The impact of Helicobacter pylori infection on low skeletal muscle mass risk in Chinese women over 40: a cross-sectional analysis
- 1Department of Medical Care Center, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 2The First School of Medicine, School of Information and Engineering, Wenzhou Medical University, Wenzhou, China
- 3Key Laboratory of Interventional Pulmonology of Zhejiang Province, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 4Department of Pulmonary and Critical Care Medicine, Quzhou People’s Hospital, The Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou, China
- 5Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
Background: Sarcopenia can lead to significant personal, social, and economic burdens. The diagnosis of sarcopenia heavily relies on the identification of Low Skeletal Muscle Mass (LSMM), which is an independent predictor of frailty, disability, and increased risk of death among seniors. Women have physiologically lower levels of skeletal muscle mass than men, and female sarcopenia appears to be more influenced by menopause. They also tend to have higher body fat levels than man, which increases the risk of sarcopenia obesity. On another front, it’s also recognized that humans are largely prone to Helicobacter pylori (H. pylori) infection, with global prevalence rates often surpassing 50%. Nevertheless, the interconnection between H. pylori infection and LSMM remains relatively unexplored. Hence, our study specifically targeted women as the research population and sought to explore several risk factors for LSMM. Additionally, we delved into the potential correlation between LSMM and H. pylori infection in women, hoping to gain insights into potential preventative measures or treatment options that may enhance the quality of life for women affected by sarcopenia.
Methods: We conducted a cross-sectional study among women aged over 18 years undergoing physical examination. We performed 13C-urea breath test (UBT) for diagnosis of H. pylori infection and Bioelectrical impedance analysis (BIA) for the assessment of LSMM. Logistic regression models were used to analyze the associations of H. pylori infection with LSMM.
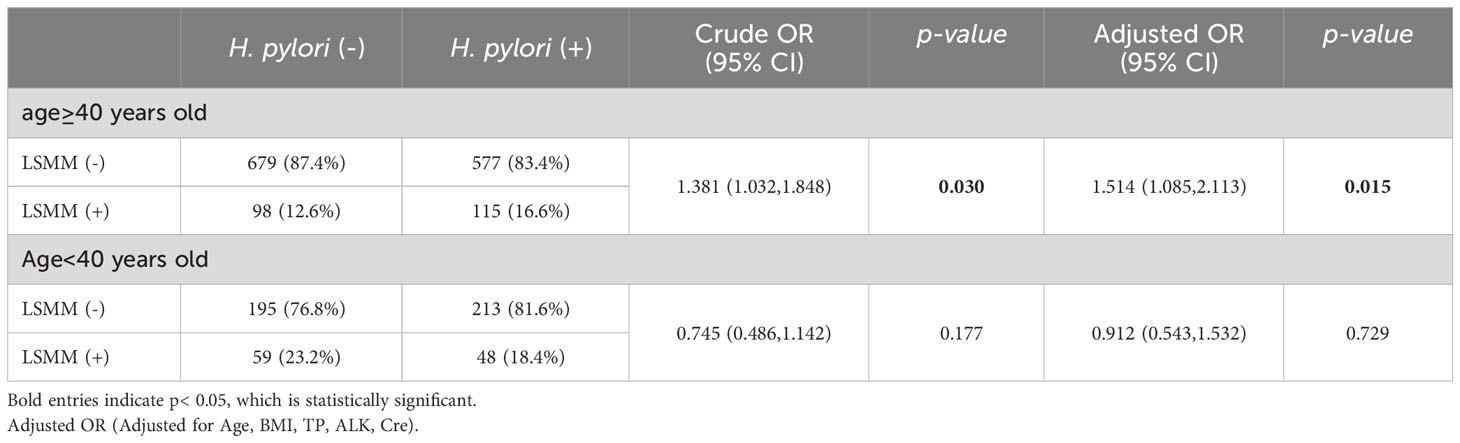
Results: This study enrolled 1984 Chinese women who were undergoing health check-ups. A univariate logistic regression analysis did not reveal a direct correlation between H. pylori infection and LSMM among this female population (OR=1.149, 95% CI 0.904-1.459, p=0.257). Yet, upon dividing the participants into age-based subgroups, an evident link was observed between H. pylori infection and LSMM in women aged 40 or above (OR=1.381, 95%CI 1.032-1.848, p= 0.030). After adjusting for variables including Age, BMI, TP, ALK, Cre, this relationship remained statistically relevant (OR=1.514, 95%CI 1.085-2.113, p= 0.015).
Conclusions: Women who are over 40 years old and currently infected with H. pylori have an increased risk of developing LSMM. Therefore, timely treatment for H. pylori eradication is recommended for this group of women to reduce the occurrence of LSMM.
Introduction
Helicobacter pylori (H. pylori) is a gram-negative, curved bacillus that was discovered and identified by Marshall and Warren in 1983 from gastric mucosa (Marshall and Warren, 1984). Studies have confirmed that H. pylori has coexisted with humans for 58,000 years (Linz et al., 2007), and can be found in approximately half of the world’s population with infection levels reaching over 70% in developing countries (Bravo et al., 2018). And The analysis of 410,879 participants from 73 countries showed that the global prevalence of H. pylori infection in women was 42.7% (Zamani et al., 2018). The association between H. pylori and conditions such as gastric cancer, peptic ulcer, and gastric lymphoma has been widely acknowledged (Hunt et al., 2011). However, recent studies have revealed a growing correlation between H. pylori and various extra-gastric disorders (Franceschi and Gasbarrini, 2007). These include a positive association with non-alcoholic fatty liver and adults metabolic syndrome (Chen et al., 2015; Buzás, 2020), as well as an inverse association with asthma, reflux disease, and allergic disorders (Cover and Blaser, 2009).
Identified as a muscular disorder in the International Classification of Disease (ICD-10: M62) (Lj and Mo, 2017), sarcopenia is typically distinguished by decreased muscle mass, reduced muscular strength, and impaired physical functioning (Chen et al., 2021). The diminished presence of skeletal muscle tissue is a vital element in the identification of sarcopenia, associated with numerous contributors like reduced physical exertion, ongoing inflammation, age-related hormonal alterations, genetic influences, and more (Sk, 2020). Furthermore, Low Skeletal Muscle Mass (LSMM) was considered an autonomous predictor of decreased overall survival in multiple diseases (Walowski et al., 2020). Asia is a rapidly aging region with a large population, and therefore the estimated impact of sarcopenia on this region is substantial. However, due to the lack of standard diagnosis criteria and different target populations used in studies, the prevalence of LSMM varies greatly between studies (Fielding et al., 2011; Chen et al., 2014; Petermann-Rocha et al., 2022). The prevalence of sarcopenia varies between 10% and 27% when using different classifications and cutoff points. According to the European Working Group on Sarcopenia 2 (EWGSOP2) definition, the prevalence of sarcopenia is higher in men (11% vs. 2%). Conversely, as defined by the International Sarcopenia Working Group, the prevalence of sarcopenia is higher in women (17% vs. 12%) (Petermann-Rocha et al., 2022). Analyzing 41 studies and a total of 34,955 participants, another systematic review and meta-analysis showed that among community-dwelling individuals, the prevalence of sarcopenia was 11% in men and 9% in women (Papadopoulou et al., 2020). However, according to several studies, women have physiologically lower levels of skeletal muscle mass than men (Churchward-Venne et al., 2014; Zhang et al., 2021). A cross-sectional study reported that positive serum H. pylori infectious markers are correlated with sarcopenia and low muscle quantity (Wu and Chen, 2021). Another study involving 1061 women reported that the risk of low skeletal muscle mass is reduced in older adults women aged 60 and over who receive Helicobacter pylori eradication therapy (Baeg et al., 2015). Skeletal muscle mass begins to decline as early as age 40 and decreases by approximately 30%–50% between ages 40 and 80 (Faulkner et al., 2007). What’s more, LSMM was sex-specific, especially women LSMM appears to be strongly associated with menopause (Yang et al., 2019; Geraci et al., 2021). Therefore, further exploration of the relationship between menopause and low skeletal muscle mass holds significant meaning. In spite of the existing knowledge, comprehensive investigations probing the connection between H. pylori and low skeletal muscle mass (LSMM) are scarce. In light of this, this investigation is centered around the female demographic. And the purpose of our study is to determine whether there is a correlation between H. pylori infection and LSMM, specifically within a demographic of adult Chinese women. This information could potentially shed light on the interactions between these factors and contribute to a broader understanding of the disease progression and make it possible to take proactive steps to intervene early in order to halt or slow the progression of the disease.
