Different phenotype of left atrial function impairment in patients with hypertrophic cardiomyopathy and hypertension: comparison of healthy controls
- 1Department of Radiology, The First Affiliated Hospital of Anhui Medical University, Hefei, China
- 2Cardiac Imaging Center, The First Affiliated Hospital of Anhui Medical University, Hefei, China
- 3Department of Magnetic Resonance Imaging, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 4Key Laboratory of Cardiovascular Imaging (Cultivation), Chinese Academy of Medical Sciences, Beijing, China
Background: The impairment of atrial function and atrial-ventricular coupling in diseases with left ventricular (LV) hypertrophy has been increasingly recognized. This study compares left atrium (LA) and right atrium (RA) function, as well as LA-LV coupling, in patients with hypertrophic cardiomyopathy (HCM) and hypertension (HTN) with preserved LV ejection fraction (EF), using cardiovascular magnetic resonance feature tracking (CMR-FT).
Methods: Fifty-eight HCM patients, 44 HTN patients, and 25 healthy controls were retrospectively enrolled. LA and RA functions were compared among the three groups. LA-LV correlations were evaluated in the HCM and HTN groups.
Results: LA reservoir (LA total EF, ɛs, and SRs), conduit (LA passive EF, ɛe, SRe), and booster pump (LA booster EF, ɛa, SRa) functions were significantly impaired in HCM and HTN patients compared to healthy controls (HCM vs. HTN vs. healthy controls: ɛs, 24.8 ± 9.8% vs. 31.3 ± 9.3% vs. 25.2 ± 7.2%; ɛe, 11.7 ± 6.7% vs. 16.8 ± 6.9% vs. 25.5 ± 7.5%; ɛa, 13.1 ± 5.8% vs. 14.6 ± 5.5% vs. 16.5 ± 4.5%, p < 0.05). Reservoir and conduit functions were more impaired in HCM patients compared to HTN patients (p < 0.05). LA strains demonstrated significant correlations with LV EF, LV mass index, LV MWT, global longitudinal strain parameters, and native T1 in HCM patients (p < 0.05). The only correlations in HTN were observed between LA reservoir strain (ɛs) and booster pump strain (ɛa) with LV GLS (p < 0.05). RA reservoir function (RA ɛs, SRs) and conduit function (RA ɛe, SRe) were significantly impaired in HCM and HTN patients (p < 0.05), while RA booster pump function (RA ɛa, SRa) was preserved.
Conclusions: LA functions were impaired in HCM and HTN patients with preserved LV EF, with reservoir and conduit functions more affected in HCM patients. Moreover, different LA-LV couplings were apparent in two different diseases, and abnormal LA-LV coupling was emphasized in HTN. Decreased RA reservoir and conduit strains were evident in both HCM and HTN, while booster pump strain was preserved.
1. Introduction
Hypertrophic cardiomyopathy (HCM) and hypertension (HTN) are two common cardiovascular diseases involving left ventricular hypertrophy (LVH), characterized by distinct histological changes of replacement fibrosis and diffuse interstitial fibrosis (1, 2). Myocardial hypertrophy and diastolic dysfunction of the left ventricle (LV) are the main manifestations of these diseases (2, 3). Hemodynamically, the left atrium (LA) plays a crucial role in modulating LV diastolic filling through the following basic functional elements: (1) reservoir function (collection of pulmonary venous return during ventricular systole); (2) conduit function (passage of blood to the LV during early diastole); and (3) contractile booster pump function (augmentation of ventricular filling during late diastole). LA remodeling and dysfunction have been increasingly recognized and are closely related to atrial fibrillation and the development of cardiovascular disease, as demonstrated by conventional indices such as LA size and volume (4–7) and the latest strain measurements (8, 9). Deformation studies have shown that LA functions, particularly reservoir and conduit functions, are impaired prior to LA enlargement in the early stages of HCM and HTN (8–12). However, most of these findings have been reported in separate studies and have rarely been compared together.
Although both HCM and HTN present with pathological LVH, they are two distinct diseases with specific pathophysiological mechanisms. HCM is the most common inheritable heart disorder activated via genetic pathways (13, 14), while HTN is an acquired chronic disease initiated by pressure-related LV diastolic dysfunction triggered by increased afterload (12, 15). Further exploration of LA-LV coupling in these two different diseases has gradually gained attention but has not been sufficiently investigated thus far. We hypothesize that impaired LA-LV coupling plays a key role in LA dysfunction.
On the other hand, the right heart, previously considered a “dispensable” part of the heart, has recently become a research hotspot in pathophysiological conditions such as heart failure and pulmonary hypertension (16). Numerous studies have revealed that right ventricular (RV) hypertrophy and diastolic dysfunction are essential components of cardiac damage in HCM and systemic hypertension, possibly secondary to ventricular interaction (2, 17). However, data focusing on right atrial (RA) function in diseases with LVH are limited, even though RA plays a critical role in modulating RV diastolic filling (18). It has been reported that RA deformation is significantly impaired in hypertensive patients who are untreated or ineffectively treated using echocardiographic speckle tracking (STE) (19). CMR-feature tracking (CMR-FT) is a novel offline technique for myocardial deformation evaluation based on routinely acquired balanced steady-state free precession sequence (SSFP) cine images. It offers a larger field of view encompassing the four chambers and has superior reproducibility compared to STE (20, 21). Recent studies have demonstrated that CMR-FT can be used for RA strain in various diseases (22–24).
Therefore, the present study aims to compare LA function between patients with HCM and HTN with preserved EF and further explores LA-LV coupling in these two diseases. We also investigate the feasibility of CMR-FT in RA deformation assessment and whether early RA dysfunction can be detected in HCM and HTN patients.
2. Materials and methods
2.1. Patient population
We retrospectively enrolled 58 consecutive HCM and 44 HTN patients between January 2019 and November 2022. The HCM inclusion criteria were as follows: CMR demonstrating LVH (maximal wall thickness ≥15 mm in adults or ≥13 mm in adults with relatives who had HCM) without other hypertrophy-causing diseases (25). HTN was defined as a SBP >140 mmHg and/or a DBP >90 mmHg based on at least two office readings (26). HTN patients included individuals receiving antihypertensive treatment and newly diagnosed patients. SBP and DBP measurements were obtained during hospitalization or outpatient visits. The exclusion criteria were: (1) claustrophobia, impaired renal function, pacemaker/defibrillator devices, or other metallic implants; (2) LV EF <50%; (3) atrial fibrillation; (4) history of septal myectomy or alcoholic septal ablation; (5) coronary artery stenosis >50% confirmed by coronary CT angiography or coronary angiography; (6) severe valvular diseases. Twenty-five normotensive subjects (11 females, 14 males) with no history of cardiovascular disease and normal physical examination results were selected as the control group. The local institutional ethics committee approved this study, and all subjects provided written informed consent.
2.2. CMR protocol
2.2.1. Image acquisition
All CMR images were obtained using two clinical 3.0-T MR scanners (Magnetom Prisma, Siemens, Erlangen, Germany and Ingenia, Philips Healthcare, Best, the Netherlands). Transverse dark blood images were acquired with the following parameters: slice thickness: 8 mm; TR: 882 ms; TE: 40 ms; FOV: 344 mm × 343 mm. Balanced SSFP breath-held cine images were acquired in the two-chamber, three-chamber, four-chamber, and 8–11 equidistant short-axis planes, covering the entire LV and RV. Typical imaging parameters included: slice thickness: 8 mm; TR: 3.4 ms; TE: 1.1–1.5 ms; FOV: 360 mm × 315 mm; spatial resolution: 1.3 mm × 1.3 mm × 8.0 mm; flip angle: 60–70°; temporal resolution 42 ms.
