The influence of pair duration on reproductive success in the monogamous ‘Alalā (Hawaiian crow, Corvus hawaiiensis)
- San Diego Zoo Wildlife Alliance, Volcano, HI, United States
Conservation breeding program practitioners select potential mates in an attempt to maximize pair compatibility and maintain genetic diversity. Therefore, pair duration, or the number of breeding seasons that individuals retain the same mate, is practitioner-determined in these settings. There is a critical need to evaluate whether pair duration influences reproductive success in ex situ assurance populations, particularly for socially monogamous species. The ‘Alalā (Hawaiian crow, Corvus hawaiiensis) is a monogamous forest bird that is currently extinct in the wild. Today, ‘Alalā exist only in human care for intensive conservation breeding. We analyzed breeding program data from 2018-2021 to determine the effects of ‘Alalā pair duration and age on reproduction (nest building, egg laying, hatching, and fledging). We found that pair duration does not influence reproductive outcomes, and thus practitioners can be more proactive when re-pairing birds. Female and male age, on the other hand, influenced the probability of nest building, clutch production, and overall reproductive success. Nest building and clutch production probabilities were high (near 1) and stable as females aged from 2 to ~ 12 years old, declining sharply thereafter. In males, overall reproductive success (from building robust nests to rearing at least one nestling to fledge) increased with age from 2 to ~ 9 years old, peaked and reached an asymptote with males ≳ 9 to ~ 13 years old, and decreased in males ≳ 13 years old. Thus, integrating age into the pair selection process will increase the likelihood of achieving conservation goals. To our knowledge, we are the first to utilize empirical pair duration results to provide specific management recommendations for mate selection in an avian conservation breeding program. Our findings have critical utility for guiding ‘Alalā pairing decisions, and more broadly underscore the importance of evaluating mate retention and selection protocols in other conservation breeding programs.
Introduction
Conservation breeding is an important tool for animal conservation used to save species from extinction and achieve species recovery goals by establishing ex situ assurance populations often for reintroduction and translocation. Breeding practitioners are inherently faced with limited resources and small sample sizes (in terms of the total number of individuals available to breed), as well as sparse data on the species prior to being brought into human care which are typically collected during a time when wild populations were already in marked decline. Thus, practitioners must make husbandry decisions while being immersed in uncertainty. Yet, with an adaptive management framework, conservation breeding practitioners can continually adjust and refine decisions as new information becomes available. Specifically, by recording and analyzing detailed data on reproductive outcomes, as well as their potential drivers, practitioners can take a scientific approach to guide and adapt management decisions for their unique programs (Heinrichs et al., 2019).
A major challenge in conservation breeding programs is identifying potential mates that will successfully produce offspring. Mate selection can involve a “hands-off” approach by providing animals with the opportunity to choose their own mate from a pool of contenders (e.g., in mate choice studies; Ihle et al., 2015; Martin-Wintle et al., 2019; Munson et al., 2020). Other approaches may involve pairing individuals based on criteria such as whether a pair would positively contribute to the genetic health of the population, assuming offspring are produced (e.g., by minimizing inbreeding and retaining founder representation; Montgomery et al., 1997; Ballou et al., 2010; Ivy and Lacy, 2012). Alternatively pairs may be chosen based on assessments of behavioral compatibility (e.g., based on personality type; Smith and Blumstein, 2008; Martin-Wintle et al., 2017; Faust and Goldstein, 2021), or a hybrid approach that considers both genetic and behavioral compatibility. For species that cannot be housed in a communal setting, practitioners must make difficult decisions about when to divorce and re-pair previously selected mates, which becomes necessary, particularly if the pair has been repeatedly unsuccessful at breeding. When faced with these decisions, practitioners can draw knowledge from the animal’s life history, such as its mating system. When working with a monogamous species with strong mate fidelity, long-term mate retention may be desirable. In contrast, for species that are not monogamous or have part-time partnerships (Black, 1996), practitioners may re-pair potential breeders more frequently or allow individuals to have simultaneous access to multiple potential mates. Despite the mating system of the species, in the context of conservation breeding, it is vital to determine if, when, and how often to re-pair animals to maximize productivity. Critical to this decision is assessing the effects of mate retention (referred to as pair duration in this study). However, the effects of pair duration in conservation breeding programs (in monogamous species) have not been thoroughly studied, particularly in birds.
Previous work across numerous taxa of socially monogamous species with strong mate fidelity, predominantly in the wild, has shown that individuals that retain the same mate for longer periods of time have higher reproductive success. There are many probable mechanisms explaining the positive relationship, or association, between pair duration and better reproductive outcomes (e.g., resource-based, reproductive performance, and mate familiarity hypotheses underpinning pair fidelity, reviewed in Leu et al., 2015). While the resource-based hypothesis may be irrelevant to birds in ex situ breeding programs, as individuals in these settings do not face the same resource limitations as their wild counterparts, the reproductive performance and mate familiarity hypotheses may apply. The reproductive performance hypothesis predicts that older and more experienced pairs are more likely to achieve reproductive success than younger, inexperienced pairs, and thus mate retention is preferable to divorce (i.e., splitting from an established mate to seek another) in the context of reproductive success (Leu et al., 2015). The mate familiarity hypothesis predicts better reproductive outcomes in pairs that retain the same mates because familiarity with one another enables pairs to breed more efficiently with coordinated reproductive behaviors (Leu et al., 2015). For instance, in Australian sleepy lizards (Tiliqua rugosa), pairs in which mates were more familiar with one another tended to breed earlier than unfamiliar pairs (Leu et al., 2015). Likewise, gray wolves (Canis lupus) that kept the same mate for longer periods of time had higher apparent offspring survival (Ausband, 2019). Other work has suggested that birds with more familiar or compatible mates better coordinate incubation and provisioning of young (Spoon et al., 2006 but see Ihle et al., 2019 who found that coordination of mates did not improve offspring condition or survival). Moreover, experimental work with bearded reedlings (Panurus biarmicus) found that pairs that were together longer were better coordinated, bred earlier, and had higher hatching and fledgling success than newly formed pairs (Griggio and Hoi, 2011). This pattern has also emerged in peregrine falcons (Falco peregrinus) living in human care, where longer-term mates produced more fledglings during their lifetime than birds that did not retain their mates (Clum, 1995).
