Tracing the trajectories of SARS-CoV-2 variants of concern between December 2020 and September 2021 in the Canary Islands (Spain)
- 1Research Unit, Hospital Universitario Nuestra Señora de Candelaria, Santa Cruz de Tenerife, Spain
- 2Genomics Division, Instituto Tecnológico y de Energías Renovables, Santa Cruz de Tenerife, Spain
- 3Servicio de Microbiología, Hospital Universitario Nuestra Señora de Candelaria, Santa Cruz de Tenerife, Spain
- 4Servicio de Microbiología, Hospital Universitario Insular de Gran Canaria, Las Palmas de Gran Canaria, Spain
- 5Laboratorio de Inmunología Celular y Viral, Unidad de Farmacología, Facultad de Medicina, Universidad de La Laguna, San Cristóbal de La Laguna, Spain
- 6CIBER de Enfermedades Respiratorias, Instituto de Salud Carlos III, Madrid, Spain
- 7Faculty of Health Sciences, University of Fernando Pessoa Canarias, Las Palmas de Gran Canaria, Spain
Several variants of concern (VOCs) explain most of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic waves in Europe. We aimed to dissect the spread of the SARS-CoV-2 VOCs in the Canary Islands (Spain) between December 2020 and September 2021 at a micro-geographical level. We sequenced the viral genome of 8,224 respiratory samples collected in the archipelago. We observed that Alpha (B.1.1.7) and Delta (B.1.617.2 and sublineages) were ubiquitously present in the islands, while Beta (B.1.351) and Gamma (P.1/P.1.1) had a heterogeneous distribution and were responsible for fewer and more controlled outbreaks. This work represents the largest effort for viral genomic surveillance in the Canary Islands so far, helping the public health bodies in decision-making throughout the pandemic.
Introduction
The continuous emergence and international widespread of variants of concern (VOCs) of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have revealed the occurrence of multiple viral spike gene mutations that promote increased transmissibility and some degree of immune escape (Tao et al., 2021). In response, the public health system activated the organization of centers for routine viral genome surveillance starting in mid-January 2021 in our laboratories, which were later distinguished as the Reference Centre for the Network of COVID-19 Genomic Surveillance in the Canary Islands.
We have previously tracked the entrance and surge of one of the three most widely distributed VOCs, B.1.1.7 [Pango lineage nomenclature (Rambaut et al., 2020)], or Alpha variant (WHO, 2022), in December 2020 in Tenerife (Alcoba-Florez et al., 2021) due to the island direct flight connections with the United Kingdom where it may have originated. This lineage has been associated with increased transmissibility (Davies et al., 2021; Volz et al., 2021) compared to the previously circulating variants, a feature that has been linked to the presence of the mutation N501Y in the Spike (S) receptor-binding domain (RBD). Other VOCs that have also emerged in late 2020 are B.1.351, or the Beta variant, and P.1/P.1.1, or the Gamma variant, firstly identified in South Africa (Tegally et al., 2021) and in Brazil (Faria et al., 2021), respectively. These lineages have been associated with increased transmission (Faria et al., 2021; Pearson et al., 2021; Tegally et al., 2021) and carry multiple mutations affecting the RBD, most importantly the N501Y (such as Alpha), as well as K417N/K417T and E484K that reduce the effectiveness of some vaccines (Garcia-Beltran et al., 2021) and specific monoclonal antibody treatments (Greaney et al., 2021; Harvey et al., 2021). Later in time, lineage B.1.617.2, or the Delta variant, was identified in India where it was responsible for a surge of coronavirus disease 2019 (COVID-19) cases in April 2021 (Dhar et al., 2021). The Delta (B.1.617.2) variant presented additional mutations, such as the L452R in the RBD and the P618R, and has been characterized by higher transmissibility (Campbell et al., 2021b) and reduced sensitivity to antibody neutralization (Planas et al., 2021; Wall et al., 2021) and vaccine effectiveness (Lopez Bernal et al., 2021; Planas et al., 2021). From the Delta variant, numerous sublineages have emerged, reporting the presence of additional mutations such as the Y145H and the Y222V in the N-terminal domain of the S protein found in the AY.4.2 lineage (Gangavarapu et al., 2022), which was suggested to be more transmissible than the original variant (Public Health England, 2021). Delta and its sublineages became prevalent and completely displaced Alpha worldwide throughout the summer of 2021 (WHO, 2022).
Here, we describe the introduction and temporal evolution of these four VOCs in the Canary Islands (Spain). The archipelago is formed by eight islands, four occidental (Tenerife, La Palma, La Gomera, and El Hierro) belonging to the Tenerife province and four oriental islands (Gran Canaria, Fuerteventura, Lanzarote, and La Graciosa) belonging to the Gran Canaria province (Figure 1). Throughout the study period (18 December 2020 to 29 September 2021), there were a total of 70,401 reported COVID-19 cases and 489 COVID-19 deaths in the archipelago, with the islands of Tenerife and Gran Canaria accounting for most of them (60,069 COVID-19 cases and 421 deaths) (https://opendata.sitcan.es/dataset/datos-epidemiologicos-covid-19).
