Recent advances on bifunctional catalysts for one-pot conversion of furfural to γ-valerolactone
- National and Local Joint Engineering Laboratory for New Petrochemical Materials and Fine Utilization of Resources, Key Laboratory of Chemical Biology and Traditional Chinese Medicine, Ministry of Education, Hunan Normal University, Changsha, China
γ-Valerolactone (GVL) is one of the most valuable compounds derived from furfural (FAL), which has been industrially produced from agricultural byproducts like corn cobs. It is extremely challenging to synthesize GVL from FAL efficiently via a one-pot cascade reaction due to the need for multiple active sites in a single pot. By focusing on the aspects of one-pot synthesis of GVL from FAL, the authors aim to shed light on the rational design and utilization of environmentally friendly bifunctional catalysts with high efficiency in this reaction. Perspectives regarding future research opportunities in bi- or multi-functional catalysts for one-pot GVL synthesis are also discussed.
Introduction
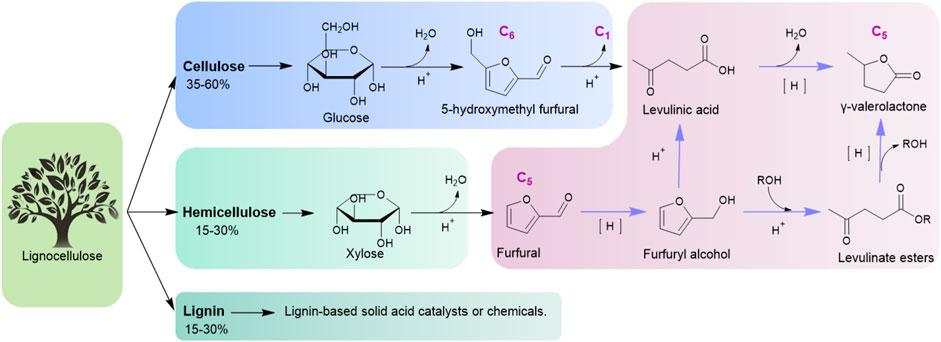
In the 21st century, transition from a fossil-fuel industry to one based on renewable resources is a great challenge in the process of sustainable development (Jakob and Hilaire, 2015). Biomass is the only renewable carbon resource with a wide range of sources, abundant reserves, short formation cycles, and low prices, which can be used as an alternative resource for fuel and chemical production (Chua et al., 2019; Liu et al., 2020; Clauser et al., 2021). γ-Valerolactone (GVL) is an important intermediate compound derived from biomass. The unique physicochemical properties of GVL enables it to have a wide range of applications as fuel, fuel additive, polymeric material precursor, and green solvent (Alonso et al., 2013; Xu et al., 2020). Figure 1 shows the production routes of GVL from lignocelluloses (Raj et al., 2021). Lignocellulosic biomass, whose main components are cellulose/hemicellulose or derived platform compounds like 5-hydroxymethyl furfural (HMF)/furfural (FAL), can be converted into GVL smoothly.
Among these routes, the approach to producing GVL from FAL has attracted much attention. That is because first, it is a carbon-balanced process and has a good carbon-atom economy. In addition, FAL is non-edible, inexpensive, and can be produced from agricultural waste corn cobs industrially (Cui et al., 2016). This makes it possible to produce GVL on a large scale by a two-step reaction from lignocellulose to FAL and then to GVL. Therefore, great efforts have been made to one-pot conversion of FAL to GVL (Osatiashtiani et al., 2017; Yu et al., 2019).
