Borrowing from the palaeolimnologists toolkit; the use of lake sediment cores in diagnosing the causes of freshwater species decline
- 1Environmental Change Research Centre, Department of Geography, University College London, London, United Kingdom
- 2Conservation Evidence Department, Wildfowl & Wetlands Trust, Gloucestershire, United Kingdom
- 3Life Sciences, Natural History Museum, London, United Kingdom
- 4NatureScot, Great Glen House, Inverness, United Kingdom
Populations of freshwater species are experiencing dramatic declines globally. Tools that facilitate the diagnosis of decline and identify management solutions and/or restoration targets are thus vital. Typically approaches taken to diagnose decline are carried out over short timescales and rely upon identifying spatial associations between presence or abundance of declining species and variables hypothesised to be driving decline. The potential to contextualise observed declines on longer time scales, with a broader range of potential explanatory variables is frequently dismissed, because of a perceived lack of existing long-term data. In this study we explore the value of incorporating a longer-term perspective to decline diagnosis using the common scoter as a case study. The number of scoter breeding in Scotland has declined substantially since the 1970s. Hypotheses for decline include a reduction in macroinvertebrate food available for females and young at the breeding lakes. In this study we apply palaeolimnological techniques to generate standardised, long-term ecological data, enabling us to characterise recent changes at four common scoter breeding lakes. Our results demonstrate that the (macroinvertebrate) food resource of common scoter has, in fact, gradually increased in abundance at all four sites from ca. 1900, and that a further statistically significant increase in macroinvertebrate abundance occurred at ca. 1970. We draw on our palaeolimnological data, to explore alternative hypotheses for common scoter decline. Increases in overall abundance across multiple algal, macrophyte and macroinvertebrate taxa, combined with specific increases in nutrient tolerant taxa, and concurrent declines in nutrient sensitive taxa indicate that the lakes have experienced enrichment within their current oligotrophic state during the last 100 years, and that this trajectory has become more marked during the period of common scoter decline. There is no evidence of changes to habitat, turbidity or increased competition from fish. In the absence of within lake changes that could be detrimental to the benthic (and generalist) feeding common scoter, we conclude that factors outside of the lake, such as increased predation, associated with afforestation in the surrounding area, are the most plausible drivers of common scoter decline. Prioritisation/testing of management solutions that address these issues are indicated.
Introduction
The restoration of threatened freshwater populations is a conservation priority (Albert et al., 2021). Globally, the average abundance of freshwater species has declined by 83% since 1970, far outnumbering those in terrestrial and marine systems (WWF, 2020). Conservation action undertaken to conserve threatened and diminishing populations relies upon diagnosis of the drivers of decline, and identification of appropriate restoration strategies (Caughley, 1994; Sayer et al., 2016b). Conventionally, approaches used to diagnose species decline have focused on spatial associations between species distributions and environmental variables that are considered important (Peery et al., 2004). However, a limitation of this approach is that associations do not equal causality. This can be a particular concern for highly mobile species such as birds, where responses to environmental variables can be driven by settlement patterns rather than direct effects. For instance, in a declining population, birds may prefer to settle in habitats with a particularly favoured environmental characteristic – such as a good food supply – creating an association with sites rich in food. But the food supply might not be the limiting factor and might have little direct effect on the demographic rates of the population.
One way of substantiating the findings from spatial based associations is to undertake experimental manipulations and measure (or model) demographic or behavioural responses (Norris, 2004), but this can be difficult and expensive (Peery et al., 2004). An alternative is to add a temporal perspective and establish whether the onset of decline is also temporally associated with a change in environmental conditions. Not only does the identification of temporal associations validate spatial associations, it also enables the effects of spatially inseparable drivers to be separated, for example where multiple pressures that may be acting upon a single or small number of sites. Temporal data are of most value when species and environmental data are of a high resolution, generated using consistent methods and cover sufficiently long-time scales. The addition of a temporal perspective, derived from long term datasets, is commonly dismissed in species decline diagnoses as it is believed that datasets covering both the temporal range, and the necessary scope of physcio-chemical and biological variables do not exist (Bonebrake et al., 2010; Mihoub et al., 2017). We argue that is a misconception for freshwaters. Such data do exist, however they are locked away in the natural archives of lake sediments, and require an approach known as palaeolimnology to retrieve and interpret them (Battarbee, 1999; Brooks et al., 2012; Gillson and Marchant, 2014; Roberts et al., 2020). Palaeolimnological data are highly standardised, and can cover a range of physico-chemical and biological variables, for time periods ranging from tens to hundreds, or even thousands of years (Willis et al., 2010). We advocate, using the example of the common scoter (Melanitta nigra), that combining spatial and temporal approaches can provide more robust evidence upon which to base decline diagnosis.
The common scoter is a migratory seaduck that breeds close to freshwater lakes in north Scotland. Whilst numbers remain stable in other parts of the north European breeding range (Keller et al., 2020), declines and site abandonment have occurred throughout Scotland and Ireland since the 1970s and 80s, leading to the species being red listed in the UK and Ireland (Stanbury et al., 2021). Common scoter nest close to oligotrophic lakes (Balmer et al., 2013). Geolocator studies in Scotland, Iceland and Norway have shown that common scoters from a single breeding site disperse throughout the wintering range, and are highly site faithful, using the same wintering grounds in successive years and often returning to nest within a few hundred metres of previous nest sites each year (I.K. Peterson pers. comm). The stability of breeding populations in other parts of the range (Keller et al., 2020; Petersen et al., 2021) suggests that the declines observed in Scotland are a consequence of local, breeding ground factors, rather than those associated with wintering grounds.
The breeding stronghold for common scoter in Scotland is the Flow Country in North Scotland, where declines have occurred less rapidly than at other sites (Gregory et al., 2002). An internationally important peatland, the Flow Country extends over 440,000 hectares, and includes hundreds of small (between 1 and 3,371 ha, mean area of 40ha), predominantly acidic, oligotrophic lakes (Underhill et al., 1998; SNH, 2001). Common scoter typically arrive in the Flow Country from their marine wintering areas in April. Groups of males and females initially move between lakes, before pairing and mating, after which the majority of the males return to the sea, whilst the females remain to nest and rear young. Common scoter are diving ducks that feed on benthic invertebrates, from marine environments during the winter and freshwater sources during the breeding season (Cramp and Simmons, 1977; Fox et al., 2003). Chironomids, trichoptera and ephemeroptera are all important components of common scoter diet in freshwaters (Bengtson, 1972; Kondratyev, 1999). Zooplankton, such as cladocera and emerging diptera, such as chironomids, have been shown to be a particularly important diet component for young birds before they are able to dive for sustained periods (Bengtson, 1971; Gardarsson and Einarsson, 1994; Fox, 2003; Gardarsson and Einarsson, 2004). Anecdotal evidence suggests that females breeding in the Flow Country continue to use multiple lakes for feeding prior to hatching. However once hatched, the female and her young are more reliant on a single, or smaller number of lakes until the young have fledged.
Common scoter were first recorded in the Flow Country in the 1850s, with the breeding population in the area reaching its highest numbers (55 pairs) between 1970 and 1980 (Underhill et al., 1998), before experiencing substantial declines to fewer than 20 pairs by 2007 (Eaton et al., 2008). Although common scoter declines are well documented in the Flow Country (Hancock, 1991; Underhill and Hughes, 1996; Hancock and Avery, 1998; Cranswick, 1999), very little data concerning changes in other environmental variables, such as physico-chemical conditions within the lakes, nesting habitat quality/quantity, predator numbers or macroinvertebrate food availability, exist for the time-period of decline, and none as a baseline for the period prior to that. What little data do exist are typically a single survey of a single variable (Lindsay et al., 1988; Coulson et al., 1995; Downie et al., 1998; NatureScot, 2014), and therefore give no insight into temporal trends and/or natural levels of variability. In 2009 a three-year study was commissioned which examined associations between contemporary common scoter lake use and a number of variables that were hypothesised as being ecologically relevant to breeding common scoter (Hancock et al., 2015). The key findings from this study were that i) female and brood presence at a lake was significantly associated with the abundance of large bodied macroinvertebrates, and ii) lakes with a higher abundance of large bodied macroinvertebrates contain more small fish (sticklebacks, Gasterosteus aculeatus) and fewer larger fish (brown trout, Salmo trutta). Hypotheses for common scoter decline were therefore refined to focus more specifically on a possible lack in the macroinvertebrate food resource (Hancock et al., 2020b).
This approach taken to diagnose the decline of the common scoter in Scotland is not unusual, and therefore is an appropriate case study for which we can examine the value of adding a temporal perspective derived from palaeolimnological data.
Palaeolimnology is reliant on the sediments within a lake being deposited in a time-depth sequence, remaining undisturbed until a core is extracted intact for analysis (Smol, 2008). The number of samples generated from a sediment core depends on the resolution at which the core is sub-sampled. Each of these slices or samples represent a time period, and can be analysed for a range of physico-chemical and biological variables, depending on the hypothesis under investigation. Physico-chemical variables such as dry weight, wet density, organic matter content (loss-on-ignition, (LOI)) and heavy metal content (determined by x-ray fluorescence (XRF) analysis) along with radiometric dating techniques such as 210Pb are used to establish that the time-depth sequence of the core is intact -which is particularly important at shallow, wind stressed sites, such as those used by common scoter in the Flow Country.