Alternatively, some individuals experience benefits to seeking a new mate instead of retaining their original mate (e.g., better option hypothesis, Ens et al., 1993). For example, in cockatiels (Nymphicus hollandicus), pair duration did not correlate with the number of eggs or nestlings produced, providing evidence against the mate familiarity hypothesis (Spoon et al., 2016). Other work has shown that, in some cases, it is beneficial for animals to acquire new mates after a successful breeding season (Kelley et al., 1999). In plovers (Charadrius spp.), for example, divorced birds produced more hatchlings than birds that retained mates, and divorce was more likely when a nest hatched successfully (Halimubieke et al., 2020). This idea has also been supported in island foxes (Urocyon littoralis) that were part of an ex situ breeding program, as well; newer pairs had a higher probability of reproductive success compared to more established pairs, a result that has important implications for unsuccessful pairs in conservation breeding programs in terms of mate selection (Calkins et al., 2013). Given these discrepant results, it remains unclear how pair duration affects reproductive success for bird species in human care.
The ‘Alalā (Hawaiian crow; Corvus hawaiiensis) (Figure 1), the only remaining endemic corvid species found in Hawai’i, is presently extinct in the wild. Attempted reintroductions that occurred in the 1990s (Kuehler et al., 1995) and from 2016-2020 (Smetzer et al., 2021) did not produce any self-sustaining wild populations. Thus, at the time of this writing, all living individuals reside in human care for intensive conservation breeding. Here, we tested whether pair duration influenced ‘Alalā reproductive success using detailed data on reproductive outcomes from 2018-2021 (Figure 1). After a sufficiently large assurance population was established, from ~ 2018 onward, (with ~ 140 living individuals), the breeding program moved away from intensive, traditional avicultural methods (involving artificial incubation and puppet-rearing offspring) to parental breeding with pairs being predominantly full-time socialized to allow for coordinated breeding behaviors to occur. The transition to parental breeding was an important shift in management intended to encourage the birds to successfully build nests, incubate eggs, and parent-rear nestlings, with important implications for animal welfare and, eventually, reintroduction to the wild (Flanagan et al., 2023).
When ‘Alalā were observed in the wild, they were observed in monogamous pairs with strong mate fidelity (Banko et al., 2002). Based on this and work with other monogamous species (e.g., Clum, 1995; Spoon et al., 2007; Griggio and Hoi, 2011; Ihle et al., 2015), we assumed that pair duration, albeit artificially imposed, would be an adequate proxy for pair compatibility, due to the fact that incompatible pairs are separated and re-paired. We therefore predicted that pair duration would be positively related to reproductive success, due to better compatibility and/or greater familiarity, where mates that were together longer would have a greater probability of engaging in nest building, producing a clutch, and achieving other downstream reproductive milestones, such as rearing nestlings to fledge. While this study design does not allow these underlying mechanisms to be fully understood, it does allow us to evaluate alternate management strategies related to maintaining pairs. Testing this hypothesis will inform practitioners’ decisions about maintaining existing pairs versus separating and re-pairing with different individuals across reproductive seasons. To our knowledge, we are the first to leverage pair duration data from an avian conservation breeding program to produce results with direct links to management recommendations for mate selection.
Methods
Summary of the conservation breeding program
‘Alalā conservation breeding is conducted at two locations in Hawai’i: the Maui Bird Conservation Center (MBCC) on Maui and the Keauhou Bird Conservation Center (KBCC) on Hawai’i Island. The breeding season starts in April and ends in August of each year. Potential mates are selected prior to the start of the breeding season on an annual basis, using genetic and demographic criteria, along with caretaker-perceived behavioral compatibility, and, more recently, mate choice (Greggor et al., 2018) and personality studies. Throughout our study, established successful mates, particularly those that have produced offspring, were kept together, as the ‘Alalā is long-lived (some individuals live for up to ~ 30 years) and was observed to have strong mate fidelity in the wild (Banko et al., 2002). Regardless of reproductive success, or lack thereof, most pairs in our study were kept together for more than one breeding season (71%), based on the rationale that some pairs may need to be together for multiple seasons prior to reaching various reproductive milestones (nest building, egg laying and incubation, hatching eggs, and rearing chicks to fledge). Some first year pairs were split up and re-paired, particularly if there were clear behavioral indicators of incompatibility observed, such as observations of highly concerning or persistent aggression (e.g., fighting), which can compromise the safety of the birds. However, it is generally unclear whether unsuccessful mates should be separated to find more suitable mates in an effort to improve breeding outcomes. All pairs reside in open-air aviaries with wire-mesh walls, and most aviaries house a single pair. Throughout our study, pairs remained socialized except when caretakers occasionally moved one member of a pair into a separate, but adjacent aviary compartment (within the same building) to administer medication to a sick bird or to address pair compatibility issues, such as severe or persistent aggression.
Reproductive data recorded
‘Alalā commonly lay 2-3 eggs per clutch, producing 2-3 clutches per breeding season, unless the pair is successful at hatching and rearing nestling(s) from their first clutch. Caretakers monitored all breeding activities in-person during daily husbandry and by closed-circuit television. ‘Alalā pairs were provided with 2-5 nest building platforms (Figure 2) in addition to an assortment of nest materials such as sticks, grasses, and coconut fibers. Throughout our study, caretakers recorded nest progress data to capture whether each pair had placed no sticks, a few sticks, many sticks, or constructed a nest with a visible nesting cup, on one or more nest platforms in their aviary, at least three times per week, beginning March 1st, until females laid their first clutch. Nests with eggs were assigned a discrete, ordinal nest quality score, ranging from 1 (worst nest; essentially no attempt at nest building) to 5 (best nest) at the time of lay (Flanagan et al., 2023). In addition to collecting data on nest progress and nest quality, caretakers monitored and recorded data on egg laying, hatching, and nestling survival. Because the conservation breeding management approach taken throughout our study was centered on parental breeding, we did not examine most eggs for signs of fertility to minimize human disturbance.

Figure 2 Examples of the nest building platforms offered to ‘Alalā. Dimensions vary among platform designs, with maximum widths ranging from 0.4-1.1 m.
