Spillover and genome selection of the gut parasite Nosema ceranae between honey bee species
- 1Honeybee Research Institute, Jiangxi Agricultural University, Nanchang, China
- 2USDA-ARS Bee Research Laboratory, BARC-East Building 306, Beltsville, MD, United States
- 3Department of Integrative Biology, The University of Texas at Austin, Austin, TX, United States
Nosema ceranae is a honey bee gut parasite that has recently spilled to another honey bee host through trading. The impact of infection on the native host is minor, which is substantial in the novel host. In this study, artificial inoculation simulated the parasite transmission from the native to the novel host. We found that the parasite initiated proliferation earlier in the novel host than in the native host. Additionally, parasite gene expression was significantly higher when infecting the novel host compared with the native host, leading to a significantly higher number of spores. Allele frequencies were similar for spores of parasites infecting both native and novel hosts. This suggests that the high number of spores found in the novel host was not caused by a subset of more fit spores from native hosts. Native hosts also showed a higher number of up-regulated genes in response to infection when compared with novel hosts. Our data further showed that native hosts suppressed parasite gene expression and arguably sacrificed cells to limit the parasite. The results provide novel insights into host defenses and gene selection during a parasite spillover event.
Introduction
Host-parasite evolution involves reciprocal genetic changes between the interacting species (Brockhurst et al., 2014). When the parasite infects a novel host, high virulence is usually observed. The parasite spillover has been substantially facilitated by international trading. The honey bee is the most important commercial pollinator. The bee wax and fruit trading have led to the global spread of the bee parasite, causing substantial damage to the apicultural industry (Idrissou et al., 2019; Liu et al., 2021). Nosema ceranae is an obligate intracellular parasite that infects the epithelial cells of honey bee midgut (Higes et al., 2007). N. ceranae is a native parasite of the Asian honey bee Apis cerana and has successfully established infection in the novel honey bee species, Apis mellifera (Fries et al., 1996; Higes et al., 2006). Using a set of genetic markers, a high level of genetic diversity was found in N. ceranae (Gómez-Moracho et al., 2014; Pelin et al., 2015). However, minor variance was found among various geographically distinct regions, suggesting human-mediated gene flow. The infection exhibits high levels of virulence in the novel honey bee host, leading to impaired flying ability, reduced life span, and suppressed immune responses (Antunez et al., 2009; Dussaubat et al., 2013; Goblirsch et al., 2013; Gage et al., 2018). However, the impact of infection was minor in the native host. As the two honey bee species share habitats in Asia and Australia, it provides an ideal opportunity to use A. mellifera, A. cerana, and N. ceranae as model organisms to study the parasite spillover.
N. ceranae was recently proposed within Vairimorpha (Tokarev et al., 2020). To be consistent with previous literatures, we kept N. ceranae. This study aims to reveal the mechanism under the reported high virulence after the parasite spillover to the novel host. Accordingly, we quantified host and parasite gene expression, covering the entire proliferation cycle. Additionally, we investigated whether a subset of parasite genotype was favored leading to the high virulence.
Results
The native host showed high mortality following infection
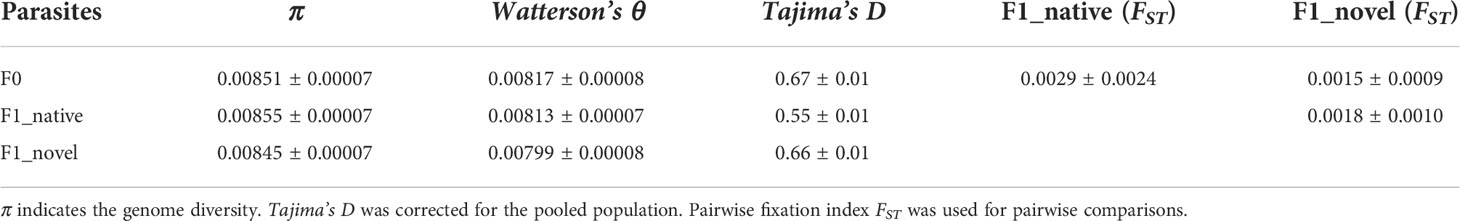
Parasite spores were not found in the uninfected group. Among the experimental groups, uninfected novel host showed the highest survival (> 98%), which was not significantly different from the uninfected native host (Log Rank, adjusted P > 0.05). Comparatively, the infected native host showed the lowest survival (87%), which was significantly lower than the other three groups (Log Rank, adjusted P < 0.001) (Figure 1B). At 14 days post-infection (dpi), 5.1 million spores were found in the novel host, which was significantly higher than those found in the native host (Kruskal Wallis test, P < 0.05) (Figure 1C).