Methods
Setting and study population
This research, characterized as a retrospective cohort study, received formal approval from the Ethics Committee associated with the First Affiliated Hospital of Wenzhou Medical University. Furthermore, all research activities and methodologies strictly adhere to the principles outlined in the Declaration of Helsinki. The study encompassed 1984 adult women aged 18 and above who satisfied certain eligibility conditions. To qualify for inclusion, the participants had to be female adults who had undergone a health check at the First Affiliated Hospital of Wenzhou Medical University from April 2016 through August 2017. Additionally, these individuals had to have completed a 13C-urea breath test and bioelectrical impedance analysis (BIA). Our research used the following exclusion criteria: (1) Those under the age of 18 were excluded from the study. (2) had a history of gastrectomy, heart failure, liver cirrhosis, cerebrovascular accident, thyroid disease, end-stage renal disease, or malignancy. (3) physical disability or injuries of hand/wrist/leg/foot in the last three months. (4) using antibiotics and traditional Chinese medicines with antibacterial effects within a month, or proton pump inhibitors (PPI), sucralfate, bismuth, etc within 2 weeks. (5) participants with deficiencies of information such as skeletal muscle mass index, H. pylori results, and serum biomarkers.
Data collection
We collected participants’ basic information, such as age, smoking and drinking status, comorbidities, and past medical history, using pre-designed questionnaires. Smoking was classified as either a current or non-current smoker (someone who has never smoked or used to smoke). Alcohol use was categorized as either a heavy drinker (someone who drinks alcohol at 1 time ≥40 g or 5 drinks, twice or more per week) or not a heavy drinker (someone who drinks less than twice per week). Blood measurements were collected after fasting for 12-16 hours and avoiding alcohol, high-protein, and high-fat foods the day before.
Several blood-based metrics were meticulously evaluated, including counts of white and red blood cells, hemoglobin levels, glucose concentration, glycosylated hemoglobin content, and levels of total protein and albumin. Additionally, measurements of enzymes, Examples of these enzymes include, but are not limited to, alanine aminotransferase and aspartate aminotransferase, in addition to alkaline phosphatase, along with γ-glutamyl transferase, were performed, as were assessments of creatinine, uric acid, total cholesterol, triglycerides, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol levels. Furthermore, anthropometric parameters were scrupulously recorded by well-trained nursing professionals utilizing uniform and standardized equipment to ensure accuracy and reliability. Blood pressure measurements, presented in millimeters of mercury (mmHg), were conducted in the morning under tranquil and relaxed conditions to minimize external influences. Body weight and height were determined, with weights reported in kilograms and heights noted in centimeters. The formula for calculating BMI (Body Mass Index) is: weight (in kilograms) divided by height squared (in meters).
Diagnosis of H. pylori infection
The 13C-urea breath test (UBT) stands out as a leading non-invasive approach for the identification of H. pylori infection. It has straightforward procedural steps and high sensitivity and specificity. The combination of these factors underscores why the 13C-UBT is often the method of choice in clinical settings for the diagnosis of H. pylori infection (Nocon et al., 2009; Gisbert and Calvet, 2013; Malfertheiner et al., 2017). After oral administration of nuclide 13C-labeled urea, the urease present in the living Helicobacter pylori can break down the nuclide-labeled urea into nuclide-labeled CO2. It is necessary to collect and detect the exhaled breath at two time points before taking the medicine and 30 minutes after taking the medicine (Chen et al., 2003; Ricci et al., 2007; Di Rienzo et al., 2013). H. pylori infection can be judged by the change in the concentration ratio of 13CO2/12CO2 in the breath sample before and after taking the medicine. Generally, Delta Over Baseline (DOB)=4 is used as the cut-off value for H. pylori infection, DOB value ≥ 4 means H. pylori positive, DOB value < 4 means H. pylori negative (Logan, 1998; Gisbert and Pajares, 2004). In this study, H. pylori infection status was detected by a 13C-UBT, with an empty stomach or fasting for at least two hours. Participants were required to stop using all kinds of antibiotics and traditional Chinese medicines with antibacterial effects for at least 4 weeks, proton pump inhibitors (PPI), bismuth, sucralfate, etc. for 2 weeks before test.
Measures and definition of LSMM
Bioelectrical impedance analysis (BIA), an approach doesn’t require invasive procedures that assesses body composition, is recognized by international guidelines as a valid alternative to whole-body dual-energy DXA. In our study, we employed a BIA device (InBody770, produced by InBody Korean Inc.) This method allows for the assessment of appendicular skeletal muscle mass (ASM). Once the ASM was established, we further computed the skeletal muscle index (SMI). This evaluation was performed by taking the previously acquired value for appendicular skeletal muscle mass and dividing it by the participant’s height squared, with the height being measured in square meters (m2). To define LSMM, we adhered to the unified recommendations put forth by the Asian Working Group for Sarcopenia (AWGS) in 2019. According to these guidelines, the thresholds of LSMM were an SMI below 7.0 kg/m2 for men and below 5.7 kg/m2 for women. These cutoff values are widely accepted and used in sarcopenia research, enabling the identification of individuals at risk or already affected by the condition (Chen et al., 2020).
Statistical analyses
All our statistical computations were performed employing a two-tailed approach and were conducted with a 5% significance level, utilizing SPSS statistical software (version 23.0 provided by IBM Corp). The normality of the continuous variables within our dataset was validated using the Kolmogorov-Smirnov test. For those variables adhering to a normal distribution, they were presented as means ± standard deviations. To discern any disparities between the two groups, we implemented the Student’s t-test. For the continuous variables that didn’t follow a normal distribution, we presented them as medians, alongside their interquartile ranges. To compare differences between these two groups, we utilized the Mann-Whitney U test. When it came to categorical variables, we expressed them as frequencies and percentages. To identify the differences between the two groups in this context, we applied the chi-square test or, when necessary, the exact method proposed by Fisher. For evaluating the ORs and their associated 95% CIs across various groups, both univariate and multivariate-adjusted calculations were made, deploying logistic regression analysis.
