Stem Cell Therapy in Chronic Periodontitis: Host Limitations and Strategies
- 1Hunan Key Laboratory of Oral Health Research and Hunan 3D Printing Engineering Research Center of Oral Care and Hunan Clinical Research Center of Oral Major Diseases and Oral Health and Xiangya Stomatological Hospital and Xiangya School of Stomatology, Central South University, Changsha, China
- 2Guanghua School of Stomatology, South China Center of Craniofacial Stem Cell Research, Sun Yat-sen University, Guangzhou, China
The treatment of chronic periodontitis is undergoing a transition from simple plaque removal and replacement with substitute materials to regenerative therapy, in which stem cells play an important role. Although stem cell-based periodontal reconstruction has been widely explored, few clinical regeneration studies have been reported. The inflammatory lesions under the impact of host factors such as local microbial–host responses, may impede the regenerative properties of stem cells and destroy their living microenvironment. Furthermore, systemic diseases, in particular diabetes mellitus, synergistically shape the disordered host-bacterial responses and exacerbate the dysfunction of resident periodontal ligament stem cells (PDLSCs), which ultimately restrain the capacity of mesenchymal stromal cells (MSCs) to repair the damaged periodontal tissue. Accordingly, precise regulation of an instructive niche has become a promising approach to facilitate stem cell-based therapeutics for ameliorating periodontitis and for periodontal tissue regeneration. This review describes host limitations and coping strategies that influence resident or transplanted stem cell-mediated periodontal regeneration, such as the management of local microbial–host responses and rejuvenation of endogenous PDLSCs. More importantly, we recommend that active treatments for systemic diseases would also assist in recovering the limited stem cell function on the basis of amelioration of the inflammatory periodontal microenvironment.
Introduction
The oral cavity is a gateway for the external world and is closely linked to systemic immunity and nutrient sensing, and the distinct symbiotic relationship between microbiota and oral tissues renders the bacteria influential to oral health importantly. For example, periodontal homeostasis is susceptible to overwhelmed bacteria–immune responses, which may dramatically call for the construction of chronic infection (1, 2). Until now, chronic periodontitis is the primary cause of tooth loss in adults. Once it occurs, the junctional epithelium migrates apically and transforms into the pocket epithelium, accompanied by a massive inflammatory infiltration culminating with impairment of bone homeostasis (3). Particularly, as oral bacteria and their components can enter the circulation through the ulcers within the periodontal pockets, periodontitis affects the occurrence and development of many systemic diseases, such as cardiovascular disease and diabetes (4, 5). Reciprocally, the morbidity and progression of periodontal inflammation are also affected by systemic conditions (6, 7). Therefore, chronic periodontitis is an important disease that is related to the whole body rather than localized infection.
The previous pathogenic understanding of periodontitis has prompted the mechanotherapy based on physically removing the bacterial plaques within periodontal pockets, including scaling and root planning, which can effectively prevent the progression of periodontitis but fail to recover the destroyed periodontal tissue (8). The subsequent introduction of guided tissue regeneration and bone substitute materials ushered in an era of periodontal regenerative therapies because of their positive effects in providing space and fundamental structure for nascent tissue regeneration, and the application of growth factors accelerates this process (9, 10). However, the inherent instability and delivery controllability of growth factors make their effects still controversial. In addition, the present regenerative periodontal therapies are usually conducted with artificial materials in surgery, which remains challenging to reduce surgical trauma and reconstruct the structural support upon massive defects (11). Therefore, current treatments of chronic periodontitis are still challenging in achieving functional recovery of the periodontium.
Over the past decades, mesenchymal stromal cells (MSCs) have journeyed from discovery to mechanistic studies and periodontal regenerative applications. For example, several studies reported the excellent capacity of transplanted periodontal ligament stem cells (PDLSCs) or adipose-derived stem cells (ADSCs) for repairing multiple periodontal lesions in animal models (12–14). Interestingly, PDLSCs appear to be better candidates for regenerative periodontal therapy than other types of MSCs because of their easy accessibility in the oral maxillofacial region (15). Furthermore, PDLSCs exhibited preferable self-renewal capacity than bone marrow MSCs and superior differentiation potential compared with other orofacial MSCs like gingival MSCs under conditioned medium (16, 17). Inspired by the excellent therapeutic potentials of PDLSCs, several preclinical and clinical studies applying PDLSCs for oral regeneration have been performed. However, the results of a recent clinical trial using autologous PDLSCs derived from the impacted tooth are not satisfactory, because there is no significant advantage to restoring the defective periodontium by transplanting PDLSCs, as the control group (cell-free group) had comparable tissue reconstruction (18). These observations appear to support the assumption that the living environment might be the primary reason affecting the regenerative process (19). Specifically, the endogenous stem cells in animal models may shape a healthy stem cell niche and possess a periodontal regenerative property but are impaired in periodontitis patients, which may explain the parallel mandibular defect regeneration in rabbits between the autologous bone graft group and the ADSC-containing group (20). Further, the artificial defects in animal models may not suffer from some decisive pathogenic factors, such as prolonged inflammatory stimuli and influences from systemic diseases (21). Therefore, mitigating host factors and recreating a beneficial microenvironment could be a promising approach for improving stem cell-based periodontal regeneration. Here, we summarize limitations from the host and coping strategies that influence resident or transplanted stem cell-mediated periodontal regeneration, such as the management of local microbial–host responses and rejuvenation of endogenous PDLSCs. More importantly, we recommend that active treatments for systemic diseases would also assist in recovering the limited stem cell function on the basis of amelioration of the inflammatory periodontal microenvironment.
