A Myo-Inositol-Inducible Expression System for Corynebacterium glutamicum and Its Application
- College of Biotechnology, Tianjin University of Science and Technology, Tianjin, China
Corynebacterium glutamicum is one of the important industrial microorganisms for production of amino acids and other value-added compounds. Most expression vectors used in C. glutamicum are based on inducible promoter (Ptac or Ptrc) activated by isopropyl-β-D-thiogalactopyranoside (IPTG). However, these vectors seem unsuitable for large-scale industrial production due to the high cost and toxicity of IPTG. Myo-inositol is an ideal inducer because of its non-toxicity and lower price. In this study, a myo-inositol-inducible expression vector pMI-4, derived from the expression vector pXMJ19, was constructed. Besides the original chloramphenicol resistance gene cat, multiple cloning sites, and rrnB terminator, the pMI-4 (6,643 bp) contains the iolRq cassette and the myo-inositol-inducible promoter PiolT1. The pMI-4 could stably replicate in the C. glutamicum host. Meanwhile, the non-myo-inositol degradation host strain C. glutamicumΔiolGΔoxiCΔoxiDΔoxiE for maintaining the pMI-4 was developed. Overexpression of hemAM and hemL using pMI-4 resulted in a significant accumulation of 5-aminolevulinic acid, indicating its potential application in metabolic engineering and industrial fermentation.
Introduction
As a non-pathogenic Gram-positive bacterium, Corynebacterium glutamicum has been widely used in industrial biotechnology for the production of several million tons of amino acids annually, especially L-glutamate, L-lysine, and L-valine (Hasegawa et al., 2012; Eggeling and Bott, 2015; Wendisch, 2020). Besides amino acids, the current product spectrum that is accessible with C. glutamicum comprises organic acids, diamines, vitamins, aromates, and alcohols (Chen et al., 2016; Chung et al., 2017; Becker et al., 2018; Kogure and Inui, 2018; Sato et al., 2020). Benefiting from the genome annotation of C. glutamicum and the availability of molecular biology techniques and tools, significant progress has been made in designing production strains by metabolic engineering via rational approaches (Ikeda and Nakagawa, 2003; Nesvera and Patek, 2011; Suzuki and Inui, 2013; Wang et al., 2021).
Overexpression of genes encoding the rate-limiting enzymes involved in pathways is one of the most efficient strategies (Nesvera and Patek, 2011; Suzuki and Inui, 2013). Several vectors for gene overexpression in C. glutamicum have been developed, such as pXMJ19, pEC-XK-99E, and pDXW-8. Most of these expression vectors are based on inducible promoters (Ptac or Ptrc) regulated by isopropyl-β-D-thiogalactopyranoside (IPTG) (Jakoby et al., 1999; Kirchner and Tauch, 2003; Xu et al., 2010; Li et al., 2020). However, IPTG is toxic to cell growth and expensive, which makes it unsuitable for industrial production (Li et al., 2020).
Myo-inositol, a water-soluble vitamin B group compound, is found to induce transcription of several genes involved in its transport and catabolism (Figure 1), such as iolT1 (encoding myo-inositol transporter), iolG (encoding myo-inositol dehydrogenase), and iolH (encoding myo-inositol isomerases/epimerase) (Krings et al., 2006; You et al., 2020). Furthermore, this induction works regardless of whether the glucose is present or not (Krings et al., 2006; Klaffl et al., 2013). Besides, compared with IPTG, myo-inositol is much cheaper and non-toxic to cells (Krings et al., 2006; Takeno et al., 2016). Above all, myo-inositol seems an alternative inducer for gene expression in C. glutamicum.
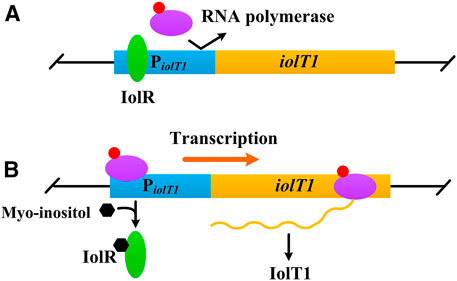
FIGURE 1. Negative control of iolT1 transcription. (A) No myo-inositol. The repressor IolR binds to the promoter of iolT1 (PiolT1) and blocks RNA polymerase from transcribing the iolT1. (B) With presence of myo-inositol. Myo-inositol as an inducer binds to the repressor IolR, resulting in its dissociation and allowing RNA polymerase to transcribe the iolT1.
In this study, a myo-inositol-inducible expression vector pMI-4 for C. glutamicum was constructed. Meanwhile, the chassis strain that is suitable for maintaining pMI-4 and unable to consume myo-inositol, C. glutamicumΔiolGΔoxiCΔoxiDΔoxiE, was developed. This constructed expression system was successfully applied to express hemAM (encoding glutamyl-tRNA reductase) and hemL (encoding glutamate-1-semialdehyde aminotransferase) for production of 5-aminolevulinic acid (5-ALA).
Materials and Methods
Bacterial Strains, Media, and Cultivation Conditions
The strains and plasmids used are listed in Table 1. Escherichia coli, used for recombinant DNA experiments, was cultured in lysogeny broth medium at 37°C. C. glutamicum ATCC 13032 and its recombinants were cultured at 30°C in Brain Heart Infusion (BHI) medium or CGXII medium. Modified CGXII medium [50 g/l glucose, 15 g/L (NH4)2SO4, 2 g/l yeast extract, 4 g/l tryptone, 1 g/l KH2PO4, 0.25 g/l MgSO4·7H2O, 42 g/l 3-morpholinopropanesulfonic acid, 10 mg/l CaCl2, 0.1 mg/l FeSO4·7H2O, 0.1 mg/l MnSO4·7H2O, 1 mg/l ZnSO4·7H2O, 0.2 mg/l CuSO4, 0.02 mg/l NiCl2·6H2O, and 0.2 mg/l biotin] was used for production of 5-ALA. When necessary, media were supplemented with 20 μg/ml chloramphenicol or 40 μg/ml kanamycin.