Biological remains in lake sediments can provide direct evidence of community composition (by reconstructing species composition and abundance), and indirect indications of the physio-chemical conditions (by complementing species composition and abundance with known physio-chemical and ecological tolerances)(Smol et al., 2001). Diatoms (Bacillariophyta) dominate freshwater algal communities and leave robust siliceous valve remains in sediment cores (Battarbee et al., 2001). Diatoms are well-studied, with distinct ecological optima and tolerances, meaning species assemblages can be used to reconstruct a range of chemical conditions including pH and nutrient levels (Bennion et al., 2010; Jones, 2013). Macrofossil analysis involves the examination of both macrophyte and macroinvertebrate remains. Aquatic plants leave a variety of identifiable remains, such as seeds, spores, leaves and fruits (Birks, 2002). Of the macroinvertebrate remains preserved in lake sediments, chironomids and cladocera are perhaps the most well-established, with known species level tolerances for a range of geochemical and biological conditions. The abundance of other macroinvertebrate groups, such as trichopera, ephemeroptera and coleoptera, can also be determined by their chitinous remains and are also commonly used in palaeolimnological research (Smol et al., 2001).
This study data from lake sediment cores is used to i) determine the extent to which macroinvertebrate food availability has changed at the lakes during, and prior to, the period of common scoter declines, ii) reconstruct ecological and physico-chemical change at the lakes since ca. 1900 and identify alternative hypothesise for common scoter decline, and iii) assess the implications of findings from i) and ii) for common scoter decline diagnosis and future conservation management prioritisation.
Materials and methods
Site selection
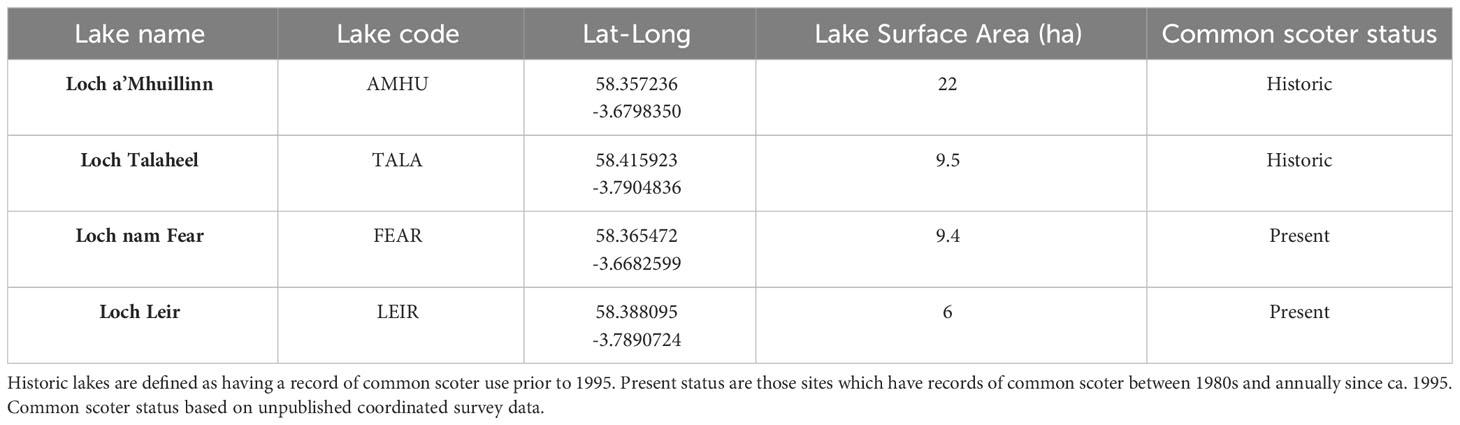
The sites selected for use in this study were chosen based upon several factors. Firstly, we identified lakes which had records of common scoter. The four Flow Country lakes ultimately selected for use in this study all had records of breeding common scoter since coordinated monitoring began in the 1980s and ad-hoc records prior to that. We then refined our list of sites by targeting those for which contemporary ecological and physico-chemical data existed. At 18 of these sites, we established the stratigraphic integrity of the sediments within the lakes by taking short sediment cores and carrying out geochemical analyses (Robson et al., 2019). This analysis showed that, despite concerns about potential surface sediment mixing in these shallow lakes, the stratigraphy (and therefore the chronology) of the sediment in the lakes was intact. The selection of our final four sites, Loch a’Mhuillinn (AMHU), Loch nam Fear (FEAR), Loch Leir (LEIR), Loch Talaheel (TALA) (Table 1), was based on the above in addition to logistical accessibility and ability to represent a mixture of landcover settings. Common scoter breeding numbers at all four study sites have declined since the 1970s. Lakes AMHU and TALA have had only intermittent records of single breeding common scoter 1980s, whereas FEAR and LEIR have annual records of use by multiple breeding birds during the same period.
Fieldwork
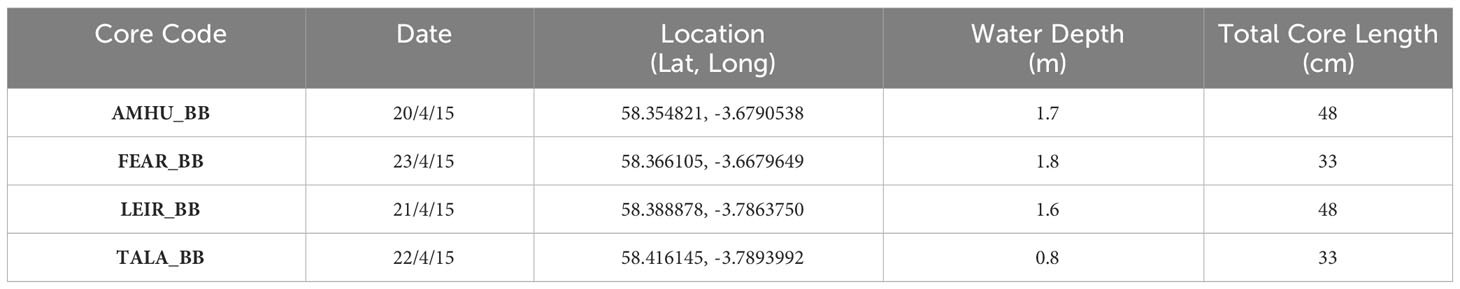
Wide bore (8cm) sediment cores (Patmore et al., 2014) were taken from the lakes using a bespoke piston corer in April 2016. Cores, of between 32 and 47 cm in length, were taken from the littoral zone of each lake (Table 2), and sliced at 0.5cm intervals into sealed plastic bags in the field. Samples were then refrigerated and stored in a cool box for transport back to the laboratory and cold room storage.
Laboratory analysis -geochemical and radiometric
There is potential for sediment resuspension and mixing in shallow, wind stressed lakes, such as our study sites. To ensure cores were not homogonised standard palaeolimnological approaches of loss on ignition (Dean, 1974) and XRF (Boyle, 2001) were first undertaken (see Supplementary Materials).
Radiometric dating (Appleby, 1997) of the cores was undertaken at the Bloomsbury Environmental Isotope Facility (BEIF) at University College London (UCL). The timescale covered by each slice of the cores was established by measuring naturally occurring lead-210 (210Pb) radionuclides in addition to artificially produced Caesium-137 (137Cs) and Americium-241 (241Am) released by nuclear weapons testing and nuclear reactor accidents (Appleby et al., 2023). The accuracy of the dates from the Constant Rate of Supply (CRS) models was further assessed by cross correlation of the LOI’s of short cores and lead profiles generated from the XRF analyses (see Supplementary Materials and (Robson, 2017).
Laboratory analysis -biological indicators
Diatom samples were prepared following standard techniques (Battarbee et al., 2001). Concentration was determined using a known volume of microspheres, and identification (a minimum of 300 valves per sample) was based on (Krammer and Lange-Bertalot, 1986; Camburn and Charles, 2000).
Palaeolimnological analysis of chironomid remains was based on Brooks et al. (2007) and involved the identification (Brooks et al., 2007; Anderson et al., 2013) of larval head capsule remains picked from a known weight of wet sediment, slide mounted in Epural.
The remains of non-chironomid macroinvertebrate and macrophyte taxa are also preserved within lake sediments, although typically in much lower quantities than chironomids or diatoms and therefore a larger amount of sediment is required to be analysed. Macrofossil samples were prepared for analysis using 25-60cm3 of wet sediment (Birks, 2007). The material was gently washed using a stack of two sieves of 355µm and 125µm mesh size, examined under a 4-40x magnification stereomicroscope and identified with reference to (Birks, 2002) and the macrofossil collection at ECRC in UCL.
Statistical analysis and data presentation
Stratigraphic plots were produced using C2 (Juggins, 2007) to visualise the trends in biological indicators over time. Abundances of diatoms, macrofossils and chironomids are expressed as abundance per cm3 of dry sediment per year.
Principal component analysis (PCA) was used to explore patterns of community change over time at each of the lakes. PCA was carried out in Canoco ver5 (ter Braak and Smilauer, 2012) on data that were log transformed and centred by species, PCA was selected as the compositional gradient of these data was 0.5 standard deviation units long (Legendre and Legrendre, 2012).