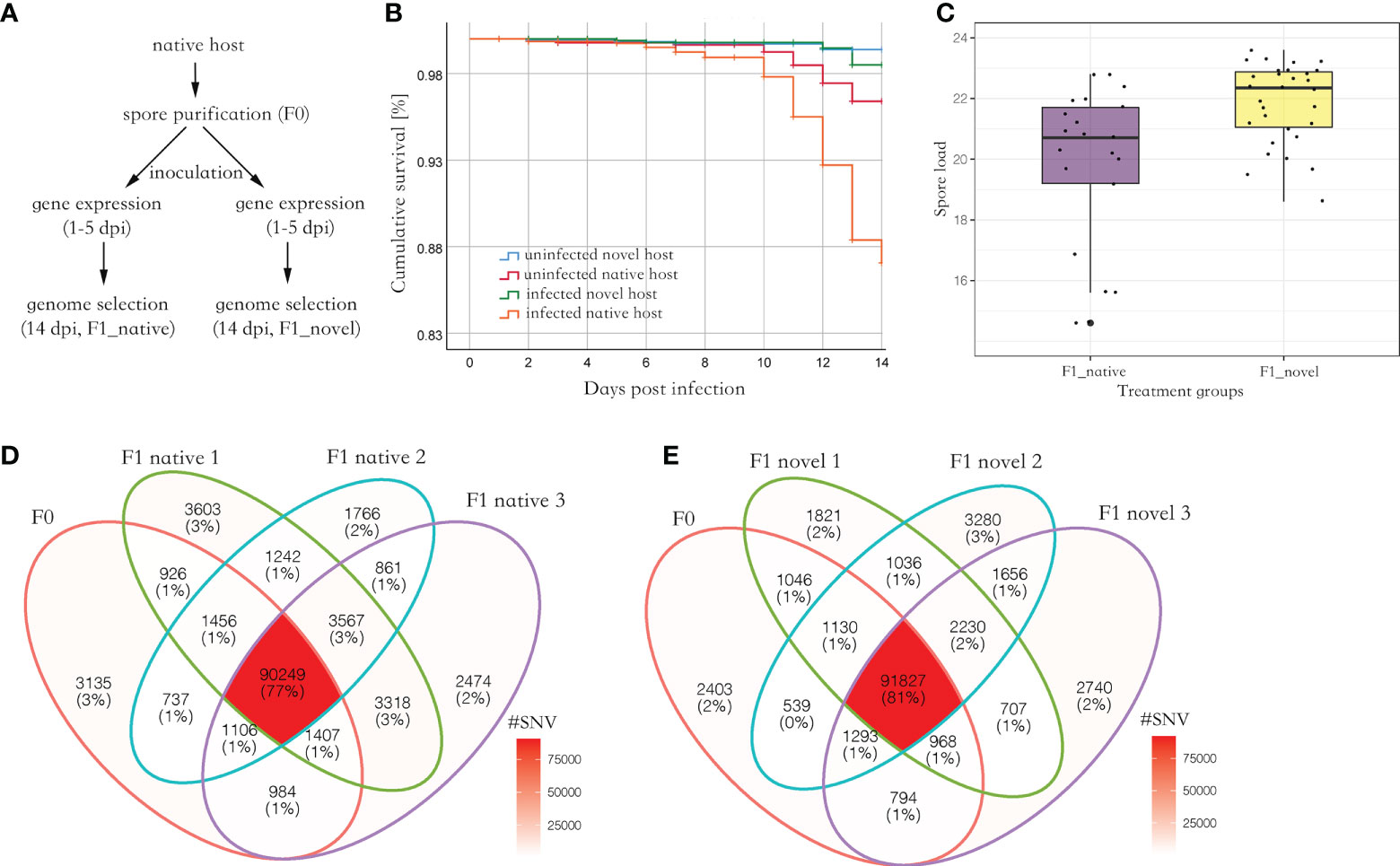
Figure 1 (A) The experimental design of this study. The spores were purified from the native host. Then the spores were inoculated to newly emerged honey bees from the native and novel host, respectively, to harvest produced spores. The gene expression (RNA-seq) of the infected bees was quantified from 1 to 5 dpi, covering a complete proliferation cycle of the parasite. Additionally, the SNVs along the parasite genome were quantified and compared between the parental and produced spores at 14 dpi. (B) The cumulative survival of honey bees. The native host infected by the parasite (F1_native) showed the highest mortality (Log Rank, adjusted P < 0.001). (C) The parasite spore load in the infected native and novel hosts. A significantly higher number of produced spores were found in the novel host compared with the native host (Kruskal Wallis test, P < 0.05). The y-axis represents the spore load which was log-transformed with base 2. (D) Venn diagram of SNVs identified in parental and produced spores in the native host. (E) Venn diagram of SNVs identified in the parental and produced spores in the novel host. Irrespective of the host species, at least 90% of parental SNVs were maintained in the produced spores. Additionally, over 50% of novel SNVs in produced spores were shared between the replicates in each host species, suggesting the mutation may not be random (Fisher’s exact test, P < 0.001).
Parasites initiated proliferation earlier in the novel than the native host
During parasite proliferation, 762 genes were significantly over-expressed in parasites infecting the novel host, which is substantially higher than the 26 genes over-expressed in the native host (Fisher’s Exact test, P < 0.001) (Figure 2A). Significantly regulated genes were enriched in genetic information processing (Figure 2B). Parasite gene expression was detected as early as one dpi in the novel host. Comparatively, the infection was not detected until three dpi in the native host (Figure 2A). Additionally, 11 genes were over expressed at three time points when the parasite proliferated in the novel host, including a polar-tube protein (G9O61_00g021150). Using the genome as background, genes related to nuclear chromatin were significantly enriched in the parasite infecting the novel host (GO:0000790, adjusted P < 0.01).