There is a series of cascading reactions involving hydrogenation, ring-opening reaction, and lactonization in this reaction. Regarding the hydrogenation process for γ-valerolactone synthesis, metal catalysts such as Ru, Pt, and Ni (Nemanashi et al., 2018; Wang Y et al., 2020; Maumela et al., 2021) are efficient for direct hydrogenation using gaseous H2 as an H-donor in FAL hydrogenation and LA/levulinate ester lactonization. Compared with the use of H2 reduction, reaction systems using alcohol as an H-donor for one-pot production of GVL from FAL via a Meerwein–Ponndorf–Verley (MPV) reduction can be effectively catalyzed by acid catalysts (He et al., 2020; Antunes et al., 2022). As the ring-opening reaction of furfuryl alcohol (FOL) is also an acidic catalytic reaction, bifunctional catalysts with Brönsted/Lewis acid active sites have been greatly developed for the GVL synthesis via a MPV reaction (Osatiashtiani et al., 2017).
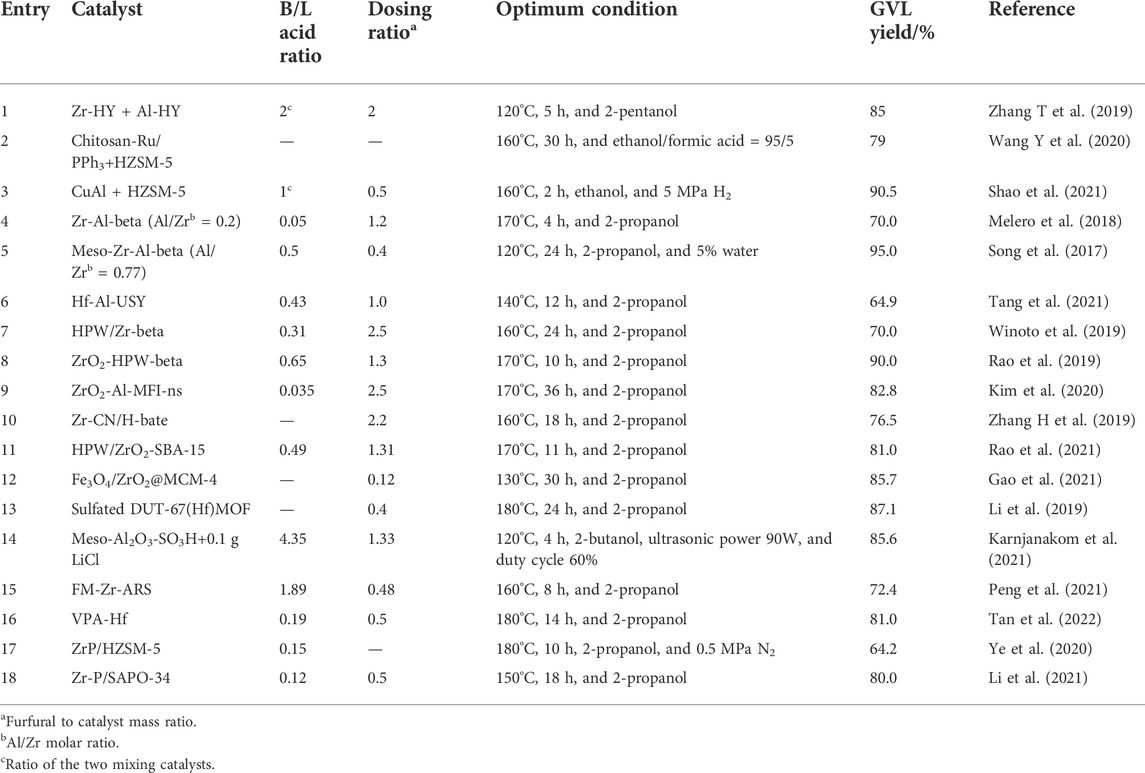
In this mini-review, the authors summarize the efficient bifunctional catalysts with metal/acid or Brönsted/Lewis acid active sites for one-pot conversion of FAL to GVL reported in the last 5 years. Table 1 lists the most efficient catalytic systems among them. Construction of acid centers as well as the supports’ structure are discussed, and catalytic reaction conditions are compared. It is hoped to provide insights into designing and developing efficient new sustainable catalysts for one-pot synthesis of GVL.
