- 1Department of Ophthalmology, Guangdong Eye Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
- 2Ophthalmology, Department of Surgery, Centre for Eye Research Australia, University of Melbourne, Melbourne, VIC, Australia
- 3State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou, China
- 4Medical Sciences Division, University of Oxford, Oxford, United Kingdom
- 5Zhongshan School of Medicine, Sun Yat-sen Universtiy, Guangzhou, China
Purpose: To assess the impact of retinopathy and systemic vascular comorbidities on the all-cause mortality in a representative U.S. sample.
Methods: A total of 5703 participants (≥40 years old) from the 2005-2008 National Health and Nutrition Examination Survey. The Early Treatment Diabetic Retinopathy Study grading scale was used to evaluate the retinopathy status. Systemic vascular comorbidities included diabetes mellitus (DM), high blood pressure (HBP), chronic kidney disease (CKD) and cardiovascular disease (CVD). Time to death was calculated as the time from baseline to either the date of death or censoring (December 31st, 2015), whichever came first. Risks of mortality were estimated using Cox proportional hazards models after adjusting for confounders and vascular comorbidities.
Results: After a median follow-up of 8.33 years (IQR: 7.50-9.67 years), there were 949 (11.8%) deaths from all causes. After adjusting for confounders, the presence of retinopathy predicted higher all-cause mortality (hazard ratio (HR), 1.41; 95% confidence interval (CI), 1.08-1.83). The all-cause mortality among participants with both retinopathy and systemic vascular comorbidities including DM (HR, 1.72; 95% CI, 1.21-2.43), HBP (HR, 1.47; 95% CI, 1.03-2.10), CKD (HR, 1.73; 95% CI, 1.26-2.39) and CVD (HR, 1.92; 95% CI, 1.21-3.04) was significantly higher than that among those without either condition. When stratified by diabetic or hypertension status, the co-occurrence of retinopathy and CKD or CVD further increased the all-cause mortality compared to those without either condition.
Conclusions: The co-occurrence of retinopathy and systemic vascular conditions predicted a further increase in the risk of mortality. More extensive vascular risk factor assessment and management are needed to detect the burden of vascular pathologies and improve long-term survival in individuals with retinopathy.
Introduction
Retinopathy commonly refers to a spectrum of signs on the fundus (e.g. microaneurysms, soft/hard exudates, and/or retinal hemorrhage) that are common in the elderly, even in those without diabetes (1). A recent pooled analysis of 22,896 individuals with diabetes reported that nearly 35% had retinopathy (2). Furthermore, previous evidence suggested that up to 15% of non-diabetic patients exhibited clinical signs of retinopathy (3–7).
Previous literature has consistently described significant associations between retinopathy and systemic vascular comorbidities, including diabetes mellitus (DM) (5), high blood pressure (HBP) (8), chronic kidney disease (CKD) (6, 9–11) and cardiovascular disease (CVD) (12–15). The presence of retinopathy has been reported to be an independent risk factor for mortality (16–27). A recent meta-analysis of 20 observational studies reported that individuals with retinopathy had a two- to four-fold increase in their risks of all-cause mortality independent of other potential risk factors (28).
To date, only a few studies have investigated the joint effects of retinopathy and systemic vascular comorbidities [such as DM (16), CKD (16, 29–31), and clinical cerebrovascular disease (16)] on mortality. These previous studies were subject to selection bias, underestimation of retinopathy, short duration of follow-up and a lack of investigation of the effects of concomitant macrovascular disorders. Given that the retina is readily viewable via non-invasive retinal imaging and that there is a well-established association between retinopathy and systemic vascular comorbidities, clarifying the precise effects of the co-occurrence of retinopathy and systemic vascular comorbidities on the risk of mortality is of great significance.
The National Health and Nutrition Examination Survey (NHANES) is a continuous, national population-based study of elderly participants in the U.S., which provides a unique opportunity to investigate the impact of retinopathy and systemic vascular comorbidities on mortality.
Methods
Sample and Population
The NHANES dataset from 2005 to 2008 was used for the current analysis. NHANES is a nationally representative survey of the non-institutionalized U.S. civilian population that includes in-person interviews and extensive clinical examinations. As described in detail elsewhere, the NHANES uses a multistage design to select participants from strata and proportions of minority populations (32). Since 1999, the NHANES has released a dataset every two years. Because of the availability of retinal imaging, two NHANES cycles (2005-2006 and 2007-2008) were merged. In total, 5,703 out of 6,797 individuals aged 40 years and older were included for the current analysis. A total of 1,093 participants were excluded due to missing information on the grading of retinopathy for both eyes. One participant was excluded because of the lack of mortality data.
The research adhered to the tenets of the Declaration of Helsinki. Written informed consent was given by every participant, and the NHANES was conducted in accordance with ethical standards. Because data used in this analysis were publicly available and de-identified, the present study received exemption from review by the institutional review board.
