Metal-Free Synthesis of N-Heterocycles via Intramolecular Electrochemical C-H Aminations
- 1School of Chemical and Environmental Engineering, Anyang Institute of Technology, Anyang, China
- 2Department of Civil and Environmental Engineering, University of Nebraska–Lincoln, Lincoln, NE, United States
N-heterocycles are key structural units in many drugs, biologically interesting molecules and functional materials. To avoid the residues of metal catalysts, the construction of N-heterocycles under metal-free conditions has attracted much research attention in academia and industry. Among them, the intramolecular electrochemical C-H aminations arguably constitute environmentally friendly methodologies for the metal-free construction of N-heterocycles, mainly due to the direct use of clean electricity as the redox agents. With the recent renaissance of organic electrosynthesis, the intramolecular electrochemical C-H aminations have undergone much progress in recent years. In this article, we would like to summarize the advances in this research field since 2019. The emphasis is placed on the reaction design and mechanistic insight. The challenges and future developments in the intramolecular electrochemical C-H aminations are also discussed.
Introduction
N-heterocycles are key structural units in many drugs, biologically interesting molecules and functional materials (Kim and Movassaghi, 2009; Meng et al., 2021; Staśkiewicz et al., 2021). Therefore, the development of robust synthetic methods for the construction of N-heterocycles has gained increased attention in academia and industry. Over the past decades, many metal-catalyzed methodologies such as Buchwald-Hartwig cross coupling (Newman and Lautens, 2010) and Ullmann reactions (Lu and Li, 2006) have provided direct routes to obtain N-heterocycles with high efficiencies. They are indeed one of the best choices for the preparation of N-heterocycles in laboratory. However, the difficulty to remove the metal residues resulted from the metal catalysts largely limits the industrial use of these metal-catalyzed methodologies. To circumvent this limitation, many metal-free methodologies have been developed for the synthesis of N-heterocycles recently (Preeti and Singh, 2021; Yang et al., 2021; Han et al., 2022; Yamamoto et al., 2022). Among them, the intramolecular C-H aminations are of high interest mainly because the prefunctionalized substrates are avoided (Tan et al., 2020). In light of the significance of N-heterocycles, the development of atom economy and environmentally benign methods for intramolecular C-H aminations continues to play a pivotal role in medicinal and material chemistry.
Organic electrosynthesis utilizes electricity as the driving force to activate substrates of interest, thus offering a sustainable way to obtain reactive intermediates for organic synthesis (Li et al., 2019; Meyer et al., 2020; Luo et al., 2021; Novaes et al., 2021; Chen et al., 2022; Qian et al., 2022; Tan et al., 2022; Wang and Xu, 2022). Recently, organic electrosynthesis has undergone considerable renaissance, and provided unique reactivities that are not accessible with traditional synthetic methodologies. With these benefits, organic electrosynthesis has widely been employed in the construction of C-C (Pollok and Waldvogel, 2020; Zhang et al., 2021), C-O (Herszman et al., 2019; Zhang et al., 2019), C-S (Zhang et al., 2020) and C-Si (Ke et al., 2021; Jiang et al., 2022) bonds in green manners. In the context of intramolecular C-H aminations, organic electrosynthesis has also showed great synthetic potentials. Many types of N-heterocycles were electrochemically synthesized under metal- and external oxidant-free conditions. Compared with the traditional metal-free methods for N-heterocycles syntheses, these electrochemical protocols feature environmentally friendly conditions and operational simplicity. In this mini-review, would like to summarize the advances in intramolecular electrochemical C-H aminations under metal-free conditions since 2019. The contents were categorized by the C (sp2)-H and C (sp3)-H aminations. Our focus is on both 1) reaction design including electrolytic conditions, catalyst choice and substrate design, and 2) mechanistic insights of intramolecular C-H aminations. Finally, the challenges and future developments in this research field are also discussed. We hope this mini-review will offer a balanced overview of the recent contributions to intramolecular electrochemical C-H aminations, and will act as a useful reference to students and scientists working in this research field.
