Effects of Dietary Glycerol Monobutyrate Supplementation on Egg Performance, Biochemical Indices, and Gut Microbiota of Aged Hens
- 1School of Life Science and Engineering, Foshan University, Foshan, China
- 2Guangdong Guangken Animal Husbandry Engineering Research Institute, Guangzhou, China
- 3Institute of Animal Science, State Key Laboratory of Livestock and Poultry Breeding, Guangdong Academy of Agricultural Sciences, Guangzhou, China
This experiment aimed to determine the effect of dietary supplementation with glycerol monobutyrate (GMB) on egg-laying performance, biochemical indicators, and gut microflora at the late stage of laying hens. A total of 252 healthy Dawu Golden Phoenix laying hens with no difference in body weight were selected and randomly divided into two groups: (1) control group (CG), corn-soybean meal diet, (2) 500 mg glycerol monobutyrate/kg added to the basal diet. Six replicates were set up for each treatment group, with 21 birds per replicate. The trial started at week 55 and lasted for 8 weeks. Compared to the control group, the supplementation with GMB increased egg weight (P = 0.03), shell thickness (P = 0.03) and decreased egg breaking rate (P = 0.04). There was no significant difference in egg production rate, feed-to-egg ratio, egg shape index, eggshell strength, and Haugh unit between the two groups. In addition, dietary GMB decreased the levels of aspartate aminotransferase (P = 0.03) and total bilirubin (P = 0.02) in serum, and increased total antioxidant capacity (P = 0.03) and total superoxide dismutase (P = 0.02). However, alpha diversity indices (Ace, Chao1, Shannon, Simpson, goods_coverage, and PD_whole tree) were not different between the two groups. Notably, dietary GMB significantly decreased the abundances of Proteobacteria at the phylum level and the abundances of Enterobacter at the genus level (P < 0.01), but there was no significant difference in the composition of other cecal microbiota. In summary, the present study revealed that supplementation with 500 mg/kg glycerol monobutyrate improved egg weight, eggshell quality, and antioxidant capacity in serum, but its effect on cecal microbiota composition was limited.
Introduction
The production performance and eggshell quality of laying hens in the late laying period (40–48 weeks old) decreased with age (Bain et al., 2016). In addition, poor health of older hens have also resulted in poor egg quality, thinner shells, increased breakage rates, reduced egg white height, and poor egg flavor (Gan et al., 2020). Short-chain fatty acids (SCFAs) are a feed additive capable of controlling poultry pathogens (Ricke, 2003). Butyric acid is a 4-carbon SCFA, its glyceride form is easy to handle because it does not have the unique unpleasant odor of free acids, and it also facilitates release into the digestive tract (Sampugna et al., 1967).
Recently published research has shown that supplementation of monobutyric acid in the diet increases egg weight and tends to reduce egg breakage in late-production Qingyuan chickens (Feng et al., 2021). Meantime, dietary glycerol monolaurate improves reproductive performance, feed efficiency, and egg quality in aged hens associated with gut microbiota alteration (Liu et al., 2020). The addition of glyceryl butyrate to the diet could improve slaughter weight and feed efficiency of broilers (Antongiovanni et al., 2007). However, there is no effect on the average daily gain and feed efficiency of broilers by dietary monobutyrate (Bedford et al., 2017). Surprisingly, the mixture of monobutyrin and tributyrin improved blood biochemical parameters in broilers, especially in lipid catabolism (Yin et al., 2016).
The healthy development of the gut is an important condition for the healthy production of the animal body. Chickens have a complex microbiota in the cecum that interacts closely with the host and ingested feed (Pan and Yu, 2014). Growing evidence also links gut microbiota and function to weight gain, feed utilization conversion, and chicken health (Angelakis, 2017). The dysbiosis of broiler chickens is caused by the prohibition of antibiotics in feed, dietary changes, and environmental stresses in modern broiler production. In the case of intestinal flora imbalance in the body, it is usually accompanied by an increase in the level of Proteobacteria (Pinacchio et al., 2018). Proteobacteria mainly consists of many gram-negative bacteria such as Vibrio cholerae, Helicobacter pylori, Salmonella, and Escherichia coli, which could result in diarrhea, gastritis, vomiting, gastrointestinal ulcers, and even death; posing a great threat to animal health (Li et al., 2021). Butyric acid has been reported to have antibacterial properties, it can diffuse through the bacterial cell membrane and dissociate within the bacterial cell, resulting in a drop in the intracellular pH of the bacterial cell and eventual death (Hanna, 2019). Derivatives of butyric acid have been added to broiler diets to replace antibiotics, with glycerol butyrate being typical. Glyceryl butyrate has been reported to maintain broiler performance when encountering coccidiosis (Leeson et al., 2005) and can reduce Salmonella Enteritidis infection and improve growth performance under stress (Zhang et al., 2011). Alpha monoglycerides of these SCFAs have been reported to have stronger antibacterial effects, and their addition to the diet may benefit chicken gut health and growth performance (Namkung et al., 2011). Many studies have been conducted on how butyrin and other forms of butyric acid affect broiler performance. However, studies on the effects of butyrin on egg quality, blood biochemical markers, and gut microbiota in laying hens are limited. This study aimed to investigate how glycerol monobutyrate affects egg quality, blood biochemical parameters, and cecal microbiota in late laying hens.
