The Effectiveness of Metagenomic Next-Generation Sequencing in the Diagnosis of Prosthetic Joint Infection: A Systematic Review and Meta-Analysis
- 1Department of Orthopedic Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Department of Clinical Sciences, Orthopedics, Faculty of Medicine, Lund University, Lund, Sweden
- 3Department of Trauma and Reconstructive Surgery, BG Trauma Center Tübingen, Siegfried Weller Institute for Trauma Research, Eberhard Karls University Tübingen, Tübingen, Germany
Background: A prosthetic joint infection (PJI) is a devastating complication following total joint arthroplasties with poor prognosis. Identifying an accurate and prompt diagnostic method is particularly important for PJI. Recently, the diagnostic value of metagenomic next-generation sequencing (mNGS) in detecting PJI has attracted much attention, while the evidence of its accuracy is quite limited. Thus, this study aimed to evaluate the accuracy of mNGS for the diagnosis of PJI.
Methods: We summarized published studies to identify the potential diagnostic value of mNGS for PJI patients by searching online databases using keywords such as “prosthetic joint infection”, “PJI”, and “metagenomic sequencing”. Ten of 380 studies with 955 patients in total were included. The included studies provided sufficient data for the completion of 2-by-2 tables. We calculated the sensitivity, specificity, and area under the SROC curve (AUC) to evaluate mNGS for PJI diagnosis.
Results: We found that the pooled diagnostic sensitivity and specificity of mNGS for PJI were 0.93 (95% CI, 0.83 to 0.97) and 0.95 (95% CI, 0.92 to 0.97), respectively. Positive and negative likelihood ratios were 18.3 (95% CI, 10.9 to 30.6) and 0.07 (95% CI, 0.03 to 0.18), respectively. The area under the curve was 0.96 (95% CI, 0.93 to 0.97).
Conclusion: Metagenomic next-generation sequencing displays high accuracy in the diagnosis of PJI, especially for culture-negative cases.
Introduction
Prosthetic joint infection (PJI), noted as a devastating complication of prosthetic joint implantation, accounts for 25% of failed knee arthroplasties and 15% of failed hip arthroplasties (Bozic et al., 2010; Rietbergen et al., 2016). PJI after joint arthroplasty has extreme adverse effects on cost and quality of life. In recent years, with the increasing number of cases, the proportion of its cost in the healthcare budgets is also increasing (Kallala et al., 2018). It is estimated that each episode of prosthetic infection costs the health service over 20, 000 pounds (Vanhegan et al., 2012).
To date, the timely and accurately diagnosis of PJI is still challenging, especially for the identification of pathogenic microorganisms. Although many methods have emerged for establishing the diagnosis, none has been universally accepted (Moojen et al., 2014). Nowadays, traditional blood testing, such as white blood cell (WBC) count, serum erythrocyte sedimentation rate (ESR), and serum C-reactive protein concentration (CRP) are being widely performed for diagnosing PJI in clinics. Nonetheless, these inflammatory markers are nonspecific for PJI, and sometimes they may even be normal in severe cases of joint infections (Nodzo et al., 2015). In addition, routine microbial culture has also been widely used to identify causative organisms in PJI, but it has a significantly high false-negative rate (Rak et al., 2013; Yoon et al., 2017). It has been reported that approximately 40% of culture-negative cases meet the clinical diagnostic criteria for PJI, which might be due to the restricted growth conditions of specific pathogens and the widespread use of antibiotics (Tande and Patel, 2014). In recent years, matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) has emerged for the identification of bacterial in clinical laboratories (Peel et al., 2015). The MALDI-TOF MS process is rapid, sensitive and economical in terms of labor and costs involved, in which microbes are identified using either intact cells or cell extracts. Although it shows high accuracy for the direct identification of Gram-negative bacteria from blood culture, the accuracy for Gram-positive bacteria is moderate (Ruiz-Aragón et al., 2018). Therefore, the research and development of a reliable technique for the diagnosis of PJI are urgent for both patients and clinicians.
In recent years, researches on PJI diagnosis have switched to mNGS. mNGS is a rapidly developing technology in terms of both pathogenic microorganism detection and data analysis. It has been shown to play important roles in the diagnosis of cancers, genetic diseases, and infectious diseases (Kwon et al., 2019; Wilson et al., 2019). Compared to PCR, mNGS does not have limitations on the detection of specific pathogens, and it can detect almost all pathogens, such as bacteria, fungi, viruses, and parasites. Furthermore, it allows thousands or even billions of DNA fragments to be sequenced independently at the same time, and its consequence is confirmed through comparison with a dedicated pathogen database (Schlaberg et al., 2017; Gu et al., 2019).
mNGS has shown high value in the diagnosis of pathogens of many infectious diseases. In a study on tuberculous meningitis, the diagnostic sensitivity of mNGS based on cerebrospinal fluid was 84.44%, which was much higher than the 22.2% of traditional cerebrospinal fluid culture (Yan et al., 2020). Another study showed that the sensitivity of mNGS was much higher than that of traditional culture in the pathogen diagnosis of mixed lung infections (97.2% vs 13.9%, P<0.01) (Wang et al., 2019).
In 2019, a systematic review discussed the sequencing assays for the diagnosis of PJI and showed a low statistical power, owing to a few studies regarding mNGS was involved in this study. Additionally, this review failed to analyze and evaluate the accuracy and diagnostic value of mNGS for PJI (Li et al., 2019). Herein, we incorporated the latest clinical trials for this systematic review to summarize published studies about mNGS. We also performed a meta-analysis to investigate its diagnostic accuracy for PJI.
Materials and Methods
The protocol for this review was registered with the PROSPERO database, registration number CRD42020193251. We strictly adhered to the standards of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) in reporting the findings of this review (Moher et al., 2015).
Search Strategy
We carefully searched for longitudinal studies (prospective or retrospective case-control, prospective cohort, retrospective cohort, case-cohort, nested-case control trials) reporting on the use of mNGS for PJI in MEDLINE, EMBASE, China National Knowledge Internet (CNKI), and Cochrane Library databases from inception to July 2021. A systematic literature search was performed to obtain all of the published articles focusing on mNGS diagnosis of PJI. Vocabulary and syntax were adjusted according to the different databases. We mainly used “prosthetic joint infection”, “periprosthetic joint infection”, “PJI”, “prosthesis-related infections”, “prosthesis infection”, “infection”, and “metagenomic sequencing”, “mNGS”, “metagenomic next-generation sequencing”, “shotgun metagenomics”, “genomics”, “genetic diagnosis”, “sequencing”, as the search target keywords. The exact retrieval strategy is demonstrated in Supplementary S1. Reference lists of the retrieved articles were manually scanned for all relevant additional studies and review articles.
Study Selection
The screening was performed in two stages, title and abstract screening, followed by full-text screening. A gold standard for diagnosing PJI has not been established, and different studies may adopt different reference standards. Among these reference standards, Musculoskeletal Infection Society (MSIS) (Parvizi et al., 2011) and Infectious Diseases Society of America (IDSA) (Osmon et al., 2013) are commonly used. We included studies with different reference standards, and then investigated the heterogeneity between MSIS and IDSA as reference standards through subgroup analysis. Two researchers independently reviewed the title and abstract of each study to select those likely to meet the inclusion criteria. In the initial stage of the screening, 10 to 12 articles were used to confirm the agreement between the researchers. To achieve at a consensus, any discrepancy was resolved by discussion or with the assistance of a third reviewer. After full-text screening, a list of excluded studies with reasons for exclusion was performed.