Evaluation of Retinopathy
The Canon CR6-45NM Ophthalmic Digital Imaging System and Canon EOS 10D digital camera (Canon USA Inc., One Canon Park, Melville, New York) were used to capture non-mydriatic retinal photographs. Two digital images per eye (one for the macula and one for the optic nerve) were taken in an almost completely dark room. Based on a modification of the Airlie House classification system (33, 34), graders in the University of Wisconsin Ocular Epidemiologic Reading Center, Madison assessed the fundus photographs. Details of image capture and grading of fundus photographs have been described in an earlier study (35). The Early Treatment Diabetic Retinopathy Study (ETDRS) grading scale was used to evaluate the retinopathy severity (33). Participants were classified as having retinopathy (level 14-51) based on the eye with the worse retinopathy level.
Assessment of Systemic Vascular Comorbidities
Systemic vascular comorbidities, including DM, HBP, CKD and self-reported history of CVD, were assessed. Identification of DM was based on self-reported medical history, use of insulin or antihyperglycaemic medications, or a glycosylated hemoglobin level exceeding 6.5% (36). Identification of HBP was based on a self-reported physician diagnosis, use of antihypertensive medications, or the average of three measurements of systolic ≥140 mmHg) and/or the average of three measurements of diastolic blood pressure ≥90 mmHg). According to the recent American Heart Association classification of HBP, we considered stage 2 HBP in the present analysis (37). An estimated glomerular filtration rate (eGFR) of less than 60 ml/min/1.73 m2 was used to as the definition for CKD (38). A self-reported physician diagnosis of coronary heart disease, myocardial infarction, congestive heart failure, angina or stroke was used to identify the self-reported history of CVD.
Mortality Data
Based on a probabilistic matching algorithm, mortality was ascertained from the National Death Index (NDI) (39). This linkage was performed by matching participants’ personal identification information, such as name, sex, date of birth, social security number, between the NHANES and the NDI datasets. Participants not found to be deceased were considered to be alive. The time between the date of the interview and the date of death or end-of-study censoring (the 31st of December, 2015), whichever came first, was calculated as the duration of follow-up.
Covariates
Gender (male and female) was included as a covariate. Ethnicity was classified into four groups: non-Hispanic white, non-Hispanic black, Mexican American, and other. Two categories of marital status included unmarried or other, and married or lived with a partner. Education level was classified into two groups: did not complete a high school degree, and completed at least a high school degree. The poverty-income ratio (PIR) was categorized into two groups: < 1.00 and ≥ 1.00 (poverty threshold) respectively. Smoking status was classified into two groups: never, and former or current smokers. Drinking status was classified into two groups: abstainer or former drinker, and current drinker. Identification of hypercholesterolaemia was based on the total cholesterol level (≥240 mg/dL) or the use of antihyperlipidemic agents. Body mass index (BMI) was a value derived from the weight in kilograms divided by the height in meters squared. A high C-reactive protein (CRP) was defined as CRP level ≥1 mg/dL. Walking disability was based on self-reported responses to the questionnaire or the requirement of special equipment to aid walking. Health status was dichotomously classified into poor or fair, and good or excellent.
Statistical Analysis
The complex, stratified design of NHANES was taken into account for all analyses. The means and standard errors (SEs), or numbers and weighted proportions were used to present the baseline characteristics of the study population where appropriate. The design-adjusted t-test or the Rao-Scott Pearson χ2 were used to compare distributions for continuous or categorical variables. We used Kaplan-Meier estimates to generate plots of survival curves among participants with retinopathy and concomitant systemic vascular disorders, and log-rank tests to compare survival distributions among these groups. We used Cox proportional hazards regression models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for survival. Furthermore, we explored the joint effects of retinopathy and systemic vascular diseases on mortality. We tested the proportional hazard assumption for each variable in the Cox regression models by creating the time-dependent covariate (interaction between the variable and survival time), with a p-value < 0.05 for the time-dependent covariate indicating a violation of the assumption. We found that all variables included in the Cox regression models were valid. Collinearity among variables was tested using the variance inflation factor (VIF) procedure, and the average value was 1.26 in the present analysis. We used Stata (ver. 14.0; StataCorp., College Station, TX) to perform all analyses. A p-value of less than 0.05 was considered statistically significant.
Results
In total, there were 6,797 individuals aged 40 years and older from the NHANES 2005-2008. Of these participants, 5,703 were included in the current analysis due to missing information on the grading of retinopathy for both eyes in 1,093 participants and missing data on vital status in 1 participant. Participants with missing data were more likely to be older, black, poorly educated, unmarried or other, have a lower level of household income and be unhealthier in terms of systemic comorbidities when compared to those with complete data. The characteristics of the excluded and the included participants are shown in Supplement Table 1.
A total of 710 participants were identified as having retinopathy (weighted prevalence: 9.75%). The mean age of these participants was 56.5 ± 0.38 years (SE), and 52.6% were women. The baseline characteristics of participants by retinopathy status are presented in Table 1. Participants identified as having retinopathy were more likely to be older, male, black, poorly educated, and lifetime abstainers/former drinkers and to have higher BMI, proportion of walking disability, poor/fair self-rated health status, and concomitant micro- and macrovascular pathologies (DM, HBP, CKD and CVD) compared to those without retinopathy. No significant difference in other characteristics was observed between these two groups.
