Alkaline Phosphatases Account for Low Plasma Levels of Inorganic Pyrophosphate in Chronic Kidney Disease
- 1Faculty of Medicine, Côte d’Azur University, Nice, France
- 2UMR 7073, Laboratory of Physiology and Molecular Medicine (LP2M), Centre National de la Recherche Scientifique, Nice, France
- 3Nephrology Department, University Hospital, Nice, France
- 4Department of General Pediatrics, Muenster University Children’s Hospital, Muenster, Germany
- 5Department of Vascular Medicine and Surgery, University Hospital, Nice, France
Introduction: Patients on dialysis and kidney transplant recipients (KTR) present the syndrome of mineral and bone disorders (MBD), which share common traits with monogenic calcifying diseases related to disturbances of the purinergic system. Low plasma levels of inorganic pyrophosphate (PPi) and ectopic vascular calcifications belong to these two conditions. This suggests that the purinergic system may be altered in chronic kidney disease with MBD. Therefore, we perform a transversal pilot study in order to compare the determinants of PPi homeostasis and the plasma levels of PPi in patients on dialysis, in KTR and in healthy people.
Patients and Methods: We included 10 controls, 10 patients on maintenance dialysis, 10 early KTR 3 ± 1 months after transplantation and nine late KTR 24 ± 3 months after transplantation. We measured aortic calcifications, plasma and urine levels of PPi, the renal fractional excretion of PPi (FePPi), nucleoside triphosphate hydrolase (NPP) and ALP activities in plasma. Correlations and comparisons were assessed with non-parametric tests.
Results: Low PPi was found in patients on dialysis [1.11 (0.88–1.35), p = 0.004], in early KTR [0.91 (0.66–0.98), p = 0.0003] and in late KTR [1.16 (1.07–1.45), p = 0.02] compared to controls [1.66 (1.31–1.72) μmol/L]. Arterial calcifications were higher in patients on dialysis than in controls [9 (1–75) vs. 399 (25–526) calcium score/cm2, p < 0.05]. ALP activity was augmented in patients on dialysis [113 (74–160), p = 0.01] and in early KTR [120 (84–142), p = 0.002] compared to controls [64 (56–70) UI/L]. The activity of NPP and FePPi were not different between groups. ALP activity was negatively correlated with PPi (r = −0.49, p = 0.001).
Discussion: Patients on dialysis and KTR have low plasma levels of PPi, which are partly related to high ALP activity, but neither to low NPP activity, nor to increased renal excretion of PPi. Further work is necessary to explore comprehensively the purinergic system in chronic kidney disease.
Introduction
Patients with chronic kidney disease (CKD) and those receiving renal replacement therapy exhibit various traits of the syndrome of mineral and bone disorders (MBD) during their lifetime (Ketteler et al., 2017). This syndrome is characterized by ectopic calcifications located in the intimal or medial arterial layers (Vervloet and Cozzolino, 2017) increasing the risk of cardiovascular-related events and mortality (Chiu et al., 2010). It is also characterized by low plasma levels of calcium, high plasma levels of inorganic phosphates (Pi) and of parathyroid hormone (PTH), and by an increased alkaline phosphatase (ALP) plasma activity (Stevens et al., 2006). Bone turn-over ranges from high to low (Kurz et al., 1994) in relation to decreased bone mineral density observed in approximately 50% of patients, resulting in a higher risk of bone fracture (Miller et al., 2005). Any combination of the aforementioned abnormalities depends on the course and management of the renal disease (Miller et al., 2005; Stevens et al., 2006; Mazzaferro et al., 2009; Chiu et al., 2010; Wolf et al., 2016).
Here, we consider inorganic pyrophosphate (PPi) in patients with end stage CKD on renal replacement therapies as a new player in the syndrome of MBD. Indeed, PPi is one of the main circulating endogenous calcification inhibitors (Back et al., 2018). Micromoles of PPi appear sufficient to inhibit the deposition of hydroxyapatite crystal on collagen activating sites in the presence of millimoles of Ca and Pi (Fleisch et al., 1966). In patients on maintenance dialysis [PPi]pl is low for unknown reasons (Lomashvili et al., 2005; O’Neill et al., 2010). Since PPi is hydrolyzed by ALP, this might be due to high ALP, because a 30% decrease of [PPi]pl is observed at the end of the dialysis session in conjunction with significantly increased plasma ALP activity (Azpiazu et al., 2018) and because high plasma ALP activity belongs to the syndrome of MBD in end stage CKD (Kurz et al., 1994; Wallace et al., 1996; Wolf et al., 2016; Haarhaus et al., 2017; Ketteler et al., 2017; Bover et al., 2018). Inorganic pyrophosphate is a key compound in the purinergic system. Actually, PPi homeostasis has been shown to primarily depend on adenosine triphosphate (ATP) metabolism. Levels of extracellular PPi are partly regulated by the ATP-binding cassette transporter, subfamily C, member 6 (ABCC6) (Le Saux et al., 2000) and by ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) (Rutsch et al., 2003). The liver is the main source of ABCC6-mediated and ENPP1-mediated extracellular PPi (Jansen et al., 2014). Another source of PPi is the secretion from its intracellular pool into the extracellular fluid by inorganic pyrophosphate transport regulator (ANKH) (Netter et al., 2004; Mitton-Fitzgerald et al., 2016). The influence of the diet on plasma PPi levels is very limited (Dedinszki et al., 2017; Pomozi et al., 2017). Finally, circulating PPi is hydrolyzed by ALP and eliminated in urine (Letavernier et al., 2019).
