI shows good performance in diagnosing DPN, which provides more information for clinical treatment. Therefore, SSI has considerable potential as a noninvasive tool for detecting DPN. Notably, the cut-off values for detecting DPN with this method still vary greatly across the included studies. Further work is required to identify the optimal cut-off value for SSI of the tibial nerve to ensure its applicability to clinical use.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YC contributed to the study design, YC and HD contributed to the literature search. YC, LH and ZJ completed the data analysis. YC and HH generated the figures and tables. YC completed the manuscript. YC and HH proofread the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Sciences and Technology project of Fujian Provincial Department (2019J01166), Innovative medical research project of Fujian Province (2018-CX-33) and The Second Affiliated Hospital of Fujian Medical University horizontal scientific research project (HX202201 and HX202202).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.934749/full#supplementary-material
References
1. Wei J, Tian J, Tang C, Fang X, Miao R, Wu H, et al. The influence of different types of diabetes on vascular complications. J Diabetes Res (2022) 2022:3448618. doi: 10.1155/2022/3448618
2. Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet (2011) 378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X
3. Carmichael J, Fadavi H, Ishibashi F, Shore AC, Tavakoli M. Advances in screening, early diagnosis and accurate staging of diabetic neuropathy. Front Endocrinol (Lausanne) (2021) 12:671257. doi: 10.3389/fendo.2021.671257
4. Neeland IJ, Patel KV. Chapter 4 - diabetes: Key markers of injury and prognosis. In: Nambi V, editor. Biomarkers in cardiovascular disease. Netherlands: Elsevier (2019). p. 41–51.
5. International Diabetes Federation. IDF diabetes atlas (2019). Available at: https://www.diabetesatlas.org (Accessed 1 May 2022).
6. Hicks CW, Selvin E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr Diabetes Rep (2019) 19(10):86. doi: 10.1007/s11892-019-1212-8
7. Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K. Microvascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol (2020) 18(2):117–24. doi: 10.2174/1570161117666190502103733
8. Feldman EL, Nave KA, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: Mechanisms, bioenergetics, and pain. Neuron (2017) 93(6):1296–313. doi: 10.1016/j.neuron.2017.02.005
9. Zhang Y, Li N, Zhao Y, Fan D. Painful diabetic peripheral neuropathy study of chinese OutPatiEnts (PDN-SCOPE): Protocol for a multicentre cross-sectional registry study of clinical characteristics and treatment in china. BMJ Open (2019) 9(4):e025722. doi: 10.1136/bmjopen-2018-025722
10. Yu Y. Gold standard for diagnosis of DPN. Front Endocrinol (Lausanne) (2021) 12:719356. doi: 10.3389/fendo.2021.719356
11. Sloan G, Shillo P, Selvarajah D, Wu J, Wilkinson ID, Tracey I, et al. A new look at painful diabetic neuropathy. Diabetes Res Clin Pract (2018) 144:177–91. doi: 10.1016/j.diabres.2018.08.020
12. Binns-Hall O, Selvarajah D, Sanger D, Walker J, Scott A, Tesfaye S. One-stop microvascular screening service: an effective model for the early detection of diabetic peripheral neuropathy and the high-risk foot. Diabetes Med (2018) 35(7):887–94. doi: 10.1111/dme.13630
13. Banik PC, Barua L, Moniruzzaman M, Mondal R, Zaman F, Ali L. Risk of diabetic foot ulcer and its associated factors among bangladeshi subjects: a multicentric cross-sectional study. BMJ Open (2020) 10(2):e034058. doi: 10.1136/bmjopen-2019-034058
14. Wang F, Zhang J, Yu J, Liu S, Zhang R, Ma X, et al. Diagnostic accuracy of monofilament tests for detecting diabetic peripheral neuropathy: A systematic review and meta-analysis. J Diabetes Res (2017) 2017:8787261. doi: 10.1155/2017/8787261
15. Feng Y, Schlösser FJ, Sumpio BE. The semmes weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vasc Surg (2009) 50(3):675–682, 682.e1. doi: 10.1016/j.jvs.2009.05.017
16. Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol (2015) 41(5):1126–47. doi: 10.1016/j.ultrasmedbio.2015.03.009
17. Wei H, Jiang HY, Li M, Zhang T, Song B. Two-dimensional shear wave elastography for significant liver fibrosis in patients with chronic hepatitis b: A systematic review and meta-analysis. Eur J Radiol (2020) 124:108839. doi: 10.1016/j.ejrad.2020.108839
18. Wee TC, Simon NG. Ultrasound elastography for the evaluation of peripheral nerves: A systematic review. Muscle Nerve (2019) 60(5):501–12. doi: 10.1002/mus.26624
19. Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound elastography: Review of techniques and clinical applications. Theranostics (2017) 7(5):1303–29. doi: 10.7150/thno.18650
20. Lima KMME, Costa Júnior JFS, Pereira WCA, Oliveira LF. Assessment of the mechanical properties of the muscle-tendon unit by supersonic shear wave imaging elastography: a review. Ultrasonography (2018) 37(1):3–15. doi: 10.14366/usg.17017
21. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ (2009) 339:b2700. doi: 10.1136/bmj.b2700
22. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
24. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol (2005) 58(9):882–93. doi: 10.1016/j.jclinepi.2005.01.016
25. Dikici AS, Ustabasioglu FE, Delil S, Nalbantoglu M, Korkmaz B, Bakan S, et al. Evaluation of the tibial nerve with shear-wave elastography: A potential sonographic method for the diagnosis of diabetic peripheral neuropathy. Radiology (2017) 282(2):494–501. doi: 10.1148/radiol.2016160135
26. Huang W. The value of shear wave elastography in diagnosis of type 2 diabetic peripheral neuropathy. Nanchang University (2018).