Results
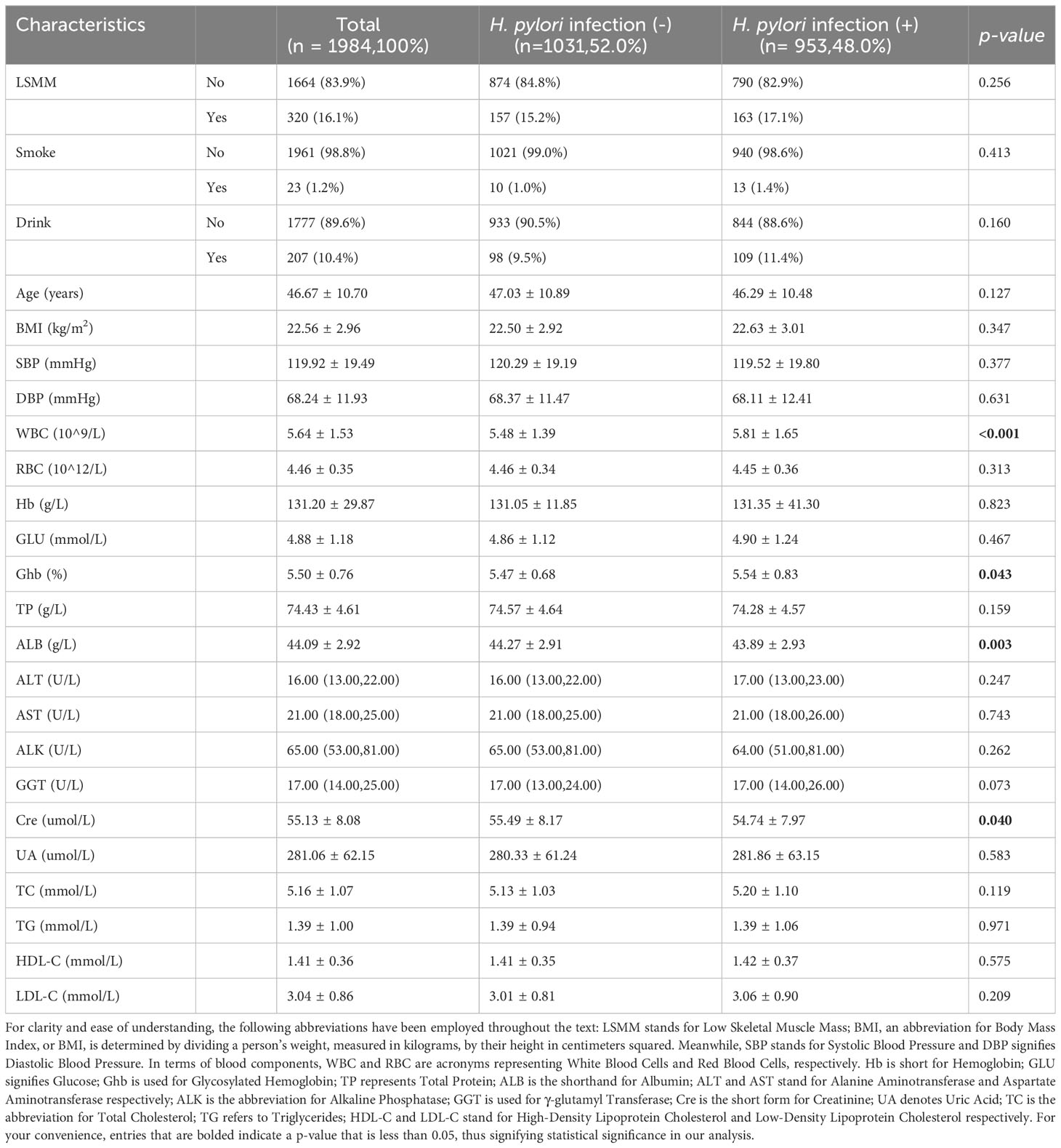
Characteristics of participants
Table 1 provides a snapshot of the primary characteristics of our study subjects, divided based on their H. pylori infection condition as identified via the 13C-urea breath test. The study included a total of 1984 fitting participants, with their average age being 46.67 ± 10.70 years. Among the study population, the mean ages of the Helicobacter pylori-negative and Helicobacter pylori-positive group were 47.03 ± 10.89, 46.29 ± 10.48. No significant age differences were observed between the two groups under study (t=1.529, p=0.127). The total incidence of H. pylori infection was found to be 48.0% (953 out of 1984 participants). The distribution of this infection did not present notable statistical disparities when comparing the group with LSMM and the group without LSMM (15.2% vs 17.1%, p=0.256). Thus, the prevalence of H. pylori infection does not seem to be biased towards one group over the other, indicating a balanced sample for our investigation. Most parameters, such as smoking status, alcohol status, age, BMI, SBP, DBP, RBC, Hb, TP, GLU, ALT, AST, ALK, UA, TC, TG, HDL-C, LDL-C showed no notable statistical disparities between individuals with H. pylori infection and those without. Nonetheless, variables such as WBC (P <0.001), Ghb (p= 0.043), ALB (p= 0.003), Cre (p= 0.040), did present statistical differences (p<0.05).
Logistic regression analyses for different markers and LSMM
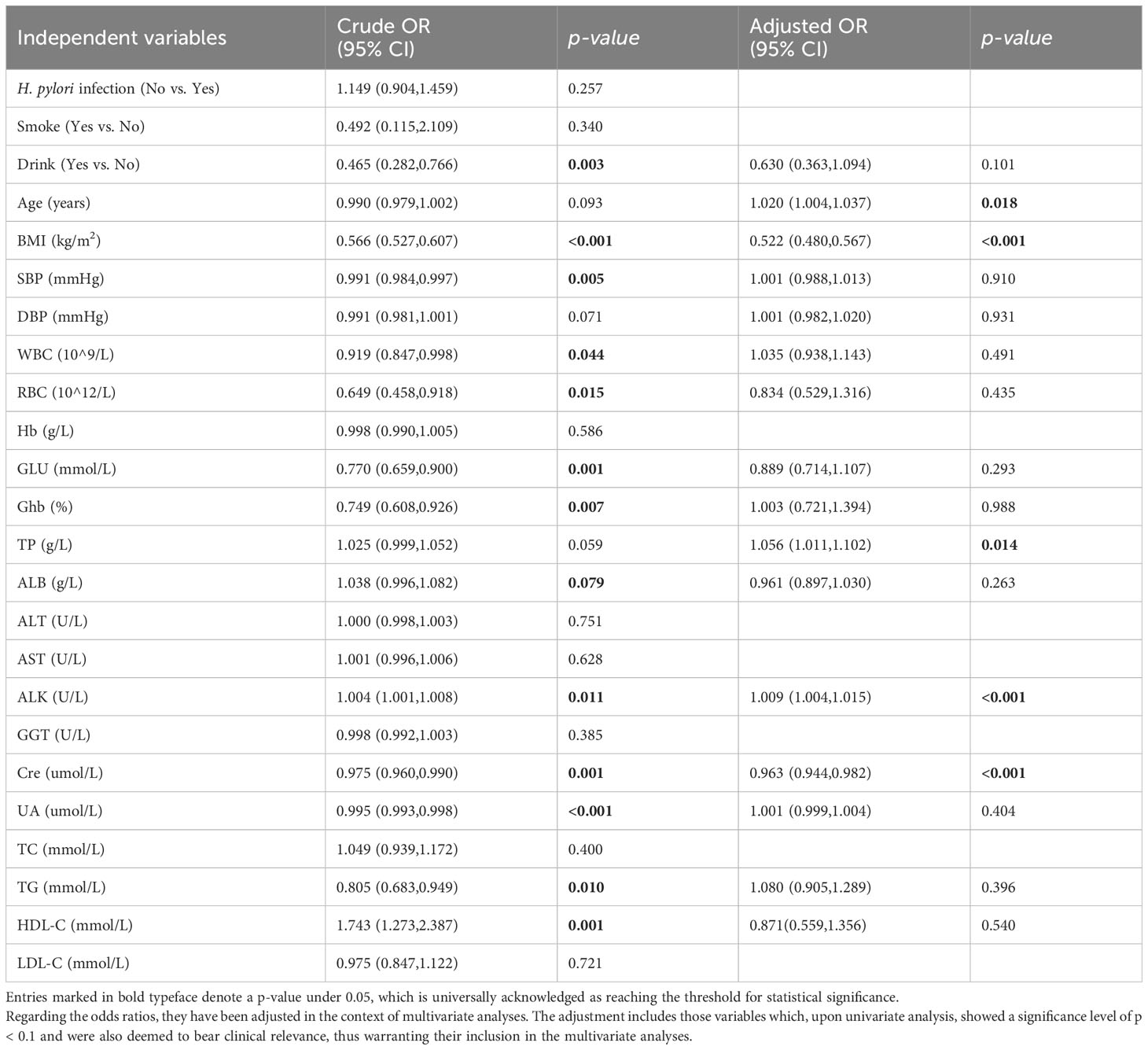
The univariate logistic regression analysis results revealed notable correlations between LSMM and various factors. These include Alcohol Consumption (OR=0.456, 95% CI 0.282-0.766, p=0.003), BMI (OR=0.566, 95% CI 0.527-0.607, p<0.001), SBP (OR=0.991, 95% CI 0.984-0.997, p=0.005), WBC (OR=0.919, 95% CI 0.847-0.998, p=0.044), RBC (OR=0.649, 95% CI 0.458-0.918, p=0.015), Glu (OR=0.770, 95% CI 0.659-0.900, p<0.001), Ghb (OR=0.749 95% CI 0.608-0.926, p=0.007), ALB (OR=1.038, 95% CI 0.996-1,082, p=0.079), ALK (OR=1.004, 95% CI 1.001-1,008, p=0.011), Cre (OR=0.975, 95% CI 0.960-0.990, p=0.001), UA (OR=0.995, 95% CI 0.993-0.998, p<0.001), TG (OR=0.805, 95% CI 0.683-0.949, p=0.010), and HDL-C (OR=1.743, 95% CI 1.273-2.387, p=0.001).
