Application of Reaction Force Field Molecular Dynamics in Lithium Batteries
- Shagang School of Iron and Steel, Soochow University, Suzhou, China
Lithium batteries are widely used in portable electronic products. Although the performance of the batteries has been greatly improved in the past few decades, limited understanding of the working mechanisms at an atomic scale has become a major factor for further improvement. In the past 10 years, a reaction force field (ReaxFF) has been developed within the molecular dynamics framework. The ReaxFF has been demonstrated to correctly describe both physical processes and chemical reactions for a system significantly larger than the one simulated by quantum chemistry, and therefore in turn has been broadly applied in lithium batteries. In this article, we review the ReaxFF studies on the sulfur cathode, various anodes, and electrolytes of lithium batteries and put particular focus on the ability of the ReaxFF to reveal atomic-scale working mechanisms. A brief prospect is also given.
Introduction
Owing to energy shortages, air pollution and population growth, intermittent energy sources (e.g., solar energy and wind power) have been utilized to substitute traditional ones, which greatly promotes the development of energy storage devices (Lv et al., 2018, 2019; Liu et al., 2020). Among these devices, due to high efficiency, zero-emission, and low noise, lithium batteries have attracted considerable attention and have already been used in various applications (Nishi, 2001; Goodenough and Kim, 2010). In the past few decades, energy density, rate capability, and cycling lifespan of lithium batteries have been significantly improved (Li et al., 2020; Sui et al., 2020; Wang et al., 2020b; Luo et al., 2021), but still do not fully meet the requirements of electric vehicles (Lu et al., 2013) and smart-grids. In order to further improve the performance of lithium batteries, a deeper understanding of the working mechanisms at an atomic scale, which to a large extent are unknown, is desired.
Molecular dynamics (MD) is a powerful atomic-scale simulation method and is suitable for studying processes such as interface reactions and microstructure evolution. MD exploits classical Newtonian functions to describe particle movement, in which interaction force between particles is calculated by potential equations/fields. To date, a large number of potential equations/fields have been developed for different systems. These equations/fields nevertheless can only describe physical interactions. Chemical reactions have to be handed over to another simulation method, i.e., quantum chemistry (QC), which however is computationally expensive and usually confined to small systems.
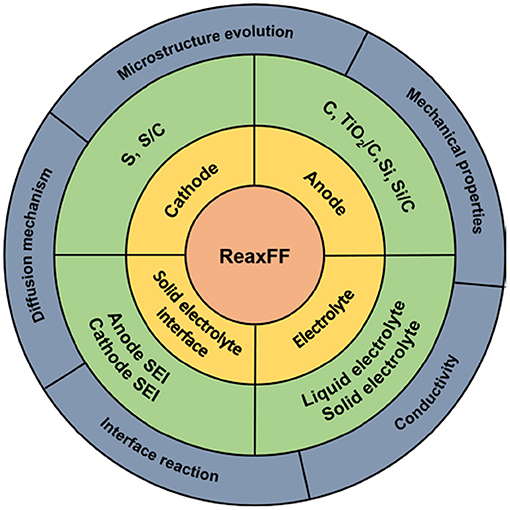
In order to increase computational efficiency for large simulation systems, van Duin et al. (2001) proposed the reaction force field (ReaxFF) to simulate chemical reactions within the MD framework (hereinafter referred to as ReaxFF simulation). The ReaxFF simulation now covers most elements contained in cathode, anode, and electrolyte materials of lithium batteries. Based on the ReaxFF simulation, the sulfur cathode, various anodes, and electrolytes of lithium batteries have been investigated, as summarized in Figure 1. In this study, we review all these works by focusing on the ability of the ReaxFF to reveal atomic-scale working mechanisms of lithium batteries.
Sulfur Cathode
Sulfur cathode is one of the most potential candidate materials for next-generation lithium batteries mainly due to is high theoretical specific capacity as 1675 mAh/g. Besides, sulfur is inexpensive and environmentally friendly. However, there are still many problems restricting commercialization of sulfur cathodes, such as huge volume expansion during cycling, poor electronic and ionic conductivities, and the polysulfide shuttle effect (Manthiram et al., 2013). In terms of simulation, the ReaxFF mainly focuses on the expansion behaviors of bulk sulfur, microstructure and composition evolution of sulfur nanoparticles, and interaction mechanisms between sulfur and carbon for a composite cathode.