27. Jiang W, Huang S, Teng H, Wang P, Wu M, Zhou X, et al. Diagnostic performance of two-dimensional shear wave elastography for evaluating tibial nerve stiffness in patients with diabetic peripheral neuropathy. Eur Radiol (2019) 29(5):2167–74. doi: 10.1007/s00330-018-5858-4
28. He Y, Xiang X, Zhu BH, Qiu L. Shear wave elastography evaluation of the median and tibial nerve in diabetic peripheral neuropathy. Quant Imaging Med Surg (2019) 9(2):273–82. doi: 10.21037/qims.2019.02.05
29. Zhao W, Guo R, Wu Y, Cao W, Lu R, Lv C, et al. Shear wave elastography in observation on tibial nerve changes in patients with diabetic peripheral neuropathy. Chin J Med Imaging Technol (2019) 35(8):1142–6. doi: 10.13929/j.1003-3289.201902015
30. Wang Y, Nie F, Wang Y, Wang T, Chen B. Evaluation of shear wave elastography for lower extremity neuropathy in patients with type 2 diabetes. Chin J Clin Res (2019) 32(9):1193–6. doi: 10.13429/j.cnki.cjcr.2019.09.010
31. Chen R, Wang XL, Xue WL, Sun JW, Dong XY, Jiang ZP, et al. Application value of conventional ultrasound and real-time shear wave elastography in patients with type 2 diabetic polyneuropathy. Eur J Radiol (2020) 126:108965. doi: 10.1016/j.ejrad.2020.108965
32. Tang Y, Chen W, Jin L, Li Q. The value of high frequency ultrasound combined with shear wave elastography in diagnosis of type 2 diabetic peripheral neuropathy. Sichuan Med J (2020) 41(12):1217–22. doi: 10.16252/j.cnki.issn1004-0501-2020.12.001
33. Su J, Xu B, Xu Q, Su Y, Cui H. Diagnostic value of tibial nerve measurement with ultrasound elastography in early type 2 diabetic peripheral neuropathy. Yi Yao Qian Yan (2020) 10(11):122–4.
34. Huang Y, Feng X, Zhu M. The value of ultrasonic shear wave elastography in diabetic peripheral neuropathy. Med J West China (2020) 32(4):567–71. doi: 10.3969/j.issn.1672-3511.2020.04.024
35. Wang F, Zheng M, Hu J, Fang C, Chen T, Wang M, et al. Value of shear wave elastography combined with the toronto clinical scoring system in diagnosis of diabetic peripheral neuropathy. Med (Baltimore) (2021) 100(35):e27104. doi: 10.1097/MD.0000000000027104
36. Li Y, Zheng M, Gao F. Shear wave elastography evaluation of lower limb nerve injury in type 2 diabetes. J Yan'an Univ (Medical Sci Edition) (2021) 19(2):25–9. doi: 10.19893/j.cnki.ydyxb.2021-0105
37. Shang J, Wang W, Feng J, Luo GG, Dang Y, Sun J, et al. Carotid plaque stiffness measured with supersonic shear imaging and its correlation with serum homocysteine level in ischemic stroke patients. Korean J Radiol (2018) 19(1):15–22. doi: 10.3348/kjr.2018.19.1.15
38. Bercoff J, Tanter M, Fink M. Supersonic shear imaging: A new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control (2004) 51(4):396–409. doi: 10.1109/tuffc.2004.1295425
39. Dyck PJ, Overland CJ, Low PA, Litchy WJ, Davies JL, Dyck PJ, et al. Signs and symptoms versus nerve conduction studies to diagnose diabetic sensorimotor polyneuropathy: Cl vs. NPhys trial. Muscle Nerve (2010) 42(2):157–64. doi: 10.1002/mus.21661
40. Burgess J, Frank B, Marshall A, Khalil RS, Ponirakis G, Petropoulos IN, et al. Early detection of diabetic peripheral neuropathy: A focus on small nerve fibres. Diagnostics (Basel) (2021) 11(2):165. doi: 10.3390/diagnostics11020165
41. Ishibashi F, Taniguchi M, Kojima R, Kawasaki A, Kosaka A, Uetake H. Elasticity of the tibial nerve assessed by sonoelastography was reduced before the development of neuropathy and further deterioration associated with the severity of neuropathy in patients with type 2 diabetes. J Diabetes Investig (2016) 7(3):404–12. doi: 10.1111/jdi.12408
Keywords: diabetic peripheral neuropathy, diagnosis, tibial nerve, stiffness measurement, supersonic shear wave imaging
Citation: Chen Y, Duan H, Huang L, Jiang Z and Huang H (2022) Supersonic shear wave imaging of the tibial nerve for diagnosis of diabetic peripheral neuropathy: A meta-analysis. Front. Endocrinol. 13:934749. doi: 10.3389/fendo.2022.934749
Received: 03 May 2022; Accepted: 01 August 2022;
Published: 02 September 2022.
Edited by:
Xinguo Hou, Qilu Hospital, Shandong University, ChinaReviewed by:
Mohammad H. Abukhalil, Al-Hussein Bin Talal University, JordanTakahisa Deguchi, Kagoshima University, Japan
Copyright © 2022 Chen, Duan, Huang, Jiang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huibin Huang, huibinhuang@aliyun.com
†These authors have contributed equally and share first authorship
 Yuping Chen
Yuping Chen Honghong Duan2†
Honghong Duan2† Huibin Huang
Huibin Huang