Statistically significant splits or zones in the stratigraphic plots were determined using constrained incremental sum-of-squares clustering (CONISS) analysis (Grimm, 1987), carried out in R (R Core Team, 2016), using the rioja package (Juggins, 2012). CONISS is based on cluster analysis, constrained by agglomeration of stratigraphically adjacent samples (Birks et al., 2012). A pairwise dissimilarity matrix is first created (in this case using Euclidean distances) and a sum-of-squares statistic calculated for each cluster, which is recalculated as clusters are merged. Stratigraphically adjacent clusters that, when merged, result in the least increase in total dispersion are identified (Legendre and Birks, 2012). The number of significant zones was determined using the broken stick model (Bennett, 1996).
Results
Stratigraphic integrity and radiometric dating of the cores
Loss on ignition and x-ray fluorescence analyses (Supplementary Materials) demonstrated that the material in the cores was not homogenised. Core chronology was determined using 210Pb dating techniques. The chronologies of the cores from AMHU, FEAR and LEIR were confirmed by the cross-correlation with Pb XRF profiles which placed 1900 at between 5 and 8 cm depth for these sites (Figure 1). There was some uncertainty with the 210Pb dates for TALA, it was not possible to fully validate the CRS modelled dates with confidence as it was difficult to identify points for cross correlation in the LOI profiles.
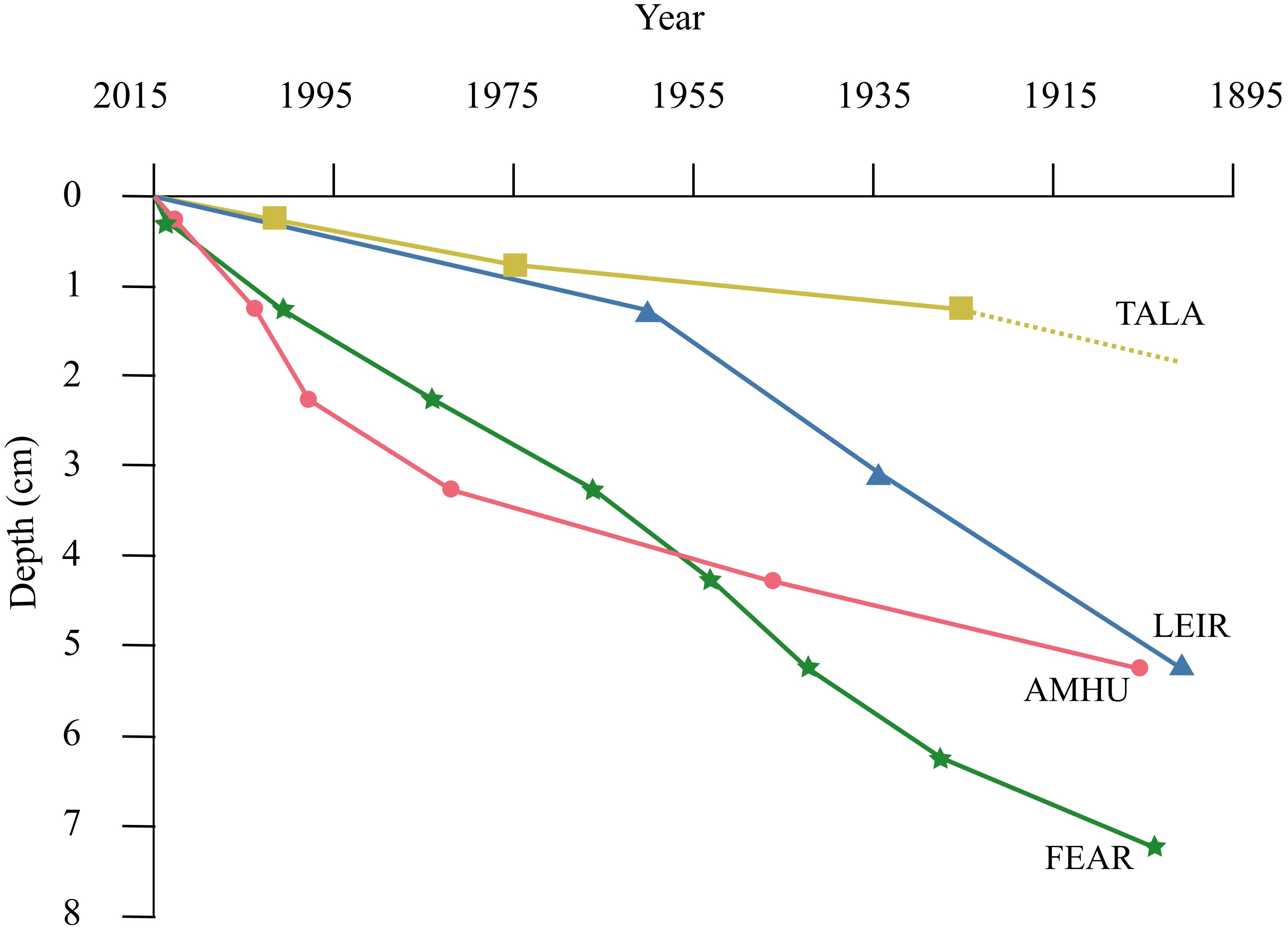
Figure 1 Age-depth profiles of sediment cores from the study lakes, derived from radiometric (210Pb) dating. Depth of 1900 for TALA inferred (dotted line) as radiometric dating was only possible with confidence to 1925 ± 15 years at 1-1.5cm depth.
Macroinvertebrate food availability
Trends in invertebrate taxa that make up a substantial component of common scoter diet are summarised in Figure 2, alongside diatoms and macrophytes (discussed below). Data on other invertebrate groups are provided in the Supplementary Materials.
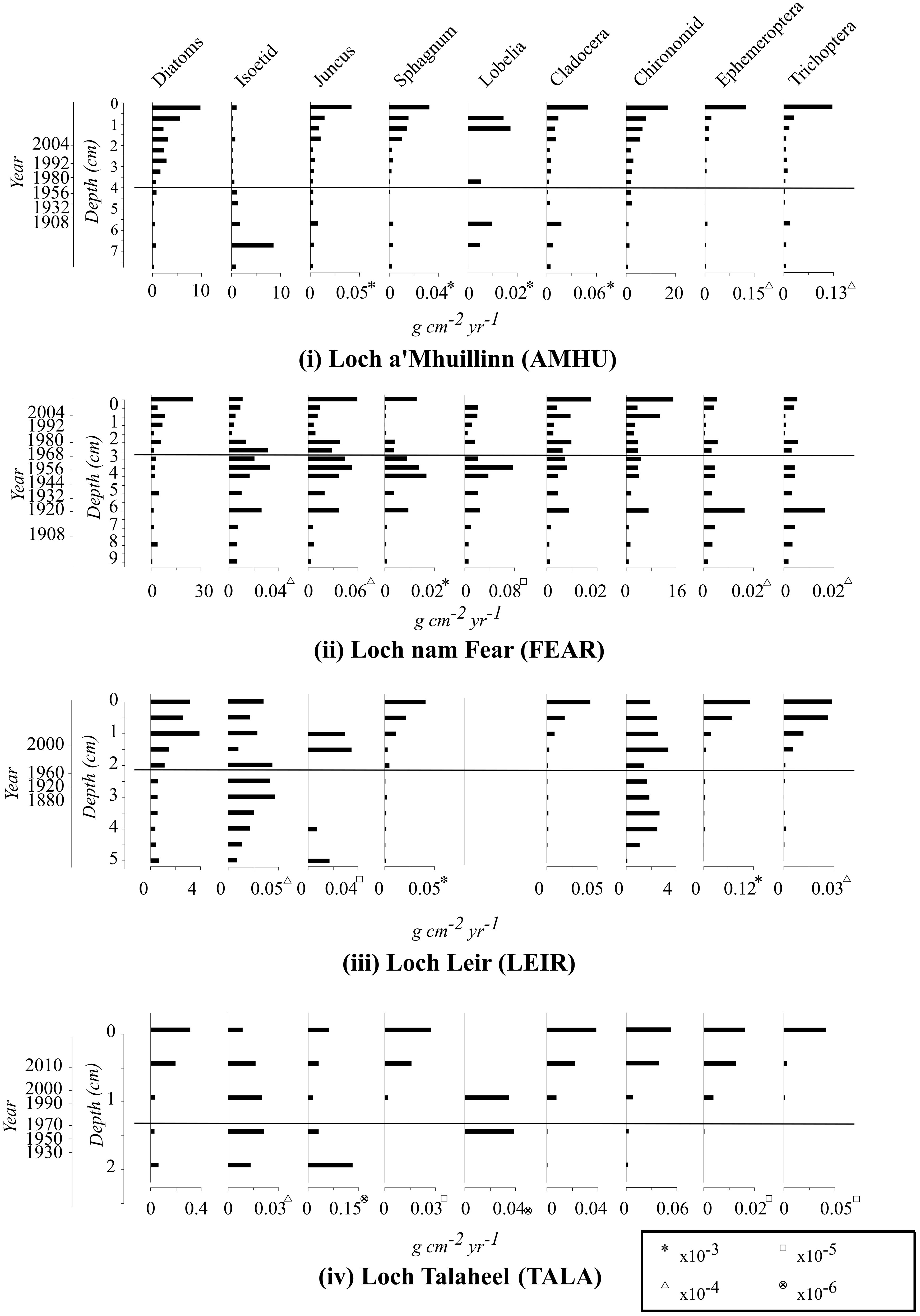
Figure 2 Temporal change in the relative abundance of selected taxa in the four study lakes over the period of increasing and subsequently declining common scoter abundance. Abundance values are mass of preserved material per unit volume of deposited sediment per year. The horizontal line represents the centroid 1970 of the period (1960s-1980s) at which common scoter decline began.