Figure 1 Geographical location of the Canary Islands with respect to the Iberian Peninsula and Northwest Africa.
The relative geographical isolation of the archipelago and its islands and their central role as a hub for international tourism and in the European migratory crisis have shaped the COVID-19 pandemic and delineated the introduction and spread of these VOCs in the territory. In fact, the first SARS-CoV-2 outbreaks in Spain were detected in La Gomera and Tenerife islands (January and February 2020, respectively; available reports from: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/Informes%20COVID-19/Informe%20COVID-19.%20N%c2%ba%201_11febrero2020_ISCIII.pdf).
Methods
Design and sample collection
The study was conducted at the University Hospital Nuestra Senãora de Candelaria (HUNSC) (Santa Cruz de Tenerife, Spain). The institutional review board approved the study (approval number: CHUNSC_2020_24). We assessed nasopharyngeal swabs from COVID-19/SARS-CoV-2 patients from 18 December 2020 to 27 September 2021. Routine COVID-19 testing in the centers was conducted using diverse commercial RT-qPCR alternatives, as described elsewhere (Alcoba-Florez et al., 2020). In line with the guidelines indicated by the ECDC (European Centre for Disease Prevention and Control, 2021) and assuming a prevalence of 10,000 positive cases per month in the territory (https://opendata.sitcan.es/dataset/datos-epidemiologicos-covid-19), we estimated the sequencing of 12,000 samples a year for accurate tracking of viral variants (at 50% accuracy in prevalence estimates).
Viral genome sequencing and classification
Samples were selected for sequencing if they showed a cycle threshold (Ct) ≤30 for any of the amplicon targets included in the COVID-19 diagnostic kits. Libraries were prepared following either the Midnight protocol v.4 (Freed et al., 2020) by means of the Rapid Barcoding kit (SQK-RBK004, Oxford Nanopore Technologies) and sequenced on a MinION and an R9.4 flow cell (Oxford Nanopore Technologies) for 6 h or the COVIDSeq Test (Illumina, Inc.) protocol based on the ARTIC V3 amplicons and sequenced on a NextSeq 550 (Illumina) instrument on High Output mode with 36-bp single- or pair-end (after 7 September 2021) reads following the procedures described elsewhere (Alcoba-Florez et al., 2021; Ciuffreda et al., 2021). Positive and negative amplification controls were included in each run (one for each fraction of 94 samples).
The RAMPART (v.1.2.0) software package was used for real-time monitoring of the MinION sequencing run. Reads were basecalled and demultiplexed with Guppy 4.2.2 (high-accuracy mode), and the ARTIC Network bioinformatics procedures (https://github.com/artic-network/artic-ncov2019) were used for read filtering (by length, 250–1,500 bp), consensus assembly, and variant calling (nanopolish workflow with maximum coverage of 200×). The COVIDSeq Test reads were processed as described elsewhere (Alcoba-Florez et al., 2021) based on the DRAGEN COVIDSeq Test v1.2.2 pipeline and the DRAGEN Lineage v3.5.3 (Illumina, Inc.). Nextclade v.0.14.3 (Aksamentov et al., 2021) was used for variant calling and functional predictions. In the final analysis, sequences having a genome coverage less than 70% of the entire SARS-CoV-2 sequence and having a QC classified as “bad” by Nextclade software were excluded. Pangolin v3.1.1 (O’Toole et al., 2021b) was used for the classification of the consensus sequences. Microbetrace v0.8.2 (Campbell et al., 2021a) was used for cluster investigation together with epidemiological data from the public health authorities.
Results
An overview of COVID-19 cases and sequenced samples in the Canary Islands throughout the study period is presented in Figures 2, S1. Of the 70,588 positive samples collected in the archipelago from 18 December 2020 to 27 September 2021, 11,956 (16.9%) were sequenced using either Illumina sequencing (11,936) or Oxford Nanopore Technologies (20). Of these, 8,224 samples passed the QC filtering steps and had an assigned lineage. In the period, 3,447 samples (41.9%) were assigned to Alpha, 138 (1.7%) to Beta, 47 (0.6%) to Gamma, and 3,262 (39.7%) to Delta and sublineages of Delta [1,066 (12.9%)], while 1,330 (16.1%) were assigned to other non-VOC lineages. Within the Delta sublineages, AY.4 (153) and AY.12 (119) were the most commonly found throughout the period. None of the Delta sublineages was assigned to AY.4.2 (also known as Delta plus).
