The First Evidence of Cryptosporidium meleagridis Infection in a Colon Adenocarcinoma From an Immunocompetent Patient
- 1Department of Biology and Medical Parasitology, Wrocław Medical University, Wrocław, Poland
- 2Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, České Budějovice, Czechia
- 3Faculty of Agriculture, University of South Bohemia, České Budějovice, Czechia
- 4Department of Genetics, Wrocław Medical University, Wrocław, Poland
- 5Department of Microbiological Sciences, North Dakota State University, Fargo, ND, United States
Objectives: The potential linkage between Cryptosporidium spp. infection and colorectal human cancer was suggested by limited reports showing higher prevalence of C. parvum and C. hominis in patients with colon cancer. Here we conducted research concerning presence of Cryptosporidium spp. in malignant tissue collected from patients with colorectal cancer.
Methods: Cancerous colon tissue samples collected from 145 non-HIV infected patients with colorectal cancer were screened for Cryptosporidium spp. by immunofluorescence antibody test and genus-specific nested polymerase chain reaction followed by sequencing.
Results: Screened pathogen was found in cancerous tissue originating from immunocompetent man with colon adenocarcinoma. Genotyping revealed presence of Cryptosporidium meleagridis. The presence of Cryptosporidium life cycle stages (oocysts and endogenous stages) in colon carcinoma tissue was confirmed by genus-specific FITC-labeling.
Conclusions: Herein, we report on a C. meleagridis infection of a colon adenocarcinoma in an immunocompetent patient. This is the first report of C. meleagridis infection in the human colon and first evidence of active development of this species in cancer tissue.
Introduction
Cryptosporidiosis, a diarrheal disease caused by species of protist parasites in the genus Cryptosporidium, can be severe and life-threatening in those with a compromised or underdeveloped immune system. More than 90% of human cases are caused by C. hominis, a species that is generally restricted to humans, and C. parvum, a species that infects a broad range of mammals. Cryptosporidium meleagridis, a species that primarily infects birds, but which also infects various mammal species, is the third most common cause of cryptosporidiosis in humans (Xiao, 2010). Estimated prevalence of human cryptosporidiosis in Poland (1.4–2.3%) has been available from limited research studies and based on the data from the National Institute of Public Health, National Institute of Hygiene in Poland and 24 findings of Cryptosporidium spp. have been reported from 2007 to 2016 (Wolska-Kusnierz et al., 2007; Bajer et al., 2008; Wesołowska et al., 2016; Bednarska et al., 2018). The majority of infection cases was caused by C. parvum and C. hominis. To date, C. felis infection has been detected in a child after liver transplantation (Bednarska et al., 2018) and C. meleagridis infection has only been confirmed in three immunodeficient patients; two children, with CD40L primary deficiency (Bajer et al., 2008) and with X linked hyper-IgM syndrome type 1 (XHIM syndrome) (Wolska-Kusnierz et al., 2007), and in a woman with AIDS suffering from persisted diarrhea (Wesołowska et al., 2016). Human infection by C. meleagridis is more frequent in some populations in Thailand or Peru. In the rest of the world, such cases have been linked to travels to endemic countries or to contact with poultry (Leoni et al., 2006; Cama et al., 2008; Elwin et al., 2012; Silverlås et al., 2012).
Worldwide, colorectal cancer is the second most frequently diagnosed cancer in females and the third in males. Major risk factors for colorectal cancer include age, personal or family history of chronic inflammatory bowel disease, gene mutations, high consumption of red or processed meat, smoking, physical inactivity, obesity and moderate to heavy alcohol consumption (Subramaniam et al., 2016). Other risk factors include bacterial, viral and parasitic infections (van Tong et al., 2017).
Epidemiological studies have shown an association between colorectal cancer and Cryptosporidium spp. infection (Osman et al., 2018; Sulzyc-Bielicka et al., 2018). Furthermore, a limited number of experimental studies have shown that C. parvum induces neoplastic changes in immunocompromised animals and in cells cultured in vitro, consistent with a role in carcinogenesis (Benamrouz et al., 2012a,b).