A disruption in the balance between PPi production and degradation results in disturbed mineralization process and low [PPi]pl in human with disorders of the purinergic system (Fish et al., 2013) and very likely in CKD. Therefore, we explored the relationship between [PPi]pl and the main compounds of the purinergic system in patients with CKD and MBD.
Patients and Methods
Patient Recruitment
For this cross-sectional pilot study, we included patients with well-defined phenotypes and controls (Figure 1). We selected 10 controls with no renal impairment, i.e., eGFR > 60 ml/min/1.73 m2, no albuminuria, normal red and white urinary cell counts and normal renal ultrasonography. We selected patients on maintenance therapy for end-stage chronic kidney disease: 10 patients on maintenance hemodialysis (HD) and on a waiting list for KT; 10 KT recipients at 2 ± 1 months post-transplantation or early KTR (EKTR); nine KT recipients at 24 ± 3 months post-transplantation or late KTR (LKTR). All patients and controls gave informed consent for the study and underwent routine medical examination and fasting blood and urine analysis. Computer tomogram (CT) scans were collected when available but were not performed for the purpose of the study. The study was approved by the institutional Ethics Committee.
Biochemical and Hormonal Assays
PPi
Pyrophosphate was measured in plasma and urine using an enzyme assay. Patient blood samples were collected in the morning after an overnight fasting period (venous punction) directly in a tube containing citrate, theophyllin, adenosine and dypiridamole (CTAD). Plasmas were ultra-filtrated at 4°C using centrisart filtration units (300 KDa cut-off, Sartorius) according to Tolouian et al. (2012). Urine samples were collected in the morning concomitantly to the blood samples. PPi quantifications were performed using a modified method based on the enzyme assay described by Jansen et al. (2014).
Briefly, this method is based on the conversion of PPi into ATP with ATP sulfurylase (Perkin Elmer, Boston MA, United States) and ATP concentrations are measured using a luminescent ATP detection kit (ATPlite®, PerkinElmer). The values were corrected using the basal ATP levels measured in each sample. Dosage accuracy was 10% with 0.12 μmol/L absolute uncertainty.
Nucleoside Triphosphate Hydrolase (NPP) Activity
Enzyme activity was measured in heparinized plasma, buffered 1:2 in 0.2 M Tris, 1.6 mM MgCl2, pH 8.1 by a previously described colorimetric assay using the synthetic substrate p-nitrophenylthymidine 5’-monophosphate (PNTM) (Rutsch et al., 2003). Protein concentration was determined using PierceTM BCATM Protein-Assay (Thermo Fisher Scientific). NPP activity was defined as 1 μmol substrate hydrolyzed per μg protein per hour.
Serum creatinine was assessed with Jaffe’s kinetic methods (IDMS standardized), serum calcium was measured by absorption spectrophotometry, serum Pi was measured with UV methods on Cobas 8000 (RocheDiagnostics, Mannheim, Germany). 25-hydroxy vitamin D (25-OH vit D) was assessed by immunologic luminescence (Diasorin®, Fallugia, Italy). Plasma ALP activity was assessed by absorbance at 450 nm of paranitrophenol at alkaline pH. Bone ALP isoform was measured using an 125I-labeled sandwich radioimmunoassay (Immunotech, Beckman-Coulter Society, Marseille, France). Bio-intact parathyroid hormone (PTH) was measured by luminescence immunoassay (Advia Centaur Intact PTH, Siemens healthcare, Tarrytown, United States). Osteocalcin was assessed by immunoassay (ECLIA, RocheDiagnostics, Mannheim, Germany) and luminescence was determined with an automated device (Liaison XL, Diasorin®, Fallugia, Italy).
Measurement of Arterial Calcification
Arterial calcification was measured on CT scans. Data were reconstructed with Aquarius© software (Tera Recon, San Mateo, CA, United States) to obtain 3-mm-thick consecutive slices. Calcification was measured with the open source Horos Software 2.0 between the right renal artery and the distal abdominal aorta according to the method described by Agatston et al. (1990). Arterial surface was computed from the length (L) and the mean value of three diameters (D) according to the following formula: L × D ×π (in cm2). Results were expressed as calcium score divided by the surface of this segment of the abdominal aorta (calcium score/cm2).
Calculations
GFR was estimated (eGFR) using the CKD-EPI formula according to the plasma creatinine level. Body mass index was calculated as weight in kg divided by height in meters squared (kg/m2). Fractional excretion of PPi (FePPi) was calculated as a percentage according to the following formula: [PPi]u × [creat]pl/[PPi]pl × [creat]u × 100.
Statistics
Data are presented as medians with interquartile ranges [25th–75th percentiles]. All statistics were conducted with GraphPad Prism 6® software. Data were compared using non-parametric tests: Kruskal Wallis test, followed by a Mann Whitney test in case of a statistically significant difference. Correlations were evaluated with Spearman’s test. A p < 0.05 was considered statistically significant.