Intramolecular Electrochemical C(sp2)-H Aminations
Indoles and indolines are widely existed in many biologically important molecules (Newhouse and Baran, 2008). In 2020, Wang and co-workers report an iodide-mediated electrochemical C (sp2)-H amination methodology for the tunable synthesis of indoles two and indolines 4 (Scheme 1) (Hu et al., 2020). This tandem reaction was proposed to proceed via the key intermediate 5. When the reaction was carried out in the presence of KSCN, the nucleophilic substitution of iodide by KSCN gives intermediate 6. The β-H elimination within 6 affords indoles 2. However, when the reaction was carried out in the presence of PhNH2 (3), the nucleophilic substitution of iodide by PhNH2 directly yields indolines 4. This protocol features metal- and external oxidant-free conditions and switchable synthesis of indoles and indolines.
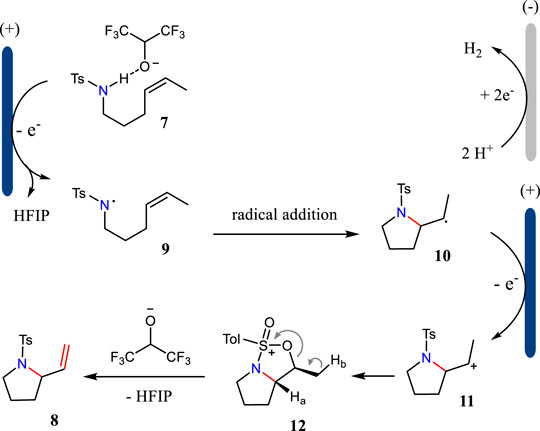
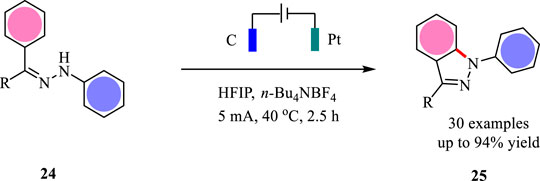
Aza-Wacker cyclization is one of the most straightforward methods to synthesize alkene-functionalized N-heterocycles. The traditional methods often relied on the Pd catalysis, thus leading to unexpected metal residues (Thomas et al., 2020). In 2021, Xu and co-workers report a new formal electrochemical aza-Wacker cyclization under continuous-flow conditions (Huang et al., 2021) (Scheme 2). This reaction was carried out with dimethylacetamide (DMA) and 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) as the mixed solvents. This newly developed protocol allows the construction of saturated N-heterocycles with up to 94% yield. The catalyst- and supporting electrolyte-free conditions make this electrochemical method more appealing to the developed methodologies. Moreover, this electrochemical condition tolerates multiple substituted alkenes well, which are challenging substrates in metal-catalyzed aza-Wacker cyclizations.
The tandem reaction sequence for the aza-Wacker cyclizations is shown in Scheme 3. First, the substrate has a SET oxidation at the anode via a proton-coupled electron-transfer (PCET) pathway to give N-centered radical 9. Then, radical nine undergoes intramolecular radical addition to alkene leading to the formation of radical 10, which has a further SET oxidation to yield intermediate 11. The intramolecular cyclization of intermediate 11 gives bicyclic cation 12, which has an elimination reaction to afford the desired product 8. The electrochemically generated hexafluoroisopropoxide plays an important role to drive the reaction.
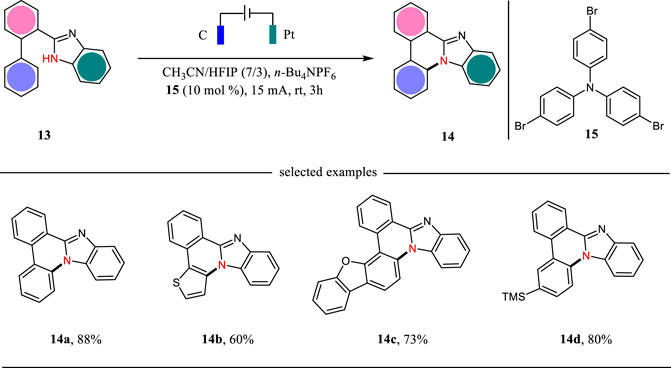
Benzimidazole-fused phenanthridines are excellent candidates as fluorescent and phosphorescent organic light-emitting diodes (Ohsawa et al., 2019). The classic methods for the synthesis of benzimidazole-fused phenanthridines relied on the Pd catalysis or hypervalent iodine oxidation reactions. In 2021, Xu, Zhang and co-workers report a triarylamine-mediated electrochemical protocol for the formation of benzimidazole-fused phenanthridines in good to excellent yields (Shi et al., 2021) (Scheme 4). The reaction was carried out in an undivided cell with carbon cloth as the anode and platinum as the cathode under CCE conditions. This reaction was proceeded through an intramolecular C-H amination sequence with hydrogen evolution as the side reaction. For some substrates, the direct electrolysis was also efficient to facilitate the intramolecular C-H amination reactions.