As displayed in Table 2, no significant relationship was found between H. pylori infection status and LSMM, corroborated by univariate analyses (p=0.257). Additionally, we conducted multivariate analyses including variables that were clinically relevant and had a P value < 0.1 in univariate analysis, such as Drink, BMI, SBP, WBC, RBC, Glu, Ghb, ALB, ALK, Cre, UA, TG and HDL-C. Our results indicated a significant association between LSMM and age (OR=1.020, 95% CI 1.004-1.037, p<0.001), BMI (OR=0.522, 95% CI 0.480-0.576, p<0.001), TP (OR=1.056, 95% CI 1.011-1.012, p=0.014), ALK (OR=1.009, 95% CI 1.004-1.015, p<0.001) and Cre (OR=0.965, 95% CI 0.947-0.984, p<0.001). To enhance the understanding of these associations, we have also visualized these findings in a forest plot (Figure 1).
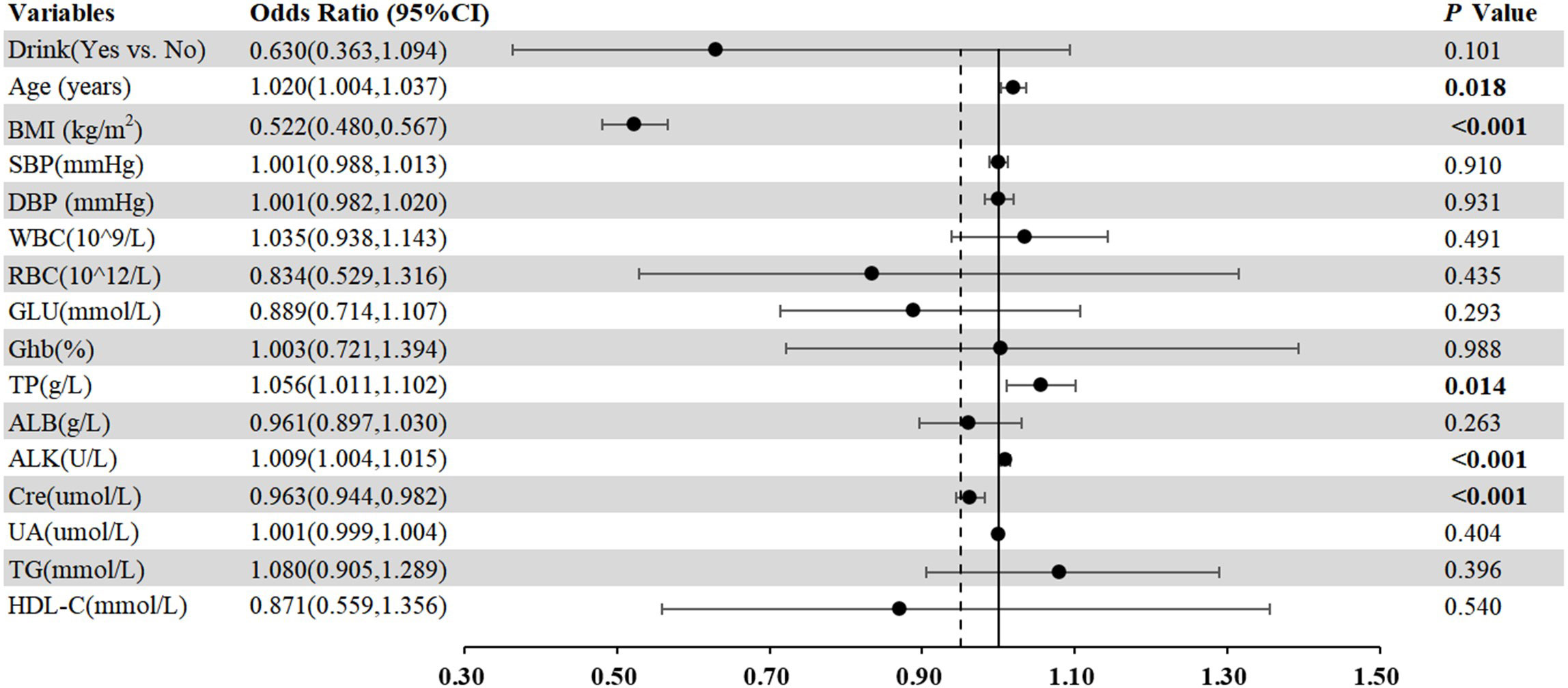
Figure 1 Forest plots of multivariate analysis with 95% CI representing pooled estimates for the association between different markers and LSMM.
The relationship between LSMM and H. pylori infection in different age group
There was not a significant disparity in the presence of LSMM when comparing the group with H. pylori infection and the group without. Nevertheless, when we dissected the data based on age brackets (as depicted in Table 3), a compelling link was found between H. pylori infection status and LSMM among females aged 40 years or older (OR=1.381, 95%CI 1.032-1.848, p = 0.030). This correlation persisted, remaining statistically significant (OR=1.514, 95%CI 1.085-2.113, p = 0.015), even after adjustments were made for several variables (Age, BMI, TP, ALK, Cre). More specifically, our analysis indicated that women aged 40 or older with H. pylori infection displayed an elevated occurrence of LSMM. Conversely, no statistical divergence was observed in relation to H. pylori infection status and LSMM in women younger than 40 years.