Results
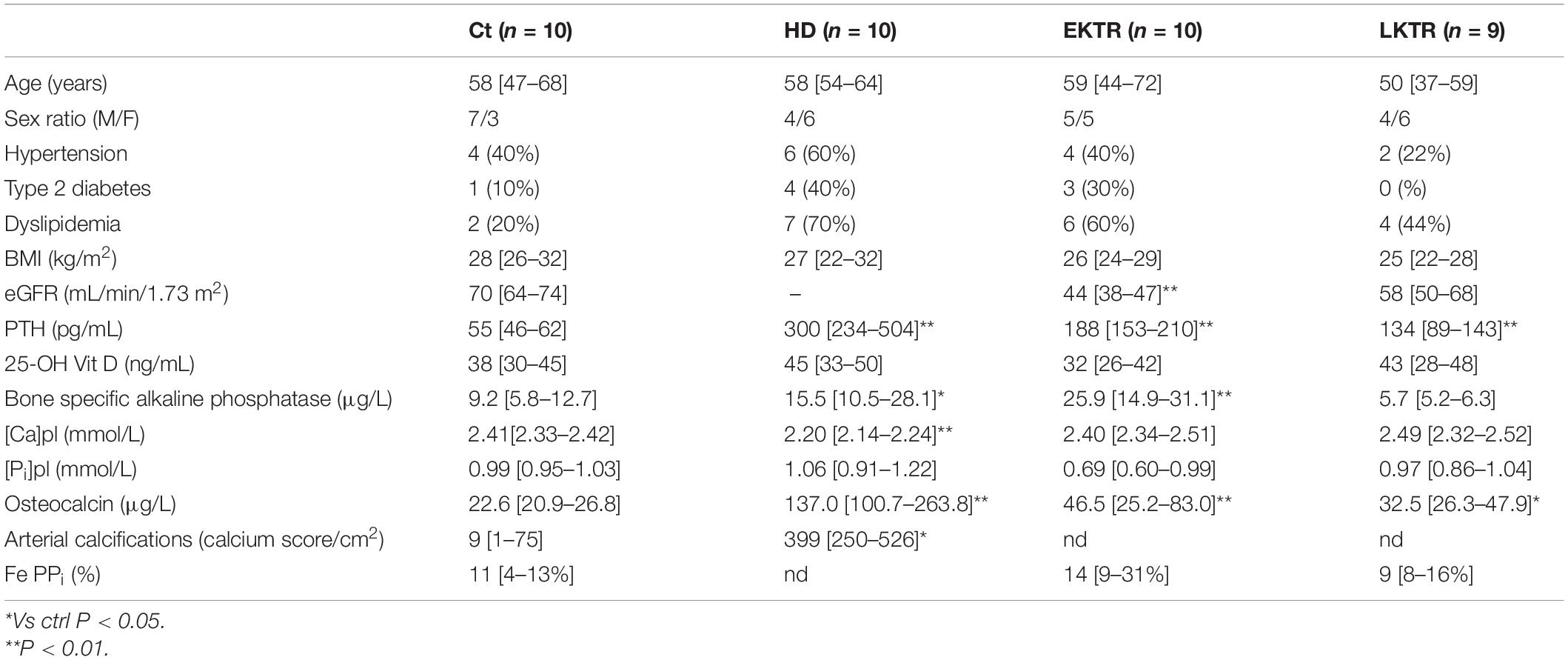
Overall, 39 patients were included in our study (Table 1). Age range and BMI were comparable in all groups. Hypertension was present in 40% of controls, 60% of HD, 40% of EKTR and 22% of LKTR. Type 2 diabetes (DT2) was present in 10% of controls, 30% of HD, 30% of EKTR whereas LKTR did not have DT2. Dyslipidemia was present in 30% of controls, 70% of HD, 60% of EKTR and 44% of LKTR. The syndrome of MBD was reflected by the degree of arterial calcification, which was significantly higher in HD than in controls; by significantly higher levels of parathyroid hormone (PTH) and osteocalcin in HD and KTR than in controls; by significantly higher bone ALP isoforms (Table 1) and higher ALP activity (Figure 2A) in HD or EKTR than in controls. Of note, two patients had very high ALP activity due to their poor compliance with treatment. All HD but one received phosphate binders and eight took 1-alpha 25-OH Vit D while most patients received cholecalciferol. Plasma levels of PPi were significantly lower in all patient groups than in controls (Figure 2B). In contrast, renal elimination of PPi was similar in all groups according to the FePPi (Table 1). Plasma NPP activity was the same in all groups (Figure 2C). Plasma ALP activity was strongly and negatively correlated to [PPi]pl (Figure 3A) and [PPi]pl was positively correlated to [Pi]pl (Figure 3B). In contrast, there was no significant correlation between plasma ALP activity and [Pi]pl (Figure 3C) nor between plasma ALP activity and CRP levels (r = 0.28, p = 0.09), nor between bone ALP isoforms and CRP levels (r = 0.18; p = 0.27).