Considering the expansion behavior and resulting change in mechanical properties of the sulfur cathode, Islam et al. (2015) simulated lithiation with different amounts of Li. The authors found that volume expansion rate and open circuit voltage curves obtained in their simulations were in good agreement with experimental findings, and both strength and fracture toughness of the cathode increased with the Li amount. Wang et al. (2017) further elucidated that this strengthening behavior resulted from a transition of the bonding network from non-bonded to covalently bonded atomic interactions. In addition, Wang et al. (2017) studied the expansion behavior under restriction conditions (suppressing in-plane expansion but allowing out-of-plane free expansion to occur). They found that the lithiation products of amorphous lithiated sulfur (a-LixS) facilitated out-of-plane inelastic deformation at a low level of in-plane stress. This finding supports the optimization strategies of sulfur cathodes encapsulated by carbon nanotubes/nanofibers (Zheng et al., 2011).
In addition to the volume expansion, the lithium polysulfide species (e.g., Li2Sn, n > 2) formed during lithium intercalation and deintercalation can also greatly affect battery performance. The microstructure and composition of Li2S nanoparticles during delithiation have been investigated by the ReaxFF. Li et al. (2018) compared the energy of four possible structures of Li2S8 nanoparticles, and concluded that the core-shell Li2S8 nanoparticles were most likely present during charging. Furthermore, via ring and fragment analyses with regard to Li2S and Li2S8 nanoparticles, it was found that a broad distribution of sulfur, lithium sulfide and lithium polysulfide species existed in both crystalline and amorphous Li2S and Li2S8 nanoparticles, which was attributed to surface atoms with significant strain, dangling bonds, and under-coordination.
Because of the ability to correctly describe intermolecular forces and Coulomb interactions, the ReaxFF has also been used to investigate interaction mechanisms between sulfur and carbon for a sulfur/graphene composite. Beltran and Balbuena (2018) studied a lithium insertion process for a mixture of 8-membered sulfur rings and graphene sheets. They found that the under-coordinated carbon atoms at the edge of the graphene could promote sulfur reduction, which caused the ring to directly reduce to short-chain sulfur, thus decreasing lithium polysulfide products. Ponce and Seminario (2020) compared the structural stability of the same composite undergoing fast and slow lithiation and found that the structure that experienced slow lithiation was more stable. These simulation results demonstrate that defects and lithiation rate can significantly affect the performance of the S/C composite.
Anodes
Anodes are required to be of large specific capacity, high electronic/ionic conductivities and good cycle stability (Wang et al., 2020). At present, typical anode materials include carbon materials, TiO2, and silicon materials. Among them, TiO2 owns a theoretical specific capacity of 336 mAh/g (close to 372 mAh/g of graphite) and stable microstructure, but its conductivity is low (Wu et al., 2019). The theoretical specific capacity of Si is as high as 4200 mAh/g, but Si suffers serious volume expansion, which is usually alleviated by combining Si with other materials to form composite anodes (Reddy et al., 2013).
Carbon Materials
Graphite and carbon nanotubes are considered to be good anode materials mainly due to their high electronic conductivity. The inevitable defects (such as vacancies, holes, and cracks) introduced during preparation however have a huge impact on these materials. The ReaxFF has been utilized to investigate defect effects on Li storage capacity, diffusion path of Li and mechanical properties of the anodes. In addition, structural stability of core-shell-structured multi-walled carbon nanotubes/TiO2 has also been studied.
The defects of a graphene sheet are thought to benefit Li storage. Raju et al. (2015) confirmed by the ReaxFF that vacancies can cause graphite to store more Li. Even with a low vacancy density of 2.1%, the maximum specific capacity of graphite was increased to 428 mAh/g. For defect-free graphite and carbon nanotubes, Li can only diffuse through the spaces between adjacent graphite layers and the center channels of carbon nanotubes, respectively. Raju et al. (2015) compared the diffusion barrier of Li across defect-free graphene and graphene with monovacancy/divacancy, and found that as vacancy number increased, the diffusion barrier decreased significantly, which indicate that the defect of vacancies provides Li with shortcut diffusion paths so as to increase charge and discharge rate.