The present study is aimed to determine the presence of Cryptosporidium spp. infection in tissue samples from colorectal tumors. We describe an active C. meleagridis infection in samples from malignant tissue of non-HIV infected immunocompetent male patient with colon adenocarcinoma. To our knowledge, this is the first report of the case of C. meleagridis infection in the colon of a human.
Patients and Methods
Samples of colic neoplasia obtained during colectomies of 145 patients, who were being treated for colorectal cancer at the First Department of Surgical Oncology, Lower Silesian Oncology Center in Wrocław (Poland) between 2009 and 2010 were screened for Cryptosporidium infection between 2017 and 2018. The inclusion criteria for of all of the patients in this study were a) primary sporadic CRC (no history of hereditary/familial CRC), (b) HIV negativity, (c) not receiving an immunosuppressive treatment, (d) no chemotherapy and/or radiotherapy before surgical resection.
Tissues of colorectal cancer from all patients were screened for the presence of specific DNA of Cryptosporidium spp. using molecular methods and presence of Cryptosporidium developmental stages using the immunofluorescence antibody test (IFA). Samples were collected intraoperatively and aseptically. All tissue samples were stored in RNAlater™ Stabilization Solution (Thermo Fisher Scientific, Carlsbad, CA, United States) and immediately frozen at −70°C. A total of 200 mg of tissue was homogenized by bead disruption for 60 s at 5.5 m/s with 0.5 mm glass beads using a Precellys 24 Instrument (Bertin Technologies, France). Genomic DNA was subsequently isolated using the Gentra Puregene Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. DNA quality was verified by NanoDrop (Thermo Fisher Scientific, Carlsbad, CA, United States) measurements and β-globin gene amplification (Pan et al., 2001). To detect Cryptosporidium specific DNA, a nested protocol was used to amplify a partial region of the small ribosomal subunit rRNA (SSU; ~830 bp) gene (Xiao et al., 1999; Jiang et al., 2005). Only samples that were positive for SSU were screened for the 60 kDa glycoprotein (gp60; ~900 bp) gene (Glaberman et al., 2001; Alves et al., 2003). The PCR conditions were as previously described (Xiao et al., 1999; Glaberman et al., 2001). Negative (molecular grade water) and positive (DNA of C. proliferans) controls were included with each PCR amplification. Secondary products were purified (QIAquick®; Gel Extraction Kit, Qiagen, Hilden, Germany) and sequencing was carried out in both directions using an ABI 3130 sequencer (Applied Biosystems, Foster City, CA, United States). Amplification and sequencing of each locus were repeated three times. The nucleotide sequences in this study were manually edited using the program ChromasPro 2.1.4 (Technelysium, Pty, Ltd., South Brisbane, Australia) and aligned with previously published sequences. Sequences of SSU and gp60 derived in this study have been deposited in GenBank under accession numbers MK311181 and MK311182.
For IFA analysis, tissue samples were thawed at laboratory temperature, mechanically homogenized using a sterile mortar and pestle, and smeared on sterile slides (ten for each labeling). The presence of Cryptosporidium oocysts was examined using differential interference contrast (DIC) and fluorescence microscopy following labeling with a fluorescein-tagged mouse monoclonal antibody that is specific for the Cryptosporidium oocyst wall (Cryptosporidium IF Test, Crypto cel, Cellabs Pty Ltd., Brookvale, Australia). Endogenous life stages were examined under DIC and fluorescence microscopy following labeling with fluorescein-tagged rat anti-Cryptosporidium parvum sporozoite polyclonal antibody, which is specific for sporozoites, merozoites, and all other intracellular reproductive stages (A600FLR-20X Sporo-Glo™, Waterborne, INC. New Orleans, LA, United States).
This study was approved by the Human Research Ethics Committee of Wrocław Medical University (agreement number KB-328/2009). Written informed consent was obtained from every participant prior to examination.
Results
Among all patients (n = 145) the mean age was 65 ± 10.2 years, and ranged between 35–88 years. Overall, the male-to-female ratio was 77 (53%) to 68 (47%). The study group included 34 patients with proximal (right part of colon inclusive of transverse colon) and 111 patients with distal (left part of colon) tumor location. Cryptosporidium meleagridis DNA was confirmed in one patient and no other species of Cryptosporidium were found in the tumor tissues of the rest of study group.
