Impact of early PCSK9 inhibitor treatment on heart after percutaneous coronary intervention in patients with STEMI: Design and rationale of the PERFECT II trial
- 1Institute of Cardiovascular Diseases, Tongren Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Cardiology, Tongren Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Collaborative Innovation Centre of Regenerative Medicine and Medical BioResource Development and Application Co-constructed by the Province and Ministry, Guangxi Medical University, Nanning, China
- 4Department of Cardiology, Shanghai Jiao Tong University Affiliated Chest Hospital, Shanghai, China
- 5Department of Cardiology, Shanghai Tenth People's Hospital, Tongji University, Shanghai, China
- 6Department of Cardiology, Shanghai Jiao Tong University School of Medicine Affiliated Ninth People's Hospital, Shanghai, China
- 7Dental Public Health, The University of Hong Kong Faculty of Dentistry, Hong Kong, China
- 8Department of Radiology, Tongren Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background and aims: Primary percutaneous coronary intervention (PPCI) is the most effective treatment strategy for ST-segment elevation myocardial infarction (STEMI). Nevertheless, dysregulated inflammation induced by myocardial reperfusion injury may increase the final infarct size and induce maladaptive myocardial remodeling. Proprotein convertase subtilisin/kexin 9 (PCSK9) inhibitor, as a novel and potent lipid-lowering drug, plays an important role in inflammation. The aim of this study is to investigate whether the early application of PCSK9 inhibitor can increase the myocardial salvage index (MSI) and improve ventricular remodeling in patients with STEMI.
Design: The PERFECT II trial is a prospective, open-label, multicenter, randomized controlled study involving 160 patients with STEMI who are scheduled to undergo PPCI. The eligible patients will be divided into PCSK9 inhibitor group and control group via the interactive web response system, at a 1:1 ratio. In the PCSK9 inhibitor group, the PCSK9 inhibitor alirocumab at a dose of 75 mg will be subcutaneously injected immediately after PPCI and administered every 2 weeks thereafter for 3 months based on conventional treatment. In the control group, conventional treatment will be administered. The primary endpoint is MSI, as measured by cardiac magnetic resonance imaging (CMR) at 1 week after PPCI. The secondary endpoints are the peak time of creatine kinase (CK)-MB and troponin I (TnI)/TnT after PPCI; the postoperative fall time of the ST segment on electrocardiography (ECG); the rate of plasma low-density lipoprotein cholesterol (LDL-C) compliance (< 1.4 mmol/L and a reduction of >50% from baseline) at 1, 3, and 6 months after PPCI; infarct size and ejection fraction (EF) measured by CMR at 6 months after PPCI; the occurrence of major adverse cardiovascular event (MACE: a composite of cardiovascular death, non-fatal myocardial infarction, stent thrombosis, repeat revascularization, stroke, and heart failure needed to be hospitalized).
Conclusions: This is the first multicenter study to investigate the effect of early application of the PCSK9 inhibitor alirocumab on MSI in patients with STEMI undergoing PPCI. The findings will provide an opportunity to explore novel ideas and methods for the treatment of acute myocardial infarction.
Trial registration: ClinicalTrials.gov, identifier: NCT05292404.
Introduction
Primary percutaneous coronary intervention (PPCI) with drug-eluting stent is an effective and common treatment strategy for acute coronary syndrome (ACS), including unstable angina (UA), non-ST-segment elevation myocardial infarction (non-STEMI), and STEMI (1). Over the past decade, the prognosis of acute myocardial infarction (AMI) has improved substantially. However, it is still the leading cause of morbidity and mortality worldwide (2). Myocardial reperfusion is imperative to salvage the ischemic myocardium in patients with STEMI; nevertheless, early restoration of blood flow itself can induce damage to the myocardium by triggering a series of sterile inflammatory responses known as myocardial ischemia-reperfusion injury (3). Crucially, the lack of effective therapy to attenuate local inflammation after myocardial infarction in patients with STEMI makes it necessary to identify a viable target for cardioprotection.