Studies were considered eligible for inclusion if they met the following criteria: (1) patients with suspected PJI following primary or revision total hip or knee arthroplasty; (2) focus on mNGS-based diagnosis of PJI; (3) the diagnosis of PJI was confirmed by MSIS or IDSA; (4) false positive (FP), true positive (TP), false negative (FN), and true negative (TN) were provided to construct the 2 × 2 contingency table. Articles were excluded based on the following criteria: (1) Irrelevant reviews, letters, personal opinions, book chapters, and meeting abstracts; (2) insufficient data to calculate sensitivity and specificity; (3) mNGS and PJI were not studied.
Quality Assessment
The quality of the included studies was evaluated by two researchers using the revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 (Whiting et al., 2011), which is comprised of four key domains that focus on patient selection, index test, reference standard, flow, and timing. Signaling questions were applied to assess the risk of bias and clinical applicability. The overall risk of bias and applicability was summarized as low, high, or unclear.
Data Extraction
Two reviewers independently extracted the data from the included studies using a standardized form. Data extraction included the following items: last name of the first author; publication year; study population and regions; false and true positives and negatives; sample site and type; reference standard and study design. To deal with absent or unclear data, we tried to contact the study authors.
Statistical Analysis
Overall pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and the corresponding 95% CI for the diagnosis of PJI were calculated using a bivariate meta-analysis framework. We also tested the pooled diagnostic value of mNGS through the summary receiver operating characteristic (SROC) curve and the area under the SROC curve (AUC). We assessed heterogeneity among the studies using the chi‐squared and I2 tests. Moreover, subgroup and sensitivity analyses were undertaken to explore the potential sources of heterogeneity. All analyses were undertaken by using RevMan 5.4 (The Nordic Cochrane Centre, The Cochrane Collaboration, London, UK 2020) and Stata 15.0 (Stata Corporation, College Station, TX, USA), and a value of P < 0.05 was considered statistically significant.
Results
The selection process was shown in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart in Figure 1. 380 relevant articles were identified for initial review by systematically searching in the aforementioned databases. Of the identified 380 articles, 253 duplicates were excluded. Then, 106 articles were excluded due to inappropriate article types (reviews, comments, or letters). After reading the remaining 21 articles in full text, seven were excluded due to insufficient data, and four were excluded due to not being an original diagnostic study. Ten studies were finally included in this meta-analysis (Ivy et al., 2018; Thoendel et al., 2018; Huang et al., 2019; Zhang et al., 2019; Cai et al., 2020; Fang et al., 2020; Huang et al., 2020; Wang et al., 2020; He et al., 2021; Yu et al., 2021).