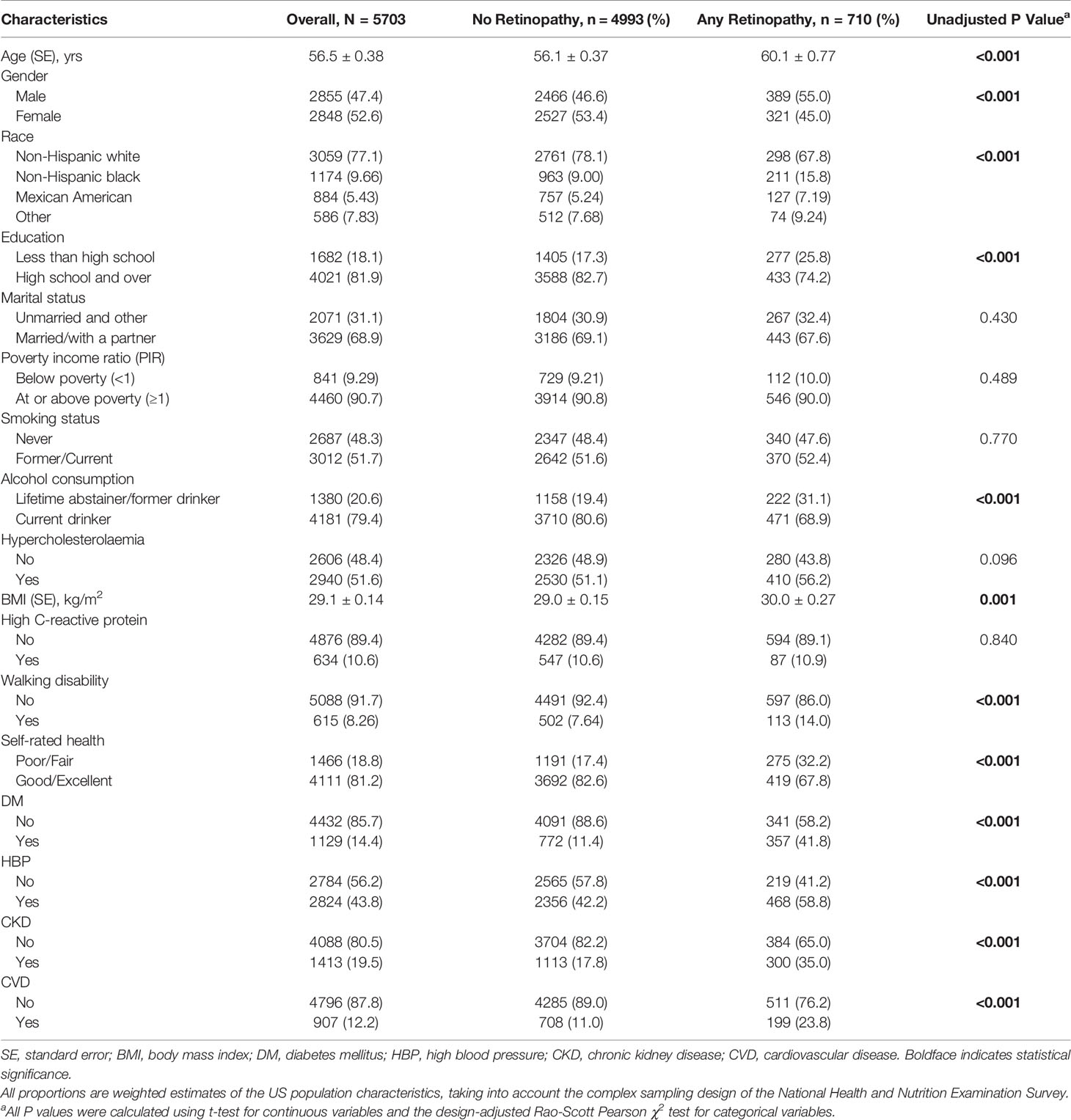
Table 1 Demographic, health-related behaviors and general health characteristics of participants with and without retinopathy.
The median duration of follow-up was 8.33 years (interquartile range [IQR]: 7.50-9.67 years). There were 949 (11.8%) deaths from all causes. Participants with retinopathy had higher all-cause mortality than those without (23.0% versus 10.5%, t-test P < 0.001). The baseline characteristics, including demographic factors, health-related behaviors and general health characteristics for participants by survival status, are presented in Table 2. Several covariates, including age, sex, ethnicity, educational attainment, types of marital status, household income level, smoking status, alcohol consumption, the level of CRP, self-rated health status, walking disability, and micro- and macrovascular disorders (DM, CKD and history of CVD), were strongly associated with all-cause mortality in age- and sex-adjusted models. Multivariate Cox regression models suggested that the presence of any degree of retinopathy at baseline was associated with higher all-cause mortality (HR, 1.41; 95% CI, 1.08-1.83; P=0.012).