Oral Microbial Infection and Host Defenses Within Periodontal Lesions
Whether involving transplanted or resident stem cells, an instructive microenvironment is a prerequisite for their function (22). The term “instructive microenvironment” within defects refers to a precise regulation between multiple cells and extracellular stimuli that provides inductive signaling to tissue regeneration. A typical example is that stem cells from human exfoliated deciduous teeth (SHED) can differentiate into sensory neurons in rat dorsal root ganglia, and SHED-originated aggregates can regenerate the functional pulp in the acellular and protective space of the pulp cavity (23). Specifically, effective root canal disinfection and the relatively closed space can allow pulp regeneration to escape bacterial interference (24), while the periodontal lesions in chronic periodontitis are usually accompanied by an imbalance of the oral microbiome and host immunity, which dramatically affects stem cell function (25, 26). Host responses to the microbiome orchestrate an inflammatory immunological niche, and we have summarized the inflammatory alteration of immune cell subsets and abnormal production of irritating cytokines, which significantly lead to irreversible periodontal inflammation (2). For example, dysbiosis in periodontitis triggers T helper (Th) 17 cells to produce inflammatory cytokines, such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β, which promote osteoclastogenesis and bone loss, and the Treg-mediated dysregulation of immunological surveillance under dysbiotic background promotes the above inflammatory cascade (Figure 1). However, recent evidence on the differentiation of bone-damaging Th17 cells has rehabilitated their role in restricting periodontal infection, although it is manifested as unacceptable tooth loss (27). Of course, during periodontitis treatment, the management of microbial–host responses is very important. For example, the application of resolvin D2 in periodontitis mice inhibits the adaptive immune responses mediated by Th1 cells, promotes M2 polarization of macrophages, and suppresses neutrophil accumulation and release of inflammatory factors, which synergistically reduces periodontal bone loss (28). Therefore, an instructive microenvironment is necessary, and balancing the interaction between the oral microflora and host defenses may be a promising approach in stem cell-mediated periodontal regeneration.
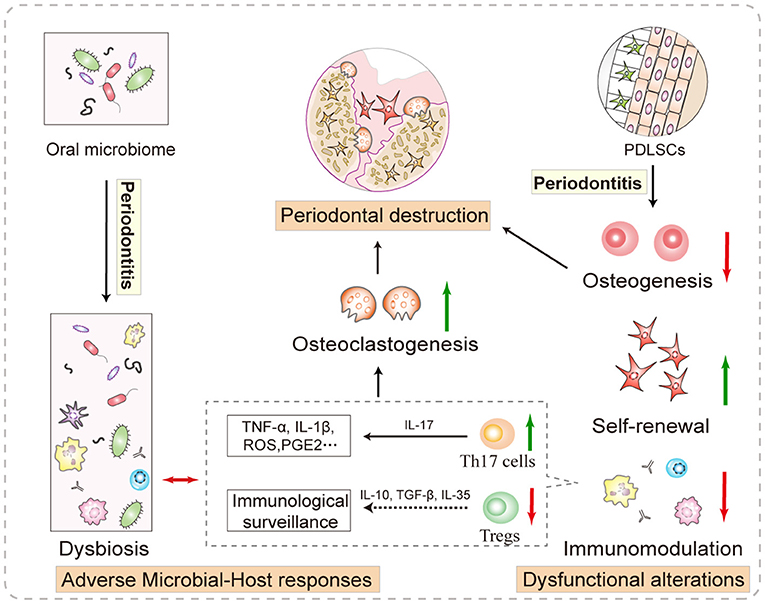
Figure 1. Local factors that promote periodontal destruction. The adverse immunity and continuous stimulation of the dysbiotic microbiome reinforce each other and create a hostile microenvironment for stem cell function. More importantly, the compensatory and decompensatory responses of resident P-PDLSCs are involved in shaping the local microenvironment. Specifically, the increased proliferation of P-PDLSCs cannot offset osteogenesis disorder, which indirectly leads to bone homeostasis imbalance. In addition, abnormal P-PDLSC immunoregulation increases Th17 cell differentiation. These IL-17-releasing cells trigger massive production of inflammatory cytokines, such as TNF-α and IL-1β, which promote osteoclastogenesis and bone loss, and the Treg-mediated dysregulation of immunological surveillance promotes the above inflammatory cascade.
The Dysfunctional Alterations of Resident PDLSCs
Except for the inevitable pulp extirpation post-infection, resident stem cells within other oral defects remain, whose functional status is closely related to tissue restoration (29). It has been suggested that transplanted stem cells may rejuvenate resident stem cells by improving the surrounding microenvironment in a paracrine or other manner, rather than facilitating tissue regeneration directly (30). However, overwhelmed inflammation leads to dysfunction in PDLSCs from periodontitis (P-PDLSCs), especially the decreased osteogenesis and immunomodulation capacities, and it is challenging for these defective cells to repair the periodontitis-damaged tissue under dysbiotic background (Figure 1). Specifically, the compensatory alterations in P-PDLSC, observed as high proliferation and migration rates, are considered a resistance response to the inflammation (31). In addition, angiogenin and basic fibroblast growth factor are expressed at higher levels in P-PDLSCs compared to healthy PDLSCs, suggesting that P-PDLSCs retain stronger angiogenesis-promoting capacity (32). However, the compensatory responses of P-PDLSCs are often insufficient to counterbalance the negative effects under continuous inflammatory stimulation, and subsequently show functional impairments as a decompensation functional phase. On the one hand, a functional study of P-PDLSCs mentioned the correlation between their impaired cell aggregates formation and osteogenesis disorder, which may lead to the inevitable progressive loss of the periodontium (33). Importantly, the lack of natural interaction between the cells may decrease the developmental signal in tissue recovery (23, 34). On the other hand, P-PDLSCs exhibit diminished immunosuppressive capability (31, 35). Typically, the P-PDLSC-induced imbalanced ratio of Th17 and Tregs facilitates the aggravation of periodontitis, the alteration thereof may be associated with the massive proinflammatory cytokine release and affected immunological surveillance (35). These studies suggest that resident P-PDLSC functional impairments may contribute to restrained regeneration in periodontitis, and the recruitment of resident stem cells applying low-intensity pulsed ultrasound and plant extracts such as resveratrol facilitated resident P-PDLSC functional recovery and reduced bone resorption in animal models (33, 36). Previously, we have reviewed affected intracellular signaling pathways and epigenetic regulation within P-PDLSCs, including but being not limited to nuclear factor-kappa B (NF-κB), Wnt, and mitogen-activated protein kinases (MAPK) pathways, as key mediators of the functional disorders of P-PDLSCs (2). For example, treatment with aspirin induces the expression of general control non-repressed protein 5 (GCN5) in P-PDLSCs, which then upregulates the expression of dickkopf-related protein 1 (DKK1) and inactivates the Wnt–β-catenin pathway indirectly, ultimately enhancing the osteogenic differentiation of P-PDLSCs (37). Similarly, the application of erythropoietin could activate the p38 MAPK pathway in P-PDLSCs and promote their bone formation property (38) (Table 1). Taken together, it is still a hotspot and future direction to restore impaired functional phenotypes of P-PDLSCs through molecular targets combined with improving the extracellular microenvironment, which has a promising clinical perspective in realizing endogenous stem cell-mediated periodontal regeneration.