To investigate the effect of myo-inositol on cell growth, C. glutamicum ATCC 13032 cells were cultivated in CGXII containing 0.1–100 mM of myo-inositol, and the optical density at 600 nm (OD600) was determined after cultivation of 48 h at 30°C with shaking at 200 rpm. To investigate the effect of myo-inositol on glucose utilization, C. glutamicumΔiolGΔoxiCΔoxiDΔoxiE cells were cultivated in CGXII medium containing 5 mM of myo-inositol or not at 30°C with shaking at 200 rpm. The final concentration of glucose was determined after cultivation of 48 h.
Construction of Strains and Plasmids
All the primers used in this study are listed in Supplementary Table S1. E. coli DH5α was used for vector construction experiments. C. glutamicum ATCC 13032 was used as a base strain for investigating the characteristics of plasmids and for construction of the non-myo-inositol-degrading host. Preparation of competent cells and transformation were performed according to the standard protocols (Sambrook and Russell, 2001; Eggeling and Bott, 2005). Strains and plasmids were constructed as follows.
The plasmid pXMJ19 was used as the backbone. The fragments amplified from pXMJ19 with primers PX-1 and PX-2 were digested by Hpa I and were subsequently self-ligated to obtain pMI-1. PiolT1 was amplified with primers PX-3 and PX-4 from genomic DNA of C. glutamicum ATCC 13032, and the resulting fragments were ligated to Hpa I-digested pMI-1 using a ClonExpress II One Step Cloning Kit, generating pMI-2. Similarly, fragments of iolR cassette amplified with primers PX-5 and PX-6 were ligated to pMI-2, resulting in pMI-3. Primers PX-7/tuf-iolR-1 and tuf-iolR-2/PX-6 were used to amplify iolR cassette (without the native promoter) and the constitutive promoter Ptuf. The fragments were fused using overlap PCR and then were ligated to pMI-2 to obtain pMI-4. Primers gfp-1 and gfp-2 were used to amplify gfp from vector pEGFP-N1, which was ligated into Hind III-digested pMI-3 and pMI-4, resulting in pMI-3-gfp and pMI-4-gfp, respectively. The fused hemAM-hemL was amplified with primers PAL-1 and PAL-2 from pAL1 (Zhang et al., 2020) and was ligated into Hind III-digested pMI-4, resulting in pMI-4-hemAM-hemL.
Primers iolG-1/iolG-2 and iolG-3/iolG-4 were used to amplify the upstream homologous arm and downstream homologous arm of iolG from C. glutamicum ATCC 13032. The fragments were fused using overlap PCR, and the resulting fragments were ligated to Xba I/EcoR I-digested pK18mobsacB, generating pK18mobsacBΔiolG; similarly, pK18mobsacBΔoxiII for deleting oxiC-oxiD-oxiE cluster as well as pK18mobsacBcgl 2000: PiolT1-pntAB for pntAB integration were constructed. Knockout of iolG and oxiC-oxiD-oxiE cluster occurred via two successive recombination events using pK18mobsacBΔiolG and pK18mobsacBΔoxiII to generate C. glutamicumΔiolGΔoxiCΔoxiDΔoxiE. AL-1 and AL-3 were constructed using the same approach.
The plasmids pMI-3-gfp and pMI-4-gfp were transformed into C. glutamicum ATCC 13032, and pMI-4-hemAM-hemL was transformed into AL-1, obtaining C. glutamicum/pMI-3-gfp, C. glutamicum/pMI-4-gfp, and AL-2, respectively.
Stability Analysis of pMI-4
C. glutamicumΔiolGΔoxiCΔoxiDΔoxiE/pMI-4 and C. glutamicumΔiolGΔoxiCΔoxiDΔoxiE/pXMJ19 were respectively inoculated in BHI medium containing chloramphenicol and were cultivated overnight at 30°C with shaking at 200 rpm as the seed. The seed was then inoculated into BHI medium without chloramphenicol and was cultivated at 30°C for 30 generations. Samples were taken every five generations. After appropriate dilution, they were spread on BHI plates with or without chloramphenicol, followed by incubation for 24 h. The ratio of colonies on BHI with chloramphenicol/colonies on BHI without chloramphenicol was used to determine the stability.
Conditions for 5-ALA Production
A single colony was transferred to 5 ml BHI medium and was cultivated overnight at 30°C with shaking at 200 rpm. Next, the culture was inoculated into a 500-ml shake flask containing 45 ml of modified CGXII medium for a 64-h incubation at 30°C, 200 rpm. To induce gene expression, myo-inositol was added at a final concentration of 0.1–5 mM when the OD600 of cell reached approximately 0.8.
Detection of GFP Activity
C. glutamicum/pMI-1-gfp, C. glutamicum/pMI-3-gfp, and C. glutamicum/pMI-4-gfp were respectively grown overnight in 24-deep-well plates containing 2 ml/well CGXII medium. Then, 20 μl of each culture were inoculated into 2 ml fresh CGXII medium and grown for 24 h. Fluorescence intensities normalized against culture OD600 (determined using a Synergy H4 Microplate Reader, BioTek, United States) were used to indicate the expression level of GFP (Peng et al., 2017).
