Reverse Epidemiology: An Experimental Framework to Drive Leishmania Biomarker Discovery in situ by Functional Genetic Screening Using Relevant Animal Models
- 1Institut Pasteur, Unité de Parasitologie Moléculaire et Signalisation, INSERM U1201, Paris, France
- 2Université Paris Diderot, Sorbonne Paris Cité, Paris, France
Leishmania biomarker discovery remains an important challenge that needs to be revisited in light of our increasing knowledge on parasite-specific biology, notably its genome instability. In the absence of classical transcriptional regulation in these early-branching eukaryotes, fluctuations in transcript abundance can be generated by gene and chromosome amplifications, which have been linked to parasite phenotypic variability with respect to virulence, tissue tropism, and drug resistance. Conducting in vitro evolutionary experiments to study mechanisms of Leishmania environmental adaptation, we recently validated the link between parasite genetic amplification and fitness gain, thus defining gene and chromosome copy number variations (CNVs) as important Leishmania biomarkers. These experiments also demonstrated that long-term Leishmania culture adaptation can strongly interfere with epidemiologically relevant, genetic signals, which challenges current protocols for biomarker discovery, all of which rely on in vitro expansion of clinical isolates. Here we propose an experimental framework independent of long-term culture termed “reverse” epidemiology, which applies established protocols for functional genetic screening of cosmid-transfected parasites in animal models for the identification of clinically relevant genetic loci that then inform targeted field studies for their validation as Leishmania biomarkers.
Introduction
Biomarkers are defined as biological characteristics that are objective and quantifiable indicators for responses to therapeutic interventions, or normal and pathogenic biological processes (Biomarkers Definitions Working Group 2001, 2001). With respect to Leishmania infection, we can distinguish direct biomarkers that are applied to determine parasite species and prevalence (e.g., parasite-specific proteins, lipids, transcripts, genetic loci), and indirect biomarkers that correspond to different correlates of the host anti-microbial response [e.g., adenosine deaminase (ADA) or cytokines such as IL-10 or TNF] (Kip et al., 2015).
Direct Leishmania biomarkers can have either purely diagnostic value (e.g., kinetoplast (k) DNA, ribosomal small sub-unit (SSU) RNA, HSP70 locus, carbohydrate antigens), or prognostic value allowing for the prediction of treatment outcome or disease evolution (e.g., dissemination in cutaneaous leishmaniasis or development of post-kala-azar dermal leishmaniasis in visceral leishmaniasis). However, despite their potentially important impact on clinical management of leishmaniasis, only few biomarker candidates with potential prognostic value are described, most of which are linked to drug resistance (Vanaerschot et al., 2012; Torres et al., 2013; Hefnawy et al., 2017; Ponte-Sucre et al., 2017). The absence of this class of markers is explained by various biological and technical constraints, some of which are linked to Leishmania genome instability that limits biomarker discovery and needs to be considered in ongoing and future biomarkers discovery campaigns.
In the absence of classical transcriptional regulation, Leishmania often regulates transcript and protein abundance by chromosome and gene copy number variations (CNVs) (Dumetz et al., 2017; Prieto Barja et al., 2017), which can drive environmental adaptation (Leprohon et al., 2009; Downing et al., 2011; Rogers et al., 2011; Brotherton et al., 2013; Mukherjee et al., 2013; Ubeda et al., 2014; Zhang et al., 2014; Laffitte et al., 2016). Our recent demonstration that karyotypic fluctuations and haplotype selection allow for fitness gain in culture reveals the importance of Leishmania genome plasticity in short-term evolutionary adaptation (Prieto Barja et al., 2017). Conceivably, the highly dynamic genomic changes occurring during culture adaptation challenge past and current protocols in Leishmania biomarker discovery, which rely on adaptation and mass-expansion of field isolates in culture prior to analysis, often resulting in loss of epidemiologically relevant, genetic signals. Here, by drawing from the current literature, we propose an alternative strategy independent of long-term culture that is based on functional genetic screening in relevant animal models. Our review provides an overview on past functional screening results and their documented success in revealing genomic loci that are under environmental selection, and advocates for Leishmania biomarker discovery by combining cosmid selection and subsequent clinical validation, an experimental framework we termed “reverse” epidemiology. In the following we summarize studies that developed and applied cosmid-based approaches to identify new Leishmania factors linked to parasite pathogenicity, tropism and drug resistance, and discuss the potential epidemiological relevance of these factors where clinical data were available.
Cosmid-Based Functional Genetic Screening in Leishmania
Various genetic methods have been successfully applied in the past to identify Leishmania genes or genetic markers that are associated with disease outcome or clinical manifestation, including whole genome sequencing (WGS) of isolates (Downing et al., 2011; Rogers et al., 2011; Leprohon et al., 2015), random amplification of polymorphic DNA (RAPD) (Bhattacharyya et al., 1993; Schönian et al., 1996; Mkada-Driss et al., 2014), or assessment of amplified fragment length polymorphisms (AFLP) (Kumar et al., 2009, 2010a; Odiwuor et al., 2011; Jaber et al., 2018). Likewise, cosmid-based functional screens have been applied to discover clinically relevant loci. This approach is based on the genetic transfer of a given cellular phenotype (e.g., drug resistance) from a donor strain to a recipient strain via transfection of a cosmid library. While currently established WGS protocols for Leishmania biomarker discovery have been applied on clinical isolates maintained in long-term culture, causing potentially important bias, cosmid-based approaches can directly reveal clinically relevant genotype-phenotype relationships, especially when applied in situ in infected animals. Even though this functional genetic approach represents a powerful tool, this technology has not been applied in a systematic way at larger scale to drive biomarker discovery.
The preparation and application of a cosmid library is a complex procedure, where genomic DNA fragments of an appropriate size are cloned into purified cosmid DNA and packaged into phages for efficient bacterial transduction, which allows for amplification of the library and assessment of its genomic coverage prior to transfection into parasites by electroporation. The generation of a first series of Leishmania shuttle cosmid vectors and the validation of a protocol that allows for genetic complementation and functional screening in these parasites using genomic cosmid libraires was established in 1993 by Beverley and collaborators (Ryan et al., 1993a) followed by Kelly and collaborators in 1994 (Kelly et al., 1994). Subsequently, this protocol was applied in various studies for the identification of Leishmania pathogenicity and drug resistance genes.