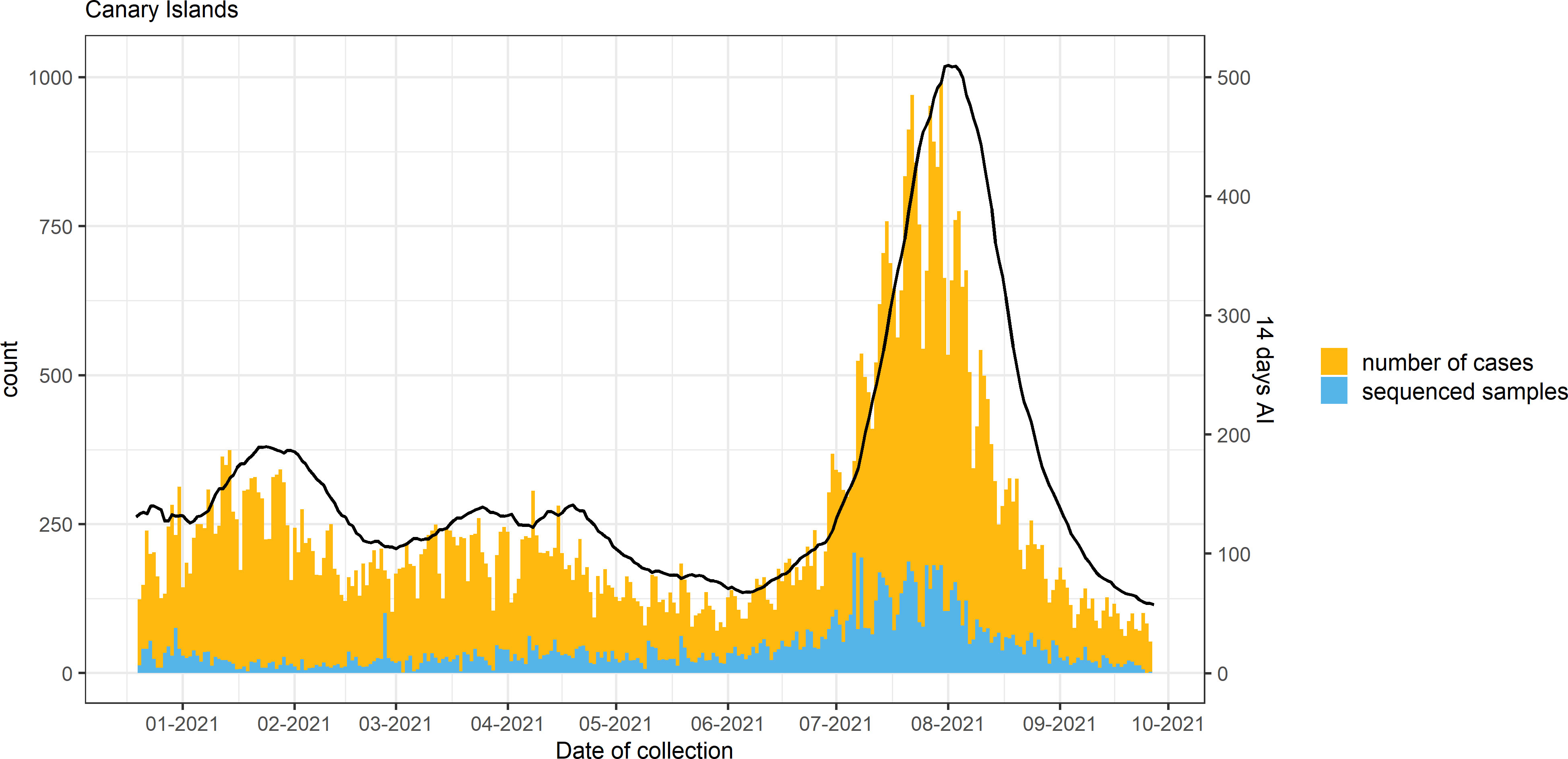
Figure 2 Number of cases, sequenced samples, and the 14-day accumulated incidence (AI; continuous line) throughout the study period in the Canary Islands archipelago.
The temporal distribution of the different VOCs in the Canary Islands is represented in Figure 3. Since there were relatively few samples sequenced from two of the smallest islands [La Gomera (n = 17) and El Hierro (n = 50)] (Figure S1) and the samples from La Graciosa were collected within those from Lanzarote, we excluded these islands from the following description. As it was previously reported for Tenerife alone (Alcoba-Florez et al., 2021), the first description of Alpha in the Canary Islands occurred around 23 December 2020 and was associated with an increase in the 14-day accumulated incidence (Figure 3A). After that, the replacement of non-VOC lineages by the Alpha variant occurred more sharply in Gran Canaria and Lanzarote than in the other islands (Tenerife, Fuerteventura, and La Palma) with almost the totality of sequenced cases belonging to Alpha already by early February 2021 (Figures 3B, C).