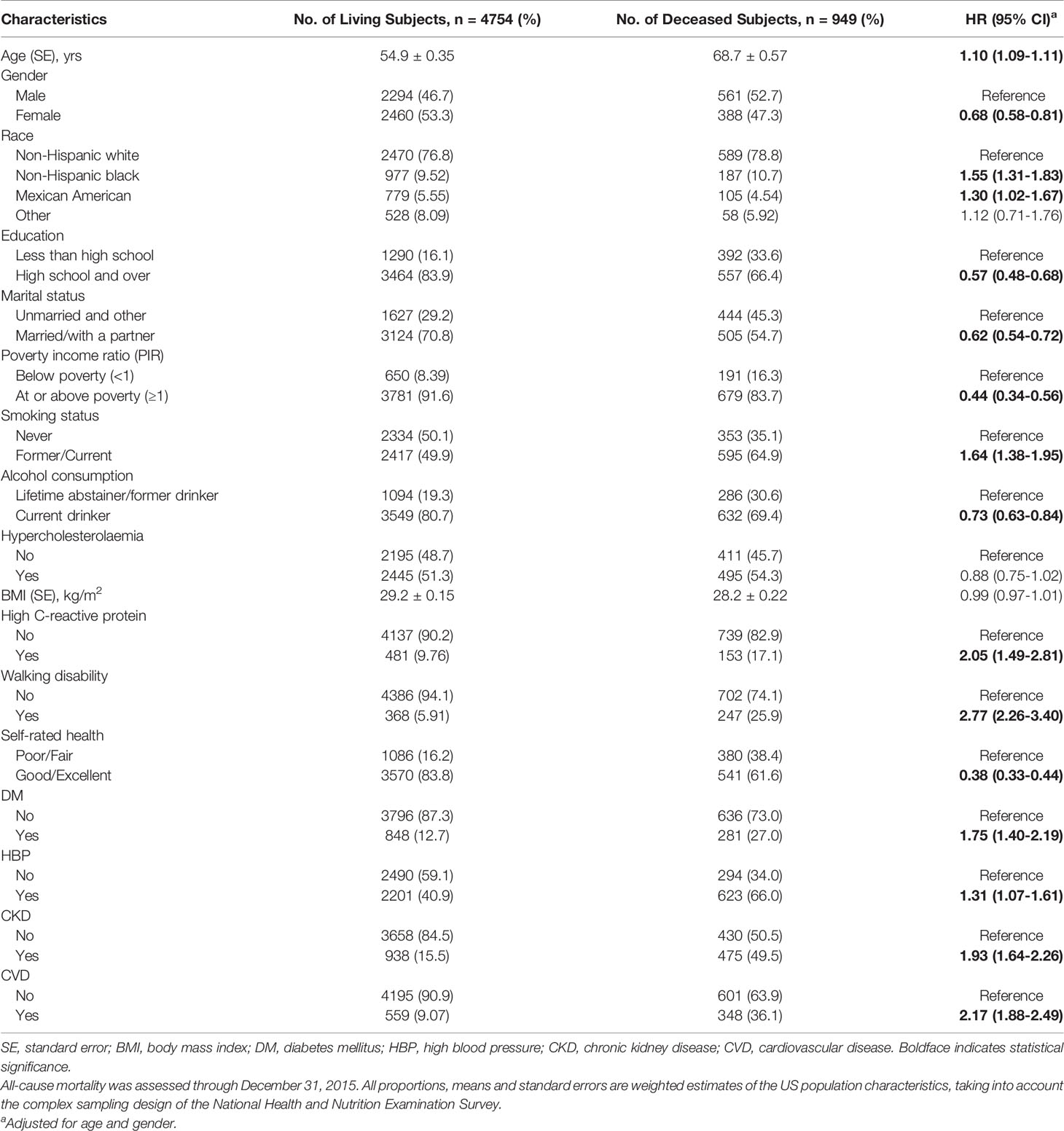
Table 2 All-cause mortality by demographic, health-related behaviors and general health characteristics.
Interactions between retinopathy and each systemic vascular disorder were assessed, and no significant interaction for DM, CKD, or CVD was found. There was a significant interaction between retinopathy and HBP in the model (P=0.041). Kaplan-Meier curves for all-cause mortality according to retinopathy and systemic vascular conditions are shown in Figure 1. Table 3 presents the synergistic impact of each vascular disorder together with retinopathy on mortality. All-cause mortality for participants with retinopathy and comorbidities such as DM (HR, 1.72; 95% CI, 1.21-2.43; P=0.003), HBP (HR, 1.47; 95% CI, 1.03-2.10; P=0.034), CKD (HR, 1.73; 95% CI, 1.26-2.39; P=0.002) and CVD (HR, 1.92; 95% CI, 1.21-3.04; P=0.007) was significantly higher than for those without any condition.

Figure 1 Kaplan-Meier curve showing all-cause mortality rate by retinopathy status and concomitant systemic vascular disorders [(A): diabetes mellitus; (B): high blood pressure; (C): chronic kidney disease] (D): history of cardiovascular disease), using the 2005-2008 National Health and Nutrition Examination Survey (NHANES). All-cause mortality was assessed through December 31, 2015. Risk of mortality was further increased among those with both retinopathy and systemic vascular diseases compared to participants without either condition.