Efficient bifunctional catalysts
Molecular sieve catalysts
Molecular sieves have regular pore structure, strong acidity, and high hydrothermal stability, which have been perceived as the most popular catalysts reported in the synthesis of GVL so far. Both physically mixed molecular sieve co-catalysts and modified bifunctional molecular sieve catalysts have been developed in one-pot synthesis of GVL from FAL. A physical mixture of Zr-HY and Al-HY zeolites was reported as a highly efficient catalyst system for the one-pot transformation of FAL to GVL using 2-pentanol as the hydrogen donor (Zhang H et al., 2019; Table 1, entry 1). GVL yields reached 85% only after 5 h of reaction at 120 C. The excellent activity of Zr-HY zeolites for the Meerwein–Ponndorf–Verley (MPV) reduction of FAL and levulinate ester was attributed to the larger pore size of HY-zeolites and stronger Lewis acidic sites. Also, Al-HY zeolites provided Brönsted acids, thus promoting furfuryl ether conversion to levulinate ester. Physically mixing H-ZSM-5 and metal catalysts was also reported as efficient metal/acid bifunctional catalytic systems in this reaction. Wang T et al. (2020) mixed chitosan-Ru/PPh3 and HZSM-5 in an ethanol/formic acid (95/5) solvent to catalyze the FAL one-pot cascade reaction (Table 1, entry 2). GVL yield was 79% after 30 h at 160 C. The unprecedented GVL yield of 90.5% has been reported in a dual catalyst system including CuAl and H-ZSM-5 in ethanol solvent and 5 MPa H2 (Shao et al., 2021; Table 1, entry 3). The relative abundance of the hydrogenation sites provided by CuAl and acidic sites provided by HZSM-5 determines the main products from furfuryl alcohol (FA) to ethyl levulinate (EL) or GVL. The biggest advantage of physically mixed catalysts is that the proportion and strength of acid sites can be independently optimized conveniently, thus avoiding complicated catalyst preparation. However, the physically mixed approach does not perform well when reusability and selectivity are concerned.
Since an appropriate proportion of Lewis and Brönsted acid plays a key role in the activity of the catalyst, molecular sieves with tunable Lewis and Brönsted acid sites prepared by doping metal ions or loading Brönsted acids have been widely reported (Li et al., 2020; Sun et al., 2022). Beta zeolite was treated in nitric acid solution and calcination was performed to remove Al, which can lead to more vacancies for the introduction of other metal species by post-impregnation (Melero, et al., 2018, Table 1, entry 4) or solid-state ion-exchange method (Song et al., 2017; Table 1, entry 5; Tang et al., 2021; Table 1, entry 6). The Zr(Hf)-modified molecular sieve catalysts showed superior catalytic activities in this reaction (Table 1, entries 4–10). In this type of dual acid site catalysts, Brönsted acidity is obtained from the Al sites and Lewis acidity is obtained from Zr or Hf sites. From the aspect of increasing catalysts’ Brönsted acid centers, phosphotungstic acid (HPW) has received attention for its strong Brönsted acidity (Frattini et al., 2017). HPW/Zr-beta and ZrO2-HPW-beta were prepared and applied in one-pot conversion of FAL in 2-propanol to give high GVL yields (Winoto et al., 2019; Table 1, entry 7; Rao et al.; Table 1, entry 8).
In addition to the effect brought by active sites, structure and morphology of the molecular sieve are also important for its catalytic efficiency. Taking ZrO2-[Al]MFI-ns 30 as an example, the MFI molecular sieve has a nano-sponge (NS) morphology, and its unique mesoporous structure allows the ZrO2 clusters (Lewis acid sites) to be confined within a few unit cell-thin porous silica-aluminate molecular sieve walls (Brönsted acid sites) (Kim et al., 2020; Table 1, entry 9). The generation of separate Brönsted and Lewis acid sites on the internal and external molecular sieves resulted in a significant enhancement of GVL yield (82.8%).