Cryptosporidium-positive patient was a 71-year-old HIV-negative man who presented with a 1-year history of bloody stools, increased difficulty with defecation, abdominal pain, flatulence, increased fatigue and unintended weight loss. He had a 20-year history of regular smoking above 2 packs of cigarettes per week, seldomly drank alcohol (median 50 g of pure alcohol per week), and had no previous medical or surgical history of note. Additionally, he had no risk factors for, or blood result anomalies suggestive of, underlying immunodeficiency. A colonoscopy identified a lesion (pre-cancerous polyp) located 20 cm from ileocecal valve. Histopathological examination of biopsy tissue showed the presence of a stage IIA adenocarcinoma, according to the TNM Classification of Malignant Tumors (Tumor, Nodes, Metastasis, TNM) of staging as maintained by the American Joint Committee on Cancer (AJCC) and Union for International Cancer Control (UICC) (Hari et al., 2013). The patient was admitted to the hospital to undergo a planned right hemicolectomy of an ascending adenocarcinoma.
The presence of Cryptosporidium life cycle stages (oocysts and endogenous stages) in colon carcinoma tissue was confirmed by genus-specific FITC-labeling (Figure 1). Phylogenetic analyses of SSU rRNA sequences revealed the presence of C. meleagridis that was identical to isolates previously reported in humans and birds (GenBank acc. Nos. AY166839 and KY352486). Subtyping of C. meleagridis isolates at the gp60 locus showed the presence of subtype family IIIg previously reported in a human from Nepal and Sweden (GenBank acc. Nos. KU852730 and KJ210619) and birds from Algeria (GenBank acc. Nos. KY352480, KY352481, JX878610).
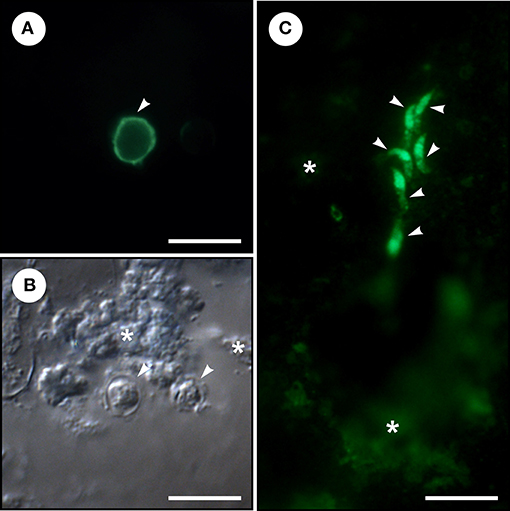
Figure 1. Microscopic examination of homogenized colonical tissue from a patient with adenocarcinoma and Cryptosporidium meleagridis infection. (A) Cryptosporidium oocyst (arrowhead) labeled with fluorescein-labeled mouse monoclonal antibody binds to Cryptosporidium oocyst wall (Cryptosporidium IF Test, Crypto cel, Cellabs Pty Ltd., Brookvale, Australia). (B) Cryptosporidium oocysts (arrowheads) under differential interference contrast and colonical tissue (asterisks). (C) Six merozoites (arrowheads) labeled with fluorescein-labeled rat anti-Cryptosporidium parvum sporozoite polyclonal antibody binds to sporozoites, merozoites, and all other intracellular reproductive stages (A600FLR-20X Sporo-Glo™, Waterborne, INC. New Orleans, LA, USA) and colonical tissue (asterisks). Bar = 10 μm.