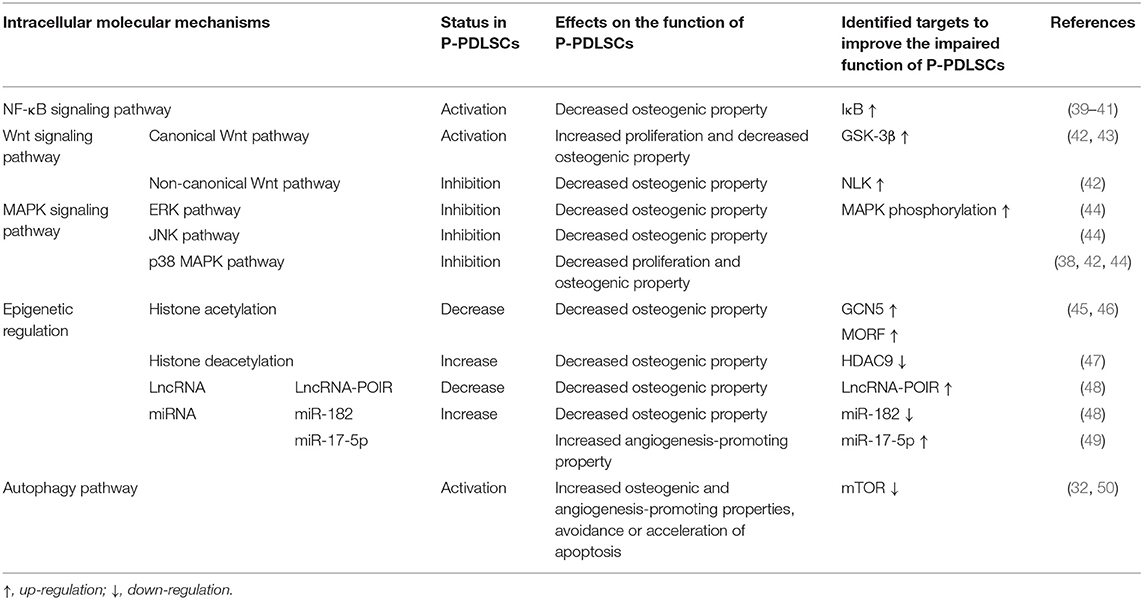
Table 1. Known molecular mechanisms underlying P-PDLSC dysfunction and targeted therapies to improve the impaired function of P-PDLSCs.
What Is the Role of Systemic Conditions When Stem Cells Work?
As the important correlation between oral and general health becomes more widely known, the positive role of systemic diseases, such as diabetes, in hindering recovery from oral defects has been recognized (51–53). Here, we summarize the impact of some systemic factors, particularly high blood glucose, on stem cell-mediated periodontal, including aggravating the microbial–host responses and exacerbating the dysfunction of resident stem cells (Figure 2).
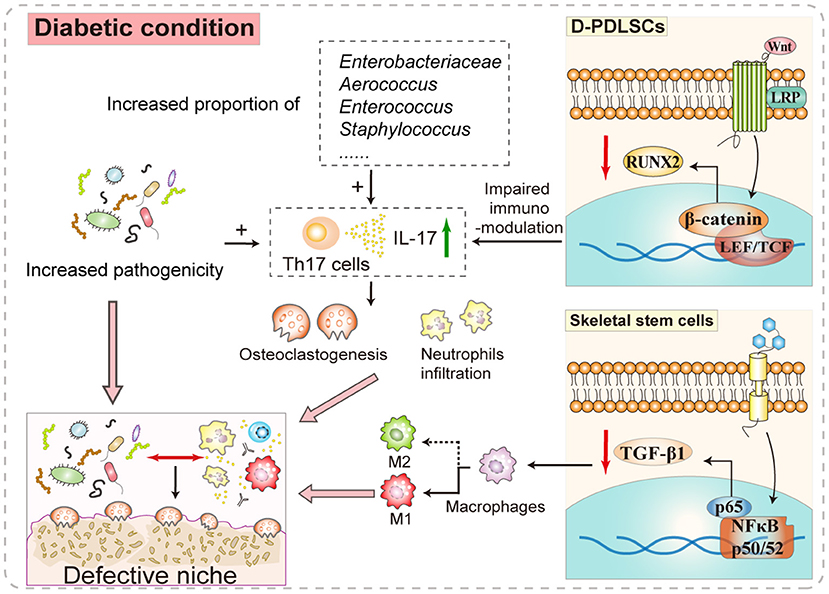
Figure 2. The participatory role of diabetes in shaping the periodontal niche. The increased release of IL-17 in the oral microenvironment of patients with diabetes-associated periodontitis caused by the readjusted proportion and increased pathogenicity of the oral microbiome forms positive feedback with the disordered P-PDLSC immunomodulation, which intensifies the local inflammatory infiltration and osteoclastic activity. Besides, certain signaling pathways in stem cells are abnormally activated in the background of hyperglycemia. For example, P-PDLSCs have more obvious osteogenic disorders under diabetic conditions, which may be related to intracellular Wnt–β-catenin pathway activation. Another example is the aberrant NF-κB signaling activation in skeletal stem cells reducing the secretion of the immunoregulatory factor TGF-β1 and increasing M1 inflammatory polarization. Therefore, systemic factors may synergistically deteriorate the local stem cell niche-like microenvironment and influence periodontal recovery.