Concerning mechanical properties, Huang et al. (2013) simulated tensile behaviors of carbon nanotubes with hole-like defects and single vacancy at different Li concentrations, and found that fracture strength decreased with an increase in Li concentration. The authors further revealed that, driven by the chemical potential difference, Li tended to accumulate around the defects and therefore weakened C-C bonds around the defects. As the defect number increased, this weakening effect became more significant due to more Li accumulation. A similar fracture mechanism was found in graphene by Yang et al. (2013).
Carbon nanotubes and TiO2 are combined to obtain core-shell multi-walled carbon nanotubes/TiO2 for good cycle performance (Yang et al., 2009). With the ReaxFF, the relationship between microstructure and performance was studied. Muñiz et al. (2016) calculated adsorption energy of TiO2 on the surface of multi-walled carbon nanotubes (MWCNT) and found that there was a strong electrostatic interaction between the two materials. The authors further focused on the thickness influence of the TiO2 shell on the microstructure, and found that at a specific thickness, MWCNT became highly symmetrical, meanwhile TiO2 became uniformly distributed. This optimal simulation microstructure agrees well with experimental observations (Cao et al., 2010).
Silicon Materials
During lithiation, Li atoms diffuse into crystalline silicon (c-Si) anode and form amorphous lithiated silicon (a-LixSi), which results in great volume expansion as well as a propagating interface between the two Si phases. In order to get insights into the effect of the volume expansion on the mechanical properties of the Si anode and atomic-scale mechanisms of both Li diffusion and Si phase transition around the interface, many ReaxFF works have been performed.
In terms of mechanical properties, Fan et al. (2013) calculated yield and fracture strength of a-LixSi under various chemomechanical loading conditions. The authors found that changes in the mechanical properties were closely related to atomic bonding types. As Li concentration increased, the anode underwent plastic softening, which was caused by a decrease in Si-Si covalent bonds and increase in Li-Li metal bonds.
Considering interface reactions, Kim et al. (2014) and Ostadhossein et al. (2015) found that Li preferentially migrated along [110] direction in bulk c-Si, and the interface parallel to (111) plane propagated at the fastest speed. Jung et al. (2015) found that for c-Si nanowires, in addition to [110] direction, Li also preferentially migrated along [112] direction. Kim et al. (2014) examined composition around the interface and determined that on the two sides of the interface were c-LiSi and a-Li15Si4.
Silicon Composite
SiO2 and Al2O3 have been used to coat Si for preventing volume expansion. These materials are thought to exhibit different mechanisms for Li diffusion into Si. Concerning SiO2 and Al2O3, Li reacts with them to form lithium silicon oxide and lithium aluminum oxide, respectively, and then diffuses across these products and Si. For carbonaceous layers, Li diffusion can only occur through defects and/or the spaces between adjacent graphite layers. For the different coating materials, many ReaxFF studies have been done.
The ReaxFF was utilized to elucidate the volume expansion inhibition effect as well as change of SiO2/Al2O3 mechanical properties during lithiation. Ostadhossein et al. (2016) studied the effect of lithiation on the mechanical properties of SiO2, and found that an increase in Li would lead to the enhancement of elastic softening and ductility of lithium silicon oxide compounds, which indicates that SiO2 can alleviate the mechanical deformation of Si. Jung et al. (2016) studied the structural evolution of core-shell-structured c-Si/a-SiO2 nanowires during lithiation, and suggested that the LixSi formed from Si nanowires was under tensile stress, resulting in expansion behavior, while the one from SiO2/Si nanowires was subjected to compressive stress, thereby suppressing expansion. Kim et al. (2016) studied structural stability of Si with SiO2/Al2O3 shells of different thickness. It was found that the 4.5 Å Al2O3 shell was most stable, which was ascribed to the gradient of Young's modulus due to the Li concentration gradient. Based on the ReaxFF results, Kim et al. (2016) and Verners and Simone (2019) proposed to use a modulus gradient coating for optimizing Si anode. Recent experimental studies have shown that this strategy is effective (Jiao et al., 2020).
