ALB-dNLR Score Predicts Mortality in Coronary Artery Disease Patients After Percutaneous Coronary Intervention
- 1Department of Cardiology, First Affiliated Hospital of Xinjiang Medical University, Urumqi, China
- 2Key Laboratory of Cardiac Injury and Repair of Henan Province, Department of Cardiology, First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Background: The influence of the albumin/derived neutrophil and lymphocyte ratio (ALB-dNLR) on the outcomes of patients with coronary artery disease (CAD) after percutaneous coronary intervention (PCI) is not known. Here, we aimed to determine the association between the ALB-dNLR score and post-PCI CAD patient outcomes.
Methods: A total of 6,050 patients from the First Affiliated Hospital of Xinjiang Medical University were enrolled between January 2008 and December 2016. These patients were divided into three groups according to their ALB-dNLR scores (0 points, n = 1,121; 1 point, n = 3,119; 2 points, n = 1,810). Mortality after PCI [all-cause (ACM) and cardiac (CM)] was taken as the primary endpoint. The prognostic value of the ALB-dNLR score was determined with the Cox proportional hazard model after adjustment for covariates.
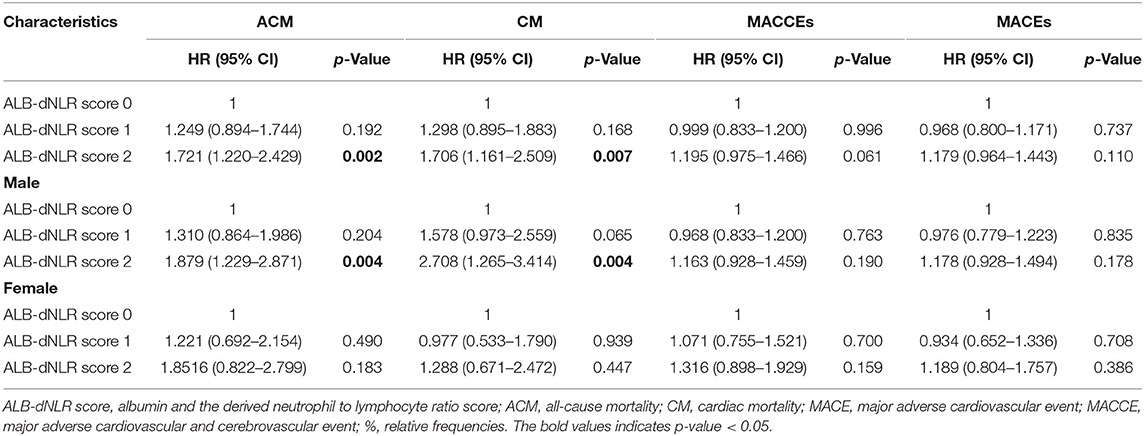
Results: The ACM and CM rates differed among participants in the three groups (P = 0.007 and P = 0.034, respectively). Multivariate Cox analysis showed that the ALB-dNLR score independently predicted both ACM [1 point vs. 0 points, HR = 1.249 (95% CI: 0.79–1.774), P = 0.215; 2 points vs. 0 points, HR = 1.777 (95% CI: 1.239–2.549), P = 0.002] and CM [1 point vs. 0 points, HR = 1.294 (95% CI: 0.871–1.922), P = 0.202; 2 points vs. 0 points, HR = 1.782 (95% CI: 1.185–1.782), P = 0.027]. We also found that among male patients in the three groups, both ACM and CM rates differed (P = 0.006 and P = 0.017, respectively). Multivariate Cox analysis showed that the ALB-dNLR score independently predicted both ACM [1 point vs. 0 points, HR = 1.237 (95% CI: 0.806–0.330), P = 0.330; 2 points vs. 0 points, HR = 1.790 (95% CI: 1.159–2.764), P = 0.009] and CM [1 point vs. 0 points HR = 1.472 (95% CI: 0.892–2.430), P = 0.130; 2 points vs. 0 points, HR = 1.792 (95% CI: 1.182–3.289), P = 0.009].
Conclusion: The ALB-dNLR score is a credible predictor for mortality in patients with CAD who have undergone PCI.
Introduction
Coronary artery disease (CAD) has high morbidity and mortality rates (1, 2). Factors involved in CAD pathogenesis include inflammation (3, 4), lipids (5, 6), and dysfunctional fibrinolysis and coagulation (7, 8). As a potential inflammatory biomarker, leukocytes have been widely recognized in assessing the risk of cardiovascular disease (9). At present, the neutrophil/lymphocyte ratio (NLR) in leukocyte subtypes has been studied as a sensitive indicator of inflammation and has become an independent predictor of cardiovascular disease (10, 11). The NLR has proven useful in the risk stratification of cardiovascular patients and is the strongest predictor of prognosis in coronary artery bypass and PCI patients (12, 13). Horne et al. (14) first proposed a significant role for NLR in stable CAD patients. Although the total white blood cell count is known to predict myocardial infarction and death in CAD patients, high neutrophil counts or low lymphocyte counts have higher predictive power, and the strongest predictor of risk is the ratio of neutrophils to lymphocytes. They believe that white blood cell differential counts are similar to high-sensitivity C-reactive protein in their capacity to be used to predict risk and is even higher than hypersensitive C-reactive protein in this regard (14).
Systemic inflammation influences the synthesis of albumin, and the level of this serum protein is thus useful as an inflammatory marker. Albumin is synthesized in the liver and is involved in the maintenance of plasma colloid osmotic pressure, metabolic material transport, and nutrition. Studies have reported that as the serum albumin concentration decreases, the incidence of cardiovascular diseases increases (15). Albumin also has an antioxidant role in host defense (16, 17). The derived neutrophil to lymphocyte ratio (dNLR) is defined as neutrophils/(leukocytes-neutrophils) (18). The degree of systemic inflammation is indicated by the numbers of circulating immune cells, including neutrophils, lymphocytes, and monocytes (19). Albumin serves as a negative acute-phase reactant, and the circulatory level of albumin decreases as a result of increased transcapillary leakage or reduced hepatic synthesis mediated by interleukin-6 and tumor necrosis factor alpha. Therefore, a low albumin level may reflect an underlying inflammatory state, which increases the risk of mortality in various disease processes, including coronary artery disease. Alternatively, hypoalbuminemia may occur in the presence of coexisting nephrotic syndrome or chronic kidney disease, which are also risk factors for mortality (20).
The albumin level may reflect an underlying inflammatory and nutritional status. The derived neutrophil-to-lymphocyte ratio (dNLR) is defined as neutrophils/(leukocytes-neutrophils) and can reflect the severity of systematic inflammation. Previous research has demonstrated that systemic inflammation and malnutrition might contribute to the progression of CAD. The ALB-dNLR score consists of the serum albumin and derived neutrophil to lymphocyte ratio, indicating nutritional status and inflammatory conditions that are associated with the disease activity of CAD patients, while there is no investigation about the associations between ALB-dNLR score and disease activity in CAD patients.
Here, we retrospectively analyzed 6,050 post-PCI CAD patients to determine the value of the ALB-dNLR score in predicting clinical outcomes over long-term follow-up period.
Methods
Participants
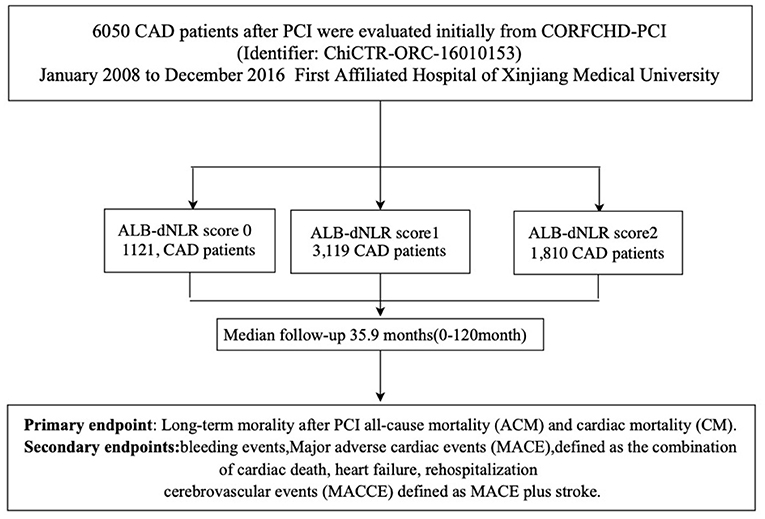
Participants were from the CORFCHD-PCI cohort (a large single-center retrospective cohort) comprising 6,050 CAD patients who had undergone PCI at the First Affiliated Hospital of Xinjiang Medical University from January 2008 to December 2016 (21). This information is registered at http://www.chictr.org.cn (identifier: ChiCTR-ORC-16010153). The selection of study subjects had strict inclusion/exclusion criteria. The inclusion criteria were acute ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation acute coronary syndrome (NSTE-ACS), and stable angina patients who had undergone coronary angiography and had at least one implanted stent. The exclusion criteria were malignant tumors, infectious diseases, hematological diseases, liver diseases, severe heart failure, congenital, rheumatic, or valvular heart disease, and severe kidney or liver dysfunction. Finally, we recruited 6,050 post-PCI CAD patients, including 1,121 patients with 0 points, 3,119 with 1 point and 1,810 with 2 points. The flowchart of the inclusion and exclusion criteria used in the selection of participants is shown in Figure 1.