Table 3 Cox proportional hazards regression models of all-cause and cardiovascular disease mortality by retinopathy status and concomitant medical conditions.
The results stratified by diabetes and hypertension status are shown in Supplement Table 2. Among non-diabetic participants, all-cause mortality was higher in those with retinopathy and CKD (HR, 1.55; 95% CI, 1.07-2.25; P=0.023). Among diabetic participants, the co-occurrence of retinopathy and CVD (HR, 2.52; 95% CI, 1.20-5.29; P=0.016) further increased all-cause mortality. For participants without hypertension, the co-occurrence of retinopathy and CVD posed a higher risk of death (HR, 3.32; 95% CI, 1.32-8.35; P=0.012). In an analysis limited to participants with hypertension, the joint effect of retinopathy and CVD (HR, 1.64; 95% CI, 1.01-2.67; P=0.047) or CKD (HR, 1.85; 95% CI, 1.35-2.55; P<0.001) predicted higher all-cause mortality than that of those without either condition.
Discussion
In this large sample of middle-aged and older adults, participants with retinopathy had a higher all-cause mortality rate. Moreover, the co-occurrence of retinopathy and systemic vascular conditions (DM, HBP, CKD, and history of CVD) further increased all-cause mortality.
Our findings are in line with previous results that reported a significant association between retinopathy and mortality (17–26). Contrary to the EURODIAB Prospective Complications Study, which found that the relationship between retinopathy and mortality can be largely explained by the presence of coexisting cardiovascular risk factors (40), we report that retinopathy was an independent risk factor for mortality after comprehensive adjustment of covariates. We postulate that the observed difference might be a result of missing fundus photographs (31%) and morbidity data (14%) in the EURODIAB Prospective Complications Study, which may have led to the underestimation of the association between retinopathy and mortality.
Only a limited number of studies have attempted to explore the joint effects of retinopathy and systemic vascular comorbidities [such as DM (16), CKD (16, 29–31), and clinical cerebrovascular disease (16)] on mortality. A more detailed investigation of systemic vascular comorbidities (DM, HBP, CKD, and CVD) in our study provides further insights into the association between retinopathy and mortality. That is, our results not only support previous findings of a higher mortality risk in individuals with retinopathy but also suggest that retinopathy in the presence of concomitant DM, HBP, CKD or CVD increases the risk of mortality even further.
Mechanisms underlying the association between retinopathy and mortality remain unknown. It has been proposed that retinopathy may be an indicator of the burden of CVD risk factors, including dyslipidemia, obesity, hypertension, and smoking (17). However, our results do not support this theory. Although we found that the distribution of CVD risk factors differed between non-retinopathy and retinopathy groups, retinopathy was found to be an independent risk factor for mortality in the fully adjusted model. Another potential explanation is that retinopathy, as a marker of abnormal microcirculation in the retina, may be an important indicator of systemic micro- and macro-vascular abnormalities (24). These micro- and macrovascular disorders may share similar pathophysiological processes with retinopathy, including endothelial dysfunction, inflammation, apoptosis, and neovascularization (41, 42). In addition, the co-occurrence of retinopathy and systemic vascular disorders may reflect a greater burden and/or severity of vascular pathologies, which may explain the further increase in mortality among the participants who have both retinopathy and systemic vascular disorders, and the marginally interaction between retinopathy and HBP.
Given that retinopathy can be readily detected via non-invasive means, our findings may have several practical implications. Firstly, our finding of a statistically significant association between retinopathy and systemic vascular abnormalities indicates that individuals with retinopathy may benefit from a comprehensive vascular assessment and should be closely monitored. Secondly, the current study and previous studies find that the co-occurrence of retinopathy and micro- or macrovascular disorders poses a further increase in all-cause mortality. Therefore, intensive vascular risk reduction may be warranted in the management of these patients.
The strengths of this analysis include the use of a large-scale sample with national representativeness, the use of a standardized objective grading protocol to assess retinopathy, access to death records, long-term follow-up and inclusion of a comprehensive range of covariates. However, several potential limitations should be considered. Firstly, retinopathy status and potential confounders adjusted for in our analysis were assessed on a single occasion. During the follow-up period, the behaviors and/or retinopathy status of the patients may have changed, which may have a direct impact on the outcome. Secondly, discrepancies may exist between the self-reported data and clinically measured data. This may cause underreporting of milder cases and subsequently lead to a bias in the analysis. Finally, participants with missing data tended to be in poorer health status, which may have resulted in selection bias.
In summary, our findings suggest that middle-aged and elderly people with retinopathy have increased all-cause mortality. Furthermore, the joint effects of retinopathy and major systemic vascular comorbidities increase the all-cause mortality further. Our results indicate that more extensive risk factor assessment and management of individuals with retinopathy may be beneficial to reduce their mortality rate, especially in patients who have both retinopathy and systemic vascular disorders.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
Author Contributions
Study concept and design: ZZ, MH, and XY. Acquisition, analysis, or interpretation: All authors. Drafting of the manuscript: ZZ and XS. Critical revision of the manuscript for important intellectual content: WW, JH, YC, MH, and XY. Statistical analysis: ZZ, XS, and WW. Obtained funding: MH. Administrative, technical, or material support: ZZ, XS, WW, MH, and XY. Study supervision: MH and XY. All authors contributed to the article and approved the submitted version.