Electrolytes
Electrolytes, as a carrier for lithium ion transport, need to be of high ionic conductivity, thermal stability, and non-reaction with electrodes (Wang et al., 2020a). The most used electrolyte for lithium batteries is high dielectric constant ethylene carbonate (EC) mixed with low viscosity chain carbonate, e.g., dimethyl carbonate (DMC), ethyl methyl carbonate (EMC), and diethyl carbonate (DEC), or carboxylate acid ester, e.g., methyl acetate (MA) (Xu, 2004). The ReaxFF has been successfully applied to investigate thermal stability and ionic conductivity of the electrolytes.
For the liquid electrolytes, high-temperature degradation reactions were studied. Gao and Lu (2019) investigated the influence of solvent type, LiPF6 salt concentration, and temperature on thermal stability of three electrolytes. It was found that DMC possessed the highest thermal stability, followed by EMC and DEC. In addition, the gas phase products of pyrolysis of these three electrolytes all contained CO2, H2, CH4, C2H4, C2H6, and CH3CH2F. These results are consistent with the experimental findings. The authors further studied the thermal degradation mechanism of the three electrolytes and found that during the decomposition process, ions tended to preferentially form PF5 and F− ions. The PF5 lewis acid promotes the subsequent degradation reactions of different electrolytes by attacking C-O bonds.
Solid Electrolyte Interface
During the first cycle of a lithium battery, the electrolyte usually reacts with the electrode to form a solid electrolyte interface (SEI) film. On one hand, the SEI film consumes active lithium and electrolyte, resulting in capacity degradation, increased battery internal resistance and reduced energy density. On the other hand, the film improves cycle stability and prevents further decomposition of electrolyte. The formation mechanisms of nanometer-thick SEI film has not been fully understood (Wang et al., 2018). Through the ReaxFF, composition of the SEI film and reaction mechanisms have been predicted.
The ReaxFF was shown to be able to simulate the formation process of the SEI film. In terms of a graphite anode, Kim et al. (2011) and Bertolini and Balbuena (2018) simulated the reaction process of Li and common electrolytes. Kim and coauthors found that the SEI film separated into an organic layer and an inorganic layer according to product composition. According to the Li distribution, Bertolini and Balbuena determined that the SEI film contained a dense phase and a porous phase. The latter was further found to be composed of a nest phase and a dispersed phase based on connectivity of Li. SEI film was also determined for cathodes. Reddivari et al. (2017) studied a reaction between LiMn2O4 and a mixed electrolyte. The authors found that the SEI film on the cathode was mainly composed of aldehydes, esters, and alcohol organic compounds, but there was no inorganic carbonate or organic lithium salt.
Regarding reaction mechanisms, Kim et al. (2011) proposed two decomposition mechanisms for reaction of DMC and EC on Li anode. Bedrov et al. (2012) revealed a competitive reaction mechanism for reaction of EC on Li anode. Most of the decomposition products are in good agreement with experimental results, while discrepancies possibly arose from deviation of Li concentration at the interface or electron injection speed.
Conclusions and Outlook
To summarize, based on the various works mentioned above, the ReaxFF has been proved to be a powerful tool for investigating lithium battery systems. The atomic-scale mechanisms revealed by the ReaxFF is expected to be useful for optimizing battery structures and also improving battery performance.
In the future, the ReaxFF simulation can be utilized for many other processes, such as reactions between Li and trace water in electrolyte, diffusion of polysulfide in electrolyte and dendrite growth at electrolyte-anode interface. ReaxFF can further discover the role of composite electrode materials and functional coatings in these processes. In particular, the ReaxFF simulation is readily extended to sodium-ion, potassium-ion and lithium-air battery systems, which are popular candidates for next-generation batteries.
Author Contributions
All authors collected and summarized literature and wrote the manuscript. JZ provided guidance and revised the manuscript.