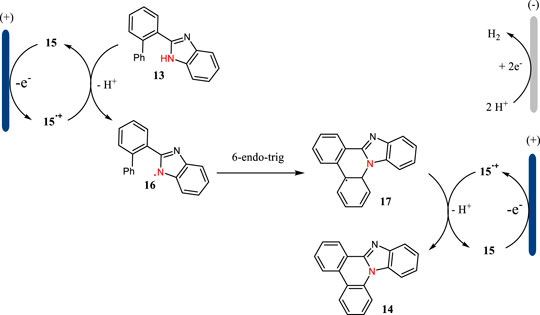
The plausible mechanism for the triarylamine-mediated C-H aminations is shown in Scheme 5. Triarylamine 15 acting as an organic mediator has a SET oxidation at the anode to give the corresponding radical cation, which then undergoes an electron transfer with 13 to afford N-centered radical 16. The radical 16 could have a 6-endo-trig cyclization to give intermediate 17, which then undergoes an indirect oxidation followed by proton releasing to yield benzimidazole-fused phenanthridine 14. Simultaneously, the proton is reduced to hydrogen gas at the cathode.
1H-indazoles as one important class of N-heterocycles showed interesting biological activities (Schmidt and Dreger, 2011). The reported methods for the synthesis of 1H-indazoles are limited by the use of metal catalysts at high temperature or stoichiometric chemical oxidants such as Oxane. To circumvent these limitations, Lei and co-workers developed an electrochemical strategy for the synthesis of 1H-indazoles under metal-free conditions (Wan et al., 2022). This intramolecular cyclization was carried out in an undivided cell with platinum plates as the electrodes and n-Bu4NBF4/DCM/HFIP as the electrolyte solution under CCE conditions. As shown in Scheme 6, a variety of 1H-indazoles were obtained in good to excellent yields. The functional groups such as cyano and ester were tolerated well under the optimal conditions. This reaction represents the first example to construct 1H-indazoles under metal- and external-oxidant conditions.
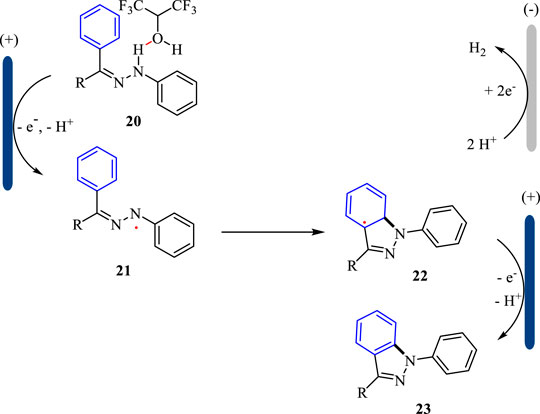
The plausible mechanism for the electrochemical synthesis of 1H-indazoles is shown in Scheme 7. With the assistance of HFIP, the substrate 20 is oxidized at the anode and loses one molecule of proton giving N-centered radical 21, which undergoes the following intramolecular cyclization to afford radical 22. Subsequently, the intermediate 22 undergoes another SET oxidation followed by deprotonation to yield 1H-indazole 23. Simultaneously, the proton is reduced to hydrogen gas at the cathode. The hydrogen-bond interaction between HFIP and substrate 20 is the key to the success of the intramolecular cyclization reaction.
At almost the same time, Zhang’s group reported a similar electrochemical protocol for the formation of 1H-indazoles via an intramolecular C-H/N-H coupling reaction (Zhang et al., 2022) (Scheme 8). Different from Lei’s protocol, this electrochemical protocol employs graphite rod as the anode and HFIP as the solvent. As shown in Scheme 9, the desired 1H-indazoles were obtained with up to 94% yield.
