Environmental allergen reduction in asthma management: an overview
- 1Faculty of Medicine, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam
- 2University Medical Center Ho Chi Minh City, Ho Chi Minh City, Vietnam
- 3Center for Molecular Biomedicine, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam
- 4University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam
Asthma is a prevalent non-communicable disease that affects both children and adults. Many patients with severe, uncontrolled asthma could not achieve total control despite using anti-asthmatic drugs. There is increasing evidence that allergy to environmental allergens, including both indoor and outdoor allergens, is associated with asthma symptoms and severe asthma. Frequently reported sensitized allergens were dust mites, cockroaches, grass pollens, molds, pets, and rodents in allergic asthma patients, although the patterns of widespread allergens differed from each country. Allergen avoidance is the cornerstone of asthma management, especially in sensitized subjects. This review summarizes environmental allergen avoidance and clarifies their effects on asthma control. Despite contrasting results about the impact of allergen exposure reduction on asthma control, several studies supported the beneficial effects of reducing asthma-related symptoms or risk of exacerbations as a nondrug therapy. Identifying environmental allergens is helpful for asthma patients, and further studies on clinically effective avoidance methods are required.
1. Introduction
Asthma is one of the most prevalent chronic, non-communicable diseases; global prevalence estimates were 9.8%–17.9% (1). The hallmarks of asthma are chronic airway inflammation, leading to recurrent respiratory symptoms, and variable expiratory airflow limitation (2). The pathogenesis of asthma is complicated by complex gene-environment interactions, leading to heterogeneity in clinical presentation. Initially, asthma was classified as non-atopic or “intrinsic” asthma and atopic or “extrinsic” asthma. Recently, asthma endotypes are divided into type 2 (T2) high or T2-low (3–5). The T2-high atopic asthma usually manifests as early onset, steroid sensitive and allergic sensitization with positive allergy skin tests and increased serum-specific IgE. Allergic asthma is predominant in early childhood, which gradually declines with advancing age. In contrast, most patients with asthma onset after 40 years of age are non-allergic (6).
Pharmacological treatment of asthma is based on anti-inflammatory drugs and bronchodilators to achieve asthma control, i.e., to reduce symptoms and prevent exacerbations (2). Despite this, some patients could not achieve complete asthma control, defined as poorly controlled or severe asthma. Individuals with severe asthma were frequently older and presented more symptoms, accompanied by limited activities and lower lung function (7). In 2019, more than 260 million people had poorly controlled asthma (8). Additionally, asthma is responsible for over 1,000 deaths a day (9–11). The rising trend of asthma leads to an increased burden on patients’ quality of life and economic burden. Asthma significantly affects patients’ quality of life physically and emotionally, in both adults and children (12, 13). The quality of life was negatively worse in patients with severe asthma (14, 15). In the United States, asthma costs increased from $53 billion to $56 billion within five years from 2002 to 2007, reaching $81.9 billion in 2013 (16, 17).
There is a need to improve asthma control and management. Multiple factors were shown to be associated with uncontrolled asthma, including allergen sensitization, socioeconomic status, and climate changes (18–21). Growing evidence shows that allergy is essential in asthma pathogenesis, particularly severe asthma, in both children and adults (7, 19, 22–25). Therefore, the identification of allergens triggering asthma symptoms may be potential to improve disease control. Generally, environmental allergens are classified as indoor allergens (house dust mites, pet dander, mold, cockroach, rodents) and outdoor allergens (mold, pollens), which would be later discussed. Low socioeconomic status (SES) has been shown to be related to worse asthma outcome as well as being a risk factor for indoor allergens sensitization (18, 26). Additionally, climate change negatively impacts pollen allergy and mold allergy (20, 27). Thus, in the context of this review, we aimed to summarize the current understanding of allergens, particularly environmental allergens, and their association with asthma. Furthermore, up-to-date evidence about means of allergen avoidance in asthma control will be subsequently discussed.
2. Overview of environmental allergens
Allergic asthma is one of the most common manifestations of reactions to indoor and outdoor allergens. The main sources of outdoor pollutants worldwide are fuel combustion from vehicular transportation, construction, agricultural operations, power plants, and industries (28). However, indoor environments also pose significant health risks, given that the majority of people spend more than 90% of their time indoors (29). Factors such as building systems, construction techniques, contaminant sources, and occupants’ behavior can affect the indoor environment. The most commonly studied risk factors for indoor pollution are environmental tobacco smoke, biomass fuel, cleaning products, and biological allergens (30). House dust mites (HDM), furred pets (primarily cat and dog dander), cockroaches, molds, plants, and rodents are the main sources of indoor allergens (31, 32). Main outdoor allergenic sources include plants (pollen, fern spores, soy dust) mold (spores, hyphae), and yeasts (33). The significant rise in allergy incidence in recent decades cannot be solely explained by genetic factors but by increasing air pollution, changing lifestyles, and interactions between biological allergens and air pollution (34, 35).
There is conflicting evidence regarding association between early exposure to mite allergen and asthma development (36). Symptoms of dust mite allergy in asthmatic children (37) and among adult asthmatics that are mite sensitive, poor pulmonary function, and aberrant bronchial reactivity (38) all correlate with the mite allergen level in their home. Seasonal changes in dust mite allergen exposure result in seasonal changes in bronchial hyperreactivity (39). According to a study on dust from primary health care centers (PHCCs) in Lisbon, Portugal in 2018, levels of dust mite Der p 1 and Fel d 1 ranged from 13.0 µg/g to 971.0 µg/g and from 7.0 µg/g to 4618.8 µg/g, respectively (40). Bases on a few studies investigated the presence of household mites in several cities in Brazil in 1998, elevated levels of Der f 1 were found in the beds of asthmatic patients (15.8 μg/g dust) and non-asthmatics (8.2 μg/g dust) (41). On the other hands, Der p 1 levels were lower in the beds of these same individuals (asthmatics—2.8 μg/g dust; non-asthmatics—4.9 μg/g dust) (41). Samples were collected from surfaces such as sofas and beds (including mattresses, bedspreads, and pillows) in 60 households during two separate periods (March and July) (42). Higher levels were found in March in these samples; Der f 1 levels were 31.7 μg/g dust in beds and 8.3 μg/g dust on sofas (42). In a US birth-cohort study of 440 children, early exposure to dust mite allergens at levels of ≥10 µg/g was linked to an increased risk of asthma at age 7 (43). Another study in Taiwan children in 2011 showed that early-life exposure to carpet at home was associated with early-onset asthma and ever-having asthma, particularly when the carpet was in the child’s bedroom (44). Additionally, an ISAAC study of 6,928 Chinese schoolchildren aged 13–14 years in 2009 found that increasing sensitization to dust mites was associated with an increased prevalence of wheezing, with high degrees of sensitization serving as a risk factor for asthma diagnosis (45).
Cockroach, dogs, cats and rodents are important indoor allergens. Dust mite allergen (Der f 1 and Der p 1), cockroach allergen (Bla g 1), cat allergen (Fel d 1), and dog allergen (Can f 1) with concentrations of 10 μg/g, 8 Units/g, 8 μg/g, and 10 μg/g, respectively, are asthma symptom thresholds for sensitive children. Additionally, the kitchen has 1.6 μg/g quantities of mouse allergen (Mus m 1) (45–50). According to a study conducted in Taiwan, exposure to dogs or any pets during early life was significantly associated with the onset of asthma before age 5 (44). Regarding rodents, a study from Netherlands reported the geometric mean of the mouse allergens and rat allergens in settled dust in positive houses was 2.5 ng/m2 (GSD 3.6) and 39.3 ng/m2, respectively (48). Additionally, mouse allergens were detectable in inner city homes of children with asthma (49).