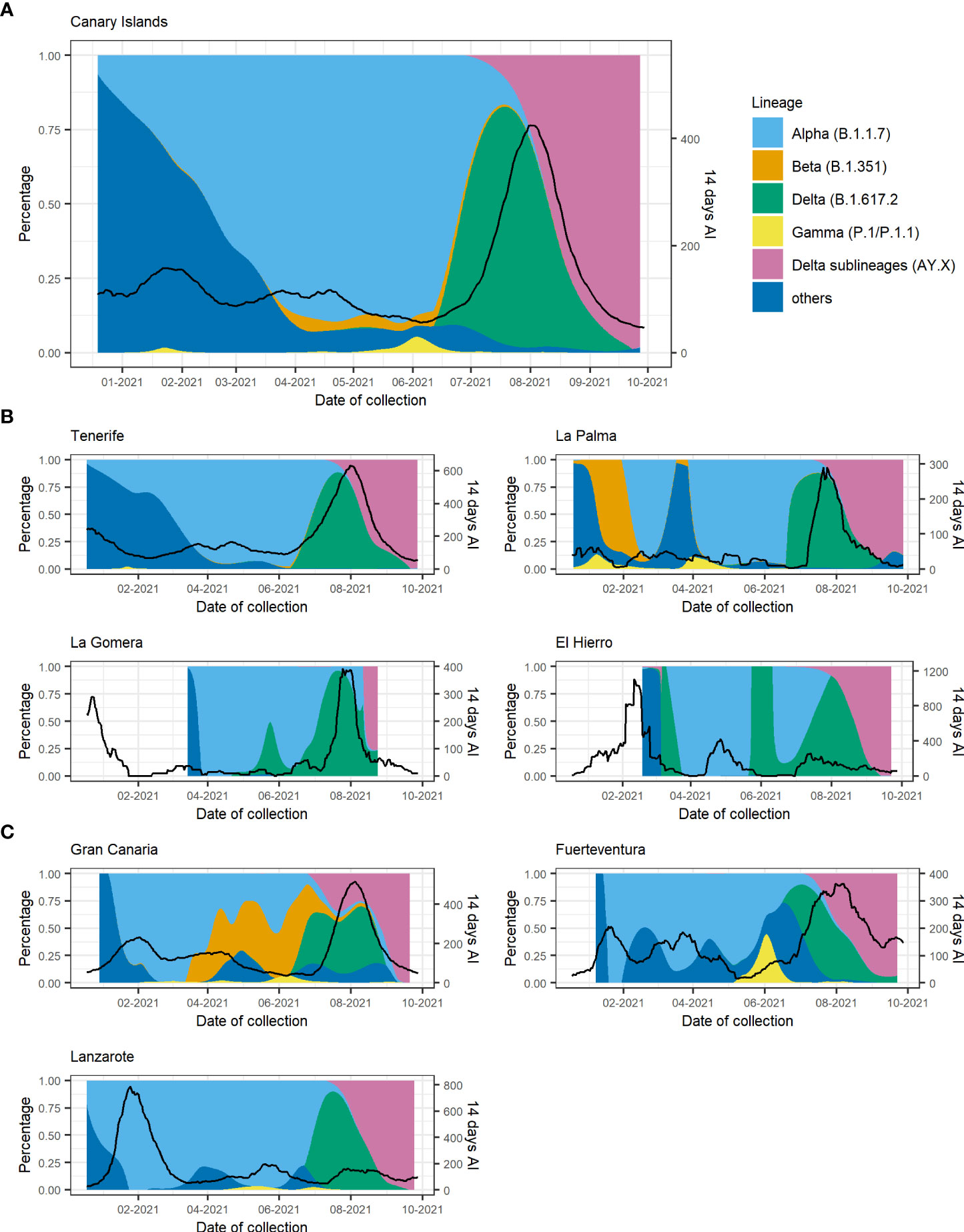
Figure 3 Proportion of variants of concern (VOCs) and the 14-day accumulated incidence throughout the study period for (A) the Canary Islands as a whole and disaggregated by island for (B) the Tenerife province and (C) the Gran Canaria province. Black lines depict the 14-day accumulated incidence. AY.X denotes the sublineages of Delta (B.1.617.2).
Apart from a first imported case in La Palma at the end of January 2021 due to a traveler returning from Cameroon, community transmission of the Beta variant in the archipelago was first identified in the island of Gran Canaria, where a large cluster of 46 cases was observed between the end of March and mid-May 2021. At the beginning of April, a second small cluster of six cases was observed in Gran Canaria with no apparent connection to the first one. In Tenerife, three small clusters of an average of four cases were observed, one at the beginning of April and two throughout June. In mid-May, a second big cluster of 58 cases was observed in Gran Canaria that lasted up to the beginning of August, when the last case of this variant was observed. Almost no cases of the Beta variant were found on any other island throughout the study period. Cases of the Gamma variant were scarce and observed in Tenerife, Gran Canaria, La Palma, and Lanzarote up to the end of May 2021, when a cluster of 24 P.1.1 cases was observed in Fuerteventura. The Fuerteventura outbreak was associated with a religious celebration involving local residents and foreign visitors and lasted up to the middle of June. An overview of the Canary Islands archipelago geography and of the heterogeneous distribution of Beta and Gamma variants in the archipelago is presented in Figure 4.
