Escitalopram-induced sinus bradycardia in coronary heart disease combined with depression: a case report and review of literature
- 1Department of Pharmacy, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Center for Clinical Pharmacy, Cancer Center, Department of Pharmacy, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, China
- 3Department of Pharmacy, The Affiliated Hospital of Hangzhou Normal University, Hangzhou, China
- 4Department of Drug Monitoring and Evaluation, Zhejiang Center for Drug and Cosmetic Evaluation, Hangzhou, China
- 5Department of Pharmacy, The People’s Hospital of Pingyang, Wenzhou, China
For patients with cardiovascular disease, using the antidepressant escitalopram may lead to unexpected adverse events. Here, a rare repeated sinus bradycardia event due to escitalopram is first reported. In an 82-year-old female patient with cardiac dysfunction using digoxin, tachycardia (average heart rate of 93 beats/min) was demonstrated by electrocardiogram (ECG). She began to take escitalopram and lorazepam due to depression, but sinus bradycardia (93.7% heart rate was <60 beats/min) and sinus arrest were first detected after 3 months. Its proportion decreased to 0.1% after discontinuation of digoxin and escitalopram for 1 day, and the rhythm returned to normal 2 weeks later. After 2 months, escitalopram was prescribed again in combination with quetiapine; then, 17.1% heart rate was <60 beats/min. After escitalopram and quetiapine withdrawal, the ECG showed the heart rhythm had normalized again. No other drug changes were made during these periods. Escitalopram was deemed to be a highly possible cause of sinus bradycardia according to its Naranjo's Algorithm score. Furthermore, literature on escitalopram-mediated cardiovascular adverse events was reviewed and analyzed. Empirically, escitalopram should be discontinued immediately if iatrogenic causes cannot be ruled out. Furthermore, ECG monitoring in escitalopram-related cardiovascular adverse events is highlighted, especially in patients receiving certain drug classes simultaneously (i.e., sinoatrial node inhibitors, antipsychotics).
1 Introduction
Escitalopram is a well-tolerated selective serotonin reuptake inhibitor (SSRI) used to treat depressive and anxiety disorders (1). It has no inhibitory effects on cholinergic receptors, histamine receptors, or adrenergic α receptors, and thus, may cause few adverse drug reactions (ADR) in patients (2). The ESC working group indicated that 15%–30% of patients with coronary heart disease also have depression, and the incidence of depression in patients with coronary heart disease is 2–3 times higher than that in the general population (3). A recent meta-analysis of randomized controlled trials revealed that the probability of cardiovascular side effects associated with antidepressants could be as high as 0.89% (4). The treatment of coronary heart disease and depression may present more complicated drug-related problems (DRP) and, thus, clinical pharmacists are urgently needed to participate in the drug treatment process.
In the present case report, escitalopram-induced sinus bradycardia and sinus arrest were reported in a patient with coronary heart disease and depression. We analyzed the etiology of escitalopram-induced sinus bradycardia under the condition of drug combination and the key points of pharmaceutical care in this population. We also conducted a review of literature on escitalopram-induced ADR other than sinus bradycardia, which is not listed in the package insert of escitalopram in patients with cardiovascular disease in order to reduce and avoid DRP.
2 Case presentation
At the first visit to our hospital, this 82-year-old female patient said she began to develop chest tightness and shortness of breath without an obvious trigger 10 years ago. These symptoms gradually worsened due to the lack of systematic diagnosis and timely treatment. She underwent coronary angiography 5 years ago, which revealed multiple vascular stenosis and lesions, and two stents were implanted in the right coronary artery.