Mesoporous silica catalysts
Mesoporous silica materials are good catalytic supports for their well-defined pore channels, large surface area, and tunable physical/chemical properties (Singh et al., 2018). ZrO2/SBA-15 and Zr-KIT were prepared by loading ZrO2 onto SBA-15 (Iglesias et al., 2018) and incorporating Zr into KIT-5 (He et al., 2019) and applied for GVL production by MPV reduction. A medium GVL yield of about 40% from FAL was reported. It may be because the acid strength and density of the catalyst is not enough.
Rao et al. (2021) reported a more efficient bifunctional mesoporous silica catalyst containing Zr and tungstophosphoric acid (TPA/ZrO2-SBA-15), which can completely convert furfural and give a GVL yield of 81%. (Table 1, entry 11). This research indicated that the acid–base properties of the catalyst were directed by the location of ZrO2 or TPA in the support, and that the catalyst with ZrO2 present inside the SBA-15 pores and TPA dispersed on the support showed the highest activity.
A magnetically recoverable multifunctional catalyst (Fe3O4/ZrO2@MCM-41) was tailored by the impregnation of ZrO2 supported on mesoporous MCM-41 coated with Fe3O4 nanoparticles for the cascade conversion of furfural (FAL) giving high yield of GVL. According to the literature, incorporation of Fe3O4 could not only impart the catalyst with a strong magnetism but also tune its acidity to promote GVL production (Gao et al., 2021; Table 1, entry 12).
Other bifunctional catalysts
Among the most active bifunctional catalytic systems reported in the last 5 years, metal–organic frameworks (MOFs), porous Al2O3, and inorganic–organic framework catalysts are notable. As MOFs have a well-defined three-dimensional crystal structure, uniform active centers, high surface area, and porosity, they can be used as both catalysts and catalyst carriers. As shown in Table 1 entry 13, sulfated DUT-67(Hf) bifunctional metal–organic framework catalyst was prepared for the preparation of GVL reaching a yield of 87.1% (Li et al., 2019). The amount of Brönsted acid centers is regulated by adjusting the concentration of the aqueous sulfuric acid solution. But this catalyst cannot be recycled due to a dramatic loss of Brönsted acid sites in the reaction. A complex acid regeneration process was required before reuse.
A mesoporous Al2O3-SO3H catalyst was prepared by grafting sulfonic acid groups onto mesoporous Al2O3 (Karnjanakom et al., 2021, Table 1 entry 14). The mesoporous structure allows the Lewis and Brönsted acid sites in the catalyst to easily transfer intermediates and thus produces the desired GVL. The addition of salt (LiCl) increased the miscibility of the substrate and intermediates in the solvent phase and therefore increased the diffusion rate of the reacting molecules in the organic phase. It was also shown that ultrasonic assistance and the presence of O2 could inhibit the formation of humus and could maintain the stability of the catalyst. The catalyst could be reused for up to twenty runs without significant change. This catalytic system replaces the high-pressure hydrothermal system with a safer and greener ultrasonic/oxygen molecular system, which provides a new idea for the future one-pot method to produce GVL.
Peng et al. (2021) (Table 1 entry 15) reported a multifunctional Zr-containing catalyst (FM-Zr-ARS) with a stable porous inorganic–organic framework that showed high catalytic activity for GVL production (72.4% yield of GVL) from FAL (Table 1, entry 14). The -O-Zr-O- network in the FM-Zr-ARS structure formed a rich content of Lewis acid–base sites. The inherent sulfonic groups in ARS gave the FM-Zr-ARS hybrid an unsaturated acid site.
In addition, coordination organophosphate-Hf polymers (VPA-Hf) were prepared and found to exhibit superior performance in the one-step conversion of furfural to γ-valerolactone with a high yield of 81.0%, with a turnover frequency of 5.0 h−1 (Ye et al., 2020, Table 1 entry 16). The environmentally friendly material zirconium phosphate (ZrP) was also used as bifunctional catalysts in this reaction and showed good catalytic activity (Ye et al., 2020, Table 1 entry 17; Li et al., 2021, Table 1 entry 18).