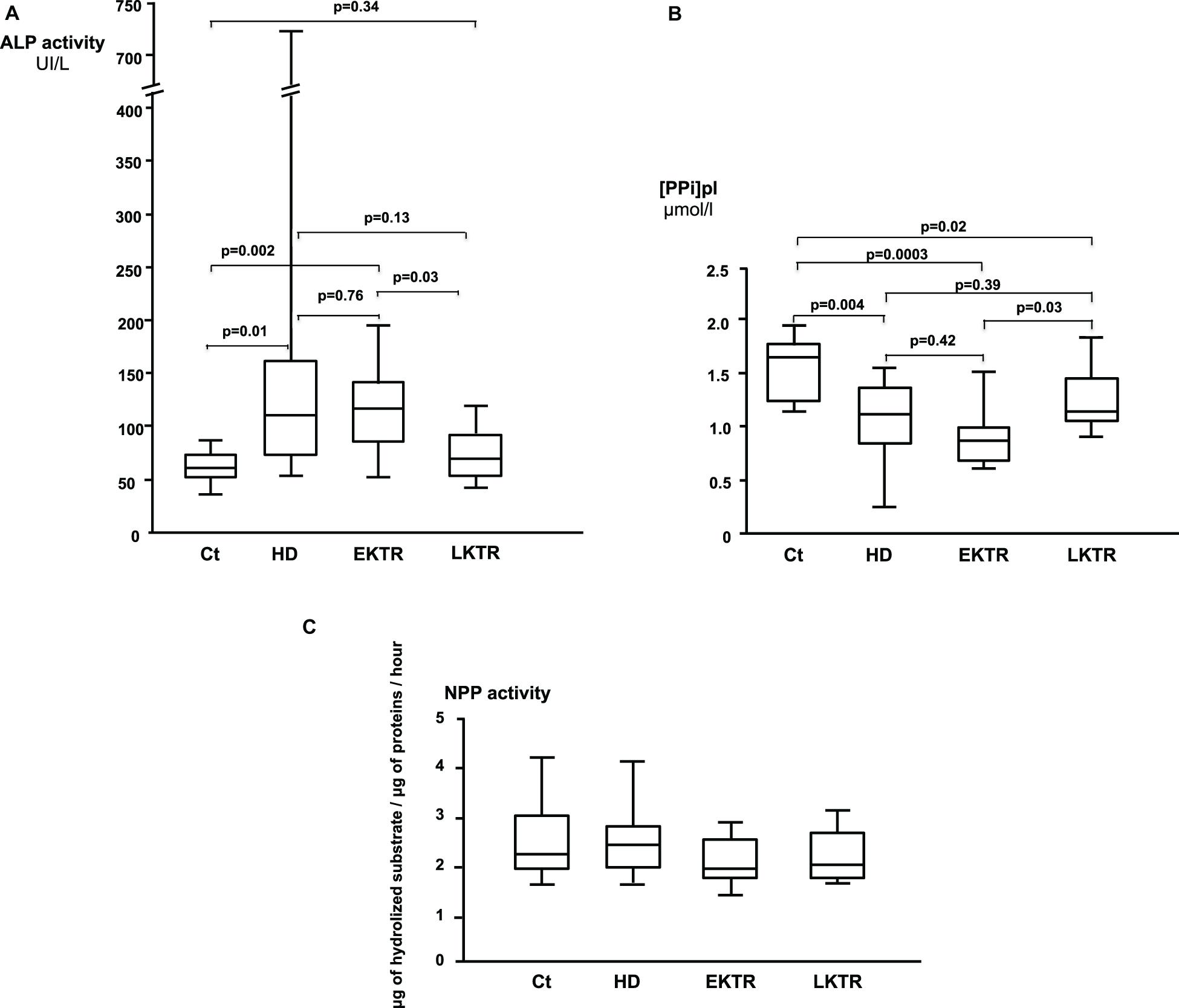
Figure 2. (A) Plasma ALP activity (Median, IQR, and highest and lowest values). (B) Plasma levels of PPi (Median, IQR, and highest and lowest values). (C) Plasma activities of NPP (Median, IQR, and highest and lowest values). There were no statistically significant differences.
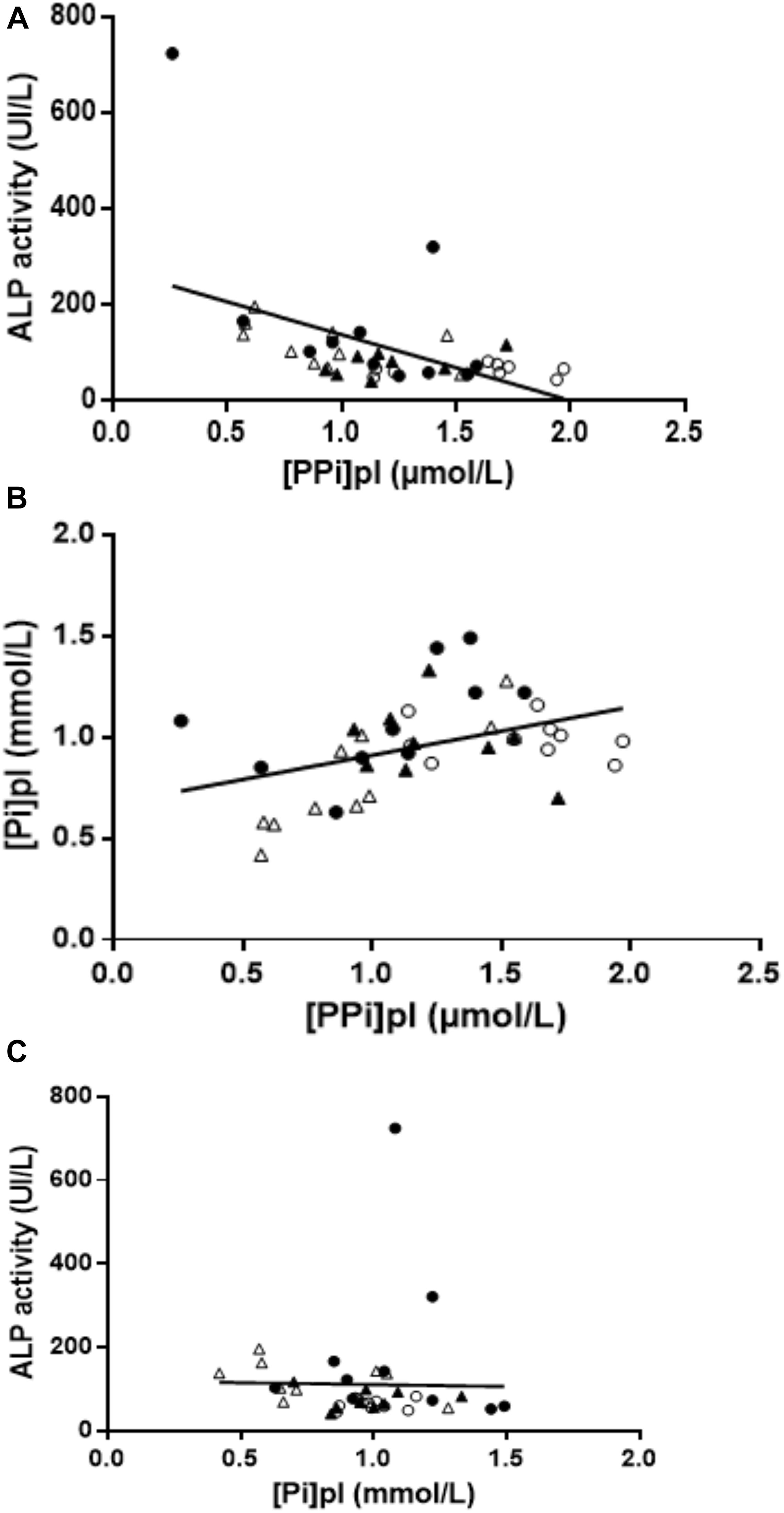
Figure 3. (A) Relationship between plasma ALP activity and [PPi]pl. Negative correlation between plasma ALP activity and [PPi]pl (r = −0.49, p = 0.001). Empty circles = controls; dark circles = HD; empty triangle = EKTR; dark triangle = LKTR. (B) Relationship between PPi and Pi. Positive correlation between [PPi]pl and [Pi]pl (r = 0.43, p = 0.006). Empty circles = controls; dark circles = HD; empty triangle = EKTR; dark triangle = LKTR. (C) Relationship between plasma ALP activity and [Pi]pl. There was no correlation between [Pi]pl and ALP activity (r = −0.23, p = 0.16). Empty circles = controls; dark circles = HD; empty triangle = EKTR; dark triangle = LKTR.
All incident or prevalent patients were asked to take part to the study unless they had an acute co-existing pathology, were below 18 years old or were incapable of giving informed consent. The inclusion of 10 patients in each group was anticipated and the patients could withdraw their consent anytime.
Patients without kidney disease after comprehensive examination were called controls. Early kidney transplant recipients (EKTR) were included at 2 ± 1 months post-transplantation. Late kidney transplant recipients (LKTR) were included at 24 ± 3 months post-transplantation. Routine medical examination and fasting blood and urine analysis were performed on a dedicated consultation for the purpose of the study.
Discussion
In the present study, we observed that [PPi]pl levels were negatively correlated to plasma ALP activity and that [PPi]pl was low in HD and remained low in KTR despite normalization of ALP activity, indicating that several mechanisms were controlling PPi homeostasis in addition to ALP.
