- Department of Ultrasound Medicine, The First Affiliated Hospital of University of Science and Technology of China Anhui Provincial Hospital, Hefei, China
Aim: Annual T1 stage papillary thyroid carcinoma (PTC) incidence rates continue to rise, yet the optimal treatment for this cancer type remains controversial. Central lymph node metastasis (CLNM) is a critical determinant in the context of treatment decision-making. While several prior studies have evaluated patients with clinica l T1a(cT1a) stage PTC, there have been fewer analyses of clinical T1b(cT1b) disease to date. The present study was thus formulated to explore predictors of CLNM in patients with cT1a and cT1b stage PTC.
Methods: A retrospective analysis of data including clinicopathological characteristics and BRAFV600E mutation status was conducted for 452 PTC patients undergoing surgical treatment. Logistic univariate and multivariate analyses were performed to identify risk factors associated with CLNM in particular patients’ characteristics and the accuracy of the established logistic regression models was evaluated using the R software platform.
Results: Respective CLNM incidence rates in cT1a and cT1b disease were 39.39% and 67.21%. Factors associated with a higher risk of CLNM among PTC(cT1a) patients included male sex, young age, tumor size, contact with capsule, and multifocality as determined through comparisons of the area under the curve for logistic regression models. Whereas male sex and age were associated with CLNM risk in PTC(cT1b) patients in univariate and multivariate analyses, age was the only risk factor associated with CLNM incidence among women with PTC(cT1b).
Conclusion: Predictors of CLNM differ between PTC patients with cT1a and cT1b stage disease, and a comprehensive assessment of these risk factors should thus be conducted when designing individualized treatment regimens for PTC patients.
Introduction
Thyroid cancer remains an important cause of global morbidity, in large part owing to the rapid increase in the incidence of papillary thyroid cancer (PTC) and papillary thyroid microcarcinoma (PTMC) (1, 2), which is defined by PTC tumors ≤ 10 mm in diameter. At present, ultrasonography (US) is recommended as an initial auxiliary approach when seeking to differentiate between benign and malignant thyroid nodules. The combination of routine health assessments and US-guided fine-needle aspiration biopsy (FNAB) approaches have contributed to significant increases in rates of PTC detection and preoperative diagnosis (3, 4). At present, the AJCC and UICC TNM staging systems for differentiated thyroid carcinoma (DTC) classify T1 stage disease based upon an intrathyroidal tumor size ≤ 2 cm, with these tumors being further subdivided into T1a (≤ 1 cm) and T1b (> 1 cm but ≤ 2 cm) stage disease (5, 6). The incidence of clinical T1 (cT1) stage PTC as defined by pre-operative imaging studies and physical exam, has steadily increased in recent years (7). As PTC has an excellent prognosis in comparison to other malignancies, particularly for PTMC, a range of therapeutic approaches are recommended to patients with PTC(cT1N0) disease including active surveillance, radiofrequency ablation, and surgery.
However, certain factors are associated with poorer PTC patients prognosis, with central lymph node metastasis (CLNM) being an important risk factor that must be considered when evaluating patient risk (8, 9), given that such metastases have been tied to increases in rates of local recurrence and the risk of a second surgery. CLNM is a common finding that affects an estimated 13.4% - 64% of PTC patients (10–12). Owing to anatomical factors including the carotid pulse and the potential for small metastases not detectable via imaging examination to be present, the preoperative diagnosis of CLNM can be challenging. Prophylactic central lymph node dissection (pCLND) in the absence of clinical evidence of CLNM is associated with higher rates of surgery-related complications including recurrent laryngeal nerve injury and permanent hypoparathyroidism. As such, radiofrequency ablation or active surveillance are generally the management approaches of choice for low-risk PTC(T1) patients without any evidence of cervical lymph node involvement (13, 14). It is thus critical that risk factors related to CLNM incidence in patients with PTC be better defined in order to guide clinical decision-making. Several studies to date have assessed PTMC(T1a) patients and identified potential clinical and US features associated with CLNM risk, including male sex, younger age multifocality, and extrathyroidal extension, but these findings have not been consistent across studies and analyses of individuals with cT1b stage disease are currently lacking (15–17).
Several genetic mutations have been linked to the pathogenesis of PTC at the molecular level, and these genes can thus be examined to guide the diagnosis and prognostic evaluation of patients with PTC. The BRAFV600E kinase mutation has been shown to be the most critical candidate biomarker in this oncogenic context owing to its ability to promote PTC onset and progression (18, 19). However, the association between BRAFV600E mutation and CLNM prevalence remains controversial (19–21). BRAFV600E mutation alone is thus not sufficient to accurately predict patient CLNM status, underscoring the need to define a panel of clinical risk factors associated with CLNM status in an effort to guide more accurate and effective patient treatment.
In the present retrospective analysis, we assessed the clinical features, preoperative ultrasonographic findings, BRAFV600E mutational status, and postoperative clinicopathological features of cT1N0 stage PTC patients in an effort to identify predictors of CLNM. Together, these results provide a valuable foundation for efforts to more precisely and accurately plan treatments for PTC patients with cT1N0 stage disease.
Materials and Methods
Patient Demographics
For the present retrospective study, we analyzed data from PTC patients who had undergone thyroid lobectomy or total thyroidectomy with routine prophylactic central lymph node dissection (pCLND) between December 2020 and January 2022. The present study was approved by Review Board of our hospital, experienced thyroid surgeons performed all surgical procedures in order to ensure the integrity of the central lymph node dissection procedure.
In an effort to offer more reliable and valuable treatment options to patients with PTC, separate univariate and multivariate analyses were conducted for individuals with stage cT1a and stage cT1b disease. Individual patient groups were separated into individuals with and without CLNM based on postoperative clinicopathological findings.
Inclusion criteria for the present study were: (1) availability of complete preoperative and postoperative data; (2) postoperative pathological confirmation of the PTC diagnosis; (3) patients had undergone unilateral thyroidectomy or total thyroidectomy and central lymph node dissection; and (4) patients had undergone FNA and BRAFV600E mutational status analyses. Patients were excluded from this study if they met the following criteria: (1) a history of neck irradiation, neck surgery, or radioactive iodide treatment; (2) an absence of histologic results pertaining to the thyroid and/or central lymph nodes; (3) pregnancy; (4) other head and neck tumors.