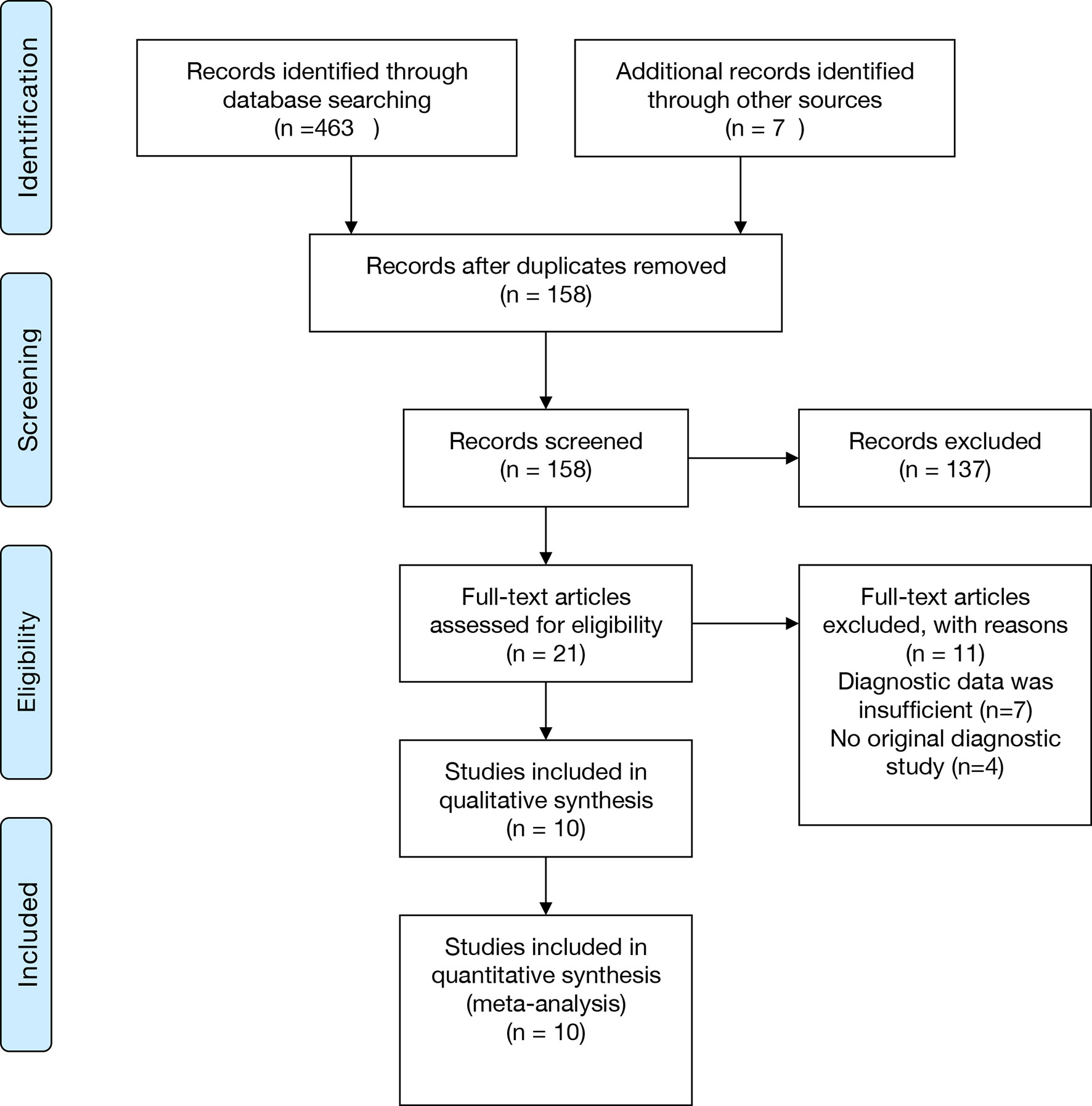
Figure 1 PRISMA flowchart. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
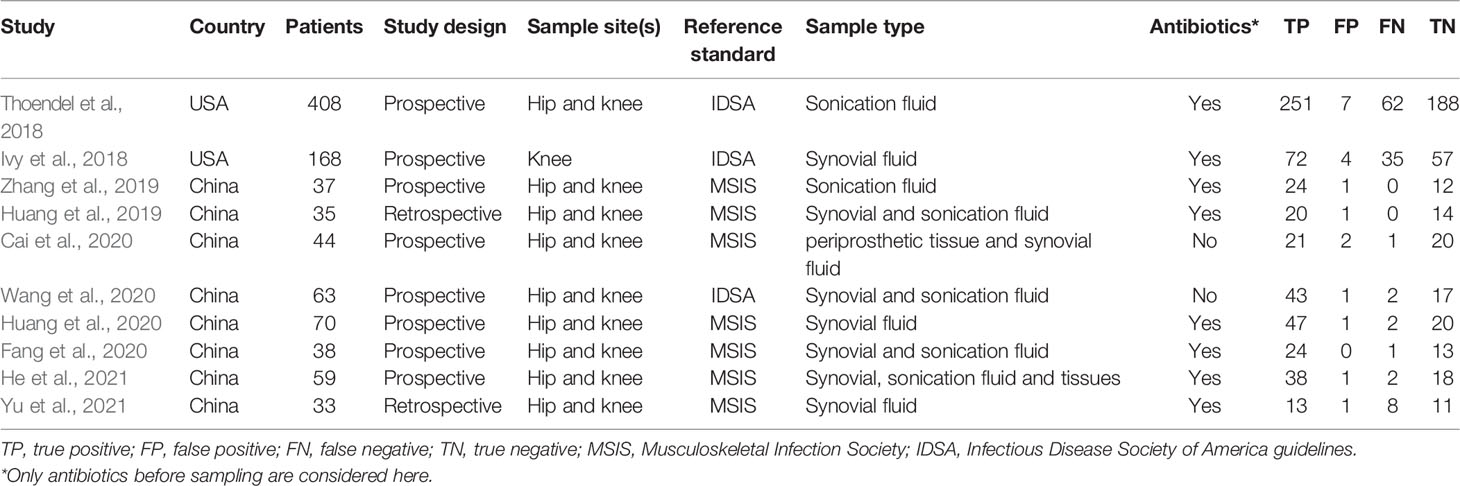
These 10 studies, including a total of 955 patients, were published between 2018 and 2021. Among the included studies, two (Huang et al., 2019; Yu et al., 2021) were conducted retrospectively, and the other studies were conducted prospectively. Eight studies (Ivy et al., 2018; Huang et al., 2019; Cai et al., 2020; Fang et al., 2020; Huang et al., 2020; Wang et al., 2020; He et al., 2021; Yu et al., 2021) collected synovial fluid samples before any clinical treatment, six studies (Thoendel et al., 2018; Huang et al., 2019; Zhang et al., 2019; Fang et al., 2020; Wang et al., 2020; He et al., 2021) obtained sonication fluid and two studies (Cai et al., 2020; He et al., 2021) selected periprosthetic tissue for mNGS. The MSIS criteria were used in seven studies (Huang et al., 2019; Zhang et al., 2019; Cai et al., 2020; Fang et al., 2020; Huang et al., 2020; He et al., 2021; Yu et al., 2021), and the other three studies (Ivy et al., 2018; Thoendel et al., 2018; Wang et al., 2020) adopted the IDSA as the only reference standard. Among the ten studies analyzed, nine studies (Thoendel et al., 2018; Huang et al., 2019; Zhang et al., 2019; Cai et al., 2020; Fang et al., 2020; Huang et al., 2020; Wang et al., 2020; He et al., 2021; Yu et al., 2021) focused on both hip and knee while one study (Ivy et al., 2018) only enrolled knee arthroplasty (Table 1). A graphical summary of the methodological assessment based on the QUADAS-2 quality assessment for the 10 studies is shown in Figures 2A, B.
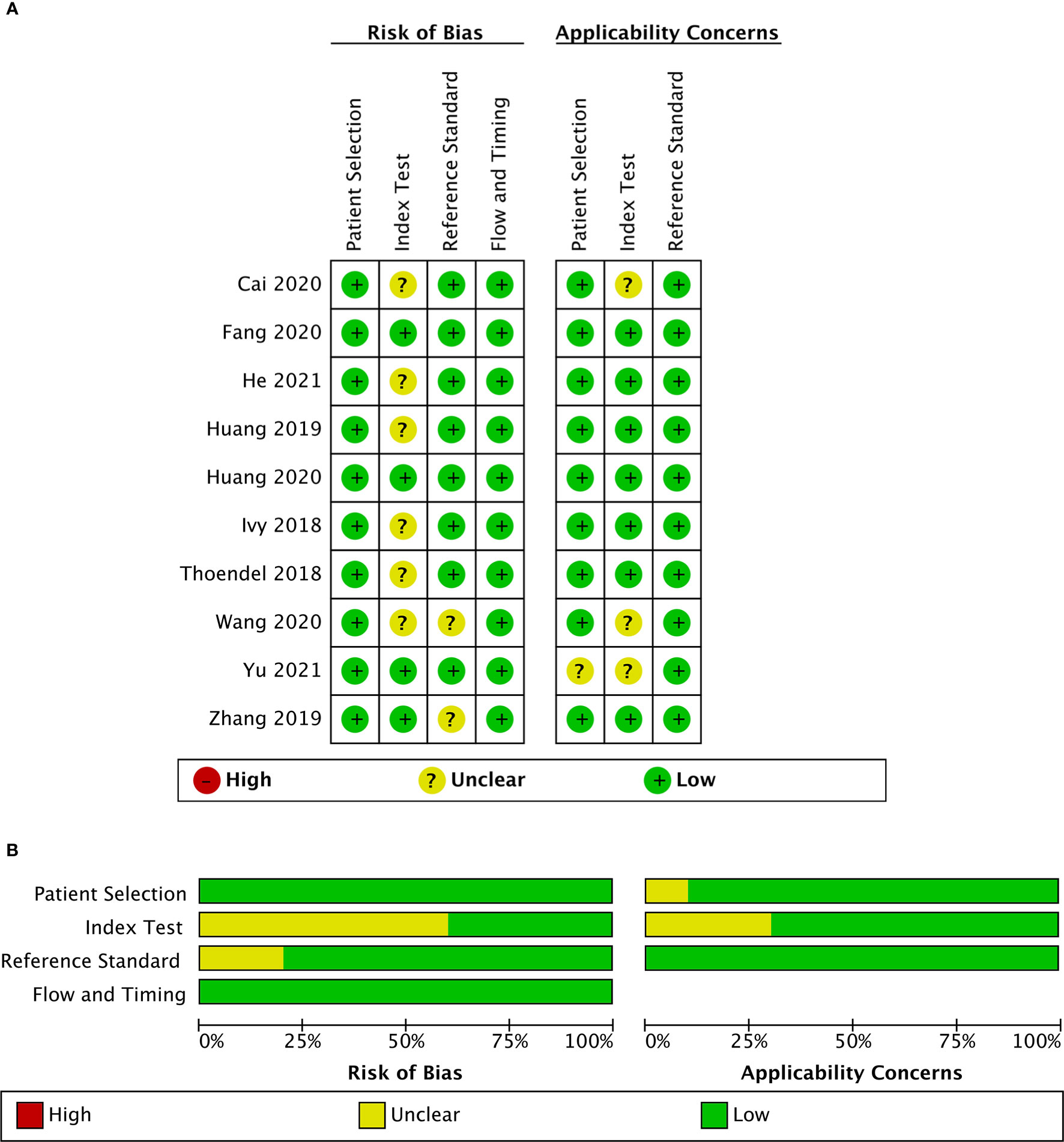
Figure 2 Risk of bias and applicability concerns summary (A); risk of bias and applicability concerns graph (B).
Included studies were assessed with the QUADAS-2 guidelines, and detailed information is shown in Supplementary Table S1. The majority of studies had a low risk of bias for patient selection, reference standard, flow, and timing. For index test bias, six studies were at an unclear risk because the information was insufficient to ensure that the index test results were interpreted without knowledge of the results of the reference standard. Most of the studies in this meta-analysis raised low concerns about applicability.
The sensitivity and specificity of mNGS for diagnosing PJI are shown in Figure 3. The pooled sensitivity was 0.93 (95% CI, 0.83–0.97), specificity was 0.95 (95% CI, 0.92–0.97), positive likelihood ratio was 18.3 (95% CI, 10.9–30.6), negative likelihood ratio was 0.07 (95% CI, 0.03–0.18), and DOR was 247 (95% CI, 84–723). Moreover, we plotted the SROC curve to evaluate diagnostic accuracy (Figure 4). AUC was 0.96 (95% CI, 0.93–0.97), suggesting a unique superior diagnostic accuracy of mNGS.