The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University and complies with the Declaration of Helsinki. As the study was a retrospective cohort study, it did not require the informed consent of patients.
Data Collection
The collected data included demographics, blood and biochemical parameters, alcohol consumption, diabetes history, hypertension, smoking status, and family history of CAD. All patients underwent routine blood and blood biochemical index testing 24 h before coronary angiography. The inspection was completed by the Department of Laboratory Medicine, the First Affiliated Hospital of Xinjiang Medical University. According to the recommendations of the American Heart Association, hypertension was defined as a history of hypertension with active treatment or at least three resting blood pressure readings ≥140/90 mm Hg and at least two independent hygiene health care visits (22). The diabetes diagnosis was proposed by the WHO in 1999: ① symptoms of diabetes, intravenous plasma glucose ≥11.1 mmol/L (200 mg/dl) at any time, ② fasting venous plasma glucose ≥7.0 mmol/L (126 mg/dl), and ③ 2-h intravenous plasma glucose ≥11.1 mmol/L in an OGTT (75 g anhydrous glucose). Regarding the above three criteria, as long as one of the three is met, any one of the three items is checked again on the following day, and the check also meets the criteria, diabetes can be diagnosed (23). Hyperlipidemia was diagnosed according to the “Guidelines for the Prevention and Treatment of Dyslipidemia in Adults in China (2016).” Smoking status was classified as current, former, or never smoked with current smokers, including regular smoking over the previous 6 months. Similarly, people who had consumed alcohol in the previous 6 months were regarded as drinkers (24).
dNLR and ALB-dNLR
Our study quotes the definition of dNLR and ALB-dNLR score used in Chen et al.'s (25) study. Briefly, the dNLR is defined as neutrophils/(leukocytes-neutrophils). Cutoff values from the ROC curve in Chen et al. (25) for dNLR and serum albumin were set at 1.37 and 37.6 g/L, respectively. However, the cutoff value of either serum albumin or dNLR was different from that in Chen et al.'s study. In our study, the cutoff values of the ROC curves for dNLR and serum albumin were 1.365 and 39.6 g/L, respectively. According to each cutoff value of serum albumin and dNLR, the patients were divided into three groups: patients with ALB-dNLR scores of 2, including participants with hypoalbuminemia ( ≤ 39.6 g/L) and high dNLR (>1.365); ALB-dNLR score of 1, including patients with either hypoproteinemia or high dNLR, and scores of 0 for patients without hypoproteinemia or high dNLR.
Endpoints
Mortality during the 10-year follow-up was taken as the primary endpoint. This included both all-cause mortality (ACM) and cardiac mortality (CM). The secondary endpoints were major adverse cardiac events (MACEs), major adverse cardiac and cerebrovascular events (MACEs), and bleeding events. MACEs included cardiac death, heart failure, reinfarction and rehospitalization. All-cause death included both cardiac death and non-cardiac death. Death was classified as all-cause death if there was a clear non-cardiac cause of death; otherwise, death was considered to be cardiac death. Stroke included a sudden onset of dizziness, dysarthria, numbness, or aphasia as a result of cerebrovascular disease (caused by bleeding, thrombosis, embolism, or aneurysm rupture) of more than 24 h (26). Reinfarction refers to acute MI that occurs within 28 days of an event or recurrent MI. When ST elevation is ≥0.1 mV or new pathological Q waves appear again in at least two consecutive leads, especially when ischemic symptoms persist for 20 min or more (27). HF was referred to as systolic HF in accordance with a previous description (28). These events were decided by a blinded committee composed of 4 cardiovascular physicians and a nurse from the CORFCHD-PCI research group. If there are disagreements in the adjudication of the events, it will be solved according to the principle of the minority obeying the majority.
Follow-Up
All post-PCI patients were followed up by six assessments over 0–120 months. Assessments were either office visits or by telephone.
Statistical Analyses
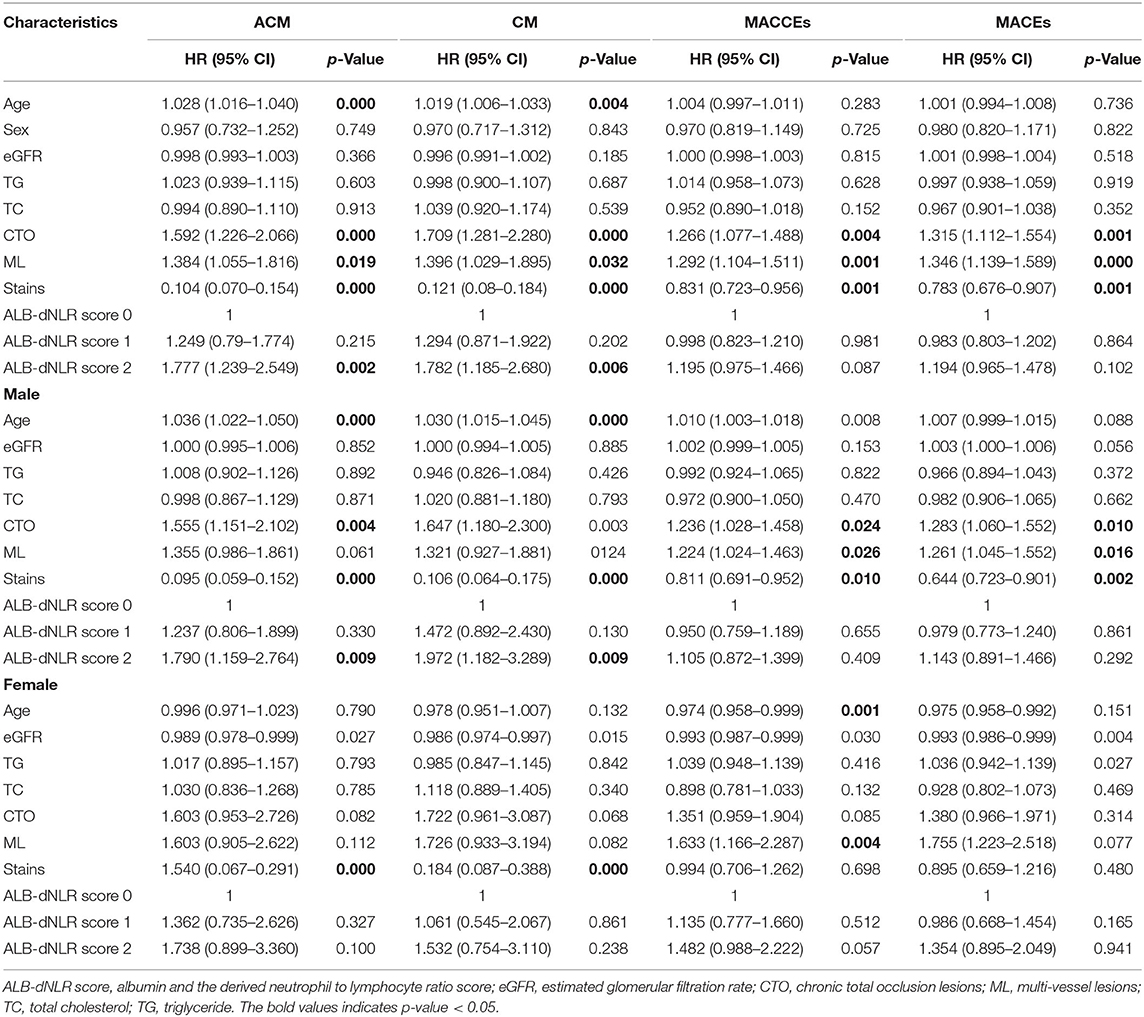
Data were analyzed using SPSS version 22.0. Continuous variables are expressed as the means ± standard deviations, and categorical variables are expressed as frequencies and percentages. Analysis used the t-test for normally distributed variables; non-normally distributed data were analyzed by the Mann–Whitney U test or Kruskal–Wallis analysis of variance. Categorical variables were investigated by the χ-square test, and the cumulative incidence of major adverse events was analyzed by Kaplan–Meier analysis. Multivariable analysis was performed to assess the prognostic value of the ALB-dNLR and adverse outcomes after adjusting for confounders, such as sex, age, estimated glomerular filtration rate (eGFR), total cholesterol (TC), triglycerides (TGs), chronic total occlusion lesions (CTOs), multivessel lesions (MLs), and stains, and to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). P <0.05 was considered significant.