Materials and Methods
Birds, Experimental Design, and Diets
A total of 252 (55-wk-old) Dawu Golden Phoenix laying hens were randomly divided into two groups (21 hens per replicate, 6 replicates per group) and fed with a basal diet or a basal diet containing 500 mg/kg of GMB. The experiment started at week 55 and lasted for 8 wk. The basal diet was formulated according to the Nutrient Requirements for laying hens (2012). During the study, the birds were housed in three-tiered cages with an overall shape like the letter A when viewed from the side and shared a room cleaned and disinfected daily, maintained at 26 ± 2°C and 60–65% humidity with a 16 h light regime. Feed (100 g per hen per day) was provided at 7:00 a.m. and 2:00 p.m. and eggs were collected at 5:00 p.m. The birds had free access to drink water. Feeding, egg collection, and weighing of eggs were conducted daily.
Productive Performance and Egg Quality
Feed intake, number of eggs, and egg breaking were recorded daily during 55–62 wk. The egg breaking rate was calculated based on the number of broken eggs. The egg production rate, feed-to-egg ratio, and average egg weight were calculated per week. In the last week of the experiment, 36 eggs from each group (6 eggs per replicate) were randomly selected. Haugh units (Egg Analyzer, Orka Food Technology Ltd., Israel), shell breaking strength (Egg Force Reader, Orka Food Technology Ltd.), and shell thickness (Eggshell Thickness Gauge, Orka Food Technology Ltd.) were determined from these 72 eggs. The egg width to length ratio was calculated as the egg shape index (%).
Serum Biochemical Indices and Antioxidant Parameters
One bird was randomly selected from each replicate and blood was collected after feed deprivation for 12 h at the end of the experiment (Feng et al., 2021). Blood samples were collected from the pterygoid vein and serum was separated after 2 h of quiescence at 4°C (Gong et al., 2021). Triglycerides, total cholesterol, total bilirubin, high-density lipoprotein, low-density lipoprotein, aspartate aminotransferase, and alanine aminotransferase in serum were detected by kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). Antioxidant parameters analyzed according to the instruction manual of the Nanjing Jiancheng Bioengineering Institute kit included malondialdehyde (MDA), total antioxidant capacity (T-AOC), total superoxide dismutase (T-SOD), and glutathione peroxidase (GSH-PX). After the blood collection, the selected chickens were sacrificed by cervical dislocation and bled. The intestinal contents of the left and right cecum were aseptically collected from each chicken in a capped vial and immediately snap frozen in liquid nitrogen. The samples were then stored at −80°C until further analysis.
Cecal Digesta DNA Extraction and 16S rRNA Sequencing Analysis
Cecal microbiota evaluation was carried on the selected six older laying hens per group at the end of the study. After being snap frozen in liquid nitrogen, the cecal contents were extracted aseptically and kept at −80°C. The cetyltrimethylammonium bromide method was used to extract total genome DNA from cecal digesta. 1.5% agarose gel electrophoresis has been used to determine DNA content and purity. The primers 341F (5′-CCTACGGGNGGCWGCAG-3′) and 805R (5′-GACTACHVGGGTATCTAATCC-3′) were used to amplify the V3/V4 region of the 16S rRNA gene. To obtain the high-quality clean reads, paired-end reads were merged using Fast Length Adjustment of Short Reads software (V1.2.7) and quality filtering on the raw sequences was conducted on a quality control pipeline using the Quantitative Insight into Microbial Ecology (QIIME) tool kit (Caporaso et al., 2010). At 97 percent similarity, high-quality sequences were grouped into operational taxa (OTUs), and representative OTU sequences were assigned taxonomy using the SILVA database (Quast et al., 2013). A Venn diagram had been used to visualize shared and unique OTUs among three groups. Package vegan was used to calculate the alpha diversity, which was then displayed utilizing GraphPad prism 9.0. A principal coordinate analysis (PCOA) based on Bray-Curtis distance was performed for beta-diversity analysis, with statistical significance evaluated using the R package's permutational multivariate analysis of variance (t-test). Linear discriminant analysis effect size and STAMP with t-test were being used to investigate differences in microbiota relative abundances (Parks et al., 2014).