Reproductive outcomes evaluated
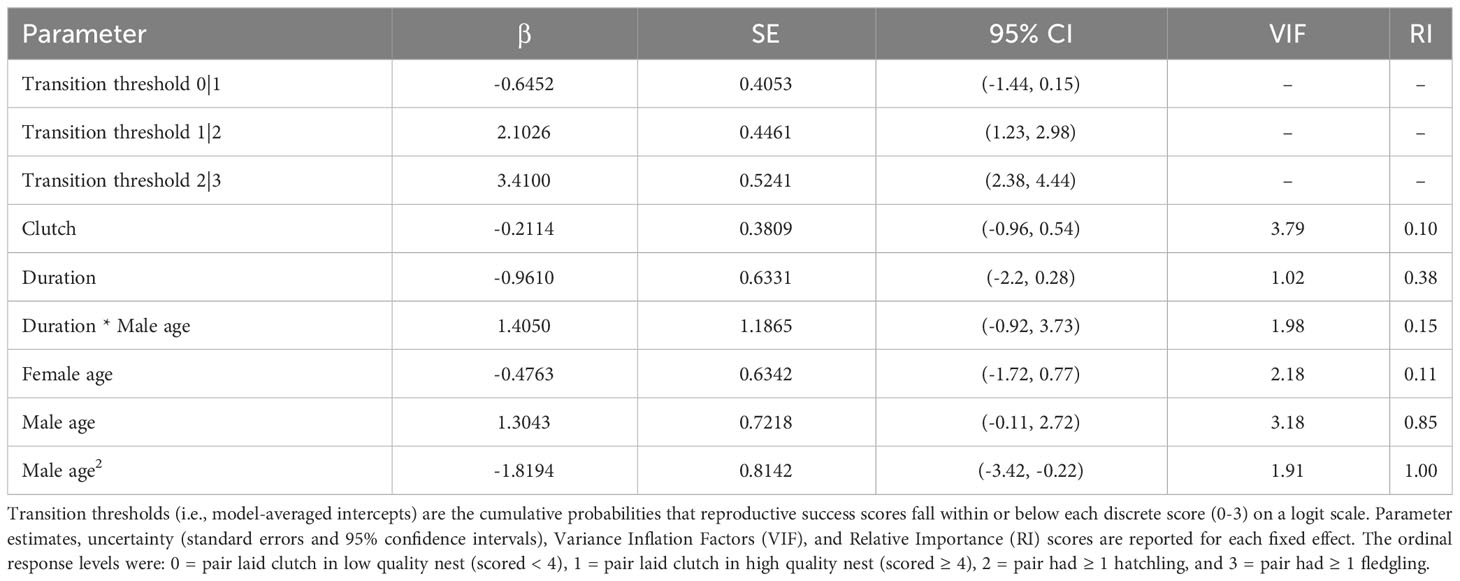
We tested whether pair duration predicted nest building, production of one or more clutches, and overall reproductive success (in pairs with a minimum of one clutch). We examined potential pair duration effects on nest building to capture evidence of breeding behaviors, or lack thereof, particularly in pairs that did not necessarily produce clutches (but pairs with clutches were also included in this analysis). We operationalized nest building attempts as nests containing a minimum of “many sticks.” Although we currently do not have sufficient data to formally analyze differences in reproductive outcomes associated with the various nest platform types provided, preliminary assessments suggest that nest building behaviors do not vary systematically with platform type. Because not all females consistently lay from year-to-year, we also investigated potential pair duration effects on the probability of clutch production. Overall reproductive success was measured by assigning each pair a discrete, ordinal “success” score ranging from 0-3: 0 = pair laid a clutch of eggs in a low-quality nest (scored < 4), 1 = pair laid a clutch of eggs in a high-quality nest (scored ≥ 4), 2 = pair had ≥ 1 hatchling, and 3 = pair had ≥ 1 fledgling (at ~ 60 days after hatch).
Statistical analyses
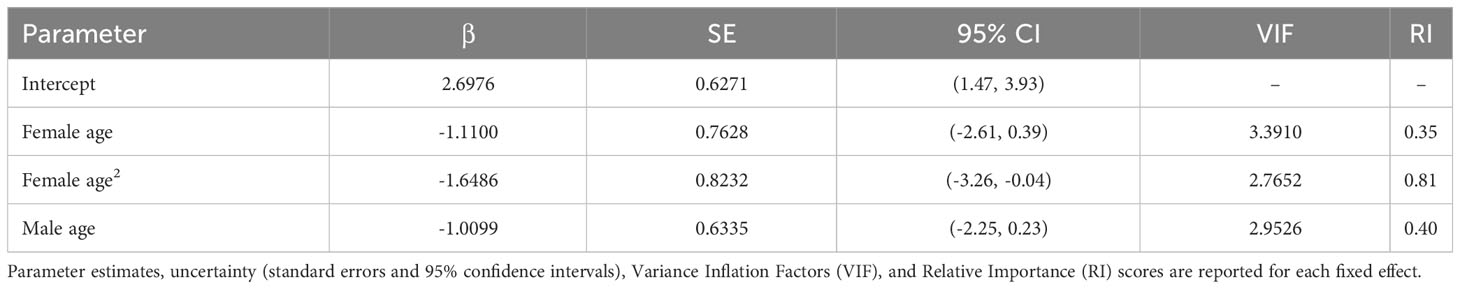
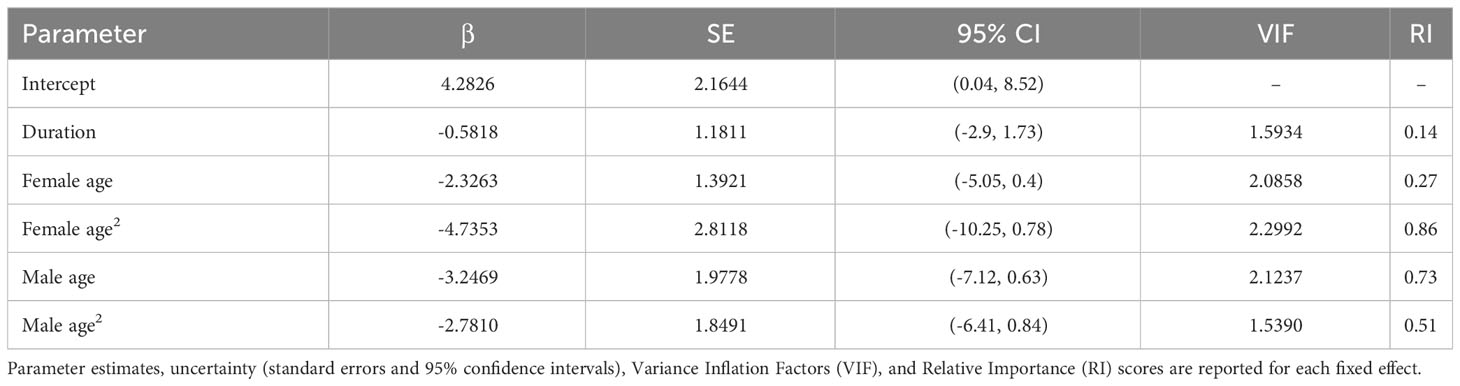
All of our analyses were conducted in R Studio (R Core Development Team, 2023). We constructed global models for the nest building (n = 161 observations, 75 dyads, and 4 breeding seasons), clutch production (n = 164 observations, 75 dyads, and 4 breeding seasons), and overall reproductive success analyses (n = 168 observations, 52 dyads, and 4 breeding seasons). All global models included breeding season (year) and dyad (pair identity) as random effects. We ran separate models with pair duration as a numeric fixed effect (number of years paired) and as a binary fixed effect (i.e., whether a pair had ≥ 1 prior breeding season together), the latter of which was intended to capture if simply having experience together as a pair impacts reproductive outcomes (vs. the temporal extent of experience or years paired). To incorporate potential age effects, we included the age of the birds and age2, based on the assumption that reproductive outcomes may vary nonlinearly with age, in addition to age × pair duration interaction terms. In addition to the age covariates, we included clutch number in the model of overall reproductive success to account for the possibility that earlier/later clutches may be associated with varying levels of reproductive success (e.g., in terms of hatching or nestling survival to fledge). All fixed effects were standardized with the arm package (Gelman and Su, 2018), and multicollinearity was evaluated with variance inflation factors (VIF), calculated in the car package (Fox and Weisberg, 2011). We used binomial generalized linear mixed models (GLMMs) for the analyses of nest building and clutch production, both fitted with a logit link function. As the reproductive success scores were ordinal, we used a cumulative linked mixed model (CLMM) for this analysis. We checked model assumptions with the DHARMa (Hartig, 2022) and ordinal (Christensen, 2018) packages for the nest building and clutch production GLMMs and the reproductive success CLMM, respectively.