Conventional and Color Doppler Ultrasonography
A sonographer with over 10 years of experience performed all imaging. Patients were directed to lie in the supine position with dorsal flexion of the head, and malignant thyroid node findings were identified via conventional US as per the TI-RADS criteria or according to the ATA guidelines (22, 23). Thyroid nodule size was assessed based on the maximum tumor diameter, with the presence or absence of the following features additionally being assessed (yes/no): aspect ratio>1, defined border, contact with the capsule, regular margin, microcalcification, and multifocality. After conventional grayscale ultrasonography, color Doppler ultrasound imaging was performed to detect the presence or absence of perinodular and intramodular vascular distributions.
Fine Needle Aspiration, BRAFV600E Detection, Surgical Methods
For FNA procedures, patients were directed to lie on their back with their neck hyperextended to ensure sufficient exposure of the puncture site. Very fine needles (23G) were then repeatedly inserted into the suspect node under ultrasonographic guidance to facilitate the acquisition of sufficient tissue. A portion of the collected tissue was used for cytological diagnosis, while the remainder was stored at 2-8°C. BRAFV600E mutational status was assessed following DNA extraction via real-time fluorescent quantitative polymerase chain reaction.
Unilatearl thyroidectomy and isthmusectomy were performed when the tumor was located on a single lobe. Total thyroidectomy was performed when there were bilateral tumors, presence of extrathyroidal extension during intraoperative examination. Ipsilateral pCLND was performed when the tumor was unilateral, bilateral pCLND was performed in bilateral tumors. The boundary of pCLND was defined by the carotid arteries laterally, hyoid bone superiorly and suprasternal notch inferiorly, including pre- and paratracheal nodes, relaryngeal lymph nodes, perithyroidal nodes, and lymph nodes along recurrent laryngeal nerves. Parathyroid gland and recurrent laryngeal nerve were protected during procedure.
Statistical Analysis
SPSS 26 (IBM Corporation, NY, USA) was used for statistical analyses. Categorical data were reported as frequencies and compared using chi-squared tests or Fisher’s exact test, while quantitative data are given as means ± standard deviation (SD) and compared via independent sample t-tests or Mann-Whitney U-tests when normally and non-normally distributed, respectively.
Univariate and multivariate logistic regression analyses were used to compare clinical characteristics, thyroid US features, and BRAFV600E mutation status, with P < 0.05 as the threshold of significance. Those risk factors related to CLNM in univariate and multivariate analyses were employed to establish logistic regression models, with area under the curve (AUC) values being compared to confirm model accuracy. All such analyses were performed using the pROC package for the R software platform (R Foundation; v 4.1.2).
Results
Patient Characteristics
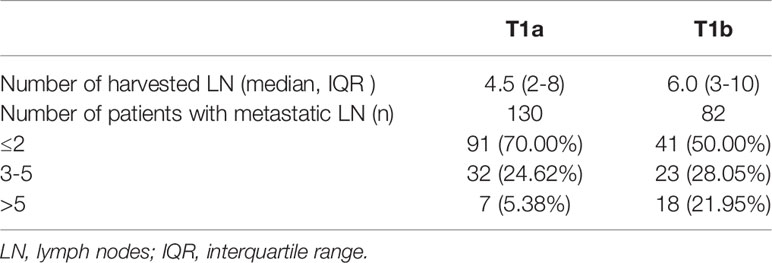
In total, this retrospective analysis incorporated data from 452 patients (113 male, 339 female), all of whom were preoperatively confirmed to have PTC and underwent US-guided FNA. The median age of these patients was 42.64 ± 11.13 years, and all had cT1N0M0 stage disease, including 330 patients (73.01%) with stage cT1a disease and 122 patients (26.99%) with stage cT1b disease. Overall, 46.9%(212/452) of patients had occult central lymph node metastases, with a significant difference in CLNM rates between males (61.95%; 70/113) and females (41.89%; 142/339) (P<0.01). The overall BRAFV600E mutation rate in these patients was 88.27% (399/452), with no significant differences in this rate when comparing males (86.73%; 98/113) and females (88.79%; 301/339) (P=0.555). The rate of Bethesda III, V, VI in cT1a stage was 8.48% (28/330), 23.64%(78/330), 67.88%(224/330), respectively, and 2.46%(3/122), 12.30%(15/122), 85.24%(104/122) in cT1b stage, respectively. No patients exhibited temporary hematoma or other complications following FNA, transient hypoparathyroidism occured in bilateral CLND and the incidence was 31.25% (25/80), serum parathyroid hormone returned to normal at post-operation one week or 2 months. There was no permanent hypoparathyroidism and recurrent laryngeal nerve injury after operation. There were no significant differences in maximum measured dimension between US results (9.09 ± 4.32 mm) and pathological specimens (9.52 ± 5.76 mm)(p=0.246). There were also no significant differences in disease duration, which was defined as the time that from ultrasound detection of nodules to surgery, when comparing patients with and without CLNM in the cT1a and cT1b groups.