Statistical Analysis
All data were expressed as means ± SD, using an independent samples t-test with SPSS 25.0 software (SPSS, Inc., Chicago, IL), including fixed effects treatments in the model, and presented using GraphPad Prism version 9 (GraphPad Software, La Jolla, CA). A P < 0.05 was considered significant (P < 0.05, P < 0.01), and 0.05 < P < 0.10 was discussed as tendencies.
Results
Effect of Dietary GMB on Productive Performance and Egg Quality
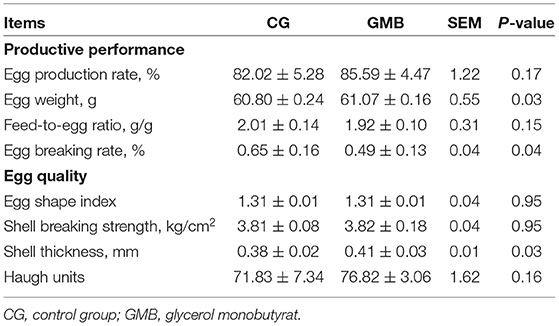
In this study, the egg weight and shell thickness of the GMB group were significantly higher than that of the control group (Table 1; P < 0.05). There was no significant difference between the two groups in egg shape index, eggshell strength, and Haugh unit. However, the egg breaking rate of the treatment group decreased by 0.16% compared with the control group (Table 1; P < 0.05). These results indicated that dietary GMB could improve egg weight and shell thickness and reduce egg breaking rate in the late laying period.
Effect of Dietary GMB on Serum Biochemical Indices and Antioxidant Parameters
The aspartate aminotransferase and total bilirubin of the treatment group were significantly lower than that of the control group (Table 2; P < 0.05). Compared with the control group, the alanine aminotransferase, in the GMB group tended to decrease (Table 2; P = 0.056). The total antioxidant capacity and total superoxide dismutase of the GMB group were significantly increased (Table 2; P < 0.05), suggesting that part of the antioxidant parameters of laying hens could be improved by GMB supplementation.
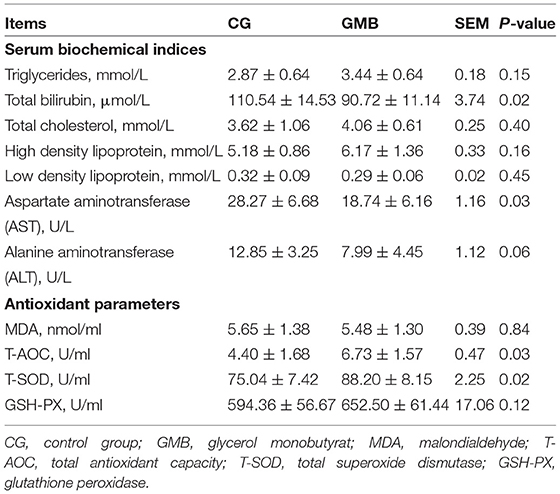
Table 2. Effects of glycerol monobutyrat supplementation on serum biochemical indices and antioxidant parameters.
Effect of Dietary GMB on Microbial Composition of Cecal Contents
A total of 2,481 operational taxonomic units (OTUs) were received from the two groups, and 1,029 and 396 OTUs were, respectively, found only in the control group and GMB group (Figure 1A). Alpha diversity indices including Ace, Chao1, Shannon, Simpson, goods_coverage, and PD_whole tree were not affected by GMB supplementation (Table 3). The Bray-Curtis similarity method was used for PCOA analysis. The first principal component (PCOA1) and the second principal component (PCOA2) explained 77.33 and 9.11% of the microbial diversity variation, respectively (Figure 1B). The principal coordinate analysis diagram shows that the samples in the control group and GMB group were densely clustered and not far away from each other. The taxonomic analysis reflected that the structure of cecal flora did not change after GMB treatment. At the phylum level, Bacteroidetes (>40%) and Firmicutes (>32%) are the first 2 most predominant phylum (Figure 1C). Proteobacteria were significantly reduced in the GMB group (Figure 1E; P < 0.01), while other phyla did not change significantly. The core gut microbiota in both groups was similar at the genus level. At the genus level, Bacteroidota dominates (>35%), followed by Firmicutes (>32%), Fusobacteriota (>2%), and unidentified_Bacteria (>2%) and the rest genera listed were all below 2% (Figure 1D). The supplementation of glycerol monobutyrate did not affect microbiota composition at genus levels other than Enterobacter whereas the GMB supplementation significantly reduced the relative abundance (Figure 1E; P < 0.01).