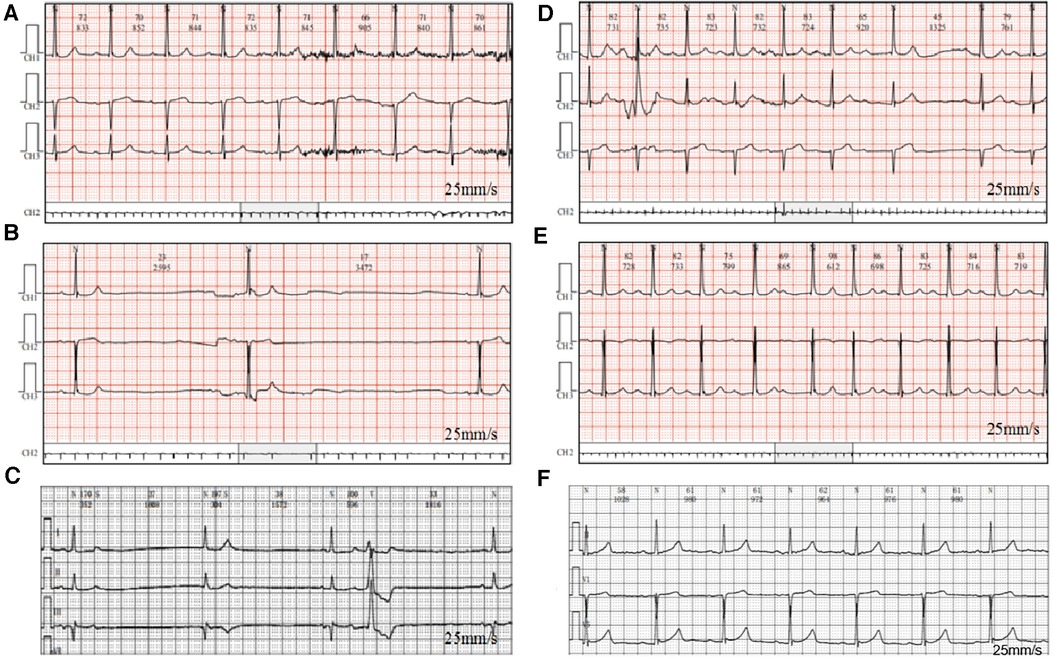
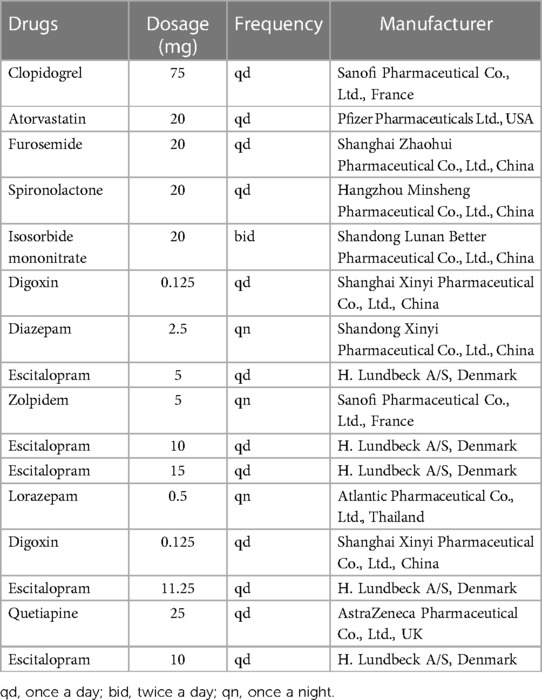
She attended our hospital again 6 months ago due to chest pain and shortness of breath accompanied by nocturnal paranoia and edema of the lower extremities. The admission diagnosis included coronary atherosclerotic heart disease, status after coronary stent implantation, and NYHA III cardiac function. Coronary artery calcium was detected by electron-beam computed tomography. Atherosclerotic plaques in the proximal left anterior descending branch and mixed plaques in the proximal left circumflex branch showed mild to moderate lumen stenosis. Meanwhile, the liver enzyme levels that reflected liver function and the creatinine level that reflected renal function were examined. We found that the levels of glutamic-pyruvic transaminase (17 U/L), glutamic oxalacetic transaminase (20 U/L), glutamyl transpeptidase (21 U/L), alkaline phosphatase (92 U/L), total bilirubin (10 μmol/L), and creatinine (98.5 μmol/L) were all within the normal range in our hospital. However, the patient did not have an echocardiogram examined or cardiac markers tested. The 24-h Holter ECG monitoring of the patient revealed sinus rhythm accompanied by ST segment changes and atrial tachycardia but without bradycardia or sinus pause/arrest. Then, the patient was treated with symptomatic supportive treatments including clopidogrel, atorvastatin, furosemide, spironolactone, and isosorbide mononitrate. Due to the symptoms of cardiac dysfunction (difficulty breathing, limited physical activity, and fluid retention) and atrial tachycardia, digoxin was also prescribed on day 1. All the drugs used for treating the patient are shown in Figure 1. She developed symptoms of insomnia and was first diagnosed with depression; she began diazepam treatment on day 2. The next day, electrocardiogram (ECG) detection showed tachycardia and second-degree atrioventricular block type I with an average heart rate of 93 beats/min, but with no sinus bradycardia event (Figure 2A). On day 5, diazepam was replaced by zolpidem, while escitalopram (5 mg once a day) was first prescribed in the treatment due to unimproved symptoms (sleep disturbances and anxiety). On day 7, zolpidem was replaced by lorazepam, and the dose of escitalopram was increased to 10 mg once a day. On day 14, the dose of escitalopram was increased to 15 mg once a day until day 92. Laboratory test results were normal, including potassium and magnesium serum levels. A detailed description of the use of drugs during the onset of sinus bradycardia is shown in Table 1.