Real-Time Quantitative PCR
The transcriptional level of gfp, hemAM, and pntAB was quantified using real-time quantitative PCR. Total RNA was extracted after 4 h of induction by myo-inositol using RNAiso Plus (Takara Bio Inc., Dalian, China), and cDNA was obtained by reverse transcription with a PrimeScript RT Reagent Kit (Takara Bio Inc., Dalian, China). Gene transcription levels were measured using SYBR Premix Ex TaqTM II (Takara Bio Inc., Dalian, China), and 16S rDNA was used as an internal control. Data were analyzed using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
Analytical Procedure
Cell growth was monitored by measuring the OD600. Concentrations of 5-ALA were measured with modified Ehrlich’s reagent (Mauzerall and Granick, 1956). Briefly, the supernatants of cultures were reacted with acetylacetone (in sodium acetate buffer, pH 4.8, 1.0 M) at 80°C for 20 min. The absorbance at 553 nm was measured 30 min after adding of the modified Ehrlich’s reagent. C. glutamicum cells grown in CGXII medium for 24 h were harvested by centrifugation at 4°C and 13,000×g for 10 min. The intracellular NADPH was quantified using an Enzychrom NADP+/NADPH assay kit, following the manufacturer’s instruction. The myo-inositol concentrations were determined by HPLC (Bio-Rad Aminex HPX-87H column) with 5 mM H2SO4 as the mobile phase and a refractive index detector (Lu et al., 2018).
Statistical Analysis
Statistical significance was determined by one-way analysis of variance, followed by Dunnett’s multiple comparison test. The data are average of three biological replicates with error bars representing standard deviation. Results with p values of less than or equal to 0.05 were considered significant.
Results and Discussion
Construction of Myo-Inositol-Inducing Expression Vector pMI-4
To verify the non-toxicity of myo-inositol to cell growth, C. glutamicum ATCC 13032 cells were cultivated in a medium containing different concentrations of myo-inositol (0.1–100 mM). The biomass of the cells under each concentration of myo-inositol exhibited no difference and was similar with those in the medium without myo-inositol (Supplementary Figure S1), indicating the non-toxicity of myo-inositol to cell growth. The myo-inositol-inducing vector pMI-4 was constructed for use in C. glutamicum as shown in Figure 2. The plasmid pXMJ19, carrying the chloramphenicol resistance gene cat and the inducible promoter Ptac, was used as the backbone (Jakoby et al., 1999). Herein, PiolT1 of C. glutamicum, the promoter of myo-inositol transport protein encoding gene iolT1, was used. PiolT1 is a myo-inositol-inducing promoter, which is repressed by the repressor IolR (encoded by iolR) in the absence of myo-inositol, but was active in the presence of myo-inositol (Figure 1). Initially, the Ptac and lacIq cassette were removed from pXMJ19, generating pMI-1 (5,281 bp). Subsequently, the PiolT1 was introduced to the upstream of multi-cloning sites in pMI-1, resulting in pMI-2 (5,488 bp). Finally, the iolR cassette from C. glutamicum ATCC 13032 was introduced to the upstream of PiolT1 in pMI-2, resulting in pMI-3 (6,750 bp).
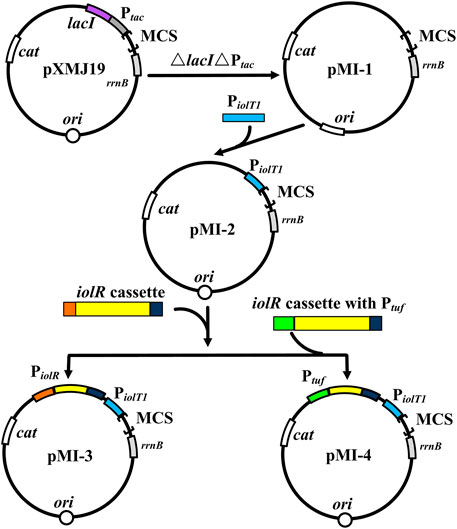
FIGURE 2. Construction of pMI-4. MCS, multiple cloning sites; cat, chloramphenicol resistance gene; rrnB, the transcriptional terminator; Ptac, tac promoter; lacI, repressor LacI of Ptac-encoding gene; ori, the origin of replication for C. glutamicum; PiolT1, iolT1 promoter; iolR cassette, fragment comprises promoter, encoding sequence, and terminator of iolR; Ptuf, promoter of the gene encoding elongation factor TU.
In order to investigate whether pMI-3 was induced by myo-inositol, the gfp gene encoding green fluorescent protein was introduced to pMI-1 and pMI-3, resulting in pMI-1-gfp (as a control) and pMI-3-gfp, respectively. These two plasmids were respectively transformed into C. glutamicum ATCC 13032 to generate C. glutamicum/pMI-1-gfp and C. glutamicum/pMI-3-gfp. The GFP activities in C. glutamicum/pMI-1-gfp and C. glutamicum/pMI-3-gfp were determined. As shown in Figure 3A, GFP activity was hardly detected in the control strain C. glutamicum/pMI-1-gfp neither in the presence nor the absence of myo-inositol. In contrast, C. glutamicum/pMI-3-gfp exhibited significant GFP fluorescence (fluorescence/OD600, 167.1) in the presence of myo-inositol, indicating that pMI-3 was myo-inositol inducible.