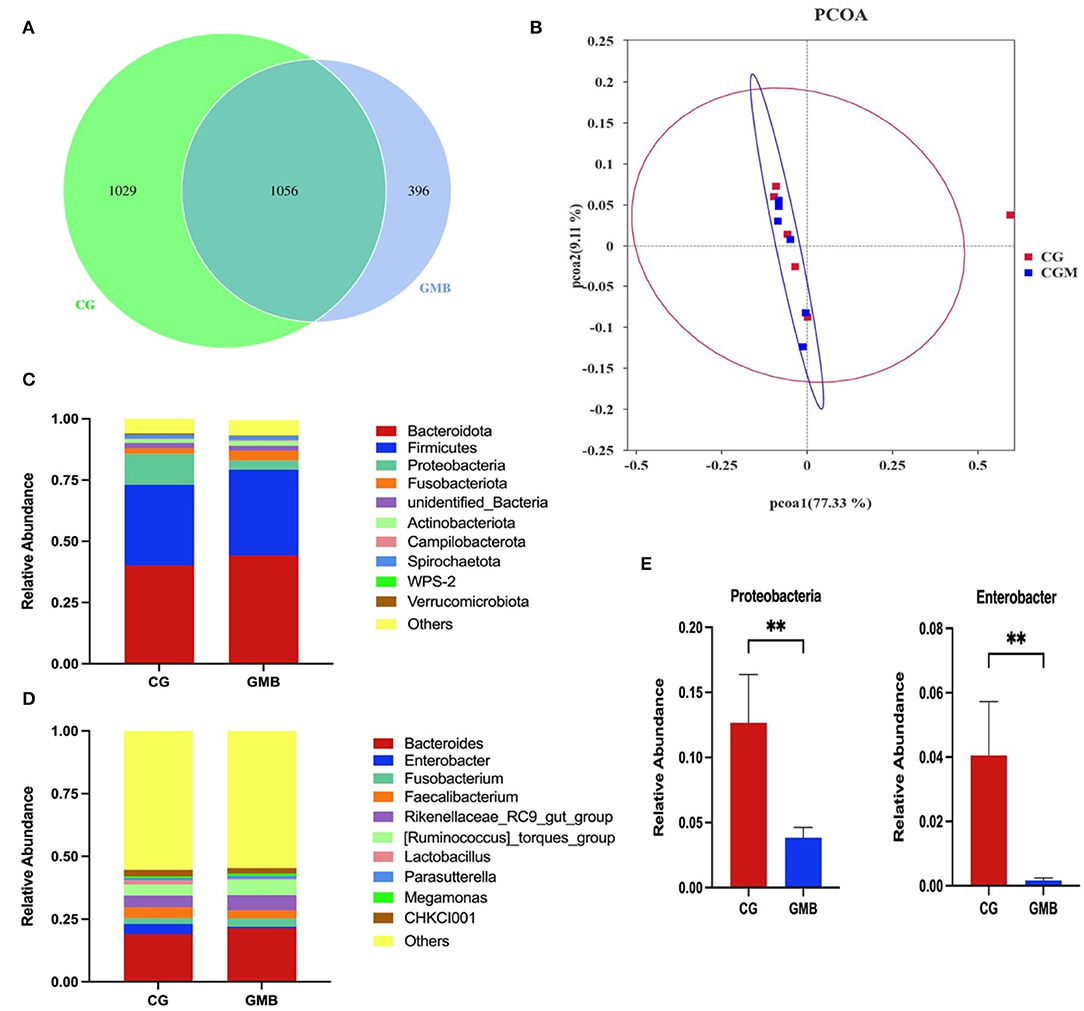
Figure 1. Effects of dietary glycerol monobutyrat on the microbial composition of cecal contents. (A) Venn diagram of the number of operational taxonomic units (OTUs); (B) Principal coordinate analysis (PCOA) of the cecal microbiota; (C) Phylum-level taxonomic composition of the cecal microbiota; (D) Genus-level taxonomic composition of the cecal microbiota. (E) Relative abundance of Proteobacteria and Enterobacter. CG, control group; GMB, glycerol monobutyrat. Asterisks indicate significant differences (**P < 0.01).
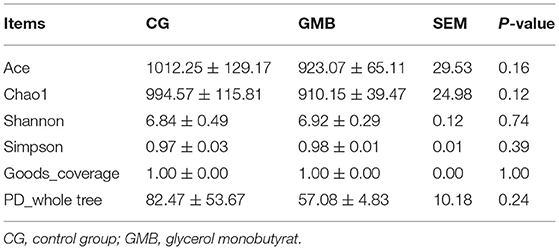
Table 3. Effects of glycerol monobutyrat supplementation on alpha diversity indexes of cecal microbiota.