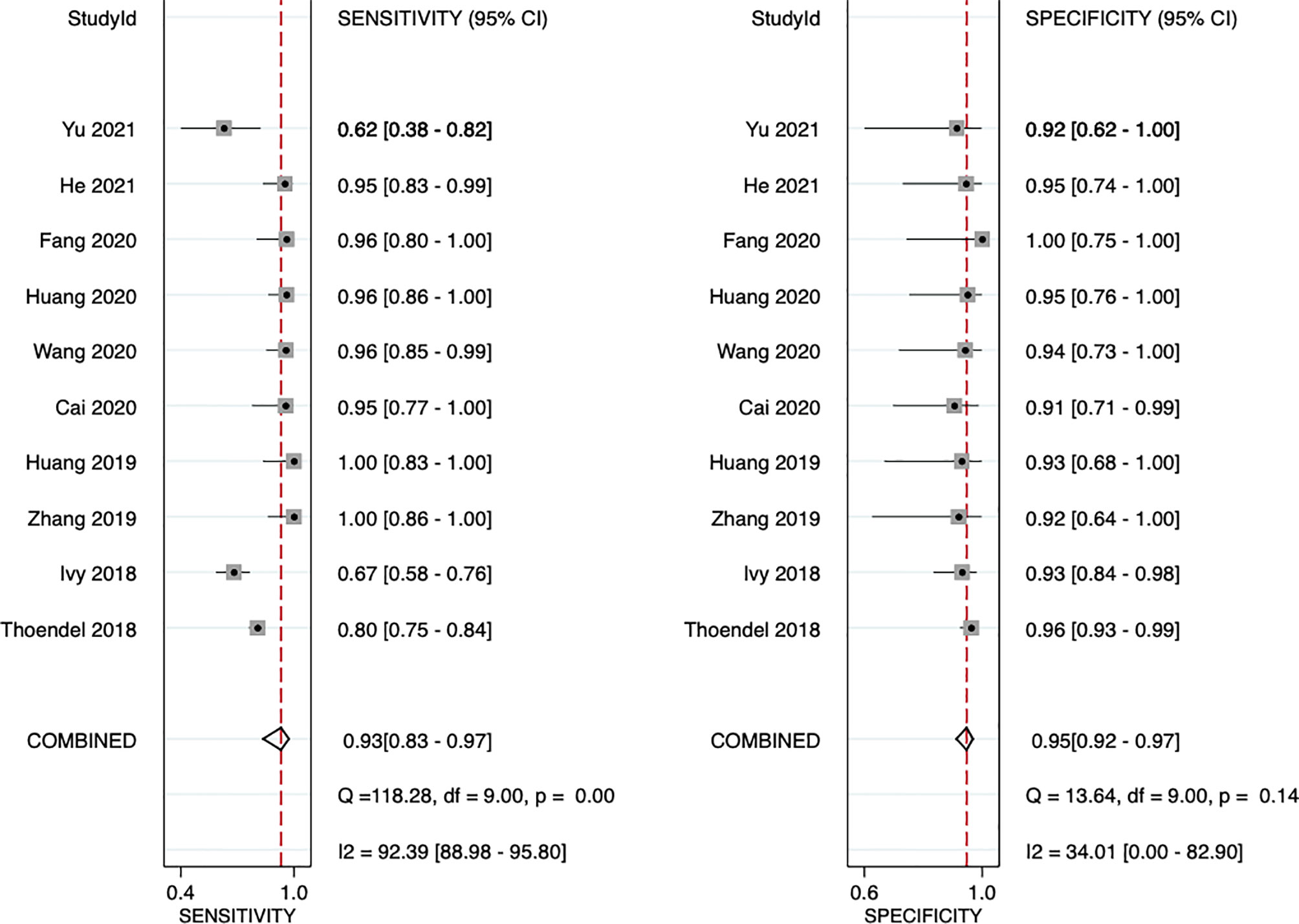
Figure 3 Forest plots for studies on overall mNGS used in the diagnosis of PJI. CI, confidence interval.
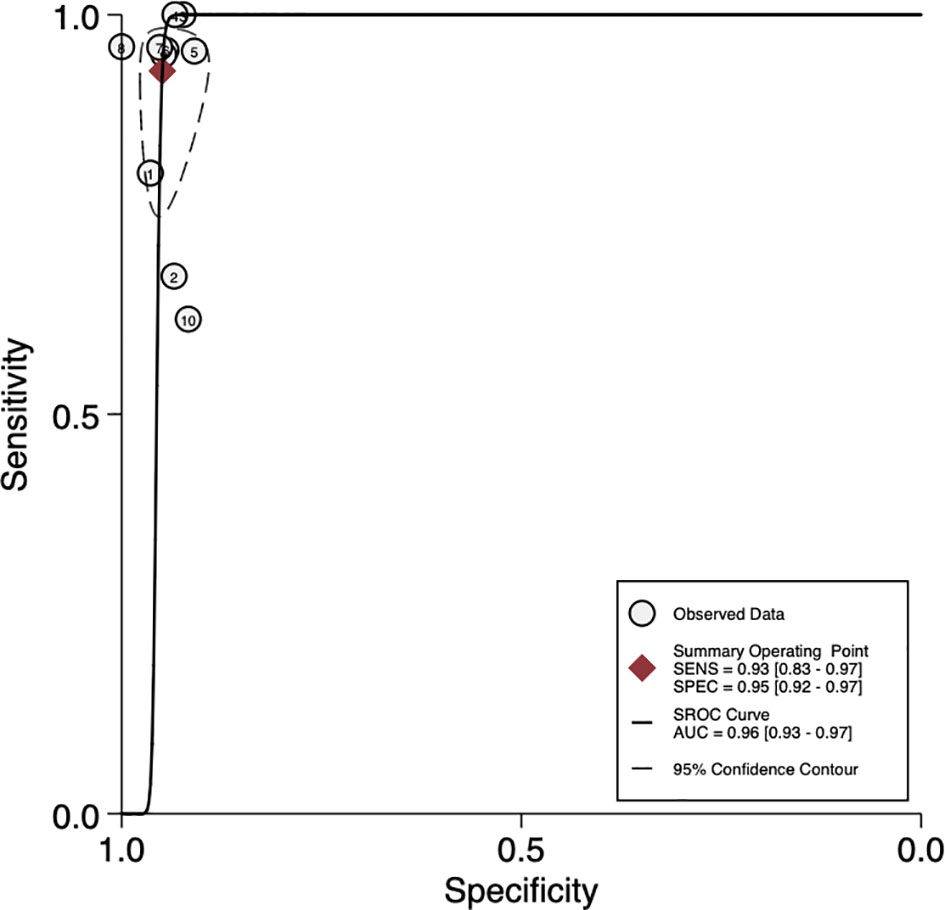
Figure 4 Summary receiver operator characteristic (SROC) curves based on mNGS. AUC, area under the curve; SENS, sensitivity; SPEC, specificity.