2.2.2. Image analysis
Conventional size parameters were obtained from dark blood images and balanced SSFP cine images. LV EDD was measured at the papillary level of LV short-axis cine images, and RV EDD was measured on the extension cord of the left measuring line. MWT was defined as end-diastolic wall thickness selected in the thickest segment from the LV short-axis cines without involving trabeculations from both ventricles. AP diameters of LA and RA were measured on transverse dark blood images.
Functional and strain analyses were performed on balanced SSFP cine images using dedicated software (cvi42; Circle Cardiovascular Imaging Inc., Calgary, Canada, version 5.5). The following LV functional parameters were calculated from all of the phases on the short-axis and three long-axis cine images in end-systole and end-diastole: LV EDV, LV ESV, LV EF, and LV mass. The following RV functional parameters were calculated from all of the phases on the short-axis and four-chamber cine images in end-systole and end-diastole: RV EDV, RV ESV, RV EF, and RV mass. Papillary muscles were included in the volume and excluded from the mass calculations.
LV and RV strain measurements were carried out based on long-axis images by tracing endocardial and epicardial borders at end-systole and end-diastole. Endocardial and epicardial borders were semi-automatically detected and manually corrected, excluding the papillary muscles. Then, all frames throughout the entire cardiac cycle were propagated. LV GLS was derived from two-, three-, and four-chamber views, and RV GLS was derived from a four-chamber view. Associated peak global systolic and diastolic strain rates (SRs) were obtained simultaneously. Native T1 mapping of LV was obtained from the basal and mid-ventricular short-axis sections before contrast medium administration.
LA volumes were calculated using the biplane area–length method (27). Manual tracking of the LA outline and length was performed in two- and four-chamber views, excluding pulmonary veins and the LA appendage. RA volumes were calculated using the single-plane area–length method (28). Manual tracking of the RA area and length was performed in a four-chamber view (24). The LA volumes indexed based on BSA were assessed at LV end-systole (LAV max), at LV diastole before LA contraction (LAV pac), and at late LV diastole after LA contraction (LAV min). The RA volumes indexed based on BSA were assessed at RV end-systole (RAV max), at RV diastole before RA contraction (RAV pac), and at late RV diastole after RA contraction (RAV min). Bi-atrial total, passive, and booster EFs were defined according to the following equations: EF total = (Vmax − Vmin) × 100%/Vmax; EF passive = (Vmax − Vpac) × 100%/Vmax; and EF booster = (Vpac − Vmin) × 100%/Vpac.
For strain analysis, LA endocardial and epicardial borders were tracked in two- and four-chamber views (Figures 1A,B). RA endocardial and epicardial borders were tracked in a four-chamber view (Figure 1C). The atrial borders were manually delineated in end-systole and end-diastole and then propagated to all frames automatically. Bi-atrial global longitudinal strain parameters were evaluated as ɛs (total strain, reflective of atrial reservoir function during ventricle systole), ɛe (passive strain, reflective of atrial conduit function during early ventricle diastole), and ɛa (active strain, reflective of atrial booster pump function during late ventricle diastole). Accordingly, their corresponding strain rate parameters were obtained as SRs (peak positive strain rate), SRe (peak early negative strain rate), and SRa (late peak negative strain rate). Five HCM and four HTN patients were excluded due to poor LA or RA tracking quality.
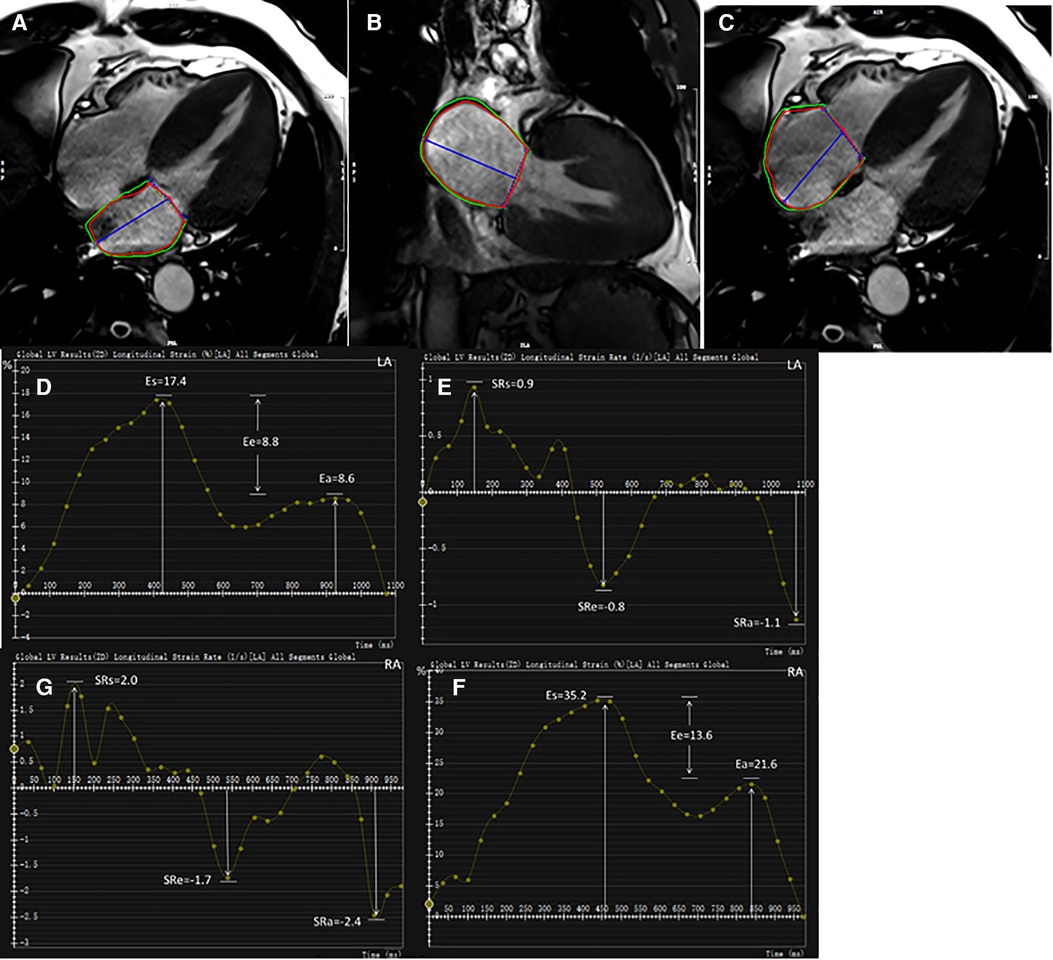
Figure 1. Representative HCM example for bi-atrial CMR feature tracking analysis. LA endocardial and epicardial contours at end-diastole shown on two- and four-chamber views (A,B). RA endocardial and epicardial borders at end-diastole shown on four-chamber view (C). Strain and strain rate parameters were obtained from strain curves (D,F) and strain rate curves (E,G), respectively. LA, left atrium; RA, right atrium; ɛs, total strain; ɛe, passive strain; ɛa, active strain; SRs, total strain rate; SRe, passive strain rate; SRa, active strain rate.