The set of submodels utilized in model averaging were derived from the global models using the dredge function in the MuMIn package (Barton, 2018). Model averaging included all submodels within 2 AICc of the most parsimonious model (i.e., the model with the lowest AICc score), and was conducted with the natural average method. We used the relative importance (RI) scores generated from model averaging to guide inferences made from the results, which we limited to fixed effects with high RI scores (≳ 0.8).
Results
‘Alalā pair duration ranged from 0 to 10 consecutive breeding seasons across the dyads included in our study (2.5 ± 0.2 SE). Males and females in our study were 2-19 (9.6 ± 0.3 SE) and 2-20 (8.9 ± 0.3 SE) years old, respectively. We removed year as a random effect from the global model of nest building because near 0 variance was associated with this term, causing model singularity. Male age × pair duration was removed from our clutch production analysis (with pair duration as a numeric effect), as this interaction term had VIF > 5; however, we were able to retain these interactions in the model with pair duration as a binary fixed effect (all fixed effects had VIF < 5 in this analysis).
We did not find any relationships between pair duration and nest building, clutch production, or overall reproductive success (Tables 1–3), regardless of whether pair duration was treated as a numeric (0-10 years) or binary (pair had some or no experience together in consecutive breeding seasons) fixed effect. As such, the results presented here are from the models that utilized pair duration as a numeric effect, but the results from models with a binary structure for pair duration are provided in the Supplementary Material, in addition to all submodels used in model averaging.
Although we did not detect relationships between pair duration and reproductive outcomes, we found some evidence suggesting female age impacted the probability of nest building and laying ≥ 1 clutch (Tables 1, 2; Figure 3). Specifically, the probability of nest building and clutch production was relatively high (near 1) and stable as females aged from 2 to ~ 12 years old, declining sharply thereafter. For pairs with a minimum of one clutch, we found that male age had an important effect on overall reproductive success (Table 3; Figure 4). Reproductive success scores increased as males aged from 2 to ~ 9 years old (across all ordered success score categories), reached a peak and an asymptote with males ≳ 9 to ~ 13 years old, and subsequently decreased in males ≳ 13 years old (Figure 4).
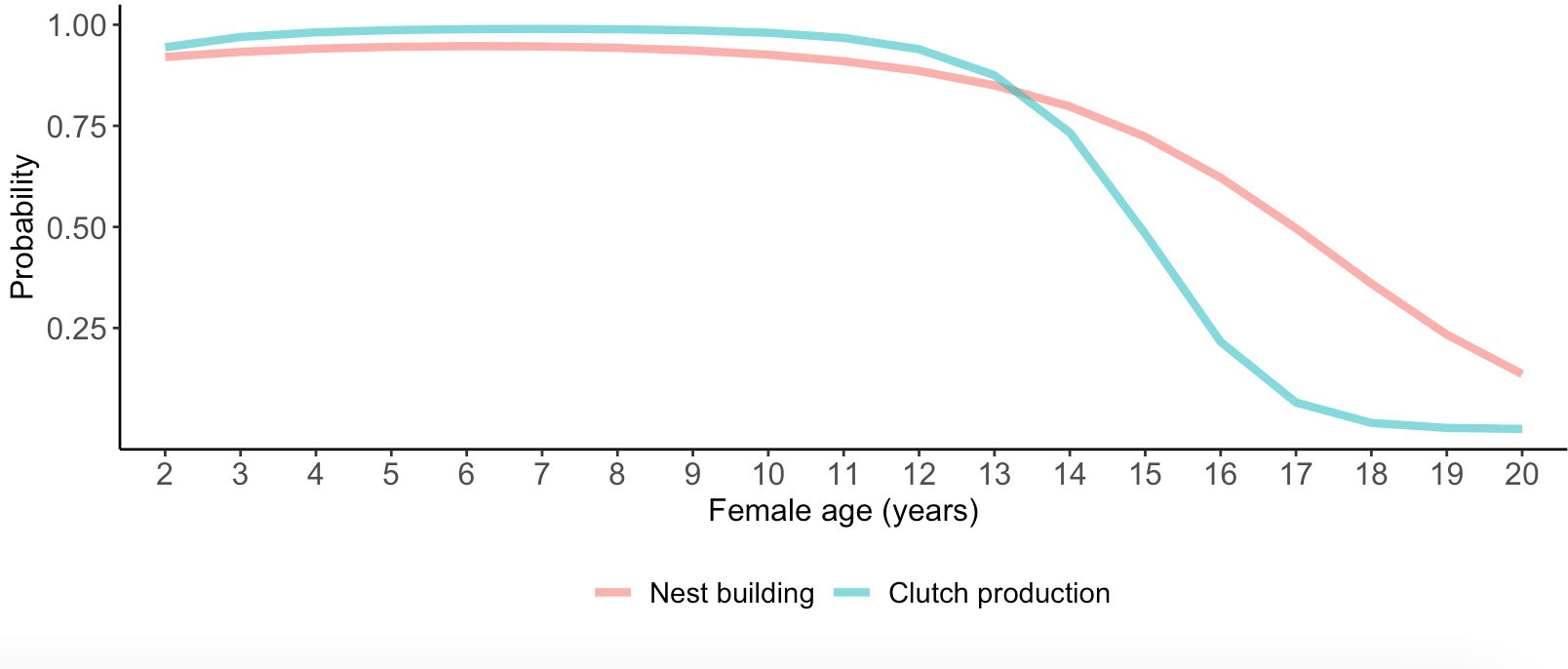
Figure 3 Female age effects on the probability of laying ≥ 1 clutch. Predictions in this figure were calculated using the model-averaged intercept and slopes of age and age2 to illustrate quadratic age effects, and back-transformed using the invlogit function to facilitate interpretability (Gelman and Su, 2018). The probability estimates were calculated with all other parameters in the model being at their means.