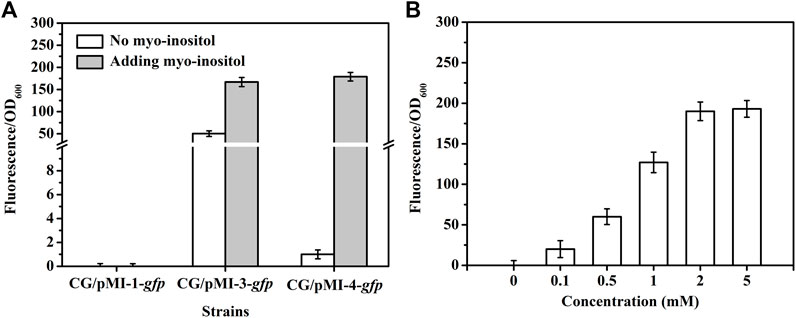
FIGURE 3. GFP fluorescence intensities in C. glutamicum strains. (A) GFP fluorescence intensities in C. glutamicum strains harboring different vectors in the absence (white) or presence of myo-inositol (gray). CG/pMI-1-gfp, CG/pMI-3-gfp, and CG/pMI-4-gfp indicate C. glutamicum/pMI-1-gfp, C. glutamicum/pMI-3-gfp, and C. glutamicum/pMI-4-gfp, respectively. C. glutamicum/pMI-1-gfp was used as a control. In this strain, GFP was not expressed because pMI-1-gfp lacks a promoter. The vector pMI-3-gfp contains PiolT1 and iolR cassette with its native promoter PiolR, and the vector pMI-4-gfp contains PiolT1 and iolRq cassette with the constitutive Ptuf. (B) The effect of myo-inositol concentrations on GFP expression in C. glutamicum/pMI-4-gfp. C. glutamicum/pMI-4-gfp was induced by myo-inositol at different concentrations.
Notably, considerable GFP fluorescence of C. glutamicum/pMI-3-gfp was also observed in the absence of myo-inositol (fluorescence/OD600, 50.3), indicating the leaky expression of GFP. It was probably due to the negative auto-regulation of iolR gene (Krings et al., 2006; Klaffl et al., 2013). To verify our hypothesis, the native promoter in iolR cassette was replaced with the constitutive promoter tuf to generate iolRq cassette, which was then inserted into pMI-2 (resulting in pMI-4, 6,643 bp). The gfp gene was introduced to pMI-4, generating pMI-4-gfp, and was subsequently transformed into C. glutamicum ATCC 13032 to generate C. glutamicum/pMI-4-gfp. As shown in Figure 3A, GFP of C. glutamicum/pMI-4-gfp was only active in the presence of myo-inositol (fluorescence/OD600, 179.3). This result indicated the non-leaky expression of GFP in the pMI-4-gfp with iolRq.
To further investigate the characteristics of pMI-4, the effect of myo-inositol concentration on GFP expression in C. glutamicum/pMI-4-gfp was determined. As shown inFigure 3B, the GFP fluorescence intensities were reinforced with the increase of myo-inositol concentration and then reached a constant level (approximately 190), indicating the dose-dependent effects of myo-inositol on GFP expression when the myo-inositol concentration was below 2 mM.
To investigate the stability of pMI-4 in C. glutamicum, it was transformed into C. glutamicum ATCC 13032 to generate C. glutamicum/pMI-4, and C. glutamicum/pXMJ19 was used a control. As shown in Figure 4, no significant difference in the stability of the vectors pMI-4 (approximately 96%–88%) and pXMJ19 was observed after 30 generations without chloramphenicol.
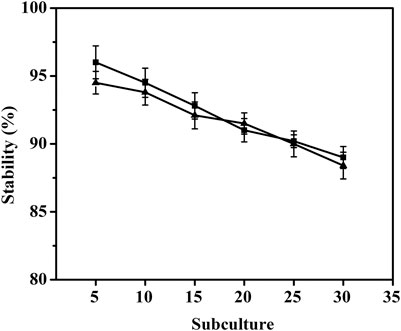
FIGURE 4. Stability of the vector pMI-4. Filled square (■) represents C. glutamicum/pMI-4, and filled triangle (▲) represents C. glutamicum/pXMJ19.
Blocking Myo-Inositol Degradation Pathway in C. glutamicum
To further test the applicability of pMI-4, the transcriptional level of gfp in C. glutamicum/pMI-4-gfp was detected during the fermentation. As shown in Figure 5A, the transcriptional level of gfp was gradually increased during the first 16 h of fermentation, but sharply declined after then. It was supposed that myo-inositol was utilized by cells, and the lowered concentration resulted in the recovery of IolR depression (Krings et al., 2006). Then, the concentration of myo-inositol was detected, and its consumption was found to start at 8 h (Figure 5A). Therefore, it was essential to block the degradation of myo-inositol to maintain its concentration at a constant level.
