- 1Department of Endocrinology, Key Laboratory of Endocrinology of Ministry of Health, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Science, Beijing, China
- 2Department of Endocrinology, the First Affiliated Hospital of Fujian Medical University, Fuzhou, China
Purpose: This study aimed to investigate the skeletal outcomes of patients with osteogenesis imperfecta (OI) who received bisphosphonate (BP) treatment and entered drug holiday after achieving an age- and sex-specific bone mineral density (BMD) reference.
Methods: Patients with OI receiving BP treatment were enrolled when they entered drug holidays of BPs. The skeletal outcomes were evaluated in detail during the drug holiday, including BMD, X-ray of the bone, bone fracture incidence, and bone turnover biomarkers. The pathogenic mutations of OI were identified by next-generation sequencing and confirmed by Sanger sequencing.
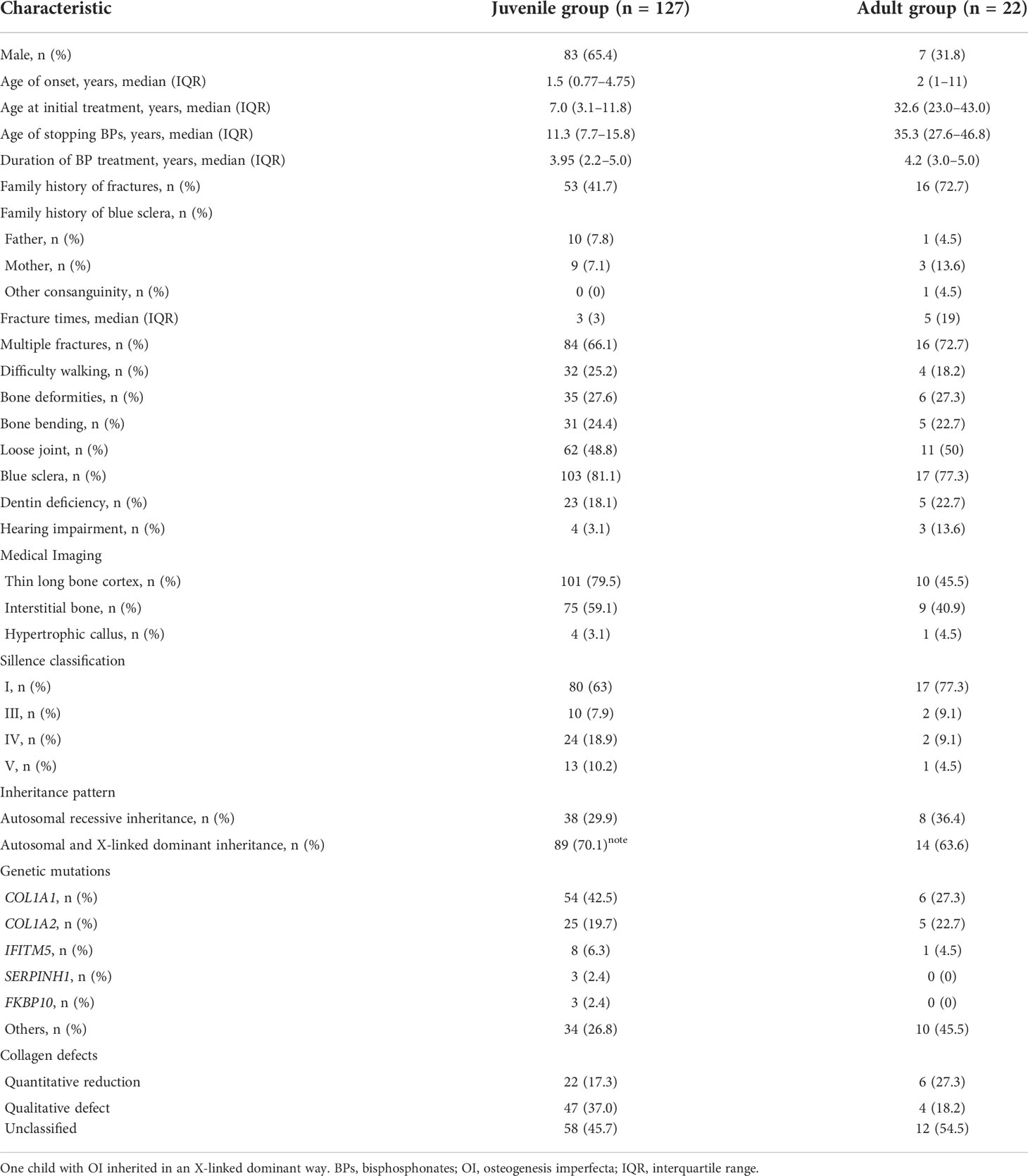
Results: A total of 149 OI patients (127 juveniles and 22 adults) who entered drug holidays after nearly 4 years of BP treatment were included. Areal BMD at the lumbar spine increased from 0.934 ± 0.151 to 0.990 ± 0.142 g/cm2 and was stable in the second (1.029 ± 0.176 g/cm2) and third years (1.023 ± 0.174 g/cm2) of BP drug holidays, and BMD at the femoral neck, trochanter, and total hip had no significant change, but it was gradually inferior to that of the same-gender juveniles in the second and third years of the drug holiday. BMD at the lumbar spine and proximal hip did not change and was inferior to that of the same-gender adults. The average time of fractures fluctuated from 0.18 to 0.08 per year in juveniles, while only one adult suffered from a fracture during BP drug holidays. Bone turnover markers were in the normal range, except for a mildly high level of β-carboxy-terminal cross-linked telopeptide of type 1 collagen in the juvenile group. A total of 17 (11.4%) patients received BP retreatment because of bone loss during the drug holiday. OI type III and type IV and COL1A2 mutation were correlated to a longer duration of BP treatment to enter drug holidays (all p < 0.05). Old age at initial treatment (OR, 1.056) and OI type III (OR, 10.880) were correlated to a higher risk of BP retreatment.
Conclusions: OI patients will undergo nearly 4 years of BP treatment to achieve drug holidays. During the 3 years of the drug holiday, the patients’ BMD is stable, and fracture incidence does not increase significantly. Patients are more inclined to need retreatment during drug holidays owing to the late start of BP treatment and more severe OI phenotypes.
Introduction
Osteogenesis imperfecta (OI) is an inherited skeletal dysplasia characterized by bone fragility and skeletal deformities, with an estimated incidence of 1 in 10,000–20,000 neonates (1, 2). OI also leads to dental and craniofacial abnormalities, muscle weakness, hearing loss, and respiratory and cardiovascular complications (1). The majority of OI patients are associated with pathogenic variants in COL1A1 and COL1A2, the encoding genes of type I collagen, and the minority of OI patients are related to mutations in other genes that are involved in type I collagen biosynthesis or osteoblast differentiation or bone mineralization (3). The clinical classification of OI includes type I to type V, of which the severity broadly ranges from nearly asymptomatic cases with a normal life span to severe bone deformities, mobility impairment, and even perinatal mortality (4).