Funding
The authors are grateful to the financial support from the Natural Science Foundation of China (51874204).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Bedrov, D., Smith, G. D., and van Duin, A. C. (2012). Reactions of singly-reduced ethylene carbonate in lithium battery electrolytes: a molecular dynamics simulation study using the ReaxFF. J. Phys. Chem. A 116, 2978–2985. doi: 10.1021/jp210345b
Beltran, S. P., and Balbuena, P. B. (2018). Formation of multilayer graphene domains with strong sulfur-carbon interaction and enhanced sulfur reduction zones for lithium-sulfur battery cathodes. Chemsuschem 11, 1970–1980. doi: 10.1002/cssc.201702446
Bertolini, S., and Balbuena, P. B. (2018). Buildup of the solid electrolyte interphase on lithium-metal anodes: reactive molecular dynamics study. J. Phys. Chem. C 122, 10783–10791. doi: 10.1021/acs.jpcc.8b03046
Cao, F. F., Guo, Y. G., Zheng, S. F., Wu, X. L., Jiang, L. Y., Bi, R. R., et al. (2010). Symbiotic coaxial nanocables: facile synthesis and an efficient and elegant morphological solution to the lithium storage problem. Chem. Mater. 22, 1908–1914. doi: 10.1021/cm9036742
Fan, F. F., Huang, S., Yang, H., Raju, M., Datta, D., Shenoy, V. B., et al. (2013). Mechanical properties of amorphous LixSi alloys: a reactive force field study. Model. Simul. Mat. Sci. Eng. 21:074002. doi: 10.1088/0965-0393/21/7/074002
Gao, T., and Lu, W. (2019). Mechanism and effect of thermal degradation on electrolyte ionic diffusivity in Li-ion batteries: a molecular dynamics study. Electrochim. Acta 323:134791. doi: 10.1016/j.electacta.2019.134791
Goodenough, J. B., and Kim, Y. (2010). Challenges for rechargeable Li batteries. Chem. Mater. 22, 587–603. doi: 10.1021/cm901452z
Huang, X., Yang, H., Liang, W. T., Raju, M., Terrones, M., Crespi, V. H., et al. (2013). Lithiation induced corrosive fracture in defective carbon nanotubes. Appl. Phys. Lett. 103:153901. doi: 10.1063/1.4824418
Islam, M. M., Ostadhossein, A., Borodin, O., Yeates, A. T., Tipton, W. W., Hennig, R. G., et al. (2015). ReaxFF molecular dynamics simulations on lithiated sulfur cathode materials. Phys. Chem. Chem. Phys. 17, 3383–3393. doi: 10.1039/C4CP04532G
Jiao, X., Yin, J., Xu, X., Wang, J., Liu, Y., Xiong, S., et al. (2020). Highly energy-dissipative, fast self-healing binder for stable Si anode in lithium-ion batteries. Adv. Funct. Mater. 2005699. doi: 10.1002/adfm.202005699. [Epub ahead of print].
Jung, H., Lee, M., Yeo, B. C., Lee, K. R., and Han, S. S. (2015). Atomistic observation of the lithiation and delithiation behaviors of silicon nanowires using reactive molecular dynamics simulations. J. Phys. Chem. C 119, 3447–3455. doi: 10.1021/jp5094756
Jung, H., Yeo, B. C., Lee, K. R., and Han, S. S. (2016). Atomistics of the lithiation of oxidized silicon (SiOx) nanowires in reactive molecular dynamics simulations. Phys. Chem. Chem. Phys. 18, 32078–32086. doi: 10.1039/C6CP06158C
Kim, S. P., Datta, D., and Shenoy, V. B. (2014). Atomistic mechanisms of phase boundary evolution during initial lithiation of crystalline silicon. J. Phys. Chem. C 118, 17247–17253. doi: 10.1021/jp502523t
Kim, S. P., van Duin, A. C. T., and Shenoy, V. B. (2011). Effect of electrolytes on the structure and evolution of the solid electrolyte interphase (SEI) in Li-ion batteries: a molecular dynamics study. J. Power Sources 196, 8590–8597. doi: 10.1016/j.jpowsour.2011.05.061
Kim, S. Y., Ostadhossein, A., van Duin, A. C., Xiao, X., Gao, H., and Qi, Y. (2016). Self-generated concentration and modulus gradient coating design to protect Si nano-wire electrodes during lithiation. Phys. Chem. Chem. Phys. 18, 3706–3715. doi: 10.1039/C5CP07219K