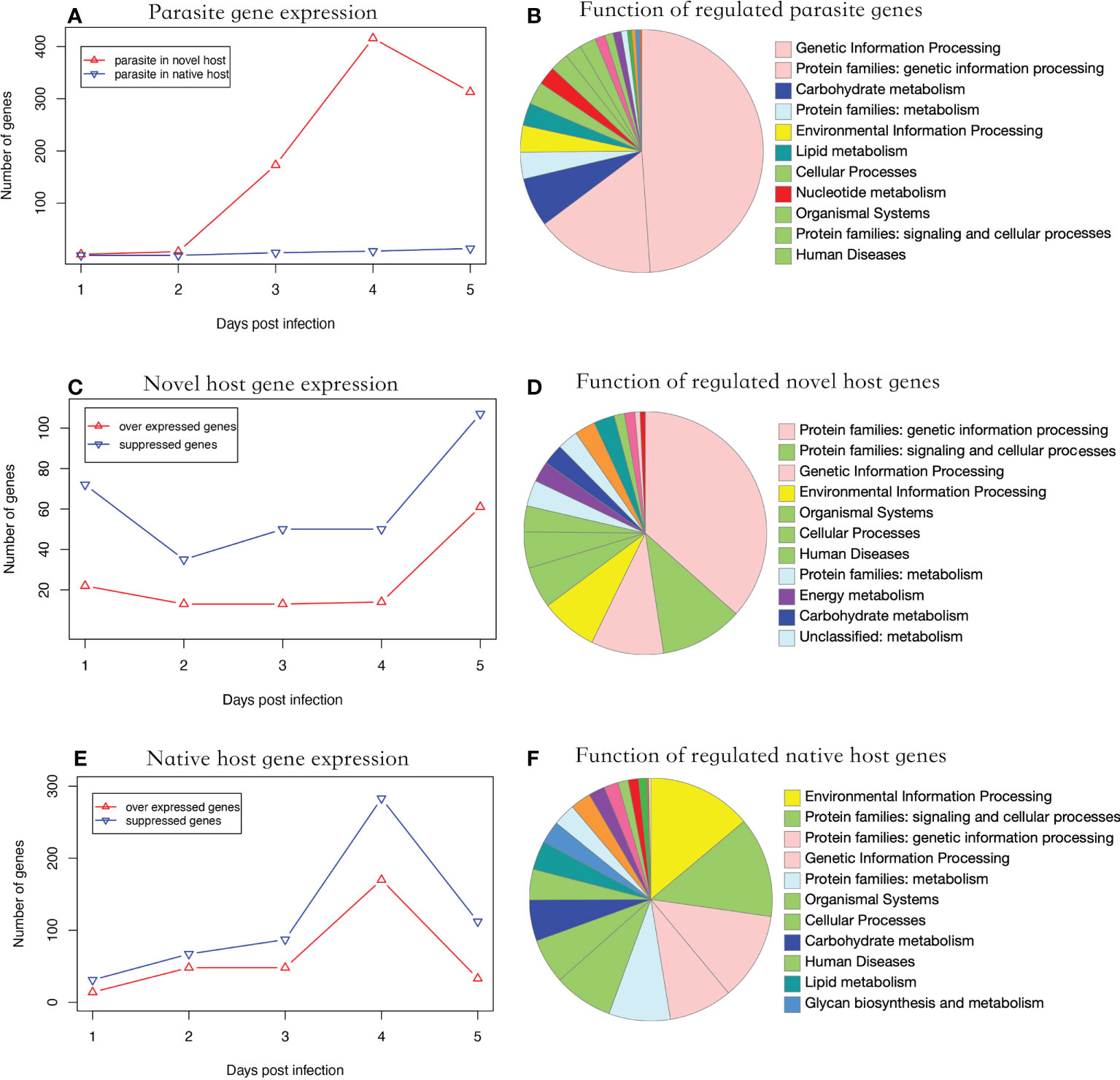
Figure 2 Functional analysis of regulated genes. (A) parasite gene expression profile in two hosts. The parasite showed higher gene expression levels in the novel than the native host (Fisher’s Exact Test, P < 0.001). (B) Putative function of significantly regulated parasite genes. (C) Number of significantly regulated genes of the novel host. The number of suppressed genes in the novel host was significantly higher than in over-expressed ones (Paired t-test, P < 0.01). (D) Putative function of the significantly regulated novel host genes. (E) Number of significantly regulated genes of the native host. The genes showed a suppressed expression pattern. (F) Putative function of the significantly regulated native host genes. In (B, D, E) the color of the functional category was used in global pathway maps and genome maps of KEGG. The functional category on the right was indicated in the pie chart in clockwise order.
The native host showed more robust responses to parasite infection than the novel host
In the novel host, the number of suppressed genes was two-fold higher than over-expressed genes from one to five dpi (Figure 2C, Paired t-test, P < 0.01), which were enriched in genetic information processing (Figure 2D). In the native host, the number of suppressed genes was slightly higher than over-expressed ones but not statistically significant during the entire experimental period (Figure 2E, Paired t-test, P = 0.057). The significantly regulated genes were enriched in environmental information processing (Figure 2F). In honey bees, the number of genes significantly regulated after infection was significantly higher in the native host (719 genes) than in the novel host (342 genes; Fisher’s exact test, P < 0.001). Using the genome as the background, genes related to the cell cycle (GO:0000082, adjusted P < 0.001) were enriched in the novel host and those related to the regulation of transcription (GO:0006357, adjusted P < 0.001) were enriched in the native host.