There is no evidence of a reduction in invertebrate food availability for common scoters since the period of common scoter decline in the 1970s. At all four sites invertebrate abundance increased towards the top of the cores (Figure 2). Chironomids, cladocera, ephemeroptera and trichoptera all showed similar, gradual increases at three of the four sites. At FEAR there was a distinct peak in all macroinvertebrate taxa at ca. 1920, following which abundance remained relatively stable or increased. Between-site comparisons indicate that the abundance of macroinvertebrates is lower in TALA relative to the other three lakes.
Long term changes in lake status relative to common scoter abundance
The total abundance of diatoms increased towards the top of all four cores (Figure 2). Diatom species data (Supplementary Materials) indicated that taxa typical of oligotrophic lakes dominate the cores especially small benthic species of Staurosira, Staurosirella and Pseudostaurosira (formerly Fragilaria spp.); Achnanthes, Aulacoseira, Naviculacae and Eunotia species. Commonly occurring Fragilaria sensu lato species include Staurosira construens var. venter, Fragilaria exigua and Staurosira elliptica. Similarly the 16 macrophyte taxa recorded in the cores, including Lobelia dortmanna, Najas flexilis, Littorella uniflora and aquatic mosses such as Fontinalis antipyretica (Supplementary Materials) are all commonly associated with oligotrophic lake conditions. All of the macrophyte species identified in the cores were also recorded in the contemporary macrophyte surveys (Robson et al., 2019), with the exception of Najas flexilis remains found in the LEIR core, which had not been recorded previously at the site.
Whilst the taxa at the sites remain those characteristic of oligotrophic sites, multi taxa ordination analysis (Figure 3) indicates there have been substantial shifts in both community composition and abundance covering the time period of common scoter decline (1970-2015). At all of the sites, the oldest samples (from the core bottoms) are associated with a low abundance of invertebrate, diatom and cladocera remains, and are more closely associated with communities dominated by Isoëtes spp. of macrophyte and low abundance of invertebrate groups. This suggests that historically, 1900 and earlier, there were low levels of nutrient availability at the sites. Common scoter populations peaked in the 1970s and declined thereafter. At around the 1970s a shift towards a more productive lake state is indicated by a shift towards a community associated with greater overall abundance of invertebrate and diatom taxa, and fewer nutrient sensitive taxa, such as Isoëtes spp. An increase in productive state at this time period is further supported by the species data (Supplementary Materials) in which increases in planktonic diatom taxa (such as the diatoms Asterionella formosa and Aulacoseira ambigua), more typical of more eutrophic waters, are observed in the more recent sediments. These increases are concurrent with decreases in other nutrient sensitive taxa, such as the chironomid Diamesinae.
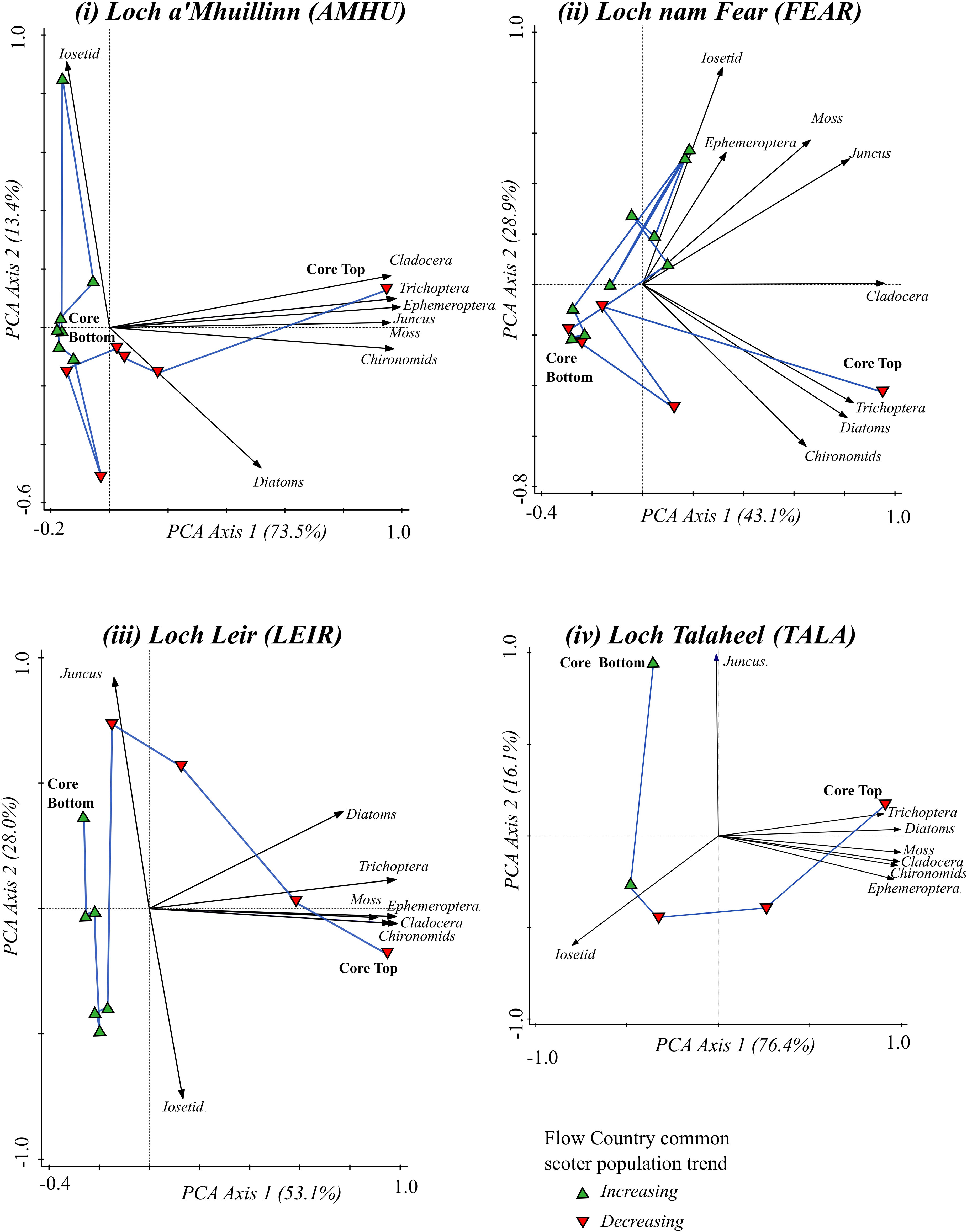
Figure 3 Temporal changes in community composition of the four study lakes over the period of increasing and subsequently declining common scoter abundance. Data are from multi-taxa Principal Component Analyses (PCA). Black arrows show PC1 and PC2 scores of the selected taxa. Blue lines join PCA scores of the loch communities for each successive time-slice in the core, from the period of scoter increase (green triangles) through to the period of scoter decline (red triangles).
The stratigraphic plots (Figure 2) clearly show a change in community structure in the lakes since ca. 1900. Ordination analysis indicates substantial community change occurred around the period common scoter began to decline, ca 1970s (Figure 3). The results of the CONISS analysis are summarised in Figure 4, which shows statistically significant change points along the core. CONISS analysis was carried out for diatoms, macrophytes and macroinvertebrates separately to explore taxon specific responses to potential state changes. Separating this analysis also allowed us to explore the extent of commonalities between the timings of significant zones for different groups of taxa. Across all sites and taxa between two to three significant zones were identified. At each of the four sites a statistically significant split was identified for the diatom communities at or around the 1970s +/- ca. 10 years (Figure 4). At some sites a concurrent shift was identified in the macrophyte and/or invertebrate communities, however in some cases the macroinvertebrate and/or macrophyte shift occurred more recently, ca. 1990 onwards.