Airborne fungi are both indoor and outdoor allergens, with major indoor fungi are Penicillium, Cladosporium, Aspergillus and outdoor species are Cladosporium, Penicillium, Aspergillus, and Alternaria (50). This study also found that exposure to mold odor during early life was associated with the development of asthma after 5 years of age, while visible mold exposure during this period was linked to ever having asthma (44). Building dampness and molds have also been linked to respiratory and asthma-related health outcomes, with specific molds such as Aspergillus and Penicillium shown to increase the risk of current asthma in children (51).
In terms of pollen—an important source of outdoor allergens, pollen counts can vary significantly throughout the day and be influenced by weather conditions in short time intervals (52, 53). Consequently, people who suffer from allergies often monitor published pollen counts and modify their behavior based on these fluctuations. A particular study revealed that a greater quantity of total pollen was collected at a height of 1.5 meters above the ground (25,204 grains) compared to 35 m (16,218 grains) or 70 m (14,408 grains) (52–54). A study involving 98 children diagnosed with allergic rhinitis and asthma found that nasal scores exhibited a linear increase in relation to pollen counts, ranging from 0 to 30 grains/m3 (55). Subsequently, symptoms escalated at a quicker pace until the counts reached 80 grains/m3 (55). Temperature and precipitation were demonstrated to affect the airborne levels of pollen and mold spores (56).
Children and teenagers in population-based research in southern Vietnam showed a high incidence of cockroach and house dust mite sensitivity, but much lower rates of sensitivity to mold and pollen (57, 58). In the south of Vietnam, dust mites and cockroaches were the most common allergen sensitizers among patients with chronic respiratory diseases. That relocation from rural to urban areas increased the incidence of dust mite sensitization (59). Our current study demonstrated that house dust mite [Dermatophagoides farinae (Df), Dermatophagoides pteronyssinus (Dp), Blomica tropicalis (Bt)] remains major allergens in adult asthma as well as pediatric asthma in Vietnam (unpublished data). Thus, inhalant allergens are major allergens to patients with respiratory diseases, including asthma, and means of allergen prevention can benefit asthma control.
3. Association of environmental allergens with asthma control
Many factors, such as adherence, intrinsic factors, and environmental exposures, contribute to poor asthma control (60). Various studies demonstrated the associations between different types of allergens, including outdoor and indoor allergens, with the development and severity of allergic diseases, including asthma (61, 62), and lack of control of severe asthma was positively correlated to the co-existence of moderate-to-severe rhinitis (63, 64). A cross-sectional study in 12,743 patients showed the ratio of the uncontrolled group was 34.71% 8,517 patients had a history of allergies (64). Allergen exposures increased the risk of asthma exacerbation and the development of fixed airflow limitation (64).
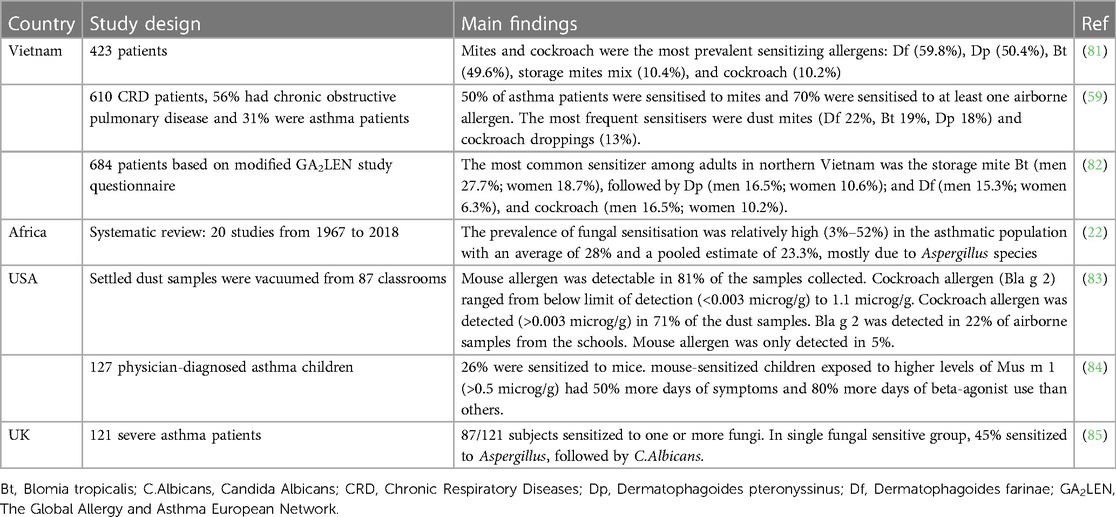
Several studies demonstrated that the inner-city home environment characterized by high airborne pollutants and higher levels of mice and cockroach were associated with more significant asthma morbidity (65–67). HDM Der p 1 allergens were proven as an independent trigger of asthma, and pet allergen levels were associated with greater asthma severity (68). Pet allergen exposure was also related to difficult-controlled asthma (69). In Table 1, we summarized the studies demonstrating the association between environmental allergens with respiratory diseases, including asthma and other allergic diseases related to asthma. Atopic sensitization is a risk factor for asthma, and the interaction between rhinovirus and high titer IgE, especially with dust mites, enhanced the risk of asthma exacerbation (70, 71). Fungal sensitization, particularly Aspergillus, was associated with asthma symptoms, increased risk of exacerbations, and severe asthma (19, 22, 23). There is increasing evidence about the relationship between indoor dampness and visible mold, especially mold odor, with the risk of the onset of asthma (72). A suitable environment, including temperature, substrate, and high indoor humidity level or water damage, is necessary for indoor mold growth, leading to increased spores of some species that can cause asthma and exacerbate symptoms (73, 74). Outdoor fungal exposure, especially Alternaria and Cladosporium, was associated with persistent, severe asthma and the severity of asthma exacerbations (75–78). Children with asthma usually have comorbid allergic diseases, with severe rhinitis may be a significant driver of severe asthma. Studies also demonstrated the association between severe asthma exacerbation with allergies to foods, pollen, and pets (24). Especially cats and dogs are assumed to enhance endotoxin-associated asthma and wheezing (79, 80).
Notably, the allergens patterns could vary between studies depending on climate, humidity, and geography. Low SES and climate change could play detrimental effects on airborne allergen levels. Asthmatic patients with a lower SES were more likely to engage in poor health behaviors, including higher rates of smoking, obesity, a higher number of pack-years, and excessive dietary fat intake (18, 86). Furthermore, low SES patients may have higher exposures to some allergens, as studies have shown the increased cockroach and mouse allergens levels in the houses where subjects with low SES lived in (87–89). As discussed above, climate change variables average weather conditions such as global warming, air pollution from traffic and industry, and more frequent extreme climate events effected in dampness proliferation of molds, pollen and fungi concentrations, which were suggested to be related to exacerbation rate, morbidity, and mortality of asthma and respiratory diseases (90–92).
Compared to Western countries, the profile of allergen sensitization in Eastern countries was slightly different (Table 1). Given the close relationship between allergens and asthma, measures to reduce the impact of environmental allergens are essential to lessen the severity of asthma in susceptible patients (93).