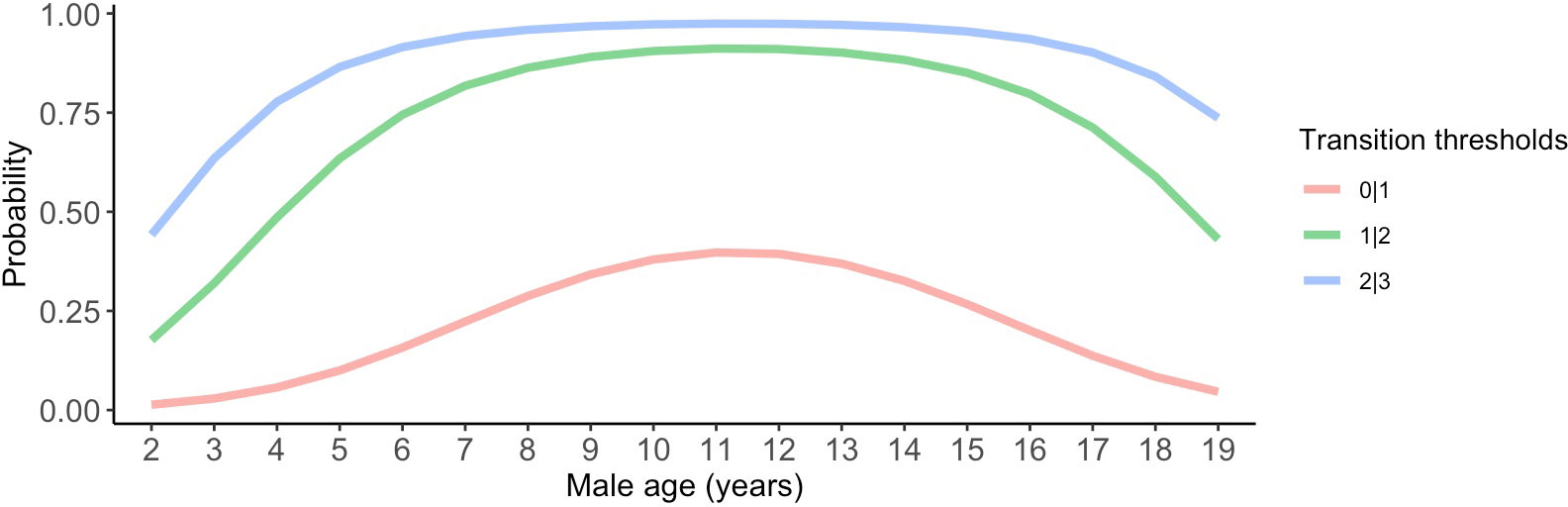
Figure 4 Male age effects on reproductive success scores. Predictions in this figure were calculated using the model-averaged intercept and slopes of age and age2 to illustrate quadratic age effects, and back-transformed using the invlogit function to facilitate interpretability (Gelman and Su, 2018). Ordinal response levels include: 0 = pair produced a clutch in a low-quality nest (scored < 4), 1 = pair produced a clutch in a high-quality nest (scored ≥ 4), 2 = pair had ≥ 1 hatchling, and 3 = pair had ≥ 1 fledgling. The probability estimates were calculated with all other parameters in the model held at their means.
Discussion
We tested whether pair duration in the critically endangered ‘Alalā influenced reproductive outcomes across four breeding seasons during which a parental breeding management approach was adopted for the species. Our results clearly show that pair duration did not impact the probability of nest building, clutch production, or downstream reproductive outcomes, including the successful rearing of nestlings to fledge. Although senescence was not the focus of our study, we found that female age influenced the probability of nest building and clutch production, and male age affected overall reproductive success.
Our results suggesting that pair duration is a poor predictor of reproductive outcomes are, at first glance, somewhat surprising given that the ‘Alalā is a monogamous species, with a history of lifetime pair bonds in the wild (Banko et al., 2002). However, it is important to keep in mind that all ‘Alalā pairs in the breeding program were practitioner-selected. Moreover, detecting pair duration effects on reproductive outcomes may be difficult given the dataset analyzed. For example, pair duration was not an experimental treatment assigned randomly to ‘Alalā pairs, so our conclusions may be confounded by other factors related to caretaker decisions to split pairs. There are two fundamental reasons why caretakers may decide to split a pair: behavioral signs of incompatibility such as aggression and failure to demonstrate reproductive behavior or output. To the extent that a bias exists in these decisions, the bias would support leaving pairs together for longer periods of time if they have greater success. It is difficult to disentangle cause and effect, as pairs may be left together for longer periods of time because they are reproductively successful, or pairs may be reproductively successful because they are left together for longer periods of time. However, this potential bias actually makes our inferences about the management decisions more robust, as the bias should skew the data in favor of higher reproductive success for pairs kept together for longer periods. Yet, our results do not support this hypothesis and in fact we found no relationship between pair duration and reproductive success despite having the odds stacked in favor of higher reproductive success with longer pair duration. Our main conclusion that there is little to be gained from leaving unsuccessful pairs together is therefore more strongly supported in the face of this bias from confounding variables. Moreover, pair duration may not translate well as a “pair bond” and may therefore provide an inaccurate measure of mate compatibility, nor does it capture the biological effects derived from familiarity. In the context of conservation breeding, familiarity may be unimportant, or masked by, the effects of incompatibility, particularly if many pairs in the flock simply tolerate one another but are not motivated to mate, incubate, and/or rear chicks as a pair. Ongoing work on mate choice and compatibility in this population will provide valuable information on improving the compatibility of pairs in the flock (Greggor et al., unpublished data). Another extension of this work could involve testing whether the amount of time that has passed since an individual has been separated from its partner, without immediate re-pairing, influences its ability to form a strong preference and/or stable bond with a new mate (e.g., Harbert et al., 2020). However, all females in our care are immediately re-paired after any mate separations, so this test would only be relevant to the males in our population (i.e., because there are more males than females in the flock).