Minor impact of host species on the parasite genome selection
On average, 74,002,415 reads (150bps paired reads, over 1,000 times genome coverage) were aligned to the N. ceranae genome in a library, which accounted for 97.4% of total sequenced reads. In F0 spores, 99,999 Single Nucleotide Variants (SNVs) were identified. In the F1_native group, 105,768 (F1_native_1), 100,983 (F1_native_2) and 103,965 (F1_native_3) SNVs were identified (Figure 1D). Comparatively, 100,765 (F1_novel_1), 101,506 (F1_novel_2) and 100,772 (F1_novel_3) SNVs were identified in the F1_novel group (Figure 1E). Out of 99,999 SNVs in F0 spores, 90,249 and 91,827 SNVs were found in all three replicates in both native and novel hosts. Over 90% of the parental SNVs were found in spores produced by the infection, irrespective of the host species. The numbers of maintained SNVs were independent of host species (Fisher’s exact test, P > 0.05). The genome differentiation index (FST) within species was slightly smaller than between the host species (Table 1). Within the produced spores, the native host showed greater genome differentiation than the novel host when compared with the parental spores. The parental and produced spores showed similar genome diversity (π) and Watterson’s θ, irrespective of the host species (Table 1). Tajima’s D was positive in all isolates. The coefficient S was quantified for the maintained SNVs from F0 to F1 spores. Among the top 1% loci with the highest allele frequency variation, the loci were highly congruent across replicates and the host species, which was significantly higher than random (Fisher’s Exact test, P < 0.001). Among those loci, 50 genes were identified, which were significantly enriched in ATP binding (GO:0005524, adjusted P < 0.001) and cell proliferation (GO: 0010971, adjusted P < 0.01).
Discussion
Understanding the co-evolution between hosts and their parasites is particularly important in ecology and conservation biology (Gómez and Nichols, 2013; Paplauskas et al., 2021). Most empirical and theoretical studies of host-parasite interactions were conducted in a paired host and parasite (Rabajante et al., 2016; Best et al., 2017; Ebert and Fields, 2020). In natural conditions, the parasite may infect and spill among multiple hosts (Bailey et al., 2009). Consequently, parasite transmission and diversity depend on the relative degree of host abundance and susceptibility (Hardy and Cook, 2010; Neiman and Fields, 2016). N. ceranae is an intracellular gut parasite of honey bees transmitted through the fecal-oral route. Transmission heavily depends on direct (such as trophallaxis among colony members) and indirect (via commonly visited flowers) contact among honey bees (Graystock et al., 2015). As a part of its range, N. ceranae has spilled from Asian honey bees to European honey bees in instances where the shifting dynamic depends on the relative abundance of the host species’ numbers (Hovestadt et al., 2019). Previous studies found that the infection showed a high virulence in the novel host (Eiri et al., 2015; Gage et al., 2018; Paris et al., 2018). In this study, we found that the parasite N. ceranae produced a significantly higher number of spores in novel hosts compared with the native host. This suggests that novel hosts cannot suppress parasite proliferation at the individual honey bee level, as we previously found for the colony level (Wu et al., 2022). Additionally, we found that native hosts showed higher mortality. Thus, native hosts might limit parasite growth by sacrificing infected nest members, a trait observed in a honey bee strain selected for N. ceranae tolerance (Huang et al., 2012). A similar phenomenon has also been observed in the native host towards the mite infestation, wherein infected larvae were sacrificed to limit mite reproduction (Page et al., 2016).
Using a set of markers, N. ceranae diversity has been reported to be higher within honey bee colonies than among neighboring colonies (Gómez-Moracho et al., 2014; Gómez-Moracho et al., 2015). Previously, we found that the genetic diversity of N. ceranae was substantially higher in habitats where the native and novel honey bee species co-exist (Ke et al., 2021). This study found minor disparities in genetic diversity variation across replicates. In our data, π was slightly higher than Watterson’s θ, leading to a small positive Tajima’s D, which suggests a small number of low-frequency alleles in all parasite isolates. The rare loci might have a strong tendency to be lost, and the genome was under balancing selection (Fu and Li, 1993; Stajich and Hahn, 2005). The fixation index FST reflects the overall genome differentiation. In our data, the FST was slightly higher within a species than between the two host species, which suggests host-specific adaptation shaped the genome evolution, even though the effect was not strong. In host-parasite studies, allele frequency fluctuation can provide insights into virulence and infection (Campbell et al., 2013; Papkou et al., 2019). In our study, the impact of host species on allele frequency variation was minor, which might reflect their close phylogenetic relationship.