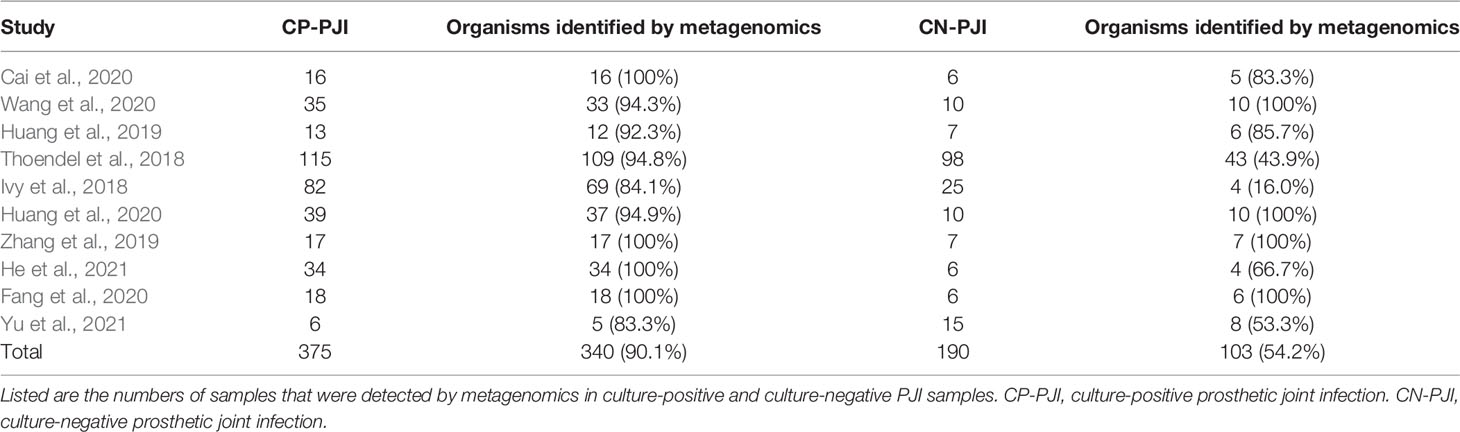
The performance of mNGS in both culture-positive and culture-negative is indicated in Table 2. In all 565 specimens tested in all publications, 375 (66.4%) were culture-positive and 190 (33.6%) were culture-negative. In 375 culture-positive specimens, the pathogens identified by culture were also detected by metagenomics in 340 (90.1%) cases. In 190 specimens considered as culture-negative, potential pathogens were detected in 103 (54.2%) using metagenomics.
For sensitivity analysis, the goodness of fit and bivariate normality showed that a random-effects bivariate model is suitable (Figures 5A, B). Influence analysis identified that the studies of Thoendel et al. (Thoendel et al., 2018), Ivy et al. (Ivy et al., 2018), and Yu et al. (Yu et al., 2021) were the most dominant studies in weight (Figure 5C). Outlier detection implied that the studies of Ivy et al. (Ivy et al., 2018) and Yu et al. (Yu et al., 2021) might be the reason for the heterogeneity (Figure 5D). The Spearman correlation coefficient of sensitivity and 1-specificity was 0.418, and the P-value was 0.229, indicating that heterogeneity may not be caused by the threshold effect (Supplementary S2). We conducted an univariable meta-regression analysis based on the characteristics of the ten studies to explore the potential sources of heterogeneity. We found that sensitivity was affected by ethnicity, sample site, and study design, while specificity was influenced by ethnicity, sample type, and reference standard (Figure S1).
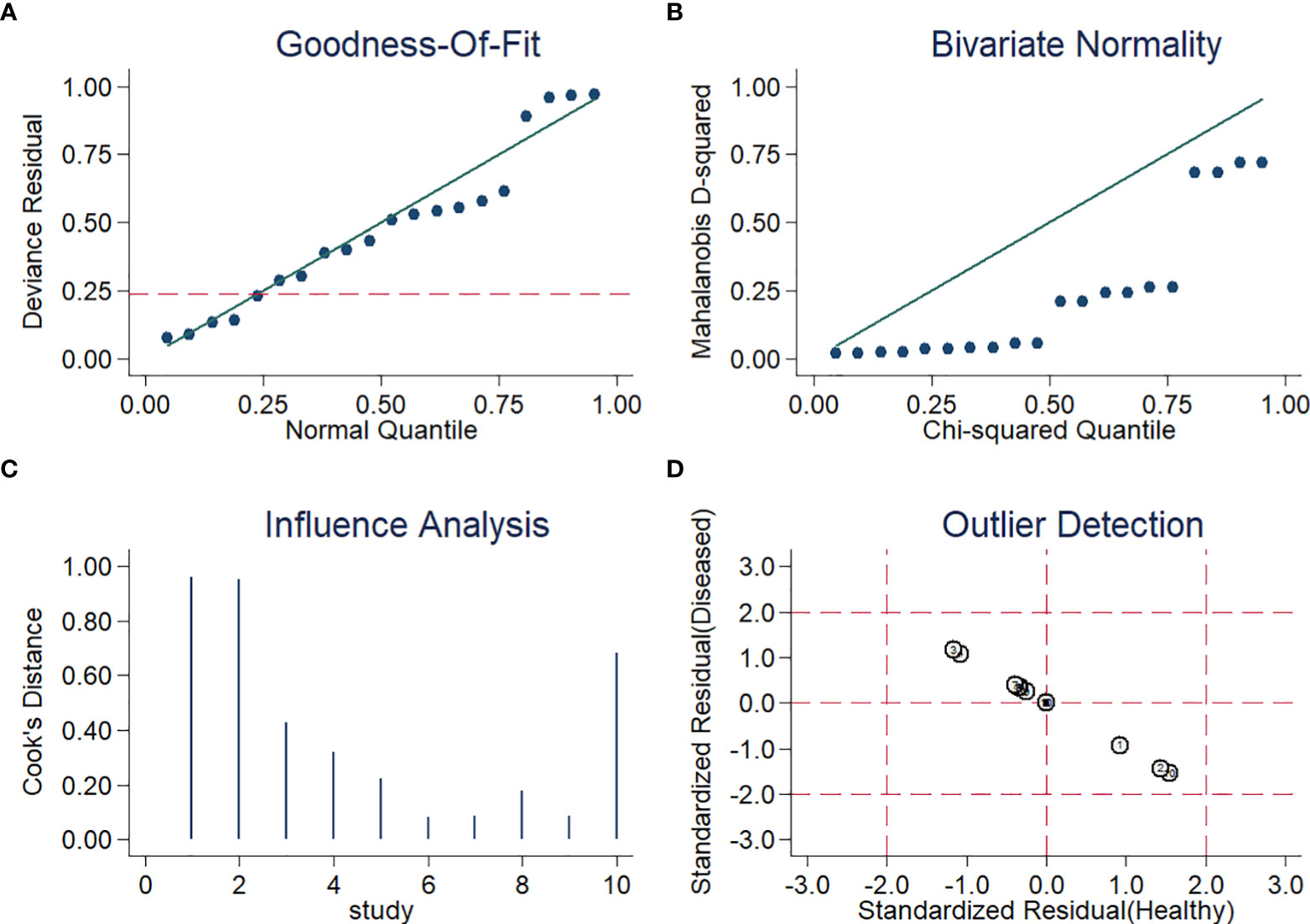
Figure 5 Diagram of (A) Goodness-of-fit (B) Bivariate normality (C) Influence analysis (D) Outlier detection.
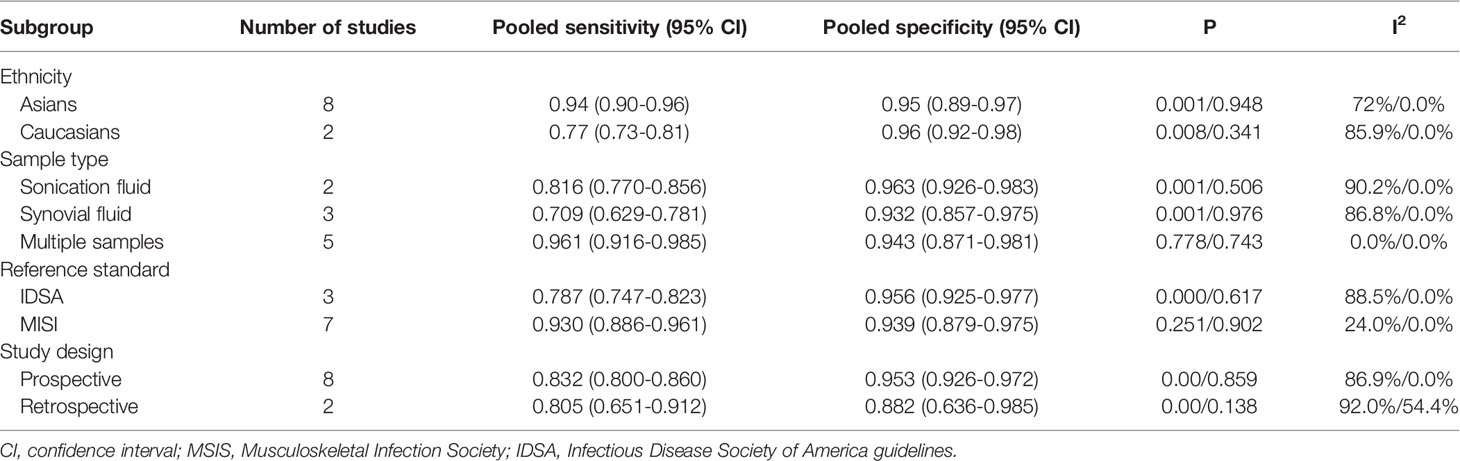
We performed subgroup analysis according to the results of univariate meta-regression to further investigate the sources of heterogeneity. If I2 < 50%, or P > 0.05, heterogeneity in this subgroup was defined as low. Between these subgroup analyses, ethnicity, sample type, and reference standard showed low heterogeneity (Table 3).