4. Measures of environmental allergen controls
4.1. House dust mite
House dust mites (HDMs) are associated with an increased prevalence of perennial allergic rhinitis at lower concentrations and asthma at higher concentrations (94). The main HDM species include Dermatophagoides farinae, Dermatophagoides pteronyssinus, Euroglyphus maynei, and Blomia tropicalis. The most significant concentration of HDMs is detected in bedding, especially with increasing temperature and humidity during sleep, in fabric-covered furniture, carpets, and soft toys, as these areas provide an ideal food source in the form of exfoliated human skin (95). There have been numerous proposed measures to avoid dust mites, such as using mattress and pillow encasings, employing high-efficiency particulate air filtration (HEPA) vacuum cleaners, utilizing air purification systems, employing acaricides, controlling humidity, and physically removing mite reservoirs:
- Usage of suitable materials for covering pillows and mattresses: Fine woven fabrics with a pore size of less than 6–10 mm are recommended for pillow and mattress covers. Front-loading washing machines, which do not completely submerge goods in water, are less successful than toploading washing machines at removing mites from blankets when using cold water (96). To eradicate dust mites from blankets, use hot water in a front-loading, high-efficiency washing machine (96). All other materials in the bed should be regularly washed and dried to kill mites. The use of detergent, bleach, and repeated washing can also be important (97, 98).
- Humidity control: Lowering humidity levels can effectively inhibit dust mite growth; however, it is essential to maintain a consistent relative humidity well below 50% for this approach to be successful (61). The effectiveness of different methods to achieve such humidity levels varies depending on house design and climate conditions. In regions with temperate climates and high relative humidity, portable dehumidifiers have not demonstrated any significant impact on the presence of dust mites or the levels of mite allergens, although air conditioning and central dehumidification capable of lowering humidity below 50% can be of benefit (99).
- Use of vacuum cleaners with (high-efficiency particulate air cleanser) HEPA filters: To reduce exposure to dust mite allergen-containing particles, it is advised to regularly vacuum using cleaners equipped with HEPA filtration or opt for a central vacuum system with sufficient filtration that vents to the outside (100, 101). It is recommended to use vacuum cleaners equipped with a HEPA filtration system to trap dust mite fecal pellets effectively within the vacuum bag to remove HDMs from carpets and furniture (100, 101). While HEPA filters may not be highly effective for reducing airborne mite exposure due to the fact that mite allergens only become airborne briefly following disturbance of a soft substrate, they can still be beneficial in specific situations, although less so for mite allergens than for the smaller particles of animal dander allergens (102).
- Cleaning carpets and upholstered furniture: The majority of mite avoidance strategies were designed with the assumption that individual patients primarily encounter house dust mites (HDM) during their time spent in bed, on the floor, or on upholstered furniture (99). However, it is now suggested that for younger children who spend the majority of their time at home, with more hours spent in bed or on the floor than adults, the main mite reservoirs and sources of exposure might be mattresses and carpets (103). On the other hand, other sources of HDM might have a significant impact on adults’ exposure to these allergens (103). If possible, it is best to remove carpeting, especially in the bedroom, leaving a smooth wood or other wipeable floor. Acaricides applied to carpets have not shown clinical benefit. As mentioned, vacuuming with a vacuum cleaner with a HEPA will remove some of the mite allergen, but it cannot remove the mites themselves (99).
4.2. Cockroach
The two most frequently encountered cockroach species in domestic homes and public buildings are Blatella germanica (German cockroach) and Periplaneta americana (American cockroach). When disrupted, cockroach protein, similar to dust mite allergen, becomes airborne and settles down rapidly (104). Although most studies on cockroaches and asthma have focused on urban areas, cockroaches can also infest buildings in suburban and rural locations, regardless of the socioeconomic status of their occupants (105). The main allergens associated with cockroaches, namely Per a1, Bla g1, and Bla g2, have been found commonly in floor dust, as well as in kitchen cabinets, bathrooms, and basements (106). To reduce cockroach allergens, measures such as blocking entry points, eliminating sources of food, water, and shelter, and using traps and insecticides can be taken (43). HEPA vacuuming and covering mattresses can also help eliminate reservoirs of allergens (43). Gel bait insecticides, particularly those containing fipronil or indoxacarb, and hiring a professional pest control service have been proven effective interventions, while sprays should be avoided (107).
4.3. Grass
Grass pollens have been classified into 8 classes based on their immunologic properties and contain 20–40 distinct antigens (108). Recent studies suggest that grass pollens are the primary cause of allergic reactions, as they can trigger the rupture of pollen grains, releasing hundreds of toxic starch granules that are small enough (<3 µm) to penetrate and disturb small airways (109). Group I allergens are particularly relevant, as they provoke a reaction in 90%–95% of grass pollen-allergic patients during skin testing (110). Exposure to outdoor tree and grass pollens and fungal spores has been linked to increased allergic illnesses, including asthma (111). Although controlling outdoor pollen levels is challenging, reducing exposure can be achieved by spending less time outdoors, mainly when counts are high and in the morning (112). Besides that, indoor incursion of outdoor allergens can be reduced by closing windows, using air conditioning and high-efficiency particulate arrestor filters, and frequently washing surfaces (112). Additionally, those who are allergic to grass should avoid mowing pollinating grass. Reductions in allergy symptoms have been noticed by employing respirators, goggles, or face masks outdoors (113, 114).
4.4. Molds
Molds can grow and spread in both indoor and outdoor environments. There are several common types of molds, including Cladosporium species, basidiospores, spores of the Penicillium/Aspergillus type, and Alternaria spp (25). Most mold allergens are encountered by inhaling mold spores of varying sizes and shapes, ranging from 2 to 250 mm, with many being respirable (115). Floods, leaks, condensation, and household molds are primary indoor sources of mold growth (116). Additionally, molds can grow in aeration/conditioning ducts and water pipes (116). Reducing indoor mold exposure is essential to asthma management in sensitized patients. These interventions may include removing mold from hard surfaces, preventing rainwater intrusion, installing ventilation systems in areas such as attics, kitchens, laundry rooms, and basements, repairing plumbing leaks and water seepage by filling gaps and cracks around the foundation, cleaning exterior guttering, and so on (80). Control of indoor relative humidity under 50% by increasing ventilation and covering cold surfaces, washing with detergent, contaminated carpets and removing wallpaper can be taken to discourage mold growth and mold remediation (117). In addition, the US National Institute of Occupational Safety and Health (NIOSH) recommends using an N-95 mask or higher when removing visible mold, while the US Environmental Protection Agency (EPA) advises seeking assistance from an experienced contractor for mold removal (118). Furthermore, building materials that are extensively contaminated with mold should be replaced. While frequent vacuuming may help lower mold spores in dust, replacing rugs with alternative flooring options appears to be more effective (119).
4.5. Pets and rodents (cats, dogs, mice)
The major allergen sources from animals include dander (desquamated epithelium), saliva, urine, hair, and feathers, which produce allergens unique to each species. The main source of cat allergen, especially Fel d1, is cat saliva, also in the sebaceous glands and urine of male cats (117). It can be transported through the air by particles larger than 2.5 µm and can remain airborne for long periods (117). Besides, dog allergen, particularly Can f1 and Can f2, is present in dander, saliva, urine, and serum (117). In addition, sensitivity to rodent dander may develop in laboratory workers due to occupational exposure. Still, but the allergenic protein in rodent urine can also contribute to allergic reactions in homes infested with rodents Once asthma is established and sensitization is confirmed, the current general approach is to avoid exposure by removing pets from the home. However, it may take several months (20–24 weeks) to to significantly reduce allergen levels once the pets are removed (120). When the air cleaner was used with the cat in the room, the quantity of the Inhaled Fel d 1 was reduced by up to one-third (121). Several studies demonstrated that air filtration and mechanical washing with detergents also effectively remove cat and dog allergens and indoor air particles (102, 122). Moreover, positive results were obtained from investigating the effectiveness of using a HEPA air cleaner to reduce airborne cat allergens (121). It can be challenging to avoid exposure to cat allergens as they can be present in public places, around pet owners, and even at school. Thus, it is vital to have comprehensive knowledge of potential exposures to other furry animals. Although avoiding the animal is the best option if there is a proven relationship between exposure and symptoms, it is often not feasible and can have a significant emotional impact (117).