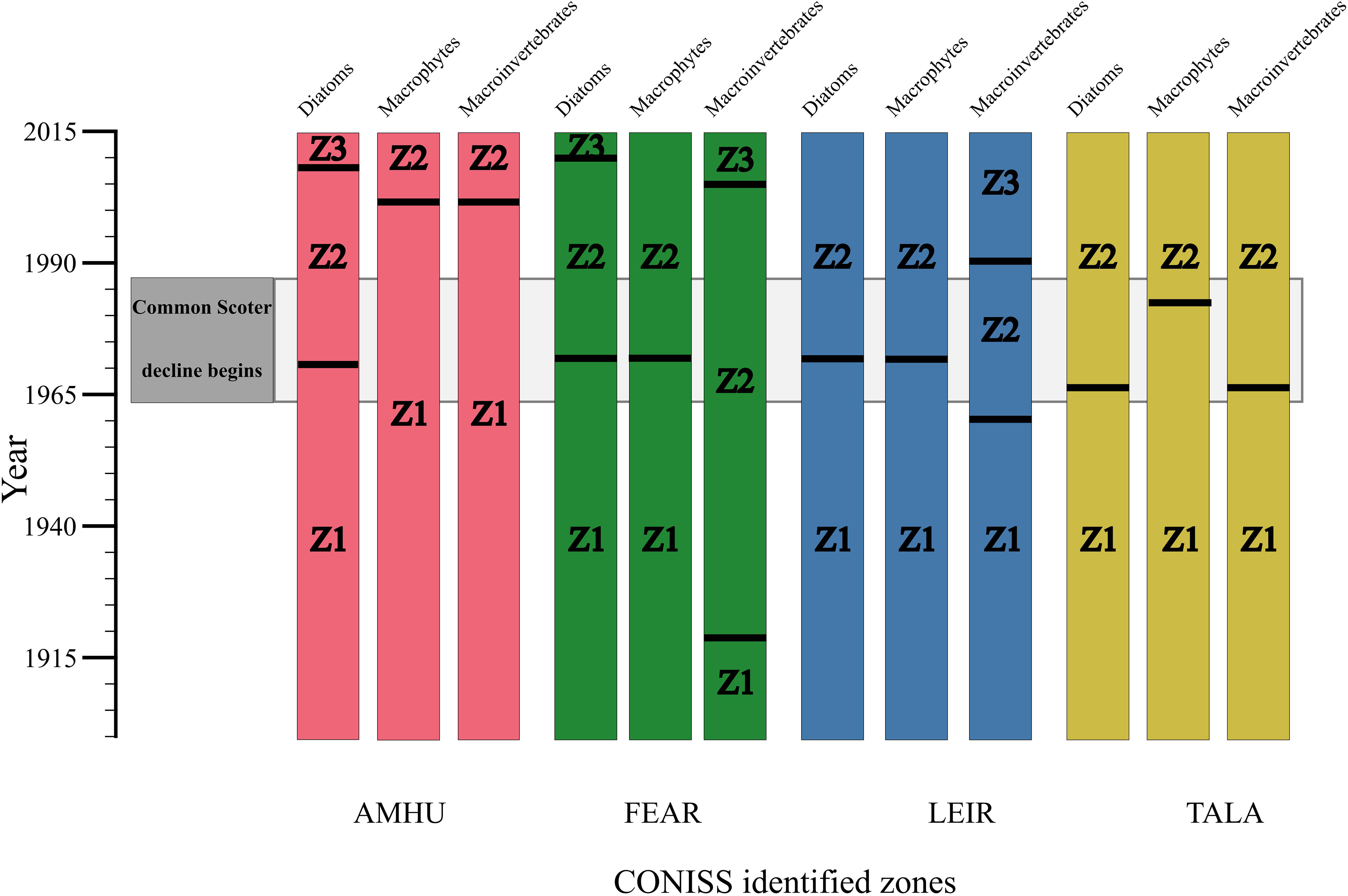
Figure 4 Change-points in community composition in the four study lakes, 1890-2015. Black lines represent significant change points for diatom, macrophyte and macroinvertebrate communities determined by hierarchical cluster analysis using constrained incremental sum-of-squares clustering (CONISS) with Euclidian distances.
Discussion
Data from lake sediment cores has shown that macroinvertebrate abundance in Flow Country lakes has increased since ca. 1900, indicating that there is more macroinvertebrate food available for common scoter now than there was in the recent past. Following a period of steadily increasing abundance in the early part of the twentieth century (during which time common scoter numbers were also increasing), a stepped increase in macroinvertebrate abundance is demonstrated at all study sites at/around the 1970s -coinciding with the time common scoter declines began. Contemporary ecological studies have demonstrated a spatial correlation between common scoter lake use and the abundance of large-bodied macroinvertebrates (Hancock et al., 2015). However, the addition of a temporal perspective, using palaeolimnology, has demonstrated that common scoter numbers have declined even at sites where macroinvertebrate abundance has increased. There is no evidence that the foraging effort of either common scoter females or ducklings varies throughout the season (Hancock et al., 2019), indicating that there were not periods during which food was more difficult to obtain, as has been suggested elsewhere (Fox et al., 2015). Our findings suggest that whilst large-bodied macroinvertebrate abundance may be driving the current distribution of common scoter on Flow Country lakes, as the population contracts towards the best sites, a reduction in food is not driving the observed breeding population decline.
Data from palaeolimnology may have refuted our original hypothesis for decline, however an advantage of taking a multi-indicator palaeolimnological approach is that the wealth of ecological information generated can be used to identify alternative hypotheses for decline.
Palaeolimnological data from algae, plants and invertebrates indicate a shift towards communities typical of more productive lakes, containing more nutrient tolerant taxa and fewer nutrient intolerant taxa. Whilst there is generally a pattern of gradually increasing productivity between 1900 and 1970, this trend is accelerated in the post 1970 period, when common scoter decline began.
Common scoter typically breed in areas of low breeding wildfowl density, suggesting they may be poor competitors for resources. As diving ducks, common scoter feed primarily in benthic habitats where their main competitors (other than diving wildfowl) are fish. In the Flow Country brown trout (Salmo trutta) are the lakes’ apex aquatic predators and have been recorded in the majority of the lakes (Crawford, 1991), particularly those that are popular with breeding common scoter (Hancock et al., 2023). It has been hypothesised that alterations in the abundance and/or structure of brown trout populations, resulting from fish stocking and/or reduced management for angling, could have implications for lake ecology at multiple trophic levels, including common scoter that are competing for the same invertebrate food resources (Hancock et al., 2020b; Hancock et al., 2023). When fish stocking occurs its effects are visible in the palaeolimnological record of a site (Jeppesen et al., 1996; Davidson et al., 2003). The total abundance of invertebrates is reduced (Schilling et al., 2009), and in particular those taxa sensitive to predation (Sayer et al., 2016a; Eloranta et al., 2022). No decline in macroinvertebrate abundance was observed in our sites, indeed invertebrate abundance was shown to have increased at the sites over the period of common scoter decline. We did not observe a decline in taxa particularly sensitive to fish predation (such as planktonic cladocera and/or the diptera chaoboridae) and indeed as common scoter are generalist feeders (Bengtson, 1971; Cramp and Simmons, 1977; Fox, 2003; Fancourt, 2016), it is unlikely that a change in invertebrate composition would be driving decline, when overall abundance of invertebrates has been shown to have increased.
A significant land use change in the Flow Country during the 1970s and 1980s was the introduction of commercial forestry, which led to thousands of hectares of peatland being planted with non-native tree species, primarily Sitka spruce (Picea sitchensis) and Lodgepole pine (Pinus contorta) (Stroud et al., 1988). There are two primary mechanisms via which afforestation of the lake catchments could negatively influence breeding common scoter; either by changing water chemistry (particularly water clarity) which has been shown to be correlated with abundance of breeding waterbirds in other studies (Fox et al., 2020) or by providing habitat for predators directly feeding on the birds themselves (Holopainen et al., 2021; Holopainen et al. (submitted) 1).
Ploughing, drainage and fertiliser application are typically required for plantations to be successful on deep peat, and have all been shown to affect the physical, hydrological and chemical characteristics of nearby waterbodies (Miller et al., 1996; Prévost et al., 1999; Cummins and Farrell, 2003; Räsänen et al., 2007; Ramchunder et al., 2009; McElarney et al., 2010; Drinan et al., 2013a; Drinan et al., 2013b) Afforestation and subsequent deforestation has occurred to different extents in the catchments of all of the study sites. The hydrological complexities of water movement in ombrotrophic peatlands, particularly in the surface and subsurface areas of the peat, mean the extent to which afforestation affects nearby waterbodies can be variable and complex (Howson et al., 2021a; Gaffney et al., 2021; Howson et al., 2021b; Shah et al., 2021). The compositional shifts in lake communities demonstrated in this study coincide with the timing of afforestation, which took place between the 1970 and 1990s, and are consistent with the changes in communities (such as the appearance of Asterionella sp.) documented at other boreal lakes (Turkia et al., 1998). Whilst declines in species such as Isoëtes spp could indicate a decrease in water clarity, no significant change is observed in the proportion of benthic to planktonic diatoms, or in other submerged macrophytes such as mosses and lobelia. This suggests Isoëtes spp decline is more likely caused by nutrient increases than substantial reductions in water clarity.
Besides altering food availability, afforestation of the [previously treeless (Charman, 2002)] Flow Country, could impact ground-nesting birds, such as the common scoter, in other ways. Prime among these is that forestry can support an increased density of generalist predators and ground-nesting waterbird populations are known to be vulnerable to high levels of nest and chick predation (Roos et al., 2018). Indeed Pöysä and Linkola (2021) found that predation had a greater impact than eutrophication on the abundance of breeding waterbird species in other north European boreal wetlands. In our Flow Country study area, Hancock et al. (2020a) showed that the density of mammalian predators was substantially higher in bogs that were near forestry plantations compared to open, treeless bog areas. We suggest that increases in nest predation, resulting from increased predator densities that arise from afforestation is a plausible alternative cause of scoter decline that merits investigation.
This study has provided evidence of the effects of plantation forestry on peatland lakes, with signs of rapid eutrophication evident in the sediment core records associated with the post tree planting period. However, there is no indication that afforestation has resulted in within lake changes (such as a reduction in food availability or changes to habitat) that are likely to have affected breeding common scoter. Management solutions that focus solely on within lake interventions, such as increasing invertebrate abundance in the lakes (Hancock et al., 2023), should therefore be complemented by the testing of mitigation measures that address pressures associated with an increased predator population that are supported by plantation forests.