Proprotein convertase subtilisin/kexin 9 (PCSK9), which is mainly secreted by the liver, can increase the concentration of serum low-density lipoprotein cholesterol (LDL-C) by promoting LDL receptor (LDLR) degradation (4). The plasma concentration of PCSK9 is significantly increased in patients with AMI (5). Recent evidence suggests that PCSK9 contributes to inflammation beyond cholesterol regulation. In atherosclerosis progression, PCSK9 activates proinflammatory cytokine production and affects oxidative modifications within atherosclerotic lesions (6, 7). In addition, in animal models of AMI, PCSK9 is up-regulated mostly in the region bordering the infarct area and determines the development of infarct size, cardiac function, and autophagy (8). Additionally, the concentration of serum PCSK9 is associated with the future risk of cardiovascular disease (9). PCSK9 inhibitors like alirocumab are frequently performed to reduce the concentration of LDL-C and lower the rate of adverse cardiovascular events (10). Moreover, application of alirocumab and the anti-PCSK9 AT04A vaccine in APOE*3Leiden.CETP transgenic mice reduces vascular inflammation and atherosclerotic lesions (11, 12). Our team found that PCSK9 knockout mice showed ameliorated myocardial inflammation and heart failure after myocardial infarction.
We recently conducted a randomized pilot study (PERFECT trial, NCT04731105) in 20 patients with anterior myocardial infarction accompanied by chest pain that began within 24 h. In this study, a single 75-mg dose of alirocumab was subcutaneously injected immediately after PPCI and lead to a trend toward an increase in the myocardial salvage index (MSI) compared with the control group (0.50 ± 0.16 vs. 0.41 ± 0.15; p = 0.21).
To further confirm the results of the PERFECT trial, we designed this multicenter, randomized controlled study to investigate whether early application of alirocumab in patients with STEMI can increase the MSI 1 week after injection and decrease the infarct size 6 months later. The MSI, as assessed by cardiac magnetic resonance imaging (CMR) with late gadolinium enhancement, is considered to be the gold-standard method to assess of infarct size (13). Thus, we will use the MSI, instead of infarct size, as the primary endpoint in this study.
Methods
Study objectives
The primary objective of this study is to explore whether the early application of the PCSK9 inhibitor alirocumab after successful PPCI increases the MSI, limits infarct size, and improves ventricular remodeling in patients with STEMI.
Study design
The PERFECT II trial is a multicenter, prospective, open-label, randomized controlled clinical trial. The centers of this study include Tongren Hospital, Chest Hospital, Tenth People's Hospital, and Ninth People's Hospital in Shanghai. STEMI patients with an onset of within 24 h who are scheduled to undergo PPCI will be randomized in a 1:1 fashion to receive alirocumab (PCSK9 inhibitor treatment group) or conventional treatment (control group); each group will include 80 eligible patients. The study flow chart is shown in Figure 1.
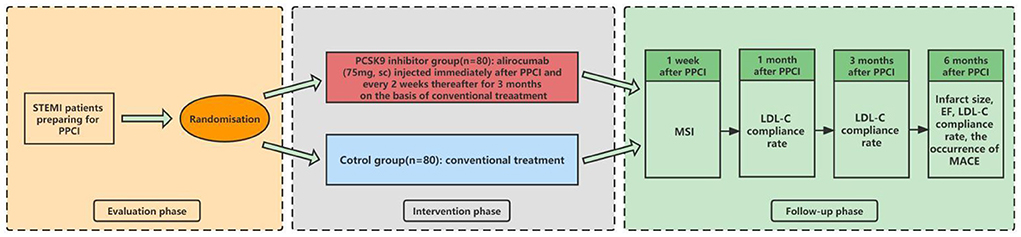
Figure 1. Study flow chart. STEMI, ST-segment elevation myocardial infarction; PPCI, primary percutaneous coronary intervention; sc, subcutaneous; MSI, myocardial salvage index; LDL-C, low-density lipoprotein cholesterol; EF, ejection faction; MACE, major adverse cardiovascular event.