Although age was not the focus of this study, we detected relationships between age and reproductive outcomes. The probabilities of nest building and clutch production were similar in pairs with females from 2 to 12 years old. However, nest building and clutch production probabilities notably declined after females reached ~ 13 years old. Moreover, male age influenced overall reproductive success in pairs with a minimum of one clutch: pairs with males aged ≳ 9 to ~ 13 years attained higher reproductive success scores compared to pairs with males from 2 to ~ 8 years old and pairs with older males, ≳ 13 years old. Our findings therefore suggest that female senescence impacts the earlier stages of reproduction, and male age has a role in influencing nest quality and downstream reproductive success, including hatching and nestling survival to fledge, perhaps due to their role in assisting their mates with nest building, feeding females at the nest during the egg incubation and rearing stages, and cooperatively rearing nestlings. There is support for these findings demonstrating the importance of age in the literature. Recent work with monogamous mountain chickadees (Poecile gambeli) showed that age or an individual’s breeding experience (vs. pair duration) affected parental investment; more experienced pairs produced eggs earlier and raised heavier chicks compared to inexperienced pairs (Pitera et al., 2021). This finding was largely driven by female age/experience, since experienced females initiated egg laying earlier and laid larger clutches than inexperienced breeding females (Pitera et al., 2021). Similarly, reproductive performance of brown thornbills (Acanthiza pusilla) improved with age but not with repeated breeding attempts with the same partner (Green, 2001). Researchers have proposed several restraint hypotheses (cost of reproduction, residual reproductive value hypotheses) and constraint hypotheses (selection, breeding experience, and breeding age hypotheses) to explain the pattern that performance improves with age until “middle age” (reviewed in Robertson and Rendell, 2001). For example, it is possible that birds acquire critical, non-breeding-related skills as they age (until they reach senescence or otherwise reach older age with reduced breeding experience), such as self-maintenance or foraging (Robertson and Rendell, 2001). However, given that the birds in our study do not need to compete for food with conspecifics (aside from with their mate), this is an unlikely scenario (Griggio and Hoi, 2011). Alternatively, or additionally, younger females could balance the costs of current reproduction against the probability of future reproduction, resulting in lower reproductive success when they are younger (reviewed in Fowler, 1995). Moreover, in our study, after age 7-12 for females and ≳ 13 for males, reproductive success declined; “middle age” reproductive success decline has been well-documented in other bird species (e.g., short-tailed shearwater (Puffinus tenuirostris), Wooller et al., 1990; Seychelles warbler (Acrocephalus sechellen), Komdeur, 1996). Here, we did not explore the influence of breeding experience on reproductive outcomes, but future work could categorize pairs based on breeding experience (e.g., experienced-experienced, inexperienced-inexperienced, and experienced-inexperienced), following an approach similar to Lv et al. (2016), to disentangle the effects of breeding experience and age.
The findings of our study have several important implications for the ‘Alalā conservation breeding program. First, increasing compatibility across a higher proportion of the pairs in the flock is paramount. It is possible that long-term pairings, given the monogamous mating system of the species, could increase reproductive success if the potential mates selected are indeed keen to breed. The problem of having too many moderately compatible or incompatible pairs in the program is a challenge that we hope to help resolve through ongoing mate choice studies (Greggor et al., unpublished data). Moreover, since our findings indicate that pair duration does not lead to higher productivity, birds can be more aggressively re-paired with other potential mates. While we aim to have all pairs established ahead of each breeding season, going forward, we may take a last-minute pivot approach to pairing, by finding more suitable mates for members of newly selected pairs that are not exhibiting promising signs of breeding early in the breeding season (such as nest building or at least some potential pair bonding behaviors such as perch sharing, allopreening, and allofeeding). Moreover, we recommend that age be integrated into the ‘Alalā pair selection process, to the fullest extent possible, based on the results of this study. Although releasing pairs has been considered as a future option, there are currently no immediate plans to release established ‘Alalā pairs to the wild. Of course, reproduction in the wild comprises a vastly different system than breeding in human care; thus, we suggest that, in addition to routine monitoring, researchers study any future released pairs to understand if there are pair duration effects on reproductive success in the wild.
Our findings with ‘Alalā broadly highlight the importance of testing the effectiveness of mate selection practices in other conservation breeding programs to ensure that practitioners have the information they need to make evidence-based decisions for their unique breeding programs. It would appear that leaving unsuccessful ‘Alalā pairs together long-term takes the form of a Concorde Fallacy (i.e., sunk cost fallacy) (sensu Curio, 1987): clearly there is no empirical rationale to avoid “wasting” previous investment in establishing a pair, as it has little predictive value of future success. We suspect that many practitioners in avian conservation breeding programs with species that have similar life history characteristics as ‘Alalā are reluctant to separate mated pairs, fearing the loss of investment in developing a pair bond. We suspect equally that these decisions are too frequently made without sufficient evidence. As ex situ conservation assumes a more prominent role in the Anthropocene extinction crisis (Dirzo et al., 2014), it is incumbent upon us to develop efficient and effective breeding programs. These programs are costly conservation tools (Conde et al., 2011), and judicious decision-making is required before commencing an ex situ conservation program (McGowan et al., 2017). Once established, these programs need to produce offspring to fulfill their roles as assurance populations and as sources for translocation, or risk losing support and funding. Researchers can help bridge the science-practitioner gap (Beier et al., 2017; Greggor et al., 2021) by conducting thorough analyses of the data available in many of these breeding programs, determining what is working and what is not; this is the best path toward a more evidence-based ex situ conservation strategy and practice that can contribute optimally to broader conservation goals.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The data used in this study was collected as part of the ongoing ‘Alalā conservation breeding program. ‘Alalā conservation breeding is presently conducted under U.S. Fish and Wildlife Service permit TE060179-6, State of Hawai‘i Department of Land and Natural Resources permit WL21-08, and San Diego Zoo Wildlife Alliance IACUC 22-011. The animal study was approved by the San Diego Zoo Wildlife Alliance Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
LB: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing, Investigation. AF: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing, Investigation. BM: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – review & editing, Supervision. RS: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The National Fish and Wildlife Foundation, Dr. and Mrs. Richard Robbins representing the Max and Yetta Karasik Family Foundation, and anonymous donors provided financial support to conduct studies to improve conservation breeding outcomes. Financial support, particularly for the conservation breeding of ‘Alalā was provided by the U.S. Fish and Wildlife Service, State of Hawai’i Division of Forestry and Wildlife, anonymous donors, and San Diego Zoo Wildlife Alliance.