Intramolecular Electrochemical C(sp3)-H Aminations
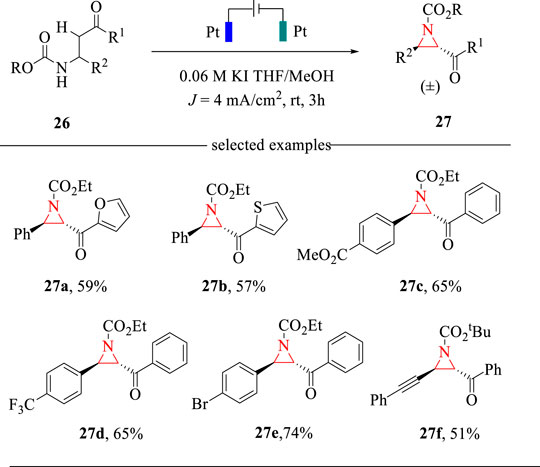
Aziridines are key structural units in many bioactive natural products, thus attracting much attention towards their facile syntheses (Miyazaki et al., 2015). The classical methods relied on the cycloaddition strategy to access aziridines (Degennaro et al., 2014). However, the expensive nitrene or carbene precursors and harsh reaction conditions are always needed. Encouraged by recent progress on halide-mediated electrochemical transformations (Lian et al., 2021) and our effort on the electrochemical cyclization for the synthesis of 2-oxazolines (Wang et al., 2018), we recently developed a KI-mediated intramolecular C-H amination reaction for the synthesis of trans-2,3-disubstituted aziridines (Wang et al., 2019) (Scheme 9). The electrochemical cyclization reaction was carried out in an undivided cell with Pt plate as the electrodes and THF/MeOH as the mixed solvents. This intramolecular C-H amination reaction allows the construction of 2,3-disubstituted aziridines in moderate yields with exclusively trans selectivities. This method also represents the first example of electrochemical synthesis of aziridines via intramolecular C-H amination strategies.
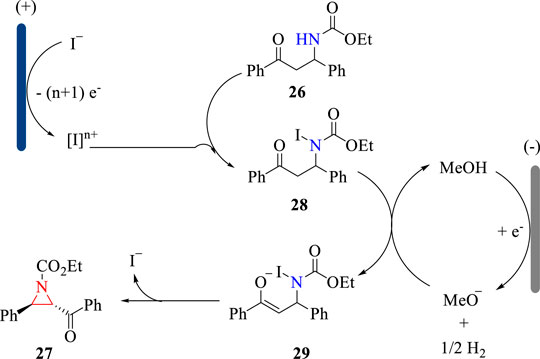
The electrochemical C-H amination reaction was proposed to proceed via the oxidative reaction sequence. As shown in Scheme 10, KI is oxidized to hypervalent iodine, which undergoes an iodination reaction with substrate 26 to afford intermediate 28. Then, intermediate 28 is deprotonated by electrogenerated MeO− to deliver intermediate 29, which undergoes the intramolecular cyclization to give aziridine 27 in a trans selectivity. The electrogenerated MeO− acts as a strong base to drive the cyclization reaction and thus obviating the addition of external bases.
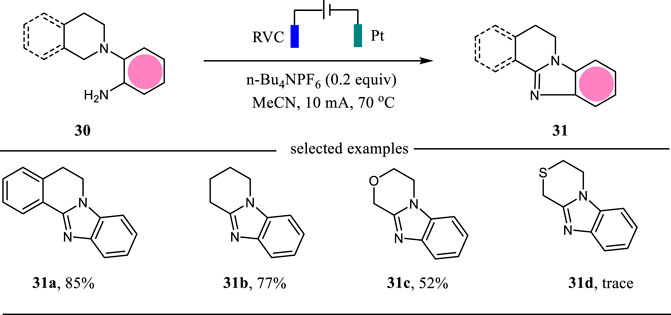
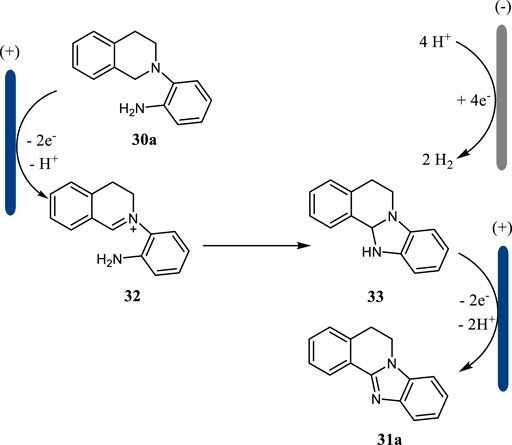
Benzimidazoles are valuable N-heterocycles possessing important biological and pharmacological activities (Hegde et al., 2015). The classical methods to access benzimidazoles relied on the condensation reaction at high temperatures. The intramolecular C-H amination reactions need Ir catalyst or 3-chloroperbenzoic acid (m-CPBA) as the oxidant (Sun et al., 2015). In 2019, Tang, Pan and co-workers reported an electrochemical C (sp3)-H amination protocol for the construction of benzimidazoles in moderate to excellent yields (Li A. et al., 2021) (Scheme 11). This intramolecular C-H amination reaction was carried out in an undivided cell with RVC as the anode and Pt as the cathode and CH3CN as the solvent. It is noteworthy that this electrochemical protocol showed an excellent functional group tolerance.