The life cycle of N. ceranae is approximately four days (Higes et al., 2007; Gisder et al., 2011). In our data, the proliferation initiated earlier when infecting the novel host. As a result, more spores were found in the novel host. In the parasite genome, 2,280 genes were annotated. Out of those, 762 genes were significantly up-regulated when infecting the novel host, which was broadly involved in DNA/RNA replication. Notably, the polar tube protein was constantly up-regulated, which is essential to attach to the host cell membrane and is vital to initiate the infection (Han et al., 2017). When the expression level of the polar tube protein was suppressed, the number of produced spores was substantially reduced (Rodriguez-Garcia et al., 2018). After the inoculation, the native host strongly responded to the infection. It is known that N. ceranae suppresses apoptosis in infected honey bees and that bees with accelerated apoptosis are more tolerant of infection (Higes et al., 2013; Kurze et al., 2015). In our data, cell-cycle genes were suppressed in the novel host, suggesting that infected cells can’t initiate apoptosis to limit parasite proliferation. Thus, apoptosis may reflect an important strategy used by native hosts to limit N. ceranae infection.
Materials and methods
Host and parasite sources
The honey bee, Apis cerana, is the native host of the parasite Nosema ceranae, and the honey bee Apis mellifera is the novel host of the parasite N. ceranae. This study simulated parasite dispersal from native to novel host species by artificially inoculating the two honey bee species with N. ceranae spores. First, the parasite spores were purified from the native host, A. cerana. Then, the purified spores were used to inoculate the novel and native host separately to harvest produced spores by infection (Figure 1A). Overall, three sources of parasite spores were collected and analyzed, including parental spores isolated from native hosts (F0), spores produced by infection in the native hosts (F1_native), and spores produced in the novel hosts (F1_novel). Additionally, the parasite gene expression (RNA-seq) in the two hosts was quantified from one to five dpi, which covers a complete parasite proliferation cycle.
Parasite isolation, inoculation, and sample collection
To harvest sufficient number of spores, 200 honey bee foragers were randomly collected from four A. cerana colonies that were heavily infected with N. ceranae in the experimental apiary. Midguts of the honey bees were dissected and homogenized to isolate N. ceranae spores using centrifugation (Fries et al., 2013). The spores were further purified using Percoll gradient centrifugation and the species status of N. ceranae was confirmed by species-specific primers using conventional PCR (Chen et al., 2013; Fries et al., 2013). The pooled spores represented local parasite diversity and served as a common source for inoculations.
Frames with emerging brood were removed from colonies of the native (A. cerana) and novel host (A. mellifera), which were kept in an incubator (34 ± 1°C, 60% relative humidity). For each honey bee species, 150 newly emerged honey bee workers were individually fed with 2 µl of sucrose solution containing 105 N. ceranae spores isolated from the native host. An additional 150 newly emerged native and novel host honey bee workers were fed with 2 µl sucrose solution as an uninfected control. During the experiment, cohorts were divided into three cups containing 50 bees each and maintained on 50% sucrose solution ad libitum in the incubator. Dead bees were recorded and removed daily. Three bees were collected from one to five dpi from each cup at 24h intervals. The midgut was dissected for RNA extraction with TriZol and sequenced using Illumina Hiseq 2000. At 14 dpi, the remaining honey bees were harvested for spore counting. The spores of the same species were pooled for DNA extraction using CTAB (cetyl trimethylammonium bromide) (Chen et al., 2013), which were also sequenced using Illumina Hiseq 2000. For each treatment, three replicates were performed.
Gene expression and SNV analysis
For genetic diversity analysis, a DNA library for F0 spores, three DNA libraries for F1_native spores (F1_native_1, F1_native_2 and F1_native_3) and three DNA libraries for F1_novel spores (F1_novel_1, F1_novel_2 and F1_novel_3) were sequenced. DNA sequencing reads were aligned to the N. ceranae genome (Ncer 3.0, GCA_004919615.1) using BWA with default parameters (Li and Durbin, 2009; Huang et al., 2021). The SNVs were identified and annotated using the Picard-GATK-SNPEFF pipeline (Van der Auwera et al., 2013). The SNVs found in F0 spores but not in F1 spores were defined as lost SNVs. The SNVs found in F1 spores but not F0 spores were defined as novel SNVs. The SNVs found in both F0 and F1 spores were defined as maintained SNVs. For the RNA-seq analysis, three RNA libraries for the uninfected native host, three RNA libraries for the uninfected novel host, three RNA libraries for the infected native host, and three RNA libraries for the infected novel host were sequenced per day from 1 to 5 dpi. The RNA reads were aligned to the parasite (N. ceranae, Ncer 3.0, GCA_004919615.1), the native host (honey bee A. ceranae, HAv3.1, GCA_003254395.2), and the novel host (honey bee A. mellifera, ApisCC1.0, GCA_002290385.1) with Hisat2 with default parameters (Kim et al., 2015; Diao et al., 2018; Wallberg et al., 2019). The variance of the three replicates was used to calculate the within-group variance to determine significantly regulated genes using EdgeR (Robinson et al., 2010). The protein sequences of significantly regulated genes were quired to NCBI non-redundant database and KEGG to infer putative biological function and pathways (Kanehisa and Goto, 2000). GO terms were retrieved using EggNOG-mapper, and enrichment analysis was performed using TopGO (Alexa and Rahnenfuhrer, 2021; Cantalapiedra et al., 2021). In total, 7 DNA and 60 RNA libraries were sequenced and analyzed.