Future directions
Despite alarming statistics indicating the rapid degradation of freshwater habitats and an increasing number of species losses (WWF, 2020), resources and funding opportunities for wetland conservation continue to be limited (Gordon et al., 2020). An evidence-based approach, that ensures effective resource allocation and ongoing best practice, is therefore vital. Both palaeolimnological and contemporary ecological approaches have their strengths and limitations, however we would advocate strongly that a combined approach can bring the strengths of both approaches to the fore. The value of palaeolimnology to wetland conservation is beginning to be recognised (Froyd and Willis, 2008; Davies and Bunting, 2010; Goodenough and Webb, 2022), however its use in applied conservation management scenarios is still limited (Chambers et al., 2007; Sayer et al., 2010; Brooks et al., 2012; Davidson et al., 2013; Bishop et al., 2019; Roberts et al., 2020). The limited uptake of palaeolimnology for conservation has been attributed to a lack of understanding between respective fields (Davies and Bunting, 2010), perhaps not surprising given the different approaches taken to study design and analysis. As was the case here, cross-disciplinary collaborations between experts are key. Typically, palaeolimnological studies are descriptive in nature -examining multiple indicators that provide a narrative of change over time for a site and its catchment. Generalisation from such studies to inform wider conservation management practice is difficult without a spatial context in which to view any site-specific idiosyncrasies. However, by identifying hypotheses at an early stage, and selecting a small number of sites and indicators accordingly, the implications of both contemporary conditions and longer-term change can be more robustly assessed. There is an added value of integrating palaeolimnological datasets into decline diagnosis approaches. If the spatial association does not turn out to be causal, there is a wealth of data which can be used to develop and explore alternative hypotheses.
Using the case of the common scoter this study has highlighted a limitation of only using spatial comparisons to diagnose species decline. Where there are multiple putative pressures that could be causing decline, and especially where these pressures are pervasive across an area, then a temporal perspective, such as that provided by palaeolimnology, may give novel insights into the causes of decline.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
HR: writing-original draft, analysis, visualization. HR, VJ, SB, CS, and GH: methodology, investigation, analysis. All: study conception, writing-reviewing editing the manuscript. VJ, GH, AD, and SB: funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This Research was funded by a University College London Impact Studentship, with co-funding from The Wildfowl & Wetlands Trust (WWT), The Natural History Museum (NHM) and NatureScot.
Acknowledgments
We would especially like to thank all the landowners who gave permission to access the study sites. We gratefully acknowledge the fieldwork support provided by RSPB Forsinard reserve, Nigel Cameron, Jono Reeves, and Ian Patmore. Thanks to Handong Yang of BEIF at UCL for carrying out the radiometric dating of the cores.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2023.1161732/full#supplementary-material
Footnotes
- ^ Holopainen, S., Miettinen, E., Väänänen, V.-M., Nummi, P., and Pöysä, H. (submitted) Balancing between predation risk and food by boreal breeding ducks. Ecology and Evolution
References
Albert J. S., Destouni G., Duke-Sylvester S. M., Magurran A. E., Oberdorff T., Reis R. E., et al. (2021). Scientists’ warning to humanity on the freshwater biodiversity crisis. Ambio 50, 85–94. doi: 10.1007/S13280-020-01318-8
Anderson T., Cranston P. S., Epler J. H. (2013). Chironomidae of the Holarctic Region (Scandinavian Entomology & Systematic Entomology Ltd: Insect Systematics & Evolution Supplement No.66).
Appleby P. (1997). “Dating of sediments and determination of sedimentation rate,” in Proceedings of a seminar held Helsinki, 2-3 April 1997.
Appleby P. G., Piliposyan G., Weckström J., Piliposian G. (2023). Delayed inputs of hot 137Cs and 241Am particles from Chernobyl to sediments from three Finnish lakes: implications for sediment dating. J. Paleolimnol. 69 (4), 293–303. doi: 10.1007/s10933-022-00273-6
Balmer D., Gillings S., Caffrey B., Swann B., Downie I., Fuller R. (2013). Bird Altas 2007-2011: The breeding and wintering birds of Britain and Ireland (Theford: BTO Books).
Battarbee R. (1999). “The importance of palaeolimnology to lake restoration,” in The Ecological Bases for Lake and Reservoir Management: Proceedings of the Ecological Bases for Management of Lakes and Reservoirs Symposium, held 19–22 March 1996, Leicester, United Kingdom (pp. 149–159). Springer Netherlands.
Battarbee R., Carvalho L., Jones V. J., Flower R. J., Cameron N. G., Bennion H. (2001). “Diatoms,” in Tracking Environmental Change Using Lake Sediments Volume 3: Terrestrial, Algal, and Siliceous Indicators. Eds. Smol J. P., Last W. M., Birks H. J. B. (Dordrecht: Kluwer Academic Publishers).
Bengtson S. A. (1971). Variations in clutch-size in ducks in relation to the food supply. Ibis 113, 523–526. doi: 10.1111/j.1474-919X.1971.tb05190.x
Bengtson S. A. (1972). Reproduction and fluctuations in the size of duck populations at Lake Myvatn, Iceland. Oikos 23, 35–58. doi: 10.2307/3543925
Bennett K. (1996). Determination of the number of zones in a biostratigraphical sequence. New Phytol. 132, 155–170. doi: 10.1111/j.1469-8137.1996.tb04521.x
Bennion H., Sayer C., Tibby J., Carrick H. (2010). Diatoms as indicators of environmental change in shallow lakes. In: Stoermer EF, Smol JP (eds) The diatoms: applications for the environmental and earth sciences, 2nd edn. (Cambridge, UK: Cambridge University Press).
Birks H. (2002). Plant macrofossils. In: Birks H. J. B., Last W. M. (eds) Tracking Environmental Change Using Lake Sediments: Terrestrial, algal, and siliceous indicators. (Dordrecht: Kluwer Academic Publishers) 3, pp. 49–74.
Birks H. H. (2007). Encyclopedia of Quaternary Science, Encyclopedia of Quaternary Science (Amsterdam, The Netherlands: Elsevier). doi: 10.1016/B0-44-452747-8/00215-5
Birks J., Lotter A., Juggins S., Smol J. (2012). Tracking environmental change using lake sediments: data handling and numerical techniques (Berlin/Heidelberg, Germany: Springer Netherlands).
Bishop I. J., Bennion H., Sayer C. D., Patmore I. R., Yang H. (2019). Filling the “data gap”: Using paleoecology to investigate the decline of Najas flexilis (a rare aquatic plant). Geo: Geography and Environment 6 (2), e00081. doi: 10.1002/geo2.81
Bonebrake T. C., Christensen J., Boggs C. L., Ehrlich P. R. (2010). Population decline assessment, historical baselines, and conservation. Conserv. Lett. 3, 371–378. doi: 10.1111/J.1755-263X.2010.00139.X
Boyle J. F. (2001). “Inorganic geochemical methods in palaeolimnology,” in Last W. N., Smol J. P. eds. Tracking Environmental Change Using Lake Sediments. (Dordrecht: Kluwer Academic Publishers) pp. 83–141.
Brooks S. J., Jones V. J., Telford R. J., Appleby P. G., Watson E., McGowan S., et al. (2012). Population trends in the Slavonian grebe Podiceps auritus (L.) and Chironomidae (Diptera) at a Scottish loch. J. Paleolimnol. 47, 631–644. doi: 10.1007/s10933-012-9587-4
Brooks S. J., Langdon P. G., Heiri O. (2007). The identification and use of Palaearctic Chironomidae larvae in paleoecology., QRA Technical Guide No.10 (London: Quanternary Research Association).
Camburn K. E., Charles D. F. (2000). Diatoms of Low-Alkalinity Lakes in the Northeastern United States (Philadelphia, PA: Academy of Natural Sciences of Philadelphia Special Publication).
Chambers F. M., Mauquoy D., Gent A., Pearson F., Daniell J. R. G., Jones P. S. (2007). Palaeoecology of degraded blanket mire in South Wales: Data to inform conservation management. Biol. Conserv. 137, 197–209. doi: 10.1016/j.biocon.2007.02.002
Coulson J., Bauer L., Butterfield J., Downie I., Cranna L., Smith C. (1995). The invertebrates of the northern Scottish Flows, and a comparison with other peatland habitats. In Thompson D. B., Hester A. J., Usher M. B. Eds. Heaths and moorland: cultural landscapes. Edinburgh, UK: Office Books (TSO), 74–94.
Cramp S., Simmons K. E. L. (1977). “Handbook of the birds of europe the middle east and north africa, the birds of the western paeartctic,” in Ostrich to Ducks, vol. Volume 1. (Oxford: Oxford University Press).
Cranswick P. (1999). Regional status reports Status and distribution of common scoter Melanitta nigra and velvet scoter M. fusca in the United Kingdom. (Gloucestershire, UK: WWT Report), 59–65.
Crawford L. (1991). Caithness & Sutherland, Trout loch country (Wick: North of Scotland Newspapers).