This intramolecular C (sp3)-H amination was proposed to proceed via an intramolecular oxidative cyclization mechanism (Scheme 12). The substrate 30a undergoes a two-electron oxidation followed by deprotonation to give imine 32, which then cyclizes to afford intermediate 33. The further anodic oxidation of intermediate 33 followed by deprotonation lead to the formation of benzimidazole 31a. Simultaneously, the proton is reduced to hydrogen gas at the cathode. This intramolecular C (sp3)-H amination strategy may find more applications in the synthesis of other types of N-heterocycles.
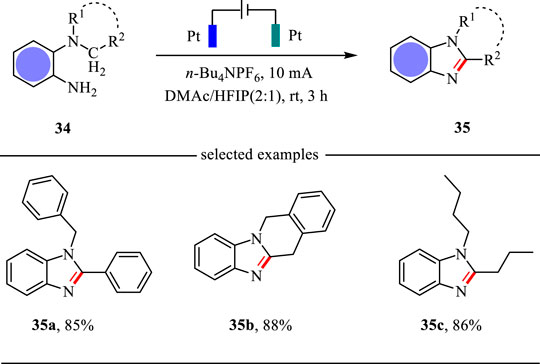
Following the similar reaction mechanism, Tang, Zhou and co-workers reported an electrochemical C (sp3)-H amination strategy for the construction of benzimidazoles in excellent yields (Li J. et al., 2021) (Scheme 13). This intramolecular C-H amination reaction was carried out in an undivided cell with Pt as the electrodes and N, N-dimethylacetamide (DMAc)/HFIP as the mixed solvents. This electrochemical strategy features mild conditions and wide substrate scope.
Summary and Outlook
In recent years, the intramolecular electrochemical C-H aminations have emerged as powerful synthetic tools for the synthesis of N-heterocycles under metal-free conditions. This mini-review highlights the recent contributions to the field of metal-free synthesis of N-heterocycles via intramolecular electrochemical C-H aminations since 2019. The related contents were categorized by the C (sp2)-H and C (sp3)-H aminations. The detailed mechanisms for the C-H aminations were discussed to shed light on the reaction design principle for these electrochemical transformations. Although significant advances in this research field have been made, some challenges persist. First, large amounts of supporting electrolytes are necessary for the reported intramolecular electrochemical C-H aminations. Considering the trend of green synthesis, the use of continuous-flow electrochemistry under supporting electrolyte-free conditions is one of the directions in this domain. Second, compared with the well-established intramolecular electrochemical C (sp2)-H aminations, C (sp3)-H aminations only have limited successful examples. The development of new strategies based on intramolecular electrochemical C (sp3)-H aminations would provide new opportunities to access N-heterocycles of interest. This field will soon find more opportunities in the synthesis of functional materials and drugs on academic and even industrial level.
Author Contributions
HW designed this proposal and wrote the manuscript. YZ, HX, and JZ collected the related literature data. CJ revised the manuscript. All authors contributed to the final version of the manuscript.