Moreover, The Deeks’ funnel plot asymmetry test of pooled DOR with a P-value of 0.20 indicated no significant publication bias (Figure S2).
Discussion
Although mNGS has demonstrated an encouraging value in the diagnosis of pathogens of various infectious diseases, especially for diagnosing tuberculous meningitis and chlamydia psittaci pneumonia, consensus for its clinical application of PJI diagnosis has still not yet been achieved (Chen et al., 2020; Yan et al., 2020). A former meta-analysis suggested that sequencing assays have the potential to improve the clinical diagnosis of PJI, especially for culture-negative cases, but the diagnostic value and accuracy of mNGS in PJI were still unclear (Li et al., 2019). According to our literature search, no previous systematic review or meta-analysis about mNGS in the diagnosis of PJI has been published, which makes it necessary to explore and fill this gap.
Our findings suggested that mNGS had a high accuracy in PJI diagnostics, with a pooled sensitivity of 0.93, a pooled specificity of 0.95, and an AUC of 0.96. The pooled PLR was 18.3, indicating that the probability of an accurate diagnosis of PJI increased by 18.3-fold with positive mNGS testing. Moreover, NLR was 0.07, implying that the probability of a PJI decreased by 93% when the studied mNGS was negative.
Li et al. (Li et al., 2019) showed that the sensitivity, specificity, and AUC of sequencing assays were 0.81, 0.94, and 0.94, respectively. The pooled sensitivity and specificity were both lower than the data of our study (0.81 vs 0.93; 0.94 vs 0.95). The AUC, which is usually used to indicate overall accuracy, was also lower than our study (0.94 vs. 0.96), supporting the idea that mNGS might be more effective in the diagnosis of PJI than other sequencing assays. There are several potential reasons for the higher sensitivity and AUC in our study: our study only focused on the diagnostic accuracy of mNGS, while Li et al. used different sequencing methods, including Sanger sequencing, Sequencing by Synthesis and NGS methods. mNGS technology can simultaneously and independently detect pathogens and multiple target genes in the same clinical samples without the need of pre-amplify target sequences (Gu et al., 2019). The ability of mNGS to effectively identify most pathogens in the joint fluid of PJI may have contributed to this result.
In another study of broad-range PCR-based (BR-PCR) diagnosis of PJI (Wang et al., 2020), the pooled sensitivity and specificity were 0.82 and 0.94, respectively, which were both also lower than in our analysis (0.82 vs 0.93; 0.94 vs 0.95). These results were likely caused by different sequencing procedures between mNGS and BR-PCR. The outstanding advantage of mNGS is unbiased sampling, which can broadly identify known and unexpected pathogens and even discover new organisms in an unbiased approach (Gu et al., 2019). BR-PCR is based on the V3-V4 region of 16S rDNA, which can only identify some pathogens at the genus level and may miss the causative pathogens in polymicrobial infection and fungal infections (Dabrowski et al., 2017).
According to subgroup analysis, the effectiveness of mNGS in the diagnosis of PJI among Asians seems to have a better sensitivity than that of Caucasians (0.94 vs. 0.77), while the specificity in Caucasians was slightly higher than in Asians (0.96 vs. 0.95). In fact, the total number of Caucasians studies was much larger than that of Asians (576 vs. 379) and a different platform was used to perform mNGS in the included study. We assume that this may cause the significant difference in sensitivity and specificity between Asians and Caucasians. Therefore, it is necessary to carry out more high-quality clinical trials of different ethnicities to explore racial differences in mNGS. Besides, the significant differences among sample types were considered as the main source of heterogeneity in specificity. Sequencing of sonication fluid seems to have a better specificity than other sample types, while multiple sample types sequencing had better sensitivity than other sample types. In fact, the ultrasonic lysis method can peel the biofilm from the prosthesis surface, increasing the microbial load in the lysate and improving the probability of microbial detection (Huang et al., 2019). Further, compared to the thickened joint fluids that are difficult to centrifuge, ultrasonic lysis fluids could achieve a 20-fold higher concentration of microbial cells after centrifugation and increase the sensitivity of diagnosis. Nevertheless, the ultrasonic lysis procedure may introduce exogenous microbial cells and nucleic acid fragments. Therefore, the additional pathogenic bacteria detected in the ultrasonic lysate should be further verified by specific PCR or other methods to exclude the possibility of exogenous contamination.
Our results showed that the sensitivity of MSIS was better than IDSA (0.930 vs. 0.787), while the specificity of MSIS was lower than IDSA (0.939 vs. 0.956). However, some information important for determining the cases with low virulence levels may be missed by using different reference standards and therefore resulting in the wrong grouping method. For that reason, a common and widely accepted reference standard should be established to help to minimize classification bias.
The main pathogenic microorganisms of PJI obtained by mNGS were Staphylococcus epidermidis (25.1%, 139/553) and Staphylococcus aureus (17.5%, 97/553), which is similar to the common microbiological causes of PJI reported by Tande et al. (Tande and Patel, 2014). It is noteworthy that metagenomics is able to detect most pathogens identified by culture (90.1%) as well as many that were not detected by culture. This occurs particularly in the culture-negative PJI group in which potential pathogens were detected in 54.2% of cases. This result supported the idea that mNGS is a powerful tool to identify PJI pathogens that are difficult to detect in culture-negative infections. Importantly, mNGS will become more accurate and offer more comprehensive microbiologic diagnosis as the technology evolves.
Helping clinical decision-making is the most important value of mNGS. Likelihood ratios and post-test probabilities are useful for clinicians, as they could show the probability that a patient has or does not have PJI, given a negative or positive test result. We also summarized the positive likelihood ratios and negative likelihood ratios to judge the clinical applicability of mNGS for diagnosis (Figure S3). PLR >10 and NLR <0.1 represent a high diagnostic accuracy (Wacker et al., 2013). We found that the articles of mNGS from Wang et al. (Wang et al., 2020), Huang et al. (Huang et al., 2020), Fang et al. (Fang et al., 2020), and He et al. (He et al., 2021) had high diagnostic accuracy and clinical applicability. When the pre-test probability was set at 50%, the post-test probability for a positive test result was 95%. When the negative likelihood ratio was set at 0.07, the post-test probability was reduced to 7% for a negative test result (Figure S4).
mNGS offers a novel approach to diagnose clinical infectious diseases and address current pitfalls in clinical management. Although the valuable insights of mNGS have already been derived, its use in the diagnosis of PJI is still in its infancy and many challenges still exist (Han et al., 2019). In particular, it is difficult to detect pathogenic virulence and drug sensitivity, which limits its role in guiding the rational selection of antibiotics. Another challenge is no comprehensive and unified background bacteria identification strategy, making interpretation of the sequencing results difficult. It seems inevitable to mix microbial gene sequences during sampling and laboratory testing, which makes it difficult to identify the real pathogen. Moreover, the high cost and lack of timeliness also limit the clinical applications of this technology.