Population genetic analysis and statistics
Watterson’s θ, genome diversity π, and corrected Tajima’s D values were calculated using Popoolation (Kofler et al., 2011a). The fixation index FST was calculated using Popoolation2 (Kofler et al., 2011b). Survival was analyzed with the Kaplan-Meier procedure using SPSS and adjusted for FDR using R (R Core Team, 2013). The spore load variance was analyzed using the Kruskal Wallis test using R (R Core Team, 2013). Paired t-tests were used to compare the number of up and downregulated genes with R (R Core Team, 2013). As the common parental spores were inoculated to harvest produced spores, one sample t-test was used to compare the number of SNVs in parental and produced spores using R (R Core Team, 2013).
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA784016; https://www.ncbi.nlm.nih.gov/, PRJNA820988.
Author contributions
XW conducted the experiment. QH designed the experiment. XW, JE, YC, and QH organized the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the initiation package of Jiangxi Agricultural University (050014/923230722) and the National Natural Science Foundation of China #32060778.
Acknowledgments
We appreciate Dr. Lizhen Zhang for technical support and Prof. Nancy Moran for revising the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alexa, A., Rahnenfuhrer, J. (2021). TopGO: Enrichment analysis for gene ontology. R Packag. version 2.46.0.
Antunez, K., Martin-Hernandez, R., Prieto, L., Meana, A., Zunino, P., Higes, M. (2009). Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ. Microbiol. 11, 2284–2290. doi: 10.1111/j.1462-2920.2009.01953.x
Bailey, R., Schönrogge, K., Cook, J. M., Melika, G., Csóka, G., Thuróczy, C., et al. (2009). Host niches and defensive extended phenotypes structure parasitoid wasp communities. PloS Biol. 7, e1000179. doi: 10.1371/journal.pbio.1000179
Best, A., Ashby, B., White, A., Bowers, R., Buckling, A., Koskella, B., et al. (2017). Host–parasite fluctuating selection in the absence of specificity. Proc. R. Soc B Biol. Sci. 284, 20171615. doi: 10.1098/rspb.2017.1615
Brockhurst, M. A., Chapman, T., King, K. C., Mank, J. E., Paterson, S., Hurst, G. D. D. (2014). Running with the red queen: the role of biotic conflicts in evolution. Proc. R. Soc B Biol. Sci. 281, 20141382. doi: 10.1098/rspb.2014.1382
Campbell, S. E., Williams, T. A., Yousuf, A., Soanes, D. M., Paszkiewicz, K. H., Williams, B. A. P. (2013). The genome of Spraguea lophii and the basis of host-microsporidian interactions. PloS Genet. 9, e1003676. doi: 10.1371/journal.pgen.1003676
Cantalapiedra, C. P., Hernández-Plaza, A., Letunic, I., Bork, P., Huerta-Cepas, J. (2021). eggNOG-mapper v2: Functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol. Biol. Evol. 38, 5825–5829. doi: 10.1093/molbev/msab293
Chen, Y., Pettis, J. S., Zhao, Y., Liu, X., Tallon, L. J., Sadzewicz, L. D., et al. (2013). Genome sequencing and comparative genomics of honey bee microsporidia, Nosema apis reveal novel insights into host-parasite interactions. BMC Genomics 14, 451. doi: 10.1186/1471-2164-14-451
Core Team, R. (2013) R: A language and environment for statistical computing. r found. stat. comput. Vienna, Austria. Available at: http://www.R-project.org/.
Diao, Q., Sun, L., Zheng, H., Zeng, Z., Wang, S., Xu, S., et al. (2018). Genomic and transcriptomic analysis of the Asian honeybee Apis cerana provides novel insights into honeybee biology. Sci. Rep. 8. doi: 10.1038/s41598-017-17338-6
Dussaubat, C., Maisonnasse, A., Crauser, D., Beslay, D., Costagliola, G., Soubeyrand, S., et al. (2013). Flight behavior and pheromone changes associated to nosema ceranae infection of honey bee workers (Apis mellifera) in field conditions. J. Invertebr. Pathol. 113, 42–51. doi: 10.1016/j.jip.2013.01.002
Ebert, D., Fields, P. D. (2020). Host–parasite co-evolution and its genomic signature. Nat. Rev. Genet. 21, 754–768. doi: 10.1038/s41576-020-0269-1
Eiri, D. M., Suwannapong, G., Endler, M., Nieh, J. C. (2015). Nosema ceranae can infect honey bee larvae and reduces subsequent adult longevity. PloS One 10, e0126330. doi: 10.1371/journal.pone.0126330
Fries, I., Chauzat, M.-P., Chen, Y.-P., Doublet, V., Genersch, E., Gisder, S., et al. (2013). Standard methods for Nosema research. J. Apic. Res. 52, 1–28. doi: 10.3896/IBRA.1.52.1.14
Fries, I., Feng, F., da Silva, A., Slemenda, S. B., Pieniazek, N. J. (1996). Nosema ceranae n. sp. (Microspora, nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis cerana (Hymenoptera, apidae). Eur. J. Protistol. 32, 356–365. doi: 10.1016/S0932-4739(96)80059-9
Fu, Y. X., Li, W. H. (1993). Statistical tests of neutrality of mutations. Genetics 133, 693–709. doi: 10.1093/genetics/133.3.693
Gage, S. L., Kramer, C., Calle, S., Carroll, M., Heien, M., DeGrandi-Hoffman, G. (2018). Nosema ceranae parasitism impacts olfactory learning and memory and neurochemistry in honey bees (Apis mellifera). J. Exp. Biol. 221, jeb161489. doi: 10.1242/jeb.161489