Aggravating the Microbial–Host Responses
The remodeled oral microbiome and aggravated host adverse immunity under systemic diseases have drawn much research attention, which may hinder stem cell-based therapy. For example, the pathogenicity of the oral microbiome under diabetic conditions has been increased, as inoculating similar bacteria into diabetic mice led to greater inflammation than that in the control group (54). Further, elevated blood glucose may alter the resident microbiome composition and pathogenicity. Specifically, the bacteria from diabetic mice induced more IL-17 production, which may be responsible for the increased neutrophil infiltration and osteoclast formation in germ-free mice (55). The theory that glucose influences the oral microbiome composition may only hold under the periodontitis background, as diabetic people and healthy controls have similar salivary microbiomes (56). Generally, systemic diseases that interfere with oral microbiome establishment are common (53), e.g., reduced microbial diversity and a higher proportion of pathogenic bacteria can be detected in patients with periodontitis with systemic lupus erythematosus (57). Further complicating this item, systemic diseases also aggravate the host's inappropriate immunity to oral bacteria (55, 57, 58). Typically, the NF-κB signaling pathway in skeletal stem cells from diabetic patients has activated aberrantly, which reduces the expression of transforming growth factor-β (TGF-β1) and inhibits M2 polarization of anti-inflammatory macrophages (59), Additionally, high blood glucose can increase the expression of bone-resorbing cytokines, such as receptor activator for NF-κB ligand (RANKL), in periodontal fibroblasts and promote osteoclastogenesis in alveolar bone (55, 58). In short, the establishment of chronic periodontitis is a dynamic and holistic process, whereby distinct but disturbed host defenses are engaged at different stages.
Therefore, local injection of anti-IL-17 antibody suppressed the pathogenicity of oral bacteria under diabetes, manifested as reduced neutrophil infiltration and bone resorption (55). Further, active treatments for systemic diseases would also assist periodontal regeneration based on the amelioration of the inflammatory niche and maintenance of the instructive environment. For example, metformin hypoglycemic therapy and rheumatoid arthritis treatment restored the dynamic balance of the oral microbiome, thereby relieving periodontitis (56, 60). In addition, bindarit suppressed the persistently elevated chemokine (C-C motif) ligand 2 in diabetes-associated periodontitis, which decreased proinflammatory monocyte recruitment and alleviated periodontitis (61).
Exacerbating the Dysfunction of Resident PDLSCs
The impact of systemic diseases on resident stem cells has been proposed for years. Recently, a study on P-PDLSCs under diabetes (D-PDLSCs) has provided evidence of their impaired osteogenic differentiation, while the underlying mechanism may differ from that of simple P-PDLSCs (39). It appears that high blood glucose readjusts the intracellular cascade effects of NF-κB signaling in D-PDLSCs, as inhibiting the NF-κB signaling increases the P-PDLSC osteogenesis, whereas it does not upregulate D-PDLSC osteogenesis (2). Surprisingly, suppressing Wnt–β-catenin signaling reversed the osteogenic potential of D-PDLSCs, which is consistent with that of simple P-PDLSCs (39, 48). However, inhibiting NF-κB signaling in diabetic mice reduced osteoclast numbers and alveolar bone loss (58). Hence, further studies can explore the balance of Wnt–β-catenin and NF-κB signaling in D-PDLSCs to achieve optimal periodontal recovery. Until now, few studies have established the functional status and reparative role of resident PDLSCs under systemic diseases, and more interventions are still required for rejuvenating the resident PDLSCs and achieving periodontal regeneration.
Perspectives on Current Strategies for Stem Cell-Mediated Periodontitis Therapy
In the past 20 years, there has been considerable progress in stem cell-mediated oral tissue reconstruction, and promoting resident or transplanted stem cells has demonstrated therapeutic prospects (62, 63). However, until now, only the pulp has been clinically regenerated, which may be due to the applied young SHED aggregates and the natural shape of the pulp cavity, while the results of a recent clinical trial using autologous healthy PDLSCs derived from the impacted tooth are not satisfactory (18, 23). Of note, pulpitis is a localized disease that is rarely affected by persistent host factors, and the pulp cavity confers physical protection for pulp regeneration (64). Other than pulpitis, however, periodontal lesions lack protective space for stem cell function and are closely linked to systemic inflammation, which may partially explain the difficulty in their regeneration. Accordingly, a reasonable breakthrough for ensuring periodontal regeneration could be the improvement of adverse effects from local or systemic factors. For example, decreasing the pathogenicity of oral bacteria and suppressing the recruitment of proinflammatory monocyte by using small molecule drugs can alleviate diabetes-associated periodontitis (55, 61). Furthermore, active treatments for systemic diseases, such as diabetes and rheumatoid arthritis, would aid recovery based on ameliorating the bacterial inflammatory responses and stem cell status (56, 60). However, most strategies for intervening microbial-host responses are based on mechanistic in vitro or preclinical studies, which require further clinical verification, presenting more challenges for future treatment.
The unsatisfactory outcomes in autologous healthy PDLSCs-mediated periodontal regeneration may also attribute to the functional condition of endogenous PDLSCs, as the endogenous PDLSCs in animal models may possess the periodontal regenerative property but are impaired in periodontitis patients. Previous studies on PDLSCs from discovery to mechanistic studies and regenerative applications may set an emerging direction to recruit and mobilize endogenous P-PDLSCs for periodontal regeneration (Figure 3). As current studies have revealed the molecular mechanisms involved in the inflammatory responses of P-PDLSCs, restoration and/or mobilization of P-PDLSCs can be targetedly achieved by using small molecule drugs, herbal extracts, and accessory extracellular vesicle (EV) products (2). However, it should be noted that precise delivery of drugs to modulate PDLSCs in vivo remain as a major challenge and a potential direction in this field. In this regard, as programmed cell death protein 1 (PD1) represents a functional surface marker of orofacial MSCs, targeted techniques such as aptamers may serve as effective tools for precise modulation (65). More importantly, the self-assemble SHED aggregates in pulp regeneration may initiate the redevelopment process, implicating the significance of natural signals inductive of tissue regeneration (23, 66). Notably, induction between stem cell subsets may evolve a natural condition for periodontal regeneration, as shown by the improved expression of extracellular matrix and bone-related genes and the regeneration of complex periodontium-like structures in vivo when using composite cell sheets composed of PDLSCs and jaw bone marrow MSCs (67). Therefore, the activation of resident P-PDLSCs and prudent administration of cellular interaction are necessary, but require multiple considerations to ensure the natural process of stem cell-mediated periodontal recovery, which is a lesson we should learn from the clinical success of pulp regeneration.