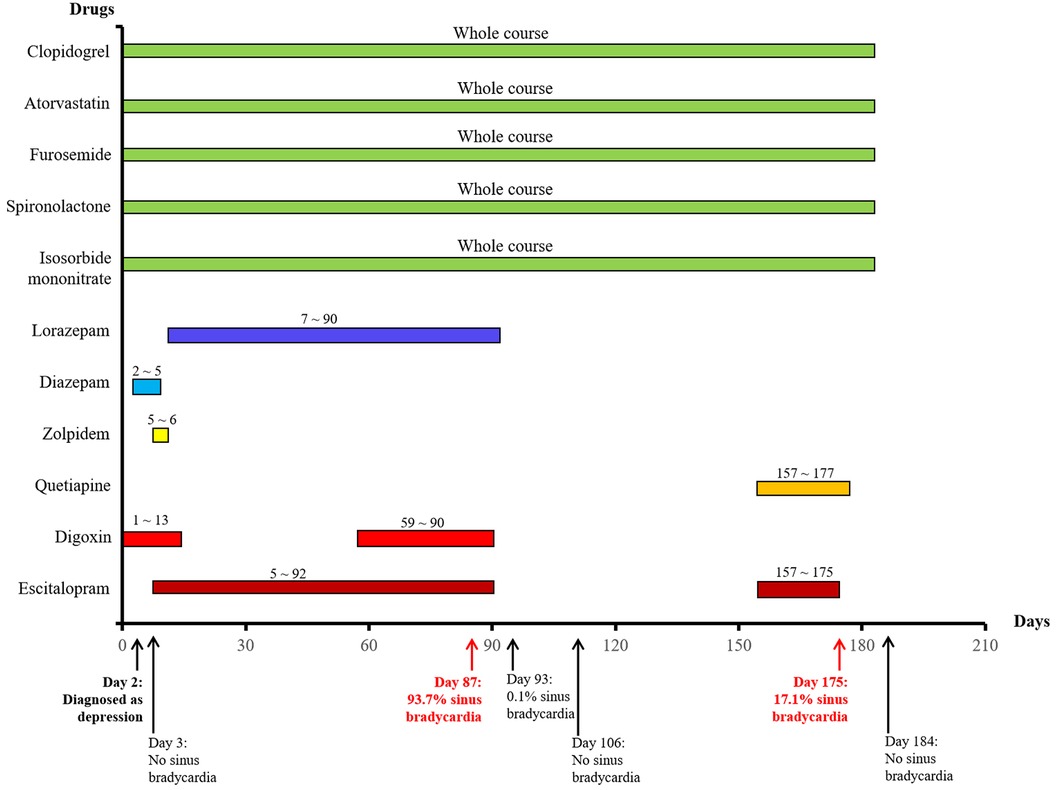
Figure 1. The used drugs and sinus bradycardia events by time in a patient with coronary heart disease.
On day 87, the patient developed a substantial proportion of bradycardia below the focused level (heart rate <60 beats/min) and sinus arrest, with second-degree type II sinus atrial block with an average heart rate of 53 beats/min by the second ECG detection (Figure 2B). The duration of sinus bradycardia (heart rate <60 beats/min) was 93.7% of the total time. The slowest heart rate was 32 beats/min, while the fastest heart rate was 69 beats/min. However, the ECG report revealed similar percentages of low heart rate in the daytime and nighttime (sleep) periods. On day 90, lorazepam was suspended but the use of escitalopram was continued. On day 92, escitalopram was also suspended. The next day, we rechecked the ECG and it showed continued sinus arrest with first-degree atrioventricular block with an average heart rate of 67 beats/min (Figure 2C). Surprisingly, sinus bradycardia significantly decreased from 93.7% (before escitalopram discontinuation) to 0.1% (after escitalopram discontinuation). The doctors concluded that escitalopram might have been the cause of the sinus bradycardia events, and then escitalopram was discontinued. On day 106, sinus bradycardia disappeared but the atrioventricular conduction remained, showing prolonged PR and second-degree Mobitz type I blockage (Figure 2D). Furthermore, we found that the levels of glutamic-pyruvic transaminase (8 U/L), glutamic oxalacetic transaminase (13 U/L), glutamyl transpeptidase (14 U/L), alkaline phosphatase (99 U/L), total bilirubin (8.1 μmol/L), and creatinine (103.8 μmol/L) were all within the normal range in our hospital.
On day 157, the patient felt anxiety and subsequently purchased escitalopram (11.25 mg every day) and quetiapine to treat anxiety outside of our hospital. Unfortunately, the patient said she experienced chest distress and shortness of breath again with worse symptoms, accompanied by fatigue and conscious numbness of the limbs on day 173. The dynamic ECG in the local hospital showed sinus rhythm, paroxysmal atrial fibrillation, occasional R-R long interval, paired and short atrial tachycardia in atrial premature beats, and dual ventricular premature beats. After symptomatic treatment, she showed no significant improvement and was admitted to our hospital again. During this hospitalization, escitalopram and quetiapine were continued, but the dose of escitalopram was decreased to 10 mg on day 175 and then discontinued, while quetiapine was stopped on the third day. On day 175, the duration of sinus bradycardia was 17.1% of the total monitoring times, accompanied by intermittent first-degree atrioventricular block by dynamic ECG monitoring (Figure 2E). The slowest heart rate was 52 beats/min, while the fastest heart rate was 98 beats/min, with an average heart rate of 74 beats/min according to ECG detection. Meanwhile, the ECG report revealed that the bradycardia events mainly occurred from 22:00 to 05:00. One week after escitalopram discontinuation, the last ECG showed the sinus rhythm with an average heart rate of 60 beats/min and first-degree atrioventricular block (Figure 2F). Furthermore, we detected that the levels of glutamic-pyruvic transaminase (13 U/L), glutamic oxalacetic transaminase (17 U/L), glutamyl transpeptidase (14 U/L), alkaline phosphatase (124 U/L), total bilirubin (11 μmol/L), and creatinine (76.4 μmol/L) were all within the normal range in our hospital. The levels of cardiac troponin I (0.007 μg/L) and B natriuretic peptide (45.1 pg/ml) were also in the normal range. The patient obtained a Naranjo's Algorithm ADR probability score of 7, which is used to assess whether there is a causal relationship between an adverse drug experience and a drug, using a simple questionnaire to assign probability scores (5). If the total Naranjo's Algorithm score lies between 5 and 8, the causal relationship is “highly possible”; if the total score is greater than or equal to 9, the causal relationship is “definite”. Thus, escitalopram use was deemed to be a “highly possible” cause of the patient's sinus bradycardia based on the Naranjo's Algorithm ADR probability score.