2.3. Reproducibility
Intra-observer reproducibility for global strain and SR parameters was assessed in 30 randomly selected subjects (10 HCM patients, 10 HTN patients, and 10 healthy controls) with a four-week interval between analyses (H.L. with four years of cardiovascular MRI experience). Inter-observer reproducibility was assessed in the same 30 subjects by comparing results from a second experienced observer (T.W. with five years of cardiovascular MRI experience).
2.4. Statistical analysis
Continuous variables were represented as means ± standard deviations. Categorical variables were represented as numbers and percentages. Comparisons of continuous variables among the three groups were performed using one-way ANOVA. Comparisons between the two groups (HCM vs. controls, HTN vs. controls, and HCM vs. HTN) were performed using independent t-test for normally distributed data or Mann–Whitney U-test for non-normally distributed data. Categorical variables were evaluated using chi-square test or Fisher's exact test. Pearson's or Spearman's correlation analysis was performed to investigate the relationship between LA strains and LV parameters. The correlation was considered weak if r was <0.5, moderate if r was between 0.5 and 0.7, and strong if r was >0.7 (29). Intra- and inter-observer variabilities of the atrial strain indices were evaluated using the Bland–Altman test. Reproducibility analysis was performed using intra-class correlation coefficients (ICCs) for absolute agreement and coefficients of variation (CoVs). All statistical analyses were performed with the IBM SPSS Statistical program for Windows (version 21.0, Armonk, NY), MedCalc software (version 15.0, Mariakerke, Belgium), and GraphPad Prism 8.0. p-Values of <0.05 were considered significant.
3. Results
3.1. Population characteristics
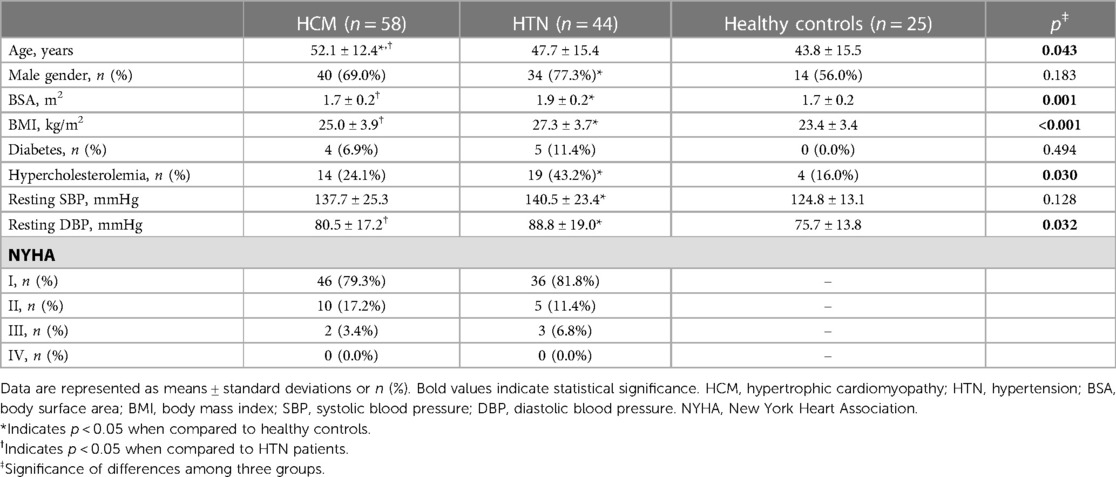
Table 1 lists the population characteristics of the study. BSA, BMI, and resting DBP were significantly higher in HTN patients than in HCM patients and healthy controls (all p < 0.05). No significant difference was observed in the rate of diabetes, resting SBP, and NYHA distribution between HCM and HTN patients.
3.2. Conventional functional and strain parameters of LV and RV
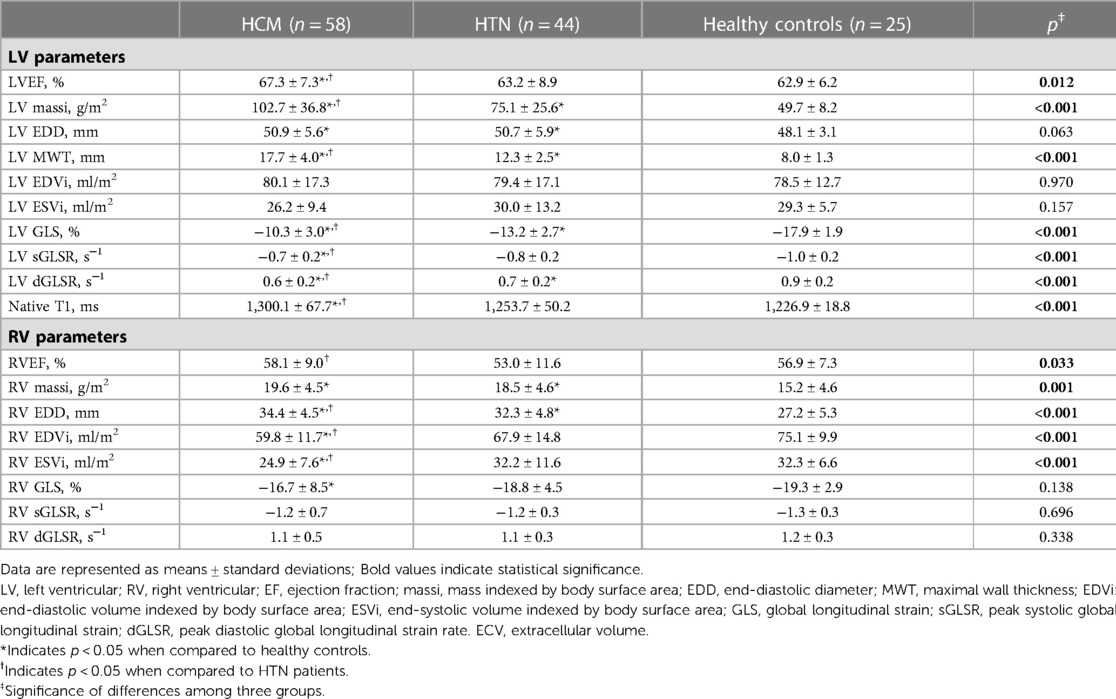
HCM and HTN patients exhibited higher LV massi, LV EDD, LV MWT, RV massi, and RV EDD compared to healthy controls (all p < 0.05; Table 2). HCM patients had higher levels of LV EF, LV massi, LV MWT, RV EF, and RV EDD, as well as lower RV EDVi and RV ESVi compared to HTN patients and healthy controls (all p < 0.05).
Impaired LV GLS and peak diastolic global longitudinal strain rate (dGLSR) were observed in HCM and HTN patients compared to healthy controls. Furthermore, HCM patients had lower levels of GLS, sGLSR, and dGLSR compared to HTN patients (HCM vs. HTN vs. healthy controls: LV GLS, −10.3 ± 3.0% vs. −13.2 ± 2.7% vs. −17.9 ± 1.9%, all p < 0.05). Native T1 was elevated in HCM patients, but no significant difference was noted in HTN patients compared to healthy controls (HCM vs. HTN vs. healthy controls: 1,300.1 ± 67.7 ms vs. 1,253.7 ± 50.2 ms vs. 1,226.9 ± 18.8 ms, p < 0.05). Impaired RV GLS was observed in HCM patients, but no significant difference was found in HTN patients (HCM vs. HTN vs. healthy controls: −16.7 ± 8.5% vs. −18.8 ± 4.5% vs. −19.3 ± 2.9%, p > 0.05).