Study population
Patients with STEMI who are scheduled to undergo PPCI will be evaluated for enrollment. The diagnosis of STEMI will be based on 2017 European Society of Cardiology guidelines (14). The onset of STEMI must be within 24 h, and participants must be aged 18 to 80, inclusive but not 18. Importantly, written informed consent will be obtained before enrollment. Patients who meet these enrollment criteria will also be judged against the exclusion criteria. For example, patients who are allergic to PCSK9 inhibitors and aspirin (which is an important component of conventional treatment) will be excluded. To exclude the effect of pregnancy on the results of this trial and the effect of drugs on pregnant women, patients who are pregnant, breast feeding, or planning to become pregnant in the next 6 months will not be considered for inclusion. To maximize the completeness of follow-up, we intend to select patients with a life expectancy of >6 months; therefore, the following patients will be excluded: patients with severe hepatic or renal insufficiency and patients with malignant tumors. Patients who have undergone previous revascularization or treatment with PCSK9 inhibitors previously will also be excluded also. Meanwhile, patients who are deemed unsuitable for participation in this study by the investigator will not be eligible for inclusion. In all cases, the final decision will be made by the investigator based on clinical factors and a review of the initial angiography results. The detailed inclusion and exclusion criteria are presented in Table 1.
Randomization
Randomization procedures will be conducted strictly before PPCI. Eligible patients will be randomized 1:1 to the PCSK9 inhibitor group or the control group and stratified by age (≤ 60/>60; 2 categories), gender (female/male; 2 categories), time from symptom onset (< 3/3–24 h; 2 categories) using the interactive web response system (IWRS) after providing written informed consents.
Interventions and follow-up
After grouping, enrolled patients will undergo PPCI with new-generation drug-eluting stents. Immediately after PPCI, patients grouped in the PCSK9 inhibitor group will be injected with alirocumab subcutaneously at a dose of 75 mg after the PPCI, followed by injections at the same dose every 2 weeks for 3 months on the basis of conventional treatment. Patients in the control group will receive conventional treatment in accordance with the published guidelines, including dual anti-platelet therapy and statins (15). Patients will be evaluated at 1 week and at 1, 3, and 6 months after the index PPCI, and the occurrence of clinical events will be monitored. As the primary endpoint, the MSI will be measured by CMR at 1 week after PPCI. As the key secondary endpoints, the infarct size and ejection fraction (EF) will also be measured by CMR at 6 months after PPCI. Additionally, the peak time of creatine kinase (CK)-MB and troponin I (TnI)/TnT, as well as the postoperative fall time of the ST segment on electrocardiography (ECG) will be recorded. Plasma LDL-C compliance rate (< 1.4 mmol/L and reduction >50% of baseline) and the occurrence of major adverse cardiovascular event (MACE: a composite of cardiovascular death, non-fatal myocardial infarction, stent thrombosis, repeat revascularization, stroke, and heart failure needed to be hospitalized) will be recorded at 1, 3, and 6 months after PPCI (16). Besides, medications at discharge will be recorded, especially those that affect cardiac remodeling. The investigators will follow up with the patients by office visits or telephone contact. The data collected during all follow-ups will be submitted to the original documents. If any patient is admitted to a non-research hospital again, every effort will be made to obtain the original data from the hospital. For all re-infarctions, ECG and myocardial enzyme data will be obtained and recorded. The following events will lead to termination of the patient's follow-up: (1) death; (2) voluntary withdrawal; (3) evacuation of patients by the investigators based on clinical manifestations; and (4) loss to follow-up (informal withdrawal). The follow-up schedule is shown in Figure 1 and Table 2.