Correlations Between Clinicopathological Parameters, Ultrasonographic Features, and CLNM in PTC (cT1a)
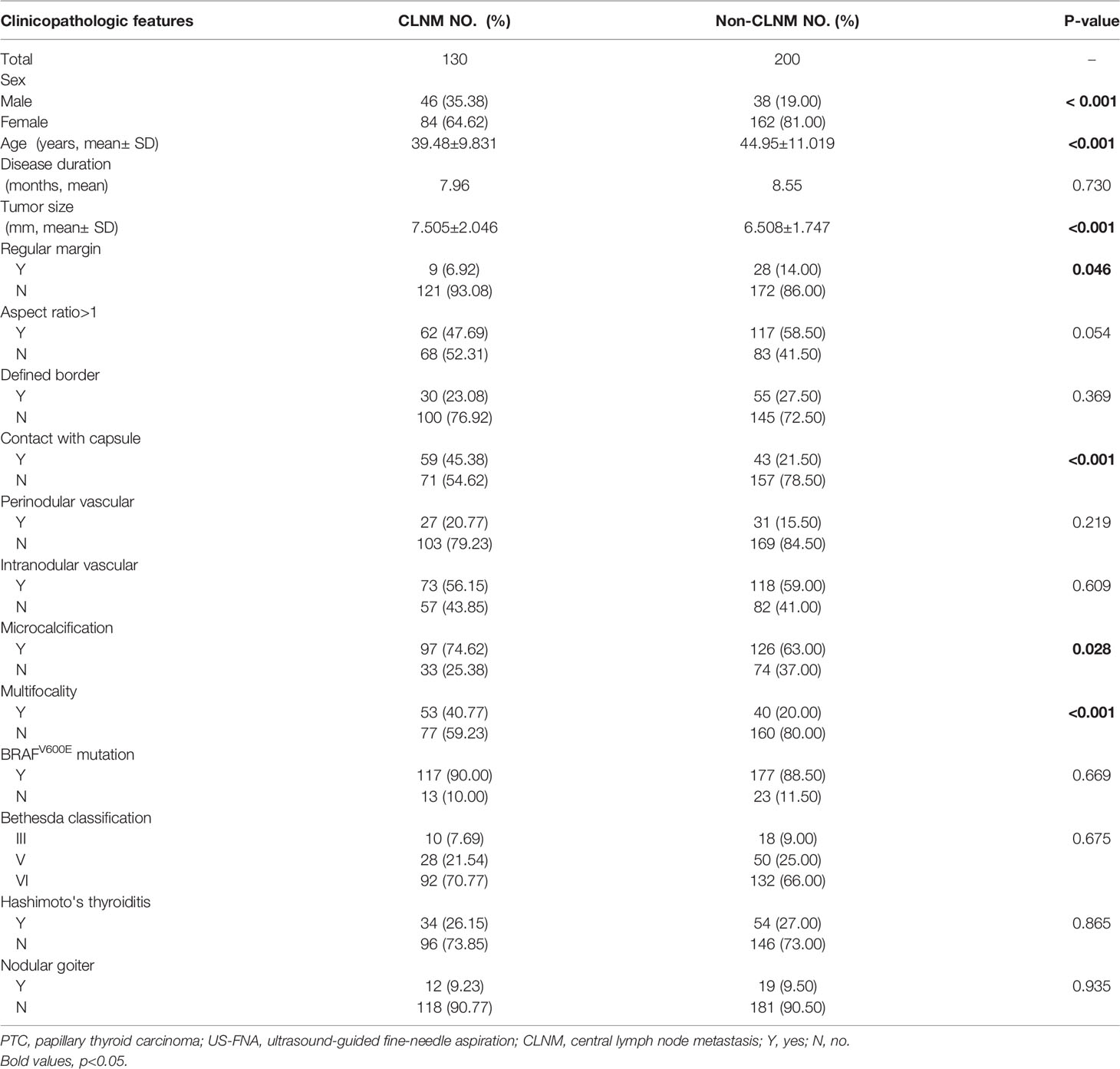
The association between PTC(cT1a) patient clinicopathological findings, US features, and CLNM status was next assessed. The overall CLNM incidence in the PTC(cT1a) patient population was 39.39% (130/330), with respective incidence rates of 54.76% (46/84) and 34.15% (84/246) in male and female patients. The median number of central neck lymph nodes (CNLN) harvested was 4.5(2-8), the rate of CLNM number>5 was 5.38%(7/130) in cT1a stage patients (Table 1). Factors significantly associated with CLNM status included male sex (P < 0.001), age (P < 0.001), tumor size (P < 0.001), contact with capsule (P < 0.001), regular margin (P=0.046), microcalcification (P=0.028), and multifocality (P < 0.001) (Table 2). Univariate analyses indicated CLNM to be significantly associated with risk factors including sex (P=0.001), age (P < 0.001), tumor size (P < 0.001), contact with capsule (P < 0.001), microcalcification (P < 0.001), and multifocality (P < 0.001). For the multivariate analyses, six variables statistically significant in the univariate analyses were included in the logistic regression model. In our model, multivariate analyses further confirming sex (P=0.003, 95%CI 1.331-4.198), age (P < 0.001, 95%CI 1.027-1.077), tumor size (P < 0.001, 95%CI 0.667-0.879), contact with capsule (P=0.021, 95%CI 1.110-3.679), and multifocality (P=0.015, 95%CI 1.155-3.906) to be independent predictors for CLNM (Table 3).
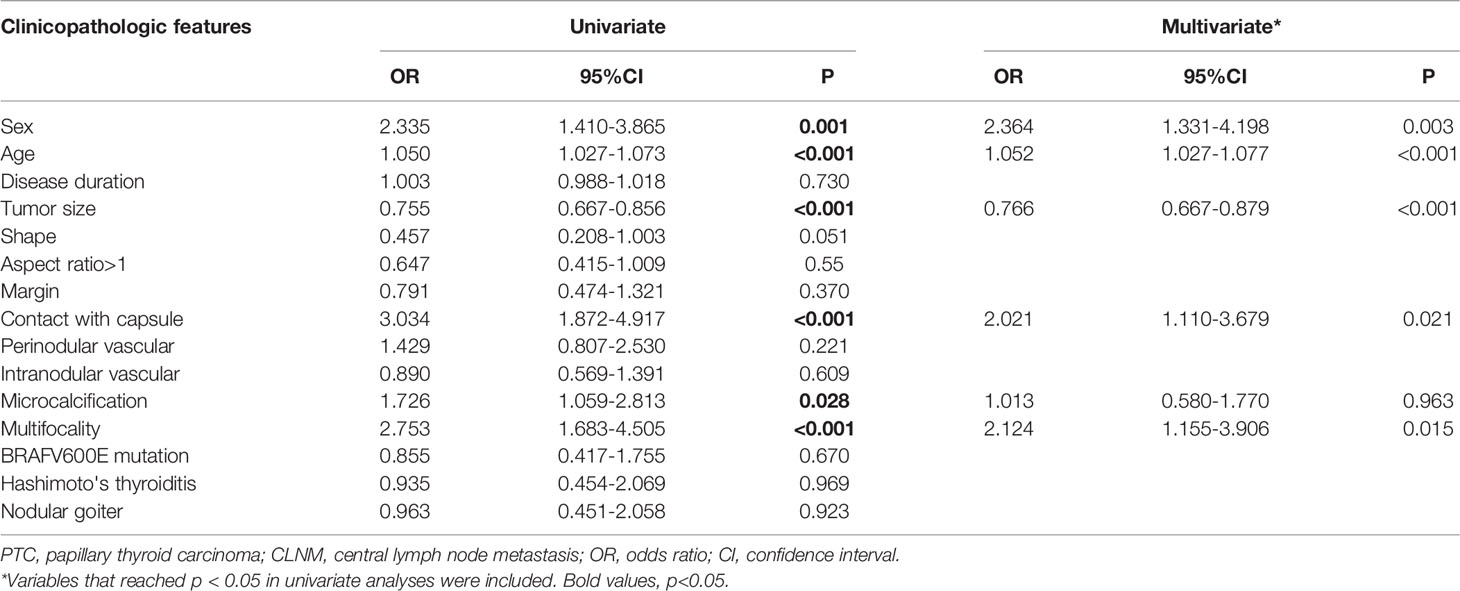
Table 3 Univariate and multivariate analyses to compare the high-risk factors of CLNM in PTC (cT1aN0).