Li, L. J., Xia, L. F., Yang, H. P., Zhan, X. H., Chen, J., Chen, Z. Y., et al. (2020). Solid-state synthesis of lanthanum-based oxides Co-coated LiNi 0.5 Co 0.2 Mn 0.3 O 2 for advanced lithium ion batteries. J. Alloys Compd. 832:154959. doi: 10.1016/j.jallcom.2020.154959
Li, Y., Romero, N. A., and Lau, K. C. (2018). Structure-property of lithium-sulfur nanoparticles via molecular dynamics simulation. ACS Appl. Mater. Interfaces 10, 37575–37585. doi: 10.1021/acsami.8b09128
Liu, Z., Li, L., Chen, J., Yang, H., Xia, L., Chen, J., et al. (2020). Effects of chelating agents on electrochemical properties of Na0.9Ni0.45Mn0.55O2 cathode materials. J. Alloys Compd. 855:157485. doi: 10.1016/j.jallcom.2020.157485
Lu, L. G., Han, X. B., Li, J. Q., Hua, J. F., and Ouyang, M. G. (2013). A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 226, 272–288. doi: 10.1016/j.jpowsour.2012.10.060
Luo, Z., Zhou, Z., He, Z., Sun, Z., Zheng, J., and Li, Y. (2021). Enhanced electrochemical performance of Li1. 2Mn0. 54Ni0.13Co0.13O2 cathode by surface modification using La–Co–O compound. Ceram. Int. 47, 2656–2664. doi: 10.1016/j.ceramint.2020.09.114
Lv, Z. S., Li, W. L., Yang, L., Loh, X. J., and Chen, X. D. (2019). Custom-Made Electrochemical Energy Storage Devices. ACS Energy Lett. 4, 606–614. doi: 10.1021/acsenergylett.8b02408
Lv, Z. S., Tang, Y. X., Zhu, Z. Q., Wei, J. Q., Li, W. L., Xia, H. R., et al. (2018). Honeycomb-lantern-inspired 3D stretchable supercapacitors with enhanced specific areal capacitance. Adv. Mater. 30:1805468. doi: 10.1002/adma.201805468
Manthiram, A., Fu, Y. Z., and Su, Y. S. (2013). Challenges and prospects of lithium-sulfur batteries. Acc. Chem. Res. 46, 1125–1134. doi: 10.1021/ar300179v
Muñiz, J., Rincón, M. E., and Acevedo-Peña, P. (2016). The role of the oxide shell on the stability and energy storage properties of MWCNT@TiO2 nanohybrid materials used in Li-ion batteries. Theor. Chem. Acc. 135:181. doi: 10.1007/s00214-016-1940-7
Nishi, Y. (2001). Lithium ion secondary batteries; past 10 years and the future. J. Power Sources 100, 101–106. doi: 10.1016/S0378-7753(01)00887-4
Ostadhossein, A., Cubuk, E. D., Tritsaris, G. A., Kaxiras, E., Zhang, S., and van Duin, A. C. (2015). Stress effects on the initial lithiation of crystalline silicon nanowires: reactive molecular dynamics simulations using ReaxFF. Phys. Chem. Chem. Phys. 17, 3832–3840. doi: 10.1039/C4CP05198J
Ostadhossein, A., Kim, S. Y., Cubuk, E. D., Qi, Y., and van Duin, A. C. (2016). Atomic insight into the lithium storage and diffusion mechanism of SiO2/Al2O3 electrodes of lithium ion batteries: reaxff reactive force field modeling. J. Phys. Chem. A 120, 2114–2127. doi: 10.1021/acs.jpca.5b11908
Ponce, V., and Seminario, J. M. (2020). Lithiation of sulfur-graphene compounds using reactive force-field molecular dynamics simulations. J. Electrochem. Soc. 167:100555. doi: 10.1149/1945-7111/ab9ccf
Raju, M., Ganesh, P., Kent, P. R. C., and van Duin, A. C. T. (2015). Reactive force field study of Li/C systems for electrical energy storage. J. Chem. Theory Comput. 11, 2156–2166. doi: 10.1021/ct501027v
Reddivari, S., Lastoskie, C., Wu, R. F., and Zhang, J. L. (2017). Chemical composition and formation mechanisms in the cathode-electrolyte interface layer of lithium manganese oxide batteries from reactive force field (ReaxFF) based molecular dynamics. Front. Energy 11, 365–373. doi: 10.1007/s11708-017-0500-8
Reddy, M. V., Rao, G. V. S., and Chowdari, B. V. R. (2013). Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 113, 5364–5457. doi: 10.1021/cr3001884
Sui, Y., Zhou, J., Wang, X., Wu, L., Zhong, S., and Li, Y. (2020). Recent advances in black-phosphorus based materials for electrochemical energy storage. Mater. Today (in press). doi: 10.1016/j.mattod.2020.09.005. [Epub ahead of print].