3.3. Volumetric and strain parameters of LA and RA
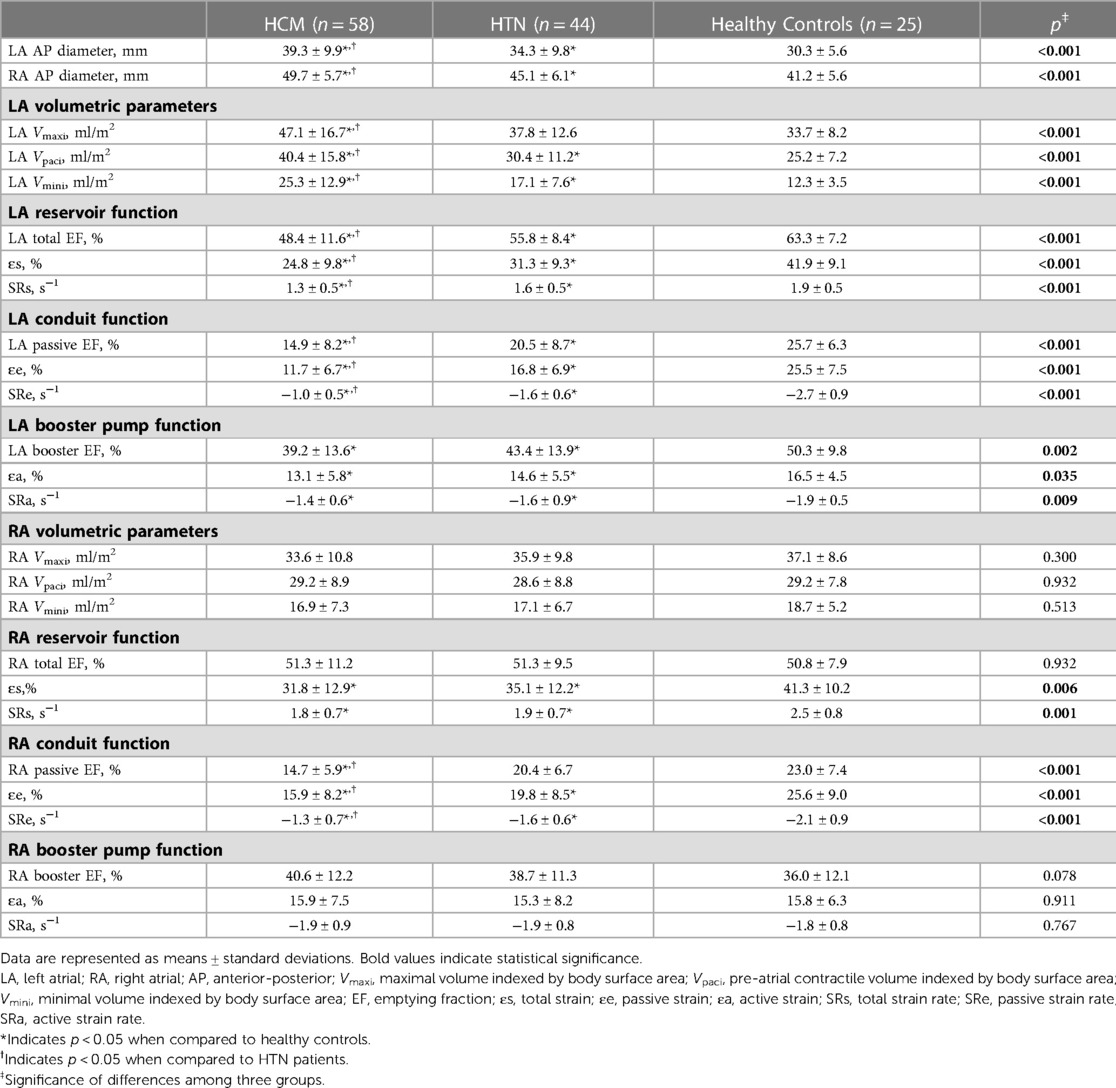
HCM and HTN patients had larger AP diameters of LA and RA compared to healthy controls, with HCM patients showing the highest values (p < 0.05). Table 3 displays the volumetric and strain parameters of LA and RA. HCM patients had the largest LA volumes, including Vmaxi, Vpaci, and Vmini, followed by HTN patients and healthy controls (p < 0.05).
LA reservoir function (LA total EF, ɛs, SRs), conduit function (LA passive EF, ɛe, SRe), and booster pump function (LA booster EF, ɛa, SRa) were significantly reduced in HCM and HTN patients compared to healthy controls (HCM vs. HTN vs. healthy controls: ɛs, 24.8 ± 9.8% vs. 31.3 ± 9.3% vs. 25.2 ± 7.2%; ɛe, 11.7 ± 6.7% vs. 16.8 ± 6.9% vs. 25.5 ± 7.5%; ɛa, 13.1 ± 5.8% vs. 14.6 ± 5.5% vs. 16.5 ± 4.5%, p < 0.05). Moreover, LA reservoir function (LA total EF, ɛs, SRs) and conduit function (LA passive EF, ɛe, SRe) were more impaired in HCM patients compared to HTN patients (p < 0.05; Figure 2).
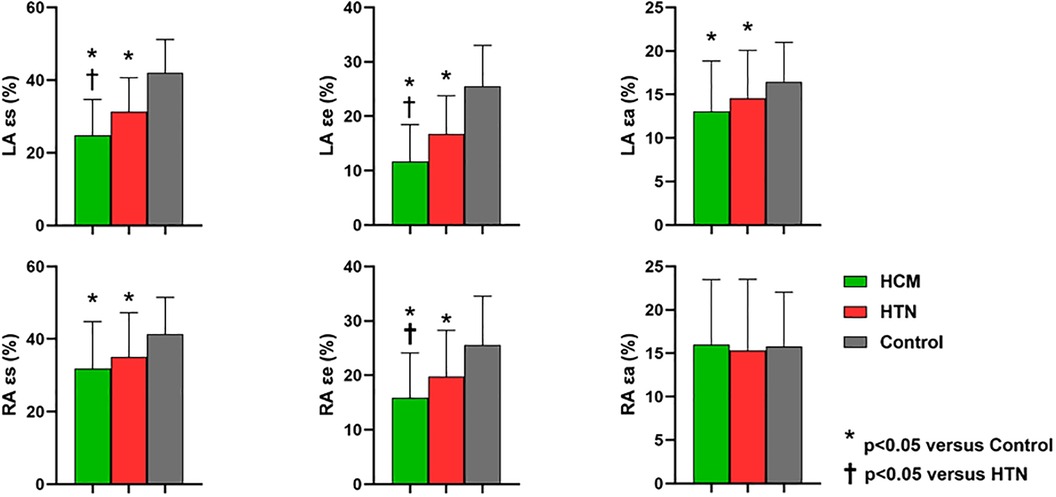
Figure 2. Comparisons of left and right atrial global strain parameters among HCM, HTN, and healthy control groups. LA, left atrial; RA, right atrial; ɛs, total strain; ɛe, passive strain; ɛa, active strain; SRs, total strain rate; SRe, passive strain rate; SRa, active strain rate. *Indicates p < 0.05 when compared to healthy controls; †indicates p < 0.05 when compared to HTN patients.