Discussion
In the production process of laying hens, egg production, and egg quality are the most direct and important economic issues. Eggshell thickness and eggshell strength are very important for egg quality. Thicker eggshells have a great impact on the egg breaking rate, and also make the eggs have good pressure resistance, which is convenient for long-distance transportation and storage. Dawu Golden Phoenix laying hens will be culled after 72 weeks when the egg production rate is below 80% (Li, 2021). In the present study, our results showed that dietary supplementation with GMB did not significantly improve egg production and reduce the feed-to-egg ratio. The effects of glyceryl butyrate supplementation on broiler performance vary widely. Bedford et al. (2017) observed that the average daily gain and feed conversion ratio in broilers were not significantly difference by dietary monobutyric acid levels ranging from 500 ppm to 3,000 ppm. Feng et al. (2021) reported that the addition of 250 mg/kg of monobutyric acid did not affect performance and feed conversion ratio. However, some researchers have observed improved growth performance (Antongiovanni et al., 2007). Hu and Guo (2007) observed weight gain in broilers from 0 to 21 days. Nollet et al. (2002) found that 500 mg/kg of sodium butyrate supplementation did not affect average egg weight, but improved egg production and feed conversion. When assessing the effects of butyrate supplementation, different responses were attributable to supplementation levels, dietary composition, age, and health status (Cerisuelo et al., 2014). More researchers have observed that dietary butyrate is beneficial to eggshell quality and thus reduces egg breakage. Feng et al. (2021) observed that monobutyric acid could improve egg weight and reduce egg breaking rate, but had no significant effect on eggshell strength and eggshell thickness. Hanna (2019) observed an increased effect of butyrate (550 mg/kg) on eggshell strength. Butyrate (added at 185 mg/kg) can enhance eggshell strength and reduce the number of deformed eggs in older hens (Sengor et al., 2007). Hu and Guo (2007) noted butyric acid is a SCFA and has water-fat amphiphilicity and can be absorbed and utilized by intestinal epithelial cells in the intestine, thereby providing more energy for the body, which may be the reason for improving egg quality. In this study, egg weight was significantly improved and egg breaking rate was reduced in the treatment group, suggesting the GMB has a more pronounced effect on improving the value and quality of eggs.
The presence of serum enzymes and their levels in serum can provide some indication of the extent of organ or tissue damage. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin are important indicators for evaluating the liver function of poultry because they are synthesized in the liver. Dietary supplementation of coated sodium butyrate could significantly reduce serum ALT and total bilirubin contents and serum AST contents of 44-week-old black-bone breeder hens at 36 weeks of age (Xing, 2017). Oral administration of tributyrin can significantly decrease the levels of serum alanine aminotransferase, aspartate aminotransferase, and total bilirubin in rats with liver injury caused by endotoxin (Miyoshi et al., 2011). This is consistent with the results found in our study that dietary supplementation with 500 mg/kg monobutyrin can significantly reduce the content of aspartate aminotransferase and total bilirubin, as well as the trend of decreasing alanine aminotransferase. However, sodium butyrate supplementation did not significantly reduce aspartate aminotransferase in serum, but alanine aminotransferase decreased (Elnesr et al., 2019). This study and previous studies have shown that butyrin can reduce alanine aminotransferase in host animals, but the mechanism of action is not clear. Supplementation with butyrin has been reported to reduce serum triglyceride and total cholesterol concentrations (Yang et al., 2018). Broilers supplemented with a mixture of monobutyric acid and tributyric acid had lower serum cholesterol levels compared to controls (Bedford et al., 2017). However, these results were not observed in our study. Reasons for different results may be related to the form of butyric acid derivative supplementation, dosage, environmental factors, and animal species.
Antioxidant processes in animal bodies are critical to improving animal health, growth, production, and economic benefits. T-SOD and GSH-PX are important antioxidant enzymes in organisms, which play an important role in scavenging superoxide radicals, and peroxides and preventing or reducing the formation of hydroxyl radicals. T-AOC is a comprehensive index to measure the body's antioxidant function, and the level of MDA content reflects the degree of lipid peroxidation mediated by oxygen free radicals. The supplementation with sodium butyrate can significantly increase the serum T-SOD, GSH-PX, and catalase, and reduce the content of MDA in broilers under hot conditions (Lan et al., 2020). Supplementation with tributyric acid can improve the T-AOC of broiler breeder ovaries and most parts, and reduce MDA, which may also be the possible reason for the increase of the effect of tributyric acid on protein quality (Wang et al., 2021). Likewise, previous studies have shown that supplementation with sodium butyrate increases T-SOD activity and reduces MDA levels in breeders (Alhaj et al., 2018). In our study, we observed that serum T-AOC and T-SOD were also significantly increased by GMB while MDA and GSH-PX did not differ significantly, suggesting that GMB could more effectively protect laying hens from oxidative damage.