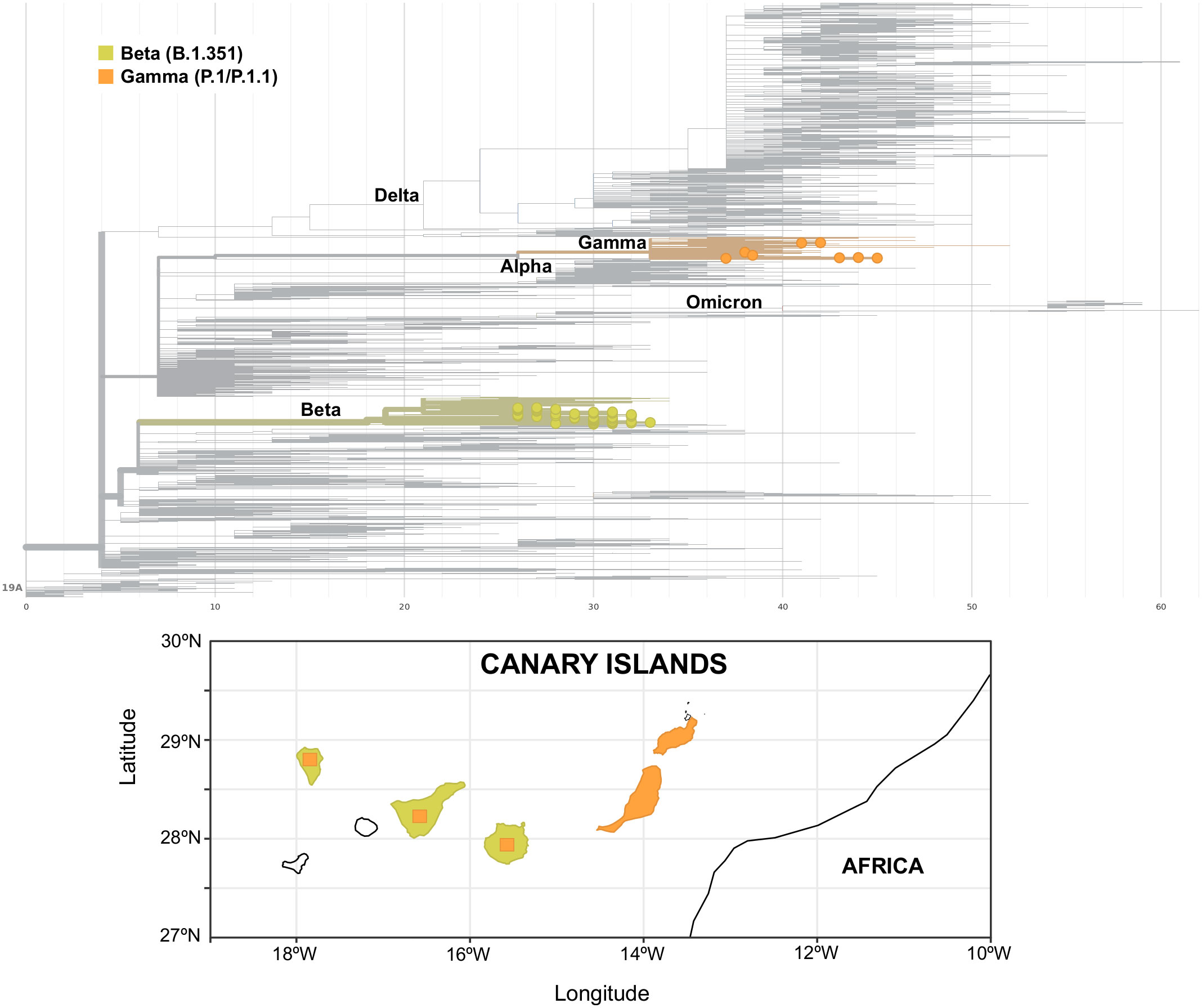
Figure 4 Phylogenetic tree of Beta (yellow) and Gamma (orange) variants (upper panel) and their distribution in the islands of the archipelago (lower panel). Square areas are proportional to the presence of the variant in each island.
Alpha, Beta, and Gamma lineages were replaced by the Delta variant that started circulating in Tenerife at the beginning of June 2021 (the first case detected in Tenerife on the 17th of May) followed by its identification in all other islands probably due to multiple introductions of this variant in the archipelago. The presence of Delta was associated with a rapid surge of COVID-19 cases in all islands when the archipelago entered the fifth wave, and the highest number of cases was reached since the beginning of the pandemic. From the end of July 2021, the previous heterogeneous distribution of VOCs in the different islands converged to a similar figure, with Delta becoming prevalent and almost completely displacing all other variants circulating in the archipelago. At the beginning of August, the sublineages of Delta (mainly AY.4 and AY.12) started circulating in the islands, completely superseding Delta by the end of September 2021.