High plasma ALP activity was a major determinant for low [PPi]pl in our study (Figure 2A). This was expected for the following reasons: 1/the elimination of PPi depends on its hydrolysis by tissue non-specific alkaline phosphatase (TNAP), encoded by the ALPL gene (Favre et al., 2017; Whyte, 2017), 2/ALP activity increases at the end of a dialysis session as compared to its baseline level and this results in 30% decrease of [PPi]pl (Azpiazu et al., 2018), 3/pharmacological blockade of ALP activity restored [PPi]pl in uremic mice (Tani et al., 2020). Plasma ALP activity and plasma levels of bone ALP isoforms followed the same pattern in our patients, in accordance with the overproduction of TNAP in CKD (Bover et al., 2018; Nizet et al., 2020). In contrast to our findings, Lomashvili et al. observed no correlation between plasma ALP activity and [PPi]pl among 38 patients on maintenance dialysis (Lomashvili et al., 2005). This discrepancy might result from the broader range of plasma ALP activity in our cohort, including values both within and above normal limits. Since we found no statistically significant relationship between ALP activity and CRP, in contrast to published reports (Haarhaus et al., 2017), the degree of inflammation did not appear to influence ALP activity nor bone ALP isoforms in our patients. Here, high ALP activity belonged to the syndrome of MBD because high plasma ALP activity matched elevated bone ALP isoforms, was associated with high levels of osteocalcin and PTH (Table 1), as already described (Kurz et al., 1994; Wolf et al., 2016), and with the presence of arterial calcifications, in line with the literature (London et al., 2005).
We observed a positive correlation between [PPi]pl and [Pi]pl (Figure 2C), which is consistent with other reports from three independent cohorts of patients on maintenance dialysis (Lomashvili et al., 2005; O’Neill et al., 2010; Villa-Bellosta et al., 2016). This led to the hypothesis that [Pi]pl could exert a negative feedback on ALP activity according to data observed ex vivo (Fernley and Walker, 1967; Coburn et al., 1998). However, our data were not in favor of such a negative feedback of [Pi]pl on ALP activity, as the latter was not correlated to [Pi]pl (Figure 2B). Consequently, we suggest that this balance may depend on undetermined cellular processes.