There is increasing evidence that animal performance is closely related to the modulation of gut microbiota by feed supplementation (Pan and Yu, 2014). Relatively few studies have been done on the effect of glycerol monobutyrate supplementation on the cecal microbiota. Yang et al. (2018) noted that supplementation with 3,000 ppm butyrate altered the gut microbiota. Alpha diversity index and microbiota composition at the phylum level were affected by dietary supplementation with unprotected butyrate (Moquet, 2018). In the present study, alpha diversity indices including Ace, Chao1, Shannon, Simpson, goods_coverage, and PD_whole tree were not affected by glycerol monobutyrate supplementation. The principal coordinates analysis chart shows that the samples in the control group and the GMB group are relatively densely clustered, which suggests that there is not much difference between the two groups of bacteria. One of the possible reasons for the bacteriostatic or bactericidal effect of butyrate is that the dissociation of SCFAs in the bacterial cytoplasm disrupts the proton motive force across the membrane and reduces the pH of the cytoplasm (Moquet, 2018). Proteobacteria mainly consists of many gram-negative bacteria such as Vibrio cholerae, Helicobacter pylori, Salmonella, and Escherichia coli, which could result in diarrhea, gastritis, vomiting, gastrointestinal ulcers, and even death, posing a great threat to animal health (Liu et al., 2021). Most researchers' research on butyrin has focused on its antibacterial and bacteriostatic effects, such as on Salmonella and Escherichia coli. The small chain fatty acid butyrate has been reported to reduce Salmonella (Rebollada-Merino et al., 2020). Zhang et al. (2011) reported that sodium butyrate prevents growth reduction in birds challenged by Salmonella Enteritidis. Metabolic disorders of gut microbiota are often accompanied by an increase in Proteobacteria (Shin et al., 2015). Overall, although the caecal microbiota composition did not change much, the Proteobacteria and Enterobacter were significantly less affected by GMB. This suggests that GMB may maintain or even improve the homeostasis of the cecal microbial system by reducing the relative abundance of Proteobacteria and Enterobacter. One of the potential mechanisms by which the significant reduction of harmful bacteria-enriched Proteobacteria may improve egg quality.
Conclusions
This study showed that dietary supplementation with 500 mg/kg of GMB improved egg weight, eggshell quality, serum antioxidant capacity, and reduced egg breaking rate in late laying hens. In the cecal microbiota, GMB supplementation had no effect on the composition of the microbiota and the Alpha diversity index except that it significantly reduced the relative abundance of Proteobacteria and Enterobacter. These findings shed new light on the use of GMB as a functional ingredient to improve the production cycle and egg quality of laying hens, which has important implications for the healthy development of the laying hen industry. However, the mechanism and optimal ratio of GMB need to be further explored.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics Statement
The animal study was reviewed and approved by the Ethical Committee and conducted under the supervision of the Institutional Animal Care and Use Committee of Foshan University (Foshan, China).
Author Contributions
HZ designed the experiment. GX and LG finished the statistical analysis of all data and the original draft written. LZ and YY conducted the animal feeding and the sample analysis. XY and QQ participated in the sample collection. All authors read and approved the final manuscript.