The relationship between different age group and markers
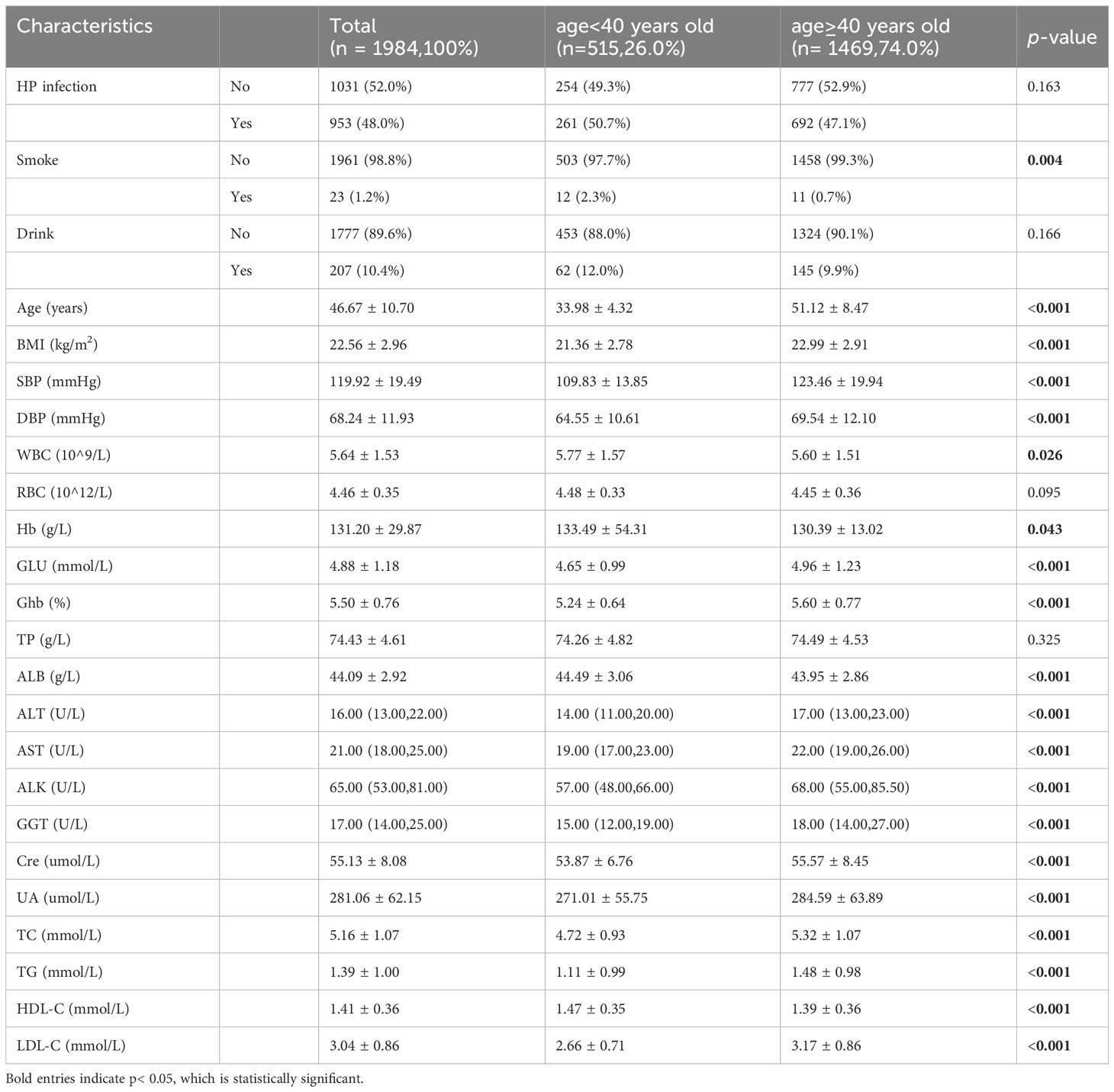
We stratified the study population according to age and further analyzed the baseline characteristics, such as metabolic syndrome related indicators (triglycerides, HDL-C, blood pressure, hyperglycaemia). As delineated in Table 4, significant variances were observed in several markers such as smoking habits, age, BMI, blood pressure, glucose levels, white blood cell count, hemoglobin, liver function tests (ALT, AST, ALK, and GGT), creatinine levels, uric acid levels, and lipid profiles (TC, TG, HDL-C, and LDL-C) across different age groups.
Discussion
In summary, our study has shed light on several critical risk factors associated with LSMM. Through rigorous univariate and multivariate logistic regression analysis, we had identified age, BMI, TP, ALK and Cre as significant risk factors that are closely linked to this disease. These results represent significant additions and provide further insights into the study of risk factors in LSMM. Nonetheless, the crux of this study reveals a meaningful association between H. pylori infection and LSMM in Chinese women aged over 40 years. This implies that women within this demographic who are infected with H. pylori could have an elevated likelihood of LSMM development. The potential mechanisms explaining the harmful effects of H. pylori infection on LSMM could be as follows. Numerous studies had suggested that inflammation response might play vital role in loss of skeletal muscle (Ryall et al., 2008; Londhe and Guttridge, 2015; Dhillon and Hasni, 2017; Howard et al., 2020). For instance, research examining the relationship between older adults sarcopenia and inflammatory factors revealed that patients with sarcopenia had significantly increased circulating concentrations of IL-6 and TNFα (Bian et al., 2017). Additional research has suggested that TNF-α and IL-6 play a significant role in the inflammatory response of skeletal muscle, with elevated levels of IL-6 being associated with reductions in both muscle mass and strength (Wang et al., 2017). The release of the Vacuolating cytotoxin A (VacA) protein plays a crucial role in H. pylori infection, as it initiates the generation of multiple proinflammatory cytokines. These include macrophage-inflammatory protein-1α, TNF-α, along with interleukins such as IL-1β, IL-6, IL-10, and IL-13 (Watanabe et al., 2010; Figueiredo et al., 2014). Additionally, the impact of early environment on growth and development may have long-term effects on humans. A prospective cohort study strongly pointed out that H. pylori infection may hinder linear growth in preschool children (Bravo et al., 2003), leading to reduced absorption of important nutrients and slow growth in children, ultimately resulting in low skeletal muscle mass in older adults individuals (Franceschi et al., 2014). What’s more, ghrelin and leptin may also be factors contributing to the association between H. pylori and LSMM, as H. pylori has been shown to affect their secretion (Meng et al., 2016). Leptin, a hormone secreted by adipose tissue, performs a pivotal function in various physiological processes. These include inflammation regulation, insulin sensitivity, appetite regulation, and the deposition of fat (Lin et al., 2018), while ghrelin can increase muscle mass through stimulating appetite, increasing food intake, promoting myocyte differentiation and fusion, among other ways (Baeg et al., 2015). And several studies showed decreased levels of ghrelin and leptin in patients with LSMM (Kohara et al., 2011; Serra-Prat et al., 2015).
However, there was no statistically significant variation observed among women of all ages. Further analysis conducted on subgroups revealed that LSMM was only linked to H. pylori infection in women aged 40 years and above. Maybe sex hormones were responsible for age-specific differences. Some researches had demonstrated the protective effect of certain steroids against H. pylori infection (Hosoda et al., 2011; Ohtani et al., 2011; Fong and Wang, 2021). For example, Kouichi Hosoda discovered that the growth of H. pylori could be inhibited by estradiol and progesterone. Among the two hormones, progesterone exhibited the strongest antimicrobial effect (Hosoda et al., 2011). In a study conducted by P Fong, the protective effect of oral contraceptives against H. pylori infection was investigated. The results indicated that women who were using oral contraceptives had a significantly reduced risk of acquiring H. pylori infection in comparison to those who did not use them (Fong and Wang, 2021). As women reach the age of 40, their levels of free estradiol decline significantly, which weakens the protective effect of hormones on them (Runolfsdottir et al., 2015).This may lead to a more vulnerable state in older women, resulting in greater muscle decline following H. pylori infection. Moreover, after analyzing the study population, we found that there were significant differences in metabolic syndrome related indicators, including BMI, blood pressure, TC, TG between different age groups. This may also explain why a significant link between H. pylori infection and LSMM was only observed in women aged 40 or older. Metabolic syndrome is also called syndrome X or insulin resistance syndrome, which is a cluster of metabolic abnormalities including abdominal obesity, dysglycemia, elevated blood pressure, low HDL cholesterol and high triglycerides (Saklayen, 2018; Lemieux and Després, 2020). Metabolic syndrome is a group of conditions that raise risk of serious health problems such as coronary heart disease, type 2 diabetes, stroke, and even all-cause mortality (Isomaa et al., 2001; Grundy, 2008). Many studies have linked sarcopenia to various metabolic disorders, such as high blood pressure, dyslipidemia, dysglycemia (Doğan et al., 2012; Baek et al., 2014; Nishikawa et al., 2021). Several previous studies have revealed a relationship between metabolic syndrome and sarcopenia. Metabolic syndrome may be associated with muscle loss through a complex interplay of multiple factors, including pro-inflammatory cytokines, insulin resistance, mitochondrial dysfunction and oxidative stress (Sanada et al., 2012; Moon, 2014; Rubio-Ruiz et al., 2019; Gonzalez et al., 2021).