Results
Baseline Data
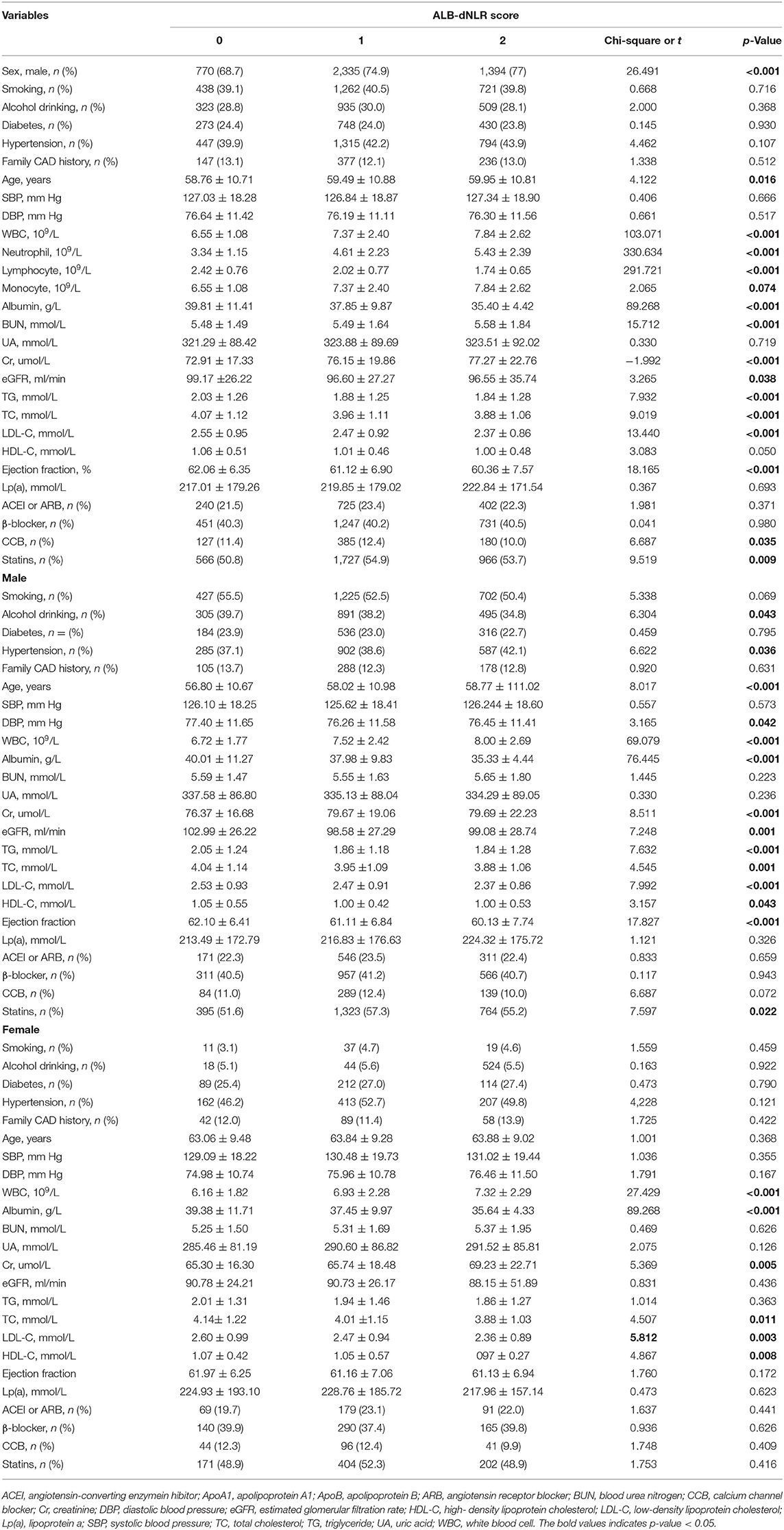
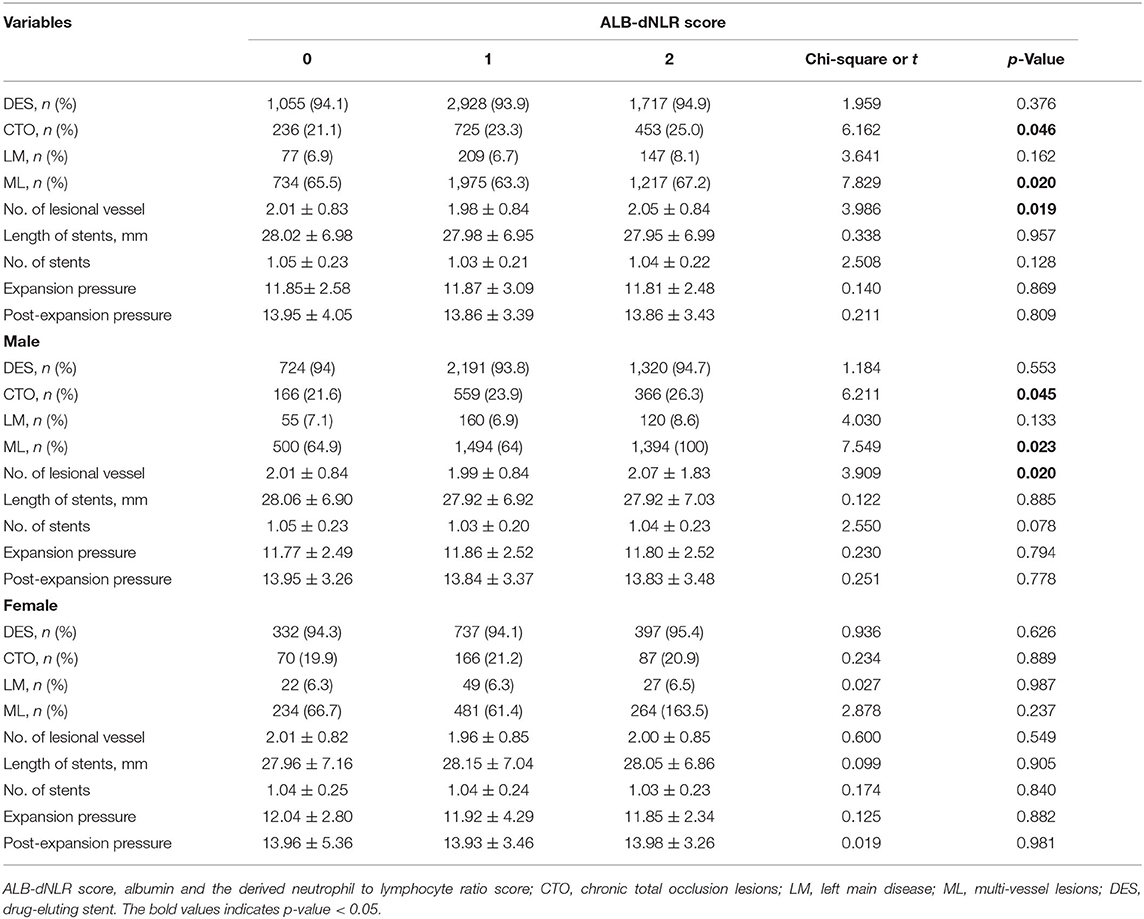
The optimal cutoff value (dNLR = 1.365 and serum albumin = 39.6 g/L) was defined according to the receiver operating curve (ROC). The patients were divided into three groups according to ALB-dNLR scores: 0 points (n = 1,121), 1 point (n = 3,119), and 2 points (n = 1,810). Many variables were significantly different among the three groups. As shown in Table 1, compared with the other two groups, in the ALB-dNLR 2-point group, there were more men (p < 0.001), older age (p = 0.016), higher creatinine (Cr) (p < 0.001), higher WBC (p < 0.001), higher neutrophils (p < 0.001), and higher BUN (p < 0.001). Albumin (p < 0.001), lymphocytes (p < 0.001), estimated glomerular filtration rate (eGFR) (p = 0.038), triglycerides (TGs) (p = 0.009), total cholesterol (TC) (p < 0.001), low-density lipoprotein cholesterol (LDL-C) (p < 0.001) and ejection fraction (p < 0.001) were significantly lower than those in the other two groups. Calcium channel blockade (CCB) (p = 0.035) and statins (p = 0.009) were also significantly different among the three groups. Other baseline data did not differ (all P > 0.05). As shown in Table 2, the characteristics of chronic total occlusion (CTO) (P = 0.046), multivessel lesions (MLs) (P = 0.020) and the number of lesional vessels (P = 0.019) differed significantly among the three groups.