It is noteworthy that ZrOCl2 as an acid/base bifunctional catalyst showed remarkable catalytic activity in the production of GVL (He et al., 2020). A maximum GVL yield of 63.3 and 52.1% was achieved from furfuryl alcohol and furfural at 200°C, respectively. [ZrO(OH)2]n• xH2O species and Brönsted acid species H+ were derived from in situ hydrolysis of ZrOCl2•8H2O. This research may provide a new idea for the construction of bifunctional catalysts.
Conclusion and outlook
At present, researchers continue to explore various bifunctional or multifunctional high-efficiency catalysts for the one-pot production of GVL from FAL. To avoid the high pressure and high cost associated with direct hydrogenation (H2), alcohols, especially 2-propanol, are considered hydrogen donors for the production of GVL via MPV reduction reactions. In terms of bifunctional catalysts, molecular sieve catalysts with Brönsted/Lewis acid active sites are in mainstream GVL production. The highly active Lewis acid centers are mainly Zr or its cognate main group of Hf-based genera. Acting as Brönsted acids are Al-beta molecular sieves, H-ZSM-5 molecular sieves, HPW, zirconium phosphate, and sulfonic acid groups. The supports with a well-defined structure, well-developed pores, and an easily adjustable acid center will be more favorable for this reaction.
Though rapid development in developing efficient catalytic systems for one-pot conversion of FAL to GVL has been made, there are still some challenges. For example, humins are a big problem in the one-pot reaction, which decrease GVL yields and deactivate catalysts by deposition. Future research should also vigorously focus on exploring new bifunctional catalysts with high efficiency, low cost, energy saving, and environmental protection. Synergistic acid–base bifunctional catalysts can be developed to explore new catalytic systems. The plausible catalytic mechanism is worthy to be understood. Finally, one-pot synthesis of GVL from more upstream raw materials (e.g., lignocellulose, hemicellulose, or xylose) by using synergistic catalysis of Brönsted acid and Lewis acid is highly expected.
Author contributions
JW: investigation, methodology, writing—original draft preparation, data curation, and formal analysis. ZX: methodology and formal analysis. ZH: validation and discussion. QX: writing—review and editing, conceptualization, project administration, funding acquisition, and supervision. DY: conceptualization, project administration, funding acquisition, and supervision.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alonso, D. M., Wettstein, S. G., and Dumesic, J. A. (2013). Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem. 15 (3), 584. doi:10.1039/c3gc37065h
Antunes, M. M., Silva, A. F., Fernandes, A., Pillinger, M., Ribeiro, F., Valente, A. A., et al. (2022). Renewable bio-based routes to gamma-valerolactone in the presence of hafnium nanocrystalline or hierarchical microcrystalline zeotype catalysts. J. Catal. 4, 56–71. doi:10.1016/j.jcat.2021.12.022
Chua, S. Y., A/P Periasamy, L., Goh, C. M. H., Tan, Y. H., Mujawar, M. N., Kansedo, J., et al. (2019). Biodiesel synthesis using natural solid catalyst derived from biomass waste - a review. J. Ind. Eng. Chem. 81, 41–60. doi:10.1016/j.jiec.2019.09.022