Although RA volumetric parameters, including Vmaxi, Vpaci, and Vmini, showed no significant differences among the three groups, RA reservoir strain (ɛs, SRs) and conduit strain (ɛe, SRe) were significantly impaired in HCM and HTN patients compared to healthy controls (HCM vs. HTN vs. healthy controls: ɛs, 31.8 ± 12.9% vs. 35.1 ± 12.2% vs. 41.3 ± 10.2%; ɛe, 15.9 ± 8.2% vs. 19.8 ± 8.5% vs. 25.6 ± 9.0%, p < 0.05). In addition, RA conduit function (passive EF, ɛe, SRe) was more impaired in HCM patients compared to HTN patients (p < 0.05). However, RA booster pump function (RA booster EF, ɛa, SRa) was preserved in both HCM and HTN patients (p > 0.05).
3.4. LA-LV coupling
Significant correlations were found between LA reservoir strain (ɛs), conduit strain (ɛe), and booster pump strain (ɛa) with LV EF, LV massi, LV MWT, global longitudinal strain parameters, and native T1 in HCM patients (p < 0.05; Table 4, Figure 3). The only correlations present in HTN were those between LA reservoir strain (ɛs) and booster pump strain (ɛa) with LV GLS (p < 0.05; Table 4).
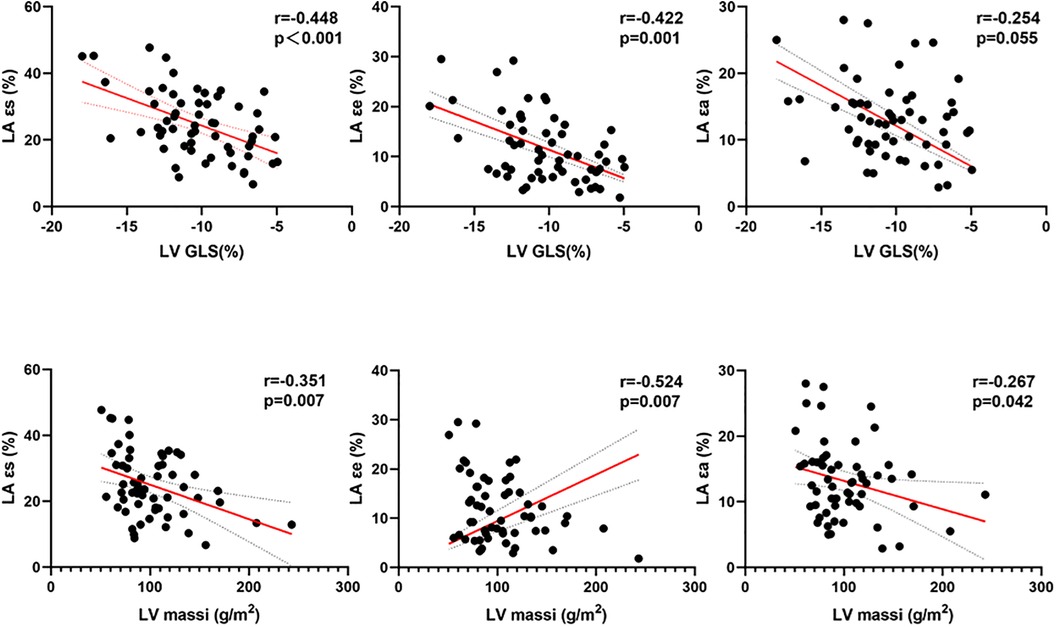
Figure 3. Correlations between LA strains and LV massi and LV GLS in patients with HCM. LA, left atrial; ɛs, total strain; ɛe, passive strain; ɛa, active strain; LV, left ventricular; massi, mass indexed by body surface area; GLS, global longitudinal strain.
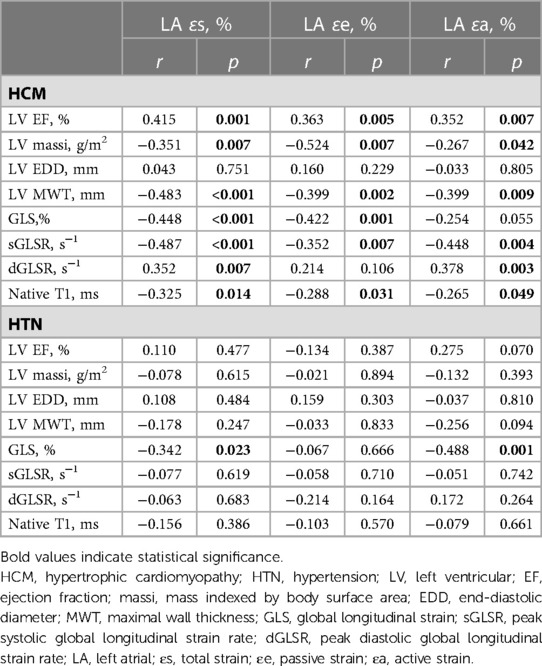
Table 4. Correlations between LA strains and LV functional and deformation parameters in HCM and HTN patients.
3.5. Intra-observer and inter-observer reproducibility
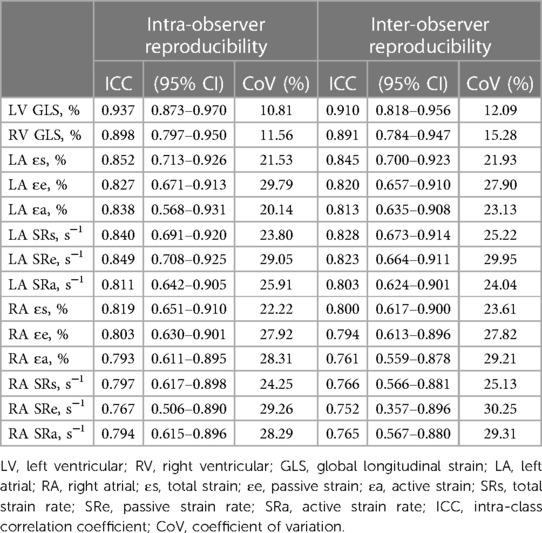
Table 5 summarizes the ICCs and CoVs of LA and RA global strain and SR parameters derived using CMR-FT. Bland–Altman plots for global strain measurements of LA and RA are shown in Figure 4. Both LA and RA strains and SR parameters demonstrated good intra- and inter-observer reproducibility values (intra ICC: 0.794–0.852, inter ICC: 0.752–0.845). Intra- or inter-observer analysis results for LA strains and SRs showed much higher reproducibility than RA strains and SRs. The lowest reproducibility was observed in RA SRe at the inter-observer level (ICC: 0.752).