Cosmid-Based Identification of Novel Leishmania Pathogenicity Factors
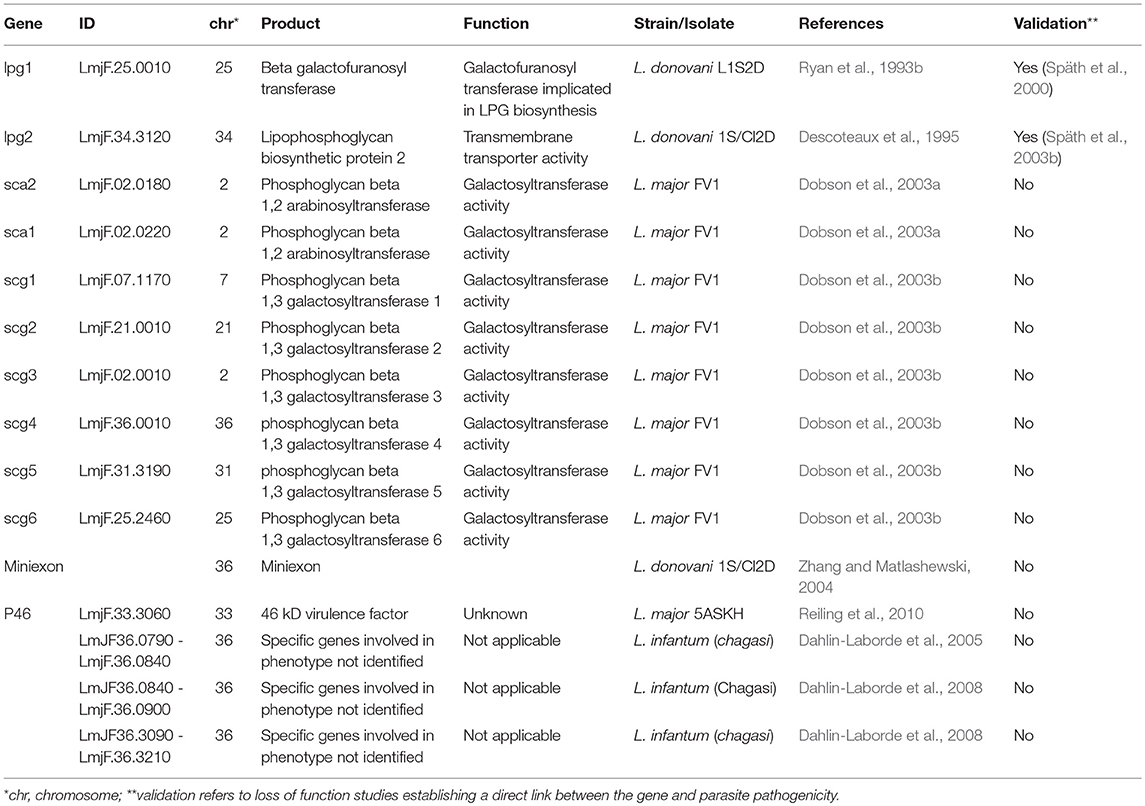
Key for Leishmania infectivity is the capacity of procyclic promastigotes to undergo differentiation into infectious metacyclic promastigotes able to resist to complement lysis encountered inside the mammal host following parasite transmission (Sacks and Perkins, 1984; Franke et al., 1985; Howard et al., 1987). Parasite resistance has been largely attributed to the surface glycolipid lipophosphoglycan (LPG), a major Leishmania virulence factor essential for L. major promastigote virulence (Späth et al., 2000, 2003a), that undergoes important modifications during metacyclogenesis (Sacks et al., 1990; Mcconville et al., 1992; Sacks, 2001). LPG biosynthetic genes and their virulence functions have been genetically identified combining cosmid screens with functional null mutant analysis and virulence assessment in macrophages and mice. LPG deficient mutants were generated by chemical mutagenesis, isolated by their failure to agglutinate in the presence of lectin (King and Turco, 1988), transfected with a cosmid library prepared from L. donovani, and screened for restoration of LPG expression using either lectin- or antibody-based agglutination assays revealing the two first LPG biosynthetic genes, a galactofuranose transferase encoded by the gene lpg1 (Ryan et al., 1993b), and an UdP galactose transporter encoded by lpg2 (Descoteaux et al., 1995). The virulence functions of both genes were confirmed in subsequent studies in L. major lpg1 and lpg2 null mutants (Späth et al., 2000, 2003b). Few years later, by combining cosmid library transfection and antibody panning, Dobson et al. identifed genes encoding arabinosyl- and galactosyltransferases that mediate developmental modifications of LPG during metacyclogenesis (Dobson et al., 2003a,b).
Cosmid-based functional screening has also been applied to gain insight into pathways that govern complement resistance in promastigotes revealing genes that are likely linked to metacyclogenesis. Based on the observation that decrease in resistance to complement lysis is a consequence of long-term maintenance in culture (Lincoln et al., 2004), Dahlin-Laborde et al. used genomic DNA from animal-derived Leishmania infantum (chagasi) promastigotes to construct a cosmid library that was transfected into long-term cultured parasites. The transfected parasites were subjected to complement lysis allowing for the selection of seven different cosmids that conferred increased complement resistance albeit at lower levels compared to short-term cultured control parasites. In-depth analysis of two cosmids revealed genomic fragments of L. infantum chromosome 36 (Dahlin-Laborde et al., 2005), with two sub-regions encoding, respectively, 5 and 13 genes shown to be critical for the phenotype, including an ADP-ribosylation factor-like protein and an ATP-dependent RNA helicase (Dahlin-Laborde et al., 2008). Cosmid screens were further applied by the Matlashewski team to identify virulence and visceralization factors using libraries prepared with genomic DNA from L. donovani transfected in L. major promastigotes. Transfectants expressing the heterologous library were inoculated into mice by tail vein or footpad injections and cosmids were recovered from parasites that established infection in spleen (type I), skin (type III), or both (type II). Subsequent analysis of individual ORFs by transgenic expression and infection validated an ORF encoding for an unknown protein and a 4.4 kb miniexon gene array on chromosome 36 (Zhang and Matlashewski, 2004). Unlike in L. major, overexpression of the miniexon region in L. braziliensis led to complete virulence attenuation in a hamster model (de Toledo et al., 2009), suggesting species-specific functions of this array. This is further supported by the genetic divergence of this array between new world and old world dermotropic species (Fernandes et al., 1994), which is used as a diagnostic signal for parasite genotyping (Serin et al., 2005; Ovalle-Bracho et al., 2016).
A final example documenting the power of cosmid-based approaches in identifying putative Leishmania virulence factors is represented by a complementation screen conducted using a cosmid library derived from an attenuated HSP100 null mutant that spontaneously recovered infectivity and/or pathogenicity in mice, likely by the amplification of a compensatory locus (Reiling et al., 2006). A screen conducted in mice using cosmid-transfected HSP100 null mutants and subsequent validation experiments revealed P46 as a new virulence factor (Reiling et al., 2010). A follow-up study by Bifeld et al applied a phylogenetic approach on 20 clinical isolates comparing P46 amino acid sequences thus establishing a strong correlation between P46 isoforms and their geographical origin. Transgenic parasites over-expressing three different P46 isoforms in a L. major lab strain were co-injected in BALB/c and C57BL/6 mice. Selection of different isoforms according to the mouse strain suggested that the P46 genetic polymorphism may be linked to parasite adaptation to genetically distinct, region-specific host reservoirs (Bifeld et al., 2015; Table 1).