5. Effectiveness of environmental allergen avoidance on asthma control
5.1. House dust mite
Various methods for HDM avoidance have been introduced; however, the effectiveness of those measures is still controversial.
While allergen immunotherapy to HDM was recommended in managing uncontrolled asthma, most meta-analyses found that HDM control in the living environment did not affect asthmatic management or prevention (123–127). Consequently, HDM avoidance was not broadly recommended in the guidelines for asthma management, including Global Initiative For Asthma (GINA) (128). Nevertheless, several considerations should be taken when analyzing the systematic review and meta-analysis data. The combination of different study populations (e.g., adults and children, HDM avoidance measures (e.g., single and multiple methods), period of intervention (e.g., long and short periods), outcomes (e.g., asthmatic symptoms, hospitalization, lung function, quality of life, etc.) in meta-analyses could affect the final result (129, 130). Notably, clinical trials available in the literature were predominantly conducted in mild-to-moderate asthma and/or well-controlled asthma patients, which could blunt the effectiveness of the allergen avoidance interventions.
In adult asthmatic patients, the use of impermeable bed covers and/or pillow covers as the only intervention was found to be not effective in preventing allergic symptoms, suggesting the need for a multi-faceted approach. In a clinical trial with 1,122 adult asthmatic patients, although the concentration of HDM in mattress dust was lower in the intervention group compared to the control group, morning peak expiratory flow rate, mean reduction in steroid dose, symptom scores and quality of life were not significantly different between the two study groups (131). Similar findings were demonstrated in other clinical trials (132, 133). Another study involving adult patients with allergic rhinitis and HDM sensitization also found that using bed encasing had no effect on improving rhinitis-specific visual analog scale as well as mean daily symptom score (134). Notably, two aspects need to be considered simultaneously when conducting studies to investigate the effects of the allergen avoidance methods: the reduction of allergen concentration and the improvement of respiratory allergy symptoms (135). Avoidance measures that fail to reduce allergen levels obviously cannot be expected to reduce allergy symptoms.
In contrast, studies in children with asthma have showed different findings. A randomized trial in 241 children aged 3–17 with asthma demonstrated that using impermeable bedcover reduced HDM concentration in mattress dust and the prevalence of hospital administration due to severe asthma exacerbation (71). In another study, mattress, and pillow encasings also reduced HDM allergen concentrations and the dose of inhaled steroids used by asthmatic patients (136). A multifaceted home-based intervention, including the use of allergen-proof bedcovers and fragrance-free cleaning products as well as avoidance of indoor smoking, showed a significant reduction in daily asthma symptoms, nighttime awakening, and steroid use in children with asthma (137). Other studies in children also found that control of HDM allergens by using single or multiple methods could provide a beneficial effect on asthma management (138–140).
Interestingly, a recent study found that staying at home during COVID-19 pandemic improved asthma control and reduced inhaled corticosteroid use in children with asthma and HDM sensitization (141). This finding suggests that air pollution could be an important confounding factor to consider when investigating the effectiveness of in-door allergen avoidance.
5.2. Cockroach
Interventions in most clinical trials were successful in reducing indoor cockroach allergen; however, the effectiveness in asthma management was controversial. Wood et al. (142) demonstrated that utilization of abamectin and professional house cleaning significantly reduced Bla g1 amount in house dusts; however, the concentration was still above 8 U/g (the level associated with asthma symptoms). Results from the Inner-City Asthma Study (ICAS) demonstrated that interventions to concomitantly eliminate multiple indoor allergens (including HDM, cockroach, pet, mold) and tobacco smoke significantly reduced Der f1, Fel d1, and Bla g1 levels, which was associated with a reduction in asthmatic symptoms and morbidity for 2 years of the study (143). Another study showed that insecticide bait application significantly reduced number of cockroaches, which was associated with a lower asthma morbidity in children living in these houses (144).
In contrast, a clinical trial with multifaceted interventions significantly reduced the levels of indoor allergens; however, the cockroach allergen level (Bla g2) reduction was similar in control and intervention groups (145). In National Cooperative Inner-City Asthma Study (NCICAS), cleaning house and insecticide utilization significantly reduced cockroach allergen Bla g1 level for 2 months, but the allergen levels were increased to the baseline level at the end of the study. The compliance of the study subjects with the cleaning instructions was likely poor (146).
5.3. Grass
Besides grass pollen immunotherapy, grass pollen avoidance is highlighted for asthma management to prevent exacerbation (112–114). Due to the limited time window of pollen peak occurrences, susceptible individuals should be advised to stay home and avoid outdoor exposure (1, 2). However, there is inconclusive evidence on clinical outcomes. Masks effectively prevent pollen exposure and reduce nasal and conjunctival symptoms (147). The upper respiratory tract is closely related to asthma symptoms (148). In a meta-analysis, the summary effect estimates for a 10 grains/m3 increase in pollen exposure demonstrated a 2% increase in the risk of any allergic or asthmatic symptoms, 1%, 7%, and 11% increase in the risk of lower respiratory symptoms, upper respiratory symptoms, and ocular symptoms, respectively (3). Thus, even short-term pollen exposure increased the risks of asthma symptoms (3). There is a need for studies to personalize the exposure assessment to pollen with asthma control. The AAAAI Aerobiology Committee developed guidelines for hypoallergenic landscape plant selection in pollen-related allergic patients (4). Given that the grass pollen differs from each area depending on the local ecosystem, further studies in various geographical regions are necessary.
Grass avoidance is stressed in specific cases where the seasonal change is closely related to allergic and asthma symptoms. Epidemic thunderstorm asthma is characterized by acute asthma triggered after a thunderstorm (5–7). The incidences of respiratory admissions have been linked to the concentration of airborne allergens in the atmosphere, in association with environmental factors such as rainfall, temperature, and aerosols (5, 8). To prevent epidemic thunderstorm asthma, thunderstorm forecasts, and pollen counts are major factors in issuing public health warnings (7, 8). A case report showed cross-reactivity between Bermuda grass pollen with multiple grains (9). Aside from pharmacological therapy for grass-allergic patients, measures to prevent grass pollen exposure remain mandatory.
5.4. Molds
Outdoor allergens sensitive patients should limit outdoor activities in high levels seasons (149). In a systematic review including 12 studies with 8,028 participants, the intervention group with repairing houses or mold-damaged offices showed a reduction in asthmatic and respiratory symptoms compared to the control group in adults and children. However, the evidence was moderate to very low-quality (150). In another study, asthmatic children living in a home with indoor mold were distributed into the remediation group and control group. The remediation group received household repairs (e.g., water infiltration reduction, water-damaged building materials removals, and alterations of heating/ventilation/air-conditioning), while the control group was educated about home cleaning. Although there were no changes in total and outdoor mold indices at the end of the study, children in the remediation group significantly reduced symptoms days and decreased exacerbations compared with control asthmatics (151). Similarly, a global allergen avoidance method conducted by an indoor environment counselor enhanced patients’ lung function, and reduced the number of asthma-related hospitalizations, and the use of anti-asthmatic medication (152). Given the close association between fungal exposure and severe asthma, methods to avoid molds should be noted for vulnerable subjects.