3 Discussion
Cardiovascular disease is one of the leading causes of death worldwide (6, 7). Depression has been shown to have a causal relationship with the occurrence and prognosis of cardiovascular disease (8–10). Furthermore, depression after cardiovascular disease has been reported to be associated with an increased risk of adverse events (11, 12). Compared with traditional treatments, Psycho-Cardiology therapy improved depression symptoms and long-term outcomes in patients with coronary heart disease (13). It suggests the importance of synergistic treatment of depression and cardiovascular diseases.
Escitalopram is widely used in primary care for a variety of psychiatric disorders, such as depression and anxiety (14). It was well tolerated in clinical practice, but it also led to various adverse effects, including the common neurological, psychopathic, and gastrointestinal ADR, as well as the recent reports on escitalopram-derived hepatitis and epistaxis, which were rarely mentioned (14, 15). Reporting ADR can inform the rational use of escitalopram and further improve patient drug management. In this study, we reported a rare case of repeated sinus bradycardia due to escitalopram in combination with other drugs.
The patient experienced the first sinus bradycardia event, sinus arrest, after raising the escitalopram dose from 5 mg to 15 mg per day, and returned to normal after the interruption of escitalopram. In the beginning, the patient was prescribed digoxin because of cardiac dysfunction. A sinus rhythm of 93 beats/min was demonstrated by the first dynamic ECG detection but with no sinus bradycardia evidence 2 days later. The patient then began to take escitalopram accompanied by lorazepam due to her new depression diagnosis, but sinus bradycardia (93.7% of heart rate was <60 beats/min) was detected by the second ECG 3 months later. Surprisingly, the proportion of sinus bradycardia significantly decreased to 0.1% after escitalopram discontinuation for 1 day and the rhythm returned to normal with no sinus bradycardia 2 weeks later. After 2 months, escitalopram was prescribed again in combination with quetiapine, and then another sinus bradycardia event occurred. However, the bradycardia disappeared 1 week after escitalopram withdrawal. Scoring according to the Naranjo Algorithm probability scale revealed a highly probable relationship between escitalopram and sinus bradycardia.
DRP including drug-induced ADR interferes with expected clinical treatment outcomes and should also be an important risk management factor to increase patient safety (16, 17). Multiple studies have been devoted to evaluating the harmful effects of antipsychotic drugs during the treatment of nervous disorders (18, 19). From the pharmacovigilance system of EudraVigilance, 8% of individual ADR reports were escitalopram, while the proportion was 13% from the Food and Drug Administration Adverse Events Reporting System (20). In a retrospective analysis of a tertiary hospital, escitalopram (6.1%) had the highest incidence of adverse reactions among antidepressants (21). Further evidence indicated that escitalopram was grouped as CYP2C19 substrate, which is associated with ADR related to modulating the autonomic nervous system, seizure, and pain (22). Thus, differences in the CYP2C19 genotype can affect the serum concentration of escitalopram, thus affecting the ADR. This may be one reason for escitalopram-induced adverse cardiac reactions, but the CYP2C19 genotype was not analyzed in the patient in this study. Furthermore, the arrhythmia caused by escitalopram may be attributed to its cardiotoxic metabolite S-didesmethylcitalopram (23).