In addition, several limitations of this meta-analysis should be emphasized. It is hard to elucidate whether the sample site had a decisive influence on diagnostic accuracy since the raw data were not provided in the published articles and we cannot divide the data into hip and knee to eliminate heterogeneity. Future studies should focus on the differences in diagnostic accuracy associated with potential sources of heterogeneity, including different arthroplasty sites. Secondly, the gold standard for diagnosing PJI has not been established and we included studies according to different reference standards, which may result in misdiagnosis for PJI (Liu et al., 2018). Thirdly, an antibiotic-free interval before sampling may enhance the ability to detect the causative organism, but through our univariable meta-regression and subgroup analysis, we still could not conclude that antibiotics were the main source of heterogeneity. Finally, studies with positive results are more likely to be published, which can amplify the overall diagnostic accuracy.
Conclusions
To the best of our knowledge, our study is the first meta−analysis that evaluates the clinical usability of mNGS in the diagnosis of PJI. Our study indicated that mNGS has a superior diagnostic accuracy for PJI and may be particularly useful for culture-negative cases. This systematic review provides effective support for the diagnostic performance of mNGS, which can provide clinicians with recommendations for accurate and effective diagnosis of PJI and antibiotics treatment. Meanwhile, large‐sized and good‐quality studies should be conducted to verify our results and to confirm the clinical value of mNGS in PJI patients.
Author Contributions
JX and TC were responsible for the idea and concept of the paper. JT and TC built the database. JT and YY analyzed the data. JT wrote the manuscript. YL, SE, and AN critically reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2022.875822/full#supplementary-material
Supplementary Figure 1 | Univariable meta-regression.
Supplementary Figure 2 | The Deeks’ funnel plot of the pooled DOR. DOR, diagnostic odds ratio; ESS, effective sample size.
Supplementary Figure 3 | Summary of positive likelihood ratio and negative likelihood ratio for the diagnosis of PJI. LLQ, left lower quadrant; LRN, likelihood ratio negative; LRP, likelihood ratio positive; LUQ, left upper quadrant; RLQ, right lower quadrant; RUQ, right upper quadrant.
Supplementary Figure 4 | Fagan nomogram of the mNGS for the diagnosis of PJI.
References
Bozic, K. J., Kurtz, S. M., Lau, E., Ong, K., Chiu, V., Vail, T. P., et al. (2010). The Epidemiology of Revision Total Knee Arthroplasty in the United States. Clin. Orthop. Relat. Res. 468 (1), 45–51. doi: 10.1007/s11999-009-0945-0
Cai, Y., Fang, X., Chen, Y., Huang, Z., Zhang, C., Li, W., et al. (2020). Metagenomic Next Generation Sequencing Improves Diagnosis of Prosthetic Joint Infection by Detecting the Presence of Bacteria in Periprosthetic Tissues. Int. J. Infect. Dis. 96, 573–578. doi: 10.1016/j.ijid.2020.05.125
Chen, X., Cao, K., Wei, Y., Qian, Y., Liang, J., Dong, D., et al. (2020). Metagenomic Next-Generation Sequencing in the Diagnosis of Severe Pneumonias Caused by Chlamydia Psittaci. Infection 48 (4), 535–542. doi: 10.1007/s15010-020-01429-0
Dabrowski, P., Jurkiewicz, J., Czernicki, Z., Koszewski, W., Jasielski, P. (2017). Polymerase Chain Reaction Based Detection of Bacterial 16S rRNA Gene in the Cerebrospinal Fluid in the Diagnosis of Bacterial Central Nervous System Infection in the Course of External Cerebrospinal Fluid Drainage. Comparison With Standard Diagnostics Currently Used in Clinical Practice. Neurol. Neurochir. Pol. 51 (5), 388–394. doi: 10.1016/j.pjnns.2017.06.013
Fang, X., Cai, Y., Shi, T., Huang, Z., Zhang, C., Li, W., et al. (2020). Detecting the Presence of Bacteria in Low-Volume Preoperative Aspirated Synovial Fluid by Metagenomic Next-Generation Sequencing. Int. J. Infect. Dis. 99, 108–116. doi: 10.1016/j.ijid.2020.07.039
Gu, W., Miller, S., Chiu, C. Y. (2019). Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu. Rev. Pathol. 14, 319–338. doi: 10.1146/annurev-pathmechdis-012418-012751
Han, D., Li, Z., Li, R., Tan, P., Zhang, R., Li, J. (2019). mNGS in Clinical Microbiology Laboratories: On the Road to Maturity. Crit. Rev. Microbiol. 45 (5-6), 668–685. doi: 10.1080/1040841x.2019.1681933
He, R., Wang, Q., Wang, J., Tang, J., Shen, H., Zhang, X. (2021). Better Choice of the Type of Specimen Used for Untargeted Metagenomic Sequencing in the Diagnosis of Periprosthetic Joint Infections. Bone Joint J. 103-b (5), 923–930. doi: 10.1302/0301-620x.103b5.Bjj-2020-0745.R1
Huang, Z., Li, W., Lee, G. C., Fang, X., Xing, L., Yang, B., et al. (2020). Metagenomic Next-Generation Sequencing of Synovial Fluid Demonstrates High Accuracy in Prosthetic Joint Infection Diagnostics: mNGS for Diagnosing PJI. Bone Joint Res. 9 (7), 440–449. doi: 10.1302/2046-3758.97.Bjr-2019-0325.R2
Huang, Z. D., Zhang, C. J., Li, W. B., Fang, X. Y., Zhang, C. F., Yang, B., et al. (2019). Detection of Periprosthetic Joint Infection Pathogens by Metagenomic Next⁃Generation Sequencing. Chin. J. Orthop. 39, 944–953. doi: 10.3760/cma.j.issn.0253⁃2352.2019.15.005
Ivy, M. I., Thoendel, M. J., Jeraldo, P. R., Greenwood-Quaintance, K. E., Hanssen, A. D., Abdel, M. P., et al. (2018). Direct Detection and Identification of Prosthetic Joint Infection Pathogens in Synovial Fluid by Metagenomic Shotgun Sequencing. J. Clin. Microbiol. 56 (9), e00402-18. doi: 10.1128/JCM.00402-18
Kallala, R., Harris, W. E., Ibrahim, M., Dipane, M., McPherson, E. (2018). Use of Stimulan Absorbable Calcium Sulphate Beads in Revision Lower Limb Arthroplasty: Safety Profile and Complication Rates. Bone Joint Res. 7 (10), 570–579. doi: 10.1302/2046-3758.710.Bjr-2017-0319.R1
Kwon, M., Seo, S. S., Kim, M. K., Lee, D. O., Lim, M. C. (2019). Compositional and Functional Differences Between Microbiota and Cervical Carcinogenesis as Identified by Shotgun Metagenomic Sequencing. Cancers (Basel) 11 (3). doi: 10.3390/cancers11030309
Liu, K., Fu, J., Yu, B., Sun, W., Chen, J., Hao, L. (2018). Meta-Analysis of Sonication Prosthetic Fluid PCR for Diagnosing Periprosthetic Joint Infection. PLoS One 13 (4), e0196418. doi: 10.1371/journal.pone.0196418