Discussion
In this study, microscopic methods have demonstrated the presence of C. meleagridis endogenous stages (merozoites) in adenocarcinoma tissue in the colon. To our knowledge this is the first report of C. meleagridis in the human colon, and the identification of merozoites indicates an active infection. In the only other study to date in humans, C. meleagridis infection was localized to the ileocaecal valve, a sphincter muscle that separates the small and large intestine, in a person who was immunocompromised following a hematopoietic stem cell transplantation (Kagawa et al., 2018). Minor C. meleagridis infection has been reported in the colon of birds (chickens and turkeys) and mice; however, the infection intensity was low relative to that in the small intestine, which was the major site of infection (Pavlásek, 1994; Akiyoshi et al., 2003). Several recent studies have shown an increased prevalence of Cryptosporidium in patients with intestinal cancers. For example, in Poland, Cryptosporidium coproantigen was significantly more prevalent in patients with colorectal cancer than in a control group without malignant changes (Sulzyc-Bielicka et al., 2018). Similarly, Osman et al. (2018) showed that DNA and oocysts of C. parvum and C. hominis were more prevalent in digestive biopsies of patients with diagnosed colon neoplasia/adenocarcinoma compared to patients without digestive neoplasia. Moreover, according to Berahmat et al. (2017) the rate of cryptosporidiosis in children with cancer undergoing chemotherapy was higher (3.8%) than the one in the control group (0%). Although, while using an immunosuppressed mouse model, chronic C. parvum infection has been reported to cause neoplastic changes in the small intestine, suggesting a potential role as a carcinogen (Benamrouz et al., 2012a,b), the contribution of Cryptosporidium spp. to cancer etiology has been largely unexplored (Cheeseman et al., 2016). Taking into account the fact that among 145 examined patients in our study only one was infected with C. meleagridis, we consider this infection as opportunistic. Considering that human adenocarcinoma usually takes above 10 years to be diagnosed (Subramaniam et al., 2016), it is more likely that the C. meleagridis infection in the present study has resulted from the tumor presence. Our patient infected with C. meleagridis subtype IIIg, has not reported direct contact with poultry and wild birds, and the source of infection remains unknown. Nevertheless, the occurrence of this subtype as well as the other C. meleagridis subtypes (IIIb, IIIc, IIIe, IIIf, IIIh and IIIi) in humans, indicates that they are susceptible to almost all subtypes of C. meleagridis (Abal-Fabeiro et al., 2013; Wang et al., 2013). Future studies should examine characteristics of carcinoma tissue that could increase susceptibility to Cryptosporidium infection, including altered mucin expression and loss of epithelial cell integrity (Nath and Mukherjee, 2014; Cheeseman et al., 2016).
Although our results have shown that C. meleagridis might inhabit pathologically changed tumor tissue in human colon, there is still no evidence that this apicomplexan parasite should be considered as carcinogenic agent to humans according to International Agency for Research on Cancer (IARC).
Author Contributions
ŻK and MKi designed research. ŻK, MKv, PK, PL, BS, and MKi performed research. ŻK, MKv, PK, AH, MS, PL, JM, and MKi analyzed data. ŻK, MKv, JM, and MKi wrote the manuscript. AH and MS revised the manuscript for important intellectual content.
Funding
This work was supported by the Grant for Young Scientists of Wrocław Medical University, Poland [STM.A060.17.038] and the Czech Science Foundation [GACR 18-12364S]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abal-Fabeiro, J. L., Maside, X., Bello, X., Llovo, J., and Bartolomé, C. (2013). Multilocus patterns of genetic variation across Cryptosporidium species suggest balancing selection at the gp60 locus. Mol. Ecol. 22, 4723–4732. doi: 10.1111/mec.12425
Akiyoshi, D. E., Dilo, J., Pearson, C., Chapman, S., Tumwine, J., and Tzipori, S. (2003). Characterization of Cryptosporidium meleagridis of human origin passaged through different host species. Infect. Immun. 71, 1828–1832. doi: 10.1128/IAI.71.4.1828-1832.2003
Alves, M., Xiao, L., Sulaiman, I., Lal, A. A., Matos, O., and Antunes, F. (2003). Subgenotype analysis of Cryptosporidium isolates from humans, cattle, and zoo ruminants in Portugal. J. Clin. Microbiol. 41, 2744–2747. doi: 10.1128/JCM.41.6.2744-2747.2003