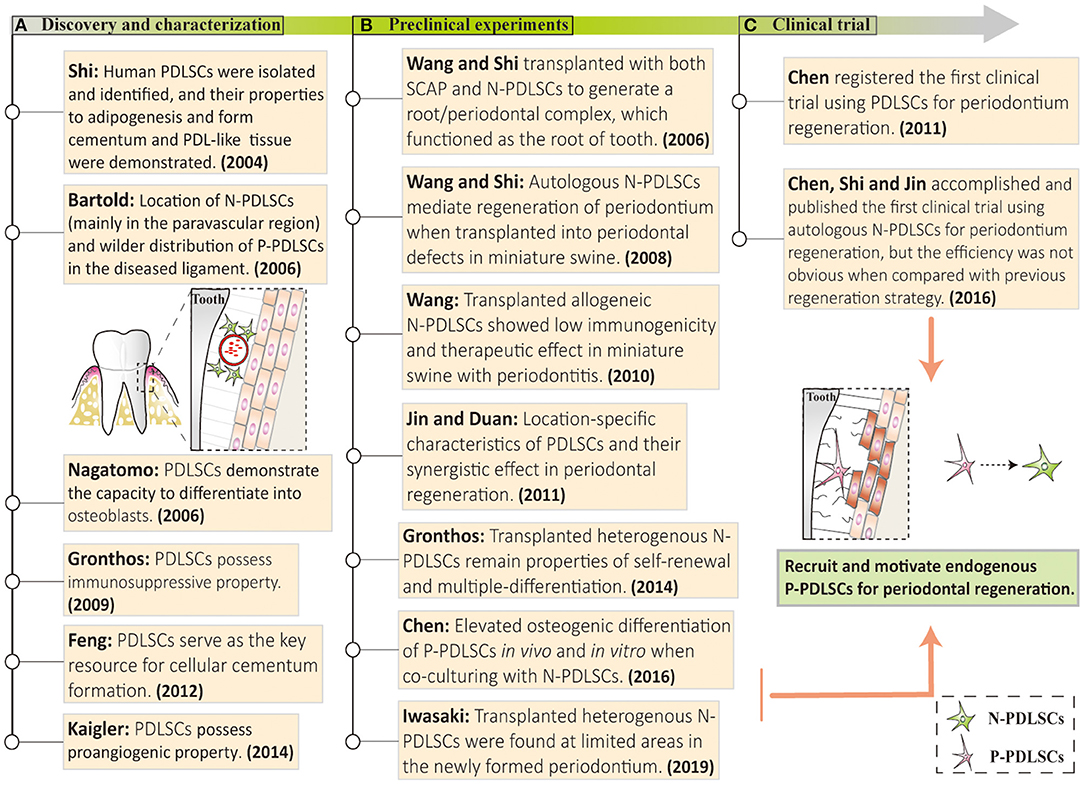
Figure 3. Discovery and application of PDLSCs in vitro and in vivo. (A) Studies over the past decade have described the excellent therapeutic potential of PDLSCs, including self-renewal, angiogenesis, immunomodulation, and multiple differentiation properties. (B) Some animal experiments based on PDLSCs transplantation have been carried out and achieved satisfactory periodontal regeneration. (C) A clinical trial of PDLSC-mediated periodontitis treatments has been successfully conducted, but the effects of repairing periodontium were not noticeable. These studies above may set an emerging direction to recruit and mobilize endogenous P-PDLSCs for periodontal regeneration. N-PDLSCs, normal PDLSCs; P-PDLSCs, periodontitis-derived PDLSCs.
Conclusions
Chronic periodontitis exerts a great influence on the patient's life quality and interpersonal communication. The current treatment for periodontitis is moving toward the goal of functional periodontal regeneration, in which MSCs play an integral role in future therapy. Importantly, MSCs may shape a distinct niche with surrounding extracellular matrix and cytokines, which is related to stem cell fate. Therefore, the prerequisite for stem cell therapy in chronic periodontitis is the adjustment of local stem cell niche, which refers to the improvement of the systemic condition and the abnormal bacterial-host responses (Figure 4). Beyond that, continuous inflammation in the periodontal tissue induces functional impairments of endogenous P-PDLSCs, which contribute to the disturbed stem cell niche and restrained regeneration in periodontitis. Therefore, the functional condition of endogenous PDLSCs may be a key approach in periodontal regeneration (Figure 4). Although prompting endogenous stem cells to treat periodontitis has been validated in animal models, it is difficult to precisely improve the function of therapeutic stem cells and the mutual induction of cell subsets, and more in-depth research is still needed for functional periodontal regeneration.
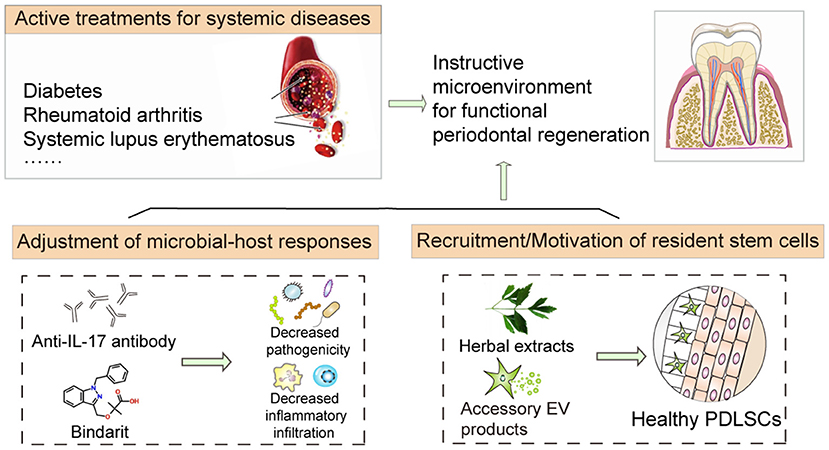
Figure 4. Coping strategies mitigating host factors for improving stem cell therapy. The application of biological products, including small molecular drugs, herbal extracts, and accessory extracellular vesicle products, can effectively improve the extracellular environment and promote stem cell function, thus alleviating periodontal defects in periodontitis. Significantly, active treatments for systemic diseases would also assist stem cell-mediated regeneration based on the amelioration of the inflammatory niche and maintenance of the instructive environment.