van Duin, A. C. T., Dasgupta, S., Lorant, F., and Goddard, W. A. (2001). ReaxFF: a reactive force field for hydrocarbons. J. Phys. Chem. A 105, 9396–9409. doi: 10.1021/jp004368u
Verners, O., and Simone, A. (2019). Characterization of the structural response of a lithiated SiO2 / Si interface: a reactive molecular dynamics study. Mech. Mater. 136:103030. doi: 10.1016/j.mechmat.2019.04.001
Wang, A. P., Kadam, S., Li, H., Shi, S. Q., and Qi, Y. (2018). Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion batteries. Npj Comput. Mater. 4:15. doi: 10.1038/s41524-018-0064-0
Wang, M., Yu, J., and Lin, S. (2017). Lithiation-Assisted Strengthening Effect and Reactive Flow in Bulk and Nanoconfined Sulfur Cathodes of Lithium-Sulfur Batteries. J. Phys. Chem. C 121, 17029–17037. doi: 10.1021/acs.jpcc.7b05446
Wang, R., Dai, X., Qian, Z., Sun, Y., Fan, S., Xiong, K., et al. (2020a). In situ surface protection for enhancing stability and performance of LiNi0.5Mn0.3Co0.2O2 at 4.8 V: the working mechanisms. ACS Mater. Lett. 2, 280–290. doi: 10.1021/acsmaterialslett.9b00476
Wang, R., Sun, Y., Yang, K., Zheng, J., Li, Y., Qian, Z., et al. (2020b). One-time sintering process to modify xLi(2)MnO(3) (1-x)LiMO2 hollow architecture and studying their enhanced electrochemical performances. J. Energy Chem. 50, 271–279. doi: 10.1016/j.jechem.2020.03.042
Wang, R. H., Dai, X. Y., Qian, Z. F., Zhong, S. K., Chen, S., Fan, S. T., et al. (2020). Boosting lithium storage in free-standing black phosphorus anode via multifunction of nanocellulose. ACS Appl. Mater. Inter. 12, 31628–31636. doi: 10.1021/acsami.0c08346
Wu, L., Zheng, J., Wang, L., Xiong, X. H., Shao, Y. Y., Wang, G., et al. (2019). PPy-encapsulated SnS2 nanosheets stabilized by defects on a TiO2 support as a durable anode material for lithium-ion batteries. Angew. Chem. Int. Ed. 58, 811–815. doi: 10.1002/anie.201811784
Xu, K. (2004). Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4417. doi: 10.1021/cr030203g
Yang, H., Huang, X., Liang, W. T., van Duin, A. C. T., Raju, M., and Zhang, S. L. (2013). Self-weakening in lithiated graphene electrodes. Chem. Phys. Lett. 563, 58–62. doi: 10.1016/j.cplett.2013.01.048
Yang, Z. G., Choi, D., Kerisit, S., Rosso, K. M., Wang, D. H., Zhang, J., et al. (2009). Nanostructures and lithium electrochemical reactivity of lithium titanites and titanium oxides: a review. J. Power Sources 192, 588–598. doi: 10.1016/j.jpowsour.2009.02.038
Keywords: lithium battery, ReaxFF, molecular dynamics, simulation, SEI
Citation: Shi Z, Zhou J and Li R (2021) Application of Reaction Force Field Molecular Dynamics in Lithium Batteries. Front. Chem. 8:634379. doi: 10.3389/fchem.2020.634379
Received: 27 November 2020; Accepted: 11 December 2020;
Published: 13 January 2021.
Edited by:
Renheng Wang, Shenzhen University, ChinaReviewed by:
Zhisheng Lv, Nanyang Technological University, SingaporeChenyang Zhao, Shenzhen University, China
Zhenjiang He, Central South University, China
Copyright © 2021 Shi, Zhou and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Zhou, jzhou@suda.edu.cn
 Zhihao Shi
Zhihao Shi Jian Zhou
Jian Zhou Runjie Li
Runjie Li