Study endpoints
The primary endpoint is MSI measured by CMR at 1 week after the index PPCI. The secondary endpoints are the peak time of CK-MB and TnI/TnT after PPCI; the postoperative fall time of the ST segment on ECG; the rate of plasma LDL-C compliance (< 1.4 mmol/L and a reduction of >50% from baseline) at 1, 3, and 6 months after PPCI; infarct size and EF measured by CMR at 6 months after PPCI; the occurrence of MACE.
Sample size calculation
Referring to our prior small sample size study, the MSI was higher in the PCSK9 inhibitor group (mean: 0.50; standard deviation [SD]: 0.16) than in the control group (mean: 0.41; SD: 0.15). To guarantee 90% power of the study at a significance level of 0.05, at least 64 patients per group will be needed. Considering a dropout rate of 20%, the required sample size is 160 (80 patients in each group). PASS 15 was used to calculate the sample size.
Statistical analysis
Continuous variables (including the primary endpoint) will be expressed as mean (SD) or median (interquartile rage) and will be compared using the t-test or the Mann-Whitney U test, as appropriate. Categorical variables will be expressed as counts and percentages and compared using the χ2 test or Fisher's exact test, as appropriate. We plan to assess the consistency of the treatment effect on the primary endpoint among three pre-specified subgroups that will be analyzed individually and then in a multivariate model. The following subgroup analyses are planned: age: < 60 years vs. ≥60 years; sex: female vs. male; duration from symptom onset to study drug infusion: < 3 h vs. 3–24 h. Safety analyses will include tabulation of the type and frequency of all adverse events and severe adverse events. All primary and secondary endpoints will be analyzed on an intention-to-treat basis (all patients will be analyzed as part of their designated treatment group) and protocol-based premise (analyses will only be performed as part of their designated treatment group when the patient received their designated treatment). For the intention-to-treat analysis, all patients who have provided written informed consent and who have been randomized in the study will be included in the analysis sample, regardless of whether they have received the correct treatment or whether there is crossover. For the protocol-based analysis, only registered patients who received the specified treatment will be included in the analysis sample. Patients who are lost to follow-up will be eliminated at the time of the last known contact. P values and confidence intervals will be two-sided with a significance level of 0.05.
Ethics and dissemination
This trial has been approved by the ethics committee of Tongren Hospital, Shanghai Jiao Tong University School of Medicine (2022-006-01). The study protocol and informed consent prior to patient participation will be approved by the ethics committee. No changes to the protocol or informed consent will be allowed without the approval of the ethics committee. As required by the ethics committee, the investigator will report the progress of the study until completion. Additionally, any protocol amendments and associated informed consent changes will be submitted to the ethics committee and must be approved in writing prior to implementation. Enrolled patients can withdraw from the trial at anytime during the process of the study. The privacy of patients will be strictly maintained during and after the study. Questions related to privacy and secrecy are listed in the informed consent form. All data will only be accessed by the study staff and will be kept strictly confidential. In addition, the data will be protected in locked cabinets at the participating clinical centers. An independent data monitoring committee (IDMC) will review the security data, including data on death, myocardial infarction, stroke, and other adverse events. The IDMC has the right to recommend suspension of enrollment or termination of the research based on safety considerations. The executive committee will make a final decision on the early termination of the study based on the recommendations.
The results will be disseminated at several research conferences and as published articles in peer-reviewed journals after approval from the study sponsors.
Discussion
Although early revascularization by PPCI is essential for myocardial myocardium, large infarctions frequently occur in patients with STEMI. Consequently, mortality remains high, and many patients develop complications, such as heart failure, after STEMI. Local and severe sterile inflammation is one of the main causes of ischemia–reperfusion injury (17). Despite some prior studies not meeting the expected goals (18–20), depressing inflammation directly or indirectly in the early stage of STEMI is a feasible strategy to protect the myocardium and limit infarct size to improve outcomes.