In this case, when the patient received escitalopram accompanied by digoxin and lorazepam for 1 month, the patient developed sinus bradycardia. Prior to this, there was no evidence of sinus bradycardia. Costa and colleagues showed that the oral intake of lorazepam did not affect heart rates (24). Digoxin had a narrow therapeutic margin and potentially led to severe cardiovascular adverse effects. Other drugs that lengthen the QT interval or slow cardiac conduction may induce the cardiac adverse effects of digoxin (25). However, the abnormality of ECG described obvious alleviation when both digoxin and escitalopram were stopped for this patient on day 90. To our surprise, sinus bradycardia disappeared when escitalopram and digoxin were discontinued for 2 weeks, suggesting that escitalopram and digoxin may have induced the ADR. Later, the sinus bradycardia event was rediscovered when escitalopram was combined with quetiapine to treat depression but disappeared again when escitalopram and quetiapine were suspended. From this perspective, we inferred the conclusion that the cause of sinus bradycardia in this patient might have been the combined effect of escitalopram and other drugs that may cause bradycardia or antipsychotic drugs. Until now, emerging data on case series on escitalopram-induced typical ADR in patients with depression and cardiovascular disease have been continuously reported (Table 2). In addition to bradycardia during escitalopram use, the first-degree atrioventricular block and second-degree type I atrioventricular block were also detected by electrocardiogram in this case. In vivo study has demonstrated that escitalopram treatment could cause a decrease in heart rate, which manifests as a significant decrease in sympathetic components and a significant increase in parasympathetic components of the autonomic nervous imbalance (38). This may be a possible pathogenesis of escitalopram-related bradycardia. Furthermore, we found that higher therapeutic doses of escitalopram (15 mg, qd) led to more serious sinus bradycardia (93.7% sinus bradycardia) than escitalopram (11.25 mg, qd), which was associated with 17.1% sinus bradycardia. This indicates that lowering therapeutic doses may help to prevent adverse events.
Citalopram consists of two stereostructures, one stereoisomer is S-Citalopram (escitalopram) and the other isomer is R-Citalopram (no drug activity), each of which is 50%. The cross-sectional registry study found that the prevalence of QT-prolonging drugs included citalopram and escitalopram, which represent 14.5% and 3.9% of the study population, respectively (39), confirming that escitalopram had fewer adverse reactions than citalopram. However, a recent systematic review and meta-analysis involving 1,141 patients (573 experimental and 568 control) in randomized controlled trials showed that citalopram has positive effects on the left ventricular ejection fraction and N-terminal pro-B-type natriuretic peptide in patients with depression combined with chronic heart failure; meanwhile, no obvious adverse drug reactions were observed (40). It highlights the importance of real-world case studies as an addition to randomized controlled trials in the discovery of ADR.
The metabolism of escitalopram is mainly mediated by cytochrome CYP 2C19 in the liver. Thus, liver function impairment would reduce the metabolic clearance of escitalopram, which may have greater treatment effects, but the adverse effects are also greater. Oral digoxin is excreted through the kidney, and the blood concentration of digoxin increases with the decrease of the glomerular filtration rate. Therefore, decreased renal function can increase the blood concentration of digoxin and increase the risk of drug poisoning. Both liver and kidney function were within normal range before the initial use of escitalopram and after the suspension of escitalopram. Though we did not follow up on these tests on the day of bradycardia, we could infer from the available data that the liver and kidney function should be normal during escitalopram use.
Until now, there have been no official notices or expert guidelines regarding ECG monitoring in patients taking SSRIs like escitalopram, probably due to the relative rarity of severe arrhythmias. In an early study in 2000, the first case of sinus bradycardia was caused by pure citalopram overdose (800 mg), lasting up to 6 days with severe hypotension and intermittent syncope (41). The blood level of escitalopram during the bradycardia process would definitively prove whether the dose of escitalopram is too high in this case. However, the blood concentration of escitalopram was not investigated due to the lack of its availability. A baseline ECG is usually done before the initiation of antidepressant therapy with SSRI.
However, there may be other possible causes of bradyarrhythmia that cannot be excluded. Firstly, we do not know if the patient has sick sinus syndrome (SSS) or pre-existing sinus node dysfunction, which may lead to possible intermittent episodes. Secondly, the degenerative process of dual locations of the conducting system (sinus node and atrioventricular node) in elderly patients may also be the reason, as the ECGs in this case implied that there were dual conduction abnormalities located in the sinus node and atrioventricular node. Escitalopram may only attack a single site. Thirdly, the bradycardia disappeared after escitalopram and digoxin were discontinued during the first bradycardia attack. Previously, 93.7% of sinus bradycardia was observed, but the effect of digoxin cannot be ruled out because we did not monitor the blood concentration of digoxin. The second bradycardia occurred when the patient was not taking digoxin, but only 17.1% of sinus bradycardia was present. Eliminating the influence of detection timing, this may suggest that the combination of escitalopram with other drugs, such as digoxin, may counteract the additive effect of escitalopram-induced bradycardia. Fourthly, the patient had coronary heart disease, and bradycardia could have been due to sinoatrial node ischemia. In addition, the dynamic imbalance of myocardial demand and coronary blood supply may also lead to intermittent ischemia. Nevertheless, the records of angina symptoms and cardiac biomarkers (troponin level) were lacking and only one test was reported on the level of cardiac troponin I, which was a normal value. Last but not least, during these two bradycardia events, we did not test the patient's thyroid function in time to rule out the possibility of bradycardia associated with hypothyroidism. This is the shortcoming and limitation of real-world research like this study.
Although there are many possibilities that may affect bradycardia, physicians should remain aware of cardiac ADR in patients with cardiovascular diseases, and clinical pharmacists should provide full-process pharmaceutical care for screening and timely treatment of cardiovascular adverse events. Pharmacokinetic interactions between over-the-counter drugs and antidepressants including escitalopram may further increase the severity of side effects of the latter, which, clinically, is worth highlighting (42). It is also recommended that early and close ECG surveillance is necessary for early identification and treatment of arrhythmias induced by escitalopram.