Cosmid-Based Identification of Leishmania Drug Resistance Genes
Since 1999, screening of cosmid libraries has been used as a gain-of-function strategy to identify drug resistance or drug tolerance genes (reviewed in Clos and Choudhury, 2006). Beverley and collaborators established the first proof-of-principle of this approach culturing cosmid transfected L. major parasites under pressure of the drugs methotrexate and tubercidin, which resulted in the selection of the known resistance genes DHFR-TS, PTR1, and TOR (Cotrim et al., 1999). The same study identified a new gene encoding a 63 kDa hypothetical protein located on chromosome 31 termed tubercidin-resistant protein (TRP) that is conserved in Leishmania and co-localizes in the endoplasmic reticulum in stationary phase promastigotes (Aoki et al., 2016).
Functional complementation has also been a powerful tool for the identification of transporters that can alter drug efficacy. The biopterin transporter bt1, previously named ORF G (Kundig et al., 1999), and the miltefosine (MIL) transporter LdMT (Perez-Victoria et al., 2003) were identified using Leishmania tarentolae transfected with a heterologous L. mexicana cosmid library selected under methotrexate pressure (showing that bt1 can confer resistance), and L. donovani MIL resistant parasites transfected with a L. donovani wild-type cosmid library subjected to MIL selection (showing that a non-mutated LdMT can restore susceptibility). Likewise, the cosmid approach was applied to screen for genes mediating resistance to two inhibitors of ergosterol biosynthesis, terbinafine, and itraconazole, which resulted in the selection of nine different cosmids, some of which conferred cross-resistance to both drugs, and the identification of squalene synthase 1 (SQS1) as an itraconazole resistance gene (Cotrim et al., 1999).
This approach has been recently applied to directly identify clinically relevant drug resistance loci by heterologous screening. Clos and collaborators prepared cosmid libraries from antimony SbIII/SbV resistant or SbIII sensitive/SbV resistant L. braziliensis field isolates that were transfected into SbIII sensitive/SbV resistant promastigotes. Culture under drug pressure selected for cosmids carrying a genomic fragment of chromosome 20, which also conferred drug resistance when transfected into L. infantum (Nühs et al., 2014). A competition assay with full-length or truncated derivatives of the cosmid insert validated ARM58 as a SbIII resistance gene. A more recent study performed by the same group with cosmid-transfected L. infantum extended this finding to the neighboring genes and defined a cluster of three genes, ARM58, ARM56 (previously named ARM58rel), and HSP23 at the telomere of the chromosome 34 that confer increased resistance of intracellular amastigotes against SbV (Tejera Nevado et al., 2016). Using a L. infantum cosmid library, the same team revealed a protein termed P299 that conferred increased resistance of intracellular amastigotes to MIL and reduced promastigote sensitivity to MIL and SbIII, but not pentamidin (Choudhury et al., 2008). Another gene—today annotated as cysteine leucine-rich protein (CLrP, LinJ.34.0570)—was revealed causing antimony resistance in L. tarentolae transfected with a cosmid library prepared from arsenite and SbIII resistant parasites (Brochu et al., 2004), and in L. infantum axenic amastigotes (Genest et al., 2008). Brochu et al. also reported members of the HSP70 protein family as important genes contributing to antimony tolerance, supporting recent phylogenetic evidence that HSP70 family members may allow parasite environmental adaptation with potential important consequences for drug susceptibility (Drini et al., 2016).
Recent work by the Ouellette team coupled cosmid selection and next generation sequencing for drug resistance and drug target gene discovery, proposing a high-throughput capable screening strategy the authors referred to as Cos-Seq (Gazanion et al., 2016). Screening cosmid transfected L. infantum against SbIII, amphotericin B, MIL, paramomycin or pentamidin revealed 64 enriched loci, including 12 common to at least two anti-leishmanial drugs, suggesting the existence of multi-drug resistance genes. This study validated 6 known and uncovered 7 new resistance genes in promastigotes, including two new genes causing methotrexate resistance both encoding for phosphatase 2C-like proteins (LinJ.34.2310 and LinJ.34.2320), one hypothetical protein with leucine-rich repeats causing both pentamidin and paromomycin resistance (LinJ.06.1010), a serine/threonine phosphatase causing SbIII resistance (LinJ.12.0610), and phospholipid-translocating ATPase (LinJ30.2270) and C-8 sterol isomerase (LinJ.29.2250) that were revealed screening for MIL resistance (Table 2).
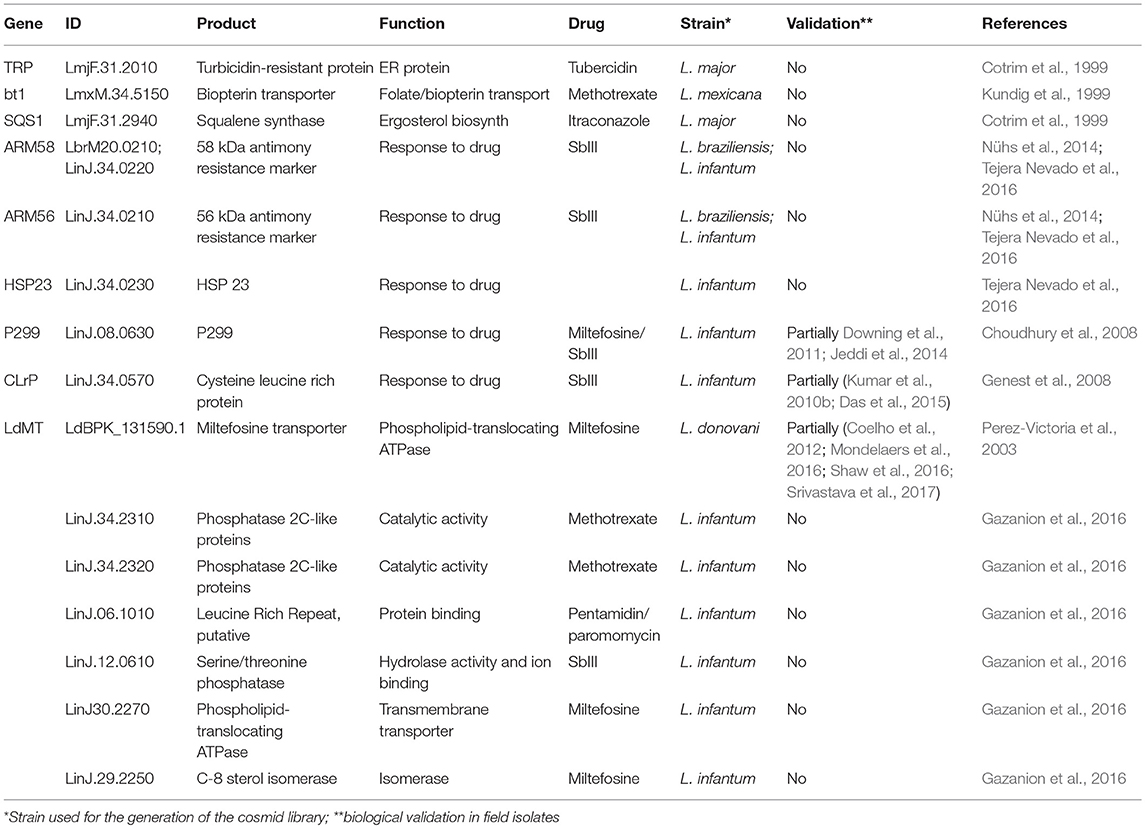
Table 2. Genes identified by cosmid-based approach linked to Leishmania drug resistance or susceptibility.