Treatment for OI is a great challenge, which is primarily supportive and symptomatic, including management with medications, physical therapy, and even orthopedic interventions to improve bone strength, reduce fracture risk, and improve mobility (5, 6). Bisphosphonates (BPs) are the most commonly used medications for OI, which can increase bone mineral density (BMD), reduce bone fracture risk, and lead to the reshaping of the vertebra (7–10), but the optimal duration of BP therapy in OI patients is unknown. Studies have shown that the therapeutic benefits for OI patients from BP treatment are more apparent in the first 2 to 4 years (11). Otherwise, iatrogenic osteopetrosis has been described with excessive treatment, and long-term BP therapy is associated with an increased risk of atypical femoral fracture in patients with OI (12, 13). Thus, many clinical concerns about BP treatment are worthy of investigation in OI patients, including when to enter the drug holiday of BPs, how long the drug holiday should hold, and how the skeletal outcomes during BP discontinuation.
Therefore, we aimed to investigate the skeletal outcomes during the drug holiday of BPs and their associated factors for OI patients when they entered the drug holiday.
Materials and methods
Study participants
Patients were diagnosed with OI if they had either a history of at least one non-traumatic or low-impact fracture and an age-adjusted and sex-adjusted areal BMD Z-score of –1.0 or less for either total body or lumbar spine sites, or an adjusted areal BMD Z-score of –2.0 or less irrespective of a history of fractures (14, 15). For patients without a family history of non-traumatic fracture, a diagnosis of OI was made if they had more than one non-traumatic fracture and at least a kind of extra-skeletal manifestations or with a genetic diagnosis of OI (14, 15).
The study comprised patients with OI who received BP treatment (alendronate or ibandronate or zoledronic acid) between the years 2003 and 2019 in the Endocrinology Department of Peking Union Medical College Hospital (PUMCH) and who discontinued BP treatment after achieving the age- and sex-specific normal BMD of juveniles (16, 17) and adults (18), termed drug holiday (19).
The study was approved by the ethics committee of PUMCH. Written informed consent was obtained from the patient or legal guardian of the patients before they participated in this study.
Data collection
The medical history was collected in detail, including the age of onset; the clinical information of bone pain, bone fracture, and bone deformity; and a family history of OI. The bone, joint, sclera, ears, and teeth were examined carefully. Detailed information about fractures, including time of the initiation, site, degree of trauma, frequency, and radiological evidence of fracture, was collected. The frequency of clinical fracture was calculated as the number of clinical fractures/disease courses. Bone deformities were evaluated, including limb bending, thoracic deformity, scoliosis, and pelvic deformity (20). The height of the juvenile was measured using a Harpenden stadiometer (Seritex Inc., Farmingdale, NJ, USA) and adjusted to age- and sex-specific Z-scores on the basis of reference data from the Chinese National Centers for Disease Control and Prevention (21). For patients who were unable to stand, their height was replaced by a body length in a supine position. Serious events were observed, including new bone fractures, osteonecrosis of the jaw, and atypical femoral fracture. Delayed fracture healing was also recorded during the follow-up.
Biochemical measurements
Blood samples were obtained after overnight fasting for at least 8 h. The serum concentrations of calcium (Ca), phosphate (P), total alkaline phosphatase (ALP), alanine aminotransferase (ALT), and creatinine (Cr) were measured using an automatic biochemical analyzer (ADVIA 1800, Siemens Inc., Munich, Germany). The serum levels of β-cross-linked C-telopeptide of type I collagen (β-CTX), N-terminal propeptide of type I precollagen (P1NP), 25-hydroxyvitamin D (25OHD), and intact parathyroid hormone (PTH) were measured with an automated electrochemiluminescence system (Roche Diagnostics, Basel, Switzerland). The biochemical measurements were completed in the central laboratory of PUMCH.
Bone mineral density and radiographic assessments
The BMD at the lumbar spine, femoral neck, trochanter, and total hip was measured by dual-energy X-ray absorptiometry (Lunar Prodigy Advance, GE Healthcare, Chicago, IL, USA). The BMD phantom scan was performed daily and detected no significant machine drifts during the 5-year study. The areal BMD values were converted into age- and sex-specific Z-scores using reference data from previous studies (16–18). The radiologic views of the skull, spine, hip, and limb were performed by the radiologists of PUMCH.
Detection of pathogenic mutation of osteogenesis imperfecta patients
The pathogenic mutations of OI were detected using a panel for next-generation sequencing (NGS) (Illumina HiSeq2000 platform, Illumina, Inc., San Diego, CA, USA), which covered 20 known candidate genes of OI (COL1A1, COL1A2, IFITM5, SERPINF1, FKBP10, CRTAP, P3H1, PPIB, SERPINH1, BMP1, PLOD2, SP7, TMEM38B, WNT1, CREB3H1, SPARC, PLS3, P4HB, SEC24D, and MBTPS2). The experimental procedures followed a previously described protocol (15). The mutations identified by NGS were further confirmed by Sanger sequencing.
Classification of osteogenesis imperfecta
Patients with OI were classified into subtypes based on Sillence classification and molecular diagnosis (22): type I, mild phenotype; type II, perinatally lethal; type III, a severe form with progressive deformity; type IV, with moderate severity; and type V, characterized by calcification of the forearm interosseous membrane, radial head dislocation, and hyperplastic callus formation (23). No patients with OI type II were included in this study because of perinatal death. According to molecular diagnosis, genetic mutations leading to an early stop codon or frameshift in COL1A1 were regarded as the quantitative reduction group (haploinsufficiency). Mutations causing amino acid substitutions in the triple-helical domain of COL1A1 or COL1A2 were classified into the qualitative defect group. As the effects of splice site mutation were difficult to predict, we did not include splice site mutations in the analyses (15).
Statistical analysis
Normally distributed data, such as BMD and height, were presented as mean ± standard deviation, while those with abnormal distribution were expressed as medians (interquartile ranges (IQRs)), or proportions. The differences among each time of BMD, height, and so on, during BP discontinuation were analyzed using a generalized linear mixed model. Multiple linear and binary logistic regression analyses were used to analyze related factors of the BP treatment course and restart BP treatment. A p-value of less than 0.05 indicated a statistically significant difference. The statistical analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA). Graphs were drawn using GraphPad Prism software version 6.0.