4 Conclusion
To the best of our knowledge, this is a rare report of a repeated sinus bradycardia event induced by escitalopram in combination with other drugs. The possibility of pharmacodynamic interactions between escitalopram and the particular drug classes (i.e., sinoatrial node inhibitors, antipsychotics) may pose a higher risk of cardiac adverse effects. Therefore, patients receiving these drug combinations should be provided with surveillance measures, including routine ECG monitoring to screen for any possible cardiovascular adverse events to facilitate early detection.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of Zhejiang Provincial People's Hospital (No. QT2022416). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
J-NS designed this study. J-NS, L-CL and WS wrote the original draft. J-NS and WS performed the analysis of medication. L-CL, X-QL, YH and Y-YX reviewed the literature and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Ministry of Education Industry-University Cooperative Education Project (No. 22087043124451), Zhejiang Provincial CONBA Hospital Management Soft Science Research Project (No. 2019ZHA-KEB313), Zhejiang Provincial Natural Science Foundation of China (No. LYY19H280006), National Natural Science Foundation of China (Nos. 72204073, 81503129), Research Project of Health Commission of Zhejiang Province (No. 2023KY491), and Zhejiang Provincial Science Technology Projects of Traditional Chinese Medicine (No. 2021ZB174).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Strumila R, Lengvenyte A, Olie E, Courtet P, Guillaume S. Escitalopram should be investigated in anorexia nervosa: rationale and review of mechanisms. J Psychopharmacol. (2022) 36(9):1016–9. doi: 10.1177/02698811221118340
2. Godi SM, Singh LK. Escitalopram-induced skin rash: dermatitis medicamentosa. Indian J Dermatol. (2022) 67(1):93. doi: 10.4103/ijd.ijd_1140_20
3. Vaccarino V, Badimon L, Bremner JD, Cenko E, Cubedo J, Dorobantu M, et al. Depression and coronary heart disease: 2018 position paper of the ESC working group on coronary pathophysiology and microcirculation. Eur Heart J. (2020) 41(17):1687–96. doi: 10.1093/eurheartj/ehy913
4. Guo S, Chen L, Cheng S, Xu H. Comparative cardiovascular safety of selective serotonin reuptake inhibitors (SSRIs) among Chinese senile depression patients: a network meta-analysis of randomized controlled trials. Medicine (Baltimore). (2019) 98(22):e15786. doi: 10.1097/MD.0000000000015786
5. Mosquera JE, Torres N, Restrepo J, Ruz-Pau C, Suryanarayanan S. Linagliptin-induced pancreatitis. AACE Clin Case Rep. (2020) 6(1):e37–9. doi: 10.4158/ACCR-2019-0307
6. Meza-Alvarado JC, Page RA, Mallard B, Bromhead C, Palmer BR. VEGF-A related SNPs: a cardiovascular context. Front Cardiovasc Med. (2023) 10:1190513. doi: 10.3389/fcvm.2023.1190513
7. Valipour M, Irannejad H, Emami S. Papaverine, a promising therapeutic agent for the treatment of COVID-19 patients with underlying cardiovascular diseases (CVDs). Drug Dev Res. (2022) 83(6):1246–50. doi: 10.1002/ddr.21961
8. Huangfu N, Lu Y, Ma H, Hu Z, Cui H, Yang F. Genetic liability to mental disorders in relation to the risk of hypertension. Front Cardiovasc Med. (2023) 10:1087251. doi: 10.3389/fcvm.2023.1087251
9. Krämer RM, Moissl AP, Lorkowski S, Krämer BK, Lehtimäki T, Mishra BH, et al. High genetic risk for depression as an independent risk factor for mortality in patients referred for coronary angiography. Front Cardiovasc Med. (2023) 10:1125151. doi: 10.3389/fcvm.2023.1125151
10. Shen R, Zhao N, Wang J, Guo P, Shen S, Liu D, et al. Association between level of depression and coronary heart disease, stroke risk and all-cause and cardiovascular mortality: data from the 2005–2018 national health and nutrition examination survey. Front Cardiovasc Med. (2022) 9:954563. doi: 10.3389/fcvm.2022.954563
11. Li J, Jiang C, Liu R, Lai Y, Li L, Zhao X, et al. Prognostic value of post-discharge depression in patients recently hospitalized with acute heart failure. Front Cardiovasc Med. (2022) 9:858751. doi: 10.3389/fcvm.2022.858751
12. Liu Q, Yin H, Jiang C, Xu M, Liu Y, Liu A, et al. Underestimated prognostic value of depression in patients with obstructive coronary artery disease. Front Cardiovasc Med. (2022) 9:961545. doi: 10.3389/fcvm.2022.961545