We observed that KT did not result in normal levels of [PPi]pl 2 years after renal graft, despite recovery of a normal glomerular filtration rate and restoration of ALP activity to normal levels. Indeed, shortly after KT (2 ± 1 months), the low [PPi]pl was in line with enhanced hydrolysis of PPi due to raised plasma ALP activity. However, 2 years after KT, plasma ALP activity was normal and could not explain low [PPi]pl levels. In light of the knowledge derived from studies of generalized arterial calcification of infancy (GACI), a rare disease characterized by low [PPi]pl due to an inactivating mutation of ENPP1 (Rutsch et al., 2003), we measured NPP plasma activity which was within normal values (Figure 1). Since PPi is excreted in urine, we measured its renal elimination as reflected by FePPi and found no increased PPi excretion (Table 1). According to the mechanisms involved in pseudoxanthoma elasticum, due to mutations in ABCC6 accounting for low [PPi]pl (Le Saux et al., 2000) and vascular calcification (Leftheriotis et al., 2013), it could be that the production of PPi related to ABCC6 was decreased in our patients. Indeed, experimental data from uremic rats showed that low [PPi]pl resulted from low ABCC6 protein expression in the kidneys and in the liver (Lau et al., 2014). Furthermore, ANKH controls [PPi]pl levels in uremic rats (Zhao et al., 2012; Chen et al., 2019). However, in humans, mutations of ANKH account for craniometaphyseal dysplasia and, to the best of our knowledge, [PPi]pl levels was not yet measured in this condition (Chen et al., 2011).
This study is the first one, to the best of our knowledge, exploring the crosstalk between the purinergic system and mineral and bone disorder in CKD. The low [PPi]pl in KTR and the inverse relationship between ALP activity and [PPi]pl are new observations. However, this study is limited by the low number of patients and by the lack of association between biological and clinical data. Larger studies are needed to confirm our observations.
Conclusion
In conclusion, low [PPi]pl is partly related to its high level of hydrolysis due to elevated plasma ALP activity in patients with MBD induced by CKD. This is not the only mechanism because [PPi]pl remains low although plasma ALP activity is restored to normal levels after kidney transplantation. In addition to many experimental data, our pilot clinical study suggests that the purinergic system should be comprehensively explored in CKD in order to shed light on the complex pathogenesis of MBD (Back et al., 2018).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The studies involving human participants were reviewed and approved by Comite de Protection des Personnes Sud Mediterranee III CHU de Nimes. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
AL selected the patients, performed the clinical and biological investigations, determined the plasma levels of PPi. IR and CD set up the dosage’s method for PPi, performed the dosages and provided their scientific expertises to the study. FR and YN performed the assays for NPP1 activity and provided their scientific expertises to the study. ER measured the arterial calcifications. SV referred the patients on maintenance dialysis and provided his medical expertise to the study. AS referred the transplanted patients and provided his medical expertise to the study. GL provided the scientific background for the design of the study and validated the measurements of arterial calcifications. GF delineated the protocol of the study and performed the clinical and biological investigations. All authors significantly contribute to the redaction of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with several of the authors FR, YN, and GL.
Funding
This work was sponsored by the “Centre Hospitalier Universitaire de Nice” (Nice University Hospital) for regulatory and ethics approval. This work was supported by a grant from the Delegation of Clinical Research and the Innovation of University Hospital of Nice “2018.” The number of the trial is 2018-A00140-55 (clinicaltrial.gov).
Acknowledgments
We are indebted to the probands and to the patients for their kind participation and to Céline Fernandez, Nadia Ben Hassen-Dakhlaoui, and Kevin Zorzi for their skilled monitoring of the study. We thank Drs. Tamas Aranyi and Flora Szeri for their contributions to the preliminary discussions regarding this work.
References
Agatston, A. S., Janowitz, W. R., Hildner, F. J., Zusmer, N. R., and Viamonte, M. Jr., et al. (1990). Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 15, 827–832. doi: 10.1016/0735-1097(90)90282-t
Azpiazu, D., Gonzalez-Parra, E., Egido, J., and Villa-Bellosta, R. (2018). Hydrolysis of extracellular pyrophosphate increases in post-hemodialysis plasma. Sci. Rep. 8:11089. doi: 10.1038/s41598-018-29432-29434