Funding
HW thanks the funding from the Natural Science Foundation of Henan Province (222300420102), and the start-up funding from Anyang Institute of Technology (BSJ2021049) and Postdoctoral Innovation Practice Base in Anyang Institute of Technology (BHJ2021009). CJ thanks the start-up funding from University of Nebraska−Lincoln.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Chen, C., Wang, X., and Yang, T. (2022). Recent Updates on Electrogenerated Hypervalent Iodine Derivatives and Their Applications as Mediators in Organic Electrosynthesis. Front. Chem. 10, 883474. doi:10.3389/fchem.2022.883474
Degennaro, L., Trinchera, P., and Luisi, R. (2014). Recent Advances in the Stereoselective Synthesis of Aziridines. Chem. Rev. 114, 7881–7929. doi:10.1021/cr400553c
Han, Q., Xu, K., Tian, F., Huang, S., and Zeng, C. (2022). A Practical Transamidation Strategy for the N-Deacylation of Amides. Chin. J. Org. Chem. 42, 1123–1128. doi:10.6023/cjoc202112007
Hegde, M., Sharath Kumar, K. S., Thomas, E., Ananda, H., Raghavan, S. C., and Rangappa, K. S. (2015). A Novel Benzimidazole Derivative Binds to the DNA Minor Groove and Induces Apoptosis in Leukemic Cells. RSC Adv. 5, 93194–93208. doi:10.1039/C5RA16605E
Herszman, J. D., Berger, M., and Waldvogel, S. R. (2019). Fluorocyclization of N-Propargylamides to Oxazoles by Electrochemically Generated ArIF2. Org. Lett. 21, 7893–7896. doi:10.1021/acs.orglett.9b02884
Hu, K., Zhang, Y., Zhou, Z., Yang, Y., Zha, Z., and Wang, Z. (2020). Iodine-Mediated Electrochemical C(sp2)-H Amination: Switchable Synthesis of Indolines and Indoles. Org. Lett. 22, 5773–5777. doi:10.1021/acs.orglett.0c01821
Huang, C., Li, Z. Y., Song, J., and Xu, H. C. (2021). Catalyst‐ and Reagent‐Free Formal Aza‐Wacker Cyclizations Enabled by Continuous‐Flow Electrochemistry. Angew. Chem. Int. Ed. 60, 11237–11241. doi:10.1002/anie.202101835
Jiang, Y., Xu, K., and Zeng, C. (2022). Electrophotocatalytic Si-H Activation Governed by Polarity-Matching Effects. CCS Chem. 4, 1796–1805. doi:10.31635/ccschem.021.202101010
Ke, J., Liu, W., Zhu, X., Tan, X., and He, C. (2021). Electrochemical Radical Silyl‐Oxygenation of Activated Alkenes. Angew. Chem. Int. Ed. 60, 8744–8749. doi:10.1002/anie.202016620
Kim, J., and Movassaghi, M. (2009). Biogenetically Inspired Syntheses of Alkaloid Natural Products. Chem. Soc. Rev. 38, 3035–3050. doi:10.1039/B819925F
Li, A., Li, C., Yang, T., Yang, Z., Liu, Y., Li, L., et al. (2021a). Electrochemical Synthesis of Benzo[d]imidazole via Intramolecular C(sp3)-H Amination. J. Org. Chem. doi:10.1021/acs.joc.1c01842
Li, J., Zhang, S., and Xu, K. (2021b). Recent Advances towards Electrochemical Transformations of α-keto Acids. Chin. Chem. Lett. 32, 2729–2735. doi:10.1016/j.cclet.2021.03.027
Li, Q.-Y., Cheng, S.-Y., Tang, H.-T., and Pan, Y.-M. (2019). Synthesis of Rutaecarpine Alkaloids via an Electrochemical Cross Dehydrogenation Coupling Reaction. Green Chem. 21, 5517–5520. doi:10.1039/c9gc03028j
Lian, F., Xu, K., and Zeng, C. (2021). Indirect Electrosynthesis with Halogen Ions as Mediators. Chem. Rec. 21, 2290–2305. doi:10.1002/tcr.202100036
Lu, H., and Li, C. (2006). General and Highly Efficient Synthesis of 2-Alkylideneazetidines and β-Lactams via Copper-Catalyzed Intramolecular N-Vinylation. Org. Lett. 8, 5365–5367. doi:10.1021/ol062274i