To further confirm the accuracy of these risk factors, we next established two regression models based upon the respective results of univariate and multivariate analyses and we then compared AUC values for these two models (Figure 1). The results of this comparison suggested the regression model established based upon multivariate analyses to be more accurate.
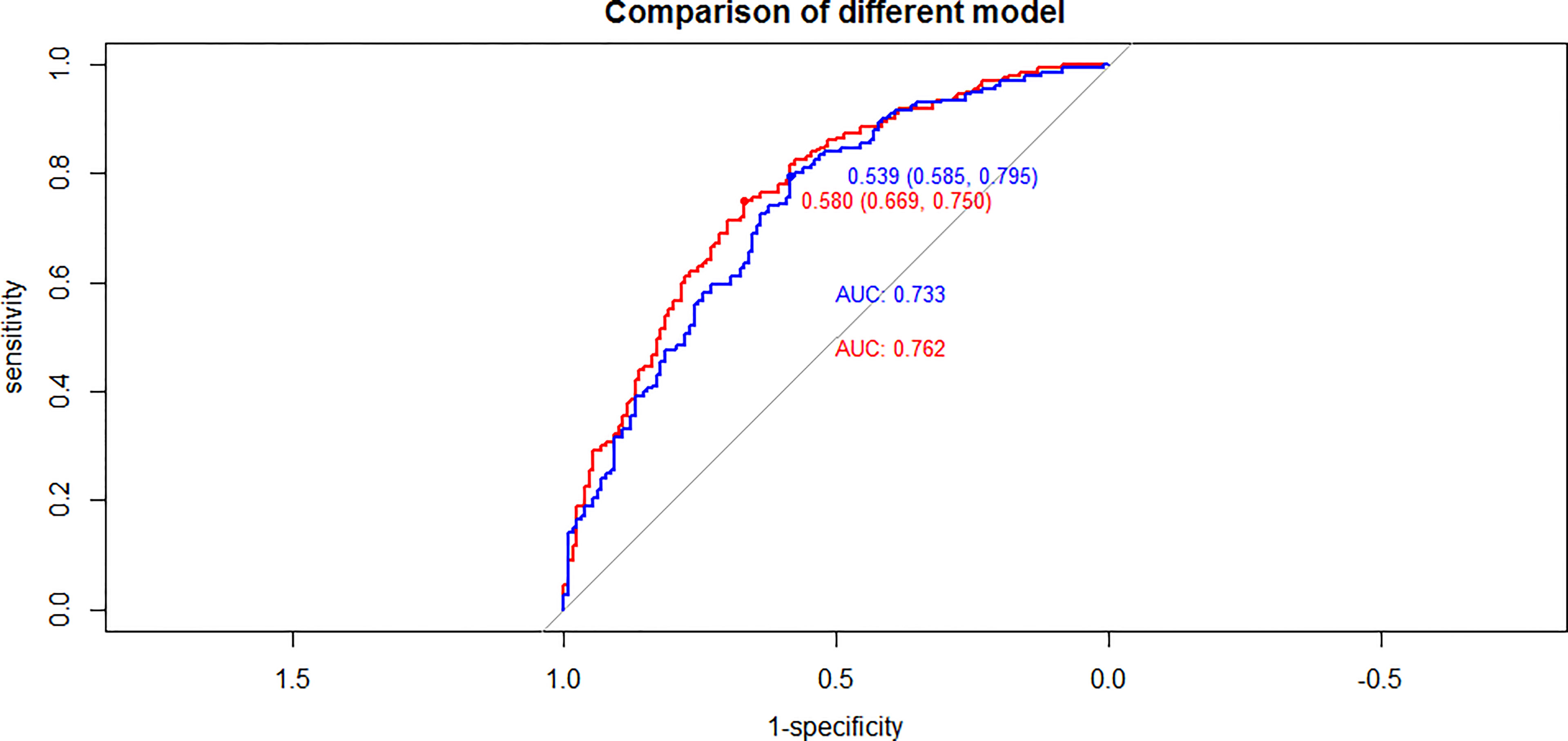
Figure 1 Compared of ROC curves between univariate and multivariate analyses results in PTC(cT1a) patients (red line: multivariate analyses results, blue line: univariate analyses results).
Given that both age and tumor size were identified as risk factors associated with CLNM incidence in PTC(cT1a) patients, efforts to more accurately define tumor size and age cut-off values are warranted to better predict CLNM. ROC curve analyses conducted based upon the above analyses revealed the optimal cut-off values for age and maximum tumor to be 44.5 years and 7.25 mm, respectively.
BRAFV600E was positive in 294(89.09%) patients in the present study cohort, of whom 117 (90.00%) patients with CLNM and 177 (88.50%) patients without CLNM were positive for this BRAFV600E mutation. As such, while this BRAFV600E mutation was common among PTC(cT1a) patients, it was not significantly related to CLNM incidence. Univariate analyses further revealed a significant association between Hashimoto’s thyroiditis (HT) and BRAFV600E mutation status.