Our results underscore the significance of incorporating these elements when investigating the link between H. pylori and LSMM across various age brackets. Future studies can utilize these findings as a foundation to delve deeper into the age-specific disparities associated with the influence of H. pylori infection on muscle deterioration, and to pinpoint potential preventive measures or treatments to alleviate its consequences.
Up until now, there has been scant investigation into the correlation between H. pylori infection and LSMM. That being said, in a study conducted with a sample size of 3453 American individuals aged 60 and over indicated an association between positive markers of H. pylori infection in serum and the presence of sarcopenia along with reduced muscle mass (Wu and Chen, 2021). Although this study had a larger number of participants, it only included American people aged ≥60 years. Our study included Asian populations, which are different from the ethnic groups they studied. What’s more, based on the available evidence, it is estimated that more than 1 in every 10 young adults from various ethnicities may have sarcopenia (Jung et al., 2023). Thus, it is imperative to associate sarcopenia not only with frail older patients but also with younger patients. So, we selected the physical examination population aged >18 years as the inclusion population.
Another study compared healthy, asymptomatic women who had received H. pylori eradication treatment with those who were HP IgG positive but had not undergone eradication therapy. This study found that older adults women who underwent H. pylori eradication had a reduced risk of LSMM (Baeg et al., 2015). It is worth mentioning that both of these studies utilized serological testing as the method for determining the status of H. pylori infection. While this method is commonly used, it requires validation for specific locations and may produce false results due to cross-reactivity. Additionally, serology antibody levels often persist for several years after therapy, which can make it difficult to accurately assess current infection status and disease outcomes (Katelaris et al., 2023). In contrast, our study utilized the 13C-urea breath test to diagnose active H. pylori infection, which is considered a reliable technique for detecting active infections (Huh and Kim, 2018). It is widely recognized that urea breath tests (UBTs) are more accurate than other non-invasive tests in patients who have not undergone gastrectomy (Katelaris et al., 2023).Furthermore, it is important to acknowledge that our research has some limitations. Firstly, due to its retrospective nature, there may have been some selection bias in the study population. Additionally, we did not collect data on certain critical factors related to LSMM, such as muscle strength and gait speed. And we also did not collect some important data such as sex hormone levels. Hence, future research endeavors should strive to incorporate a more extensive sample size and meticulous data collection in order to delve deeper into the association between H. pylori infection and LSMM. By doing so, we can gain a more complete understanding of this complex issue and develop better strategies for managing and preventing LSMM in particular individuals.
Conclusions
Our study did not conclusively show a connection between H. pylori infection and LSMM among all women. Interestingly, our findings indicated a potential link between H. pylori infection and LSMM in women aged over 40. It suggests that there is a certain correlation between menopause and low skeletal muscle mass in women. And this underscores the significance of early detection and remediation of H. pylori infection as a possible tactic for warding off LSMM in this demographic. A more profound exploration is required to decipher the mechanisms behind this association and how it fluctuates across distinct populations. The importance of pursuing this research topic lies in forming more potent approaches to managing and mitigating LSMM, especially in high-risk individuals due to factors like age or sex. In summary, while our study didn’t present definitive proof, it hinted at a possible association between H. pylori infection and LSMM in certain demographics. Future research should strive to delve deeper into this relationship, factoring in elements such as dietary habits, physical activity level and sex hormone levels, to achieve a holistic understanding of this concern.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XX: Writing – original draft, Formal analysis, Conceptualization, Methodology. YQ: Formal analysis, Writing – original draft, Conceptualization, Investigation. KJ: Data curation, Formal analysis, Resources, Writing – original draft. JC: Resources, Validation, Writing – original draft. JF: Data curation, Writing – original draft. CC: Conceptualization, Writing – review & editing, Funding acquisition, Supervision. ZZ: Supervision, Writing – review & editing, Conceptualization, Project administration.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the following grants: National Key Research and Development Program of China grants 2016YFC1304000 (CC); The National Natural Science Foundation of China 82170017 (CC); Zhejiang Provincial Key Research and Development Program 2020C03067 (CC).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Baeg, M. K., Choi, M.-G., Ko, S.-H., Lim, C.-H., Kim, J. S., Cho, Y. K., et al. (2015). Elderly women who received Helicobacter pylori-eradicating therapy have reduced risk of low skeletal muscle mass. Clin. Interv Aging 10, 1771–1777. doi: 10.2147/CIA.S95007
Baek, S. J., Nam, G. E., Han, K. D., Choi, S. W., Jung, S. W., Bok, A. R., et al. (2014). Sarcopenia and sarcopenic obesity and their association with dyslipidemia in Korean elderly men: the 2008-2010 Korea National Health and Nutrition Examination Survey. J. Endocrinol. Invest. 37, 247–260. doi: 10.1007/s40618-013-0011-3
Bian, A.-L., Hu, H.-Y., Rong, Y.-D., Wang, J., Wang, J.-X., Zhou, X.-Z. (2017). A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur. J. Med. Res. 22, 25. doi: 10.1186/s40001-017-0266-9
Bravo, D., Hoare, A., Soto, C., Valenzuela, M. A., Quest, A. F. (2018). Helicobacter pylori in human health and disease: Mechanisms for local gastric and systemic effects. World J. Gastroenterol. 24, 3071–3089. doi: 10.3748/wjg.v24.i28.3071
Bravo, L. E., Mera, R., Reina, J. C., Pradilla, A., Alzate, A., Fontham, E., et al. (2003). Impact of Helicobacter pylori infection on growth of children: a prospective cohort study. J. Pediatr. Gastroenterol. Nutr. 37, 614–619. doi: 10.1097/00005176-200311000-00021
Buzás, G. M. (2020). Helicobacter pylori and non-alcoholic fatty liver disease. Minerva Gastroenterol. Dietol 66, 267–279. doi: 10.23736/S1121-421X.20.02671-9
Chen, T.-P., Hung, H.-F., Chen, M.-K., Lai, H.-H., Hsu, W.-F., Huang, K.-C., et al. (2015). Helicobacter pylori infection is positively associated with metabolic syndrome in Taiwanese adults: a cross-sectional study. Helicobacter 20, 184–191. doi: 10.1111/hel.12190
Chen, T.-S., Chang, F.-Y., Chen, P.-C., Huang, T. W., Ou, J. T., Tsai, M.-H., et al. (2003). Simplified 13C-urea breath test with a new infrared spectrometer for diagnosis of Helicobacter pylori infection. J. Gastroenterol. Hepatol. 18, 1237–1243. doi: 10.1046/j.1440-1746.2003.03139.x
Chen, Z., Li, W.-Y., Ho, M., Chau, P.-H. (2021). The prevalence of sarcopenia in Chinese older adults: meta-analysis and meta-regression. Nutrients 13, 1441. doi: 10.3390/nu13051441
Chen, L. K., Liu, L. K., Woo, J., Assantachai, P., Auyeung, T.-W., Bahyah, K. S., et al. (2014). Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Directors Assoc. 15, 95–101. doi: 10.1016/j.jamda.2013.11.025
Chen, L.-K., Woo, J., Assantachai, P., Auyeung, T.-W., Chou, M.-Y., Iijima, K., et al. (2020). Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir Assoc. 21, 300–307.e2. doi: 10.1016/j.jamda.2019.12.012
Churchward-Venne, T. A., Breen, L., Phillips, S. M. (2014). Alterations in human muscle protein metabolism with aging: Protein and exercise as countermeasures to offset sarcopenia. Biofactors 40, 199–205. doi: 10.1002/biof.1138
Cover, T. L., Blaser, M. J. (2009). Helicobacter pylori in health and disease. Gastroenterology 136, 1863–1873. doi: 10.1053/j.gastro.2009.01.073
Dhillon, R. J. S., Hasni, S. (2017). Pathogenesis and management of sarcopenia. Clin. Geriatr. Med. 33, 17–26. doi: 10.1016/j.cger.2016.08.002
Di Rienzo, T. A., D’Angelo, G., Ojetti, V., Campanale, M. C., Tortora, A., Cesario, V., et al. (2013). 13C-Urea breath test for the diagnosis of Helicobacter pylori infection. Eur. Rev. Med. Pharmacol. Sci. 17 (Suppl 2), 51–58.