Acknowledgments
We are very grateful to current and former ‘Alalā caretakers without whom this work would not have been possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the opinions or policies of the U.S. Government or the National Fish and Wildlife Foundation and its funding sources. Mention of trade names or commercial products does not constitute their endorsement by the U.S. Government, or the National Fish and Wildlife Foundation or its funding sources.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2024.1303239/full#supplementary-material
References
Ausband D. E. (2019). Pair bonds, reproductive success, and rise of alternate mating strategies in a social carnivore. Behav. Ecol. 30, 1618–1623. doi: 10.1093/beheco/arz126
Ballou J., Lees C., Faust L., Long S., Lynch C., Lackey L., et al. (2010). “Demographic and genetic management of captive populations,” in Wild Mammals in Captivity. Eds. Kleiman D., Thompson K., Kirk-Baer C. (Chicago: University of Chicago Press).
Banko P. C., Ball D. L., Banko W. E. (2002). “Hawaiian crow Corvus hawaiiensis,” in The Birds of North America. Eds. Poole A., Gill F. (Ithaca, NY, USA: Cornell Lab of Ornithology).
Barton K. (2018). MuMIn: Multi-Model Inference. R package version 1.42.1. Available at: https://CRAN.R-project.org/package=MuMIn.
Beier P., Hansen L. J., Helbrecht L., Behar D. (2017). A how-to guide for coproduction of actionable science. Conserv. Lett. 10 (3), 288–296. doi: 10.1111/conl.12300
Black J. M. (1996). “Introduction: pair bonds and partnerships,” in Partnerships in Birds: The Study of Monogamy. Ed. Black J. M. (Oxford: Oxford University Press), 3–20.
Calkins E. S., Fuller T. K., Asa C. S., Sievert P. R., Coonan T. J. (2013). Factors influencing reproductive success and litter size in captive island foxes. J. Wildlife Manage. 77, 346–351. doi: 10.1002/jwmg.492
Christensen R. H. B. (2018). ordinal - Regression Models for Ordinal Data. R package version 2018.4-19. Available at: http://www.cran.r-project.org/package=ordinal/.
Clum N. J. (1995). Effects of aging and mate retention on reproductive success of captive female peregrine falcons. Integr. Comp. Biol. 35, 329–339. doi: 10.1093/icb/35.4.329
Conde D. A., Flesness N., Colchero F., Jones O. R., Scheuerlein A. (2011). An emerging role of zoos to conserve biodiversity. Science 331, 1390–1391. doi: 10.1126/science.1200674
Curio E. (1987). Animal decision-making and the ‘Concorde fallacy’. Trends Ecol. Evol. 2 (6), 148–152. doi: 10.1016/0169-5347(87)90064-4
Dirzo R., Young H. S., Galetti M., Ceballos G., Isaac N. J. B., Collen B. (2014). Defaunation in the anthropocene. Science 345, 401–406. doi: 10.1126/science.1251817
Ens B. J., Safriel U. N., Harris M. P. (1993). Divorce in the long-lived and monogamous oystercatcher, Haematopus ostralegus: incompatibility or choosing the better option? Anim. Behav. 45, 1199–1217. doi: 10.1006/anbe.1993.1142
Faust K. M., Goldstein M. H. (2021). The role of personality traits in pair bond formation: pairing is influenced by the trait of exploration. Behaviour 158, 447–478. doi: 10.1163/1568539X-bja10076
Flanagan A. M., Masuda B., Komarczyk L., Kuhar A., Farabaugh S., Swaisgood R. R. (2023). Adapting conservation breeding techniques using a data-driven approach to restore the ‘Alalā (Hawaiian crow, Corvus hawaiiensis). Zoo Biol. 42, 834–839. doi: 10.1002/zoo.21794
Fowler G. S. (1995). Stages of age-related reproductive success in birds: Simultaneous effects of age, pair-bond duration, and reproductive experience. Amer. Zool. 35, 318–328.
Fox J., Weisberg S. (2011). An R companion to Applied Regression. 2nd ed. (Thousand Oaks, CA: Sage).
Gelman A., Su Y. S. (2018). arm: Data Analysis Using Regression and Multilevel/Hierarchical Models. R package version 1.10-1. Available at: https://CRAN.R-project.org/package=arm.
Green D. J. (2001). Nordic Society Oikos The Influence of Age on Reproductive Performance in the Brown Thornbill Published by: Blackwell Publishing on behalf of Nordic Society Oikos influence of age on reproductive performance in the Brown Thornbill. Oikos 32, 6–14.
Greggor A. L., Berger-Tal O., Swaisgood R. R., Cooke S. J., DeVault T. L., Fernandez-Juricic E., et al. (2021). Using change models to envision better applications of animal behavior research in conservation management and beyond. Front. Conserv. Sci. 2 (7). doi: 10.3389/fcosc.2021.653056
Greggor A. L., Vicino G. A., Swaisgood R. R., Fidgett A., Brenner D., Kinney M. E., et al. (2018). Animal welfare in conservation breeding: Applications and challenges. Front. Vet. Sci. 5, 1–6. doi: 10.3389/fvets.2018.00323
Griggio M., Hoi H. (2011). An experiment on the function of the long-term pair bond period in the socially monogamous bearded reedling. Anim. Behav. 82, 1329–1335. doi: 10.1016/j.anbehav.2011.09.016
Halimubieke N., Kupán K., Valdebenito J. O., Kubelka V., Carmona-Isunza M. C., Burgas D., et al. (2020). Successful breeding predicts divorce in plovers. Sci. Rep. 10. doi: 10.1038/s41598-020-72521-6
Harbert K. J., Pellegrini M., Gordon K. M., Donaldson Z. R. (2020). How prior pair-bonding experience affects future bonding behavior in monogamous prairie voles. Horm. Behav. 126. doi: 10.1016/j.yhbeh.2020.104847
Hartig F. (2022). DHARMa - Residual Diagnostics for Hierarchical (Multi-Level/Mixed). R package version 0.4.6. Available at: https://CRAN.R-project.org/package=DHARMa.