Correlations Between Clinicopathological Parameters, Ultrasonographic Features, and CLNM in PTC (cT1b)
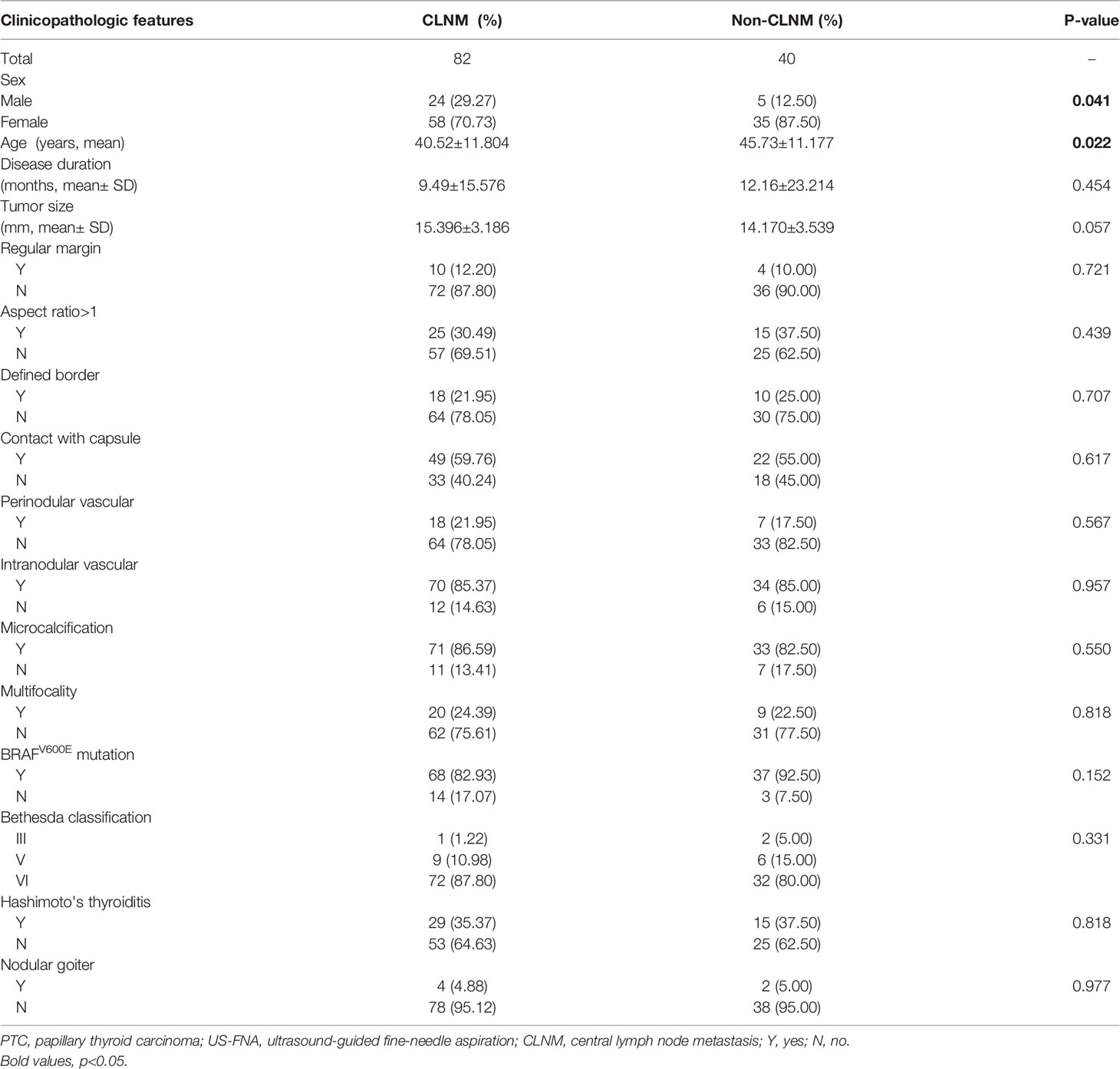
Next, the risk factors associated with CLNM among PTC(cT1b) patients were identified. Overall CLNM incidence in this patient population was 67.21% (82/122), with respective 82.76% (24/29) and 62.37% (58/93) incidence rates in male and female patients, respectively. The median number of CNLN dissected was 6.0(3-10) and the rate of CLNM number> 5 was 21.95% (18/82) in cT1b stage patients (Table 1). Accordingly, male sex (P=0.041) was associated with CLNM incidence, as was age (P=0.022), wheras tumor size reached borderline statistical significance (p=0.057) (Table 4). In univariate analysis, male sex, and age were significantly associated with CLNM. For the multivariate analyses, variables (sex and age) were included in the logistic regression model, multivariate analyses further confirming sex(p=0.042, 95%CI 1.041-8.848), age(p=0.023, 95%CI 1.006-1.077) to be risk factors associated with CLNM incidence (Table 5).
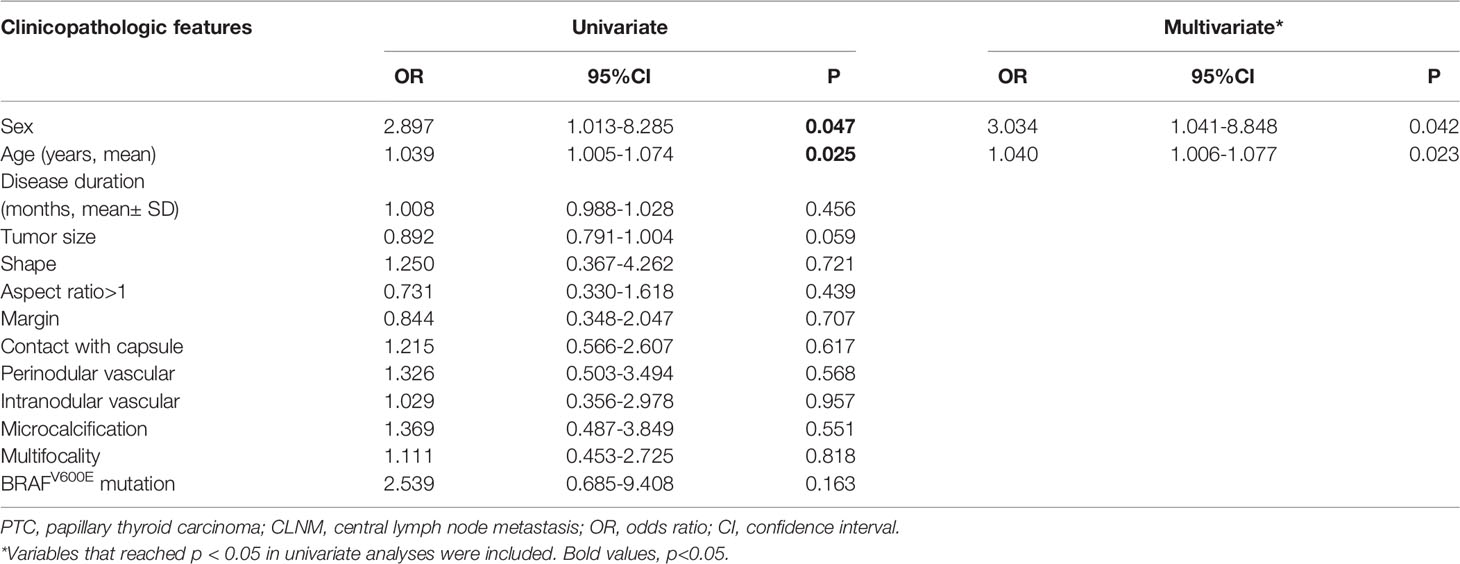
Table 5 Univariate and multivariate analyses to compare the high-risk factors of CLNM in PTC (cT1bN0).