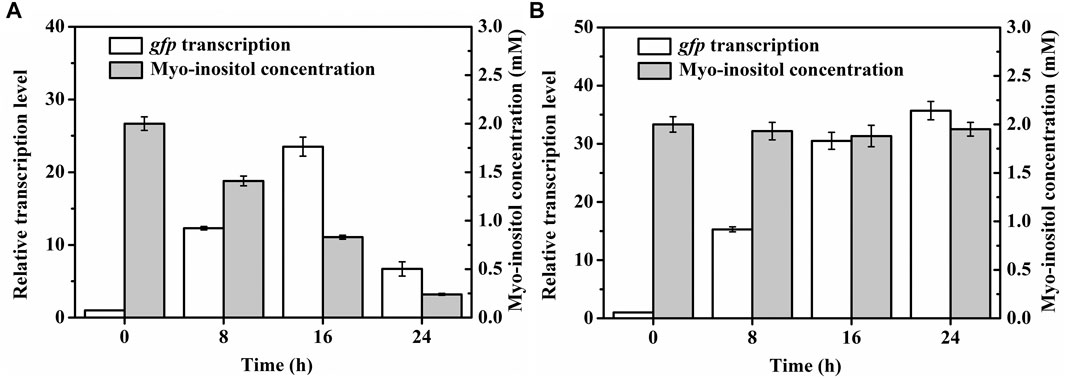
FIGURE 5. Transcriptional levels of gfp in C. glutamicum strains during the cultivation process. (A) Transcriptional levels of gfp in C. glutamicum/pMI-4-gfp and the myo-inositol concentrations during the cultivation process. (B) Transcriptional levels of gfp in C. glutamicumΔiolGΔoxiCΔoxiDΔoxiE/pMI-4-gfp and the myo-inositol concentrations during the cultivation process.
Myo-inositol dehydrogenase is the first enzyme responsible for degradation of myo-inositol (Krings et al., 2006). There are four myo-inositol dehydrogenase isozymes (encoded by iolG, oxiC, oxiD, and oxiE, respectively) in C. glutamicum. These four genes were deleted in C. glutamicum ATCC 13032. The generated C. glutamicumΔiolGΔoxiCΔoxiDΔoxiE was cultivated on CGXII containing myo-inositol (20 g/l) as carbon source to investigate its ability of myo-inositol utilization. As shown in Figure 6, unlike the wild-type C. glutamicum ATCC13032, C. glutamicumΔiolGΔoxiCΔoxiDΔoxiE was not able to grow on myo-inositol. Whereas, its growth on glucose was hardly affected compared with C. glutamicum ATCC13032, indicating that deleting the myo-inositol dehydrogenase isozymes resulted in the disability of myo-inositol degradation but did not affect the growth on glucose. To investigate the effect of myo-inositol on glucose utilization by C. glutamicumΔiolGΔoxiCΔoxiDΔoxiE, the cells were cultivated in CGXII medium containing 5 mM of myo-inositol (the maximum dose for induction). No significant differences in cell growth and glucose consumption were observed between the cells cultivated with and without myo-inositol (Supplementary Figure S2). So, it was inferred that glucose metabolism may not be negatively affected under the myo-inositol concentration for induction.
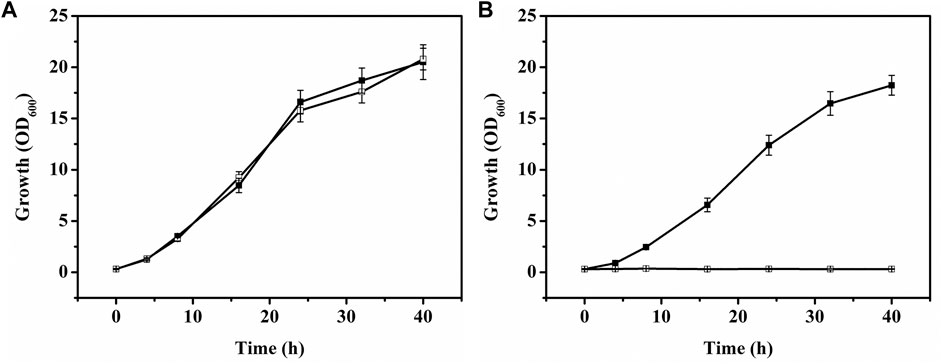
FIGURE 6. Growth of C. glutamicum strains on glucose or myo-inositol. (A) Growth of C. glutamicum ATCC 13032 [filled square (■)] and C. glutamicumΔiolGΔoxiCΔoxiDΔoxiE [white square (□)] on glucose. (B) Growth of C. glutamicum ATCC 13032 [filled square (■)] and C. glutamicumΔiolGΔoxiCΔoxiDΔoxiE [white square (□)] on myo-inositol.
The plasmid pMI-4-gfp was transformed into the C. glutamicumΔiolGΔoxiCΔoxiDΔoxiE, and the consumption of myo-inositol as well as the transcription level of gfp was determined. As shown in Figure 5B, no consumption of myo-inositol by C. glutamicumΔiolGΔoxiCΔoxiDΔoxiE/pMI-4-gfp was detected during the cultivation process. Meanwhile, a continuous increase in transcriptional levels of gfp was observed in this strain. These results further indicated that the degradation of myo-inositol was successfully blocked by deleting the myo-inositol dehydrogenase-encoding genes, and this resulted in constantly reinforced transcriptional level of gfp.
Application of the Myo-Inositol-Inducing Expression System for Producing 5-ALA
To investigate the application of the myo-inositol-inducing expression system, it was used to overexpress the key genes for production of 5-ALA. Initially, to enhance the α-ketoglutarate supply for the synthesis of 5-ALA, the ppc (encoding phosphoenolpyruvate carboxylase) and gltA (encoding citrate synthase) in C. glutamicumΔiolGΔoxiCΔoxiDΔoxiE was overexpressed by replacing the native promoters with the constitutive promoter tuf, resulting in AL-1. The glutamyl-tRNA reductase (encoded by hemA) and glutamate-1-semialdehyde aminotransferase (encoded by hemL) are rate-limiting enzymes for 5-ALA synthesis (Yu et al., 2015). The mutant hemAM (two Lys residues inserted between Thr-2 and Leu-3) from Salmonella arizona (Yu et al., 2015) and hemL from E. coli were proven conducive for efficient production of 5-ALA. Therefore, these two genes were introduced into pMI-4. The generated pMI-4-hemAM-hemL was subsequently transformed into AL-1, resulting in AL-2. At the same time, pMI-4 and pAL1 (pXMJ19 harboring hemAM and hemL) was transformed into AL-1 to obtain AL-CK1 and AL-CK2, which were used as controls.