Gisder, S., Mockel, N., Linde, A., Genersch, E. (2011). A cell culture model for Nosema ceranae and Nosema apis allows new insights into the life cycle of these important honey bee-pathogenic microsporidia. Environ. Microbiol. 13, 404–413. doi: 10.1111/j.1462-2920.2010.02346.x
Goblirsch, M., Huang, Z. Y., Spivak, M. (2013). Physiological and behavioral changes in honey bees (Apis mellifera) induced by Nosema ceranae infection. PloS One 8, e58165. doi: 10.1371/journal.pone.0058165
Gómez-Moracho, T., Bartolomé, C., Bello, X., Martín-Hernández, R., Higes, M., Maside, X. (2015). Recent worldwide expansion of Nosema ceranae (Microsporidia) in Apis mellifera populations inferred from multilocus patterns of genetic variation. Infect. Genet. Evol. 31, 87–94. doi: 10.1016/j.meegid.2015.01.002
Gómez-Moracho, T., Maside, X., Martin-Hernandez, R., Higes, M., Bartolome, C. (2014). High levels of genetic diversity in Nosema ceranae within Apis mellifera colonies. Parasitology 141, 475–481. doi: 10.1017/S0031182013001790
Gómez, A., Nichols, E. (2013). Neglected wild life: Parasitic biodiversity as a conservation target. Int. J. Parasitol. Parasites Wildl. 2, 222–227. doi: 10.1016/j.ijppaw.2013.07.002
Graystock, P., Goulson, D., Hughes, W. O. H. (2015). Parasites in bloom: flowers aid dispersal and transmission of pollinator parasites within and between bee species. Proc. Biol. Sci. 282, 20151371. doi: 10.1098/rspb.2015.1371
Han, B., Polonais, V., Sugi, T., Yakubu, R., Takvorian, P. M., Cali, A., et al. (2017). The role of microsporidian polar tube protein 4 (PTP4) in host cell infection. PloS Pathog. 13, e1006341. doi: 10.1371/journal.ppat.1006341
Hardy, N. B., Cook, L. G. (2010). Gall-induction in insects: evolutionary dead-end or speciation driver? BMC Evol. Biol. 10, 257. doi: 10.1186/1471-2148-10-257
Higes, M., Garcia-Palencia, P., Martin-Hernandez, R., Meana, A. (2007). Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 94, 211–217. doi: 10.1016/j.jip.2006.11.001
Higes, M., Juarranz, A., Dias-Almeida, J., Lucena, S., Botias, C., Meana, A., et al. (2013). Apoptosis in the pathogenesis of nosema ceranae (Microsporidia: Nosematidae) in honey bees (Apis mellifera). Environ. Microbiol. Rep. 5, 530–536. doi: 10.1111/1758-2229.12059
Higes, M., Martin, R., Meana, A. (2006). Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J. Invertebr. Pathol. 92, 93–95. doi: 10.1016/j.jip.2006.02.005
Hovestadt, T., Thomas, J. A., Mitesser, O., Schönrogge, K. (2019). Multiple host use and the dynamics of host switching in host–parasite systems. Insect Conserv. Divers. 12, 511–522. doi: 10.1111/icad.12374
Huang, Q., Kryger, P., Le Conte, Y., Moritz, R. F. A. (2012). Survival and immune response of drones of a nosemosis tolerant honey bee strain towards n. ceranae infections. J. Invertebr. Pathol. 109, 297–302. doi: 10.1016/j.jip.2012.01.004
Huang, Q., Wu, Z. H., Li, W. F., Guo, R., Xu, J. S., Dang, X. Q., et al. (2021). Genome and evolutionary analysis of Nosema ceranae: A microsporidian parasite of honey bees. Front. Microbiol. 12, 645353. doi: 10.3389/fmicb.2021.645353
Idrissou, F. O., Huang, Q., Yañez, O., Neumann, P. (2019). International beeswax trade facilitates small hive beetle invasions. Sci. Rep. 9, 10665. doi: 10.1038/s41598-019-47107-6
Kanehisa, M., Goto, S. (2000). KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. doi: 10.1093/nar/28.1.27
Ke, L., Yan, W. Y., Zhang, L. Z., Zeng, Z. J., Evans, J. D., Huang, Q. (2021). Honey bee habitat sharing enhances gene flow of the parasite Nosema ceranae. Microb. Ecol. doi: 10.1007/s00248-021-01827-3
Kim, D., Langmead, B., Salzberg, S. L. (2015). HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357. doi: 10.1038/nmeth.3317
Kofler, R., Orozco-terWengel, P., De Maio, N., Pandey, R. V., Nolte, V., Futschik, A., et al. (2011a). PoPoolation: A toolbox for population genetic analysis of next generation sequencing data from pooled individuals. PloS One 6, e15925. doi: 10.1371/journal.pone.0015925
Kofler, R., Pandey, R. V., Schlötterer, C. (2011b). PoPoolation2: identifying differentiation between populations using sequencing of pooled DNA samples (Pool-seq). Bioinformatics 27, 3435–3436. doi: 10.1093/bioinformatics/btr589
Kurze, C., Le Conte, Y., Dussaubat, C., Erler, S., Kryger, P., Lewkowski, O., et al. (2015). Nosema tolerant honeybees (Apis mellifera) escape parasitic manipulation of apoptosis. PloS One 10, e0140174. doi: 10.1371/journal.pone.0140174
Li, H., Durbin, R. (2009). Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics 25, 1754–1760. doi: 10.1093/bioinformatics/btp324
Liu, Y., Han, W., Gao, J., Su, S., Beaurepaire, A., Yañez, O., et al. (2021). Out of Africa: novel source of small hive beetles infesting Eastern and Western honey bee colonies in China. J. Apic. Res. 60, 108–110. doi: 10.1080/00218839.2020.1816686
Neiman, M., Fields, P. (2016). Antagonistic interspecific coevolution (Oxford, USA: Academic Press), 93–100. ed. R. M. B. T.-E. @ of E. B. Kliman.