13. Chen X, Zeng M, Chen C, Zhu D, Chen L, Jiang Z. Efficacy of psycho-cardiology therapy in patients with acute myocardial infarction complicated with mild anxiety and depression. Front Cardiovasc Med. (2023) 9:1031255. doi: 10.3389/fcvm.2022.1031255
14. Wabont G, Ferret L, Houdre N, Lepied A, Bene J, Cousein E. Escitalopram-induced hepatitis: a case report. World J Clin Cases. (2022) 10(8):2468–73. doi: 10.12998/wjcc.v10.i8.2468
15. AlJhani SA. Escitalopram-induced epistaxis: a case report. J Taibah Univ Med Sci. (2021) 16(6):938–42. doi: 10.1016/j.jtumed.2021.06.004
16. Chen W, Zhang H, Jiang J, Zhang X, Ding J, Liu Y, et al. Application of comprehensive pharmaceutical care program in identifying and addressing drug-related problems in hospitalized patients with osteoporosis. BMC Health Serv Res. (2022) 22(1):1438. doi: 10.1186/s12913-022-08862-x
17. Yunusa I, Rashid N, Abler V, Rajagopalan K. Comparative efficacy, safety, tolerability, and effectiveness of antipsychotics in the treatment of dementia-related psychosis (DRP): a systematic literature review. J Prev Alzheimers Dis. (2021) 8(4):520–33. doi: 10.14283/jpad.2021.48
18. Pruccoli J, Rosa S, Bergonzini L, Parmeggiani A. Lithium treatment in children and adolescents with anorexia nervosa: clinical use, side effects and tolerability. Riv Psichiatr. (2022) 57(4):198–202. doi: 10.1708/3855.38385
19. Woroń J, Siwek M, Gorostowicz A. Adverse effects of interactions between antidepressants and medications used in treatment of cardiovascular disorders. Psychiatr Pol. (2019) 53(5):977–95. doi: 10.12740/PP/OnlineFirst/96286
20. Chiappini S, Vickers-Smith R, Guirguis A, Corkery JM, Martinotti G, Schifano F. A focus on abuse/misuse and withdrawal issues with selective serotonin reuptake inhibitors (SSRIs): analysis of both the European EMA and the US FAERS pharmacovigilance databases. Pharmaceuticals (Basel). (2022) 15(5):565. doi: 10.3390/ph15050565
21. Ambwani S, Dutta S, Mishra G, Lal H, Singh S, Charan J. Adverse drug reactions associated with drugs prescribed in psychiatry: a retrospective descriptive analysis in a tertiary care hospital. Cureus. (2021) 13(11):e19493. doi: 10.7759/cureus.19493
22. Eugene AR. Optimizing drug selection in psychopharmacology based on 40 significant CYP2C19- and CYP2D6-biased adverse drug reactions of selective serotonin reuptake inhibitors. PeerJ. (2019) 7:e7860. doi: 10.7717/peerj.7860
23. Schreffler SM, Marraffa JM, Stork CM, Mackey J. Sodium channel blockade with QRS widening after an escitalopram overdose. Pediatr Emerg Care. (2013) 29(9):998–1001. doi: 10.1097/PEC.0b013e3182a314b7
24. Costa A, D'Angelo A, Ramusino MC, Perini G, Bosone D, Derosa G, et al. Effects of oral administration of alprazolam and lorazepam as hypnotics on cardiovascular parameters in hypertensive patients. J Clin Psychopharmacol. (2021) 41(2):191–5. doi: 10.1097/JCP.0000000000001362
25. Kasper S. Choosing among second-generation antidepressant treatments for depressed patients with cardiac diseases. Int J Psychiatry Clin Pract. (2019) 23(2):134–48. doi: 10.1080/13651501.2018.1519080
26. Kumar S, Gayle JA, Mogalapalli A, Hussain ST, Castiglioni A. Escitalopram induced torsade de pointes and cardiac arrest in a patient with surgically treated mitral valve prolapse. Cureus. (2020) 12(12):e11960. doi: 10.7759/cureus.11960
27. Fischer A, Connor AT, Machenzie KM, Shaw RJ. Selective serotonin reuptake inhibitors and tardive dyskinesia: a case report of escitalopram use in a cardiac and liver transplant patient. J Clin Psychopharmacol. (2020) 40(6):626–7. doi: 10.1097/JCP.0000000000001285
28. Pressman E, Penn D, Patel NJ. Intracranial hemorrhage from meningioma: 2 novel risk factors. World Neurosurg. (2020) 135:217–21. doi: 10.1016/j.wneu.2019.10.173
29. Yamagishi T, Tanabe T, Fujita H, Miyazaki K, Yukawa T, Sugiyama K, et al. Conventional cardiopulmonary resuscitation-induced refractory cardiac arrest due to latent left ventricular outflow tract obstruction due to a sigmoid septum: a case report. J Med Case Rep. (2018) 12(1):229. doi: 10.1186/s13256-018-1767-z