5.5. Pets and rodents
Although removing pets from homes is the most advisable, effective long-term strategy to reduce airway responsiveness in asthmatic patients with pet allergy in many studies, it may take at least 20 weeks to decline settled dust as small particles after pet removal (80, 120, 153). There are conflicting results on the effects of pet avoidance in reducing asthma control. HEPA filtration has not been proven to reduce asthma symptoms significantly in people with pet-allergic asthma without pet removals (154). In another study, asthmatic adults, who were sensitized to pets and lived with pets in a shared home, the effect of HEPA was similar (155). Thus, in case of inefficient patient education, allergen interventions, and pharmacotherapy, further studies on allergen immunotherapy should be warranted for pet-allergic patients (156, 157).
The multifaceted integrated pest management intervention (IPM) significantly reduced allergen levels by at least 75% (158). Other approaches (e.g., air purifiers, allergen-proof mattresses, and pillow encasements) may be effectively decrease the concentration of mouse and other animal allergens (159–161). Results from the Inner-City Asthma Study (ICAS) demonstrated that home rodent-specific environmental interventions (e.g., a HEPA air filter placed in the bedroom, filling rodent access points, setting traps, education) reduced mouse allergen levels, asthma-related sleep disorders and limited activities (65). Another randomized trial of the IPM effect in 350 sensitized/exposed asthmatic children showed that prebronchodilator/postbronchodilator forced expiratory flow increased over one year after the mouse allergen concentration was reduced up to 75% (162). In contrast, in The Mouse Allergen and Asthma Intervention Trial (MAAIT) study, 361 mouse-sensitive and exposed children were administered to receive IPM plus pest management education or education alone. There were no significant differences in maximal symptom days between the two groups, although a substantial reduction at 90% of mouse allergen was noted in both groups (163). The lowered level of mouse allergens was associated with fewer hospitalizations and acute care visits (163). Similar findings were demonstrated in other clinical trials (164).
6. Conclusion
Much evidence advocates for the strong association between asthma with an allergy to environmental allergens, including outdoor and indoor allergens, particularly in severe asthma. Dust mites, cockroaches, grass, molds, pets, and rodents are the most reported allergens. Allergen avoidance is recommended in managing allergic asthma patients, and several methods have been suggested. However, measures to avoid environmental allergens can be difficult to achieve and require lifestyle modification. Although the clinical effects are inconclusive, environmental allergens avoidance may still benefit patients to some extent, with safe and less adverse effects compared to other pharmacological treatments. Therefore, identifying the sensitized allergens in patients with asthma is crucial, followed by exposure prevention methods. Further trial studies are necessary to elucidate the effectiveness of individually tailored environmental control practices in patients with asthma, specifically those with severe asthma.
Author contributions
DP, K-ML, DT, and HL participated in writing the manuscript. TT drafted the outline, finalized the manuscript, and supervised the process. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Song P, Adeloye D, Salim H, Dos Santos JP, Campbell H, Sheikh A, et al. Global, regional, and national prevalence of asthma in 2019: a systematic analysis and modelling study. J Glob Health. (2022) 12:04052. doi: 10.7189/jogh.12.04052
2. Global Initiative for Asthma. Global Strategy for Asthma management and Prevention (2022 update).
3. Kuruvilla ME, Lee FEH, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. (2019) 56:219–33. doi: 10.1007/s12016-018-8712-1
4. de Llano L P, Rivas D D, Cid N B, Robles I M. Phenotype-guided asthma therapy: an alternative approach to guidelines. J Asthma Allergy. (2021) 14:207–17. doi: 10.2147/JAA.S266999
5. Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. (1999) 160(3):1001–8. doi: 10.1164/ajrccm.160.3.9812110
6. Pakkasela J, Ilmarinen P, Honkamäki J, Tuomisto LE, Andersén H, Piirilä P, et al. Age-specific incidence of allergic and non-allergic asthma. BMC Pulm Med. (2020) 20(1):1–9. doi: 10.1186/s12890-019-1040-2
7. Rönnebjerg L, Axelsson M, Kankaanranta H, Backman H, Rådinger M, Lundbäck B, et al. Severe asthma in a general population study: prevalence and clinical characteristics. J Asthma Allergy. (2021) 14:1105–15. doi: 10.2147/JAA.S327659
8. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396(10258):1204–22. doi: 10.1016/S0140-6736(20)30925-9
9. Anagnostou K, Harrison B, Iles R, Nasser S. Risk factors for childhood asthma deaths from the UK eastern region confidential enquiry 2001–2006. Prim Care Respir J. (2012) 21(1):71–7. doi: 10.4104/pcrj.2011.00097
10. Association BT. Death from asthma in two regions of England. British Thoracic Association. Br Med J (Clin Res Ed). (1982) 285(6350):1251–5. doi: 10.1136/bmj.285.6350.1251
12. Kharaba Z, Feghali E, El Husseini F, Sacre H, Abou Selwan C, Saadeh S, et al. An assessment of quality of life in patients with asthma through physical, emotional. Social, and occupational aspects. A Cross-sectional study. Front Pub Health. (2022) 10:883784. doi: 10.3389/fpubh.2022.883784
13. Kouzegaran S, Samimi P, Ahanchian H, Khoshkhui M, Behmanesh F. Quality of life in children with asthma versus healthy children. Open Access Maced J Med Sci. (2018) 6(8):1413–8. doi: 10.3889/oamjms.2018.287
14. Ali R, Ahmed N, Salman M, Daudpota S, Masroor M, Nasir M, et al. Assessment of quality of life in bronchial asthma patients. Cureus. (2020) 12(10):e10845. doi: 10.7759/cureus.10845
15. Song HJ, Blake KV, Wilson DL, Winterstein AG, Park H. Health-related quality of life and health utilities of mild, moderate, and severe asthma: evidence from the medical expenditure panel survey. JAA. (2021) 14:929–41. doi: 10.2147/JAA.S316278
16. Loftus PA, Wise SK. Epidemiology and economic burden of asthma. Int Forum Allergy Rhinol. (2015) 5(Suppl 1):S7–10. doi: 10.1002/alr.21547
17. Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008–2013. Ann Am Thorac Soc. (2018) 15(3):348–56. doi: 10.1513/AnnalsATS.201703-259OC