Funding
The present work was supported by Fundamental Research Funds of the State Key Laboratory of Ophthalmology (303060202400362), National Natural Science Foundation of China (82000901, 82101173), Project of Special Research on Cardiovascular Diseases (2020XXG007), and Research Foundation of Medical Science and Technology of Guangdong Province (B2021237). MH receives support from the University of Melbourne at Research Accelerator Program and the CERA Foundation. The Centre for Eye Research Australia receives Operational Infrastructure Support from the Victorian State Government. The sponsor or funding organization had no role in the design or conduct of this research.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.750017/full#supplementary-material
Abbreviations
CVD, cardiovascular disease; NHANES, National Health and Nutrition Examination Survey; DM, diabetes millitus; HBP, high blood pressure; CKD, chronic kidney disease; HR, hazard ratios; CI, confidence interval; ETDRS, Early Treatment Diabetic Retinopathy Study; NDI, National Death Index; eGFR, estimated glomerular filtration rate; ICD, International Classification of Diseases, Injuries and Causes of death; BMI, body mass index; CRP, C-reactive protein; PIR, poverty income ratio; SE, standard errors; VIF, variance inflation factors; IQR, interquartile range.
References
1. Olafsdottir E, Andersson DK, Dedorsson I, Stefansson E. The Prevalence of Retinopathy in Subjects With and Without Type 2 Diabetes Mellitus. Acta Ophthalmol (2014) 92(2):133–7. doi: 10.1111/aos.12095
2. Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care (2012) 35(3):556–64. doi: 10.2337/dc11-1909
3. Wong TY, Klein R, Sharrett AR, Manolio TA, Hubbard LD, Marino EK, et al. The Prevalence and Risk Factors of Retinal Microvascular Abnormalities in Older Persons: The Cardiovascular Health Study. Ophthalmology (2003) 110(4):658–66. doi: 10.1016/S0161-6420(02)01931-0
4. Wong TY, Barr EL, Tapp RJ, Harper CA, Taylor HR, Zimmet PZ, et al. Retinopathy in Persons With Impaired Glucose Metabolism: The Australian Diabetes Obesity and Lifestyle (Ausdiab) Study. Am J Ophthalmol (2005) 140(6):1157–9. doi: 10.1016/j.ajo.2005.07.030
5. Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, et al. Prevalence of Diabetic Retinopathy in the United States, 2005-2008. Jama (2010) 304(6):649–56. doi: 10.1001/jama.2010.1111
6. Gunnlaugsdottir E, Halldorsdottir S, Klein R, Eiriksdottir G, Klein BE, Benediktsson R, et al. Retinopathy in Old Persons With and Without Diabetes Mellitus: The Age, Gene/Environment Susceptibility–Reykjavik Study (AGES-R). Diabetologia (2012) 55(3):671–80. doi: 10.1007/s00125-011-2395-y
7. Zhu Z, Wang W, Scheetz J, Zhang J, He M. Prevalence and Risk Profile of Retinopathy in non-Diabetic Subjects: National Health and Nutrition Examination Survey 2005 to 2008. Clin Exp Ophthalmol (2019) 47(9):1173–81. doi: 10.1111/ceo.13595
8. Cheung CY, Ikram MK, Sabanayagam C, Wong TY. Retinal Microvasculature as a Model to Study the Manifestations of Hypertension. Hypertension (2012) 60(5):1094–103. doi: 10.1161/HYPERTENSIONAHA.111.189142
9. Khandekar R, Al Hassan A, Al Dhibi H, Al Bahlal A, Al-Futais M. Magnitude and Determinants of Diabetic Retinopathy Among Persons With Diabetes Registered at Employee Health Department of a Tertiary Eye Hospital of Central Saudi Arabia. Oman J Ophthalmol (2015) 8(3):162–5. doi: 10.4103/0974-620X.169889
10. Wong TY, Coresh J, Klein R, Muntner P, Couper DJ, Sharrett AR, et al. Retinal Microvascular Abnormalities and Renal Dysfunction: The Atherosclerosis Risk in Communities Study. J Am Soc Nephrol: JASN (2004) 15(9):2469–76. doi: 10.1097/01.ASN.0000136133.28194.E4
11. Wong TY, Klein R, Amirul Islam FM, Cotch MF, Couper DJ, Klein BE, et al. Three-Year Incidence and Cumulative Prevalence of Retinopathy: The Atherosclerosis Risk in Communities Study. Am J Ophthalmol (2007) 143(6):970–6. doi: 10.1016/j.ajo.2007.02.020
12. Qiu C, Cotch MF, Sigurdsson S, Garcia M, Klein R, Jonasson F, et al. Retinal and Cerebral Microvascular Signs and Diabetes: The Age, Gene/Environment Susceptibility-Reykjavik Study. Diabetes (2008) 57(6):1645–50. doi: 10.2337/db07-1455
13. Wong TY, Rosamond W, Chang PP, Couper DJ, Sharrett AR, Hubbard LD, et al. Retinopathy and Risk of Congestive Heart Failure. Jama (2005) 293(1):63–9. doi: 10.1001/jama.293.1.63