Fermentation was performed in flasks to evaluate the characteristics of AL-2 and then the performance of this myo-inositol-inducing expression system. Compared with the transcriptional level of hemAM (approximately 42-fold) in AL-CK2 induced by IPTG (1 mM), the transcriptional level of hemAM in AL-2 induced by the same dose of myo-inositol was lower (approximately 29-fold, Figure 7A). Nevertheless, the biomass of AL-2 (OD600 = 28.4) was significantly higher than that of AL-CK2 (OD600 = 24.3, Figure 7B). Furthermore, the final production of 5-ALA by AL-2 reached 0.73 g/l, slightly higher (4.2%) than that by AL-CK2 (0.71 g/l, Figure 7B). These results indicated that a stronger expression of genes sometimes was not essential for efficient production. Notably, the glucose consumption by AL-2 (30.1 g/l) was 5.6% lower than that by AL-CK2 (31.9 g/l), and this generated an increase of 9.0% in the yield of 5-ALA (24.3 VS 22.3 mg/g glucose). It was supposed that the increased cell growth and decreased glucose consumption were due to the following reasons: unlike IPTG, myo-inositol was not toxic to cell, and thus, cell growth and metabolism were not inhibited; furthermore, the constructed myo-inositol-inducible vector relieved the metabolic burden in some degree since the transcription intensity of PiolT1 was lower than that of Ptac in pXMJ19 under the same dose of inducer, which resulted in relatively lower glucose requirement and improved cell growth.
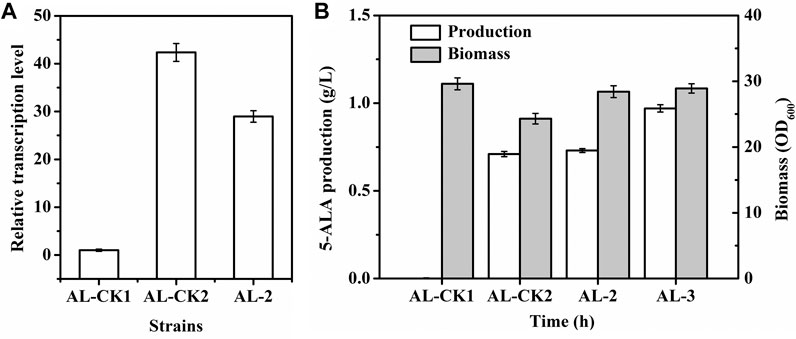
FIGURE 7. 5-ALA production, biomass, and hemAM transcription of strains. (A) hemAM transcription; (B) 5-ALA production (white) and biomass (gray). AL-CK1 was used as a control. AL-CK1, AL-1 (C. glutamicumΔiolGΔoxiCΔoxiDΔoxiEPppc:PtufPgltA:Ptuf) harboring pMI-4; AL-CK2, AL-1 harboring pAL1; AL-2, AL-1 harboring pMI-4-hemAM-hemL; AL-3, C. glutamicumΔiolGΔoxiCΔoxiDΔoxiEPppc:PtufPgltA:Ptuf cgl 2000:PiolT1-pntAB harboring pMI-4-hemAM-hemL.
The glutamyl-tRNA reductase is NADPH dependent, and enhancing NADPH regeneration has been proven to facilitate increased 5-ALA production (Zhang et al., 2020). Previous reports showed that overexpressing the pntAB gene (encoding pyridine nucleotide transhydrogenase) could improve NADPH regeneration (Kabus et al., 2007; Hao et al., 2020). To test the applicability of PiolT1, a copy of pntAB driven by PiolT1 was integrated into the genome of AL-2 (generating AL-3) so that transcription of pntAB was induced by myo-inositol. As a result, the transcription level of pntAB in AL-3 was detected to be gradually enhanced after the addition of myo-inositol and maintained constant after 16 h (Supplementary Figure S3). Moreover, the intracellular NADPH in AL-3 was significantly improved by 27.7% (from 0.65 to 0.83 μmol/g DCW). As shown in Figure 7A, the production of 5-ALA by AL-3 was increased by 32.9% (0.97 g/l) compared with AL-2. This result indicated the potential application of PiolT1 in metabolic engineering.
Besides iolT1, transcription of several genes, such as iolA (encoding aldehyde dehydrogenase) and iolC (encoding carbohydrate kinase), was detected up-regulated to different levels when C. glutamicum cells were grown on myo-inositol. These results indicated that the promoters of these genes are probably inducible by myo-inositol, and strengths of these promoters are diverse. Therefore, these promoters will be exploited for gene overexpression in the future work.
Conclusion
A myo-inositol-inducible expression vector pMI-4, containing the iolRq cassette and PiolT1, was constructed in this study. The pMI-4 was proven to stably replicate in C. glutamicum cells without antibiotic selection pressure. Furthermore, the chassis C. glutamicumΔiolGΔoxiCΔoxiDΔoxiE, which was not able to degrade myo-inositol and suitable for maintaining pMI-4, was developed as the host strain. Through overexpression of hemAM and hemL using the vector pMI-4 in the engineered chassis C. glutamicumΔiolGΔoxiCΔoxiDΔoxiE, the production of 5-ALA achieved 0.73 g/l, which indicated the potential application of this expression system in metabolic engineering and industrial production. Furthermore, the PiolT1 was proved to be applicable for overexpressing genes by integrating to genome.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding authors.