The Framework of “Reverse” Epidemiology
The examples described above are testimony to the success of cosmid-based, functional screening approaches to discover genetic loci in Leishmania that are linked to parasite virulence, tissue tropism, and drug resistance. However, even though these loci may represent potential biomarkers with important prognostic value, there are no dedicated, concerted efforts for their validation in clinically relevant settings. One exception includes CLrP, whose increased abundance on RNA and protein levels were correlated with increased Sb resistance in field isolates, albeit only a small number of isolates were used in these studies (Kumar et al., 2010b; Das et al., 2015). For other loci, clinical validation of the functional screening results can be ambiguous, with for example the MRPA and PTR1 genes of the H-locus having been either strictly, partially, or not correlated to Sb resistance in different epidemiological studies (Decuypere et al., 2005, 2012; Mittal et al., 2007; Mukherjee et al., 2007; Mukhopadhyay et al., 2011). Such divergent results may be explained by the polyclonal structure of parasite field isolates and their geographic adaptation, with different resistance mechanisms being selected in genetically distinct isolates (Decuypere et al., 2012). This possibility is supported by our recent demonstration that genetic mosaicism in an individual L. donovani strain can drive polyclonal adaptation, suggesting that different resistance mechanisms may co-exist in sub-populations of any given isolate (Prieto Barja et al., 2017). Such intra-strain specific, polyclonal fitness gain is further supported by the cosmid selection of different genetic loci in response to the same selection pressure applied on a single parasite population in vitro or during animal infection (Cotrim et al., 1999; Dahlin-Laborde et al., 2005; Gazanion et al., 2016). Indeed, such clonal phenotypic variability in a given parasite isolate has been recently documented in L. amazonensis, with important differences in culture proliferation and pathogenic potential observed in untransfected sub-clones or parasites transfected with individual cosmids selected in vivo for increased parasite infectivity (Espiau et al., 2017). Finally, other genes associated with drug resistance or susceptibility identified in cosmid screens failed to be validated in clinical studies such as LdMT, whose mutations were correlated to MIL resistance in promastigotes in culture but could not be associated with MIL resistance or treatment failure in the field (Bhandari et al., 2012). Likewise, PRP1 that has been implicated in vitro in resistance to pentamidine with reported cross-resistance to SbIII, did not show increased expression in Sb resistant field isolates (Decuypere et al., 2005, 2012).
Drawing from these examples we propose an experimental framework for the discovery of biomarker candidates by combining functional genetic screens in relevant animal models to reveal loci of interest, which then are validated by dedicated clinical and epidemiological investigations (Figure 1). In this approach, a cosmid library is prepared from parasites freshly derived from clinical isolates that show a phenotype of interest (donor strain). The gene(s) that express this phenotype are identified by transfecting a relevant recipient Leishmania strain and recovery of cosmids from transfectants that gained the phenotype under investigation. These genes can then be validated as biomarkers by quantitative PCR analysis directly applied on clinical samples. Thus, in contrast to classical biomarker discovery, where epidemiological field studies establish a correlation between a clinical phenotype and a genetic locus that then is validated in vitro or in animal studies, the epidemiological protocol we propose is in reverse from lab-based studies back to the field. Even though this approach has its drawbacks (e.g., clinical manifestations caused by gene inactivation or gene deletion cannot be revealed), it provides several interesting advantages that immediately overcome important bottlenecks in Leishmania biomarker discovery. First, it is independent of long-term culture that can have an important impact on the parasite genome thus interfering with epidemiologically relevant information. Second, the screening is performed in situ in infected animals under environmental constraints that correspond to the clinical setting, thus allowing for the selection of physiologically highly relevant loci. Third, large amounts of parasite can be recovered from different tissues of the infected animals, which can be subjected to direct and even single cell sequencing, thus informing on mechanisms of polyclonal adaptation that may be relevant to the field. Finally, this approach will overcome ethical concerns associated with applying direct genome sequencing on human tissue samples as the cosmid-identified loci will be studied in clinical samples by simple qPCR analysis.
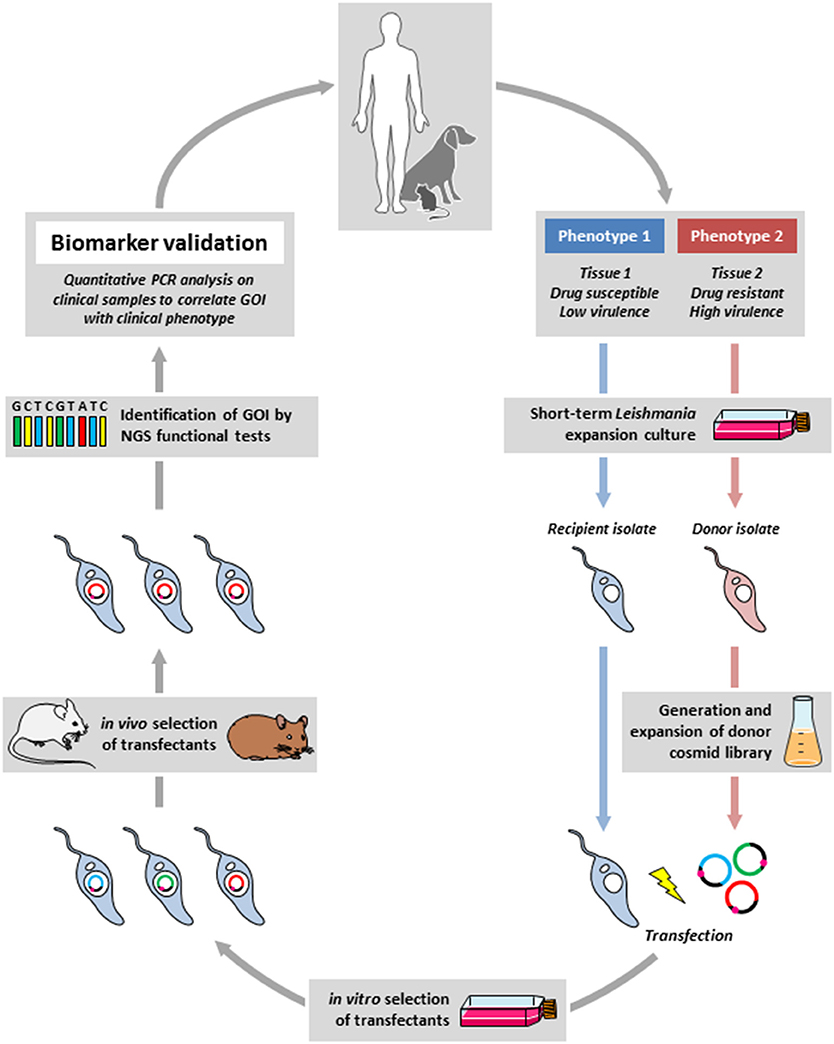
Figure 1. Outline of the reverse epidemiology framework. Field isolates from infected humans or animal reservoirs showing a defined difference in clinical phenotype (e.g., drug susceptibility) will be briefly expanded in culture, a cosmid library will be generated from the donor strain (in red) that shows the phenotype of interest (e.g., drug resistance), which then will be transfected into the recipient strain (in blue) that will be subjected to a gain-of-function screen in situ using experimental mouse or hamster infection (in the presence of drug in our example). The selected gene(s) of interested (GOI) will be identified by next generation sequencing (NGS). Correlating the identified genes with the clinical phenotype in dedicated epidemiological studies will then validate the new biomarker.