Given the observed differences between males and females with respect to CLNM rates and the higher rates of CLNM among males in this patient population, we next conducted separate analyses of risk factors associated with CLNM in male and female PTC(cT1b) patients. This approach revealed that age was the only such risk factor identified among female patients, while no risk factors were identified for males in univariate analyses. Given the association between age and CLNM status, an ROC curve analysis was performed revealing the optimal age cut-off value for predicting CLNM status in female patients to be 44.5 years old.
BRAFV600E mutation was positive in 105 (86.07%) of the analyzed cT1b PTC patients. No significant differences in BRAFV600E mutation positivity were observed when comparing CLNM (82.93%) and non-CLNM (92.50%) patients (P=0.152), suggesting that while this mutation is common among individuals with PTC (cT1b), it is not specifically related to CLNM status in this patient population. Univariate analyses revealed a significant association between BRAFV600E mutation and aspect ratio > 1 (P=0.047), contact with capsule (P=0.039), and HT (P=0.008). Multivariate analyses further confirmed BRAFV600E mutation to be significantly associated with aspect ratio > 1 (P=0.045, 95%CI 0.042-0.962) and HT (P=0.025, 95%CI 1.179-11.254), whereas it was not significantly associated with contact with capsule (P=0.081, 95%CI 0.117-1.133).
Discussion
The cervical lymph nodes are the most common site of metastasis in patients with PTC and PTMC (8), with CLNM being associated with an elevated chance of recurrent PTC and lower rates of conservative treatment for individuals diagnosed with PTMC (10, 11). In the present analysis, we observed inconsistencies in CLNM incidence between PTC patients with T1a and T1b disease (39.39% vs. 67.21%, respectively), in line with prior reports (9, 24). We also found the clinicopathological and ultrasonographic risk factors associated with CLNM in these two disease stage subgroups to differ significantly. In PTC(T1a) patients, male sex, age, tumor size, contact with capsule, and multifocality were identified as independent predictors of CLNM, whereas only age and male sex were independent predictors of CLNM among PTC(cT1b) patients. Incidence of CLNM>5 was low in cT1a stage, however, it was significantly higher in cT1b stage. Hence, we recommend that pCLND is not routinely done in cT1a stage. Together, these data have the potential to provide a valuable reference for efforts aimed at formulating more accurate surgical or non-surgical treatments for PTC patients with cT1 stage disease, thereby improving patient quality of life.
With respect to clinical characteristics, age and sex are risk factors associated with CLNM in PTC patients. Liu et al. proposed that males < 40 years of age were at an elevated risk of CLNM (25), whereas Tao et al. found being < 38 years old was associated with CLNM risk (8). Zhou et al. suggested that being male and under 30 years of age may be a more reliable cut-off when predicting the odds of CLNM in a Chinese patient population (16). However, these previous studies included patients with a range of tumor stages and failed to assess the association between age and tumor size within a given stage of diseases. Our present results suggested that an age of < 44.5 years and < 37.7 years for individuals with cT1a and cT1b disease, respectively, was associated with an increased risk of CLNM. When we conducted additional analyses of age among females with cT1b disease, being < 44.5 years was again confirmed to be associated with CLNM incidence among female patients, whereas age was not associated with CLNM risk among male patients with cT1b disease, probably due to high rate of CLNM. As such, further in-depth multi-center analyses of the association between age, sex, and CLNM are warranted, particularly for individuals with PTC(cT1b). A majority of these patients were asymptomatic with disease initially being detected via US examination, and as a result, no association between disease duration and CLNM was detected.
Tumor size has previously been linked to CLNM status, with larger tumors generally being more aggressive (21, 24, 25). In the present study, we compared differences between tumor sizes as measured via US and the actual sizes of pathological samples following surgical resection, revealing no differences between these sizes and thus confirming that US findings can be used when conducting predictive analyses. We found that tumors > 7.25 mm in size were associated with a high risk of CLNM among PTC(cT1a) patients, in line with prior research. Other US features have also been found to be associated with CLNM. For example, one meta-analysis found extrathyroidal extension to be associated with an elevated risk of CLNM among PTMC patients (12), while Wang et al. additionally conducted an analysis of 1204 patients with PTMC and found sonographic evidence of thyroid nodule microcalcification to be an independent predictor of CLNM (26). Tao et al. additionally identified a solid composition to be an independent factor of CLNM (8). While multivariate analyses in the present study revealed contact with capsule and multifocality to be independent predictors of CLNM among PTC(cT1a), no analyzed US features were related to CLNM risk in our PTC(cT1b) patient cohort. In light of these results, tumor size seems to offer greater value as a predictor of CLNM. Multifocality is commonly observed in PTC and is correlated with both CLNM and disease recurrence (27, 28), with overall reported tumor multifocality rates of 27% in a previous report (16), and 27.21% in our study, with our data confirming a correlation between such multifocality and CLNM for patients with cT1a stage disease.
Some reports have found BRAFV600E mutation status to be related to CLNM incidence, whereas other studies have failed to replicate this finding. Here, we found BRAFV600E mutation positivity to be highly prevalent among patients with both cT1a and cT1b stage disease (89.09% vs. 86.07%, respectively), with no significant difference is such positivity when comparing CLNM and non-CLNM patients in either stage subgroup, which indicated that BRAFV600E mutation by itself did not contribute to the presence of CLNM in the absence of other clinico-pathologic factors.
BRAFV600E mutation positivity was also found to be more common among PTMC patients without HT as compared to patients with comorbid HT in this study, in line with prior clinical evidence (16, 29).