Luo, Z.-X., Liu, M., Li, T., Xiong, D.-C., and Ye, X.-S. (2021). Electrochemical Bromination of Glycals. Front. Chem. 9, 796690. doi:10.3389/fchem.2021.796690
Meng, Z.-Y., Feng, C.-T., Zhang, L., Yang, Q., Chen, D.-X., and Xu, K. (2021). Regioselective C-H Phosphorothiolation of (Hetero)arenes Enabled by the Synergy of Electrooxidation and Ultrasonic Irradiation. Org. Lett. 23, 4214–4218. doi:10.1021/acs.orglett.1c01161
Meyer, T. H., Choi, I., Tian, C., and Ackermann, L. (2020). Powering the Future: How Can Electrochemistry Make a Difference in Organic Synthesis? Chem 6, 2484–2496. doi:10.1016/j.chempr.2020.08.025
Miyazaki, M., Uoto, K., Sugimoto, Y., Naito, H., Yoshida, K., Okayama, T., et al. (2015). Discovery of DS-5272 as a Promising Candidate: A Potent and Orally Active P53-MDM2 Interaction Inhibitor. Bioorg. Med. Chem. 23, 2360–2367. doi:10.1016/j.bmc.2015.03.069
Newhouse, T., and Baran, P. S. (2008). Total Synthesis of (±)-Psychotrimine. J. Am. Chem. Soc. 130, 10886–10887. doi:10.1021/ja8042307
Newman, S. G., and Lautens, M. (2010). The Role of Reversible Oxidative Addition in Selective Palladium(0)-Catalyzed Intramolecular Cross-Couplings of Polyhalogenated Substrates: Synthesis of Brominated Indoles. J. Am. Chem. Soc. 132, 11416–11417. doi:10.1021/ja1052335
Novaes, L. F. T., Liu, J., Shen, Y., Lu, L., Meinhardt, J. M., and Lin, S. (2021). Electrocatalysis as an Enabling Technology for Organic Synthesis. Chem. Soc. Rev. 50, 7941–8002. doi:10.1039/D1CS00223F
Ohsawa, T., Sasabe, H., Watanabe, T., Nakao, K., Komatsu, R., Hayashi, Y., et al. (2019). A Series of Imidazo[1,2−f]phenanthridine–Based Sky–Blue Tadf Emitters Realizing EQE of over 20. Adv. Opt. Mat. 7, 1801282. doi:10.1002/adom.201801282
Pollok, D., and Waldvogel, S. R. (2020). Electro-organic Synthesis - a 21st Century Technique. Chem. Sci. 11, 12386–12400. doi:10.1039/D0SC01848A
Preeti., , , and Singh, K. N. (2021). Metal-free Multicomponent Reactions: a Benign Access to Monocyclic Six-Membered N-Heterocycles. Org. Biomol. Chem. 19, 2622–2657. doi:10.1039/D1OB00145K
Qian, P., Xu, L., Wang, W., Zhang, L., Tang, L., Liu, J., et al. (2022). Electrochemical Synthesis of Dipyrazolo/dipyrimidine-Fused Pyridines via Oxidative Domino Cyclization of C(sp3)-H Bonds. Org. Chem. Front. 9, 1662–1667. doi:10.1039/D1QO01641E
Schmidt, A., and Dreger, A. (2011). Recent Advances in the Chemistry of Pyrazoles. Properties, Biological Activities, and Syntheses. Coc 15, 1423–1463. doi:10.2174/138527211795378263
Shi, J., Li, J., Zhao, W., Cui, M., Ni, W., Li, L., et al. (2021). Regioselective Intramolecular Sp2 C-H Amination: Direct vs. Mediated Electrooxidation. Org. Chem. Front. 8, 1581–1586. doi:10.1039/D0QO01584A
Staśkiewicz, A., Ledwoń, P., Rovero, P., Papini, A. M., and Latajka, R. (2021). Triazole-modified Peptidomimetics: an Opportunity for Drug Discovery and Development. Front. Chem. 9, 674705. doi:10.3389/fchem.2021.674705
Sun, X., Lv, X.-H., Ye, L.-M., Hu, Y., Chen, Y.-Y., Zhang, X.-J., et al. (2015). Synthesis of Benzimidazoles via Iridium-Catalyzed Acceptorless Dehydrogenative Coupling. Org. Biomol. Chem. 13, 7381–7383. doi:10.1039/C5OB00904A
Tan, C., Liu, Y., Liu, X., Jia, H., Xu, K., Huang, S., et al. (2020). Stereoselective Synthesis of Trans-aziridines via Intramolecular Oxidative C(sp3)-H Amination of β-amino Ketones. Org. Chem. Front. 7, 780–786. doi:10.1039/C9QO01489F