In conclusion, our reverse epidemiology approach exploits genetic amplification for biomarker discovery and thus mimics the very mechanism that has been linked to Leishmania genomic adaptation and fitness gain in the field and in culture (Dumetz et al., 2017; Prieto Barja et al., 2017). Cosmid-based functional genetic screening in situ linked to clinical validation thus represents a powerful framework that can fill an important gap in the currently rather desolate state of Leishmania biomarker discovery, which is challenged by the absence of robust protocols for direct tissue sequencing of parasites in human clinical samples, and the genetic bias caused by parasite long-term culture applied in current epidemiological investigations.
Author Contributions
LP wrote the chapter on the use of cosmid libraries regarding virulence and tropism, PP wrote the chapter on the use of cosmid libraries regarding drug resistance, GS corrected the manuscript and wrote introduction and the last chapter detailing the experimental framework.
Funding
This work was supported by funds from the Institute Pasteur International Direction strategic fund to the LeiSHield project and the Laboratoire d'Excellence Integrative Biology of Emerging Infectious Diseases (Grant no. ANR-10-LABX-62-IBEID). LP was supported by the Ph.D. fellowship from the Ministère de l'Enseignement Supérieur, de la Recherche et de l'Innovation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aoki, J. I., Coelho, A. C., Muxel, S. M., Zampieri, R. A., Sanchez, E. M., Nerland, A. H., et al. (2016). Characterization of a novel endoplasmic reticulum protein involved in tubercidin resistance in Leishmania major. PLoS Negl. Trop. Dis. 10:e0004972. doi: 10.1371/journal.pntd.0004972
Bhandari, V., Kulshrestha, A., Deep, D. K., Stark, O., Prajapati, V. K., Ramesh, V., et al. (2012). Drug susceptibility in Leishmania isolates following miltefosine treatment in cases of visceral leishmaniasis and post kala-azar dermal leishmaniasis. PLoS Negl. Trop. Dis. 6:e1657. doi: 10.1371/journal.pntd.0001657
Bhattacharyya, R., Singh, R., Hazra, T. K., and Majumder, H. K. (1993). Application of polymerase chain reaction with specific and arbitrary primers to identification and differentiation of Leishmania parasites. FEMS Microbiol. Lett. 114, 99–104. doi: 10.1111/j.1574-6968.1993.tb06557.x
Bifeld, E., Chrobak, M., Zander, D., Schleicher, U., and Schönian G, Clos, J. (2015). Geographical sequence variation in the Leishmania major virulence factor P46. Infect. Genet. Evol. 30, 195–205. doi: 10.1016/j.meegid.2014.12.029
Biomarkers Definitions Working Group 2001 (2001). Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 69, 89–95. doi: 10.1067/mcp.2001.113989
Brochu, C., Haimeur, A., and Ouellette, M. (2004). The heat shock protein HSP70 and heat shock cognate protein HSC70 contribute to antimony tolerance in the protozoan parasite Leishmania. Cell Stress Chaper. 9, 294–303. doi: 10.1379/CSC-15R1.1
Brotherton, M. C., Bourassa, S., Leprohon, P., Legare, D., Poirier, G. G., Droit, A., et al. (2013). Proteomic and genomic analyses of antimony resistant Leishmania infantum mutant. PLoS ONE 8:e81899. doi: 10.1371/journal.pone.0081899
Choudhury, K., Zander, D., Kube, M., Reinhardt, R., and Clos, J. (2008). Identification of a Leishmania infantum gene mediating resistance to miltefosine and SbIII. Int. J. Parasitol. 38, 1411–1423. doi: 10.1016/j.ijpara.2008.03.005
Clos, J., and Choudhury, K. (2006). Functional cloning as a means to identify Leishmania genes involved in drug resistance. Mini Rev. Med. Chem. 6, 123–129. doi: 10.2174/138955706775476028
Coelho, A. C., Boisvert, S., Mukherjee, A., Leprohon, P., Corbeil, J., and Ouellette, M. (2012). Multiple mutations in heterogeneous miltefosine-resistant Leishmania major population as determined by whole genome sequencing. PLoS Negl. Trop. Dis. 6:e1512. doi: 10.1371/journal.pntd.0001512
Cotrim, P. C., Garrity, L. K., and Beverley, S. M. (1999). Isolation of genes mediating resistance to inhibitors of nucleoside and ergosterol metabolism in Leishmania by overexpression/selection. J. Biol. Chem. 274, 37723–37730. doi: 10.1074/jbc.274.53.37723
Dahlin-Laborde, R. R., Scolaro, E. J., Romine, N. M., Ramer-Tait, A. E., Lei, S. M., and Beetham, J. K. (2008). Characterization of DNA sequences that confer complement resistance in Leishmania chagasi. Ann. N. Y. Acad. Sci. 1149, 347–351. doi: 10.1196/annals.1428.099
Dahlin-Laborde, R. R., Yu, T. P., and Beetham, J. K. (2005). Genetic complementation to identify DNA elements that influence complement resistance in Leishmania chagasi. J. Parasitol. 91, 1058–1063. doi: 10.1645/GE-477R.1
Das, S., Shah, P., Tandon, R., Yadav, N. K., Sahasrabuddhe, A. A., Sundar, S., et al. (2015). Over-expression of cysteine leucine rich protein is related to SAG resistance in clinical isolates of Leishmania donovani. PLoS Negl. Trop. Dis. 9:e0003992. doi: 10.1371/journal.pntd.0003992
de Toledo, J. S., Junqueira Dos Santos, A. F., Rodrigues De Moura, T., Antoniazi, S. A., Brodskyn, C., Indiani De Oliveira, C., et al. (2009). Leishmania (Viannia) braziliensis transfectants overexpressing the miniexon gene lose virulence in vivo. Parasitol. Int. 58, 45–50. doi: 10.1016/j.parint.2008.09.006
Decuypere, S., Rijal, S., Yardley, V., De Doncker, S., Laurent, T., Khanal, B., et al. (2005). Gene expression analysis of the mechanism of natural Sb(V) resistance in Leishmania donovani isolates from Nepal. Antimicrob. Agents Chemother. 49, 4616–4621. doi: 10.1128/AAC.49.11.4616-4621.2005
Decuypere, S., Vanaerschot, M., Brunker, K., Imamura, H., Muller, S., Khanal, B., et al. (2012). Molecular mechanisms of drug resistance in natural Leishmania populations vary with genetic background. PLoS Negl. Trop. Dis. 6:e1514. doi: 10.1371/journal.pntd.0001514