Bajer, A., Bednarska, M., Cacciò, S. M., Wolska-Kuśnierz, B., Heropolitanska-Pliszka, E., Bernatowska, E., et al. (2008). Genotyping of Cryptosporidium isolates from human clinical cases in Poland. Parasitol. Res. 103, 37–42. doi: 10.1007/s00436-008-0924-5
Bednarska, M., Jankowska, I., Pawelas, A., Piwczynska, K., Bajer, A., Wolska-Kuśnierz, B., et al. (2018). Prevalence of Cryptosporidium, Blastocystis, and other opportunistic infections in patients with primary and acquired immunodeficiency. Parasitol. Res. 117, 2869–2879. doi: 10.1007/s00436-018-5976-6
Benamrouz, S., Conseil, V., Creusy, C., Calderon, E., Dei-cas, E., and Certad, G. (2012a). Parasites and malignancies, a review, with emphasis on digestive cancer induced by Cryptosporidium parvum (Alveolata: Apicomplexa). Parasite 19, 101–115. doi: 10.1051/parasite/2012192101
Benamrouz, S., Guyot, K., Gazzola, S., Mouray, A., Chassat, T., Delaire, B., et al. (2012b). Cryptosporidium parvum infection in SCID mice infected with only one oocyst: qPCR assessment of parasite replication in tissues and development of digestive cancer. PLoS ONE 7:e51232. doi: 10.1371/journal.pone.0051232
Berahmat, R., Mahami-Oskouei, M., Rezamand, A., Spotin, A., Aminisani, N., Ghoyounchi, R., et al. (2017). Cryptosporidium infection in children with cancer undergoing chemotherapy: how important is the prevention of opportunistic parasitic infections in patients with malignancies? Parasitol. Res. 116, 2507–2515. doi: 10.1007/s00436-017-5560-5
Cama, V. A., Bern, C., Roberts, J., Cabrera, L., Sterling, C. R., Ortega, Y., et al. (2008). Cryptosporidium species and subtypes and clinical manifestations in children, Peru. Emerg. Infect. Dis. 14, 1567–1574. doi: 10.3201/eid1410.071273
Cheeseman, K., Certad, G., and Weitzman, J. B. (2016). [Parasites and cancer: is there a causal link?]. Med. Sci. 32, 867–873. doi: 10.1051/medsci/20163210020
Elwin, K., Hadfield, S. J., Robinson, G., and Chalmers, R. M. (2012). The epidemiology of sporadic human infections with unusual cryptosporidia detected during routine typing in England and Wales, 2000–2008. Epidemiol. Infect. 140, 673–683. doi: 10.1017/S0950268811000860
Glaberman, S., Sulaiman, I. M., Bern, C., Limor, J., Peng, M. M., Morgan, U., et al. (2001). A multilocus genotypic analysis of Cryptosporidium meleagridis. J. Eukaryot. Microbiol. (Suppl.), 19S−22S. doi: 10.1111/j.1550-7408.2001.tb00439.x
Hari, D. M., Leung, A. M., Lee, J.-H., Sim, M.-S., Vuong, B., Chiu, C. G., et al. (2013). AJCC cancer staging manual 7th edition criteria for colon cancer: do the complex modifications improve prognostic assessment? J. Am. Coll. Surg. 217, 181–190. doi: 10.1016/j.jamcollsurg.2013.04.018
Jiang, J., Alderisio, K. A., and Xiao, L. (2005). Distribution of Cryptosporidium genotypes in storm event water samples from three watersheds in New York. Appl. Environ. Microbiol. 71, 4446–4454. doi: 10.1128/AEM.71.8.4446-4454.2005
Kagawa, K., Fujino, H., Miki, H., Sogabe, K., Takahashi, M., Maruhashi, T., et al. (2018). Cryptosporidiosis in a transplant recipient with severe intractable diarrhea: detection of Cryptosporidium oocysts by intestinal biopsies. Transpl. Infect. Dis. 20, e12826. doi: 10.1111/tid.12826
Leoni, F., Amar, C., Nichols, G., Pedraza-Díaz, S., and McLauchlin, J. (2006). Genetic analysis of Cryptosporidium from 2414 humans with diarrhoea in England between 1985 and 2000. J. Med. Microbiol. 55, 703–707. doi: 10.1099/jmm.0.46251-0
Nath, S., and Mukherjee, P. (2014). MUC1: A multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med. 20, 332–342. doi: 10.1016/j.molmed.2014.02.007
Osman, M., Benamrouz, S., Guyot, K., Baydoun, M., Frealle, E., Chabe, M., et al. (2018). High association of Cryptosporidium spp. infection with colon adenocarcinoma in lebanese patients. PLoS ONE 12:e0189422. doi: 10.1371/journal.pone.0189422
Pan, L., Milligan, L., Michaeli, J., Cesarman, E., and Knowles, D. M. (2001). Polymerase chain reaction detection of Kaposi's sarcoma-associated herpesvirus-optimized protocols and their application to myeloma. J. Mol. Diagn. 3, 32–38. doi: 10.1016/S1525-1578(10)60647-2
Pavlásek, I. (1994). [Localization of endogenous developmental stages of Cryptosporidium meleagridis Slavin, 1955 (Apicomplexa: Cryptosporidiidae) in birds]. Vet. Med. (Praha). 39, 733–742.