Author Contributions
ZZ contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. MD and MH contributed to conception, design, and interpretation and critically revised the manuscript. JT contributed to conception, design, and data interpretation and critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the work.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81500812 to JT), Key Research and Design Program of Hunan Province (2020SK2056 to JT), the Fundamental Research Funds for the Central Universities of Central South University (2021zzts0948 to ZZ), and the Postgraduate Scientific Research Innovation Project of Hunan Province (CX20210326 to ZZ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Deng ZL, Szafranski SP, arek M, Bhuju S, Wagner-Dobler I. Dysbiosis in chronic periodontitis: key microbial players and interactions with the human host. Sci Rep. (2017) 7:3703. doi: 10.1038/s41598-017-03804-8
2. Zhang Z, Deng M, Hao M, Tang J. Periodontal ligament stem cells in the periodontitis niche: inseparable interactions and mechanisms. J Leukoc Biol. (2021) 110:565–76. doi: 10.1002/JLB.4MR0421-750R
3. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. (2017) 3:17038. doi: 10.1038/nrdp.2017.38
4. Bui FQ, Almeida-da-Silva CLC, Huynh B, Trinh A, Liu J, Woodward J, et al. Association between periodontal pathogens and systemic disease. Biomed J. (2019) 42:27–35. doi: 10.1016/j.bj.2018.12.001
5. O'Boyle C, Haley MJ, Lemarchand E, Smith CJ, Allan SM, Konkel JE, et al. Ligature-induced periodontitis induces systemic inflammation but does not alter acute outcome after stroke in mice. Int J Stroke. (2020) 15:175–87. doi: 10.1177/1747493019834191
6. Khocht A, Albandar JM. Aggressive forms of periodontitis secondary to systemic disorders. Periodontol (2000). (2014) 65:134–48. doi: 10.1111/prd.12015
7. Biosse Duplan M, Hubert A, Le Norcy E, Louzoun A, Perry A, Chaussain C, et al. Dental and periodontal manifestations of glycogen storage diseases: a case series of 60 patients. J Inherit Metab Dis. (2018) 41:947–53. doi: 10.1007/s10545-018-0182-3
8. Lu H, Zhao Y, Feng X, He L, Meng H. Microbiome in maintained periodontitis and its shift over a single maintenance interval of 3 months. J Clin Periodontol. (2019) 46:1094–104. doi: 10.1111/jcpe.13177
9. Sculean A, Nikolidakis D, Nikou G, Ivanovic A, Chapple ILC, Stavropoulos A. Biomaterials for promoting periodontal regeneration in human intrabony defects: a systematic review. Periodontology (2000). (2015) 68:182–216. doi: 10.1111/prd.12086
10. Niu Y, Li Q, Ding Y, Dong L, Wang C. Engineered delivery strategies for enhanced control of growth factor activities in wound healing. Adv Drug Deliv Rev. (2019) 146:190–208. doi: 10.1016/j.addr.2018.06.002
11. Xu Y, Zhao S, Weng Z, Zhang W, Wan X, Cui T, et al. Jelly-inspired injectable guided tissue regeneration strategy with shape auto-matched and dual-light-defined antibacterial/osteogenic pattern switch properties. ACS Appl Mater Interfaces. (2020) 12:54497–506. doi: 10.1021/acsami.0c18070
12. Liu Y, Zheng Y, Ding G, Fang D, Zhang C, Bartold PM, et al. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells. (2008) 26:1065–73. doi: 10.1634/stemcells.2007-0734
13. Ding G, Liu Y, Wang W, Wei F, Liu D, Fan Z, et al. Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells. (2010) 28:1829–38. doi: 10.1002/stem.512
14. Mrozik KM, Wada N, Marino V, Richter W, Shi S, Wheeler DL, et al. Regeneration of periodontal tissues using allogeneic periodontal ligament stem cells in an ovine model. Regen Med. (2013) 8:711–23. doi: 10.2217/rme.13.66
15. Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. (2004) 364:149–55. doi: 10.1016/S0140-6736(04)16627-0
16. Song JS, Kim SO, Kim SH, Choi HJ, Son HK, Jung HS, et al. In vitro and in vivo characteristics of stem cells derived from the periodontal ligament of human deciduous and permanent teeth. Tissue Eng Part A. (2012) 18:2040–51. doi: 10.1089/ten.tea.2011.0318
17. Yang H, Gao L, An Y, Hu C, Jin F, Zhou J, et al. Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions. Biomaterials. (2013) 34:7033–47. doi: 10.1016/j.biomaterials.2013.05.025
18. Chen FM, Gao LN, Tian BM, Zhang XY, Zhang YJ, Dong GY, et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther. (2016) 7:33. doi: 10.1186/s13287-016-0288-1
19. Sui BD, Hu CH, Liu AQ, Zheng CX, Xuan K, Jin Y. Stem cell-based bone regeneration in diseased microenvironments: challenges and solutions. Biomaterials. (2019) 196:18–30. doi: 10.1016/j.biomaterials.2017.10.046
20. Mehrabani D, Khodakaram-Tafti A, Shaterzadeh-Yazdi H, Zamiri B, Omidi M. Comparison of the regenerative effect of adipose-derived stem cells, fibrin glue scaffold, and autologous bone graft in experimental mandibular defect in rabbit. Dent Traumatol. (2018) 34:413–20. doi: 10.1111/edt.12435
21. Kantarci A, Hasturk H, Van Dyke TE. Animal models for periodontal regeneration and peri-implant responses. Periodontol (2000). (2015) 68:66–82. doi: 10.1111/prd.12052
22. Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G, Goncalves RM. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front Immunol. (2018) 9:2837. doi: 10.3389/fimmu.2018.02837
23. Xuan K, Li B, Guo H, Sun W, Kou X, He X, et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med. (2018) 10:eaaf3227. doi: 10.1126/scitranslmed.aaf3227