Discussion
In this work, we dissected the spread of VOCs in the Canary Islands archipelago in the period between mid-December 2020 and late September 2021. Through genome sequencing and lineage assignment of more than 8,000 SARS-CoV-2-positive nasopharyngeal swab samples, we were able to track the spatiotemporal distribution of the variants Alpha, Beta, Gamma, and Delta in the archipelago. We observed that the viral sequences detected in the islands were characterized by a diverse epidemiological background and variant distribution throughout the study, which can be largely explained by their geographical isolation and heterogeneous population size. While Alpha spread ubiquitously across the archipelago early after its first appearance in the island of Tenerife, Beta and Gamma variants were observed only in some of the islands and not in others. Beta was observed in the archipelago from late January up to August 2021, and its spatial distribution was mainly circumscribed to the islands of Gran Canaria and Tenerife, even though it was already present in several European countries by mid-January (O’Toole et al., 2021a), representing up to 20% and 8% of cases in Austria and France, respectively, at that moment (Hodcroft, 2021). Cases associated with the Gamma variant were also relatively few, with a small cluster of cases observed in Tenerife in late January 2021, followed by isolated cases in the other islands. The largest Gamma cluster was a P.1.1 cluster observed in Fuerteventura. Both Beta and Gamma did not spread throughout the archipelago such as Alpha did, something that was also observed in other European countries (Hodcroft, 2021). We can attribute this to two factors: 1) Alpha was largely spread in Europe and worldwide at that time, something that increases the likelihood of multiple introductions of this variant in the different islands of the archipelago; 2) travel restrictions were in place during the early months of 2021 (up to May 2021) and limited the spread of Beta and Gamma once they were introduced. When Delta emerged, it was associated with a surge of cases in the archipelago throughout the summer, mimicking what occurred in the United Kingdom (Riley et al., 2021) and in the rest of Europe (Hodcroft, 2021). We attribute this not only to the intrinsically higher transmissibility of the Delta variant compared to the previously circulating VOCs (Campbell et al., 2021b) but also to the concurrent lifting of COVID-19 restrictions in the archipelago and nationwide. Since August 2021, we observed the emergence of diverse Delta sublineages (AY.4 and AY.12), similar to what occurred worldwide throughout the summer (Angeletti et al., 2021; Eales et al., 2021).
In summary, as a response to the COVID-19 pandemic, we established a network for genomic surveillance of SARS-CoV-2 in the Canary Islands based on two sequencing technologies, leveraging homogeneous and centralized processing of samples, efficient sequencing workflows, rigorous data quality control, and accurate sequence classification. To do so, we relied on both previously existing capacities and human resources in the involved laboratories and the rapid dissemination of information over VOCs and open-source bioinformatics tools made available by researchers worldwide. We managed to sequence more than 15% of COVID-19 cases of the archipelago in the study period, aligning our sequencing strategy to the WHO and the ECDC recommendations. We recognize sampling bias as a limitation of our study, with sample collection being not proportional to the number of cases in all islands. In fact, the island of Tenerife always had the highest percentage of sequenced samples mainly because the network was physically located in the island and therefore sample logistics was easier. Despite this, we managed to efficiently identify and track all VOCs in the Canary Islands since December 2020 and promptly inform the public health authorities in the region.
Data availability statement
Sequences in FASTA format have been deposited in GISAID with EPI_ISL codes ranging in the following intervals: 14072942-14073924, 14076844-14076860, 14091532-14092460, 14094040-14094110, 14094631-14095630, 14100149-14101148, 14102007-14103006, and 14103347-14103889.
Ethics statement
The study was conducted at the University Hospital Nuestra Senãora de Candelaria (HUNSC) (Santa Cruz de Tenerife, Spain). The institutional review board approved the study (approval number: CHUNSC_2020_24). Written informed consent for participation was not provided by the participants’ legal guardians/next of kin because: For Public Health reasons linked to the COVID-19 emergency.
Author contributions
JA-F and CF planned the experiments and supervised the project. LC, RG-M, DG-MdA, HG-C, HR-P, AI-C, IDM-M, TT-N, OD-G, and JL-S collected the samples and conducted the sequencing experiments. LC, JL-S, AV-F, and CF performed the analysis and interpreted the results. CF obtained the funding. LC, JL-S, and CF drafted the first version of the manuscript and prepared the figures. All authors contributed to manuscript revision and read and approved the submitted version.
Funding
This work was supported by Cabildo Insular de Tenerife (grants CGIEU0000219140 and “Apuestas científicas del ITER para colaborar en la lucha contra la COVID-19”); the agreement with Instituto Tecnológico y de Energías Renovables (ITER) to strengthen scientific and technological education, training research, development and innovation in Genomics, Personalized Medicine and Biotechnology (grant number OA17/008); Instituto de Salud Carlos III (grant numbers FI18/00230 and PI20/00876) and Ministerio de Ciencia e Innovación (grant number RTC-2017-6471-1), co-funded by the European Regional Development Fund (ERDF), “A way of making Europe” from the European Union. The funders had no role in the study design, collection, analysis and interpretation of data, in the writing of the manuscript or in the decision to submit the manuscript for publication.