Results
Patients’ characteristics at bisphosphonate discontinuation
A total of 149 patients with OI received BP treatment and entered drug holiday, 127 of whom received BP treatment while they were still juveniles, designated as the juvenile group, while 22 patients received initial treatment during adulthood, designated as the adult group. These patients either received alendronate (12 in the juvenile group and 6 in the adult group) or zoledronate (95 in the juvenile group and 9 in the adult group) or were sequentially treated with alendronate or ibandronate and then with zoledronate (20 in the juvenile group and 7 in the adult group). The detailed follow-up records are shown in Figure 1. The median age of OI onset, age at initial treatment, and duration of BP treatment were 1.5 years (IQR 0.77–4.75 years), 7.0 years (IQR 3.1–11.8 years), and 3.95 years (IQR 2.2–5.0 years) in the juvenile group and were 2.0 years (IQR 1–11 years), 32.7 years (IQR 23.0–43.0 years), and 4.2 years (IQR 3.0–5.0 years) in the adult group, respectively, as shown in Table 1. Up to now, the time when OI patients entered drug holidays was as follows: 21 patients within 1 year, 23 patients within 2 years, 33 patients within 3 years, and 72 patients over 3 years (Figure 1). One patient had the longest follow-up of 7 years after BP discontinuation.
The pathogenic variants of OI were identified in the majority of the patients. Sixty patients carried COL1A1 mutation, 30 with COL1A2 mutation, 9 with IFITM5 mutation, 3 with SERPINF1 mutation, 3 with FKBP10 mutation, 2 with PLOD2 mutation, and 2 with WNT1 mutation, and mutations in TMEM38B, CRTAP, PLS3, and P4HB were found in one patient. Non-mutation was detected in 36 OI patients (Table 1).
Skeletal outcomes after bisphosphonate discontinuation
After BP discontinuation, the height of juveniles with OI increased from 139.72 ± 24.28 to 145.51 ± 25.43, 147.05 ± 20.60, and 148.77 ± 19.38 cm in the first, second, and third years of drug holiday, respectively, but the juveniles had lower height Z-scores, which suggested that the OI patients were shorter than their peers. The heights of adults had no obvious change during the drug holidays (Figures 2A, B). Meanwhile, no significant change was observed in the serum levels of β-CTX, Ca, P, 25OHD, and PTH during the 3 years of the drug holidays in juveniles and adults, except for mildly increased β-CTX levels in juveniles (Figures 3A–F). Liver and kidney functions were normal during the 3 years of BP discontinuation (Supplementary Figures 1A, B). No patients suffered from osteonecrosis of the jaw and atypical femoral fracture during the whole drug holiday.
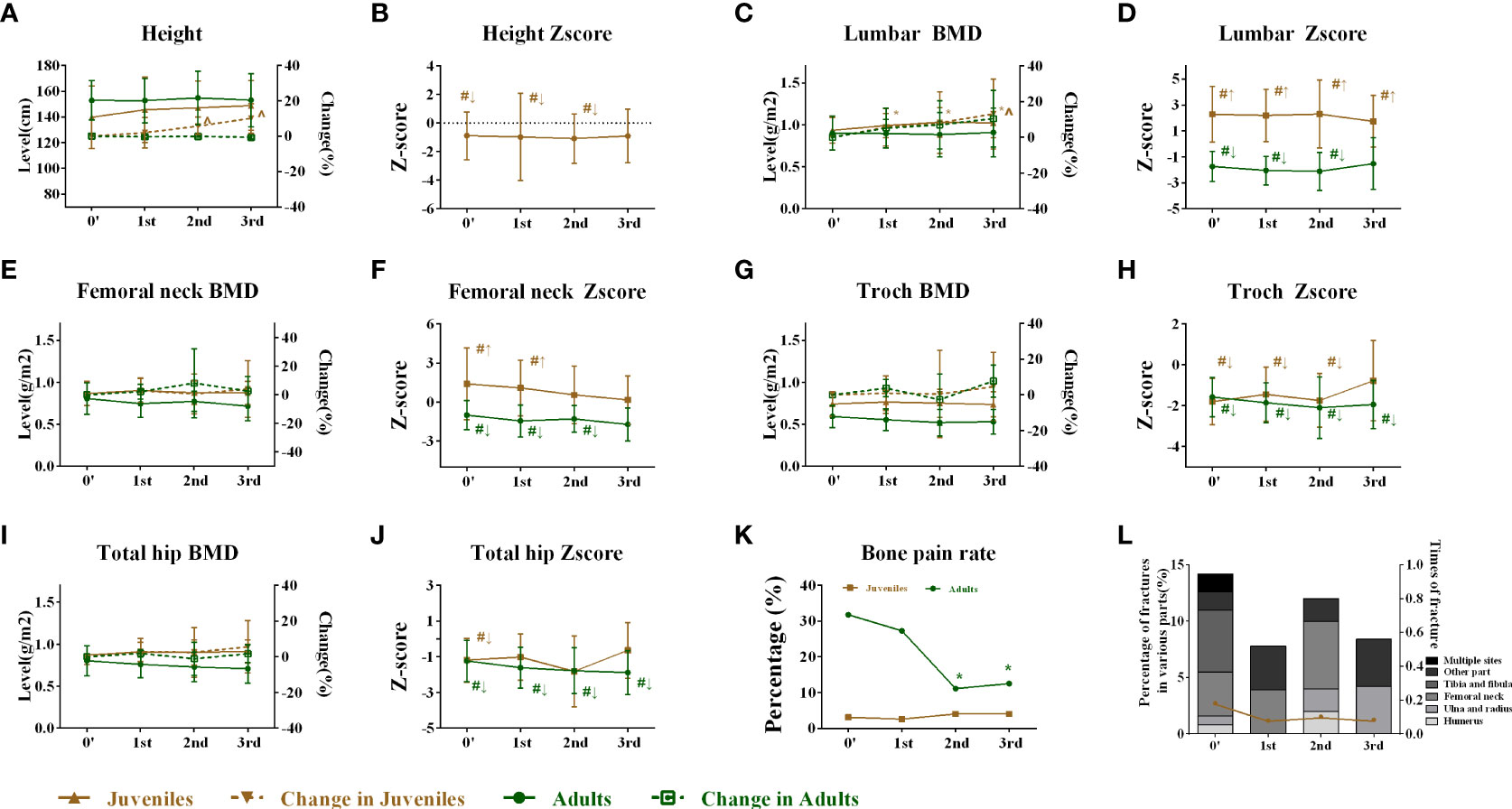
Figure 2 Changes in height and skeletal outcomes after bisphosphonate discontinuation. (A) Changes in height during the follow-up. (B) Changes in the height Z-score during the follow-up. (C) Changes in the lumbar BMD during the follow-up. (D) Changes in the lumbar Z-score during the follow-up. (E) Changes in the femoral neck BMD during the follow-up. (F) Changes in the femoral neck Z-score during the follow-up. (G) Changes in the trochanter BMD during the follow-up. (H) Changes in the trochanter Z-score during the follow-up. (I) Changes in the total hip BMD during the follow-up. (J) Changes in the total hip Z-score during the follow-up. (K) Changes in the bone pain rate during the follow-up. (L) Changes in average times per year of fractures and fracture rates during the follow-up. *Level compared with baseline after bisphosphonate discontinuation, p < 0.05. †Level compared with the first year after bisphosphonate discontinuation, p < 0.05. &Level compared with the second year after bisphosphonate discontinuation, p < 0.05. ^Change rate compared with 0′ after bisphosphonate discontinuation, p < 0.05. #Level compared with average Z-score of their peers (value = 0). ↑Superior to their same-sex peers. ↓Inferior to the same-sex peers. BMD, bone mineral density.