Author Contributions
CZ and NL designed the research, performed data analysis, and wrote the manuscript. NL, WZ, and HX constructed the expression vectors and the host strain and investigated the characteristic of the vectors. YL, MW, and JM constructed the 5-ALA producing strains. YM and JW performed the fermentation assays. CZ and NC supervised the research. All authors contributed to the article and approved the submitted version.
Funding
This research was financially supported by the National Key Research and Development Program of China (2021YFC2101802), the Natural Science Foundation of China (32170049, 31300069, and 31770053), the Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (TSBICIP-KJGG-009), the Training program of innovation and entrepreneurship for undergraduates (202110057132 and 202110057314), and the China Postdoctoral Science Foundation Funded Project (2017M611170 and 2018T110662).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2021.746322/full#supplementary-material
References
Becker, J., Rohles, C. M., and Wittmann, C. (2018). Metabolically Engineered Corynebacterium Glutamicum for Bio-Based Production of Chemicals, Fuels, Materials, and Healthcare Products. Metab. Eng. 50, 122–141. doi:10.1016/j.ymben.2018.07.008
Chen, Z., Huang, J., Wu, Y., Wu, W., Zhang, Y., and Liu, D. (2016). Metabolic Engineering of Corynebacterium Glutamicum for the Production of 3-Hydroxypropionic Acid from Glucose and Xylose. Metab. Eng. 39, 151–158. doi:10.1016/j.ymben.2016.11.009
Chung, S.-C., Park, J.-S., Yun, J., and Park, J. H. (2017). Improvement of Succinate Production by Release of End-Product Inhibition in Corynebacterium Glutamicum. Metab. Eng. 40, 157–164. doi:10.1016/j.ymben.2017.02.004
Eggeling, L., and Bott, M. (2015). A Giant Market and a Powerful Metabolism: L-Lysine provided by Corynebacterium Glutamicum. Appl. Microbiol. Biotechnol. 99, 3387–3394. doi:10.1007/s00253-015-6508-2
Hao, Y., Ma, Q., Liu, X., Fan, X., Men, J., Wu, H., et al. (2020). High-Yield Production of L-Valine in Engineered Escherichia C by a Novel Two-Stage Fermentation. Metab. Eng. 62, 198–206. doi:10.1016/j.ymben.2020.09.007
Hasegawa, S., Uematsu, K., Natsuma, Y., Suda, M., Hiraga, K., Jojima, T., et al. (2012). Improvement of the Redox Balance Increases L-Valine Production by Corynebacterium Glutamicum under Oxygen Deprivation Conditions. Appl. Environ. Microbiol. 78, 865–875. doi:10.1128/AEM.07056-11
Ikeda, M., and Nakagawa, S. (2003). The Corynebacterium Glutamicum Genome: Features and Impacts on Biotechnological Processes. Appl. Microbiol. Biotechnol. 62, 99–109. doi:10.1007/s00253-003-1328-1
Jakoby, M., Ngouoto-Nkili, C.-E., and Burkovski, A. (1999). Construction and Application of New Corynebacterium Glutamicum Vectors. Biotechnol. Tech. 13, 437–441. doi:10.1023/A:1008968419217
Kabus, A., Georgi, T., Wendisch, V. F., and Bott, M. (2007). Expression of the Escherichia C pntAB Genes Encoding a Membrane-Bound Transhydrogenase in Corynebacterium Glutamicum Improves L-Lysine Formation. Appl. Microbiol. Biotechnol. 75, 47–53. doi:10.1007/s00253-006-0804-9
Kirchner, O., and Tauch, A. (2003). Tools for Genetic Engineering in the Amino Acid-Producing Bacterium Corynebacterium Glutamicum. J. Biotechnol. 104, 287–299. doi:10.1016/S0168-1656(03)00148-2
Klaffl, S., Brocker, M., Kalinowski, J., Eikmanns, B. J., and Bott, M. (2013). Complex Regulation of the Phosphoenolpyruvate Carboxykinase Gene Pck and Characterization of its GntR-Type Regulator IolR as a Repressor of Myo-Inositol Utilization Genes in Corynebacterium Glutamicum. J. Bacteriol. 195, 4283–4296. doi:10.1128/JB.00265-13
Kogure, T., and Inui, M. (2018). Recent Advances in Metabolic Engineering of Corynebacterium Glutamicum for Bioproduction of Value-Added Aromatic Chemicals and Natural Products. Appl. Microbiol. Biotechnol. 102, 8685–8705. doi:10.1007/s00253-018-9289-6
Krings, E., Krumbach, K., Bathe, B., Kelle, R., Wendisch, V. F., Sahm, H., et al. (2006). Characterization of Myo -Inositol Utilization by Corynebacterium Glutamicum : the Stimulon, Identification of Transporters, and Influence on L -Lysine Formation. J. Bacteriol. 188, 8054–8061. doi:10.1128/JB.00935-06
Li, Y., Ai, Y., Zhang, J., Fei, J., Liu, B., Wang, J., et al. (2020). A Novel Expression Vector for Corynebacterium Glutamicum with an Auxotrophy Complementation System. Plasmid 107, 102476. doi:10.1016/j.plasmid.2019.102476
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 25, 402–408. doi:10.1006/meth.2001.1262
Lu, Y., Wang, L., Teng, F., Zhang, J., Hu, M., and Tao, Y. (2018). Production of Myo-Inositol from Glucose by a Novel Trienzymatic cascade of Polyphosphate Glucokinase, Inositol 1-Phosphate Synthase and Inositol Monophosphatase. Enzyme Microb. Technol. 112, 1–5. doi:10.1016/j.enzmictec.2018.01.006
Mauzerall, D., and Granick, S. (1956). The Occurrence and Determination of Delta-Amino-Levulinic Acid and Porphobilinogen in Urine. J. Biol. Chem. 219, 435–446. doi:10.1016/s0021-9258(18)65809-0
Nešvera, J., and Pátek, M. (2011). Tools for Genetic Manipulations in Corynebacterium Glutamicum and Their Applications. Appl. Microbiol. Biotechnol. 90, 1641–1654. doi:10.1007/s00253-011-3272-9
Peng, F., Wang, X., Sun, Y., Dong, G., Yang, Y., Liu, X., et al. (2017). Efficient Gene Editing in Corynebacterium Glutamicum Using the CRISPR/Cas9 System. Microb. .Cell .Fact. 16, 201. doi:10.1186/s12934-017-0814-6
Sambrook, J., and Russell, D. W. (2001). Molecular Cloning: A Laboratory Manual. Cold Spring Harbor. NY: Cold Spring Harbor Laboratory Press.