Acknowledgments
We deeply acknowledge the University Hospital Nuestra Señora de Candelaria (HUNSC) and the Instituto Tecnológico y de Energías Renovables (ITER) board of directors for their strong support and assistance in accessing diverse resources used in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be foundonline at: https://www.frontiersin.org/articles/10.3389/fcimb.2022.919346/full#supplementary-material
References
Aksamentov, I., Roemer, C., Hodcroft, E. B., Neher, R. A.. (2021). Nextclade: clade assignment, mutation calling and quality control for viral genomes. J. Open Source Softw. 6 (67), 3773. doi: 10.21105/joss.03773
Alcoba-Florez, J., Gil-Campesino, H., Artola, D. G.-M., de González-Montelongo, R., Valenzuela-Fernández, A., Ciuffreda, L., et al. (2020). Sensitivity of different RT-qPCR solutions for SARS-CoV-2 detection. Int. J. Infect. Dis. 99, 190–192. doi: 10.1016/j.ijid.2020.07.058
Alcoba-Florez, J., Lorenzo-Salazar, J. M., Gil-Campesino, H., Íñigo-Campos, A., Martínez de Artola, D. G., García-Olivares, V., et al. (2021). Monitoring the rise of the SARS-CoV-2 lineage B.1.1.7 in tenerife (Spain) since mid-December 2020. J. Infect. 82 (6), e1–e3. doi: 10.1016/j.jinf.2021.04.005
Angeletti, S., Giovanetti, M., Fogolari, M., Cella, E., De Florio, L., Lintas, C., et al. (2021). SARS-CoV-2 AY.4.2 variant circulating in Italy: Genomic preliminary insight. J. Med. Virol. doi: 10.1002/jmv.27451
Campbell, E. M., Boyles, A., Shankar, A., Kim, J., Knyazev, S., Cintron, R., et al. (2021a). MicrobeTrace: Retooling molecular epidemiology for rapid public health response. PLoS Comput. Biol. 17 (9), e1009300. doi: 10.1371/journal.pcbi.1009300
Campbell, F., Archer, B., Laurenson-Schafer, H., Jinnai, Y., Konings, F., Batra, N., et al. (2021b). Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Eurosurveillance 26 (24), 2100509. doi: 10.2807/1560-7917.ES.2021.26.24.2100509
Ciuffreda, L., Lorenzo-Salazar, J. M., Alcoba-Florez, J., Rodriguez-Pérez, H., Gil-Campesino, H., Íñigo-Campos, A., et al. (2021). Longitudinal study of a SARS-CoV-2 infection in an immunocompromised patient with X-linked agammaglobulinemia. J. Infect. Elsevier 83 (5), 607–635. doi: 10.1016/j.jinf.2021.07.028
Davies, N. G., Abbott, S., Barnard, R. C., Jarvis, C. I., Kucharski, A. J., Munday, J. D., et al. (2021). Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 372 (6538), eabg3055. doi: 10.1126/science.abg3055
Dhar, M. S., Marwal, R., Radhakrishnan, V.S., Kalaiarasan, P., Bani, J., BR, C., et al. (2021). Genomic characterization and epidemiology of an emerging SARS-CoV-2 variant in Delhi, India. Science 374 (6570), 995–999. doi: 10.1126/science.abj9932
Eales, O., Page, A. J., de Oliveira Martins, L., Wang, H., Bodinier, B., Haw, D., et al. (2021). SARS-CoV-2 lineage dynamics in England from September to November 2021: high diversity of delta sub-lineages and increased transmissibility of AY.4.2. medRxiv. doi: 10.1101/2021.12.17.21267925
European Centre for Disease Prevention and Control (2021). Sequencing of SARS-CoV-2: first update (Stockholm: ECDC). Available at: https://www.ecdc.europa.eu/sites/default/files/documents/Sequencing-of-SARS-CoV-2-first-update.pdf.
Faria, N. R., Mellan, T.A., Whittaker, C., Claro, I. M., Candido da, D. S., Mishra, S., et al. (2021). Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in manaus, Brazil. Science 372 (6544), 815–821. doi: 10.1126/science.abh2644
Freed, N. E., Vlková, M., Faisal, M. B., Silander, O. K.. (2020). Rapid and inexpensive whole-genome sequencing of SARS-CoV-2 using 1200 bp tiled amplicons and Oxford nanopore rapid barcoding. Biol. Methods Protoc. 5 (1), bpaa014. doi: 10.1093/biomethods/bpaa014
Garcia-Beltran, W. F., Lam, E.C., St. Denis, K., Nitido, A. D., Garcia, Z. H., Hauser, B. M., et al. (2021). Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 184 (9), 2372–2383.e9. doi: 10.1016/j.cell.2021.03.013
Greaney, A. J., Loes, A. N., Crawford, K. H. D., Starr, T. N., Malone, K. D., Chu, H. Y., et al. (2021). Comprehensive mapping of mutations in the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human plasma antibodies. Cell Host Microbe 29 (3), 463–476.e6. doi: 10.1016/j.chom.2021.02.003
Harvey, W. T., Carabelli, A. M., Jackson, B., Gupta, R. K., Thomson, E. C., Harrison, E. M., et al. (2021). SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 19 (7), 409–424. doi: 10.1038/s41579-021-00573-0
Hodcroft, E. B. (2021) CoVariants: SARS-CoV-2 mutations and variants of interest. Available at: https://covariants.org/. [Accessed 15 March 2022]
Lopez Bernal, J., Andrews, N., Gower, C., Gallagher, E., Simmons, R., Thelwall, S., et al. (2021). Effectiveness of covid-19 vaccines against the B.1.617.2 (Delta) variant. New Engl. J. Med. 385 (7), 585–594. doi: 10.1056/NEJMoa2108891
Gangavarapu, K., Latif, A. A., Mullen, J., Alkuzweny, M., Hufbauer, E., Tsueng, G., et al. (2022). Outbreak.info genomic reports: scalable and dynamic surveillance of SARS-CoV-2 variants and mutations. medRxiv [Preprint]. doi: 10.1101/2022.01.27.22269965
O’Toole, A., Hill, V., Pybus, O. G., Watts, A., Bogoch, I. I., Khan, K., et al. (2021a). TTracking the international spread of SARS-CoV-2 lineages B.1.1.7 and B.1.351/501Y-V2 with grinch. Wellcome Open Res. 6 (121). doi: 10.12688/wellcomeopenres.16661.2
O’Toole, Á., Scher, E., Underwood, A., Jackson, B., Hill, V., McCrone, J. T., et al. (2021b). Assignment of epidemiological lineages in an emerging pandemic using the pangolin tool. Virus Evol. 7 (2), veab064. doi: 10.1093/ve/veab064
Pearson, C. A., Russell, T. W., Davies, N., Kucharski, AJ., CMMID COVID-19 working group, Edmunds, W. J., et al. (2021). ‘Estimates of severity and transmissibility of novel south Africa SARS-CoV-2 variant 501Y.V2’ (CMMID Repository). Available at: https://cmmid.github.io/topics/covid19/sa-novel-variant.html.