Page, P., Lin, Z., Buawangpong, N., Zheng, H., Hu, F., Neumann, P., et al. (2016). Social apoptosis in honey bee superorganisms. Sci. Rep. 6, 27210. doi: 10.1038/srep27210
Papkou, A., Guzella, T., Yang, W., Koepper, S., Pees, B., Schalkowski, R., et al. (2019). The genomic basis of red queen dynamics during rapid reciprocal host–pathogen coevolution. PNAS 116, 923–928. doi: 10.1073/pnas.1810402116
Paplauskas, S., Brand, J., Auld, S. K. J. R. (2021). Ecology directs host–parasite coevolutionary trajectories across daphnia–microparasite populations. Nat. Ecol. Evol. 5, 480–486. doi: 10.1038/s41559-021-01390-7
Paris, L., El Alaoui, H., Delbac, F., Diogon, M. (2018). Effects of the gut parasite Nosema ceranae on honey bee physiology and behavior. Curr. Opin. Insect Sci. 26, 149–154. doi: 10.1016/j.cois.2018.02.017
Pelin, A., Selman, M., Aris-Brosou, S., Farinelli, L., Corradi, N. (2015). Genome analyses suggest the presence of polyploidy and recent human-driven expansions in eight global populations of the honeybee pathogen Nosema ceranae. Environ. Microbiol. 17, 4443–4458. doi: 10.1111/1462-2920.12883
Rabajante, J. F., Tubay, J. M., Ito, H., Uehara, T., Kakishima, S., Morita, S., et al. (2016). Host-parasite red queen dynamics with phase-locked rare genotypes. Sci. Adv. 2. doi: 10.1126/sciadv.1501548
Robinson, M. D., McCarthy, D. J., Smyth, G. K. (2010). edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. doi: 10.1093/bioinformatics/btp616
Rodriguez-Garcia, C., Evans, J. D., Li, W., Branchiccela, B., Li, J. H., Heerman, M. C., et al. (2018). Nosemosis control in European honey bees Apis mellifera by silencing the gene encoding Nosema ceranae polar tube protein 3. J. Exp. Biol. doi: 10.1242/jeb.184606
Stajich, J. E., Hahn, M. W. (2005). Disentangling the effects of demography and selection in human history. Mol. Biol. Evol. 22, 63–73. doi: 10.1093/molbev/msh252
Tokarev, Y. S., Huang, W.-F., Solter, L. F., Malysh, J. M., Becnel, J. J., Vossbrinck, C. R. (2020). A formal redefinition of the genera nosema and Vairimorpha (Microsporidia: Nosematidae) and reassignment of species based on molecular phylogenetics. J. Invertebr. Pathol. 169, 107279. doi: 10.1016/j.jip.2019.107279
Van der Auwera, G. A., Carneiro, M. O., Hartl, C., Poplin, R., Del Angel, G., Levy-Moonshine, A., et al. (2013). From FastQ data to high confidence variant calls: the genome analysis toolkit best practices pipeline. Curr. Protoc. Bioinforma. 43, 1–33. doi: 10.1002/0471250953.bi1110s43
Wallberg, A., Bunikis, I., Pettersson, O. V., Mosbech, M.-B., Childers, A. K., Evans, J. D., et al. (2019). A hybrid de novo genome assembly of the honeybee, Apis mellifera, with chromosome-length scaffolds. BMC Genomics 20, 275. doi: 10.1186/s12864-019-5642-0
Keywords: Nosema ceranae, honey bee, multi-host parasite, selection, allele frequency
Citation: Wei X, Evans JD, Chen Y and Huang Q (2022) Spillover and genome selection of the gut parasite Nosema ceranae between honey bee species. Front. Cell. Infect. Microbiol. 12:1026154. doi: 10.3389/fcimb.2022.1026154
Received: 23 August 2022; Accepted: 26 September 2022;
Published: 11 October 2022.
Edited by:
Chaoqun Yao, Ross University, United StatesReviewed by:
Leellen Solter, University of Illinois at Urbana–Champaign, United StatesZhiqiang Lu, Northwest A&F University, China
Copyright © 2022 Wei, Evans, Chen and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Huang, qiang-huang@live.com
 Xiuxiu Wei1
Xiuxiu Wei1  Jay D. Evans
Jay D. Evans Yanping Chen
Yanping Chen Qiang Huang
Qiang Huang