Silverlås, C., Mattsson, J. G., Insulander, M., and Lebbad, M. (2012). Zoonotic transmission of Cryptosporidium meleagridis on an organic swedish farm. Int. J. Parasitol. 42, 963–967. doi: 10.1016/j.ijpara.2012.08.008
Subramaniam, R., Mizoguchi, A., and Mizoguchi, E. (2016). Mechanistic roles of epithelial and immune cell signaling during the development of colitis-associated cancer. Cancer Res. Front. 2, 1–21. doi: 10.17980/2016.1
Sulzyc-Bielicka, V., Kołodziejczyk, L., Jaczewska, S., Bielicki, D., Safranow, K., Bielicki, P., et al. (2018). Colorectal cancer and Cryptosporidium spp. infection. PLoS ONE 13:e0195834. doi: 10.1371/journal.pone.0195834
van Tong, H., Brindley, P. J., Meyer, C. G., and Velavan, T. P. (2017). Parasite infection, carcinogenesis and human malignancy. EBioMedicine 15, 12–23. doi: 10.1016/j.ebiom.2016.11.034
Wang, L., Zhang, H., Zhao, X., Zhang, L., Zhang, G., Guo, M., et al. (2013). Zoonotic Cryptosporidium species and Enterocytozoon bieneusi genotypes in HIV-positive patients on antiretroviral therapy. J. Clin. Microbiol. 51, 557–563. doi: 10.1128/JCM.02758-12
Wesołowska, M., Szostakowska, B., Kicia, M., Sak, B., Kvac, M., and Knysz, B. (2016). Cryptosporidium meleagridis infection: the first report in Poland of its occurrence in an HIV-positive woman. Ann. Parasitol. 62, 239–241. doi: 10.17420/ap6203.58
Wolska-Kusnierz, B., Bajer, A., Caccio, S., Heropolitanska-Pliszka, E., Bernatowska, E., Socha, P., et al. (2007). Cryptosporidium infection in patients with primary immunodeficiencies. J. Pediatr. Gastroenterol. Nutr. 45, 458–464. doi: 10.1097/MPG.0b013e318054b09b
Xiao, L. (2010). Molecular epidemiology of cryptosporidiosis: an update. Exp. Parasitol. 124, 80–89. doi: 10.1016/j.exppara.2009.03.018
Keywords: Cryptosporidium meleagridis, colon cancer, adenocarcinoma, colon infection, PCR, immunofluorescence labeling
Citation: Kopacz Ż, Kváč M, Karpiński P, Hendrich AB, Sąsiadek MM, Leszczyński P, Sak B, McEvoy J and Kicia M (2019) The First Evidence of Cryptosporidium meleagridis Infection in a Colon Adenocarcinoma From an Immunocompetent Patient. Front. Cell. Infect. Microbiol. 9:35. doi: 10.3389/fcimb.2019.00035
Received: 26 November 2018; Accepted: 04 February 2019;
Published: 04 March 2019.
Edited by:
Javier Moreno, Instituto de Salud Carlos III, SpainReviewed by:
David Carmena, Centro Nacional de Microbiología, SpainPanagiotis Karanis, Qinghai University, China
Copyright © 2019 Kopacz, Kváč, Karpiński, Hendrich, Sąsiadek, Leszczyński, Sak, McEvoy and Kicia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Żaneta Kopacz, zaneta.kopacz@umed.wroc.pl
 Żaneta Kopacz
Żaneta Kopacz Martin Kváč2,3
Martin Kváč2,3  John McEvoy
John McEvoy