As shown in Tables 1, 2, among male patients, alcohol consumption, hypertension, age, DBP, WBC, albumin, Cr, eGFR, TG, TC, LDL-C, HDL-C, EF, statins, CTO, ML, and the number of lesional vessels were significantly different among the three groups (all p <0.05). Among female patients in the three groups, WBC, albumin, Cr, TC, LDL-C, and HDL-C were also significantly different (all p < 0.05).
Clinical Outcomes
All-Cause Mortality
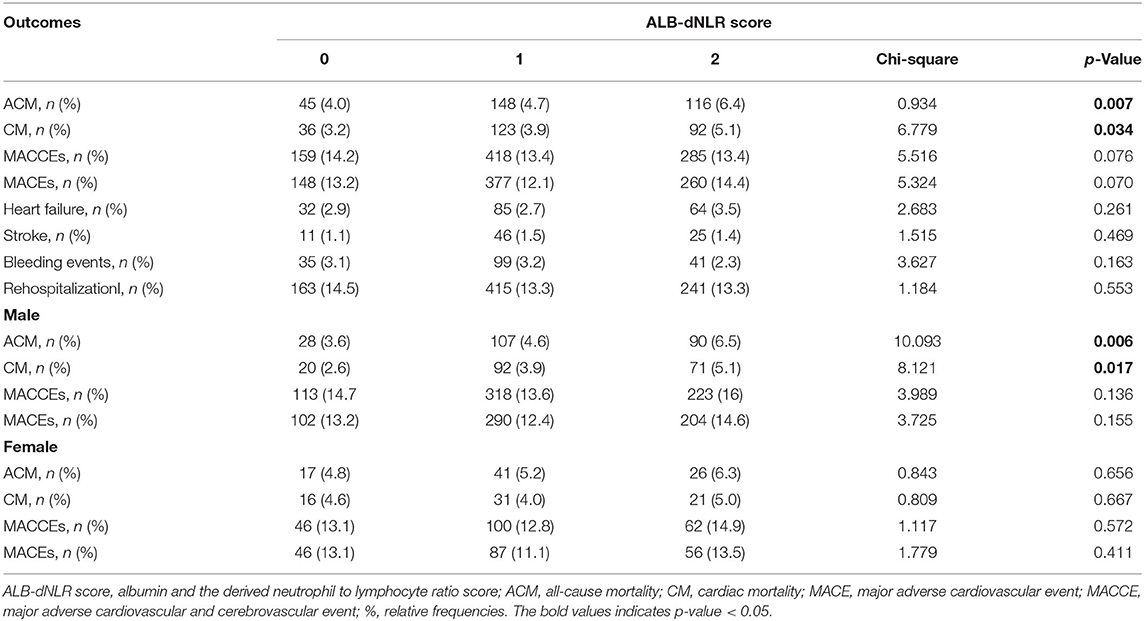
After a median follow-up period of 35.9 months, ACM occurred more frequently in the ALB-dNLR score 2 group [116 (6.4%)] than in the other two groups [0 points, 45 (4.0%) and 1 point, 1,481 (4.7%) (p = 0.007)], as shown in Table 3.
Univariate analysis showed that the risk of all-cause death increased 72.1% in the ALB-dNLR score 2-point group [adjusted HR = 1.721 (1.220–2.429), P = 0.002] compared to the 0-point group (Table 4).
Kaplan–Meier and log-rank analyses showed a significant gradual increased risk in the high ALB-dNLR group, which indicates that its long-term mortality gradually increased with increasing ALB-dNLR score (P = 0.0025, Figure 2). Multivariate analysis evaluated the prognostic value of ALB-dNLR after adjustment for age, sex, eGFR, TG, TC, statins, CTO, and ML. The multivariable Cox regression model showed that the ALB-dNLR score was independently predictive of ACM in post-PCI CAD patients. The risk of mortality increased 77.7% in the ALB-dNLR score 2-point group [adjusted HR = 1.777 (1.239–2.549), P = 0.002] compared to patients in the ALB-dNLR score 0 group (Table 5). In addition, compared to a score of 0, the risk of patients with a score of 1 increased by 24.9% [adjusted HR = 1.249 (0.79–1.774), P = 0.215].
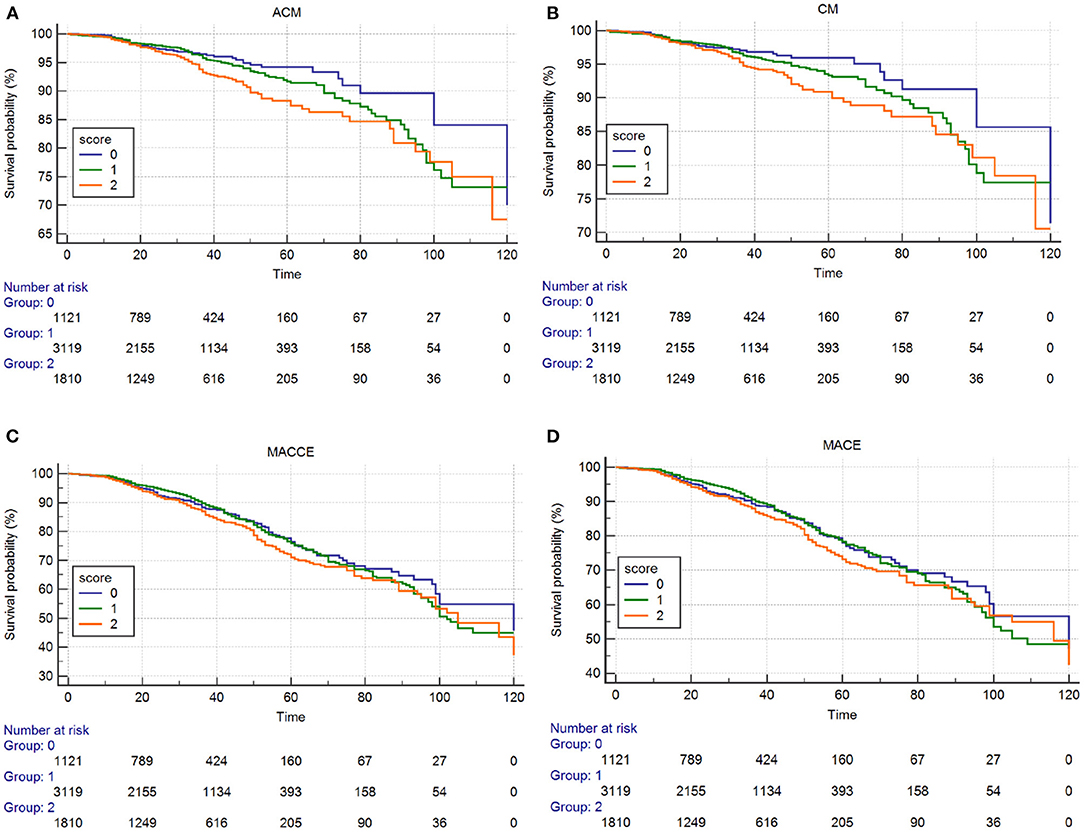
Figure 2. Cumulative Kaplan–Meier estimates of the time to the first adjudicated occurrence of primary endpoint and secondary endpoints. (A) ACM; (B) CM; (C) MACCEs; (D) MACE.
Among male patients, ACM occurred more frequently in the ALB-dNLR score 2 group [107 (6.5%)] than in the other two groups [0 points, 28 (3.6%) and 1 point, 107 (4.7%) (p = 0.006)], as shown in Table 3.