Doğan, M. H., Karadag, B., Ozyigit, T., Kayaoglu, S., Ozturk, A. O., Altuntas, Y. (2012). Correlations between sarcopenia and hypertensive target organ damage in a Turkish cohort. Acta Clin. Belg 67, 328–332. doi: 10.2143/ACB.67.5.2062685
Faulkner, J. A., Larkin, L. M., Claflin, D. R., Brooks, S. V. (2007). Age-related changes in the structure and function of skeletal muscles. Clin. Exp. Pharmacol. Physiol. 34, 1091–1096. doi: 10.1111/j.1440-1681.2007.04752.x
Fielding, R. A., Vellas, B., Evans, W. J., Bhasin, S., Morley, J. E., Newman, A. B., et al. (2011). Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir Assoc. 12, 249–256. doi: 10.1016/j.jamda.2011.01.003
Figueiredo, C. A., Marques, C. R., Costa R dos, S., da Silva, H. B. F., Alcantara-Neves, N. M. (2014). Cytokines, cytokine gene polymorphisms and Helicobacter pylori infection: friend or foe? World J. Gastroenterol. 20, 5235–5243. doi: 10.3748/wjg.v20.i18.5235
Fong, P., Wang, Q. T. (2021). Protective effect of oral contraceptive against Helicobacter pylori infection in US adult females: NHANES 1999-2000. Epidemiol. Infect. 149, e120. doi: 10.1017/S0950268821000923
Franceschi, F., Annalisa, T., Teresa, D. R., Giovanna, D., Ianiro, G., Franco, S., et al. (2014). Role of Helicobacter pylori infection on nutrition and metabolism. World J. Gastroenterol. 20, 12809–12817. doi: 10.3748/wjg.v20.i36.12809
Franceschi, F., Gasbarrini, A. (2007). Helicobacter pylori and extragastric diseases. Best Pract. Res. Clin. Gastroenterol. 21, 325–334. doi: 10.1016/j.bpg.2006.10.003
Geraci, A., Calvani, R., Ferri, E., Marzetti, E., Arosio, B., Cesari, M. (2021). Sarcopenia and menopause: the role of estradiol. Front. Endocrinol. (Lausanne) 12. doi: 10.3389/fendo.2021.682012
Gisbert, J. P., Calvet, X. (2013). Helicobacter pylori “Test-and-treat” Strategy for management of dyspepsia: A comprehensive review. Clin. Transl. Gastroenterol. 4, e32. doi: 10.1038/ctg.2013.3
Gisbert, J. P., Pajares, J. M. (2004). Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection – a critical review. Aliment Pharmacol. Ther. 20, 1001–1017. doi: 10.1111/j.1365-2036.2004.02203.x
Gonzalez, A., Simon, F., Achiardi, O., Vilos, C., Cabrera, D., Cabello-Verrugio, C. (2021). The critical role of oxidative stress in sarcopenic obesity. Oxid. Med. Cell Longev 2021, 4493817. doi: 10.1155/2021/4493817
Grundy, S. M. (2008). Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 28, 629–636. doi: 10.1161/ATVBAHA.107.151092
Hosoda, K., Shimomura, H., Hayashi, S., Yokota, K., Hirai, Y. (2011). Steroid hormones as bactericidal agents to Helicobacter pylori. FEMS Microbiol. Lett. 318, 68–75. doi: 10.1111/j.1574-6968.2011.02239.x
Howard, E. E., Pasiakos, S. M., Blesso, C. N., Fussell, M. A., Rodriguez, N. R. (2020). Divergent roles of inflammation in skeletal muscle recovery from injury. Front. Physiol. 11. doi: 10.3389/fphys.2020.00087
Huh, C. W., Kim, B.-W. (2018). [Diagnosis of helicobacter pylori infection]. Korean J. Gastroenterol. 72, 229–236. doi: 10.4166/kjg.2018.72.5.229
Hunt, R. H., Xiao, S. D., Megraud, F., Leon-Barua, R., Bazzoli, F., van der Merwe, S., et al. (2011). World gastroenterology organisation global guideline: Helicobacter pylori in developing countries. J. Dig. Dis. 12, 319–326. doi: 10.1111/j.1751-2980.2011.00529.x
Isomaa, B., Almgren, P., Tuomi, T., Forsén, B., Lahti, K., Nissén, M., et al. (2001). Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24, 683–689. doi: 10.2337/diacare.24.4.683
Jung, H. N., Jung, C. H., Hwang, Y.-C. (2023). Sarcopenia in youth. Metabolism 144, 155557. doi: 10.1016/j.metabol.2023.155557
Katelaris, P., Hunt, R., Bazzoli, F., Cohen, H., Fock, K. M., Gemilyan, M., et al. (2023). Helicobacter pylori world gastroenterology organization global guideline. J. Clin. Gastroenterol. 57, 111–126. doi: 10.1097/MCG.0000000000001719
Kohara, K., Ochi, M., Tabara, Y., Nagai, T., Igase, M., Miki, T. (2011). Leptin in sarcopenic visceral obesity: possible link between adipocytes and myocytes. PloS One 6, e24633. doi: 10.1371/journal.pone.0024633
Lemieux, I., Després, J.-P. (2020). Metabolic syndrome: past, present and future. Nutrients 12, 3501. doi: 10.3390/nu12113501
Lin, Y.-L., Wang, C.-H., Lai, Y.-H., Kuo, C.-H., Syu, R.-J., Hsu, B.-G. (2018). Negative correlation between leptin serum levels and sarcopenia in hemodialysis patients. Int. J. Clin. Exp. Pathol. 11, 1715–1723.
Linz, B., Balloux, F., Moodley, Y., Manica, A., Liu, H., Roumagnac, P., et al. (2007). An African origin for the intimate association between humans and Helicobacter pylori. Nature 445, 915–918. doi: 10.1038/nature05562
Lj, F., Mo, H.-L. (2017)Sarcopenia and the new ICD-10-CM code: screening, staging, and diagnosis considerations. In: Federal practitioner: for the health care professionals of the VA, DoD, and PHS. (Accessed November 22, 2023).