14. Grunwald JE, Ying GS, Maguire M, Pistilli M, Daniel E, Alexander J, et al. Association Between Retinopathy and Cardiovascular Disease in Patients With Chronic Kidney Disease (From the Chronic Renal Insufficiency Cohort [CRIC] Study). Am J Cardiol (2012) 110(2):246–53. doi: 10.1016/j.amjcard.2012.03.014
15. Kawasaki R, Tanaka S, Tanaka S, Abe S, Sone H, Yokote K, et al. Risk of Cardiovascular Diseases is Increased Even With Mild Diabetic Retinopathy: The Japan Diabetes Complications Study. Ophthalmology (2013) 120(3):574–82. doi: 10.1016/j.ophtha.2012.08.029
16. Fisher DE, Jonasson F, Klein R, Jonsson PV, Eiriksdottir G, Launer LJ, et al. Mortality in Older Persons With Retinopathy and Concomitant Health Conditions: The Age, Gene/Environment Susceptibility-Reykjavik Study. Ophthalmology (2016) 123(7):1570–80. doi: 10.1016/j.ophtha.2016.02.045
17. Van Hecke MV, Dekker JM, Nijpels G, Moll AC, Van Leiden HA, Heine RJ, et al. Retinopathy is Associated With Cardiovascular and All-Cause Mortality in Both Diabetic and Nondiabetic Subjects: The Hoorn Study. Diabetes Care (2003) 26(10):2958. doi: 10.2337/diacare.26.10.2958
18. Xu L, Wang YX, Xie XW, Jonas JB. Retinopathy and Mortality. The Beijing Eye Study. Graefe’s Arch Clin Exp Ophthalmol = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie (2008) 246(6):923–5. doi: 10.1007/s00417-008-0773-z
19. Sairenchi T, Iso H, Yamagishi K, Irie F, Okubo Y, Gunji J, et al. Mild Retinopathy Is a Risk Factor for Cardiovascular Mortality in Japanese With and Without Hypertension: The Ibaraki Prefectural Health Study. Circulation (2011) 124(23):2502–11. doi: 10.1161/CIRCULATIONAHA.111.049965
20. Juutilainen A, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Retinopathy Predicts Cardiovascular Mortality in Type 2 Diabetic Men and Women. Diabetes Care (2007) 30(2):292–9. doi: 10.2337/dc06-1747
21. Fuller JH, Stevens LK, Wang SL. Risk Factors for Cardiovascular Mortality and Morbidity: The WHO Mutinational Study of Vascular Disease in Diabetes. Diabetologia (2001) 44 Suppl 2:S54–64. doi: 10.1007/PL00002940
22. Cusick M, Meleth AD, Agron E, Fisher MR, Reed GF, Knatterud GL, et al. Associations of Mortality and Diabetes Complications in Patients With Type 1 and Type 2 Diabetes: Early Treatment Diabetic Retinopathy Study Report No. 27. Diabetes Care (2005) 28(3):617–25. doi: 10.2337/diacare.28.3.617
23. Wong TY, Klein R, Nieto FJ, Klein BE, Sharrett AR, Meuer SM, et al. Retinal Microvascular Abnormalities and 10-Year Cardiovascular Mortality: A Population-Based Case-Control Study. Ophthalmology (2003) 110(5):933–40. doi: 10.1016/S0161-6420(03)00084-8
24. Liew G, Wong TY, Mitchell P, Cheung N, Wang JJ. Retinopathy Predicts Coronary Heart Disease Mortality. Heart (2009) 95(5):391–4. doi: 10.1136/hrt.2008.146670
25. Mitchell P, Wang JJ, Wong TY, Smith W, Klein R, Leeder SR. Retinal Microvascular Signs and Risk of Stroke and Stroke Mortality. Neurology (2005) 65(7):1005–9. doi: 10.1212/01.wnl.0000179177.15900.ca
26. Longstreth W Jr, Larsen EK, Klein R, Wong TY, Sharrett AR, Lefkowitz D, et al. Associations Between Findings on Cranial Magnetic Resonance Imaging and Retinal Photography in the Elderly: The Cardiovascular Health Study. Am J Epidemiol (2007) 165(1):78–84. doi: 10.1093/aje/kwj350
27. Frith E, Loprinzi PD. Retinopathy and Mortality. Diabetes Spectr (2018) 31(2):184–8. doi: 10.2337/ds17-0010
28. Kramer CK, Rodrigues TC, Canani LH, Gross JL, Azevedo MJ. Diabetic Retinopathy Predicts All-Cause Mortality and Cardiovascular Events in Both Type 1 and 2 Diabetes: Meta-Analysis of Observational Studies. Diabetes Care (2011) 34(5):1238–44. doi: 10.2337/dc11-0079
29. Ricardo AC, Grunwald JE, Parvathaneni S, Goodin S, Ching A, Lash JP. Retinopathy and CKD as Predictors of All-Cause and Cardiovascular Mortality: National Health and Nutrition Examination Survey (NHANES) 1988-1994. Am J Kidney Diseases: Off J Natl Kidney Foundation (2014) 64(2):198–203. doi: 10.1053/j.ajkd.2014.01.437
30. Li YH, Sheu WH, Lee IT. Effects of Retinopathy and Chronic Kidney Disease on Long-Term Mortality in Type 2 Diabetic Inpatients With Normal Urinary Albumin or Protein: A Retrospective Cohort Study. BMJ Open (2018) 8(7):e021655. doi: 10.1136/bmjopen-2018-021655
31. Pavkov ME, Harding JL, Chou CF, Saaddine JB. Prevalence of Diabetic Retinopathy and Associated Mortality Among Diabetic Adults With and Without Chronic Kidney Disease. Am J Ophthalmol (2019) 198:200–8. doi: 10.1016/j.ajo.2018.10.019
32. Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey Data (2005). Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/nchs/nhanes.htm (Accessed February 11, 2018).