18. Cardet JC, Chang KL, Rooks BJ, Carroll JK, Celedón JC, Coyne-Beasley T, et al. Socioeconomic status associates with worse asthma morbidity among Black and Latinx adults. J Allergy Clin Immunol. (2022) 150(4):841–9. e4. doi: 10.1016/j.jaci.2022.04.030
19. Kennedy JL, Heymann PW, Platts-Mills TAE. The role of allergy in severe asthma. Clin Exp Allergy. (2012) 42(5):659–69. doi: 10.1111/j.1365-2222.2011.03944.x
20. D’Amato G, Chong-Neto HJ, Monge Ortega OP, Vitale C, Ansotegui I, Rosario N, et al. The effects of climate change on respiratory allergy and asthma induced by pollen and mold allergens. Allergy. (2020) 75(9):2219–28. doi: 10.1111/all.14476
21. Brunst KJ, Ryan PH, Lockey JE, Bernstein DI, McKay RT, Khurana Hershey GK, et al. Unraveling the relationship between aeroallergen sensitization, gender, second-hand smoke exposure, and impaired lung function. Pediatr Allergy Immunol. (2012) 23(5):479–87. doi: 10.1111/j.1399-3038.2012.01292.x
22. Kwizera R, Musaazi J, Meya DB, Worodria W, Bwanga F, Kajumbula H, et al. Burden of fungal asthma in Africa: a systematic review and meta-analysis. PloS One. (2019) 14(5):e0216568. doi: 10.1371/journal.pone.0216568
23. Al-Ahmad M, Jusufovic E, Arifhodzic N, Rodriguez T, Nurkic J. Association of molds and metrological parameters to frequency of severe asthma exacerbation. Allergy Asthma Clin Immunol. (2019) 15:29. doi: 10.1186/s13223-019-0323-8
24. Bonner K, Roberts G. Does allergy explain why some children have severe asthma? Clin Exp Allergy. (2018) 48(12):1594–605. doi: 10.1111/cea.13234
25. Twaroch TE, Curin M, Valenta R, Swoboda I. Mold allergens in respiratory allergy: from structure to therapy. Allergy Asthma Immunol Res. (2015) 7(3):205–20. doi: 10.4168/aair.2015.7.3.205
26. Cardet JC, Louisias M, King TS, Castro M, Codispoti CD, Dunn R, et al. Income is an independent risk factor for worse asthma outcomes. J Allergy Clin Immunol. (2018) 141(2):754–760.e3. doi: 10.1016/j.jaci.2017.04.036
27. Barnes CS. Impact of climate change on pollen and respiratory disease. Curr Allergy Asthma Rep. (2018) 18(11):59. doi: 10.1007/s11882-018-0813-7
28. Gerth van Wijk RG, de Graaf-in ‘t Veld C, Garrelds IM. Nasal hyperreactivity. Rhinology. (1999) 37(2):50–5. 10416248.10416248
29. Richardson G, Eick S, Jones R. How is the indoor environment related to asthma?: Literature review. J Adv Nurs. (2005) 52(3):328–39. doi: 10.1111/j.1365-2648.2005.03591.x
30. Mendell MJ. Indoor residential chemical emissions as risk factors for respiratory and allergic effects in children: a review. Indoor Air. (2007) 17(4):259–77. doi: 10.1111/j.1600-0668.2007.00478.x
31. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the world health organization, GA(2)LEN and AllerGen). Allergy. (2008) 63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x
32. Hulin M, Simoni M, Viegi G, Annesi-Maesano I. Respiratory health and indoor air pollutants based on quantitative exposure assessments. Eur Respir J. (2012) 40(4):1033–45. doi: 10.1183/09031936.00159011
33. Burge HA, Rogers CA. Outdoor allergens. Environ Health Perspect. (2000) 108(suppl 4):653–9. doi: 10.1289/ehp.00108s4653
34. Bousquet J, Annesi-Maesano I, Carat F, Léger D, Rugina M, Pribil C, et al. Characteristics of intermittent and persistent allergic rhinitis: DREAMS study group. Clin Exp Allergy. (2005) 35(6):728–32. doi: 10.1111/j.1365-2222.2005.02274.x
35. Downie SR, Andersson M, Rimmer J, Leuppi JD, Xuan W, Akerlund A, et al. Association between nasal and bronchial symptoms in subjects with persistent allergic rhinitis. Allergy. (2004) 59(3):320–6. doi: 10.1111/j.1398-9995.2003.00419.x
36. Miller JD. The role of dust mites in allergy. Clin Rev Allergy Immunol. (2019) 57(3):312–29. doi: 10.1007/s12016-018-8693-0
37. Chan-Yeung M, Manfreda J, Dimich-Ward H, Lam J, Ferguson A, Warren P, et al. Mite and cat allergen levels in homes and severity of asthma. Am J Respir Crit Care Med. (1995) 152(6 Pt 1):1805–11. doi: 10.1164/ajrccm.152.6.8520740
38. Custovic A, Taggart SC, Francis HC, Chapman MD, Woodcock A. Exposure to house dust mite allergens and the clinical activity of asthma. J Allergy Clin Immunol. (1996) 98(1):64–72. doi: 10.1016/S0091-6749(96)70227-0
39. van der Heide S, De Monchy JG, De Vries K, Dubois AE, Kauffman HF. Seasonal differences in airway hyperresponsiveness in asthmatic patients: relationship with allergen exposure and sensitization to house dust mites. Clin Exp Allergy. (1997) 27(6):627–33. doi: 10.1111/j.1365-2222.1997.tb01189.x
40. Leal M, Paciência I, Farraia M, Cavaleiro Rufo J, Castro Mendes F, Delgado L, et al. Airborne food allergen and aeroallergen levels in health care settings: an unaccounted for but potentially relevant source of exposure? J Investig Allergol Clin Immunol. (2021) 31(5):426–32. doi: 10.18176/jiaci.0623
41. Sopelete MC, Silva DA, Arruda LK, Chapman MD, Taketomi EA. Dermatophagoides farinae (der f 1) and dermatophagoides pteronyssinus (der p 1) allergen exposure among subjects living in Uberlândia, Brazil. Int Arch Allergy Immunol. (2000) 122(4):257–63. doi: 10.1159/000024407
42. Terra SA, Silva D a O, Sopelete MC, Mendes J, Sung SJ, Taketomi EA. Mite allergen levels and acarologic analysis in house dust samples in Uberaba, Brazil. J Investig Allergol Clin Immunol. (2004) 14(3):232–7. 15552718.15552718
43. Celedón JC, Milton DK, Ramsey CD, Litonjua AA, Ryan L, Platts-Mills TAE, et al. Exposure to dust mite allergen and endotoxin in early life and asthma and atopy in childhood. J Allergy Clin Immunol. (2007 Jul) 120(1):144–9. doi: 10.1016/j.jaci.2007.03.037
44. Chen YC, Tsai CH, Lee YL. Early-life indoor environmental exposures increase the risk of childhood asthma. Int J Hyg Environ Health. (2011) 215(1):19–25. doi: 10.1016/j.ijheh.2011.07.004
45. Li J, Wang H, Chen Y, Zheng J, Wong GWK, Zhong N. House dust mite sensitization is the main risk factor for the increase in prevalence of wheeze in 13- to 14-year-old schoolchildren in Guangzhou city, China. Clin Exp Allergy. (2013) 43(10):1171–9. doi: 10.1111/cea.12157
46. Platts-Mills TA, Sporik RB, Wheatley LM, Heymann PW. Is there a dose-response relationship between exposure to indoor allergens and symptoms of asthma? J Allergy Clin Immunol. (1995) 96(4):435–40. doi: 10.1016/S0091-6749(95)70284-9
47. Phipatanakul W, Eggleston PA, Wright EC, Wood RA. National coooperative inner-city asthma study. Mouse allergen. II. The relationship of mouse allergen exposure to mouse sensitization and asthma morbidity in inner-city children with asthma. J Allergy Clin Immunol. (2000) 106(6):1075–80. doi: 10.1067/mai.2000.110795