Tan, Z., He, X., Xu, K., and Zeng, C. (2022). Electrophotocatalytic C−H Functionalization of N‐Heteroarenes with Unactivated Alkanes under External Oxidant‐Free Conditions. ChemSusChem 15, e202102360. doi:10.1002/cssc.202102360
Thomas, A. A., Nagamalla, S., and Sathyamoorthi, S. (2020). Salient Features of the Aza-Wacker Cyclization Reaction. Chem. Sci. 11, 8073–8088. doi:10.1039/D0SC02554B
Wan, H., Li, D., Xia, H., Yang, L., Alhumade, H., Yi, H., et al. (2022). Synthesis of 1H-Indazoles by an Electrochemical Radical Csp2-H/N-H Cyclization of Arylhydrazones. Chem. Commun. 58, 665–668. doi:10.1039/D1CC04656J
Wang, H., Shi, J., Tan, J., Xu, W., Zhang, S., and Xu, K. (2019). Electrochemical Synthesis of Trans-2,3-disubstituted Aziridines via Oxidative Dehydrogenative Intramolecular C(sp3)-H Amination. Org. Lett. 21, 9430–9433. doi:10.1021/acs.orglett.9b03641
Wang, H., and Xu, K. (2022). Cobalta-electrocatalyzed Allylic C-H Alkylation. Chin. J. Org. Chem. 42, 1260–1261. doi:10.6023/cjoc202200021
Wang, H., Zhang, J., Tan, J., Xin, L., Li, Y., Zhang, S., et al. (2018). Electrosynthesis of Trisubstituted 2-Oxazolines via Dehydrogenative Cyclization of β-Amino Arylketones. Org. Lett. 20, 2505–2508. doi:10.1021/acs.orglett.8b00165
Yamamoto, Y., Yamakawa, C., Nishimura, R., Dong, C.-P., Kodama, S., Nomoto, A., et al. (2022). Metal-free Synthesis of 2-substituted Quinazolines via Green Oxidation of O-Aminobenzylamines: Practical Construction of N-Containing Heterocycles Based on a Salicylic Acid-Catalyzed Oxidation System. Front. Chem. 9, 822841. doi:10.3389/fchem.2021.822841
Yang, Q., Yan, X.-T., Feng, C.-T., Chen, D.-X., Yan, Z.-Z., and Xu, K. (2021). Tandem Strecker/C(sp3)-H Amination Reactions for the Construction of Cyanide-Functionalized Imidazo[1,5-A]pyridines with NH4SCN as a Cyanating Agent. Org. Chem. Front. 8, 6384–6389. doi:10.1039/D1QO01060C
Zhang, H., Wang, T., Xu, K., and Zeng, C. (2021). N-Hydroxyphthalimide-mediated Electrochemical Denitrogenation of Aroylhydrazides to Generate Acyl Radicals and Their Applications in the Syntheses of Fluorenones. J. Org. Chem. 86, 16171–16176. doi:10.1021/acs.joc.1c01262
Zhang, H., Ye, Z., Chen, N., Chen, Z., and Zhang, F. (2022). Electrochemical Dehydrogenative C-N Coupling of Hydrazones for the Synthesis of 1H-Indazoles. Green Chem. 24, 1463–1468. doi:10.1039/D1GC04534B
Zhang, J., Wang, H., Chen, Y., Xie, H., Ding, C., Tan, J., et al. (2020). Electrochemical Synthesis of Selenocyanated Imidazo[1,5-A]quinolines under Metal Catalyst- and Chemical Oxidant-free Conditions. Chin. Chem. Lett. 31, 1576–1579. doi:10.1016/j.cclet.2019.11.037
Keywords: organic electrosynthesis, C-H amination, N-heterocycle, metal-free, cyclization, N-centered radical
Citation: Wang H, Zheng Y, Xu H, Zou J and Jin C (2022) Metal-Free Synthesis of N-Heterocycles via Intramolecular Electrochemical C-H Aminations. Front. Chem. 10:950635. doi: 10.3389/fchem.2022.950635
Received: 23 May 2022; Accepted: 06 June 2022;
Published: 20 June 2022.
Edited by:
Cheng-Tao Feng, Anhui University of Chinese Medicine, ChinaCopyright © 2022 Wang, Zheng, Xu, Zou and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiqiao Wang, 20200079@ayit.edu.cn; Congrui Jin, cjin5@unl.edu
 Huiqiao Wang
Huiqiao Wang Yongjun Zheng1
Yongjun Zheng1  Congrui Jin
Congrui Jin