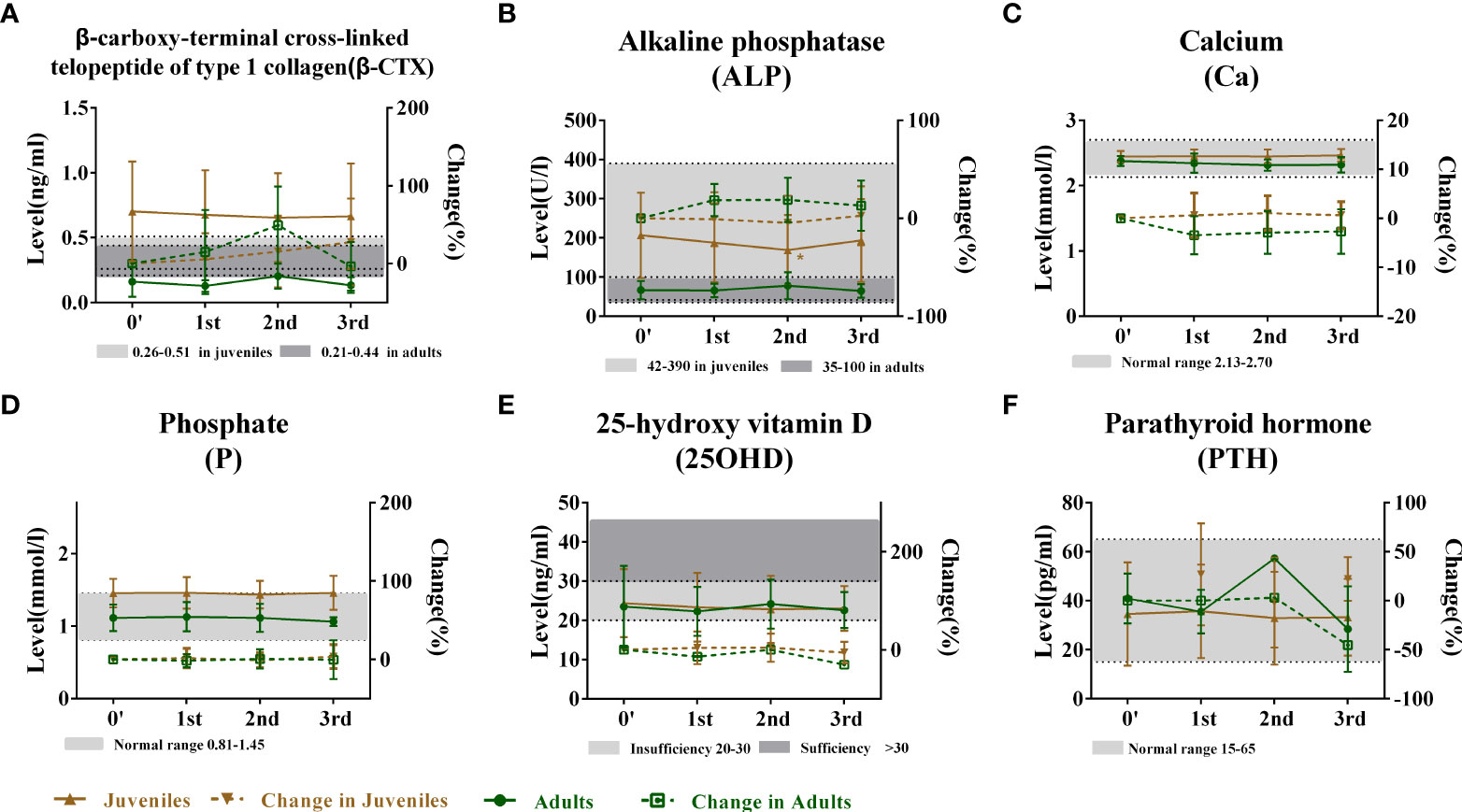
Figure 3 Changes in bone metabolic markers after bisphosphonate discontinuation. (A) Changes in the serum ALP level during the follow-up. Normal range of ALP (42–390 U/L in juvenile group and 35–100 U/L in adult group) marked by gray. (B) Changes in the serum β-CTX level during the follow-up. Normal range of β-CTX (0.26–0.51 ng/ml in juvenile group and 0.21–0.44 ng/ml in adult group) marked by gray. (C) Changes in serum Ca level during the follow-up. Normal range of Ca (2.13–2.7 mmol/L) marked by gray. (D) Changes in the serum P level during the follow-up. Normal range of P (0.81–1.45 mmol/L) marked by gray. (E) Changes in the serum 25OHD level during the follow-up. Level of 25OHD with more than 20 ng/ml marked by gray. (F) Changes in the serum PTH level during the follow-up. Normal range of PTH (15–65 pg/ml) marked by gray. *Level compared with baseline after bisphosphonate discontinuation, p < 0.05. †Level compared with the first year after bisphosphonate discontinuation, p < 0.05. &Level compared with the second year after bisphosphonate discontinuation, p < 0.05. ^Change rate compared with 0′ after bisphosphonate discontinuation, p < 0.05. ALP, alkaline phosphatase; PTH, parathyroid hormone.
In juveniles, the areal BMD at the lumbar spine increased from 0.934 ± 0.151 to 0.990 ± 0.142 g/cm2 from the baseline (0′) to the first year of BP discontinuation and was stable in the second (1.029 ± 0.176 g/cm2) and third years (1.023 ± 0.174 g/cm2) in the juveniles, with no significant change in BMD Z-score (Figures 2C, D). The femoral neck BMD of juveniles was stable, but the Z-score gradually declined during the drug holidays (Figures 2E, F). No significant change was observed in the trochanter and the total hip BMD during the 3 years of BP discontinuation. However, the BMD of the juvenile was inferior to that of the peers in trochanter during the first and second years of the drug holidays (Figures 2G–J). The bone pain incidence fluctuated from 3.1% to 4.0%, and the average times of fractures of the juveniles fluctuated from 0.18 to 0.08 per year (Figures 2K, L).