Planas, D., Veyer, D., Baidaliuk, A., Staropoli, I., Guivel-Benhassine, F., Rajah, M. M., et al. (2021). Reduced sensitivity of SARS-CoV-2 variant delta to antibody neutralization. Nature 596 (7871), 276–280. doi: 10.1038/s41586-021-03777-9
Public Health England (2021). SARS-CoV-2 variants of concern and variants under investigation in England: technical briefing 27. (UK: UKHSA), Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1029715/technical-briefing-27.pdf
Rambaut, A., Holmes, E. C., O'Toole, Á., Hill, V., McCrone, J. T., Ruis, C., et al. (2020). A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat. Microbiol. 5 (11), 1403–1407. doi: 10.1038/s41564-020-0770-5
Riley, S., Wang, H., Eales, O., Haw, D., Walters, C. E., Ainslie, K. E. C., et al. (2021). REACT-1 round 12 report: resurgence of SARS-CoV-2 infections in England associated with increased frequency of the Delta variant. medRxiv [Preprint]. doi: 10.1101/2021.06.17.21259103
Tao, K., Tzou, P. L., Nouhin, J., Gupta, R. K., de Oliveira, T., Kosakovsky Pond, S. L., et al. (2021). The biological and clinical significance of emerging SARS-CoV-2 variants. Nat. Rev. Genet. 22 (12), 757–773. doi: 10.1038/s41576-021-00408-x
Tegally, H., Wilkinson, E., Giovanetti, M., Iranzadeh, A., Fonseca, V., Giandhari, J., et al. (2021). Detection of a SARS-CoV-2 variant of concern in south Africa. Nature 592 (7854), 438–443. doi: 10.1038/s41586-021-03402-9
Volz, E., Mishra, S., Chand, M., Barrett, J. C., Johnson, R., Geidelberg, L., et al. (2021). Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature 593 (7858), 266–269. doi: 10.1038/s41586-021-03470-x
Wall, E. C., Wu, M., Harvey, R., Kelly, G., Warchal, S., Sawyer, C., et al. (2021). Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet 397 (10292), 2331–2333. doi: 10.1016/S0140-6736(21)01290-3
World Health Organization (2022). Tracking SARS-CoV-2 variants. Available at: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/. [Accessed March 15, 2022]
Keywords: SARS-CoV-2, genomic surveillance, variants of concern tracking, Canary Islands, COVID-19
Citation: Ciuffreda L, González-Montelongo R, Alcoba-Florez J, García-Martínez de Artola D, Gil-Campesino H, Rodríguez-Pérez H, Íñigo-Campos A, De Miguel-Martínez I, Tosco-Nuñez T, Díez-Gil O, Valenzuela-Fernández A, Lorenzo-Salazar JM and Flores C (2022) Tracing the trajectories of SARS-CoV-2 variants of concern between December 2020 and September 2021 in the Canary Islands (Spain). Front. Cell. Infect. Microbiol. 12:919346. doi: 10.3389/fcimb.2022.919346
Received: 13 April 2022; Accepted: 09 August 2022;
Published: 09 September 2022.
Edited by:
Davide Gibellini, University of Bologna, ItalyReviewed by:
Camila Malta Romano, University of São Paulo, BrazilJordi Serra-Cobo, University of Barcelona, Spain
Copyright © 2022 Ciuffreda, González-Montelongo, Alcoba-Florez, García-Martínez de Artola, Gil-Campesino, Rodríguez-Pérez, Íñigo-Campos, De Miguel-Martínez, Tosco-Nuñez, Díez-Gil, Valenzuela-Fernández, Lorenzo-Salazar and Flores. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlos Flores, cflores@ull.edu.es
†These authors share first authorship
‡These authors have contributed equally to this work
 Laura Ciuffreda
Laura Ciuffreda Rafaela González-Montelongo
Rafaela González-Montelongo Julia Alcoba-Florez3†
Julia Alcoba-Florez3†  Agustín Valenzuela-Fernández
Agustín Valenzuela-Fernández José M. Lorenzo-Salazar
José M. Lorenzo-Salazar Carlos Flores
Carlos Flores