Univariate analysis showed that the risk of all-cause death increased 87.9% in participants in the ALB-dNLR score 2-point group [adjusted HR = 1.879 (1.229–2.871), P = 0.004] compared to those in the 0-point group (Table 4).
The Kaplan–Meier curves and log-rank test demonstrated a significant difference in ACM in the three groups (P = 0.0036, Figure 3). After risk factor adjustment, the Cox proportional hazards model showed that the ALB-dNLR score was an independent prognostic factor for CAD patients after PCI. The risk of mortality increased 79% in the ALB-dNLR score 2-point group [adjusted HR = 1.790 (1.159–2.764), P = 0.009] compared to patients in the ALB-dNLR score 0 group (Table 5).
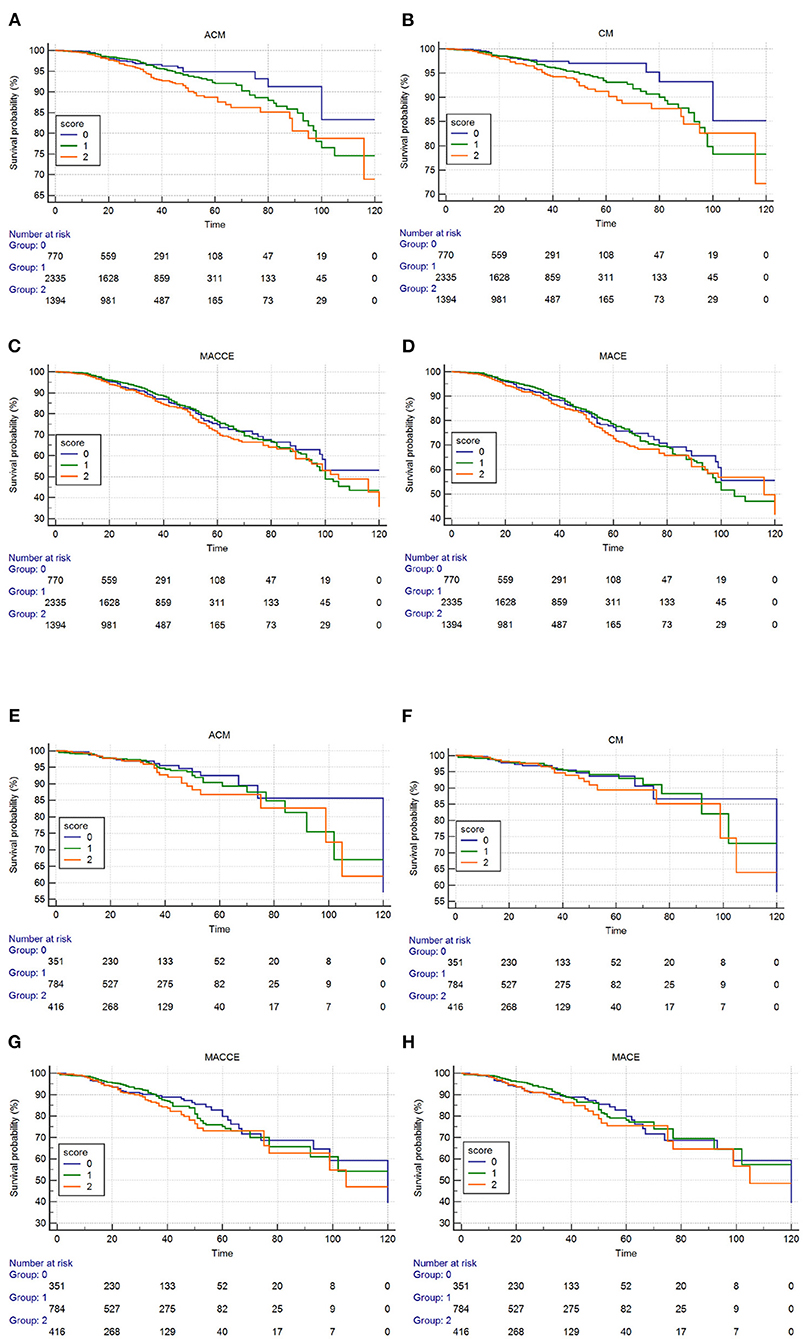
Figure 3. Cumulative Kaplan–Meier estimates of the time to the first adjudicated occurrence of primary endpoint and secondary endpoints. Male (A–D): (A) ACM; (B) CM; (C) MACCEs; (D) MACE. Female (E–H): (E) ACM; (F) CM; (G) MACCEs; (H) MACE.
Among female patients, we did not find significant differences in ACM among the three groups. Univariate analysis and multivariable Cox regression showed that the ALB-dNLR score was not independently predictive of ACM in post-PCI CAD patients.
Cardiac Mortality
CM incidence also differed significantly [36 (3.2%) vs. 123 (3.9%) vs. 92 (5.1%), P = 0.034] among participants in the three groups. CM was more frequent in the ALB-dNLR score of 2 than in the other two groups (Table 3).
Univariate analysis showed that the risk of cardiac death increased 70.6% in participants in the ALB-dNLR score 2-point group [adjusted HR = 1.706 (1.161–2.509), P = 0.007] compared to participants in the score of 0 group (Table 4).
The Kaplan–Meier curves and log-rank test demonstrated a significant difference in CM among participants in the three groups (P = 0.014, Figure 2). After risk factor adjustment, the Cox proportional hazards model showed that the ALB-dNLR score was an independent prognostic factor for CAD patients after PCI. The risk of cardiac death increased 78.2% in participants in the ALB-dNLR score 2-point group [adjusted HR = 1.782 (1.185–2.680), P = 0.006] compared to participants in the 0-point group. TC, CTO, and multivessel lesions were found to be associated with ACM, CM, MACCEs, and MACEs after potential confounding factors were adjusted (all P < 0.05, Table 5). We did not find a significant difference between the score of 0 and the score of 1 in terms of CM (HR = 1.294, 95% CI: 0.871–1.922, P = 0.202).
Among male patients in the three groups, CM incidence also differed significantly [20 (2.6%) vs. 92 (3.9%) vs. 71 (5.1%), P = 0.017]. CM was more frequent in the ALB-dNLR score of 2 than in the other two groups (Table 3).
Univariate analysis showed that the risk of cardiac death increased 1-fold in the ALB-dNLR score 2-point group [adjusted HR = 2.708 (1.265–3.414), P = 0.004] compared to the 0-point group (Table 4).
The Kaplan–Meier curves and log-rank test demonstrated a significant difference in CM among the three groups (P = 0.010, Figure 3). After risk factor adjustment, the Cox proportional hazards model showed that the ALB-dNLR score was an independent prognostic factor for CAD patients after PCI. The risk of cardiac death increased 97.2% in the ALB-dNLR score 2-point group [adjusted HR = 1.972 (1.185–3.289), P = 0.009] compared to the 0-point group (Table 5).
Among female patients, we did not find significant differences in CM among the three groups. Univariate analysis and multivariable Cox regression showed that the ALB-dNLR score was not independently predictive of CM in post-PCI CAD patients.
MACCEs and MACEs
We did not found that the incidences of MACCEs [159 (14.2%) vs. 418 (13.4%) vs. 285 (13.4%), P = 0.076], MACEs [148 (13.2%) vs. 377 (12.1%) vs. 260 (14.4%), P = 0.070], HF [32 (2.9%) vs. 85 (2.7%) vs. 64 (3.5%), P = 0.261], bleeding events [35 (3.1%) vs. 99 (3.2%) vs. 41 (2.3%), P = 0.163], stroke [11 (1.1%) vs. 46 (1.5%) vs. 25 (1.4%), P = 0.469], and rehospitalization [163 (14.5%) vs. 415 (13.3%) vs. 241 (13.3%), P = 0.083] differed significantly among participants in the three groups (Table 3).
The Kaplan–Meier curves and log-rank test demonstrated no significant difference in MACCEs and MACEs (Figure 2) among the three groups. The multivariate Cox regression model showed that the MACCEs and MACEs were not significantly different among participants in the three groups (P > 0.05, Table 5).
Among male patients and female patients, we did not find that the incidences of MACCEs and MACEs differed significantly in the three groups (Table 3). The Kaplan–Meier curves and log-rank test demonstrated no significant difference in MACCEs and MACEs (Figure 2) among the three groups. Univariate analysis and multivariate Cox regression showed that the MACCEs and MACEs were not significantly different among participants in the three groups (P > 0.05, Tables 4, 5).