Funding
This research work was supported by the Scientific Research Foundation in the Higher Education Institutions of Educational Commission of Guangdong Province (2017GCZX006), Special Foundation for Key Research Area of Educational Commission of Guangdong Province (2019KZDZX2006), Guangdong Province Modern Agriculture Poultry Industry technology system innovation team construction project (2021KJ128), Guangdong Science and Technology Innovation Strategy Special Fund (DZX20192520309), and the Discipline Construction Program of Foshan University (CGZ0400162).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alhaj, H. W., Li, Z., Shan, T., Dai, P., Zhu, P., Li, Y., et al. (2018). Effects of dietary sodium butyrate on reproduction in adult breeder roosters. Anim. Reprod. Sci. 196, 111–119. doi: 10.1016/j.anireprosci.2018.07.002
Angelakis, E. (2017). Weight gain by gut microbiota manipulation in productive animals. Microb. Pathog. 106, 162–170. doi: 10.1016/j.micpath.2016.11.002
Antongiovanni, M., Buccioni, A., Petacchi, F., Leeson, S., Minieri, S., Martini, A., et al. (2007). Butyric acid glycerides in the diet of broiler chickens: effects on gut histology and carcass composition. Ital. J. Anim. Sci. 6, 19–25 doi: 10.4081/ijas.2007.19
Bain, M. M., Nys, Y., and Dunn, I. C. (2016). Increasing persistency in lay and stabilising egg quality in longer laying cycles. What are the challenges? Br. Poult. Sci. 57, 330–338. doi: 10.1080/00071668.2016.1161727
Bedford, A., Yu, H., Squires, E. J., Leeson, S., and Gong, J. (2017). Effects of supplementation level and feeding schedule of butyrate glycerides on the growth performance and carcass composition of broiler chickens. Poult. Sci. 96, 3221–3228. doi: 10.3382/ps/pex098
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Cerisuelo, A., Marin, C., Sanchez-Vizcaino, F., Gomez, E. A., de la Fuente, J. M., Duran, R., et al. (2014). The impact of a specific blend of essential oil components and sodium butyrate in feed on growth performance and Salmonella counts in experimentally challenged broilers. Poult. Sci. 93, 599–606. doi: 10.3382/ps.2013-03528
Elnesr, S. S., Ropy, A., and Abdel-Razik, A. H. (2019). Effect of dietary sodium butyrate supplementation on growth, blood biochemistry, haematology and histomorphometry of intestine and immune organs of Japanese quail. Animal 13, 1234–1244. doi: 10.1017/S1751731118002732
Feng, X., Kong, F., Zheng, L., Qi, Q., Long, L., Gong, L., et al. (2021). Effects of monobutyrin supplementation on egg production, biochemical indexes, and gut microbiota of broiler breeders. Poult. Sci. 100:100907. doi: 10.1016/j.psj.2020.11.074
Gan, L., Zhao, Y., Mahmood, T., and Guo, Y. (2020). Effects of dietary vitamins supplementation level on the production performance and intestinal microbiota of aged laying hens. Poult. Sci. 99, 3594–3605. doi: 10.1016/j.psj.2020.04.007
Gong, L., Xiao, G., Zheng, L., Yan, X., Qi, Q., Zhu, C., et al. (2021). Effects of dietary tributyrin on growth performance, biochemical indices, and intestinal microbiota of yellow-feathered broilers. Animals 11:3425. doi: 10.3390/ani11123425
Hanna, D. E. (2019). The Effects of Butyric Acid on Performance Parameters, Egg Quality and Nutrient Utilization in Young White Leghorn Hens.
Hu, Z., and Guo, Y. (2007). Effects of dietary sodium butyrate supplementation on the intestinal morphological structure, absorptive function and gut flora in chickens. Anim. Feed Sci. Technol. 132, 240–249. doi: 10.1016/j.anifeedsci.2006.03.017
Lan, R., Zhao, Z., Li, S., and An, L. (2020). Sodium butyrate as an effective feed additive to improve performance, liver function, and meat quality in broilers under hot climatic conditions. Poult. Sci. 99, 5491–5500. doi: 10.1016/j.psj.2020.06.042
Leeson, S., Namkung, H., Antongiovanni, M., and Lee, E. H. (2005). Effect of butyric acid on the performance and carcass yield of broiler chickens. Poult. Sci. 84, 1418–1422. doi: 10.1093/ps/84.9.1418
Li, A., Yang, Y., Qin, S., Lv, S., Jin, T., Li, K., et al. (2021). Microbiome analysis reveals gut microbiota alteration of early-weaned Yimeng black goats with the effect of milk replacer and age. Microb. Cell Fact. 20, 78–78. doi: 10.1186/s12934-021-01568-5
Li, H. (2021). High-yielding red-feather pink-shell laying hens—Dawu Golden Phoenix. Hebei Agriculture,
Liu, L., Zou, Z., Yang, J., Li, X., Zhu, B., Zhang, H., et al. (2021). Jianpi Jieyu Decoction, an empirical herbal formula, exerts psychotropic effects in association with modulation of gut microbial diversity and GABA activity. Front. Pharmacol. 12:645638. doi: 10.3389/fphar.2021.645638
Liu, T., Li, C., Li, Y., and Feng, F. (2020). Glycerol monolaurate enhances reproductive performance, egg quality and albumen amino acids composition in aged hens with gut microbiota alternation. Agriculture 10:250. doi: 10.3390/agriculture10070250
Miyoshi, M., Sakaki, H., Usami, M., Iizuka, N., Shuno, K., Aoyama, M., et al. (2011). Oral administration of tributyrin increases concentration of butyrate in the portal vein and prevents lipopolysaccharide-induced liver injury in rats. Clin. Nutr. 30, 252–258. doi: 10.1016/j.clnu.2010.09.012
Moquet, P. C. (2018). Butyrate in Broiler Diets: Impact of Butyrate Presence in Distinct Gastrointestinal Tract Segments on Digestive Function, Microbiota Composition and Immune Responses. 0Wageningen University and Research.