48. Burt SA, Parramon Dolcet LI, Wouters IM. Airborne rodent allergen levels in dutch households: a pilot study. Int J Environ Res Public Health. (2019) 16(19):3736. doi: 10.3390/ijerph16193736
49. Berg J, McConnell R, Milam J, Galvan J, Kotlerman J, Thorne P, et al. Rodent allergen in Los Angeles inner city homes of children with asthma. J Urban Health. (2008) 85(1):52–61. doi: 10.1007/s11524-007-9232-0
50. Lee T, Grinshpun SA, Martuzevicius D, Adhikari A, Crawford CM, Reponen T. Culturability and concentration of indoor and outdoor airborne fungi in six single-family homes. Atmos Environ. (2006) 40(16):2902–10. doi: 10.1016/j.atmosenv.2006.01.011
51. Nguyen T, Lurie M, Gomez M, Reddy A, Pandya K, Medvesky M. The national asthma survey—New York state: association of the home environment with current asthma status. Public Health Rep. (2010) 125(6):877–87. doi: 10.1177/003335491012500615
52. Barnes C, Pacheco F, Landuyt J, Hu F, Portnoy J. The effect of temperature, relative humidity and rainfall on airborne ragweed pollen concentrations. Aerobiologia. (2001) 17(1):61–8. doi: 10.1023/A:1007693032090
53. Smith M, Jäger S, Berger U, Sikoparija B, Hallsdottir M, Sauliene I, et al. Geographic and temporal variations in pollen exposure across Europe. Allergy. (2014) 69(7):913–23. doi: 10.1111/all.12419
54. Xiao X, Fu A, Xie X, Kang M, Hu D, Yang P, et al. An investigation of airborne allergenic pollen at different heights. Int Arch Allergy Immunol. (2013) 160(2):143–51. doi: 10.1159/000339673
55. Kiotseridis H, Cilio CM, Bjermer L, Tunsäter A, Jacobsson H, Dahl A. Grass pollen allergy in children and adolescents-symptoms, health related quality of life and the value of pollen prognosis. Clin Transl Allergy. (2013) 3:19. doi: 10.1186/2045-7022-3-19
56. Paudel B, Chu T, Chen M, Sampath V, Prunicki M, Nadeau KC. Increased duration of pollen and mold exposure are linked to climate change. Sci Rep. (2021) 11(1):12816. doi: 10.1038/s41598-021-92178-z
57. Sy DQ, Thanh Binh MH, Quoc NT, Hung NV, Quynh Nhu DT, Bao NQ, et al. Prevalence of asthma and asthma-like symptoms in Dalat Highlands, Vietnam. Singapore Med J. (2007) 48(4):294–303. 17384875.17384875
58. Flohr C, Tuyen LN, Lewis S, Quinnell R, Minh TT, Liem HT, et al. Poor sanitation and helminth infection protect against skin sensitization in Vietnamese children: a cross-sectional study. J Allergy Clin Immunol. (2006) 118(6):1305–11. doi: 10.1016/j.jaci.2006.08.035
59. Chu HT, Godin I, Phuong NT, Nguyen LH, Hiep TTM, Michel O. Allergen sensitisation among chronic respiratory diseases in urban and rural areas of the south of Vietnam. Int J Tuberc Lung Dis. (2018) 22(2):221–9. doi: 10.5588/ijtld.17.0069
60. Sheehan WJ, Phipatanakul W. Difficult-to-control asthma: epidemiology and its link with environmental factors. Curr Opin Allergy Clin Immunol. (2015) 15(5):397–401. doi: 10.1097/ACI.0000000000000195
61. Baxi SN, Phipatanakul W. The role of allergen exposure and avoidance in asthma. Adolesc Med State Art Rev. (2010) 21(1):57. 20568555; 2975603.20568555
62. Sheehan WJ, Phipatanakul W. Indoor allergen exposure and asthma outcomes. Curr Opin Pediatr. (2016) 28(6):772. doi: 10.1097/MOP.0000000000000421
63. Ponte EV, Franco R, Nascimento Hd, Souza-Machado A, Cunha S, Barreto ML, et al. Lack of control of severe asthma is associated with co-existence of moderate-to-severe rhinitis. Allergy. (2008) 63(5):564–9. doi: 10.1111/j.1398-9995.2007.01624.x
64. Tomisa G, Horváth A, Szalai Z, Müller V, Tamási L. Prevalence and impact of risk factors for poor asthma outcomes in a large, specialist-managed patient cohort: a real-life study. J Asthma Allergy. (2019) 12:297–307. doi: 10.2147/JAA.S211246
65. Pongracic JA, Visness CM, Gruchalla RS, Evans R III, Mitchell HE. Effect of mouse allergen and rodent environmental intervention on asthma in inner-city children. Ann Allergy Asthma Immunol. (2008) 101(1):35–41. doi: 10.1016/S1081-1206(10)60832-0
66. Simons E, Curtin-Brosnan J, Buckley T, Breysse P, Eggleston PA. Indoor environmental differences between inner city and suburban homes of children with asthma. J Urban Health. (2007) 84:577–90. doi: 10.1007/s11524-007-9205-3
67. Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. (1997) 336(19):1356–63. doi: 10.1056/NEJM199705083361904
68. Gent JF, Belanger K, Triche EW, Bracken MB, Beckett WS, Leaderer BP. Association of pediatric asthma severity with exposure to common household dust allergens. Environ Res. (2009) 109(6):768–74. doi: 10.1016/j.envres.2009.04.010
69. Sasaki M, Yoshida K, Adachi Y, Furukawa M, Itazawa T, Odajima H, et al. Factors associated with asthma control in children: findings from a national web-based survey. Pediatr Allergy Immunol. (2014) 25(8):804–9. doi: 10.1111/pai.12316
70. Del Giacco SR, Bakirtas A, Bel E, Custovic A, Diamant Z, Hamelmann E, et al. Allergy in severe asthma. Allergy. (2017) 72(2):207–20. doi: 10.1111/all.13072
71. Murray CS, Foden P, Sumner H, Shepley E, Custovic A, Simpson A. Preventing severe asthma exacerbations in children. A randomized trial of mite-impermeable bedcovers. Am J Respir Crit Care Med. (2017) 196(2):150–8. doi: 10.1164/rccm.201609-1966OC
72. Quansah R, Jaakkola MS, Hugg TT, Heikkinen SAM, Jaakkola JJ. Residential dampness and molds and the risk of developing asthma: a systematic review and meta-analysis. PloS One. (2012) 7(11):e47526. doi: 10.1371/journal.pone.0047526
73. Pitkäranta M, Meklin T, Hyvärinen A, Nevalainen A, Paulin L, Auvinen P, et al. Molecular profiling of fungal communities in moisture damaged buildings before and after remediation-a comparison of culture-dependent and culture-independent methods. BMC Microbiol. (2011) 11(1):1–16. doi: 10.1186/1471-2180-11-235
74. Medicine I of. Damp indoor spaces and health [internet]. Washington, DC: The National Academies Press (2004). Available at: https://nap.nationalacademies.org/catalog/11011/damp-indoor-spaces-and-health
75. Barnes CS, Dowling P, Van Osdol T, Portnoy J. Comparison of indoor fungal spore levels before and after professional home remediation. Ann Allergy Asthma Immunol. (2007) 98(3):262–8. doi: 10.1016/S1081-1206(10)60716-8
76. Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. (2004) 113(2):227–34. doi: 10.1016/j.jaci.2003.11.023
77. Halonen M, Stern DA, Wright AL, Taussig LM, Martinez FD. Alternaria as a major allergen for asthma in children raised in a desert environment. Am J Respir Crit Care Med. (1997) 155(4):1356–61. doi: 10.1164/ajrccm.155.4.9105079