Descoteaux, A., Luo, Y., Turco, S. J., and Beverley, S. M. (1995). A specialized pathway affecting virulence glycoconjugates of Leishmania. Science 269, 1869–1872. doi: 10.1126/science.7569927
Dobson, D. E., Mengeling, B. J., Cilmi, S., Hickerson, S., Turco, S. J., and Beverley, S. M. (2003a). Identification of genes encoding arabinosyltransferases (SCA) mediating developmental modifications of lipophosphoglycan required for sand fly transmission of Leishmania major. J. Biol. Chem. 278, 28840–28848. doi: 10.1074/jbc.M302728200
Dobson, D. E., Scholtes, L. D., Valdez, K. E., Sullivan, D. R., Mengeling, B. J., Cilmi, S., et al. (2003b). Functional identification of galactosyltransferases (SCGs) required for species-specific modifications of the lipophosphoglycan adhesin controlling Leishmania major-sand fly interactions. J. Biol. Chem. 278, 15523–15531. doi: 10.1074/jbc.M301568200
Downing, T., Imamura, H., Decuypere, S., Clark, T. G., Coombs, G. H., Cotton, J. A., et al. (2011). Whole genome sequencing of multiple Leishmania donovani clinical isolates provides insights into population structure and mechanisms of drug resistance. Genome Res. 21, 2143–2156. doi: 10.1101/gr.123430.111
Drini, S., Criscuolo, A., Lechat, P., Imamura, H., Skalicky, T., Rachidi, N., et al. (2016). Species- and strain-specific adaptation of the HSP70 super family in pathogenic trypanosomatids. Genome Biol. Evol. 8, 1980–1995. doi: 10.1093/gbe/evw140
Dumetz, F., Imamura, H., Sanders, M., Seblova, V., Myskova, J., Pescher, P., et al. (2017). Modulation of aneuploidy in Leishmania donovani during adaptation to different in vitro and in vivo environments and its impact on gene expression. MBio 8:e00599-17. doi: 10.1128/mBio.00599-17
Espiau, B., Vilhena, V., Cuvillier, A., Barral, A., and Merlin, G. (2017). Phenotypic diversity and selection maintain Leishmania amazonensis infectivity in BALB/c mouse model. Mem. Inst. Oswaldo Cruz 112, 44–52. doi: 10.1590/0074-02760160280
Fernandes, O., Murthy, V. K., Kurath, U., Degrave, W. M., and Campbell, D. A. (1994). Mini-exon gene variation in human pathogenic Leishmania species. Mol. Biochem. Parasitol. 66, 261–271. doi: 10.1016/0166-6851(94)90153-8
Franke, E. D., Mcgreevy, P. B., Katz, S. P., and Sacks, D. L. (1985). Growth cycle-dependent generation of complement-resistant Leishmania promastigotes. J. Immunol. 134, 2713–2718.
Gazanion, E., Fernandez-Prada, C., Papadopoulou, B., and Ouellette, M. (2016). Cos-Seq for high-throughput identification of drug target and resistance mechanisms in the protozoan parasite Leishmania. Proc. Natl. Acad. Sci. U.S.A. 113, E3012–E3021. doi: 10.1073/pnas.1520693113
Genest, P. A., Haimeur, A., Legare, D., Sereno, D., Roy, G., Messier, N., et al. (2008). A protein of the leucine-rich repeats (LRRs) superfamily is implicated in antimony resistance in Leishmania infantum amastigotes. Mol. Biochem. Parasitol. 158, 95–99. doi: 10.1016/j.molbiopara.2007.11.008
Hefnawy, A., Berg, M., Dujardin, J. C., and De Muylder, G. (2017). Exploiting knowledge on Leishmania drug resistance to support the quest for new drugs. Trends Parasitol. 33, 162–174. doi: 10.1016/j.pt.2016.11.003
Howard, M. K., Sayers, G., and Miles, M. A. (1987). Leishmania donovani metacyclic promastigotes: transformation in vitro, lectin agglutination, complement resistance, and infectivity. Exp. Parasitol. 64, 147–156. doi: 10.1016/0014-4894(87)90138-X
Jaber, H. T., Hailu, A., Pratlong, F., Lami, P., Bastien, P., and Jaffe, C. L. (2018). Analysis of genetic polymorphisms and tropism in East African Leishmania donovani by amplified fragment length polymorphism and kDNA minicircle sequencing. Infect. Genet. Evol. 65, 80–90. doi: 10.1016/j.meegid.2018.07.016
Jeddi, F., Mary, C., Aoun, K., Harrat, Z., Bouratbine, A., Faraut, F., et al. (2014). Heterogeneity of molecular resistance patterns in antimony-resistant field isolates of Leishmania species from the western Mediterranean area. Antimicrob. Agents Chemother. 58, 4866–4874. doi: 10.1128/AAC.02521-13
Kelly, J. M., Das, P., and Tomas, A. M. (1994). An approach to functional complementation by introduction of large DNA fragments into Trypanosoma cruzi and Leishmania donovani using a cosmid shuttle vector. Mol. Biochem. Parasitol. 65, 51–62. doi: 10.1016/0166-6851(94)90114-7
King, D. L., and Turco, S. J. (1988). A ricin agglutinin-resistant clone of Leishmania donovani deficient in lipophosphoglycan. Mol. Biochem. Parasitol. 28, 285–293. doi: 10.1016/0166-6851(88)90013-8
Kip, A. E., Balasegaram, M., Beijnen, J. H., Schellens, J. H., De Vries, P. J., and Dorlo, T. P. (2015). Systematic review of biomarkers to monitor therapeutic response in leishmaniasis. Antimicrob. Agents Chemother. 59, 1–14. doi: 10.1128/AAC.04298-14
Kumar, A., Boggula, V. R., Misra, P., Sundar, S., Shasany, A. K., and Dube, A. (2010a). Amplified fragment length polymorphism (AFLP) analysis is useful for distinguishing Leishmania species of visceral and cutaneous forms. Acta Trop. 113, 202–206. doi: 10.1016/j.actatropica.2009.10.006
Kumar, A., Boggula, V. R., Sundar, S., Shasany, A. K., and Dube, A. (2009). Identification of genetic markers in sodium antimony gluconate (SAG) sensitive and resistant Indian clinical isolates of Leishmania donovani through amplified fragment length polymorphism (AFLP). Acta Trop. 110, 80–85. doi: 10.1016/j.actatropica.2009.01.005
Kumar, A., Sisodia, B., Misra, P., Sundar, S., Shasany, A. K., and Dube, A. (2010b). Proteome mapping of overexpressed membrane-enriched and cytosolic proteins in sodium antimony gluconate (SAG) resistant clinical isolate of Leishmania donovani. Br. J. Clin. Pharmacol. 70, 609–617. doi: 10.1111/j.1365-2125.2010.03716.x