Cummins T., Farrell E. P. (2003). Biogeochemical impacts of clearfelling and reforestation on blanket-peatland streams II. Major ions and dissolved organic carbon. For Ecol. Manage 180, 557–570. doi: 10.1016/S0378-1127(02)00649-7
Davidson T. A., Reid M. A., Sayer C. D., Chilcott S. (2013). Palaeolimnological records of shallow lake biodiversity change: exploring the merits of single versus multi-proxy approaches. J. Paleolimnol. 49, 431–446. doi: 10.1007/s10933-013-9696-8
Davidson T. A., Sayer C. D., Perrow M. R., Tomlinson M. L. (2003). Representation of fish communities by scale sub-fossils in shallow lakes: Implications for inferring percid-cyprinid shifts. J. Paleolimnol. 30, 441–449. doi: 10.1023/B:JOPL.0000007247.97059.e2
Davies A., Bunting M. (2010). Applications of palaeoecology in conservation. Open Ecol. J. 3, 54–67. doi: 10.2174/1874213001003020054
Dean W. E. (1974). Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: comparison with other methods. J. Sediment. Res. 44, 242–248. doi: 10.1306/74d729d2-2b21-11d7-8648000102c1865d
Downie I., Coulson J., Foster G., Whitfield D. (1998). Distribution of aquatic macroinvertebrates within peatland pool complexes in the Flow Country, Scotland. Hydrobiologia 377, 95–105. doi: 10.1023/A:1003267022820
Drinan T. J., Graham C. T., O’Halloran J., Harrison S. S. C. (2013a). The impact of catchment conifer plantation forestry on the hydrochemistry of peatland lakes. Sci. Total Environ. 443, 608–620. doi: 10.1016/j.scitotenv.2012.10.112
Drinan T. J., Graham C. T., O’Halloran J., Harrison S. S. C. (2013b). The impact of conifer plantation forestry on the Chydoridae (Cladocera) communities of peatland lakes. Hydrobiologia 700, 203–219. doi: 10.1007/s10750-012-1230-x
Eaton M. A., Balmer D., Burton N., Grice P. V., Musgrove A. J., Hearn R., et al. (2008). The state of the UK’s birds 2007. (UK: RSPB, BTO, WWT, CCW, EHS, NE and SNH).
Eloranta A. P., Kjærstad G., Power M., Lakka H. K., Arnekleiv J. V., Finstad A. G. (2022). Impacts of piscicide-induced fish removal on resource use and trophic diversity of lake invertebrates. Sci. Total Environ. 835, 155364. doi: 10.1016/J.SCITOTENV.2022.155364
Fancourt B. A. (2016). Diagnosing species decline: a contextual review of threats, causes and future directions for management and conservation of the eastern quoll. Wildlife Res. 43, 197–211. doi: 10.1071/WR15188
Fox A. D. (2003). Diet and habitat use of Scoters Melanitta in the western Palearctic -a brief overview. Wildfowl 54, 163–182. doi: 10.5735/086.052.0404
Fox A. D., Einar Jónsson J., Aarvak T., Bregnballe T., Kjaer Christensen T., Kuhlmann Clausen K., et al. (2015). Current and potential threats to Nordic duck populations—a horizon scanning exercise. BioOne 52, 193–220.
Fox A. D., Jørgensen H. E., Jeppesen E., Lauridsen T. L., Søndergaard M., Fugl K., et al. (2020). Relationships between breeding waterbird abundance, diversity, and clear water status after the restoration of two shallow nutrient-rich Danish lakes. Aquat. Conserv. 30, 237–245. doi: 10.1002/AQC.3260
Fox A. D., Petersen A., Frederiksen M. (2003). Annual survival and site fidelity of breeding female Common Scoter Melanitta nigra at Myvatn, Iceland 1925–58. Ibis 145, E94–E96. doi: 10.1046/j.1474-919X.2003.00162.x
Froyd C. A., Willis K. J. (2008). Emerging issues in biodiversity & conservation management: The need for a palaeoecological perspective. Quat. Sci. Rev. 27, 1723–1732. doi: 10.1016/j.quascirev.2008.06.006
Gaffney P. P. J., Hancock M. H., Taggart M. A., Andersen R. (2021). Catchment water quality in the year preceding and immediately following restoration of a drained afforested blanket bog. Biogeochemistry 153, 243–262. doi: 10.1007/S10533-021-00782-Y
Gardarsson A., Einarsson A. (1994). Responses of breeding duck populations to changes in food supply. Hydrobiologia 279–280, 15–27. doi: 10.1007/BF00027837
Gardarsson A., Einarsson A. (2004). Resource limitation of diving ducks at Myvatn: Food limits production. Aquat. Ecol. 38, 285–295. doi: 10.1023/B:AECO.0000032058.83651.2c
Gillson L., Marchant R. (2014). From myopia to clarity : sharpening the focus of ecosystem management through the lens of palaeoecology. Trends Ecol. Evol. 29 (6), 1–9. doi: 10.1016/j.tree.2014.03.010
Goodenough A. E., Webb J. C. (2022). Learning from the past: opportunities for advancing ecological research and practice using palaeoecological data. Oecologia 199, 275–287. doi: 10.1007/S00442-022-05190-Z
Gordon E. R., Butt N., Rosner-Katz H., Binley A. D., Bennett J. R. (2020). Relative costs of conserving threatened species across taxonomic groups. Conserv. Biol. 34, 276–281. doi: 10.1111/COBI.13382
Gregory R., Eaton M., Noble D., Robinson J., Arsons P., Baker H., et al. (2002). The State of the UK’s birds 2002. (UK: RSPB, JNCC, WWT, BTO).
Grimm E. C. (1987). CONISS: a fortran 77 programme for strati- graphically constrained cluster analysis by the method of incremental sum of squares. Comput. Geosci. 13, 13–35. doi: 10.1016/0098-3004(87)90022-7
Hancock M. (1991). Common Scoter in the Flow Country- results of 1991 survey, comparisons with prevuous years and suggested monitoring method. (UK: RSPB).
Hancock M., Avery M. (1998). Changes in breeding bird populations in peatlands and young forestry in north east Sutherland and Caithness between 1988 and 1995 (UK: Scottish Birds).
Hancock M. H., Klein D., Cowie N. R. (2020a). Guild-level responses by mammalian predators to afforestation and subsequent restoration in a formerly treeless peatland landscape. Restor. Ecol. 28, 1113–1123. doi: 10.1111/REC.13167
Hancock M. H., Klein D., Hughes R., Stagg P., Byrne P., Smith T. D., et al. (2023). Testing whether reducing brown trout biomass in peatland lakes increases macro-invertebrate biomass and invertivorous waterbird occurrence. Aquat. Ecol. 57, 217–240. doi: 10.1007/S10452-022-10000-Y
Hancock M., Robson H., Smith T. (2015). Correlates of lake use by breeding common scoters in Scotland. Aquat. Conserv. 26, 749–767. doi: 10.1002/aqc.2606
Hancock M. H., Robson H. J., Smith T. D., Douse A., Robson H. J., Trust W. (2019). Spatial and temporal patterns of foraging activity by breeding Common Scoters (Melanitta nigra) in Scotland. Ornis Fennica 96, 124–141.
Hancock M. H., Robson H. J., Smith T. D., Stephen A., Byrne P., MacLennan A., et al. (2020b). From a research study to a conservation partnership: Developing approaches to restoring common scoter populations. Aquat. Conserv. 30, 1770–1774. doi: 10.1002/AQC.3414
Holopainen S., Väänänen V. M., Vehkaoja M., Fox A. D. (2021). Do alien predators pose a particular risk to duck nests in Northern Europe? Results from an artificial nest experiment. Biol. Invasions 23, 3795–3807. doi: 10.1007/S10530-021-02608-2
Howson T., Chapman P., Shah N. (2021a). A comparison of porewater chemistry between intact, afforested and restored raised and blanket bogs. Sci Total Environ. 766, 144496. doi: 10.1016/j.scitotenv.2020.144496
Howson T., Chapman P., Shah N. (2021b). The effect of forest-to-bog restoration on the hydrological functioning of raised and blanket bogs. Ecohydrology 14 (7), e2334. doi: 10.1002/eco.2334
Jeppesen E., Madsen E., Jensen J. (1996). Reconstructing the past density of planktivorous fish and trophic structure from sedimentary zooplankton fossils: a surface sediment calibration data set from shallow. Freshwater 36, 115–127. doi: 10.1046/j.1365-2427.1996.00085.x
Jones V. J. (2013). Diatom Introduction. 2nd ed. (Oxford, UK: Encyclopedia of Quaternary Science), 471–480. doi: 10.1016/B978-0-444-53643-3.00218-1
Juggins S. (2007). C2 Version 1.5 Software for ecological and palaeoecological data analysis and visualisation. (Newcastle, UK: Newcastle University).
Juggins S. (2012). Rioja: analysis of Quaternary Science Data, R package version 0.7-3. (Newcastle, UK: Newcastle University).
Keller V., Herrando S., Voríšek P., Franch M., Kipson M., Milanesi P., et al. (2020). European breeding bird atlas 2: Distribution, abundance and change (Barcelona: European Bird Census Council & Lynx Edicions).
Kondratyev A. V. (1999). Foraging strategies and habitat use of sea ducks breeding in northeast Russia (Ottawa, Canada: Occasional papers -Canadian Wildlife Service), 51–58.