To assess the diagnostic ability of the ALB-dNLR in CAD patients and compare it with classical risk predictors, we generated ROC curves and compared the area under the curve (AUC) values (Figure 4). The results showed that the AUC of the ALB-dNLR +classic risk model was 0.794 (95% CI: 0.771–0.817), the AUC of the dNLR+ classic risk model was 0.720 (95% CI: 0.690–0.750) and the AUC of the ALB+ classic risk model was 0.620 (95% CI: 0.588–0.652). Therefore, we believe that the predictive performance of ALB-dNLR is stronger than that of albumin or dNLR alone.
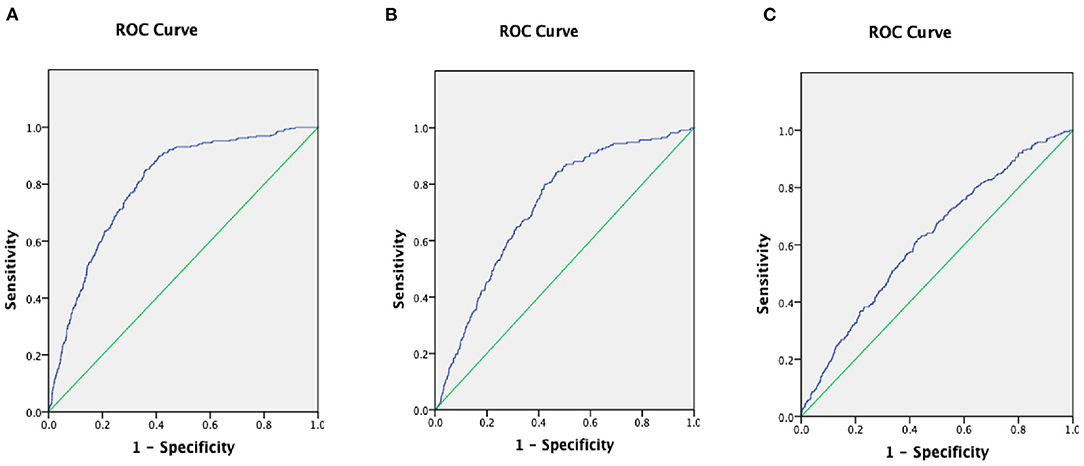
Figure 4. The areas under the curves (AUC) of AUC of ALB-dNLR +classic risk model (A), the AUC of dNLR+ classic risk model (B), and he AUC of ALB+ classic risk model (C) in CAD patients. ALB, albumin; dNLR, derived neutrophil to lymphocyte ratio.
Discussion
This retrospective study of 1,121 patients with an ALB-dNLR of 0 points, 3,119 with an ALB-dNLR of 1 point, and 1,810 with an ALB-dNLR of 2 points from the First Affiliated Hospital of Xinjiang Medical University from January 2008 to December 2016 provides compelling evidence that the incidence of mortality and cardiac death would be increased in patients with both hypoalbuminemia and high dNLR. To our knowledge, this is the first investigation of the association of ALB-dNLR with outcomes of patients who have undergone PCI.
The ALB-dNLR score measures both immune function and nutritional status. In this study, investigation of the possible association between the score and the clinical prognosis of post-PCI CAD patients demonstrated that (1) ALB-dNLR score was independently linked to adverse outcome in these patients, and (2) higher ALB-dNLR was significantly associated with an increased risk of all-cause death and cardiac death.
Serum albumin is reported to have both anti-inflammatory and immunomodulatory attributes (29), and reduced albumin synthesis caused by inflammation may have consequences for immune defense (30). Albumin levels are also influenced by nutritional status. Inflammation and malnutrition both reduce the albumin concentration by reducing its synthesis rate, while inflammation alone is associated with a higher catabolism rate, which expands nutrition by eliminating the defense mechanism that reduces the availability of protein and calories to protect the albumin pool. The adverse effects on body composition, and in extreme cases, the transfer of albumin from outside the vessel lumen, increases. What followed was a series of malignant events. Inflammation caused anorexia, reduced the effective use of protein and energy intake in the diet, and enhanced the catabolism of key albumin. The impact of inflammation on albumin levels was mainly related to low levels of albumin. Morbidity and mortality associated with albuminemia are related (31). The “TRIAGE” study found that increased inflammation indicators and increased malnutrition were independently associated with hypoalbuminemia. Low serum albumin levels, increased CRP values, and increased nutritional risk independently predicted 30-day mortality, and the area under the curve was 0.77, 0.70, and 0.75 (32). Inflammation and nutrition can promote the development of atherosclerosis (33). A meta-analysis found a 50% CAD risk with hypoalbuminemia (34). Low serum albumin levels also appear to be associated with increased CAD risk (35). A follow-up study of 8,750 AMI patients observed that low albumin levels on admission were associated with increased ACM (36).
Serum albumin was a significant parameter affecting GFR in addition to serum creatinine (37). GFR is not only a marker of the presence of conventional cardiovascular risk factors but may also have an important role in the pathogenesis of CVD, particularly CAD. GFR has numerous effects on the cardiovascular system, including inhibition of erythropoiesis and platelet function (38) and induction of volume overload (39), dyslipidemia, hypertension (40) and vascular calcification (41). In our study, we found that there were significant differences in eGFR among the three groups in baseline data (P = 0.038). However, the multivariable Cox regression model did not show that eGFR is an independent predictor of ACM, CM, MACCEs, and MACEs in post-PCI CAD patients. The dNLR is defined as the neutrophil count divided by the neutrophil count subtracted from the leukocyte count (18). The denominator includes a broad mix of monocytes and lymphocytes, which have different physiological roles. The dNLR is regarded as an indicator of inflammation and is used in a wide range of applications, including early cancer prediction, chemotherapy drug sensitivity, risk stratification of patients undergoing vascular surgery, distinction between non-alcoholic patients with severe fatty liver, and Alzheimer's disease. Earlier reports even suggested that it can be used as a diagnostic marker for appendicitis (42–45).
Inflammation is closely associated with atherosclerosis and cardiovascular disease (46, 47). Previous studies have shown that WBC counts are associated with CHD and an increased risk of ischemic disease (48). Leukocytes are key players in the vascular injury process, and total WBC counts are an indicator of the strength of this process. The Benjamin study (14) showed that total WBC counts proved to be an independent predictor of CAD, but neutrophil (N) or low lymphocyte (L) counts provided higher predictive value, with the best risk prediction given by the N/L ratio. Correlative studies have also shown (49, 50) that a higher NLR was associated with an increased risk of all-cause or cardiac death. In our study, the AUC of the ALB-dNLR+classic risk model was 0.794 (95% CI: 0.771–0.817), and the AUC of the dNLR+classic risk model was 0.720 (95% CI: 0.690–0.750). Therefore, we believe that the predictive performance of ALB-dNLR is stronger than that of dNLR alone. Compared to general WBC counts, ALB-dNLR has higher predictive power. The neutrophil to lymphocyte ratio represents the number of neutrophils in the body as a result of inflammation and is proposed as a new biomarker of systemic inflammation. The relative value of the number of lymphocytes produced by the “protection mechanism” was determined. Therefore, higher neutrophil and lymphocyte ratios represent inflammation, and inflammation can stimulate the production of neutrophils and accelerate the apoptosis of lymphocytes. Specifically, subacute inflammation in the body is important in cardiovascular disease and atherosclerosis (51).
In experimental atherosclerosis, the number of neutrophils in the blood increases and accumulates at an early stage of the injury. It helps special monocytes adhere or transport by releasing alarm proteins and other preformed granular proteins (52). Neutrophils also contain a large amount of myeloperoxidase, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and lipoxygenase, which contribute to oxidative stress, endothelial cell dysfunction, and disease growth and are the main determinants of instability.
Liu et al. (53) found that NLR is an independent risk factor for hospital mortality in COVID-19 patients (especially males). For every additional unit of NLR, the OR of male mortality after full adjustment was 1.10 (OR = 1.10; 95% CI: 1.02–1.19; P = 0.016). The study of NLR in predicting the long-term mortality of patients with non-ST-segment elevation myocardial infarction (NSTEMI) showed that after controlling for Global Registry of Acute Coronary Events risk profile scores, the average NLR level remained a significant predictor of inpatient and 4-year mortality. The hazard ratios per unit increase in the average NLR (log) increased by 1.06 (p = 0.0133) and 1.09 (p = 0.0006), respectively (54). Butt et al. (55) showed that a high NLR is an independent predictor of contrast-induced acute nephropathy in patients with acute myocardial infarction (AMI) (OR 2.03, 95% CI: 1.403–3.176, P <0.001).