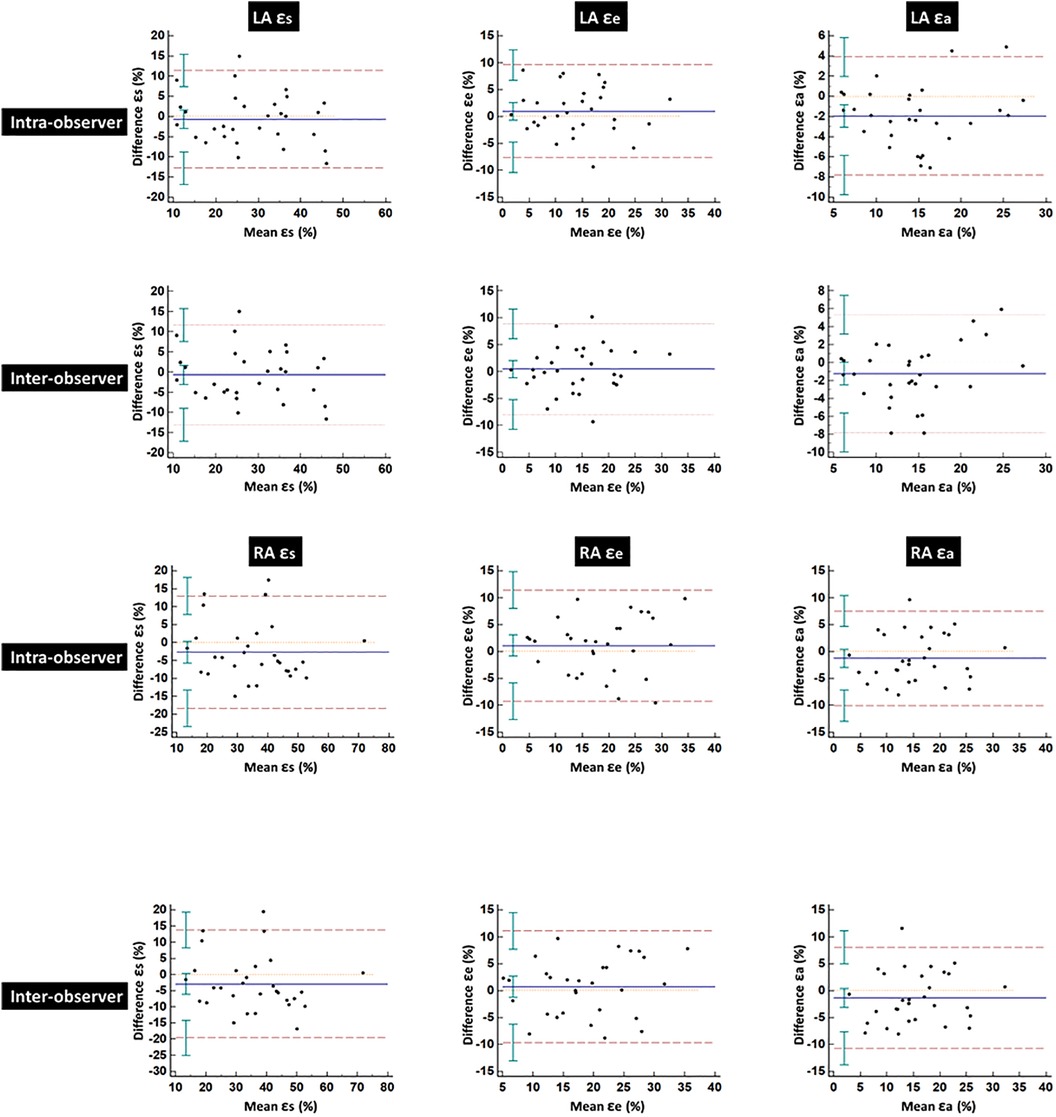
Figure 4. Bland–Altman plots for intra- and inter-observer variability obtained for global left and right atrial strains. LA, left atrial; RA, right atrial; ɛs, total strain; ɛe, passive strain; ɛa, active strain.
4. Discussion
The current study compared LA function and LA-LV coupling in patients with HCM and HTN who have preserved LV EF using CMR-FT and investigated the feasibility of CMR-FT in evaluating RA deformation. The main findings are as follows: (1) LA reservoir, conduit, and booster pump functions were impaired in both HCM and HTN patients, with reservoir and conduit functions more impaired in HCM patients; (2) HCM and HTN exhibited different LA-LV couplings, with significant correlations between LA strains (ɛs, ɛe, ɛa) and LV compliance and systolic strain parameters in HCM, while in HTN, correlations were only found between LA reservoir strain (ɛs) and booster pump strain (ɛa) with LV GLS; (3) RA reservoir and conduit strains were reduced prior to RV dysfunction in both HCM and HTN patients, while booster pump function was preserved.
Based on the structural parameter data, LA morphological remodeling was observed in our patients, as evidenced by enlarged LA size and volume. LA reservoir and conduit functions were impaired in both HCM and HTN patients, which was consistent with previous studies (21, 30, 31). The potential mechanisms are associated with increased LV wall stiffness, elevated LV filling pressure, and impaired LA-LV coupling (11, 32). Furthermore, we discovered that LA reservoir and conduit functions were more severely impaired in HCM patients compared to those with HTN, which may have been due to greater LV wall thickening and more severe diastolic dysfunction in HCM. In addition to LV diastolic dysfunction, studies have shown that LA dysfunction is also correlated with LV fibrosis (33). Contractile function, which is primarily modulated by intrinsic atrial contractility and related to LA size, has been reported to be inconsistent, with some studies reporting normal (31, 34, 35), increased (15, 36), or reduced (37) contractile function. This inconsistency may be attributed to different inclusion criteria. Impaired booster pump function was observed in both HCM and HTN patients in our study, reflecting a state of “decompensation” and a progressive stage of LA dysfunction in the study population (3). Additionally, a trend of more severe contractile function impairment was noted in HCM patients compared to those with HTN, although the difference was not statistically significant.
Interestingly, the present study showed that different atrio-ventricular interactions occurred in the two different diseases. Significant correlations were found between LA strains (ɛs, ɛe and ɛa) and LV compliance (LV massi, LV MWT) as well as systolic parameters (LV EF, GLS, sGLSR and native T1) in HCM patients. In contrast, fewer correlations were found in HTN, with the only correlation observed between LA strains (ɛs, ɛa) and impaired LV GLS. These findings indicate that HCM and HTN display different patterns of LA-LV coupling, which is in line with previous STE-based studies (8, 34). In a similar CMR study, Zhou et al. also reported that LA function correlated with the severity of LV diastolic function in HCM, but more closely with LV systolic function in HTN (31). However, another CMR study conducted by Song et al. (38) demonstrated that LV diastolic deformation indices were significantly correlated with LA reservoir and conduit function in HTN patients. The discrepancy may be ascribed to differences in the patient population stages. As previously mentioned, the impaired booster pump function indicates a progressive stage in the present study. Thus, we speculate that normal LA-LV interaction may be disrupted during a progressive stage of hypertension. Interestingly, although normal LA-LV interactions were not discovered, booster pump strain (ɛa) was demonstrated to be associated with impaired LV GLS. This finding suggests that LA contractile function might be a superior index reflecting atrio-ventricular state in the advanced stages of hypertension. In this context, we propose that future studies should pay more attention to abnormal LA-LV coupling, which may enhance our understanding of the course of hypertension.
RV hypertrophy and dysfunction are frequently observed in both HCM and systemic hypertension (39, 40). Previous studies have reported that RV global strain is also deteriorated, possibly due to ventricular interaction (2, 19). The present study data showed that HCM patients had impaired RV GLS with preserved RV EF, while RV GLS in HTN did not significantly differ from healthy controls. To the best of our knowledge, there were fewer related studies reporting on RA strain determined using CMR-FT in HCM and HTN. In the present study, RA enlargement and decreases in reservoir and conduit strains were observed in both HCM and HTN patients, demonstrating that RA structural remodeling and dysfunction can occur before RV dysfunction in the disease process. This finding is consistent with previous STE studies, which have shown that RA reservoir and conduit functions were impaired in HCM patients and that longitudinal strain was damaged in untreated and uncontrolled hypertensive patients (19, 41). The potential mechanism could involve a constant increase in RV filling pressure, similar to that in the LA and LV. Preserved RA contractile function (ɛa) and increased late diastolic SRa observed in our study patients represent a compensatory reaction to maintain stroke volume and RV filling with mild diastolic dysfunction (31).