Kundig, C., Haimeur, A., Legare, D., Papadopoulou, B., and Ouellette, M. (1999). Increased transport of pteridines compensates for mutations in the high affinity folate transporter and contributes to methotrexate resistance in the protozoan parasite Leishmania tarentolae. EMBO J. 18, 2342–2351. doi: 10.1093/emboj/18.9.2342
Laffitte, M. N., Leprohon, P., Papadopoulou, B., and Ouellette, M. (2016). Plasticity of the Leishmania genome leading to gene copy number variations and drug resistance. F1000 Res. 5:2350. doi: 10.12688/f1000research.9218.1
Leprohon, P., Fernandez-Prada, C., Gazanion, E., Monte-Neto, R., and Ouellette, M. (2015). Drug resistance analysis by next generation sequencing in Leishmania. Int. J. Parasitol. Drugs Drug Resist. 5, 26–35. doi: 10.1016/j.ijpddr.2014.09.005
Leprohon, P., Légaré, Raymond, F., Madore, E., Hardiman, G., Corbeil, J., et al. (2009). Gene expression modulation is associated with gene amplification, supernumerary chromosomes and chromosome loss in antimony-resistant Leishmania infantum. Nucleic Acids Res. 37, 1387–1399. doi: 10.1093/nar/gkn1069
Lincoln, L. M., Ozaki, M., Donelson, J. E., and Beetham, J. K. (2004). Genetic complementation of Leishmania deficient in PSA (GP46) restores their resistance to lysis by complement. Mol. Biochem. Parasitol. 137, 185–189. doi: 10.1016/j.molbiopara.2004.05.004
Mcconville, M. J., Turco, S. J., Ferguson, M. A., and Sacks, D. L. (1992). Developmental modification of lipophosphoglycan during the differentiation of Leishmania major promastigotes to an infectious stage. EMBO J. 11, 3593–3600.
Mittal, M. K., Rai, S., Ashutosh, Ravinder, Gupta, S., Sundar, S., et al. (2007). Characterization of natural antimony resistance in Leishmania donovani isolates. Am. J. Trop. Med. Hyg. 76, 681–688. Available online at: https://www.ajtmh.org
Mkada-Driss, I., Lahmadi, R., Chakroun, A. S., Talbi, C., Guerbouj, S., Driss, M., et al. (2014). Screening and characterization of RAPD markers in viscerotropic Leishmania parasites. PLoS ONE 9:e109773. doi: 10.1371/journal.pone.0109773
Mondelaers, A., Sanchez-Cañete MP, Hendrickx, S., Eberhardt, E., Garcia-Hernandez, R., Lachaud, L., et al. (2016). Genomic and molecular characterization of miltefosine resistance in Leishmania infantum strains with either natural or acquired resistance through experimental selection of intracellular amastigotes. PLoS ONE 11:e0154101. doi: 10.1371/journal.pone.0154101
Mukherjee, A., Boisvert, S., Monte-Neto, R. L., Coelho, A. C., Raymond, F., Mukhopadhyay, R., et al. (2013). Telomeric gene deletion and intrachromosomal amplification in antimony-resistant Leishmania. Mol. Microbiol. 88, 189–202. doi: 10.1111/mmi.12178
Mukherjee, A., Padmanabhan, P. K., Singh, S., Roy, G., Girard, I., Chatterjee, M., et al. (2007). Role of ABC transporter MRPA, gamma-glutamylcysteine synthetase and ornithine decarboxylase in natural antimony-resistant isolates of Leishmania donovani. J. Antimicrob. Chemother. 59, 204–211. doi: 10.1093/jac/dkl494
Mukhopadhyay, R., Mukherjee, S., Mukherjee, B., Naskar, K., Mondal, D., Decuypere, S., et al. (2011). Characterisation of antimony-resistant Leishmania donovani isolates: biochemical and biophysical studies and interaction with host cells. Int. J. Parasitol. 41, 1311–1321. doi: 10.1016/j.ijpara.2011.07.013
Nühs, A., Schafer, C., Zander, D., Trube, L., Tejera Nevado, P., Schmidt, S., et al. (2014). A novel marker, ARM58, confers antimony resistance to Leishmania spp. Int. J. Parasitol. Drugs Drug Resist. 4, 37–47. doi: 10.1016/j.ijpddr.2013.11.004
Odiwuor, S., Vuylsteke, M., De Doncker, S., Maes, I., Mbuchi, M., Dujardin, J. C., et al. (2011). Leishmania AFLP: paving the way towards improved molecular assays and markers of diversity. Infect. Genet. Evol. 11, 960–967. doi: 10.1016/j.meegid.2011.03.008
Ovalle-Bracho, C., Díaz-Toro, Y. R., and Muvdi-Arenas, S. (2016). Polymerase chain reaction-miniexon: a promising diagnostic method for mucocutaneous leishmaniasis. Int. J. Dermatol. 55, 531–539. doi: 10.1111/ijd.12910
Perez-Victoria, F. J., Gamarro, F., Ouellette, M., and Castanys, S. (2003). Functional cloning of the miltefosine transporter. a novel P-type phospholipid translocase from Leishmania involved in drug resistance. J. Biol. Chem. 278, 49965–49971. doi: 10.1074/jbc.M308352200
Ponte-Sucre, A., Gamarro, F., Dujardin, J. C., Barrett, M. P., López-Vélez, R., et al. (2017). Drug resistance and treatment failure in leishmaniasis: a 21st century challenge. PLoS Negl. Trop. Dis. 11:e0006052. doi: 10.1371/journal.pntd.0006052
Prieto Barja, P., Pescher, P., Bussotti, G., Dumetz, F., Imamura, H., Kedra, D., et al. (2017). Haplotype selection as an adaptive mechanism in the protozoan pathogen Leishmania donovani. Nat. Ecol. Evol. 1, 1961–1969. doi: 10.1038/s41559-017-0361-x
Reiling, L., Chrobak, M., Schmetz, C., and Clos, J. (2010). Overexpression of a single Leishmania major gene enhances parasite infectivity in vivo and in vitro. Mol. Microbiol. 76, 1175–1190. doi: 10.1111/j.1365-2958.2010.07130.x
Reiling, L., Jacobs, T., Kroemer, M., Gaworski, I., Graefe, S., and Clos, J. (2006). Spontaneous recovery of pathogenicity by Leishmania major hsp100-/- alters the immune response in mice. Infect. Immun. 74, 6027–6036. doi: 10.1128/IAI.00773-05
Rogers, M. B., Hilley, J. D., Dickens, N. J., Wilkes, J., Bates, P. A., Depledge, D. P., et al. (2011). Chromosome and gene copy number variation allow major structural change between species and strains of Leishmania. Genome Res. 21, 2129–2142. doi: 10.1101/gr.122945.111
Ryan, K. A., Dasgupta, S., and Beverley, S. M. (1993a). Shuttle cosmid vectors for the trypanosomatid parasite Leishmania. Gene 131, 145–150.