Clauser, N. M., González, G., Mendieta, C. M., Kruyeniski, J., Area, M. C., Vallejos, M. E., et al. (2021). Biomass waste as sustainable raw material for energy and fuels, Sustainability, 13, 794–814. doi:10.3390/su13020794
Cui, C. X., Zhang, X. M., Bi, Y. Y., Wang, Y. J., Wang, L., Wang, H. Y., et al. (2016). Estimation of corn cob resources and their development and utilization in China. Chin.agr. Res. Reg. Plan. 37 (1), 1–8. doi:10.7621/cjarrp.1005-9121.20160101
Frattini, L., Isaacs, M. A., Parlett, C. M. A., Wilson, K., Kyriakou, G., Lee, A. F., et al. (2017). Support enhanced alpha-pinene isomerization over HPW/SBA-15. Appl. Catal. B Environ. 200, 10–18. doi:10.1016/j.apcatb.2016.06.064
Gao, X. Y., Yu, X., Peng, L. C., He, L., and Zhang, J. H. (2021). Magnetic Fe3O4 nanoparticles and ZrO2-doped mesoporous MCM-41 as a monolithic multifunctional for gamma-valerolactone production directly from furfural. Fuel 300, 120996. doi:10.1016/j.fuel.2021.120996
He, J., Li, H., Xu, Y., and Yang, S. (2019). Dual acidic mesoporous KIT silicates enable one-pot production of γ-valerolactone from biomass derivatives via cascade reactions, Renew. Energy, (146), 359–370. doi:10.1016/j.renene.2019.06.105
He, J., Xu, Y. F., Yu, Z. Z., Li, H., Zhao, W. F., Wu, H. G., et al. (2020). ZrOCl2 as a bifunctional and in situ precursor material for catalytic hydrogen transfer of bio-based carboxides. Sustain. Energy Fuels 4, 3102–3114. doi:10.1039/c9se01313j
Iglesias, J., Melero, J. A., Morales, G., Paniagua, M., Hernández, B., Osatiashtiani, A., et al. (2018). ZrO2-SBA-15 catalysts for the one-pot cascade synthesis of GVL from furfural. Catal. Sci. Technol. 17 (8), 4485–4493. doi:10.1039/C8CY01121D
Jakob, M., and Hilaire, J. (2015). Climate science: Unburnable fossil-fuel reserves. Nature 517, 150–151. doi:10.1038/517150a
Karnjanakom, S., Bayu, A., Maneechakr, P., Samart, C., Kongparakul, S., Guan, G. Q., et al. (2021). Simultaneous assistance of molecular oxygen and mesoporous SO3H-alumina for a selective conversion of biomass-derived furfural to gamma-valerolactone without an external addition of H-2. Sustain. Energy Fuels 5 (16), 4041–4052. doi:10.1039/d1se00817j
Kim, K. D., Kim, J., Teoh, W. Y., Kim, J. C., Huang, J., Ryoo, R., et al. (2020). Cascade reaction engineering on zirconia-supported mesoporous MFI zeolites with tunable lewis-bronsted acid sites: A case of the one-pot conversion of furfural to gamma-valerolactone. RSC Adv. 10 (58), 35318–35328. doi:10.1039/d0ra06915a
Li, W. K., Cai, Z., Li, H., Shen, Y., Zhu, Y. Q., Li, H. C., et al. (2019). Hf-based metal organic frameworks as bifunctional catalysts for the one-pot conversion of furfural to gamma-valerolactone. Mol. Catal. 472, 17–26. doi:10.1016/j.mcat.2019.04.010
Li, W. L., Li, M. Z., Liu, H., Jia, W. L., Yu, X., Wang, S., et al. (2021). Domino transformation of furfural to γ-valerolactone over SAPO-34 zeolite supported zirconium phosphate catalysts with tunable Lewis and Bronsted acid sites. Mol. Catal. 506, 111538. doi:10.1016/j.mcat.2021.111538
Li, X. C., Yuan, X. H., Xia, G. P., Liang, J., Liu, C., Wang, Z. D., et al. (2020). Catalytic production of gamma-valerolactone from xylose over delaminated Zr-Al-SCM-1 zeolite via a cascade process. J. Catal. 392, 175–185. doi:10.1016/j.jcat.2020.10.004