Li, M., Zeng, Y., Wu, Y., Si, H., Bao, X., Shen, B. (2019). Performance of Sequencing Assays in Diagnosis of Prosthetic Joint Infection: A Systematic Review and Meta-Analysis. J. Arthroplasty 34 (7), 1514–1522.e1514. doi: 10.1016/j.arth.2019.02.044
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 4 (1), 1. doi: 10.1186/2046-4053-4-1
Moojen, D. J., Zwiers, J. H., Scholtes, V. A., Verheyen, C. C., Poolman, R. W. (2014). Similar Success Rates for Single and Multiple Debridement Surgery for Acute Hip Arthroplasty Infection. Acta Orthop. 85 (4), 383–388. doi: 10.3109/17453674.2014.927729
Nodzo, S. R., Bauer, T., Pottinger, P. S., Garrigues, G. E., Bedair, H., Deirmengian, C. A., et al. (2015). Conventional Diagnostic Challenges in Periprosthetic Joint Infection. J. Am. Acad. Orthop. Surg. 23, S18–S25. doi: 10.5435/jaaos-d-14-00385
Osmon, D. R., Berbari, E. F., Berendt, A. R., Lew, D., Zimmerli, W., Steckelberg, J. M., et al. (2013). Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 56 (1), e1–e25. doi: 10.1093/cid/cis803
Parvizi, J., Zmistowski, B., Berbari, E. F., Bauer, T. W., Springer, B. D., Della Valle, C. J., et al. (2011). New Definition for Periprosthetic Joint Infection: From the Workgroup of the Musculoskeletal Infection Society. Clin. Orthop. Relat. Res. 469 (11), 2992–2994. doi: 10.1007/s11999-011-2102-9
Peel, T. N., Cole, N. C., Dylla, B. L., Patel, R. (2015). Matrix-Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry and Diagnostic Testing for Prosthetic Joint Infection in the Clinical Microbiology Laboratory. Diagn. Microbiol. Infect. Dis. 81 (3), 163–168. doi: 10.1016/j.diagmicrobio.2014.11.015
Rak, M., Barlic-Maganja, D., Kavcic, M., Trebse, R., Cor, A. (2013). Comparison of Molecular and Culture Method in Diagnosis of Prosthetic Joint Infection. FEMS Microbiol. Lett. 343 (1), 42–48. doi: 10.1111/1574-6968.12125
Rietbergen, L., Kuiper, J. W., Walgrave, S., Hak, L., Colen, S. (2016). Quality of Life After Staged Revision for Infected Total Hip Arthroplasty: A Systematic Review. Hip. Int. 26 (4), 311–318. doi: 10.5301/hipint.5000416
Ruiz-Aragón, J., Ballestero-Téllez, M., Gutiérrez-Gutiérrez, B., de Cueto, M., Rodríguez-Baño, J., Pascual, Á. (2018). Direct Bacterial Identification From Positive Blood Cultures Using Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) Mass Spectrometry: A Systematic Review and Meta-Analysis. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 36 (8), 484–492. doi: 10.1016/j.eimc.2017.08.012
Schlaberg, R., Chiu, C. Y., Miller, S., Procop, G. W., Weinstock, G., Professional Practice, C., et al. (2017). Validation of Metagenomic Next-Generation Sequencing Tests for Universal Pathogen Detection. Arch. Pathol. Lab. Med. 141 (6), 776–786. doi: 10.5858/arpa.2016-0539-RA
Tande, A. J., Patel, R. (2014). Prosthetic Joint Infection. Clin. Microbiol. Rev. 27 (2), 302–345. doi: 10.1128/CMR.00111-13
Thoendel, M. J., Jeraldo, P. R., Greenwood-Quaintance, K. E., Yao, J. Z., Chia, N., Hanssen, A. D., et al. (2018). Identification of Prosthetic Joint Infection Pathogens Using a Shotgun Metagenomics Approach. Clin. Infect. Dis. 67 (9), 1333–1338. doi: 10.1093/cid/ciy303
Vanhegan, I. S., Malik, A. K., Jayakumar, P., Ul Islam, S., Haddad, F. S. (2012). A Financial Analysis of Revision Hip Arthroplasty: The Economic Burden in Relation to the National Tariff. J. Bone Joint Surg. Br. 94 (5), 619–623. doi: 10.1302/0301-620x.94b5.27073
Wacker, C., Prkno, A., Brunkhorst, F. M., Schlattmann, P. (2013). Procalcitonin as a Diagnostic Marker for Sepsis: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 13 (5), 426–435. doi: 10.1016/s1473-3099(12)70323-7
Wang, J., Han, Y., Feng, J. (2019). Metagenomic Next-Generation Sequencing for Mixed Pulmonary Infection Diagnosis. BMC Pulm. Med. 19 (1), 252. doi: 10.1186/s12890-019-1022-4
Wang, C. X., Huang, Z., Fang, X., Li, W., Yang, B., Zhang, W. (2020). Comparison of Broad-Range Polymerase Chain Reaction and Metagenomic Next-Generation Sequencing for the Diagnosis of Prosthetic Joint Infection. Int. J. Infect. Dis. 95, 8–12. doi: 10.1016/j.ijid.2020.03.055
Whiting, P. F., Rutjes, A. W., Westwood, M. E., Mallett, S., Deeks, J. J., Reitsma, J. B., et al. (2011). QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 155 (8), 529–536. doi: 10.7326/0003-4819-155-8-201110180-00009
Wilson, M. R., Sample, H. A., Zorn, K. C., Arevalo, S., Yu, G., Neuhaus, J., et al. (2019). Clinical Metagenomic Sequencing for Diagnosis of Meningitis and Encephalitis. N. Engl. J. Med. 380 (24), 2327–2340. doi: 10.1056/NEJMoa1803396
Yan, L., Sun, W., Lu, Z., Fan, L. (2020). Metagenomic Next-Generation Sequencing (mNGS) in Cerebrospinal Fluid for Rapid Diagnosis of Tuberculosis Meningitis in HIV-Negative Population. Int. J. Infect. Dis. 96, 270–275. doi: 10.1016/j.ijid.2020.04.048
Yoon, H. K., Cho, S. H., Lee, D. Y., Kang, B. H., Lee, S. H., Moon, D. G., et al. (2017). A Review of the Literature on Culture-Negative Periprosthetic Joint Infection: Epidemiology, Diagnosis and Treatment. Knee Surg. Relat. Res. 29 (3), 155–164. doi: 10.5792/ksrr.16.034
Yu, Y., Zhang, S. K., Lu, S. T., Tan, J., Li, Y., Xu, J. Z. (2021). Performance of Metagenomic Next⁃Generation Sequencing in Diagnosis of Prosthetic Joint Infection. Chin. J. Orthop 41 (05), 280–288. doi: 10.3760cma.j.cn121113-20200830-00532
Keywords: metagenomics, next-generation sequencing, clinical diagnosis and treatment, arthroplasty, infection disease, prosthetic joint infection
Citation: Tan J, Liu Y, Ehnert S, Nüssler AK, Yu Y, Xu J and Chen T (2022) The Effectiveness of Metagenomic Next-Generation Sequencing in the Diagnosis of Prosthetic Joint Infection: A Systematic Review and Meta-Analysis. Front. Cell. Infect. Microbiol. 12:875822. doi: 10.3389/fcimb.2022.875822
Received: 14 February 2022; Accepted: 11 May 2022;
Published: 10 June 2022.
Edited by:
Beiwen Zheng, Zhejiang University, ChinaReviewed by:
Débora Coraça-Huber, Innsbruck Medical University, AustriaHua Gao, Peking University People’s Hospital, China
Copyright © 2022 Tan, Liu, Ehnert, Nüssler, Yu, Xu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianzhong Xu, zzuxujianzhong@163.com; Tao Chen, zzuchentao@yahoo.com
 Jun Tan
Jun Tan Yang Liu
Yang Liu Sabrina Ehnert
Sabrina Ehnert Andreas K. Nüssler
Andreas K. Nüssler Yang Yu
Yang Yu Jianzhong Xu
Jianzhong Xu Tao Chen
Tao Chen