In the adults, the BMD at the lumbar spine did not change in the 3 years of the drug holidays with a lower Z-score in the first 2 years until the third year (Figures 2C, D). The femoral neck BMD and Z-score of adults remained stable during the whole drug holiday (Figures 2E, F). Similarly, no significant change was observed in the trochanter and the total hip BMD during the 3 years of BP discontinuation. However, the BMD of adults was inferior to that of peers in trochanter during the 3 years of the drug holidays (Figures 2G–J). The bone pain incidence fluctuated from 31.8% to 12.5%, while only one adult suffered from a fracture in the second year of BP discontinuation.
Related factors of bisphosphonate treatment course and restart treatment
We evaluated the related factors regarding the duration of BP treatment to enter the drug holiday. As shown in Figure 4, OI type III (β = 1.542, p = 0.018), OI type IV (β = 1.155, p = 0.014), and COL1A2 mutation (β = 1.091, p = 0.020) were positively correlated with a longer duration of BP treatment before patients entered drug holiday.
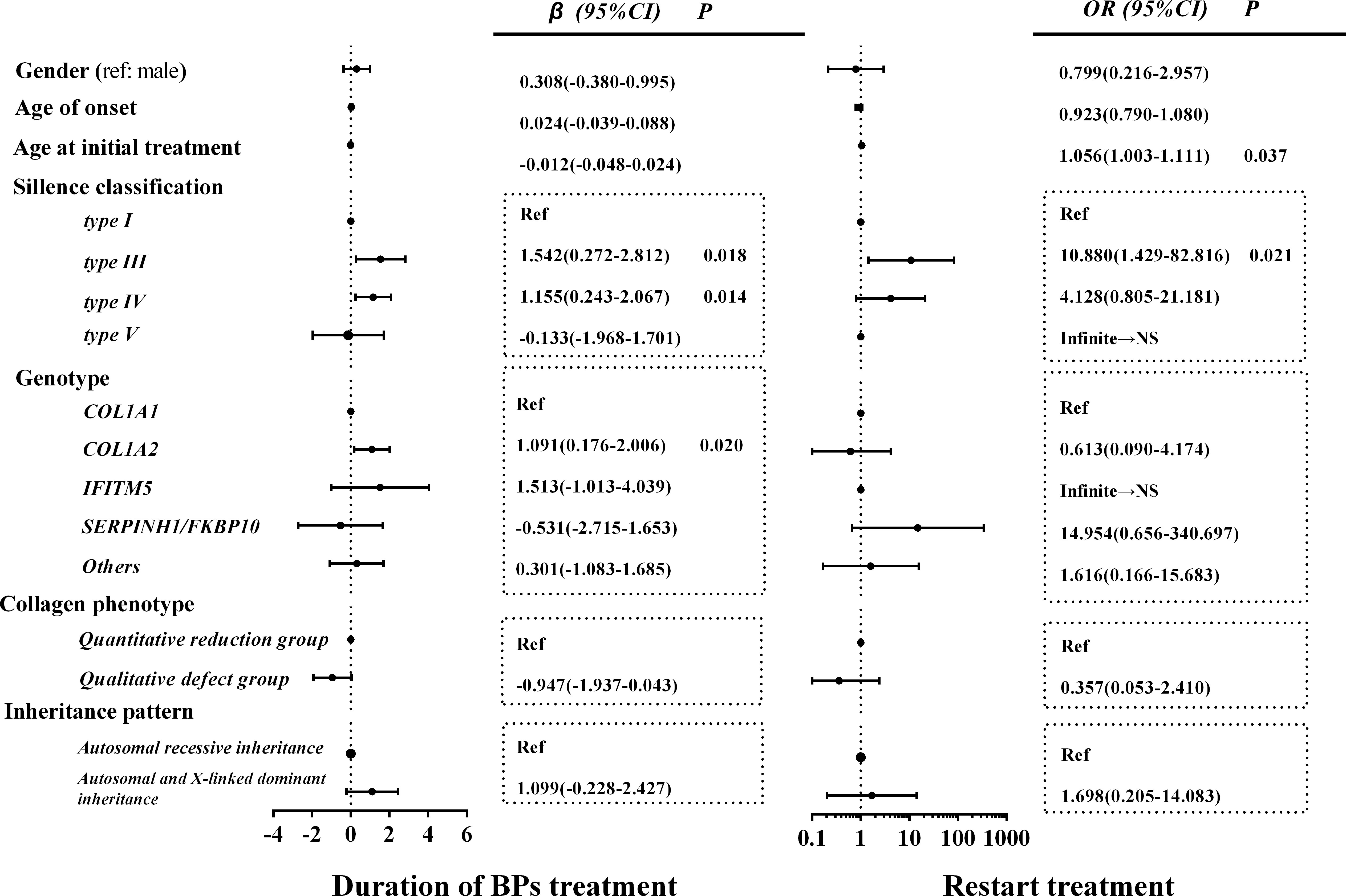
Figure 4 Related factors of BP treatment course and restart treatment of BPs. In the collagen phenotype group, those other than the quantitative and qualitative defect groups were classified as other groups to ensure that all data were fully analyzed. Data of other groups are not shown. NS, non-significant; BP, bisphosphonate.
There were 17 patients (11.4%, a median drug holiday of 2 years) who started the retreatment of BPs because of a decrease in BMD, including 12 juveniles (9.4%) and 5 adults (22.7%). The old age at initial treatment (OR, 1.056; 95% CI, 1.003–1.111) and type III OI (OR, 10.880; 95% CI, 1.429–82.816) were significantly correlated with the retreatment of BPs. However, no significant association was found between gender, age of OI onset, genotype, patterns of inheritance, and retreatment of BPs (Figure 4). The longest drug holiday was observed in an OI patient with a mutation in COL1A2, who received 2 years of zoledronic acid treatment and then entered a drug holiday. During the 7 years of the drug holiday, his lumbar and proximal hip BMD continued to increase (Supplementary Table 1). As he still had age- and sex-specific normal BMD, he did not receive retreatment.
Discussion
This was a novel clinical study to evaluate the skeletal outcome during drug holidays of BPs in a large cohort of juveniles and adults with moderate-to-severe OI. During the 3-year observation in drug holidays, the serum levels of biochemical indexes were normal, except for a mildly high level of β-CTX in juveniles. The lumbar spine BMD increased in the first year and was stable in the second and third years of drug holiday in the juveniles. The proximal hip BMD had no significant change during the drug holiday, but its Z-score tended to decline gradually during the drug holidays in juveniles. Both lumbar spine and proximal hip BMD remained stable in the adult group. New fracture incidences remained at a lower level, which did not increase during the drug holiday. Meanwhile, OI type III and type IV and COL1A2 mutation were positively correlated with a longer duration of BP treatment to achieve the drug holiday, while patients with a later onset of BP treatment and severe clinical phenotypes were associated with a higher risk of BP retreatment.