The association between PTC and HT is poorly defined, as is the association between HT and CLNM incidence. In some reports, comorbid HT and PTC have been found to be associated with a lower risk of nodal metastasis as compared to that observed for patients not affected by HT (30, 31), whereas other studies have failed to detect an association between HT and CLNM (10, 32). In the present analysis, we were unable to detect any significant association between HT and CLNM in PTC patients with either cT1a or cT1b stage. Additional molecular mechanistic studies may thus be required to more thoroughly examine the interplay between PTC, HT, and CLNM.
There are multiple limitations to the present analysis. For one, this was a retrospective study and surgery is not recommended for the majority of such patients with tumors smaller than 5 mm in clinical practice, thus resulting in the maldistribution of PTMC patients, which is concentrated tumor diameter above 5mm. Second, this was a single-center study, and the number of enrolled patients with cT1b stage disease was limited. Patients in cT1b stage are more prone to capsule invasion and high rate of CLNM number >5, further large-scale multi-center studies will be critical as a means of more accurately defining risk factors associated with CLNM in this patient population. Moreover, information about the size of the largest metastatic deposit was not described, we will recommend that pathologists provide the size of positive lymph nodes in order to provide more accurate treatment for PTC patients in future study. Third, correlation analyses examining the associations between BRAFV600E mutation status, progressive clinicopathological findings, and US features were limited by the numbers of patients with advanced-stage disease. Finally, because data on our cohort was collected fairly recently, we do not have enough followup time to determine the impact of pCLND or presence of CLNM on the overall prognosis of these PTC patients, particularly of those with cT1b disease. A prospective study with a longer duration of followup or a randomized control trial of pCLND vs no-pCLND may answer the true prognostic significance of occult CLNM in patients with cT1 disease.
Conclusion
In conclusion, the results of this study highlight significant differences between PTC patients with cT1a and cT1b disease. Among PTC(cT1a) patients, independent predictors of CLNM included male sex, age, large tumor size (> 7.25 mm), contact with capsule, and multifocality, while among PTC(cT1b) patients these independent predictors included male sex and age. A range of analyses including ultrasonography, assessments of patient clinical characteristics, and US-FNAB should be performed prior to the establishment of a definitive patient treatment plan. As not all PTC(cT1N0) lesions are alike, the formulation of appropriate individualized therapeutic regimens is dependent on comprehensive analyses of all of these pertinent risk factors.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Committee of The First Affiliated Hospital of USTC approved the present study. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Y-zZ: performed trial, data curation and analysis, write original draft. N-aH: designed and performed trial, supervision, edited draft. XY: performed trial, supervision. FJ, M-xL, X-xJ: performed trial and data collection. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int J Cancer (2019) 144(8):1941–53. doi: 10.1002/ijc.31937
2. Raze T, Lacour B, Cowppli-Bony A, Delafosse P, Velten M, Trétarre B, et al. Cancer Among Adolescents and Young Adults Between 2000 and 2016 in France: Incidence and Improved Survival. J Adolesc Young Adult Oncol (2021) 10(1):29–45. doi: 10.1089/jayao.2020.0017
3. Alexander EK. Approach to the Patient With a Cytologically Indeterminate Thyroid Nodule. J Clin Endocrinol Metab (2008) 93(11):4175–82. doi: 10.1210/jc.2008-1328
4. Haugen BR. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: What is New and What has Changed? Cancer (2017) 123(3):372–81. doi: 10.1002/cncr.30360
5. Cavalheiro BG, de Matos LL, Leite AKN, Kulcsar MAV, Cernea CR, Kowalski LP. Survival in Differentiated Thyroid Carcinoma: Comparison Between the 7th and 8th Editions of the AJCC/UICC TNM Staging System and the ATA Initial Risk Stratification System. Head Neck (2021) 43(10):2913–22. doi: 10.1002/hed.26773
6. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer stagingCA Cancer J Clin. (2017) 67(2):93–99. doi: 10.3322/caac.21388
7. Farahati J, Mäder U, Gilman E, Görges R, Maric I, Binse I, et al. Changing Trends of Incidence and Prognosis of Thyroid Carcinoma. Nuklearmedizin (2019) 58(2):86–92. doi: 10.1055/a-0859-7454
8. Tao L, Zhou W, Zhan W, Li W, Wang Y, Fan J. Preoperative Prediction of Cervical Lymph Node Metastasis in Papillary Thyroid Carcinoma via Conventional and Contrast-Enhanced Ultrasound. J Ultrasound Med (2020) 39(10):2071–80. doi: 10.1002/jum.15315
9. Zhou B, Qin J. High-Risk Factors for Lymph Node Metastasis in Contralateral Central Compartment in Unilateral Papillary Thyroid Carcinoma(Ct1n0). Eur J Surg Oncol (2021) 47(4):882–7. doi: 10.1016/j.ejso.2020.10.018
10. Jiwang L, Yahong L, Kai L, Bo H, Yuejiao Z, Haotian W, et al. Clinicopathologic Factors and Preoperative Ultrasonographic Characteristics for Predicting Central Lymph Node Metastasis in Papillary Thyroid Microcarcinoma: A Single Center Retrospective Study. Braz J Otorhinolaryngol (2022) 88(1):36–45. doi: 10.1016/j.bjorl.2020.05.004