24. Verma P, Nosrat A, Kim JR, Price JB, Wang P, Bair E, et al. Effect of residual bacteria on the outcome of pulp regeneration in vivo. J Dent Res. (2017) 96:100–6. doi: 10.1177/0022034516671499
25. Politis C, Schoenaers J, Jacobs R, Agbaje JO. Wound healing problems in the mouth. Front Physiol. (2016) 7:507. doi: 10.3389/fphys.2016.00507
26. Slots J. Primer on etiology and treatment of progressive/severe periodontitis: a systemic health perspective. Periodontol (2000). (2020) 83:272–6. doi: 10.1111/prd.12325
27. Tsukasaki M, Komatsu N, Nagashima K, Nitta T, Pluemsakunthai W, Shukunami C, et al. Host defense against oral microbiota by bone-damaging T cells. Nat Commun. (2018) 9:701. doi: 10.1038/s41467-018-03147-6
28. Mizraji G, Heyman O, Van Dyke TE, Wilensky A. Resolvin D2 restrains Th1 immunity and prevents alveolar bone loss in murine periodontitis. Front Immunol. (2018) 9:785. doi: 10.3389/fimmu.2018.00785
29. Mele L, Vitiello PP, Tirino V, Paino F, De Rosa A, Liccardo D, et al. Changing paradigms in cranio-facial regeneration: current and new strategies for the activation of endogenous stem cells. Front Physiol. (2016) 7:62. doi: 10.3389/fphys.2016.00062
30. Iwasaki K, Akazawa K, Nagata M, Komaki M, Honda I, Morioka C, et al. The fate of transplanted periodontal ligament stem cells in surgically created periodontal defects in rats. Int J Mol Sci. (2019) 20:192. doi: 10.3390/ijms20010192
31. Tang H-N, Xia Y, Yu Y, Wu R-X, Gao LN, et al. Stem cells derived from “inflamed” and healthy periodontal ligament tissues and their sheet functionalities: a patient-matched comparison. J Clin Periodontol. (2016) 43:72–84. doi: 10.1111/jcpe.12501
32. Wei W, An Y, An Y, Fei D, Wang Q. Activation of autophagy in periodontal ligament mesenchymal stem cells promotes angiogenesis in periodontitis. J Periodontol. (2018) 89:718–27. doi: 10.1002/JPER.17-0341
33. Wang YJ, Zhao P, Sui BD, Liu N, Hu CH, Chen J, et al. Resveratrol enhances the functionality and improves the regeneration of mesenchymal stem cell aggregates. Exp Mol Med. (2018) 50:1–15. doi: 10.1038/s12276-018-0109-y
34. Basu A, Rothermund K, Ahmed MN, Syed-Picard FN. Self-assembly of an organized cementum-periodontal ligament-like complex using scaffold-free tissue engineering. Front Physiol. (2019) 10:422. doi: 10.3389/fphys.2019.00422
35. Bunte K, Beikler T. Th17 Cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int J Mol Sci. (2019) 20:3394. doi: 10.3390/ijms20143394
36. Li H, Deng Y, Tan M, Feng G, Kuang Y, Li J, et al. Low-intensity pulsed ultrasound upregulates osteogenesis under inflammatory conditions in periodontal ligament stem cells through unfolded protein response. Stem Cell Res Ther. (2020) 11:215. doi: 10.1186/s13287-020-01732-5
37. Li B, Sun J, Dong Z, Xue P, He X, Liao L, et al. GCN5 modulates osteogenic differentiation of periodontal ligament stem cells through DKK1 acetylation in inflammatory microenvironment. Sci Rep. (2016) 6:26542. doi: 10.1038/srep26542
38. Wang L, Wu F, Song Y, Duan Y, Jin Z. Erythropoietin induces the osteogenesis of periodontal mesenchymal stem cells from healthy and periodontitis sources via activation of the p38 MAPK pathway. Int J Mol Med. (2018) 41:829–35. doi: 10.3892/ijmm.2017.3294
39. Liu Q, Hu CH, Zhou CH, Cui XX, Yang K, Deng C, et al. DKK1 rescues osteogenic differentiation of mesenchymal stem cells isolated from periodontal ligaments of patients with diabetes mellitus induced periodontitis. Sci Rep. (2015) 5:13142. doi: 10.1038/srep13142
40. Chen X, Hu C, Wang G, Li L, Kong X, Ding Y, et al. Nuclear factor-κB modulates osteogenesis of periodontal ligament stem cells through competition with beta-catenin signaling in inflammatory microenvironments. Cell Death Dis. (2013) 4:e510. doi: 10.1038/cddis.2013.14
41. Li C, Li B, Dong Z, Gao L, He X, Liao L, et al. Lipopolysaccharide differentially affects the osteogenic differentiation of periodontal ligament stem cells and bone marrow mesenchymal stem cells through Toll-like receptor 4 mediated nuclear factor κB pathway. Stem Cell Res Ther. (2014) 5:67. doi: 10.1186/scrt456
42. Liu N, Shi S, Deng M, Tang L, Zhang G, Liu N, et al. High levels of beta-catenin signaling reduce osteogenic differentiation of stem cells in inflammatory microenvironments through inhibition of the noncanonical Wnt pathway. J Bone Miner Res. (2011) 26:2082–95. doi: 10.1002/jbmr.440
43. Kong X, Liu Y, Ye R, Zhu B, Zhu Y, Liu X, et al. GSK3β is a checkpoint for TNF-alpha-mediated impaired osteogenic differentiation of mesenchymal stem cells in inflammatory microenvironments. Biochim Biophys Acta. (2013) 1830:5119–29. doi: 10.1016/j.bbagen.2013.07.027
44. Liang L, Zhou W, Yang N, Yu J, Liu H. ET-1 promotes differentiation of periodontal ligament stem cells into osteoblasts through ETR. MAPK, and Wnt/beta-catenin signaling pathways under inflammatory microenvironment. Mediators Inflamm. (2016) 2016:8467849. doi: 10.1155/2016/8467849
45. Xue P, Li B, An Y, Sun J, He X, Hou R, et al. Decreased MORF leads to prolonged endoplasmic reticulum stress in periodontitis-associated chronic inflammation. Cell Death Differ. (2016) 23:1862–72. doi: 10.1038/cdd.2016.74