Liu, X., Yang, W., Zhang, Q., Li, C., and Wu, H. (2020). Current approaches to alkyl levulinates via efficient valorization of biomass derivatives. Front. Chem. 8, 794. doi:10.3389/fchem.2020.00794
Maumela, M., Marx, S., and Meijboom, R. (2021). Heterogeneous Ru catalysts as the emerging potential superior catalysts in the selective hydrogenation of bio-derived levulinic acid to γ-valerolactone: Effect of particle size, solvent, and support on activity, stability, and selectivity. Catalysts 11, 292. doi:10.3390/catal.11020292
Melero, J. A., Morales, G., Iglesias, J., Paniagua, M., and Lopez-Aguado, C. (2018). Rational optimization of reaction conditions for the one-pot transformation of furfural to γ-valerolactone over Zr–Al-beta zeolite: Toward the efficient utilization of biomass. Ind. Eng. Chem. Res. 57 (34), 11592–11599. doi:10.1021/acs.iecr.8b02475
Nemanashi, M., Noh, J. H., and Meijboom, R. (2018). Hydrogenation of biomass-derived levulinic acid to γ-valerolactone catalyzed by mesoporous supported dendrimer-derived Ru and Pt catalysts: An alternative method for the production of renewable biofuels. Appl. Catal. A General 550, 77–89. doi:10.1016/j.apcata.2017.10.015
Osatiashtiani, A., Lee, A. F., and Wilson, K. (2017). Recent advances in the production of gamma-valerolactone from biomass-derived feedstocks via heterogeneous catalytic transfer hydrogenation. J. Chem. Technol. Biotechnol. 92 (6), 1125–1135. doi:10.1002/jctb.5213
Peng, Q. R., Wang, H. J., Xia, Y. M., and Liu, X. (2021). One-pot conversion of furfural to gamma-valerolactone in the presence of multifunctional zirconium alizarin red S hybrid. Appl. Catal. A General 621, 118203. doi:10.1016/j.apcata.2021.118203
Raj, T., Chandrasekhar, K., Banu, R., Yoon, J., Kumar, G., Kim, S., et al. (2021). Synthesis of γ-valerolactone (GVL) and their applications for lignocellulosic deconstruction for sustainable green biorefineries. Fuel. 303: 121333. doi:10.1016/j.fuel.2021.121333
Rao, B. S., Kumari, P. K., Koley, P., Tardio, J., and Lingaiah, N. (2019). One pot selective conversion of furfural to gamma-valerolactone over zirconia containing heteropoly tungstate supported on beta-zeolite catalyst. Mol. Catal. 466, 52–59. doi:10.1016/j.mcat.2018.12.024
Rao, B. S., YogitaLakshmi, D. D., Kumari, P. K., and Lingaiah, N. (2021). Influence of metal oxide and heteropoly tungstate location in mesoporous silica towards catalytic transfer hydrogenation of furfural to gamma-valerolactone. Sustain. Energy Fuels 5 (14), 3719–3728. doi:10.1039/d1se00340b
Shao, Y. W., Li, Q. Y., Dong, X. Y., Wang, J. Z., Sun, K., Zhang, L. J., et al. (2021). Cooperation between hydrogenation and acidic sites in Cu-based catalyst for selective conversion of furfural to gamma-valerolactone. Fuel 293, 120457. doi:10.1016/j.fuel.2021.120457
Singh, S., Kumar, R., Setiabudi, H. D., Nanda, S., and Vo, D. V. N. (2018). Advanced synthesis strategies of mesoporous SBA-15 supported catalysts for catalytic reforming applications: A state-of-the-art review. Appl. Catal. A General 559, 57–74. doi:10.1016/j.apcata.2018.04.015
Song, S., Di, L., Wu, G. J., Dai, W. L., Guan, N. J., Li, L. D., et al. (2017). Meso-Zr-Al-beta zeolite as a robust catalyst for cascade reactions in biomass valorization. Appl. Catal. B Environ. 205, 393–403. doi:10.1016/j.apcatb.2016.12.056