Namkung, H., Yu, H., Gong, J., and Leeson, S. (2011). Antimicrobial activity of butyrate glycerides toward Salmonella Typhimurium and Clostridium perfringens. Poult. Sci. 90, 2217–2222. doi: 10.3382/ps.2011-01498
Nollet, L., Janssen, G., and Arnouts, S. (2002). The Use of Sodium Butyrate (Adimix Butyrate C) in Layer Nutrition. European Poultry, 11th Conference, Bremen, Germany.
Pan, D., and Yu, Z. (2014). Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 5, 108–119. doi: 10.4161/gmic.26945
Parks, D. H., Tyson, G. W., Hugenholtz, P., and Beiko, R. G. (2014). STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30, 3123–3124. doi: 10.1093/bioinformatics/btu494
Pinacchio, C., Scheri, G. C., Statzu, M., Santinelli, L., Ceccarelli, G., Innocenti, G. P., et al. (2018). Type I/II interferon in HIV-1-infected patients: expression in gut mucosa and in peripheral blood mononuclear cells and its modification upon probiotic supplementation. J. Immunol. Res. 2018:1738676. doi: 10.1155/2018/1738676
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., et al. (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. doi: 10.1093/nar/gks1219
Rebollada-Merino, A., Ugarte-Ruiz, M., Hernández, M., Miguela-Villoldo, P., Abad, D., Rodríguez-Lázaro, D., et al. (2020). Reduction of Salmonella Typhimurium cecal colonisation and improvement of intestinal health in broilers supplemented with fermented defatted ‘alperujo’, an olive oil by-product. Animals 10:1931. doi: 10.3390/ani10101931
Ricke, S. (2003). Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult. Sci. 82, 632–639 doi: 10.1093/ps/82.4.632
Sampugna, J., Quinn, J. G., Pitas, R. E., Carpenter, D. L., and Jensen, R. G. (1967). Digestion of butyrate glycerides by pancreatic lipase. Lipids 2, 397–402. doi: 10.1007/BF02531853
Sengor, E., Yardimci, M., Cetingul, S., Bayram, I., Sahin, H., and Dogan, I. (2007). Short Communication Effects of short chain fatty acid (SCFA) supplementation on performance and egg characteristics of old breeder hens. S. Afr. J. Anim. Sci. 37, 158–163 doi: 10.4314/sajas.v37i3.4086
Shin, N. R., Whon, T. W., and Bae, J. W. (2015). Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 33, 496–503. doi: 10.1016/j.tibtech.2015.06.011
Wang, J., Zhang, H., Bai, S., Zeng, Q., Su, Z., Zhuo, Y., et al. (2021). Dietary tributyrin improves reproductive performance, antioxidant capacity, and ovary function of broiler breeders. Poult. Sci. 100:101429. doi: 10.1016/j.psj.2021.101429
Xing, W. (2017). Effect of Coated Sodium Butyrate on Production Performance, Serum Biochemical Parameters and Lipid Metabolism of Silky Breedig Hens.
Yang, X., Yin, F., Yang, Y., Lepp, D., Yu, H., Ruan, Z., et al. (2018). Dietary butyrate glycerides modulate intestinal microbiota composition and serum metabolites in broilers. Sci. Rep. 8:4940. doi: 10.1038/s41598-018-22565-6
Yin, F., Yu, H., Lepp, D., Shi, X., Yang, X., Hu, J., et al. (2016). Correction: transcriptome analysis reveals regulation of gene expression for lipid catabolism in young broilers by butyrate glycerides. PLoS ONE 11:e0162150. doi: 10.1371/journal.pone.0162150
Keywords: glycerol monobutyrate, laying hens, laying performance, egg quality, biochemical indices, microbiota
Citation: Xiao G, Zheng L, Yan X, Yang Y, Qi Q, Gong L and Zhang H (2022) Effects of Dietary Glycerol Monobutyrate Supplementation on Egg Performance, Biochemical Indices, and Gut Microbiota of Aged Hens. Front. Anim. Sci. 3:896705. doi: 10.3389/fanim.2022.896705
Received: 15 March 2022; Accepted: 19 April 2022;
Published: 11 May 2022.
Edited by:
James Levi Klotz, United States Department of Agriculture, United StatesReviewed by:
Guofeng Han, Nanjing Agricultural University, ChinaEhab El-Haroun, Cairo University, Egypt
Copyright © 2022 Xiao, Zheng, Yan, Yang, Qi, Gong and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huihua Zhang, hhzhang2@163.com
†These authors have contributed equally to this work
 Gengsheng Xiao
Gengsheng Xiao Liwei Zheng1,2†
Liwei Zheng1,2†  Qien Qi
Qien Qi Huihua Zhang
Huihua Zhang