Back, M., Aranyi, T., Cancela, M. L., Carracedo, M., Conceicao, N., Leftheriotis, G., et al. (2018). Endogenous calcification inhibitors in the prevention of vascular calcification: a consensus statement from the cost action EuroSoftCalcNet. Front. Cardiovasc. Med. 5:196. doi: 10.3389/fcvm.2018.00196
Bover, J., Urena, P., Aguilar, A., Mazzaferro, S., Benito, S., Lopez-Baez, V., et al. (2018). Alkaline phosphatases in the complex chronic kidney disease-mineral and bone disorders. Calcif. Tissue Int. 103, 111–124. doi: 10.1007/s00223-018-0399-z
Chen, B., Zhao, Y., Han, D., Zhao, B., Mao, Y., Cui, Z. K., et al. (2019). Wnt1 inhibits vascular smooth muscle cell calcification by promoting ANKH expression. J. Mol. Cell Cardio. 135, 10–21. doi: 10.1016/j.yjmcc.2019.07.008
Chen, I. P., Wang, L., Jiang, X., Aguila, H. L., and Reichenberger, E. J. (2011). A Phe377del mutation in ANK leads to impaired osteoblastogenesis and osteoclastogenesis in a mouse model for craniometaphyseal dysplasia (CMD). Hum. Mol. Genet. 20, 948–961. doi: 10.1093/hmg/ddq541
Chiu, Y. W., Adler, S. G., Budoff, M. J., Takasu, J., Ashai, J., and Mehrotra, R. (2010). Coronary artery calcification and mortality in diabetic patients with proteinuria. Kidney Int. 77, 1107–1114. doi: 10.1038/ki.2010.70
Coburn, S. P., Mahuren, J. D., Jain, M., Zubovic, Y., and Wortsman, J. (1998). Alkaline phosphatase (EC 3.1.3.1) in serum is inhibited by physiological concentrations of inorganic phosphate. J. Clin. Endocrinol. Metab. 83, 3951–3957. doi: 10.1210/jcem.83.11.5288
Dedinszki, D., Szeri, F., Kozak, E., Pomozi, V., Tokesi, N., Mezei, T. R., et al. (2017). Oral administration of pyrophosphate inhibits connective tissue calcification. EMBO Mol. Med. 9, 1463–1470. doi: 10.15252/emmm.201707532
Favre, G., Laurain, A., Aranyi, T., Szeri, F., Fulop, K., Le Saux, O., et al. (2017). The ABCC6 transporter: a new player in biomineralization. Int. J. Mol. Sci. 18:1941. doi: 10.3390/ijms18091941
Fernley, H. N., and Walker, P. G. (1967). Studies on alkaline phosphatase. Inhibition by phosphate derivatives and the substrate specificity. Biochem. J. 104, 1011–1018. doi: 10.1042/bj1041011
Fish, R. S., Klootwijk, E., Tam, F. W., Kleta, R., Wheeler, D. C., Unwin, R. J., et al. (2013). ATP and arterial calcification. Eur. J. Clin. Invest. 43, 405–412. doi: 10.1111/eci.12055
Fleisch, H., Russell, R. G., and Straumann, F. (1966). Effect of pyrophosphate on hydroxyapatite and its implications in calcium homeostasis. Nature 212, 901–903. doi: 10.1038/212901a0
Haarhaus, M., Brandenburg, V., Kalantar-Zadeh, K., Stenvinkel, P., and Magnusson, P. (2017). Alkaline phosphatase: a novel treatment target for cardiovascular disease in CKD. Nat. Rev. Nephrol. 13, 429–442. doi: 10.1038/nrneph.2017.60
Jansen, R. S., Duijst, S., Mahakena, S., Sommer, D., Szeri, F., Varadi, A., et al. (2014). ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arterioscler Thromb. Vasc. Biol. 34, 1985–1989. doi: 10.1161/ATVBAHA.114.304017
Ketteler, M., Block, G. A., Evenepoel, P., Fukagawa, M., Herzog, C. A., McCann, L., et al. (2017). Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) guideline update: what’s changed and why it matters. Kidney Int. 92, 26–36. doi: 10.1016/j.kint.2017.04.006
Kurz, P., Monier-Faugere, M. C., Bognar, B., Werner, E., Roth, P., Vlachojannis, J., et al. (1994). Evidence for abnormal calcium homeostasis in patients with adynamic bone disease. Kidney Int. 46, 855–861. doi: 10.1038/ki.1994.342
Lau, W. L., Liu, S., and Vaziri, N. D. (2014). Chronic kidney disease results in deficiency of ABCC6, the novel inhibitor of vascular calcification. Am. J. Nephrol. 40, 51–55. doi: 10.1159/000365014
Le Saux, O., Urban, Z., Tschuch, C., Csiszar, K., Bacchelli, B., Quaglino, D., et al. (2000). Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat. Genet. 25, 223–227. doi: 10.1038/76102
Leftheriotis, G., Omarjee, L., Le Saux, O., Henrion, D., Abraham, P., Prunier, F., et al. (2013). The vascular phenotype in Pseudoxanthoma elasticum and related disorders: contribution of a genetic disease to the understanding of vascular calcification. Front. Genet. 4:4. doi: 10.3389/fgene.2013.00004
Letavernier, E., Bouderlique, E., Zaworski, J., Martin, L., and Daudon, M. (2019). Pseudoxanthoma elasticum, kidney stones and pyrophosphate: from a rare disease to urolithiasis and vascular calcifications. Int. J. Mol. Sci. 20:6353. doi: 10.3390/ijms20246353
Lomashvili, K. A., Khawandi, W., and O’Neill, W. C. (2005). Reduced plasma pyrophosphate levels in hemodialysis patients. J. Am. Soc. Nephrol. 16, 2495–2500. doi: 10.1681/ASN.2004080694
London, G. M., Marchais, S. J., Guerin, A. P., and Metivier, F. (2005). Arteriosclerosis, vascular calcifications and cardiovascular disease in uremia. Curr. Opin. Nephrol. Hypertens 14, 525–531. doi: 10.1097/01.mnh.0000168336.67499.c0