BPs are the most commonly used medications for osteoporosis, which play important roles by effectively inhibiting osteoclast activities, increasing BMD, and reducing bone fracture incidence (24). Nitrogenous BPs could disrupt osteoclasts formation, survival, and cytoskeletal dynamics, and alendronate, pamidronate, and zoledronate were commonly used BPs for patients with OI. However, long-term BP treatment could increase the microcracks in the bone, thereby increasing bone fragility and the risk of atypical fracture (25, 26). The persistent effects of BPs on the bone led to the concept of a drug holiday, which was designed to minimize side effects and maximize benefits. As the mechanical properties and structure of the bone in OI patients were markedly different from those of the normal bone, bone fragility was significantly high in OI patients. Therefore, we should consider the appropriate course of BP treatment in OI patients.
Recently, the optimal duration and long-term safety of BP therapy were worthy of further investigation. A meta-analysis showed that oral or intravenous BPs increased the BMD of children and adults with OI (27). However, whether oral or intravenous BP treatment could consistently reduce fracture occurrence was controversial (27). Although specific data were not extracted, BP therapy for 1 to 3 years appeared to be beneficial, with the maximum benefits in the first year of treatment in adults and children with OI patients (27, 28). The increase in load to fracture after BP treatment came at the cost of a trend toward a decline in bone material properties, decreased strength and elastic modulus, and decreased matrix production by osteoblasts, which could be avoided by a shorter treatment duration (29). In humans, the volume of the bone increased after BP treatment, while the intrinsic material properties, stiffness, and hardness of bone tissue remained unaffected (30). However, the average length of BP treatment preceding fractures was 6.5 years without standard drug holidays in the study by Nicolaou et al. (31). Thus, in another study, drug holidays were achieved after the average treatment duration of 4.1 years (7). In our study, a median of nearly 4.0 years of BP treatment was safe according to the skeletal outcomes. However, patients with OI are often not treated in a timely manner. OI is an extremely rare disease, and both doctors and patients might have relatively insufficient knowledge of disease treatment. As a result, the patients were diagnosed with OI but did not receive timely treatment until repeated fractures and even bone deformities occurred (32).
Additionally, when to terminate BP drug holiday and restart treatment were unclear in patients with OI. Rauch et al. suggested that treatment was restarted after 15 and 16 months of cessation of pamidronate when some patients began to feel unwell and lacked stamina (33). Another study indicated that BP treatment had to be restarted owing to the decreased BMD, increased fracture rate, and recurrence of bone pain (34). In our study, most patients with OI remained stable during the first 2 years of BP discontinuation. However, 17 (11.4%) patients received the retreatment after a median 2 years of drug holiday due to the obvious decrease in BMD.
Very few studies have comprehensively evaluated the skeletal outcome of OI patients during BP discontinuation. A study reported that the lumbar spine BMD and its Z-scores decreased, while the fracture rate increased in OI patients after 1.5 years of BP discontinuation (34). Another study indicated that the effects of pamidronate discontinuation were more obvious at the radial metaphysis than at the diaphysis (35). Recently, increased lumbar spine areal BMD and a 19% decrease in the trabecular volumetric BMD at the distal metaphysis were observed after 4 years of BP discontinuation in OI patients (36). Moreover, we evaluated the changes in bone turnover markers in juveniles and adults after BP discontinuation. BP discontinuation leads to an obvious decrease in bone turnover biomarkers. As OI children were in the stage of growth and development, juveniles with OI had increased BMD and slightly increased β-CTX levels during the BP drug holiday. Interestingly, the lumbar spine BMD continued to increase, and the proximal hip BMD had no significant change during the drug holidays in juveniles. The curative effects seemed to be weaker in OI adults than in OI juveniles, which may be related to high bone remodeling and bone growth speed in juveniles. The possible mechanism of site-specific changes in juveniles’ BMD was as follows: as the spine is rich in cancellous bone, the effects of BPs on the spine BMD were more obvious than in other sites (27, 37). BPs could not alter the genetic defects of OI, and the BMD would decrease again after the long-term discontinuation of BP therapy (33). The demineralization was predominant on sites rich in cortical bone (38), and a fall in BMD was significant in the proximal hip after BP discontinuation (39). Moreover, most studies focused on the safety profile of OI patients during treatment (40). No patients suffered from osteonecrosis of the jaw and atypical bone fracture in this study. Our findings might broaden the long-term safety spectrum of BPs in OI patients.
Several factors associated with the duration to enter a drug holiday and termination of the drug holidays were investigated. We found that OI type III and type IV and COL1A2 mutation were positively correlated with a longer BP treatment duration to enter the drug holiday. Meanwhile, OI patients with later initiation of BP treatment or with the severe OI phenotype tended to require retreatment. As patients with OI type III or IV, or with a qualitative defect in type I collagen, usually had a severe phenotype, they might need a longer duration of BP treatment and need to be treated again (41, 42). As BPs were less effective in OI adults than in OI children (43, 44), the early initiation of BP treatment might have more benefits (40).
In this study, we comprehensively assessed the skeletal outcomes after BP discontinuation for the first time in a large cohort of Asian OI patients. We investigated the factors correlated with the BP treatment course to enter the drug holidays and retreatment during the drug holiday. Meanwhile, all the measurements were performed in a single center, avoiding measurement bias. However, this study had many limitations. It was a retrospective study. Patients were administered various BPs, and the duration of BP treatment and lengths of drug holidays were diverse.
Conclusions
Patients with osteogenesis imperfecta will undergo nearly 4 years of BP treatment to achieve drug holidays. During the 3 years of the drug holiday, the patients’ BMD is stable, and fracture incidence does not increase significantly. Patients are more inclined to need retreatment during the drug holidays owing to the late start of BP treatment and more severe phenotypes This study provides valuable information for the long-term rational treatment of BPs in juveniles and adults with osteogenesis imperfecta.
Data availability statement
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding authors. The datasets presented in this article are not readily available since no access to raw dataset of NGS is allowed other than the Beijing Genomics institution in charge of NGS. Requests to access the datasets should be directed to https://www.genomics.cn/contact.html.
Ethics statement
The studies involving human participants were reviewed and approved by the ethics committee of PUMCH. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
YZ collected the clinical data from the patients, analyzed the data, and wrote the manuscript. JH, XL, and LS contributed to the data collection and blood sample collection. QZ, SY, YJ, OW, WX, and XX contributed to the review of the manuscript. ML contributed to the conception and design of the research, and acquisition and interpretation of the data, and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work is supported by National Key R&D Program of China (2018YFA0800801, 2021YFC2501704), CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-C&T-B-007, 2021-I2M-1-051), National Natural Science Foundation of China (No. 81873668, 82070908), and Beijing Natural Science Foundation (7202153).