Ryan, K. A., Garraway, L. A., Descoteaux, A., Turco, S. J., and Beverley, S. M. (1993b). Isolation of virulence genes directing surface glycosyl-phosphatidylinositol synthesis by functional complementation of Leishmania. Proc. Natl. Acad. Sci. U.S.A. 90, 8609–8613.
Sacks, D. L. (2001). Leishmania-sand fly interactions controlling species-specific vector competence. Cell. Microbiol. 3, 189–196. doi: 10.1046/j.1462-5822.2001.00115.x
Sacks, D. L., Brodin, T. N., and Turco, S. J. (1990). Developmental modification of the lipophosphoglycan from Leishmania major promastigotes during metacyclogenesis. Mol. Biochem. Parasitol. 42, 225–233. doi: 10.1016/0166-6851(90)90165-I
Sacks, D. L., and Perkins, P. V. (1984). Identification of an infective stage of Leishmania promastigotes. Science 223, 1417–1419. doi: 10.1126/science.6701528
Schweynoch, C., Zlateva, K., Oskam, L., Kroon, N., Graser, Y., et al. (1996). Identification and determination of the relationships of species and strains within the genus Leishmania using single primers in the polymerase chain reaction. Mol. Biochem. Parasitol. 77, 19–29. doi: 10.1016/0166-6851(96)02572-8
Serin, M. S., Daglioglu, K., Bagirova, M., Allahverdiyev, A., Uzun, S., Vural, Z., et al. (2005). Rapid diagnosis and genotyping of Leishmania isolates from cutaneous and visceral leishmaniasis by microcapillary cultivation and polymerase chain reaction-restriction fragment length polymorphism of miniexon region. Diagn. Microbiol. Infect. Dis. 53, 209–214. doi: 10.1016/j.diagmicrobio.2005.05.007
Shaw, C. D., Lonchamp, J., Downing, T., Imamura, H., Freeman, T. M., Cotton, J. A., et al. (2016). In vitro selection of miltefosine resistance in promastigotes of Leishmania donovani from Nepal: genomic and metabolomic characterization. Mol. Microbiol. 99, 1134–1148. doi: 10.1111/mmi.13291
Späth, G. F., Epstein, L., Leader, B., Singer, S. M., Avila, H. A., Turco, S. J., et al. (2000). Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc. Natl. Acad. Sci. U.S.A. 97, 9258–9263. doi: 10.1073/pnas.160257897
Späth, G. F., Garraway, L. A., Turco, S. J., and Beverley, S. M. (2003a). The role(s) of lipophosphoglycan (LPG) in the establishment of Leishmania major infections in mammalian hosts. Proc. Natl. Acad. Sci. U.S.A. 100, 9536–9541. doi: 10.1073/pnas.1530604100
Späth, G. F., Lye, L. F., Segawa, H., Sacks, D. L., Turco, S. J., and Beverley, S. M. (2003b). Persistence without pathology in phosphoglycan-deficient Leishmania major. Science 301, 1241–1243. doi: 10.1126/science.1087499
Srivastava, S., Mishra, J., Gupta, A. K., Singh, A., Shankar, P., and Singh, S. (2017). Laboratory confirmed miltefosine resistant cases of visceral leishmaniasis from India. Parasit. Vectors 10:49. doi: 10.1186/s13071-017-1969-z
Tejera Nevado, P., Bifeld, E., Hohn, K., and Clos, J. (2016). A telomeric cluster of antimony resistance genes on chromosome 34 of Leishmania infantum. Antimicrob. Agents Chemother. 60, 5262–5275. doi: 10.1128/AAC.00544-16
Torres, D. C., Ribeiro-Alves, M., Romero, G. A., Dávila, A. M., and Cupolillo, E. (2013). Assessment of drug resistance related genes as candidate markers for treatment outcome prediction of cutaneous leishmaniasis in Brazil. Acta Trop. 126, 132–141. doi: 10.1016/j.actatropica.2013.02.002
Ubeda, J. M., Raymond, F., Mukherjee, A., Plourde, M., Gingras, H., Roy, G., et al. (2014). Genome-wide stochastic adaptive DNA amplification at direct and inverted DNA repeats in the parasite Leishmania. PLoS Biol. 12:e1001868. doi: 10.1371/journal.pbio.1001868
Vanaerschot, M., Decuypere, S., Downing, T., Imamura, H., Stark, O., De Doncker, S., et al. (2012). Genetic markers for SSG resistance in Leishmania donovani and SSG treatment failure in visceral leishmaniasis patients of the Indian subcontinent. J. Infect. Dis. 206, 752–755. doi: 10.1093/infdis/jis424
Zhang, W. W., and Matlashewski, G. (2004). In vivo selection for Leishmania donovani miniexon genes that increase virulence in Leishmania major. Mol. Microbiol. 54, 1051–1062. doi: 10.1111/j.1365-2958.2004.04327.x
Keywords: Leishmania, biomarker discovery, reverse epidemiology, cosmid screen, functional genetics
Citation: Piel L, Pescher P and Späth GF (2018) Reverse Epidemiology: An Experimental Framework to Drive Leishmania Biomarker Discovery in situ by Functional Genetic Screening Using Relevant Animal Models. Front. Cell. Infect. Microbiol. 8:325. doi: 10.3389/fcimb.2018.00325
Received: 18 June 2018; Accepted: 27 August 2018;
Published: 19 September 2018.
Edited by:
Javier Moreno, Instituto de Salud Carlos III, SpainReviewed by:
Elisa Azuara-Liceaga, Universidad Autónoma de la Ciudad de México, MexicoBellisa Freitas Barbosa, Federal University of Uberlandia, Brazil
Copyright © 2018 Piel, Pescher and Späth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gerald F. Späth, gerald.spaeth@pasteur.fr
 Laura Piel
Laura Piel Pascale Pescher
Pascale Pescher Gerald F. Späth
Gerald F. Späth