Sun, W. J., Li, H. F., Wang, X. C., and Liu, A. Q. (2022). Cascade upgrading of biomass-derived furfural to γ-valerolactone over Zr/Hf-based catalysts. Front. Chem. 10, 863674–863679. doi:10.3389/fchem.2022.863674
Tan, J. Y., Liu, Y. X., Li, M. R., Li, H., and Yang, S. (2022). One-step catalytic upgrading of bio-based furfural to gamma-valerolactone actuated by coordination organophosphate-Hf polymers. Sustain. Energy Fuels 6 (4), 484–501. doi:10.1039/d1se01476e
Tang, B., Li, S., Song, W. C., Li, Y., and Yang, E. C. (2021). One-pot transformation of furfural into gamma-valerolactone catalyzed by a hierarchical Hf-Al-USY zeolite with balanced Lewis and Bronsted acid sites. Sustain. Energy Fuels 5 (18), 4724–4735. doi:10.1039/d1se00942g
Wang T, T. L., He, J. H., and Zhang, Y. T. (2020). Production of gamma-Valerolactone from one-pot transformation of biomass-derived carbohydrates over chitosan-supported Ruthenium catalyst combined with zeolite ZSM-5. Eur. J. Org. Chem. 2020 (11), 1611–1619. doi:10.1002/ejoc.201901704
Wang Y, Y. X., Lu, Y. W., Cao, Q. E., and Fang, W. H. (2020). A magnetic CoRu-CoOX nanocomposite efficiently hydrogenates furfural to furfuryl alcohol at ambient H-2 pressure in water. Chem. Commun. 56 (26), 3765–3768. doi:10.1039/d0cc01039a
Winoto, H. P., Fikri, Z. A., Ha, J. M., Park, Y. K., Lee, H., Suh, D. J., et al. (2019). Heteropolyacid supported on Zr-Beta zeolite as an active catalyst for one-pot transformation of furfural to gamma-valerolactone. Appl. Catal. B Environ. 241, 588–597. doi:10.1016/j.apcatb.2018.09.031
Xu, R., Liu, K., Du, H. S., Liu, H. Y., Cao, X. F., Zhao, X. Y., et al. (2020). Falling leaves return to their roots: A review on the preparation of γ-valerolactone from lignocellulose and its application in the conversion of lignocellulose. ChemSusChem 13, 6461–6476. doi:10.1002/cssc.202002008
Ye, L., Han, Y. W., Bai, H., and Lu, X. B. (2020). HZ-ZrP catalysts with adjustable ratio of bronsted and Lewis acids for the one-pot value-added conversion of biomass-derived furfural. ACS Sustain. Chem. Eng. 8 (19), 7403–7413. doi:10.1021/acssuschemeng.0c01259
Yu, Z. H., Lu, X. B., Liu, C., Han, Y. W., and Ji, N. (2019). Synthesis of γ-valerolactone from different biomass-derived feedstocks: Recent advances on reaction mechanisms and catalytic systems, Renew. Sustain. Energy Rev., 112, 140–157. doi:10.1016/j.rser.2019.05.039
Zhang H, H. W., Yang, W. J., Roslan, I. I., Jaenicke, S., and Chuah, G. K. (2019). A combo Zr-HY and Al-HY zeolite catalysts for the one-pot cascade transformation of biomass-derived furfural to gamma-valerolactone. J. Catal. 375, 56–67. doi:10.1016/j.jcat.2019.05.020
Keywords: furfural, γ-valerolactone, bifunctional catalysts, transfer hydrogenation, one-pot reaction
Citation: Wang J, Xiang Z, Huang Z, Xu Q and Yin D (2022) Recent advances on bifunctional catalysts for one-pot conversion of furfural to γ-valerolactone. Front. Chem. 10:959572. doi: 10.3389/fchem.2022.959572
Received: 01 June 2022; Accepted: 06 July 2022;
Published: 09 August 2022.
Edited by:
Haian Xia, Nanjing Forestry University, ChinaCopyright © 2022 Wang, Xiang, Huang, Xu and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiong Xu, xuqiong@hunnu.edu.cn; Dulin Yin, dulinyin@126.com
 Jianhua Wang
Jianhua Wang  Qiong Xu
Qiong Xu