Legendre P., Birks H. (2012). “From classical to canonical ordination,” in Tracking Environmental Change Using Lake Sediments (Dordrecht, Netherlands: Springer International Publishing).
Legendre P., Legrendre L. (2012). Developments in Numerical Ecology (Third Edition) (Netherlands: Elsevier).
Lindsay R., Charman D. J., Everingham F., O’Reilly R. M., Palmer M. A., Rowell T. A., et al. (1988). The Flow Country: The peatlands of Caithness and Sutherland (Peterborough: Joint Nature Conservation Committee).
McElarney Y. R., Rasmussen P., Foy R. H., Anderson N. J. (2010). Response of aquatic macrophytes in Northern Irish softwater lakes to forestry management; eutrophication and dissolved organic carbon. Aquat. Bot. 93, 227–236. doi: 10.1016/j.aquabot.2010.09.002
Mihoub J. B., Henle K., Titeux N., Brotons L., Brummitt N. A., Schmeller D. S. (2017). Setting temporal baselines for biodiversity: the limits of available monitoring data for capturing the full impact of anthropogenic pressures. Sci. Rep. 7, 1 7, 1–1 7,13. doi: 10.1038/srep41591
Miller J., Anderson H., Ray D., Anderson A. (1996). Impact of some initial forestry practices on the drainage waters from blanket peatlands. Forestry 69, 193–203. doi: 10.1093/forestry/69.3.193-a
NatureScot (2014). Standing Waters Database - Scotland. Occurrence dataset. doi: 10.15468/iwllx8. accessed via GBIF.org 11/4/2014
Norris K. (2004). Managing threatened species: the ecological toolbox, evolutionary theory and declining-population paradigm. J. Appl. Ecol. 41, 413–426. doi: 10.1111/J.0021-8901.2004.00910.X
Patmore I. R., Sayer C. D., Goldsmith B., Davidson T. A., Rawcliffe R., Salgado J. (2014). Big Ben: A new wide-bore piston corer for multi-proxy palaeolimnology. J. Paleolimnol. 51, 79–86. doi: 10.1007/s10933-013-9756-0
Peery Z. M., Beissinger S. R., Newman S. H., Burkett E. B., Williams T. D. (2004). Applying the declining population paradigm: diagnosing causes of poor reproduction in the marbled murrelet. Wiley Online Library 18, 1088–1098. doi: 10.1111/j.1523-1739.2004.00134.x
Petersen I. K., Frederiksen M., Petersen A., Robson H. J., Einarsson Á., Nielsen R. D., et al. (2021). Recent increase in annual survival of nesting female Common Scoter Melanitta nigra in Iceland. J. Ornithol. 162, 135–141. doi: 10.1007/S10336-020-01818-0/METRICS
Pöysä H., Linkola P. (2021). Extending temporal baseline increases understanding of biodiversity change in European boreal waterbird communities. Biol. Conserv. 257, 109139. doi: 10.1016/J.BIOCON.2021.109139
Prévost M., Plamondon A. P., Belleau P. (1999). Effects of drainage of a forested peatland on water quality and quantity. J. Hydrol. (Amst.) 214, 130–143. doi: 10.1016/S0022-1694(98)00281-9
Ramchunder S. J., Brown L. E., Holden J. (2009). Environmental effects of drainage, drain-blocking and prescribed vegetation burning in UK upland peatlands. Prog. Phys. Geogr. 33, 49–79. doi: 10.1177/0309133309105245
Räsänen J., Kenttämies K., Sandman O. (2007). Paleolimnological assessment of the impact of logging on small boreal lakes. Limnol. Ecol. 37, 193–207. doi: 10.1016/j.limno.2006.12.003
R Core Team (2016). R: A language and environment for statistical computing. Vienna, Austria. Available at: https://www.R-project.org/.
Roberts L., Bishop I., Adams J. (2020). Anthropogenically forced change in aquatic ecosystems: reflections on the use of monitoring, archival and palaeolimnological data to inform conservation. Geo: Geography and Environment 7 (1), e00089. doi: 10.1002/geo2.89
Robson H. J. (2017). Causes of decline of common scoter (Melanitta nigra) in north Scotland: evidence from palaeolimnology. (London, UK: University College London).
Robson H. J., Jones V. J., Hilton G. M., Brooks S., Sayer C. D., Douse A. (2019). Combined palaeolimnological and ecological approach provides added value for understanding the character and drivers of recent environmental change in Flow Country lakes. (Mires and Peat) 23. doi: 10.19189/MAP.2018.OMB.386
Roos S., Smart J., Gibbons D. W., Wilson J. D. (2018). A review of predation as a limiting factor for bird populations in mesopredator-rich landscapes: a case study of the UK. Biol. Rev. 93, 1915–1937. doi: 10.1111/BRV.12426
Sayer C. D., Bennion H., Gurnell A. M., Goodyer E., Kotze D., Lindsay R. (2016b). “Restoration of freshwaters: principles and practice,” in Hughes J. ed. Freshwater ecology and conservation: approaches and techniques. (Oxford, UK: Oxford University Press), 378–403.
Sayer C. D., Davidson T. A., Jones J. I., Langdon P. G. (2010). Combining contemporary ecology and palaeolimnology to understand shallow lake ecosystem change. Freshw. Biol. 55, 487–499. doi: 10.1111/j.1365-2427.2010.02388.x
Sayer C., Davidson T., Rawcliffe R., Langdon P. (2016a). Consequences of fish kills for long-term trophic structure in shallow lakes: implications for theory and restoration. Ecosystems 19, 1289–1309. doi: 10.1007/s10021-016-0005-z
Schilling E. G., Loftin C. S., Huryn A. D. (2009). Effects of introduced fish on macroinvertebrate communities in historically fishless headwater and kettle lakes. Biol. Conserv. 142, 3030–3038. doi: 10.1016/J.BIOCON.2009.08.003
Shah N. W., Nisbet T. R., Broadmeadow S. B. (2021). The impacts of conifer afforestation and climate on water quality and freshwater ecology in a sensitive peaty catchment: A 25 year study in the upper River Halladale in North Scotland. For Ecol Manage. 502, 119616.
Smol J. P. (2008). Pollution of Lakes and Rivers. A paleoenvironmental perspective. 2nd ed. (Malden, MA, USA: Blackwell Publishing).
Smol J., Birks H., Last W. (2001). “Tracking environmental change using lake sediments,” in Zoological indicators, vol. Volume 4. (Dordrecht, Netherlands: Springer Netherlands).
Stanbury A., Eaton M., Aebischer N., Balmer D., Brown A., Douse A., et al (2021). The status of our bird populations: the fifth birds of conservation concern in the United Kingdom, Channel Islands and Isle of man and second IUCN red list assessment ofextinction risk for Great Britain. British Birds 114, 723–747. doi: 10.13140/RG.2.2.35668.73602
Stroud D. A., Reed T. M., Pienkowski M. W., Lindsay R. A. (1988). Birds, bogs and forestry - The peatlands of Caithness and Sutherland. (UK: Nature Conservancy Council).
ter Braak C. J. F., Smilauer P. (2012). Canoco 5, Windows release (5.00). Software for mutivariate data exploration, testing, and summarization. Biometris, Plant Research International, Wageningen.
Turkia J., Sandman O., Huttunen P. (1998). Palaeolimnological evidence of forestry practices disturbing small lakes in Finland. Boreal Environ. Res. 3, 45–61.
Underhill M., Gittings T., Callaghan D. (1998). Status and distribution of breeding Common Scoters Melanitta nigra nigra in Britain and Ireland in 1995. Bird Study 45 (2), 146–156.
Underhill M., Hughes B. (1996). The Status of Breeding Common Scoter Melanitta nigra nigra in Britain and Ireland in 1996: The effect of the Sea Empress Oil Spill. (UK: Countryside Council for Wales).
Willis K. J., Bailey R. M., Bhagwat S. A., Birks H. J. B. (2010). Biodiversity baselines, thresholds and resilience: testing predictions and assumptions using palaeoecological data. Trends Ecol. Evol. 25, 583–591. doi: 10.1016/J.TREE.2010.07.006
Keywords: species decline, freshwater, multidisciplinary, sediment cores, water bird, palaeolimnology, afforestation, peatland lakes
Citation: Robson HJ, Jones VJ, Brooks SJ, Sayer CD, Douse A and Hilton GM (2023) Borrowing from the palaeolimnologists toolkit; the use of lake sediment cores in diagnosing the causes of freshwater species decline. Front. Conserv. Sci. 4:1161732. doi: 10.3389/fcosc.2023.1161732
Received: 08 February 2023; Accepted: 30 October 2023;
Published: 22 November 2023.
Edited by:
Krystyna Saunders, Australian Nuclear Science and Technology Organisation, AustraliaReviewed by:
Xuhui Dong, Guangzhou University, ChinaLindsey Gillson, University of Cape Town, South Africa
Copyright © 2023 Robson, Jones, Brooks, Sayer, Douse and Hilton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hannah Jane Robson, Hannah.Robson@wwt.org.uk
 Hannah Jane Robson
Hannah Jane Robson Vivienne Jane Jones1
Vivienne Jane Jones1  Stephen John Brooks
Stephen John Brooks Carl Derek Sayer
Carl Derek Sayer Geoff Mark Hilton
Geoff Mark Hilton