Sato, N., Kishida, M., Nakano, M., Hirata, Y., and Tanaka, T. (2020). Metabolic Engineering of Shikimic Acid-Producing Corynebacterium Glutamicum from Glucose and Cellobiose Retaining its Phosphotransferase System Function and Pyruvate Kinase Activities. Front. Bioeng. Biotechnol. 8, 569406. doi:10.3389/fbioe.2020.569406
Schäfer, A., Tauch, A., Jäger, W., Kalinowski, J., Thierbach, G., and Pühler, A. (1994). Small Mobilizable Multi-Purpose Cloning Vectors Derived from the Escherichia C Plasmids pK18 and pK19: Selection of Defined Deletions in the Chromosome of Corynebacterium Glutamicum. Gene 145, 69–73. doi:10.1016/0378-1119(94)90324-7
Suzuki, N., and Inui, M. (2013). Genome Engineering of Corynebacterium Glutamicum. Berlin: Springer.
Takeno, S., Hori, K., Ohtani, S., Mimura, A., Mitsuhashi, S., and Ikeda, M. (2016). l -Lysine Production Independent of the Oxidative Pentose Phosphate Pathway by Corynebacterium Glutamicum with the Streptococcus Mutans gapN Gene. Metab. Eng. 37, 1–10. doi:10.1016/j.ymben.2016.03.007
Wang, Q., Zhang, J., Al Makishah, N. H., Sun, X., Wen, Z., Jiang, Y., et al. (2021). Advances and Perspectives for Genome Editing Tools of Corynebacterium Glutamicum. Front. Microbiol. 12, 654058. doi:10.3389/fmicb.2021.654058
Wendisch, V. F. (2020). Metabolic Engineering Advances and Prospects for Amino Acid Production. Metab. Eng. 58, 17–34. doi:10.1016/j.ymben.2019.03.008
Xu, D., Tan, Y., Huan, X., Hu, X., and Wang, X. (2010). Construction of a Novel Shuttle Vector for Use in Brevibacterium Flavum, an Industrial Amino Acid Producer. J. Microbiol. Methods 80, 86–92. doi:10.1016/j.mimet.2009.11.003
You, R., Wang, L., Shi, C., Chen, H., Zhang, S., Hu, M., et al. (2020). Efficient Production of Myo-Inositol in Escherichia C through Metabolic Engineering. Microb. Cel. Fact. 19, 109. doi:10.1186/s12934-020-01366-5
Yu, X., Jin, H., Liu, W., Wang, Q., and Qi, Q. (2015). Engineering Corynebacterium Glutamicum to Produce 5-Aminolevulinic Acid from Glucose. Microb. Cel. Fact. 14, 183. doi:10.1186/s12934-015-0364-8
Zhang, C., Li, Y., Ma, J., Liu, Y., He, J., Li, Y., et al. (2018). High Production of 4-Hydroxyisoleucine in Corynebacterium glutamicum by Multistep Metabolic Engineering. Metab. Eng. 49, 287–298. doi:10.1016/j.ymben.2018.09.008
Keywords: Corynebacterium glutamicum, expression vector, IolR, iolT1, myo-inositol, 5-aminolevulinic acid
Citation: Lu N, Zhang C, Zhang W, Xu H, Li Y, Wei M, Meng J, Meng Y, Wang J and Chen N (2021) A Myo-Inositol-Inducible Expression System for Corynebacterium glutamicum and Its Application. Front. Bioeng. Biotechnol. 9:746322. doi: 10.3389/fbioe.2021.746322
Received: 23 July 2021; Accepted: 05 October 2021;
Published: 15 November 2021.
Edited by:
Yu Wang, Tianjin Institute of Industrial Biotechnology (CAS), ChinaReviewed by:
Guoqiang Xu, Jiangnan University, ChinaSuiping Zheng, South China University of Technology, China
Copyright © 2021 Lu, Zhang, Zhang, Xu, Li, Wei, Meng, Meng, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenglin Zhang, zcl@tust.edu.cn; Ning Chen, ningch@tust.edu.cn
†These authors have contributed equally to this work
 Nan Lu
Nan Lu Chenglin Zhang
Chenglin Zhang Wenjie Zhang
Wenjie Zhang 