46. Sun J, Dong Z, Zhang Y, He X, Fei D, Jin F, et al. Osthole improves function of periodontitis periodontal ligament stem cells via epigenetic modification in cell sheets engineering. Sci Rep. (2017) 7:5254. doi: 10.1038/s41598-017-05762-7
47. Li L, Liu W, Wang H, Yang Q, Zhang L, Jin F, et al. Mutual inhibition between HDAC9 and miR-17 regulates osteogenesis of human periodontal ligament stem cells in inflammatory conditions. Cell Death Dis. (2018) 9:480. doi: 10.1038/s41419-018-0480-6
48. Wang L, Wu F, Song Y, Li X, Wu Q, Duan Y, et al. Long noncoding RNA related to periodontitis interacts with miR-182 to upregulate osteogenic differentiation in periodontal mesenchymal stem cells of periodontitis patients. Cell Death Dis. (2016) 7:e2327. doi: 10.1038/cddis.2016.125
49. Zhang Z, Shuai Y, Zhou F, Yin J, Hu J, Guo S, et al. PDLSCs regulate angiogenesis of periodontal ligaments via VEGF transferred by exosomes in periodontitis. Int J Med Sci. (2020) 17:558–67. doi: 10.7150/ijms.40918
50. An Y, Liu W, Xue P, Zhang Y, Wang Q, Jin Y. Increased autophagy is required to protect periodontal ligament stem cells from apoptosis in inflammatory microenvironment. J Clin Periodontol. (2016) 43:618–25. doi: 10.1111/jcpe.12549
51. Lim JC, Ko KI, Mattos M, Fang M, Zhang C, Feinberg D, et al. TNFα contributes to diabetes impaired angiogenesis in fracture healing. Bone. (2017) 99:26–38. doi: 10.1016/j.bone.2017.02.014
52. Graves DT, Ding Z, Yang Y. The impact of diabetes on periodontal diseases. Periodontol (2000). (2020) 82:214–24. doi: 10.1111/prd.12318
53. Teles F, Wang Y, Hajishengallis G, Hasturk H, Marchesan JT. Impact of systemic factors in shaping the periodontal microbiome. Periodontol (2000). (2021) 85:126–60. doi: 10.1111/prd.12356
54. Naguib G, Al-Mashat H, Desta T, Graves DT. Diabetes prolongs the inflammatory response to a bacterial stimulus through cytokine dysregulation. J Invest Dermatol. (2004) 123:87–92. doi: 10.1111/j.0022-202X.2004.22711.x
55. Xiao E, Mattos M, Vieira GHA, Chen S, Correa JD, Wu Y, et al. Diabetes enhances IL-17 expression and alters the oral microbiome to increase its pathogenicity. Cell Host Microbe. (2017) 22:120–8.e124. doi: 10.1016/j.chom.2017.06.014
56. Sun X, Li M, Xia L, Fang Z, Yu S, Gao J, et al. Alteration of salivary microbiome in periodontitis with or without type-2 diabetes mellitus and metformin treatment. Sci Rep. (2020) 10:15363. doi: 10.1038/s41598-020-72035-1
57. Correa JD, Calderaro DC, Ferreira GA, Mendonca SM, Fernandes GR, Xiao E, et al. Subgingival microbiota dysbiosis in systemic lupus erythematosus: association with periodontal status. Microbiome. (2017) 5:34. doi: 10.1186/s40168-017-0252-z
58. Zheng J, Chen S, Albiero ML, Vieira GHA, Wang J, Feng JQ, et al. Diabetes activates periodontal ligament fibroblasts via NF-κB in vivo. J Dent Res. (2018) 97:580–8. doi: 10.1177/0022034518755697
59. Ko KI, Syverson AL, Kralik RM, Choi J, DerGarabedian BP, Chen C, et al. Diabetes-induced NF-κB dysregulation in skeletal stem cells prevents resolution of inflammation. Diabetes. (2019) 68:2095–106. doi: 10.2337/db19-0496
60. Zhang X, Zhang D, Jia H, Feng Q, Wang D, Liang D, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. (2015) 21:895–905. doi: 10.1038/nm.3914
61. Shen Z, Kuang S, Zhang M, Huang X, Chen J, Guan M, et al. Inhibition of CCL2 by bindarit alleviates diabetes-associated periodontitis by suppressing inflammatory monocyte infiltration and altering macrophage properties. Cell Mol Immunol. (2020) 18:1–12. doi: 10.1038/s41423-020-0500-1
62. Jakobsen C, Sorensen JA, Kassem M, Thygesen TH. Mesenchymal stem cells in oral reconstructive surgery: a systematic review of the literature. J Oral Rehabil. (2013) 40:693–706. doi: 10.1111/joor.12079
63. Holly D, Klein M, Mazreku M, Zamborsky R, Polak S, Danisovic L, et al. Stem cells and their derivatives-implications for alveolar bone regeneration: a comprehensive review. Int J Mol Sci. (2021) 22:11746. doi: 10.3390/ijms222111746
64. Yu C, Abbott PV. An overview of the dental pulp: its functions and responses to injury. Aust Dent J. (2007) 52:S4–S16. doi: 10.1111/j.1834-7819.2007.tb00525.x
65. Liu Y, Jing H, Kou X, Chen C, Liu D, Jin Y, et al. PD-1 is required to maintain stem cell properties in human dental pulp stem cells. Cell Death Differ. (2018) 25:1350–60. doi: 10.1038/s41418-018-0077-8
66. Yang X, Ma Y, Guo W, Yang B, Tian W. Stem cells from human exfoliated deciduous teeth as an alternative cell source in bio-root regeneration. Theranostics. (2019) 9:2694–711. doi: 10.7150/thno.31801
Keywords: chronic periodontitis, systemic diseases, mesenchymal stromal cells, regenerative medicine, bacterial infections
Citation: Zhang Z, Deng M, Hao M and Tang J (2022) Stem Cell Therapy in Chronic Periodontitis: Host Limitations and Strategies. Front. Dent. Med. 2:833033. doi: 10.3389/fdmed.2021.833033
Received: 10 December 2021; Accepted: 31 December 2021;
Published: 24 January 2022.
Edited by:
Luyuan Jin, Capital Medical University, ChinaReviewed by:
Dawei Liu, Peking University School and Hospital of Stomatology, ChinaJuan Du, Capital Medical University, China
Copyright © 2022 Zhang, Deng, Hao and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianxia Tang, jianxiatang@csu.edu.cn
 Zhiyu Zhang
Zhiyu Zhang Mengting Deng
Mengting Deng Meng Hao
Meng Hao Jianxia Tang
Jianxia Tang