These findings are consistent with our results. Our study found that ALB-dNLR was associated with the long-term mortality of CAD patients. Elevated ALB-dNLR may be an independent risk factor for long-term death in CAD patients who have undergone PCI. Compared with normal patients, patients with hypoalbuminemia and high dNLR have higher ACM and CM. However, the ALB-dNLR score does not seem to affect the incidence of MACCEs or MACEs. The ALB-dNLR reflects albumin and dNLR. Previous research has shown that low serum albumin predicted MACE only in patients with a low cardiovascular risk profile and was not associated with cardiac outcome in patients with 3 or more traditional cardiovascular risk factors (15). Our research included traditional cardiovascular risks such as smoking, hypertension, hyperlipidemia, and diabetes. Therefore, it can be explained that ALB-dNLR is associated with mortality but not MACE. In our study, we found that an ALB-dNLR score of 2 was significantly correlated for male but not female patients. Previous studies (56) have shown that premenopausal women are less likely to develop coronary heart disease than men, but post-menopausal women are more likely to develop coronary heart disease than men. The increased risk in women is largely attributable to the loss of sex steroids, especially estrogen. Estrogen can help regulate the metabolism of lipids in the body, maintain the function of endothelial cells and, to a certain extent, inhibit the proliferation of smooth muscle cells, help dilate blood vessels, and help prevent or delay the formation of atherosclerosis in the later stage. Our study also found that the results of this study have some clinical significance and advantages. The dNLR can be quickly calculated based on routine blood examination at the time of admission and the serum albumin obtained in the biochemical examination. Coronary angiography during hospitalization and medical history can be used to evaluate whether it is a chronic occlusive disease. Based on the results of angiography and ALB-NLR, the patient's health can be evaluated. The long-term prognosis and the need for readmission to the hospital later.
Study limitations include (1) due to the retrospective design of the study, the identified association should be considered hypothesis-generating; (2) mechanistic studies and prospective clinical studies supporting the prognostic value of ALB-dNLR score are lacking; and (3) the lack of hsCRP data makes it impossible to analyze the correlation between ALB-dNLR and hsCPR.
Conclusions
ALB-dNLR is an independent risk factor of predict adverse outcomes in post-PCI CAD patients. Assessment of ALB-dNLR may help identify high risk individuals with CAD.
What Is Known About This Topic?
• Both albumin (ALB) and derived neutrophil to lymphocyte ratio (dNLR) have been shown to be involved in the pathogenesis of coronary artery disease (CAD), chronic inflammatory disease and tumor.
• It has been reported that the ALB-dNLR score is an independent predictor for adverse outcomes of tumor.
What Does This Paper Add?
• The present study indicates that ALB-dNLR score is an independent and novel predictor of adverse long-term outcomes in CAD patients who underwent PCI.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of First Affiliated Hospital of Xinjiang Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
W-JX, H-TY, and Y-YZ conceptualized the current study objectives, analyzed the data, and wrote the manuscript draft. T-TW, X-GH, and YY collected and organized data. XX and Y-TM had responsibility of the final content. All authors read and approved the final manuscript and were involved in the conception of the research plan.
Funding
This research was funded by the National Natural Science Foundation of China (82170345).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics−2014 update: a report from the American Heart Association. Circulation. (2014) 129:399–410. doi: 10.1161/01.cir.0000442015.53336.12
2. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics−2015 update: a report from the American Heart Association. Circulation. (2015) 131:e29–322. doi: 10.1161/CIR.0000000000000152
3. Christodoulidis G, Vittorio TJ, Fudim M, Lerakis S, Kosmas CE. Inflammation in coronary artery disease. Cardiol Rev. (2014) 22:279–88. doi: 10.1097/CRD.0000000000000006
4. Heusch G, Libby P, Gersh B, Yellon D, Böhm M, Lopaschuk G, et al. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. (2014) 383:1933–43. doi: 10.1016/S0140-6736(14)60107-0
5. Boulanger CM, Loyer X, Rautou PE, Amabile N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol. (2017) 14:259–72. doi: 10.1038/nrcardio.2017.7
6. Schiele F, Ecarnot F, Chopard R. Coronary artery disease: risk stratification and patient selection for more aggressive secondary prevention. Eur J Prev Cardiol. (2017) 24(3 Suppl.):88–1007. doi: 10.1177/2047487317706586
7. Gorog DA. Prognostic value of plasma fibrinolysis activation markers in cardiovascular disease. J Am Coll Cardiol. (2010) 55:2701–9. doi: 10.1016/j.jacc.2009.11.095
8. Leone A. Markers of atherosclerotic disease: what do they mean? Current opinion and future trends. Curr Pharm Des. (2016) 22:7–17. doi: 10.2174/1381612822666151109111311
9. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO III, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the American heart association. Circulation. (2003) 107:499–511. doi: 10.1161/01.CIR.0000052939.59093.45
10. Tanindi A, Erkan AF, Ekici B, Alhan A, Töre HF. Neutrophil to lymphocyte ratio is associated with more extensive, severe and complex coronary artery disease and impaired myocardial perfusion. Turk Kardiyol Dem Ars. (2014) 42:125–30. doi: 10.5543/tkda.2014.18949
11. Papa A, Emdin M, Passino C, Michelassi C, Battaglia D'Cocci F, et al. Predictive value of elevated neutrophil lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta. (2008) 395:27–31. doi: 10.1016/j.cca.2008.04.019
12. Gibson PH, Croal BL, Cuthbertson BH, Small GR, Ifezulike AI, Gibson G, et al. Preoperative neutrophil lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J. (2007) 154:995–1002. doi: 10.1016/j.ahj.2007.06.043
13. Duffy BK, Gurm HS, Rajagopal V, Gupta R, Ellis SG, Bhatt DL, et al. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol. (2006) 97:993–6. doi: 10.1016/j.amjcard.2005.10.034
14. Horne BD, Anderson JL, John JM, Weaver A, Bair TL, Jensen KR, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. (2005) 45:1638–43. doi: 10.1016/j.jacc.2005.02.054
15. Martin S, Markus E, Wolfgang M, Jasmin A, Schila S, Oliver S, et al. Serum albumin predicts cardiac adverse events in patients with advanced atherosclerosis interrelation with traditional cardiovascular risk factors. Thromb Haemost. (2004) 91:610–8. doi: 10.1160/TH03-08-0504
16. Kaneko K, Kimata T, Tsuji S, Shimo T, Takahashi M, Tanaka S, et al. Serum albumin level accurately reflects antioxidant potentials in idiopathic nephrotic syndrome. Clin Exp Nephrol. (2012) 16:411–4. doi: 10.1007/s10157-011-0578-y
17. Kinoshita H, Watanabe K, Azma T, Feng GG, Akahori T, Hayashi H, et al. Human serum albumin and oxidative stress in preeclamptic women and the mechanism of albumin for stress reduction. Heliyon. (2017) 3:e00369. doi: 10.1016/j.heliyon.2017.e00369
18. Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer. (2012) 107:695–9. doi: 10.1038/bjc.2012.292
19. Liu JX, Li A, Zhou LY, Liu XF, Wei ZH, Wang XZ, et al. Significance of combined preoperative serum Alb and dNLR for diagnosis of pancreatic cancer. Fut Oncol. (2018) 14:229–39. doi: 10.2217/fon-2017-0339
20. Chi G, Gibson CM, Liu YY, Hernandez AF, Hull RD, Cohen AT, et al. Inverse relationship of serum albumin to the risk of venous thromboembolism among acutely ill hospitalized patients: analysis from the APEX trial. Am J Hematol. (2019) 94:21–8. doi: 10.1002/ajh.25296
21. Zheng YY, Wu TT, Chen Y, Hou XG, Yang Y, Ma X, et al. Gamma-Glutamyl transferase-to-platelet ratio as a novel predictor of long-term adverse outcomes in patients after undergoing percutaneous coronary intervention: a retrospective cohort study. Thromb Haemost. (2019) 119:1021–30. doi: 10.1055/s-0039-1681103
22. Aronow WS, Fleg JL, Pepine CJ, Artinianr NT, Bakris G, Brown AS, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Soc Hypertens. (2011) 5: 259–352. doi: 10.1016/j.jacc.2011.01.008