PCSK9 mainly regulates the concentration of LDL-C by degrading the LDLR; thus, PCSK9 inhibitors have become a novel effective therapeutic strategy for lipid management in patients with hypercholesterolemia and ACS. Recent studies have indicated that PCSK9 also plays a role in inflammation. PCSK9 also mediates cardiomyocyte autophagy (8) and aggravates inflammation through the Toll-like receptor 4/nuclear factor-κB (21) and lipoprotein receptor-related protein 1 (22) pathways. Conceivably, PCSK9 may be a new target for the treatment of AMI. Recent studies have shown that PCSK9 inhibitors can reduce cardiovascular events and attenuate the risk of type 2 MI in patients with ACS (23, 24). Thus, early application of PCSK9 inhibitors may be an effective treatment strategy for patients with STEMI.
In the PERFECT II trial, we expanded the sample size, inclusion criteria, and the number of clinical centers on the basis of the PERFECT trial. Therefore, the PERFECT II trial will provide better evidence on the impact of early application of the PCSK9 inhibitor alirocumab on MSI and ventricular remodeling in patients with STEMI after PPCI.
Author contributions
LH and JX co-designed the study. SW designed the statistical plan. JX, XW, JZho, DW, YP, XX, ZS, YZ, and JZha screen and enroll participants and arrange informed consent of the participants. ZX is responsible for CMR testing. LH, JX, and XW wrote the manuscript. All authors critically reviewed and approved the final manuscript.
Funding
This work was supported by the Xinxin Heart Foundation from China Cardiovascular Association (2021-CCA-ACCESS-73).
Acknowledgments
We would like to thank the staff at the Department of Cardiology, Tongren Hospital, Shanghai Jiao Tong University School of Medicine for their support and help in this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet. (2017) 389:197–210. doi: 10.1016/S0140-6736(16)30677-8
2. Collaborators GCoD. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1151–210. doi: 10.1016/S0140-6736(17)32152-9
3. Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. (2007) 357:1121–35. doi: 10.1056/NEJMra071667
4. Shapiro MD, Tavori H, Fazio S. PCSK9: from basic science discoveries to clinical trials. Circ Res. (2018) 122:1420–38. doi: 10.1161/CIRCRESAHA.118.311227
5. Almontashiri NA, Vilmundarson RO, Ghasemzadeh N, Dandona S, Roberts R, Quyyumi AA, et al. Plasma PCSK9 levels are elevated with acute myocardial infarction in two independent retrospective angiographic studies. PLoS ONE. (2014) 9:e106294. doi: 10.1371/journal.pone.0106294
6. Ding Z, Pothineni NVK, Goel A, Lüscher TF, Mehta JL. PCSK9 and inflammation: role of shear stress, pro-inflammatory cytokines, and LOX-1. Cardiovasc Res. (2020) 116:908–15. doi: 10.1093/cvr/cvz313
7. Liu A, Frostegård J. PCSK9 plays a novel immunological role in oxidized LDL-induced dendritic cell maturation and activation of T cells from human blood and atherosclerotic plaque. J Intern Med. (2018) 284:193–210. doi: 10.1111/joim.12758
8. Ding Z, Wang X, Liu S, Shahanawaz J, Theus S, Fan Y, et al. PCSK9 expression in the ischaemic heart and its relationship to infarct size, cardiac function, and development of autophagy. Cardiovasc Res. (2018) 114:1738–51. doi: 10.1093/cvr/cvy128
9. Leander K, Mälarstig A, Van't Hooft FM, Hyde C, Hellénius ML, Troutt JS, et al. Circulating Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) predicts future risk of cardiovascular events independently of established risk factors. Circulation. (2016) 133:1230–9. doi: 10.1161/CIRCULATIONAHA.115.018531
10. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. (2015) 372:1489–99. doi: 10.1056/NEJMoa1501031
11. Kühnast S, van der Hoorn JW, Pieterman EJ, van den Hoek AM, Sasiela WJ, Gusarova V, et al. Alirocumab inhibits atherosclerosis, improves the plaque morphology, and enhances the effects of a statin. J Lipid Res. (2014) 55:2103–12. doi: 10.1194/jlr.M051326
12. Landlinger C, Pouwer MG, Juno C, van der Hoorn JWA, Pieterman EJ, Jukema JW, et al. The AT04A vaccine against proprotein convertase subtilisin/kexin type 9 reduces total cholesterol, vascular inflammation, and atherosclerosis in APOE*3Leiden. CETP mice Eur Heart J. (2017) 38:2499–507. doi: 10.1093/eurheartj/ehx260
13. Engblom H, Heiberg E, Erlinge D, Jensen SE, Nordrehaug JE, Dubois-Randé JL, et al. Sample size in clinical cardioprotection trials using myocardial salvage index, infarct size, or biochemical markers as endpoint. J Am Heart Assoc. (2016) 5:e002708. doi: 10.1161/JAHA.115.002708
14. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
15. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2018) 39:213–60. doi: 10.1093/eurheartj/ehx419
16. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. (2021) 42:3227–337. doi: 10.1093/eurheartj/ehab484
17. Heusch G, Gersh BJ. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J. (2017) 38:774–84. doi: 10.1093/eurheartj/ehw224
18. Arslan F, Smeets MB, O'Neill LA, Keogh B, McGuirk P, Timmers L, et al. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation. (2010) 121:80–90. doi: 10.1161/CIRCULATIONAHA.109.880187
19. Armstrong PW, Granger CB, Adams PX, Hamm C, Holmes D Jr, O'Neill WW, et al. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA. (2007) 297:43–51. doi: 10.1001/jama.297.1.43
20. O'Donoghue ML, Glaser R, Cavender MA, Aylward PE, Bonaca MP, Budaj A, et al. Effect of losmapimod on cardiovascular outcomes in patients hospitalized with acute myocardial infarction: a randomized clinical trial. JAMA. (2016) 315:1591–9. doi: 10.1001/jama.2016.3609
21. Tang ZH, Peng J, Ren Z, Yang J, Li TT, Li TH, et al. New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/NF-κB pathway. Atherosclerosis. (2017) 262:113–22. doi: 10.1016/j.atherosclerosis.2017.04.023
22. Potere N, Del Buono MG, Mauro AG, Abbate A, Toldo S. Low density lipoprotein receptor-related protein-1 in cardiac inflammation and infarct healing. Front Cardiovasc Med. (2019) 6:51. doi: 10.3389/fcvm.2019.00051
23. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. (2017) 376:1713–22. doi: 10.1056/NEJMoa1615664
Keywords: proprotein convertase subtilisin/kexin type 9 (PCSK9), ST-segment elevation myocardial infarction, percutaneous coronary intervention, myocardial salvage index, alirocumab
Citation: Xia J, Wang X, Zhou J, Wang D, Pang Y, Xu X, Sang Z, Zhang Y, Zhang J, Wu S, Xiao Z and Hou L (2022) Impact of early PCSK9 inhibitor treatment on heart after percutaneous coronary intervention in patients with STEMI: Design and rationale of the PERFECT II trial. Front. Cardiovasc. Med. 9:1009674. doi: 10.3389/fcvm.2022.1009674
Received: 02 August 2022; Accepted: 05 September 2022;
Published: 23 September 2022.
Edited by:
Xinyu Weng, Fudan University, ChinaCopyright © 2022 Xia, Wang, Zhou, Wang, Pang, Xu, Sang, Zhang, Zhang, Wu, Xiao and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengguang Xiao, xzg1669@shtrhospital.com; Lei Hou, hl4081@shtrhospital.com
†These authors have contributed equally to this work
 Jiachun Xia
Jiachun Xia Xinyue Wang2†
Xinyue Wang2†  Yanan Pang
Yanan Pang Yi Zhang
Yi Zhang Junfeng Zhang
Junfeng Zhang