30. Farkas AN, Marcott M, Yanta JH, Pizon AF. Bicarbonate refractory QRS prolongation and left bundle-branch block following escitalopram and lamotrigine overdose: a case report and literature review of toxic left bundle-branch block. J Clin Pharm Ther. (2018) 3(5):717–22. doi: 10.1111/jcpt.12698
31. Diken Aİ, Yalçınkaya A, Erçen Diken Ö, Aksoy E, Doğan İ, Yılmaz S, et al. Hyponatremia due to escitalopram and thiazide use after cardiac surgery. J Card Surg. (2016) 31(2):96–7. doi: 10.1111/jocs.12681
32. Singh M, LeLorier PA, Celebi MM, Glancy DL. ECG case of the month: unexpected atrioventricular conduction in high-grade atrioventricular block. Sinus rhythm; high-grade second degree atrioventricular block with a junctional escape rhythm and three capture complexes, each with right bundle branch block aberration; possible septal myocardial infarct of indeterminate age; ST-T and U wave changes suggesting hypokalemia. J La State Med Soc. (2015) 167(2):97–9.25978059
33. Martini DI, Nacca N, Haswell D, Cobb T, Hodgman M. Serotonin syndrome following metaxalone overdose and therapeutic use of a selective serotonin reuptake inhibitor. Clin Toxicol (Phila. (2015) 53(3):185–7. doi: 10.3109/15563650.2015.1009993
34. Tseng PT, Lee Y, Lin YE, Lin PY. Low-dose escitalopram for 2 days associated with corrected QT interval prolongation in a middle-aged woman: a case report and literature review. Gen Hosp Psychiatry. (2012) 34(2):210.e13–e15. doi: 10.1016/j.genhosppsych.2011.10.005
35. Baranchuk A, Simpson CS, Methot M, Gibson K, Strum D. Corrected QT interval prolongation after an overdose of escitalopram, morphine, oxycodone, zopiclone and benzodiazepines. Can J Cardiol. (2008) 24(7):e38–e40. doi: 10.1016/s0828-282x(08)70643-3
36. Page RL II, Ruscin JM, Bainbridge JL, Brieke AA. Restless legs syndrome induced by escitalopram: case report and review of the literature. Pharmacotherapy. (2008) 28(2):271–80. doi: 10.1592/phco.28.2.271
37. Kurne A, Ertugrul A, Anil Yağcioğlu AE, Yazici KM. Venous thromboembolism and escitalopram. Gen Hosp Psychiatry. (2004) 26(6):481–3. doi: 10.1016/j.genhosppsych.2004.06.003
38. Veríssimo LF, Volpini VL, Estrada VB, Matsubara NK, Gomes MV, Resstel LBM, et al. Treatment with escitalopram modulates cardiovascular function in rats. Eur J Pharmacol. (2018) 824:120–7. doi: 10.1016/j.ejphar.2018.02.003
39. Gustafsson M, Altufaili M, Sjölander M. Prevalence of drugs and drug combinations that increase risk of prolonged QT time among people with major neurocognitive disorder living in Sweden: a cross-sectional registry study. Drugs Real World Outcomes. (2023) 10(1):61–8. doi: 10.1007/s40801-022-00341-3
40. Yan L, Ai Y, Xing Y, Wang B, Gao A, Xu Q, et al. Citalopram in the treatment of elderly chronic heart failure combined with depression: a systematic review and meta-analysis. Front Cardiovasc Med. (2023) 10:1107672. doi: 10.3389/fcvm.2023.1107672
41. Rothenhäusler HB, Hoberl C, Ehrentrout S, Kapfhammer HP, Weber MM. Suicide attempt by pure citalopram overdose causing long-lasting severe sinus bradycardia, hypotension and syncopes: successful therapy with a temporary pacemaker. Pharmacopsychiatry. (2000) 33(4):150–2. doi: 10.1055/s-2000-11225
Keywords: escitalopram, case report, sinus bradycardia, depression, coronary heart disease
Citation: Li L-C, Sun W, Lv X-Q, Xu Y-Y, Hu Y and Shi J-N (2024) Escitalopram-induced sinus bradycardia in coronary heart disease combined with depression: a case report and review of literature. Front. Cardiovasc. Med. 10:1133662. doi: 10.3389/fcvm.2023.1133662
Received: 17 January 2023; Accepted: 21 December 2023;
Published: 11 January 2024.
Edited by:
Matteo Anselmino, University of Turin, ItalyReviewed by:
Sarawut Siwamogsatham, Chulalongkorn University, ThailandJarosław Woroń, Jagiellonian University Medical College, Poland
© 2024 Li, Sun, Lv, Xu, Hu and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia-Na Shi shijjnn@126.com
†These authors have contributed equally to this work
Abbreviations ADR, adverse drug reaction; bid, twice a day; DRP, drug-related problems; ECG, electrocardiogram; qd, once a day; qn, once a night; SSRI, selective serotonin reuptake inhibitor.
‡ORCID Liu-Cheng Li orcid.org/0000-0002-2511-0156 Jia-Na Shi orcid.org/0000-0002-3422-0840
 Liu-Cheng Li
Liu-Cheng Li Wen Sun
Wen Sun Xiao-Qin Lv4,†
Xiao-Qin Lv4,†  Ying Hu
Ying Hu