33. Grading Diabetic Retinopathy From Stereoscopic Color Fundus Photographs–an Extension of the Modified Airlie House Classification. ETDRS Report Number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology (1991) 98(5 Suppl):786–806.
34. Klein R, Klein BE, Magli YL, Brothers RJ, Meuer SM, Moss SE, et al. An Alternative Method of Grading Diabetic Retinopathy. Ophthalmology (1986) 93(9):1183–7. doi: 10.1016/S0161-6420(86)33606-6
35. Klein R, Meuer SM, Moss SE, Klein BE, Neider MW, Reinke J. Detection of Age-Related Macular Degeneration Using a Nonmydriatic Digital Camera and a Standard Film Fundus Camera. Arch Ophthalmol (2004) 122(11):1642–6. doi: 10.1001/archopht.122.11.1642
36. Varma R, Bressler NM, Doan QV, Gleeson M, Danese M, Bower JK, et al. Prevalence of and Risk Factors for Diabetic Macular Edema in the United States. JAMA Ophthalmol (2014) 132(11):1334–40. doi: 10.1001/jamaophthalmol.2014.2854
37. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017ACC/AHA/AAPA/ABC/ACPM/AGS/Apha/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol (2018) 71(19):e127–248. doi: 10.1016/j.jacc.2017.11.006
38. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A New Equation to Estimate Glomerular Filtration Rate. Ann Internal Med (2009) 150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
39. Loprinzi PD, Addoh O. Predictive Validity of the American College of Cardiology/American Heart Association Pooled Cohort Equations in Predicting All-Cause and Cardiovascular Disease-Specific Mortality in a National Prospective Cohort Study of Adults in the United States. Mayo Clinic Proc (2016) 91(6):763–9. doi: 10.1016/j.mayocp.2016.03.019
40. van Hecke MV, Dekker JM, Stehouwer CD, Polak BC, Fuller JH, Sjolie AK, et al. Diabetic Retinopathy is Associated With Mortality and Cardiovascular Disease Incidence: The EURODIAB Prospective Complications Study. Diabetes Care (2005) 28(6):1383–9. doi: 10.2337/diacare.28.6.1383
41. Barger AC, Beeuwkes R 3rd, Lainey LL, Silverman KJ. Hypothesis: Vasa Vasorum and Neovascularization of Human Coronary Arteries. A Possible Role in the Pathophysiology of Atherosclerosis. N Engl J Med (1984) 310(3):175–7. doi: 10.1056/NEJM198401193100307
Keywords: retinopathy, systemic vascular comorbidities, diabetes, hypertension, kidney disease, cardiovascular disease, mortality
Citation: Zhu Z, Shang X, Wang W, Ha J, Chen Y, He J, Yang X and He M (2021) Impact of Retinopathy and Systemic Vascular Comorbidities on All-Cause Mortality. Front. Endocrinol. 12:750017. doi: 10.3389/fendo.2021.750017
Received: 30 July 2021; Accepted: 01 November 2021;
Published: 18 November 2021.
Edited by:
Michal Masternak, University of Central Florida, United StatesReviewed by:
Deiana Sorina Roman, Victor Babes University of Medicine and Pharmacy, RomaniaAllancer Divino De Carvalho Nunes, University of Minnesota Twin Cities, United States
Copyright © 2021 Zhu, Shang, Wang, Ha, Chen, He, Yang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingguang He, mingguang.he@unimelb.edu.au; Xiaohong Yang, syyangxh@scut.edu.cn
 Zhuoting Zhu
Zhuoting Zhu Xianwen Shang
Xianwen Shang Wei Wang
Wei Wang Jason Ha
Jason Ha Yifan Chen4
Yifan Chen4 Jingyi He
Jingyi He Xiaohong Yang
Xiaohong Yang Mingguang He
Mingguang He