Heinrichs J. A., McKinnon D. T., Aldridge C. L., Moehrenschlager A. (2019). Optimizing the use of endangered species in multi-population collection, captive breeding and release programs. Glob. Ecol. Conserv. 17, e00558. doi: 10.1016/j.gecco.2019.e00558
Ihle M., Kempenaers B., Forstmeier W. (2015). Fitness benefits of mate choice for compatibility in a socially monogamous species. PloS Biol. 13, e1002248. doi: 10.1371/journal.pbio.1002248
Ihle M., Pick J. L., Winney I. S., Nakagawa S., Schroeder J., Burke T. (2019). Rearing success does not improve with apparent pair coordination in offspring provisioning. Front. Ecol. Evol. 7. doi: 10.3389/fevo.2019.00405
Ivy J., Lacy R. C. (2012). A comparison of strategies for selecting breeding pairs to maximize genetic diversity retention in managed populations. J. Hered. 103, 186–196. doi: 10.1093/jhered/esr129
Kelley J. L., Graves J. A., Magurran A. E. (1999). Familiarity breeds contempt in guppies. Nature 401, 661–662. doi: 10.1038/44314
Komdeur J. (1996). Influence of age on reproductive performance in the Seychelles warbler. Behav. Ecol. 7 (4), 417–425. doi: 10.1093/beheco/7.4.417
Kuehler C., Harrity P., Lieberman A., Kuhn M. (1995). Reintroduction of hand-reared alala Corvus hawaiiensis in Hawaii. Oryx 29, 261–266. doi: 10.1017/S0030605300021256
Leu S. T., Burzacott D., Whiting M. J., Bull C. M. (2015). Mate familiarity affects pairing behaviour in a long-term monogamous lizard: evidence from detailed bio-logging and a 31-year field study. Ethology 121, 760–768. doi: 10.1111/eth.12390
Lv L., Komdeur J., Li J., Scheiber I. B. R., Zhang Z. (2016). Breeding experience, but not mate retention, determines the breeding performance in a passerine bird. Behav. Ecol. 27, 1255–1262. doi: 10.1093/beheco/arw046
Martin-Wintle M. S., Shepherdson D., Zhang G., Huang Y., Luo B., Swaisgood R. (2017). Do opposites attract? Effects of personality matching in breeding pairs of captive giant pandas on reproductive success. Biol. Conserv. 207, 1–12. doi: 10.1016/j.biocon.2017.01.010
Martin-Wintle M. S., Wintle N. J. P., Díez-León M., Swaisgood R. R., Asa C. S. (2019). Improving the sustainability of ex situ populations with mate choice. Zoo Biol. 38, 119–132. doi: 10.1002/zoo.21450
McGowan P. J. K., Traylor-Holzer K., Leus K. (2017). IUCN guidelines for determining when and how ex situ management should be used in species conservation. Conserv. Lett. 10, 361–366. doi: 10.1111/conl.12285
Montgomery M. E., Ballou J. D., Nurthen R. K., England P. R., Briscoe D. A., Frankham R. (1997). Minimizing kinship in captive breeding programs. Zoo Biol. 389, 377–389. doi: 10.1002/(SICI)1098-2361(1997)16:5<377::AID-ZOO1>3.0.CO;2-7
Munson A. A., Jones C., Schraft H., Sih A. (2020). You’re just my type: mate choice and behavioral types. Trends Ecol. Evol. 35, 823–833. doi: 10.1016/j.tree.2020.04.010
Pitera A. M., Branch C. L., Sonnenberg B. R., Benedict L. M., Kozlovsky D. Y., Pravosudov V. V. (2021). Reproduction is affected by individual breeding experience but not pair longevity in a socially monogamous bird. Behav. Ecol. Sociobiol 75. doi: 10.1007/s00265-021-03042-z
R Core Development Team. (2023). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org.
Robertson R. J., Rendell W. B. (2001). A long-term study of reproductive performance in tree swallows: the influence of age and senescence on output. J. Anim. Ecol. 70, 1014–1031. doi: 10.1046/j.0021-8790.2001.00555.x
Smetzer J. R., Greggor A. L., Paxton K. L., Masuda B., Paxton E. H. (2021). Automated telemetry reveals post-reintroduction exploratory behavior and movement patterns of an endangered corvid, ‘Alalā (Corvus hawaiiensis) in Hawaii, USA. Glob. Ecol. Conserv. 26. doi: 10.1016/j.gecco.2021.e01522
Smith B. R., Blumstein D. T. (2008). Fitness consequences of personality: a meta-analysis. Behav. Ecol. 19, 448–455. doi: 10.1093/beheco/arm144
Spoon T. R., Millam J. R., Owings D. H. (2006). The importance of mate behavioural compatibility in parenting and reproductive success by cockatiels, Nymphicus hollandicus. Anim. Behav. 71, 315–326. doi: 10.1016/j.anbehav.2005.03.034
Spoon T. R., Millam J. R., Owings D. H. (2007). Behavioural compatibility, extrapair copulation and mate switching in a socially monogamous parrot. Anim. Behav. 73, 815–824. doi: 10.1016/j.anbehav.2006.10.010
Spoon T. R., Millam J. R., Owings D. H. (2016). Variation in the stability of cockatiel (Nymphicus hollandicus) pair relationships: the roles of males, females, and mate compatibility. Behaviour 141, 1211–1234. doi: 10.1163/1568539042729711
Keywords: mate compatibility, extinct in the wild, mate selection, pair bond, mate retention, aviculture
Citation: Barrett LP, Flanagan AM, Masuda B and Swaisgood RR (2024) The influence of pair duration on reproductive success in the monogamous ‘Alalā (Hawaiian crow, Corvus hawaiiensis). Front. Conserv. Sci. 5:1303239. doi: 10.3389/fcosc.2024.1303239
Received: 27 September 2023; Accepted: 08 January 2024;
Published: 25 January 2024.
Edited by:
Eliana Pintus, Czech University of Life Sciences Prague, CzechiaReviewed by:
Ali T. Qashqaei, Borderless Wildlife Conservation Society, IranVittorio Baglione, University of León, Spain
Copyright © 2024 Barrett, Flanagan, Masuda and Swaisgood. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alison M. Flanagan, alflanagan@sdzwa.org
†These authors have contributed equally to this work and share first authorship
 Lisa P. Barrett
Lisa P. Barrett Alison M. Flanagan
Alison M. Flanagan Bryce Masuda
Bryce Masuda  Ronald R. Swaisgood
Ronald R. Swaisgood