23. Report of a WHO Consultation. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organization (1999).
24. Xie X, Ma YT, Yang YN, Li XM, Zheng YY, Ma X, et al. Personalized antiplatelet therapy according to CYP2C19 genotype after percutaneous coronary intervention: a randomized control trial. Int J Cardiol. (2013) 168:3736–40. doi: 10.1016/j.ijcard.2013.06.014
25. Chen SS, Ying HJ, Du JP, Zhu XL, Shi JF, Zhang Y, et al. The association between albumin-dNLR score and disease activity in patients with rheumatoid arthritis. J Clin Lab Anal. (2019) 33:e22695. doi: 10.1002/jcla.22695
26. Park JY, Choi BG, Rha SW, Kang TS. Five-year outcomes in patients with anemia on admission undergoing a coronary intervention for acute myocardial infarction in Koreans: propensity score matching analysis. Coron Artery Dis. (2018) 29:647–51. doi: 10.1097/MCA.0000000000000657
27. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Task force for the universal definition of myocardial infarction. Third Universal definition of myocardial infarction. Nat Rev Cardiol. (2012) 9:620–33. doi: 10.1038/nrcardio.2012.122
28. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2013) 62:e147–239. doi: 10.1016/j.jacc.2013.05.020
29. Chen Z, Shao Y, Yao H, Zhuang Q, Xu R. Preoperative albumin to globulin ratio predicts survival in clear cell renal cell carcinoma patients. Oncotarget. (2017) 15162. doi: 10.18632/oncotarget.15162
30. He XB, Guo SJ, Chen D, Yang GW, Chen X, Zhang YJ, et al. Preoperative albumin to globulin ratio (AGR) as prognostic factor in renal cell carcinoma. J Cancer. (2017) 8:258–65. doi: 10.7150/jca.16525
31. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. (2004) 17:432–7. doi: 10.1111/j.0894-0959.2004.17603.x
32. Eckart A, Struja T, Kutz A, Baumgartner A, Baumgartner T, Zurfluh S, et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: a prospective study. Am J Med. (2020) 133:713–22.e7. doi: 10.1016/j.amjmed.2019.10.031
33. Stenvinkel P, Heimburger O, Paultre F, Diezfalusy U, Wang T, Bergland L, et al. Strong association between malnutrition, inflammation and atherosclerosis in chronic renal failure. Kidney Int. (1999) 55:1899–911. doi: 10.1046/j.1523-1755.1999.00422.x
34. Danesh J, Collins R, Appleby P, Peto R. Association of fbrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. (1998) 279:1477–82. doi: 10.1001/jama.279.18.1477
35. Djousse L, Rothman KJ, Cupples LA, Levy D, Ellison RC. Serum albumin and risk of myocardial infarction and all-cause mortality in the Framingham Offspring Study. Circulation. (2002) 106:2919–24. doi: 10.1161/01.CIR.0000042673.07632.76
36. Plakht Y, Gilutz H, Shiyovich A. Decreased admission serum albumin level is an independent predictor of long-term mortality in hospital survivors of acute myocardial infarction. Soroka acute myocardial infarction II (SAMI-II) project. Int J Cardiol. (2016) 219:20–4. doi: 10.1016/j.ijcard.2016.05.067
37. Levey AS, Bosch J, Lewis J, Greene T, Rogers N, Roth N, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. (1999) 130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002
38. Mezzano D, Tagle R, Panes O, Pérez M, Downey P, Muñoz B, et al. Hemostatic disorder of uremia: the platelet defect, main determinant of the prolonged bleeding time, is correlated with indices of activation of coagulation and fibrinolysis. Thromb Haemost. (1996) 76: 312–21. doi: 10.1055/s-0038-1650576
39. Guerin AP, Adda H, London GM, Marchais SJ. Cardiovascular disease in renal failure. Minerva Urol Nefrol. (2004) 56: 279–88.
40. Toto RD. Treatment of hypertension in chronic kidney disease. Semin Nephrol. (2005) 25:435–9. doi: 10.1016/j.semnephrol.2005.05.016
41. Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. (2000) 342:1478–83. doi: 10.1056/NEJM200005183422003
42. Kalay N, Dogdu O, Koc F, Yarlioglues M, Ardic I, Akpek M, et al. Hematologic parameters and angiographic progression of coronry athemsclemsis. Angiology. (2012) 63:213–7. doi: 10.1177/0003319711412763
43. Park BJ, Shim JY, Lee HR, Lee JH, Jung DH, Kim HB, et al. Relationship of neutrophil-lymphocyte ratio with arterial stiffness and coronary calcium score. Clin Chim Acta. (2011) 412:925–9. doi: 10.1016/j.cca.2011.01.021
44. Tsai JC, Sheu SH, Chiu HC, Chung FM, Chang DM, Chen MP, et al. Association of peripheral total and differential leukocyte counts with metabolic syndrome and risk of ischemic cardiovascular diseases in patients with type 2 diabetes mellitus. Diabetes Metab Res Rev. (2007) 23:111–8. doi: 10.1002/dmrr.647
45. Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. (2008) 102:653–7. doi: 10.1016/j.amjcard.2008.05.006
46. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. (1997) 336:973–9. doi: 10.1056/NEJM199704033361401
47. Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. (1999) 340:115–26. doi: 10.1056/NEJM199901143400207
48. Li WH, Pan W. The relationship of neutrophil to lymphocyte and cardiovascular diseases. Adv Cardiovasc Dis. (2014) 35:1.
49. Wada H, Dohi T, Miyauchi K, Shitara J, Endo H, Doi S, et al. Pre- procedural neutrophil-to-lymphocyte ratio and long-term cardiac outcomes after percutaneous coronary intervention for stable coronary artery disease. Atherosclerosis. (2017) 265:35–40. doi: 10.1016/j.atherosclerosis.2017.08.007
50. Bressi E, Mangiacapra F, Ricottini E, Cavallari I, Colaiori I, Di Gioia G, et al. Impact of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio on 5-year clinical outcomes of patients with stable coronary artery disease undergoing elective percutaneous coronary intervention. J Cardiovasc Transl Res. (2018) 11:517–23. doi: 10.1007/s12265-018-9829-6
51. Ernst E, Hammerschmidt DE, Bagge U, Matrai A, Dormandy JA. Leukocytes and the risk of ischemic diseases. JAMA. (1987) 257:2318–24. doi: 10.1001/jama.257.17.2318
52. Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. (2013) 339:161–6. doi: 10.1126/science.1230719
53. Liu Y, Du X, Chen J, Jin Y, Peng L, Wang HX, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. (2020) 81:e6–12. doi: 10.1016/j.jinf.2020.04.002
54. Azab B, Zaher M, Weiserbs KF, Torbey E, Lacossiere K, Gaddam S. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. (2010) 106:470–6. doi: 10.1016/j.amjcard.2010.03.062
55. Butt K, D'Souza J, Yuan C, Jayakumaran J, Nguyen M, Butt HI, et al. Correlation of the Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) with contrast-induced nephropathy in patients with acute coronary syndrome undergoing percutaneous coronary interventions. Cureus. (2020) 12:e11879. doi: 10.7759/cureus.11879
Keywords: albumin and the derived neutrophil to lymphocyte ratio score (ALB-dNLR score), coronary artery disease, percutaneous coronary intervention, mortality, all-cause mortality, cardiac mortality, major adverse cardiovascular events, major adverse cardiovascular and cerebrovascular events
Citation: Xiu W-J, Yang H-T, Zheng Y-Y, Wu T-T, Hou X-G, Jiang Z-H, Yang Y, Ma Y-T and Xie X (2022) ALB-dNLR Score Predicts Mortality in Coronary Artery Disease Patients After Percutaneous Coronary Intervention. Front. Cardiovasc. Med. 9:709868. doi: 10.3389/fcvm.2022.709868
Received: 20 July 2021; Accepted: 27 January 2022;
Published: 15 March 2022.
Edited by:
Alexandra R. Lucas, Arizona State University, United StatesReviewed by:
Gerald Chi, Beth Israel Deaconess Medical Center and Harvard Medical School, United StatesSaskia C. A. De Jager, Utrecht University, Netherlands
Copyright © 2022 Xiu, Yang, Zheng, Wu, Hou, Jiang, Yang, Ma and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Xie, xiangxie999@sina.com
†These authors have contributed equally to this work
 Wen-Juan Xiu1†
Wen-Juan Xiu1†  Hai-Tao Yang
Hai-Tao Yang Ting-Ting Wu
Ting-Ting Wu Xiang Xie
Xiang Xie