11. Dou Y, Hu D, Chen Y, Xiong W, Xiao Q, Su X. PTC Located in the Upper Pole is More Prone to Lateral Lymph Node Metastasis and Skip Metastasis. World J Surg Oncol (2020) 18(1):188. doi: 10.1186/s12957-020-01965-x
12. Qu N, Zhang L, Ji QH, Chen JY, Zhu YX, Cao YM, et al. Risk Factors for Central Compartment Lymph Node Metastasis in Papillary Thyroid Microcarcinoma: A Meta-Analysis. World J Surg (2015) 39(10):2459–70. doi: 10.1007/s00268-015-3108-3
13. Saravana-Bawan B, Bajwa A, Paterson J, McMullen T. Active Surveillance of Low-Risk Papillary Thyroid Cancer: A Meta-Analysis. Surgery (2020) 167(1):46–55. doi: 10.1016/j.surg.2019.03.040
14. Zhang M, Tufano RP, Russell JO, Zhang Y, Zhang Y, Qiao Z, et al. Ultrasound-Guided Radiofrequency Ablation Versus Surgery for Low-Risk Papillary Thyroid Microcarcinoma: Results of Over 5 Years' Follow-Up. Thyroid (2020) 30(3):408–17. doi: 10.1089/thy.2019.0147
15. Ye J, Feng JW, Wu WX, Hu J, Hong LZ, Qin AC, et al. Papillary Thyroid Microcarcinoma: A Nomogram Based on Clinical and Ultrasound Features to Improve the Prediction of Lymph Node Metastases in the Central Compartment. Front Endocrinol (Lausanne) (2022) 12:770824. doi: 10.3389/fendo.2021.770824
16. Zhou SL, Guo YP, Zhang L, Deng T, Xu ZG, Ding C, et al. Predicting Factors of Central Lymph Node Metastasis and BRAFV600E Mutation in Chinese Population With Papillary Thyroid Carcinoma. World J Surg Oncol (2021) 19(1):211. doi: 10.1186/s12957-021-02326-y
17. Zhang L, Ling Y, Zhao Y, Li K, Zhao J, Kang H. A Nomogram Based on Clinicopathological and Ultrasound Imaging Characteristics for Predicting Cervical Lymph Node Metastasis in Cn0 Unilateral Papillary Thyroid Microcarcinoma. Front Surg (2021) 8:742328. doi: 10.3389/fsurg.2021.742328
18. Gan X, Shen F, Deng X, Feng J, Lu J, Cai W, et al. Prognostic Implications of the BRAF-V600E Mutation in Papillary Thyroid Carcinoma Based on a New Cut-Off Age Stratification. Oncol Lett (2020) 19(1):631–40. doi: 10.3892/ol.2019.11132
19. Özçelik S, Bircan R, Sarıkaya Ş, Gül AE, Aydın B, Özçelik M, et al. BRAF V600E Mutation in Papillary Thyroid Cancer is Correlated With Adverse Clinicopathological Features But Not With Iodine Exposure. Endokrynol Pol (2019) 70(5):401–8. doi: 10.5603/EP.a2019.0025
20. Celik M, Bulbul BY, Ayturk S, Durmus Y, Gurkan H, Can N, et al. The Relation Between BRAFV600E Mutation and Clinicopathological Characteristics of Papillary Thyroid Cancer. Med Glas (Zenica) (2020) 17(1):30–4. doi: 10.17392/1086-20
21. Sezer A, Celik M, Yilmaz Bulbul B, Can N, Tastekin E, Ayturk S, et al. Relationship Between Lymphovascular Invasion and Clinicopathological Features of Papillary Thyroid Carcinoma. Bosn J Basic Med Sci (2017) 17(2):144–51. doi: 10.17305/bjbms.2017.1924
22. Şahin M, Oguz A, Tuzun D, Akkus G, Törün GI, Bahar AY, et al. Effectiveness of TI-RADS and ATA Classifications for Predicting Malignancy of Thyroid Nodules. Adv Clin Exp Med (2021) 30(11):1133–9. doi: 10.17219/acem/139591
23. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
24. Zhou B, Wei L, Qin J. Analyze and Compare the Predictors of Ipsilateral Central Lymph Node Metastasis in Papillary Thyroid Carcinoma With Ct1a and Ct1b Stage. Asian J Surg (2021) 44(11):1357–62. doi: 10.1016/j.asjsur.2021.02.008
25. Liu W, Cheng R, Ma Y, Wang D, Su Y, Diao C, et al. Establishment and Validation of the Scoring System for Preoperative Prediction of Central Lymph Node Metastasis in Papillary Thyroid Carcinoma. Sci Rep (2018) 8(1):6962. doi: 10.1038/s41598-018-24668-6
26. Wang WH, Xu SY, Zhan WW. Clinicopathologic Factors and Thyroid Nodule Sonographic Features for Predicting Central Lymph Node Metastasis in Papillary Thyroid Microcarcinoma: A Retrospective Study of 1204 Patients. J Ultrasound Med (2016) 35(11):2475–81. doi: 10.7863/ultra.15.10012
27. Feng JW, Qu Z, Qin AC, Pan H, Ye J, Jiang Y. Significance of Multifocality in Papillary Thyroid Carcinoma. Eur J Surg Oncol (2020) 46(10 Pt A):1820–8. doi: 10.1016/j.ejso.2020.06.015
28. Wu X, Li B, Zheng C, He X. Predicting Factors of Central Lymph Node Metastases in Patients With Unilateral Multifocal Papillary Thyroid Microcarcinoma. Gland Surg (2020) 9(3):695–701. doi: 10.21037/gs.2020.03.27
29. Kim WW, Ha TK, Bae SK. Clinical Implications of the BRAF Mutation in Papillary Thyroid Carcinoma and Chronic Lymphocytic Thyroiditis. J Otolaryngol Head Neck Surg (2018) 47(1):4. doi: 10.1186/s40463-017-0247-6
30. Liang J, Zeng W, Fang F, Yu T, Zhao Y, Fan X, et al. Clinical Analysis of Hashimoto Thyroiditis Coexistent With Papillary Thyroid Cancer in 1392 Patients. Acta Otorhinolaryngol Ital (2017) 37(5):393–400. doi: 10.14639/0392-100X-1709
31. Kim KW, Park YJ, Kim EH, Park SY, Park DJ, Ahn SH, et al. Elevated Risk of Papillary Thyroid Cancer in Korean Patients With Hashimoto’s Thyroiditis. Head Neck (2011) 33(5):691–5. doi: 10.1002/hed.21518
Keywords: papillary thyroid carcinoma, BRAFV600E\ mutation, central lymph node metastasis, risk factors, surgery
Citation: Zhao Y-z, He N-a, Ye X-j, Jin F, Li M-x and Jiang X (2022) Analysis of Risk Factors Associated With Central Lymph Node Metastasis in Papillary Thyroid Carcinoma With cT1N0 Stage. Front. Endocrinol. 13:880911. doi: 10.3389/fendo.2022.880911
Received: 22 February 2022; Accepted: 02 May 2022;
Published: 03 June 2022.
Edited by:
Laura Boucai, Memorial Sloan Kettering Cancer Center, United StatesReviewed by:
Giulia Sapuppo, University of Catania, ItalyLilah Morris-Wiseman, Johns Hopkins Medicine, United States
Copyright © 2022 Zhao, He, Ye, Jin, Li and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nian-an He, henianan71@163.com
 Yin-zhu Zhao
Yin-zhu Zhao Nian-an He
Nian-an He Xian-jun Ye
Xian-jun Ye