There are some limitations to our study. First, the study sample size was relatively small, and the cohort predominantly consisted of Chinese participants. Therefore, these findings require further validation and confirmation in larger-scale studies with a more diverse population. Second, subgroup analysis was not performed in this study due to its small sample size. The HCM cohort included patients with and without obstruction of the LV outflow tract, and patients with and without LV hypertrophy were included in the HTN cohort. Third, since some of our patients did not undergo the hematocrit (HCT) laboratory test on the day of the CMR examination, extracellular volume fraction (ECV) data could not be obtained. Lastly, due to the thin RA wall and the tricuspid valve attachment point not being as clearly visible as the bicuspid valve, tracking the RA endocardial and epicardial borders was more challenging than tracking those of the LA. Furthermore, while the LA outline was tracked on two long-axis views, the RA outline was tracked on only one long-axis view. Consequently, RA strains and SRs demonstrated weaker intra- and inter-observer reproducibility compared to the LA in the present study.
5. Conclusion
This study demonstrated the feasibility of using CMR-FT in evaluating RA deformation and provided insight into the differences in LA function and LA-LV coupling between patients with HCM and HTN. LA reservoir, conduit, and booster pump functions were impaired in both HCM and HTN patients, with reservoir and conduit functions more impaired in HCM patients. HCM and HTN exhibited different patterns of LA-LV coupling, which may have clinical implications for understanding the disease course and developing targeted therapies. Furthermore, RA structural remodeling and dysfunction were found to occur before RV dysfunction in both HCM and HTN patients. Future larger-scale studies with diverse populations are needed to validate and confirm these findings, potentially leading to improved understanding and management of these diseases.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the First Affiliated Hospital of AnHui Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HL: Conceptualization, Methodology, Software, Formal Analysis, Writing—Original Draft. HW: Resources, Supervision. TW: Software. CJ: Resources, Data collection. ML: Conceptualization, Methodology, Supervision. BL: Validation, Supervision. All authors contributed to the article and approved the submitted version.
Acknowledgements
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tseng WY, Dou J, Reese TG, Wedeen VJ. Imaging myocardial fiber disarray and intramural strain hypokinesis in hypertrophic cardiomyopathy with MRI. J Magn Reson Imaging. (2006) 23(1):1–8. doi: 10.1002/jmri.20473
2. Yang L, Zhang L, Cao S, Gao C, Xu H, Song T, et al. Advanced myocardial characterization in hypertrophic cardiomyopathy: feasibility of CMR-based feature tracking strain analysis in a case-control study. Eur Radiol. (2020) 30(11):6118–28. doi: 10.1007/s00330-020-06922-6
3. Soullier C, Niamkey JT, Ricci JE, Messner-Pellenc P, Brunet X, Schuster I. Hypertensive patients with left ventricular hypertrophy have global left atrial dysfunction and impaired atrio-ventricular coupling. J Hypertens. (2016) 34(8):1615–20. doi: 10.1097/HJH.0000000000000971
4. Cameli M, Lisi M, Focardi M, Reccia R, Natali BM, Sparla S, et al. Left atrial deformation analysis by speckle tracking echocardiography for prediction of cardiovascular outcomes. Am J Cardiol. (2012) 110(2):264–9. doi: 10.1016/j.amjcard.2012.03.022
5. Guttmann OP, Rahman MS, O'Mahony C, Anastasakis A, Elliott PM. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: systematic review. Heart. (2014) 100(6):465–72. doi: 10.1136/heartjnl-2013-304276
6. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2015) 16(3):233–70. doi: 10.1093/ehjci/jev014
7. Debonnaire P, Joyce E, Hiemstra Y, Mertens BJ, Atsma DE, Schalij MJ, et al. Left atrial size and function in hypertrophic cardiomyopathy patients and risk of new-onset atrial fibrillation. Circulation Arrhythmia and Electrophysiology. (2017) 10(2):e004052. doi: 10.1161/CIRCEP.116.004052
8. Iio C, Inoue K, Nishimura K, Fujii A, Nagai T, Suzuki J, et al. Characteristics of left atrial deformation parameters and their prognostic impact in patients with pathological left ventricular hypertrophy: analysis by speckle tracking echocardiography. Echocardiography. (2015) 32(12):1821–30. doi: 10.1111/echo.12961
9. Demir M, Aktas I, Yildirim A. Left atrial mechanical function and stiffness in patients with nondipper hypertension: a speckle tracking study. Clin Exp Hypertens. (2017) 39(4):319–24. doi: 10.1080/10641963.2016.1246566
10. Miyoshi H, Oishi Y, Mizuguchi Y, Iuchi A, Nagase N, Ara N, et al. Effect of an increase in left ventricular pressure overload on left atrial-left ventricular coupling in patients with hypertension: a two-dimensional speckle tracking echocardiographic study. Echocardiography. (2013) 30(6):658–66. doi: 10.1111/echo.12117
11. Miyoshi H, Oishi Y, Mizuguchi Y, Iuchi A, Nagase N, Ara N, et al. Early predictors of alterations in left atrial structure and function related to left ventricular dysfunction in asymptomatic patients with hypertension. J Am Soc Hypertens. (2013) 7(3):206–15. doi: 10.1016/j.jash.2013.02.001
12. Li L, Chen X, Yin G, Yan W, Cui C, Cheng H, et al. Early detection of left atrial dysfunction assessed by CMR feature tracking in hypertensive patients. Eur Radiol. (2020) 30(2):702–11. doi: 10.1007/s00330-019-06397-0
13. Kim JB, Porreca GJ, Song L, Greenway SC, Gorham JM, Church GM, et al. Polony multiplex analysis of gene expression (PMAGE) in mouse hypertrophic cardiomyopathy. Science. (2007) 316(5830):1481–4. doi: 10.1126/science.1137325
14. Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. (2015) 65(12):1249–54. doi: 10.1016/j.jacc.2015.01.019
15. Yang L, Qiu Q, Fang SH. Evaluation of left atrial function in hypertensive patients with and without left ventricular hypertrophy using velocity vector imaging. Int J Cardiovasc Imaging. (2014) 30(8):1465–71. doi: 10.1007/s10554-014-0485-x
16. Gorter TM, van Melle JP, Rienstra M, Borlaug BA, Hummel YM, van Gelder IC, et al. Right heart dysfunction in heart failure with preserved ejection fraction: the impact of atrial fibrillation. J Card Fail. (2018) 24(3):177–85. doi: 10.1016/j.cardfail.2017.11.005
17. Kawut SM, Barr RG, Lima JA, Praestgaard A, Johnson WC, Chahal H, et al. Right ventricular structure is associated with the risk of heart failure and cardiovascular death: the multi-ethnic study of atherosclerosis (MESA)–right ventricle study. Circulation. (2012) 126(14):1681–8. doi: 10.1161/CIRCULATIONAHA.112.095216
18. Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, Brady M. Hypertrophic cardiomyopathy: a review. Anesth Analg. (2015) 120(3):554–69. doi: 10.1213/ANE.0000000000000538
19. Tadic M, Cuspidi C, Suzic-Lazic J, Andric A, Stojcevski B, Ivanovic B, et al. Is there a relationship between right-ventricular and right atrial mechanics and functional capacity in hypertensive patients? J Hypertens. (2014) 32(4):929–37. doi: 10.1097/HJH.0000000000000102
20. Zareian M, Ciuffo L, Habibi M, Opdahl A, Chamera EH, Wu CO, et al. Left atrial structure and functional quantitation using cardiovascular magnetic resonance and multimodality tissue tracking: validation and reproducibility assessment. J Cardiovasc Magn Reson. (2015) 17:52. doi: 10.1186/s12968-015-0152-y
21. Leng S, Tan RS, Zhao X, Allen JC, Koh AS, Zhong L. Validation of a rapid semi-automated method to assess left atrial longitudinal phasic strains on cine cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson. (2018) 20(1):71. doi: 10.1186/s12968-018-0496-1
22. Steinmetz M, Broder M, Hosch O, Lamata P, Kutty S, Kowallick JT, et al. Atrio-ventricular deformation and heart failure in ebstein's anomaly—a cardiovascular magnetic resonance study. Int J Cardiol. (2018) 257:54–61. doi: 10.1016/j.ijcard.2017.11.097
23. Truong VT, Palmer C, Young M, Wolking S, Ngo TNM, Sheets B, et al. Right atrial deformation using cardiovascular magnetic resonance myocardial feature tracking compared with two-dimensional speckle tracking echocardiography in healthy volunteers. Sci Rep. (2020) 10(1):5237. doi: 10.1038/s41598-020-62105-9
24. Vos JL, Leiner T, van Dijk APJ, van der Zwaan HB, Sieswerda GT, Snijder RJ, et al. Right atrial and ventricular strain detects subclinical changes in right ventricular function in precapillary pulmonary hypertension. Int J Cardiovasc Imaging. (2022):1–12. doi: 10.1007/s10554-022-02555-6
25. Authors/Task Force m, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European society of cardiology (ESC). Eur Heart J. (2014) 35(39):2733–79. doi: 10.1093/eurheartj/ehu284
26. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. (2018) 39(33):3021–104. doi: 10.1093/eurheartj/ehy339
27. Hudsmith LE, Cheng AS, Tyler DJ, Shirodaria C, Lee J, Petersen SE, et al. Assessment of left atrial volumes at 1.5 tesla and 3 tesla using FLASH and SSFP cine imaging. J Cardiovasc Magn Reson. (2007) 9(4):673–9. doi: 10.1080/10976640601138805
28. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American society of echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European association of echocardiography, a branch of the European society of cardiology. J Am Soc Echocardiogr. (2005) 18(12):1440–63. doi: 10.1016/j.echo.2005.10.005
29. Akoglu H. User's guide to correlation coefficients. Turk J Emerg Med. (2018) 18(3):91–3. doi: 10.1016/j.tjem.2018.08.001
30. Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73(15):1961–77. doi: 10.1016/j.jacc.2019.01.059
31. Zhou D, Yang W, Yang Y, Yin G, Li S, Zhuang B, et al. Left atrial dysfunction may precede left atrial enlargement and abnormal left ventricular longitudinal function: a cardiac MR feature tracking study. BMC Cardiovasc Disord. (2022) 22(1):99. doi: 10.1186/s12872-022-02532-w
32. Yang Y, Yin G, Jiang Y, Song L, Zhao S, Lu M. Quantification of left atrial function in patients with non-obstructive hypertrophic cardiomyopathy by cardiovascular magnetic resonance feature tracking imaging: a feasibility and reproducibility study. J Cardiovasc Magn Reson. (2020) 22(1):1. doi: 10.1186/s12968-019-0589-5
33. Pedrizzetti G, Claus P, Kilner PJ, Nagel E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson. (2016) 18(1):51. doi: 10.1186/s12968-016-0269-7
34. Badran HM, Faheem N, Elnoamany MF, Kenawy A, Yacoub M. Characterization of left atrial mechanics in hypertrophic cardiomyopathy and essential hypertension using vector velocity imaging. Echocardiography. (2015) 32(10):1527–38. doi: 10.1111/echo.12885
35. Kowallick JT, Silva Vieira M, Kutty S, Lotz J, Hasenfu G, Chiribiri A, et al. Left atrial performance in the course of hypertrophic cardiomyopathy: relation to left ventricular hypertrophy and fibrosis. Invest Radiol. (2017) 52(3):177–85. doi: 10.1097/RLI.0000000000000326
36. Kowallick JT, Kutty S, Edelmann F, Chiribiri A, Villa A, Steinmetz M, et al. Quantification of left atrial strain and strain rate using cardiovascular magnetic resonance myocardial feature tracking: a feasibility study. J Cardiovasc Magn Reson. (2014) 16(1):60. doi: 10.1186/s12968-014-0060-6
37. Fujimoto K, Inoue K, Saito M, Higashi H, Kono T, Uetani T, et al. Incremental value of left atrial active function measured by speckle tracking echocardiography in patients with hypertrophic cardiomyopathy. Echocardiography. (2018) 35(8):1138–48. doi: 10.1111/echo.13886
38. Song Y, Li L, Chen X, Shao X, Lu M, Cheng J, et al. Early left ventricular diastolic dysfunction and abnormal left ventricular-left atrial coupling in asymptomatic patients with hypertension: a cardiovascular magnetic resonance feature tracking study. J Thorac Imaging. (2022) 37(1):26–33. doi: 10.1097/RTI.0000000000000573
39. Cuspidi C, Negri F, Giudici V, Valerio C, Meani S, Sala C, et al. Prevalence and clinical correlates of right ventricular hypertrophy in essential hypertension. J Hypertens. (2009) 27(4):854–60. doi: 10.1097/HJH.0b013e328324eda0
40. Rosca M, Calin A, Beladan CC, Enache R, Mateescu AD, Gurzun MM, et al. Right ventricular remodeling, its correlates, and its clinical impact in hypertrophic cardiomyopathy. J Am Soc Echocardiogr. (2015) 28(11):1329–38. doi: 10.1016/j.echo.2015.07.015
Keywords: atrial function, feature tracking, hypertrophic cardiomyopathy, hypertension, magnetic resonance imaging
Citation: Li H, Wang H, Wang T, Jin C, Lu M and Liu B (2023) Different phenotype of left atrial function impairment in patients with hypertrophic cardiomyopathy and hypertension: comparison of healthy controls. Front. Cardiovasc. Med. 10:1027665. doi: 10.3389/fcvm.2023.1027665
Received: 25 August 2022; Accepted: 26 April 2023;
Published: 10 May 2023.
Edited by:
Christos Bourantas, Queen Mary University of London, United KingdomReviewed by:
Haikun Qi, ShanghaiTech University, ChinaHeng Ma, Yantai Yuhuangding Hospital, China
Constantina Aggeli, National and Kapodistrian University of Athens, Greece
© 2023 Li, Wang, Wang, Jin, Lu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minjie Lu coolkan@163.com Bin Liu lbhyz321@126.com
 Hongwen Li
Hongwen Li Haibao Wang1
Haibao Wang1  Minjie Lu
Minjie Lu