Acknowledgments
We appreciate the patients and their families for participating in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.901925/full#supplementary-material
References
1. Marom R, Rabenhorst BM, Morello R. Osteogenesis imperfecta: An update on clinical features and therapies. Eur J Endocrinol (2020) 183(4):R95–r106. doi: 10.1530/eje-20-0299
2. Marini JC, Forlino A, Bächinger HP, Bishop NJ, Byers PH, Paepe A, et al. Osteogenesis imperfecta. Nat Rev Dis Primers (2017) 3:17052. doi: 10.1038/nrdp.2017.52
3. Claeys L, Storoni S, Eekhoff M, Elting M, Wisse L, Pals G, et al. Collagen transport and related pathways in osteogenesis imperfecta. Hum Genet (2021) 140(8):1121–41. doi: 10.1007/s00439-021-02302-2
4. Etich J, Rehberg M, Eckes B, Sengle G, Semler O, Zaucke F. Signaling pathways affected by mutations causing osteogenesis imperfecta. Cell signal (2020) 76:109789. doi: 10.1016/j.cellsig.2020.109789
5. Fassier FR. Osteogenesis imperfecta-who needs rodding surgery? Curr Osteoporos Rep (2021) 19(3):264–70. doi: 10.1007/s11914-021-00665-z
6. Sakka SD, Cheung MS. Management of primary and secondary osteoporosis in children. Ther Adv Musculoskelet Dis (2020) 12:1759720x20969262. doi: 10.1177/1759720x20969262
7. Vuorimies I, Mäyränpää MK, Valta H, Kröger H, Toiviainen-Salo S, Mäkitie O. Bisphosphonate treatment and the characteristics of femoral fractures in children with osteogenesis imperfecta. J Clin Endocrinol Metab (2017) 102(4):1333–9. doi: 10.1210/jc.2016-3745
8. Xu XJ, Ma DD, Lv F, Wang JY, Liu Y, Xia WB, et al. The clinical characteristics and efficacy of bisphosphonates in audlt patients with osteogenesis impergecta. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol (2016) 22(11):1267–76. doi: 10.4158/ep151184.Or
9. Li LJ, Zheng WB, Zhao DC, Yu W, Wang O, Jiang Y, et al. Effects of zoledronic acid on vertebral shape of children and adolescents with osteogenesis imperfecta. Bone (2019) 127:164–71. doi: 10.1016/j.bone.2019.06.011
10. Idolazzi L, Fassio A, Viapiana O, Rossini M, Adami G, Bertoldo F, et al. Treatment with neridronate in children and adolescents with osteogenesis imperfecta: Data from open-label, not controlled, three-year Italian study. Bone (2017) 103:144–9. doi: 10.1016/j.bone.2017.07.004
11. Rauch F, Travers R, Plotkin H, Glorieux FH. The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. J Clin Invest (2002) 110(9):1293–9. doi: 10.1172/jci15952
12. Hegazy A, Kenawey M, Sochett E, Tile L, Cheung AM, Howard AW. Unusual femur stress fractures in children with osteogenesis imperfecta and intramedullary rods on long-term intravenous pamidronate therapy. J Pediatr Orthop (2016) 36(7):757–61. doi: 10.1097/bpo.0000000000000552
13. Vasanwala RF, Sanghrajka A, Bishop NJ, Högler W. Recurrent proximal femur fractures in a teenager with osteogenesis imperfecta on continuous bisphosphonate therapy: Are we overtreating? J Bone Miner Res Off J Am Soc Bone Miner Res (2016) 31(7):1449–54. doi: 10.1002/jbmr.2805
14. Bishop N, Adami S, Ahmed SF, Antón J, Arundel P, Burren CP, et al. Risedronate in children with osteogenesis imperfecta: A randomised, double-blind, placebo-controlled trial. Lancet (London England) (2013) 382(9902):1424–32. doi: 10.1016/s0140-6736(13)61091-0
15. Liu Y, Asan, Ma D, Lv F, Xu X, Wang J, et al. Gene mutation spectrum and genotype-phenotype correlation in a cohort of Chinese osteogenesis imperfecta patients revealed by targeted next generation sequencing. Osteoporos Int J established as result coop between Eur Found Osteoporos Natl Osteoporos Found USA (2017) 28(10):2985–95. doi: 10.1007/s00198-017-4143-8
16. Xu H, Zhao Z, Wang H, Ding M, Zhou A, Wang X, et al. Bone mineral density of the spine in 11,898 Chinese infants and young children: A cross-sectional study. PLoS One (2013) 8(12):e82098. doi: 10.1371/journal.pone.0082098
17. Khadilkar AV, Sanwalka NJ, Chiplonkar SA, Khadilkar VV, Mughal MZ. Normative data and percentile curves for dual energy X-ray absorptiometry in healthy Indian girls and boys aged 5-17 years. Bone (2011) 48(4):810–9. doi: 10.1016/j.bone.2010.12.013
18. Zhang ZQ, Ho SC, Chen ZQ, Zhang CX, Chen YM. Reference values of bone mineral density and prevalence of osteoporosis in Chinese adults. Osteoporos Int J established as result coop between Eur Found Osteoporos Natl Osteoporos Found USA (2014) 25(2):497–507. doi: 10.1007/s00198-013-2418-2
19. Fink HA, MacDonald R, Forte ML, Rosebush CE, Ensrud KE, Schousboe JT, et al. Long-term drug therapy and drug discontinuations and holidays for osteoporosis fracture prevention: A systematic review. Ann Internal Med (2019) 171(1):37–50. doi: 10.7326/m19-0533
20. Sato A, Ouellet J, Muneta T, Glorieux FH, Rauch F. Scoliosis in osteogenesis imperfecta caused by Col1a1/Col1a2 mutations - genotype-phenotype correlations and effect of bisphosphonate treatment. Bone (2016) 86:53–7. doi: 10.1016/j.bone.2016.02.018
21. Li H, Ji CY, Zong XN, Zhang YQ. Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years. Chin J Pediatr (2009) 47(7):487–92. doi: 10.3760/cma.j.issn.0578-1310.2009.07.003
22. Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet (1979) 16(2):101–16. doi: 10.1136/jmg.16.2.101
23. Jovanovic M, Guterman-Ram G, Marini JC. Osteogenesis imperfecta: Mechanisms and signaling pathways connecting classical and rare oi types. Endocr Rev (2021) 43(1):61–90. doi: 10.1210/endrev/bnab017
24. Malmgren B, Tsilingaridis G, Monsef-Johansson N, Qahtani ZHA, Dahllöf G, Åström E. Bisphosphonate therapy and tooth development in children and adolescents with osteogenesis imperfecta. Calcif Tissue Int (2020) 107(2):143–50. doi: 10.1007/s00223-020-00707-1