Mazzaferro, S., Pasquali, M., Taggi, F., Baldinelli, M., Conte, C., Muci, M. L., et al. (2009). Progression of coronary artery calcification in renal transplantation and the role of secondary hyperparathyroidism and inflammation. Clin. J. Am. Soc. Nephrol. 4, 685–690. doi: 10.2215/CJN.03930808
Miller, P. D., Roux, C., Boonen, S., Barton, I. P., Dunlap, L. E., and Burgio, D. E. (2005). Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the cockcroft and gault method: a pooled analysis of nine clinical trials. J. Bone Miner. Res. 20, 2105–2115. doi: 10.1359/JBMR.050817
Mitton-Fitzgerald, E., Gohr, C. M., Bettendorf, B., and Rosenthal, A. K. (2016). The role of ANK in calcium pyrophosphate deposition disease. Curr. Rheumatol. Rep. 18:25. doi: 10.1007/s11926-016-0574-z
Netter, P., Bardin, T., Bianchi, A., Richette, P., and Loeuille, D. (2004). The ANKH gene and familial calcium pyrophosphate dihydrate deposition disease. Joint Bone Spine 71, 365–368. doi: 10.1016/j.jbspin.2004.01.011
Nizet, A., Cavalier, E., Stenvinkel, P., Haarhaus, M., and Magnusson, P. (2020). Bone alkaline phosphatase: an important biomarker in chronic kidney disease - mineral and bone disorder. Clin. Chim. Acta 501, 198–206. doi: 10.1016/j.cca.2019.11.012
O’Neill, W. C., Sigrist, M. K., and McIntyre, C. W. (2010). Plasma pyrophosphate and vascular calcification in chronic kidney disease. Nephrol. Dial Transplant 25, 187–191. doi: 10.1093/ndt/gfp362
Pomozi, V., Brampton, C., van de Wetering, K., Zoll, J., Calio, B., Pham, K., et al. (2017). Pyrophosphate supplementation prevents chronic and acute calcification in ABCC6-Deficient mice. Am. J. Pathol. 187, 1258–1272. doi: 10.1016/j.ajpath.2017.02.009
Rutsch, F., Ruf, N., Vaingankar, S., Toliat, M. R., Suk, A., Hohne, W., et al. (2003). Mutations in ENPP1 are associated with ‘idiopathic’ infantile arterial calcification. Nat. Genet. 34, 379–381. doi: 10.1038/ng1221
Stevens, L. A., Coresh, J., Greene, T., and Levey, A. S. (2006). Assessing kidney function–measured and estimated glomerular filtration rate. N. Engl. J. Med. 354, 2473–2483. doi: 10.1056/NEJMra054415
Tani, T., Fujiwara, M., Orimo, H., Shimizu, A., Narisawa, S., Pinkerton, A. B., et al. (2020). Inhibition of tissue-nonspecific alkaline phosphatase protects against medial arterial calcification and improves survival probability in the CKD-MBD mouse model. J. Pathol. 250, 30–41. doi: 10.1002/path.5346
Tolouian, R., Connery, S. M., O’Neill, W. C., and Gupta, A. (2012). Using a filtration technique to isolate platelet free plasma for assaying pyrophosphate. Clin. Lab. 58, 1129–1134.
Vervloet, M., and Cozzolino, M. (2017). Vascular calcification in chronic kidney disease: different bricks in the wall? Kidney Int. 91, 808–817. doi: 10.1016/j.kint.2016.09.024
Villa-Bellosta, R., Gonzalez-Parra, E., and Egido, J. (2016). Alkalosis and dialytic clearance of phosphate increases phosphatase activity: a hidden consequence of hemodialysis. PLoS One 11:e0159858. doi: 10.1371/journal.pone.0159858
Wallace, B. H., Lott, J. A., Griffiths, J., and Kirkpatrick, R. B. (1996). Isoforms of alkaline phosphatase determined by isoelectric focusing in patients with chronic liver disorders. Eur. J. Clin. Chem. Clin. Biochem. 34, 711–720.
Whyte, M. P. (2017). Hypophosphatasia: an overview for 2017. Bone 102, 15–25. doi: 10.1016/j.bone.2017.02.011
Wolf, M., Weir, M. R., Kopyt, N., Mannon, R. B., Von Visger, J., Deng, H., et al. (2016). A prospective cohort study of mineral metabolism after kidney transplantation. Transplantation 100, 184–193. doi: 10.1097/TP.0000000000000823
Keywords: alkaline phosphatase activity, hemodialysis, kidney transplant, pyrophosphate, purinergic mechanisms, mineral and bone disorder (CKD-MBD)
Citation: Laurain A, Rubera I, Duranton C, Rutsch F, Nitschke Y, Ray E, Vido S, Sicard A, Lefthériotis G and Favre G (2020) Alkaline Phosphatases Account for Low Plasma Levels of Inorganic Pyrophosphate in Chronic Kidney Disease. Front. Cell Dev. Biol. 8:586831. doi: 10.3389/fcell.2020.586831
Received: 24 July 2020; Accepted: 16 November 2020;
Published: 03 December 2020.
Edited by:
Herve Kempf, UMR 7365 Ingénierie Moléculaire et Physiopathologie Articulaire (IMOPA), FranceReviewed by:
David Magne, Université de Lyon, FranceSaid Kamel, INSERM U1088 Mécanismes Physiopathologiques et Conséquences des Calcifications Cardiovasculaires: Rôle des Remodelages Cardiovasculaires et Osseux, France
Copyright © 2020 Laurain, Rubera, Duranton, Rutsch, Nitschke, Ray, Vido, Sicard, Lefthériotis and Favre. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guillaume Favre, favre.g@chu-nice.fr
 Audrey Laurain
Audrey Laurain Isabelle Rubera2
Isabelle Rubera2  Yvonne Nitschke
Yvonne Nitschke Guillaume Favre
Guillaume Favre