Logan, R. P. (1998). Urea breath tests in the management of Helicobacter pylori infection. Gut 43 Suppl 1, S47–S50. doi: 10.1136/gut.43.2008.s47
Londhe, P., Guttridge, D. C. (2015). Inflammation induced loss of skeletal muscle. Bone 80, 131–142. doi: 10.1016/j.bone.2015.03.015
Malfertheiner, P., Megraud, F., O’Morain, C. A., Gisbert, J. P., Kuipers, E. J., Axon, A. T., et al. (2017). Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 66, 6–30. doi: 10.1136/gutjnl-2016-312288
Marshall, B. J., Warren, J. R. (1984). Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1, 1311–1315. doi: 10.1016/s0140-6736(84)91816-6
Meng, W.-P., Wang, Z.-Q., Deng, J.-Q., Liu, Y., Deng, M.-M., Lü, M.-H. (2016). The role of H. pylori cagA in regulating hormones of functional dyspepsia patients. Gastroenterol. Res. Pract. 2016, 7150959. doi: 10.1155/2016/7150959
Moon, S.-S. (2014). Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009-2010. Endocr. J. 61, 61–70. doi: 10.1507/endocrj.ej13-0244
Nishikawa, H., Fukunishi, S., Asai, A., Yokohama, K., Ohama, H., Nishiguchi, S., et al. (2021). Sarcopenia, frailty and type 2 diabetes mellitus (Review). Mol. Med. Rep. 24, 854. doi: 10.3892/mmr.2021.12494
Nocon, M., Kuhlmann, A., Leodolter, A., Roll, S., Vauth, C., Willich, S. N., et al. (2009). Efficacy and cost-effectiveness of the 13C-urea breath test as the primary diagnostic investigation for the detection of Helicobacter pylori infection compared to invasive and non-invasive diagnostic tests. GMS Health Technol. Assess. 5, Doc14. doi: 10.3205/hta000076
Ohtani, M., Ge, Z., García, A., Rogers, A. B., Muthupalani, S., Taylor, N. S., et al. (2011). 17 β-estradiol suppresses Helicobacter pylori-induced gastric pathology in male hypergastrinemic INS-GAS mice. Carcinogenesis 32, 1244–1250. doi: 10.1093/carcin/bgr072
Papadopoulou, S. K., Tsintavis, P., Potsaki, P., Papandreou, D. (2020). Differences in the prevalence of sarcopenia in community-dwelling, nursing home and hospitalized individuals. A systematic review and meta-analysis. J. Nutr. Health Aging 24, 83–90. doi: 10.1007/s12603-019-1267-x
Petermann-Rocha, F., Balntzi, V., Gray, S. R., Lara, J., Ho, F. K., Pell, J. P., et al. (2022). Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J. cachexia sarcopenia Muscle 13, 86–99. doi: 10.1002/jcsm.12783
Ricci, C., Holton, J., Vaira, D. (2007). Diagnosis of Helicobacter pylori: invasive and non-invasive tests. Best Pract. Res. Clin. Gastroenterol. 21, 299–313. doi: 10.1016/j.bpg.2006.11.002
Rubio-Ruiz, M. E., Guarner-Lans, V., Pérez-Torres, I., Soto, M. E. (2019). Mechanisms underlying metabolic syndrome-related sarcopenia and possible therapeutic measures. Int. J. Mol. Sci. 20, 647. doi: 10.3390/ijms20030647
Runolfsdottir, H. L., Sigurdsson, G., Franzson, L., Indridason, O. S. (2015). Gender comparison of factors associated with age-related differences in bone mineral density. Arch. Osteoporos 10, 214. doi: 10.1007/s11657-015-0214-7
Ryall, J. G., Schertzer, J. D., Lynch, G. S. (2008). Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology 9, 213–228. doi: 10.1007/s10522-008-9131-0
Saklayen, M. G. (2018). The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 20, 12. doi: 10.1007/s11906-018-0812-z
Sanada, K., Iemitsu, M., Murakami, H., Gando, Y., Kawano, H., Kawakami, R., et al. (2012). Adverse effects of coexistence of sarcopenia and metabolic syndrome in Japanese women. Eur. J. Clin. Nutr. 66, 1093–1098. doi: 10.1038/ejcn.2012.43
Serra-Prat, M., Papiol, M., Monteis, R., Palomera, E., Cabré, M. (2015). Relationship between plasma ghrelin levels and sarcopenia in elderly subjects: A cross-sectional study. J. Nutr. Health Aging 19, 669–672. doi: 10.1007/s12603-015-0550-8
Sk, P. (2020). Sarcopenia: A contemporary health problem among older adult populations. Nutrients 12, 1293. doi: 10.3390/nu12051293
Walowski, C. O., Braun, W., Maisch, M. J., Jensen, B., Peine, S., Norman, K., et al. (2020). Reference values for skeletal muscle mass - current concepts and methodological considerations. Nutrients 12, 755. doi: 10.3390/nu12030755
Wang, J., Leung, K.-S., Chow, S. K.-H., Cheung, W.-H. (2017). Inflammation and age-associated skeletal muscle deterioration (sarcopaenia). J. Orthop Translat 10, 94–101. doi: 10.1016/j.jot.2017.05.006
Watanabe, T., Asano, N., Fichtner-, Gorelick, P. L., Tsuji, Y., Matsumoto, Y., et al. (2010). NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type I IFN and activation of the ISGF3 signaling pathway. J. Clin. Invest. 120, 1645–1662. doi: 10.1172/JCI39481
Wu, S.-E., Chen, W.-L. (2021). Detrimental relevance of Helicobacter pylori infection with sarcopenia. Gut Pathog. 13, 67. doi: 10.1186/s13099-021-00464-y
Yang, L., Smith, L., Hamer, M. (2019). Gender-specific risk factors for incident sarcopenia: 8-year follow-up of the English longitudinal study of ageing. J. Epidemiol. Community Health 73, 86–88. doi: 10.1136/jech-2018-211258
Zamani, M., Ebrahimtabar, F., Zamani, V., Miller, W. H., Alizadeh-Navaei, R., Shokri-Shirvani, J., et al. (2018). Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol. Ther. 47, 868–876. doi: 10.1111/apt.14561
Keywords: low skeletal muscle mass, 13C-urea breath test, Helicobacter pylori infection, risk factors, female
Citation: Xu X, Qian Y, Jin K, Chen J, Fu J, Chen C and Zhu Z (2024) The impact of Helicobacter pylori infection on low skeletal muscle mass risk in Chinese women over 40: a cross-sectional analysis. Front. Cell. Infect. Microbiol. 13:1289909. doi: 10.3389/fcimb.2023.1289909
Received: 07 September 2023; Accepted: 08 December 2023;
Published: 03 January 2024.
Edited by:
Dania AlQasrawi, Mayo Clinic Florida, United StatesReviewed by:
Joseph Vaccaro, University of Central Florida, United StatesAriege Bizanti, University of Central Florida, United States
Copyright © 2024 Xu, Qian, Jin, Chen, Fu, Chen and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zaisheng Zhu, zhuzaisheng@wmu.edu.cn; Chengshui Chen, chenchengshui@wmu.edu.cn
†These authors have contributed equally to this work
 Xiaohui Xu
Xiaohui Xu Yidan Qian
Yidan Qian Kejia Jin
Kejia Jin Junpeng Chen
Junpeng Chen Jiayue Fu
Jiayue Fu Chengshui Chen
Chengshui Chen Zaisheng Zhu
Zaisheng Zhu