25. Starr J, Tay YKD, Shane E. Current understanding of epidemiology, pathophysiology, and management of atypical femur fractures. Curr Osteoporos Rep (2018) 16(4):519–29. doi: 10.1007/s11914-018-0464-6
26. Farlay D, Rizzo S, Ste-Marie LG, Michou L, Morin SN, Qiu S, et al. Duration-dependent increase of human bone matrix mineralization in long-term bisphosphonate users with atypical femur fracture. J Bone miner Res Off J Am Soc Bone Miner Res (2021) 36(6):1031–41. doi: 10.1002/jbmr.4244
27. Dwan K, Phillipi CA, Steiner RD, Basel D. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev (2016) 10(10):Cd005088. doi: 10.1002/14651858.CD005088.pub4
28. Rijks EB, Bongers BC, Vlemmix MJ, Boot AM, van Dijk AT, Sakkers RJ, et al. Efficacy and safety of bisphosphonate therapy in children with osteogenesis imperfecta: A systematic review. Horm Res Paediatr (2015) 84(1):26–42. doi: 10.1159/000381713
29. Uveges TE, Kozloff KM, Ty JM, Ledgard F, Raggio CL, Gronowicz G, et al. Alendronate treatment of the brtl osteogenesis imperfecta mouse improves femoral geometry and load response before fracture but decreases predicted material properties and has detrimental effects on osteoblasts and bone formation. J Bone Miner Res Off J Am Soc Bone Miner Res (2009) 24(5):849–59. doi: 10.1359/jbmr.081238
30. Weber M, Roschger P, Fratzl-Zelman N, Schöberl T, Rauch F, Glorieux FH, et al. Pamidronate does not adversely affect bone intrinsic material properties in children with osteogenesis imperfecta. Bone (2006) 39(3):616–22. doi: 10.1016/j.bone.2006.02.071
31. Nicolaou N, Agrawal Y, Padman M, Fernandes JA, Bell MJ. Changing pattern of femoral fractures in osteogenesis imperfecta with prolonged use of bisphosphonates. J Child Orthop (2012) 6(1):21–7. doi: 10.1007/s11832-011-0380-0
32. Lai Y, Lu W, Mao H, Zhang Y, Ming WK, Wu Y. Knowledge, attitude and practices regarding health self-management among patients with osteogenesis imperfecta in China: An online cross-sectional survey. BMJ Open (2021) 11(9):e046286. doi: 10.1136/bmjopen-2020-046286
33. Rauch F, Munns C, Land C, Glorieux FH. Pamidronate in children and adolescents with osteogenesis imperfecta: Effect of treatment discontinuation. J Clin Endocrinol Metab (2006) 91(4):1268–74. doi: 10.1210/jc.2005-2413
34. Andiran N, Alikasifoglu A, Gonc N, Ozon A, Kandemir N, Yordam N. Cyclic pamidronate therapy in children with osteogenesis imperfecta: Results of treatment and follow-up after discontinuation. J Pediatr Endocrinol Metab JPEM (2008) 21(1):63–72. doi: 10.1515/jpem.2008.21.1.63
35. Rauch F, Cornibert S, Cheung M, Glorieux FH. Long-bone changes after pamidronate discontinuation in children and adolescents with osteogenesis imperfecta. Bone (2007) 40(4):821–7. doi: 10.1016/j.bone.2006.11.020
36. Robinson ME, Trejo P, Palomo T, Glorieux FH, Rauch F. Osteogenesis imperfecta: Skeletal outcomes after bisphosphonate discontinuation at final height. J Bone Miner Res Off J Am Soc Bone Miner Res (2019) 34(12):2198–204. doi: 10.1002/jbmr.3833
37. Vom Scheidt A, Hemmatian H, Püschel K, Krause M, Amling M, Busse B. Bisphosphonate treatment changes regional distribution of trabecular microstructure in human lumbar vertebrae. Bone (2019) 127:482–7. doi: 10.1016/j.bone.2019.07.003
38. Chappard C, Houillier P, Paillard M. Bone status in primary hyperparathyroidism. Joint Bone Spine (2001) 68(2):112–9. doi: 10.1016/s1297-319x(00)00240-2
39. Lyu H, Zhao SS, Yoshida K, Tedeschi SK, Xu C, Nigwekar SU, et al. Comparison of teriparatide and denosumab in patients switching from long-term bisphosphonate use. J Clin Endocrinol Metab (2019) 104(11):5611–20. doi: 10.1210/jc.2019-00924
40. Palomo T, Fassier F, Ouellet J, Sato A, Montpetit K, Glorieux FH, et al. Intravenous bisphosphonate therapy of young children with osteogenesis imperfecta: Skeletal findings during follow up throughout the growing years. J Bone miner Res Off J Am Soc Bone Miner Res (2015) 30(12):2150–7. doi: 10.1002/jbmr.2567
41. Barber LA, Abbott C, Nakhate V, Do AND, Blissett AR, Marini JC. Longitudinal growth curves for children with classical osteogenesis imperfecta (Types iii and iv) caused by structural pathogenic variants in type I collagen. Genet Med Off J Am Coll Med Genet (2019) 21(5):1233–9. doi: 10.1038/s41436-018-0307-y
42. Erbaş İM, İlgün Gürel D, Manav Kabayeğit Z, Koç A, Ünüvar T, Abacı A, et al. Clinical, genetic characteristics and treatment outcomes of children and adolescents with osteogenesis imperfecta: A two-center experience. Connect Tissue Res (2021) 63(4):349–58. doi: 10.1080/03008207.2021.1932853
43. Shi CG, Zhang Y, Yuan W. Efficacy of bisphosphonates on bone mineral density and fracture rate in patients with osteogenesis imperfecta: A systematic review and meta-analysis. Am J Ther (2016) 23(3):e894–904. doi: 10.1097/mjt.0000000000000236
Keywords: skeletal outcomes, bisphosphonate, drug holiday, osteogenesis imperfecta, long-term therapy
Citation: Zhang Y, Hu J, Lin X, Sun L, Yan S, Zhang Q, Jiang Y, Wang O, Xia W, Xing X and Li M (2022) Skeletal outcomes of patients with osteogenesis imperfecta during drug holiday of bisphosphonates: a real-world study. Front. Endocrinol. 13:901925. doi: 10.3389/fendo.2022.901925
Received: 22 March 2022; Accepted: 10 August 2022;
Published: 26 September 2022.
Edited by:
Federico Baronio, IRCCS AOU S.Orsola-Malpighi, ItalyReviewed by:
Cui Aiyong, Xi’an Honghui Hospital, ChinaReggie Charles Hamdy, McGill University, Canada
Copyright © 2022 Zhang, Hu, Lin, Sun, Yan, Zhang, Jiang, Wang, Xia, Xing and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mei Li, limeilzh@sina.com
†ORCID: Mei Li, orcid.org/0000-0001-7270-4902
 Yongze Zhang1,2
Yongze Zhang1,2 Sunjie Yan
Sunjie Yan Qian Zhang
Qian Zhang Weibo Xia
Weibo Xia Mei Li
Mei Li