78. Caillaud D, Cheriaux M, Charpin D, Chaabane N, Thibaudon M. Moisissures extérieures et santé respiratoire. Rev Mal Respir. (2018) 35(2):188–96. doi: 10.1016/j.rmr.2018.01.001
79. Mendy A, Wilkerson J, Salo PM, Cohn RD, Zeldin DC, Thorne PS. Exposure and sensitization to pets modify endotoxin association with asthma and wheeze. J Allergy Clin Immunol Pract. (2018) 6(6):2006–2013.e4. doi: 10.1016/j.jaip.2018.04.009
80. Ahluwalia SK, Matsui EC. Indoor environmental interventions for furry pet allergens, pest allergens, and mold: looking to the future. J Allergy Clin Immunol Pract. (2018) 6(1):9–19. doi: 10.1016/j.jaip.2017.10.009
81. Trinh TH, Nguyen PT, Tran TT, Pawankar R, Pham DL. Profile of aeroallergen sensitizations in allergic patients living in Southern Vietnam. Front Allergy. (2022) 3:1058865. doi: 10.3389/falgy.2022.1058865
82. Lâm HT, Ekerljung L, Bjerg A, Văn Tường N, Lundbäck B, Rönmark E. Sensitization to airborne allergens among adults and its impact on allergic symptoms: a population survey in northern Vietnam. Clin Transl Allergy. (2014) 4:1–9. doi: 10.1186/2045-7022-4-1
83. Chew G, Correa J, Perzanowski M. Mouse and cockroach allergens in the dust and air in northeastern United States inner-city public high schools. Indoor air. (2005) 15(4):228–34. doi: 10.1111/j.1600-0668.2005.00363.x
84. Matsui EC, Eggleston PA, Buckley TJ, Krishnan JA, Breysse PN, Rand CS, et al. Household mouse allergen exposure and asthma morbidity in inner-city preschool children. Ann Allergy Asthma Immunol. (2006) 97(4):514–20. doi: 10.1016/S1081-1206(10)60943-X
85. O’driscoll B, Powell G, Chew F, Niven R, Miles J, Vyas A, et al. Comparison of skin prick tests with specific serum immunoglobulin E in the diagnosis of fungal sensitization in patients with severe asthma. Clinical & Experimental Allergy. (2009) 39(11):1677–83. doi: 10.1111/j.1365-2222.2009.03339.x
86. Hulshof KF, Brussaard JH, Kruizinga AG, Telman J, Löwik MR. Socio-economic status, dietary intake and 10y trends: the Dutch National Food Consumption Survey. Eur J Clin Nutr. (2003) 57(1):128–37. doi: 10.1038/sj.ejcn.1601503
87. Forno E, Celedon JC. Asthma and ethnic minorities: socioeconomic status and beyond. Curr Opin Allergy Clin Immunol. (2009) 9(2):154-60. doi: 10.1097/aci.0b013e3283292207
88. Wang C, Abou El-Nour MM, Bennett GW. Survey of pest infestation, asthma, and allergy in low-income housing. J Community Health. (2008) 33(1):31–9. doi: 10.1007/s10900-007-9064-6
89. Kitch BT, Chew G, Burge HA, Muilenberg ML, Weiss ST, Platts-Mills TA, et al. Socioeconomic predictors of high allergen levels in homes in the greater Boston area. Environ Health Perspect. (2000) 108(4):301–7. doi: 10.1289/ehp.00108301
90. Verlato G, Calabrese R, Marco Rd. Correlation between asthma and climate in the European community respiratory health survey. Arch Environ Health Int J. (2002) 57(1):48–52. doi: 10.1080/00039890209602916
91. Eguiluz-Gracia I, Mathioudakis AG, Bartel S, Vijverberg SJ, Fuertes E, Comberiati P, et al. The need for clean air: the way air pollution and climate change affect allergic rhinitis and asthma. Allergy. (2020) 75(9):2170–84. doi: 10.1111/all.14177
92. Thibaudon M, Besancenot J. Outdoor aeroallergens and climate change. Rev Mal Respir. (2021) 38(10):1025–36. doi: 10.1016/j.rmr.2021.08.007
93. Custovic A, Murray CS, Gore RB, Woodcock A. Controlling indoor allergens. Ann Allergy Asthma Immunol. (2002) 88(5):432–42. doi: 10.1016/S1081-1206(10)62378-2
94. Zhan X, Li C, Xu H, Xu P, Zhu H, Diao J, et al. Air-conditioner filters enriching dust mites allergen. Int J Clin Exp Med. (2015) 8(3):4539–44.26064381
95. Vogel P, Bosco SMD, Ferla NJ. Mites and the implications on human health. Nutr Hosp. (2014) 31(2):944–51. doi: 10.3305/nh.2015.31.2.7772
96. Miller JD. Differences in mite survival in blankets washed in top-loading vs. front-loading washing machines.
97. Choi SY, Lee IY, Sohn JH, Lee YW, Shin YS, Yong TS, et al. Optimal conditions for the removal of house dust mite, dog dander, and pollen allergens using mechanical laundry. Ann Allergy Asthma Immunol. (2008) 100(6):583–8. doi: 10.1016/S1081-1206(10)60060-9
98. Arlian LG, Vyszenski-Moher DL, Morgan MS. Mite and mite allergen removal during machine washing of laundry. J Allergy Clin Immunol. (2003) 111(6):1269–73. doi: 10.1067/mai.2003.1547
99. Portnoy J, Miller JD, Williams PB, Chew GL, Miller JD, Zaitoun F, et al. Environmental assessment and exposure control of dust mites: a practice parameter. Ann Allergy Asthma Immunol. (2013) 111(6):465–507. doi: 10.1016/j.anai.2013.09.018
100. Wilson JM, Platts-Mills TAE. Home environmental interventions for house dust Mite. J Allergy Clin Immunol Pract. (2018) 6(1):1–7. doi: 10.1016/j.jaip.2017.10.003
101. Gore RB, Durrell B, Bishop S, Curbishley L, Woodcock A, Custovic A. High-efficiency vacuum cleaners increase personal mite allergen exposure, but only slightly. Allergy. (2006) 61(1):119–23. doi: 10.1111/j.1398-9995.2005.00946.x
102. Maya-Manzano JM, Pusch G, von Eschenbach C E, Bartusel E, Belzner T, Karg E, et al. Effect of air filtration on house dust mite, cat and dog allergens and particulate matter in homes. Clin Transl Allergy. (2022) 12(4):e12137. doi: 10.1002/clt2.12137
103. Zuiani C, Custovic A. Update on house dust mite allergen avoidance measures for asthma. Curr Allergy Asthma Rep. (2020) 20(9):50. doi: 10.1007/s11882-020-00948-y
104. Mahesh PA, Kummeling I, Amrutha DH, Vedanthan PK. Effect of area of residence on patterns of aeroallergen sensitization in atopic patients. Am J Rhinol Allergy. (2010) 24(5):e98–103. doi: 10.2500/ajra.2010.24.3529
105. Portnoy J, Chew GL, Phipatanakul W, Williams PB, Grimes C, Kennedy K, et al. Environmental assessment and exposure reduction of cockroaches: a practice parameter. J Allergy Clin Immunol. (2013) 132(4):802–808.e1-25. doi: 10.1016/j.jaci.2013.04.061
106. Maio S, Simoni M, Baldacci S, Sherrill D, Viegi G. Indoor air pollution and airway disease. In: Pawankar R, Holgate ST, Rosenwasser LJ, editors. Allergy frontiers: Epigenetics, allergens and risk factors. Tokyo: Springer Japan (2009). p. 387–401. (Allergy Frontiers). (Cited 2023 May 4). doi: 10.1007/978-4-431-72802-3_22
107. Silva JM, Camara AA, Tobias KRC, Macedo IS, Cardoso MRA, Arruda E, et al. A prospective study of wheezing in young children: the independent effects of cockroach exposure, breast-feeding and allergic sensitization. Pediatr Allergy Immunol. (2005) 16(5):393–401. doi: 10.1111/j.1399-3038.2005.00308.x
108. Singh M, Hays A. Indoor and outdoor allergies. Prim Care. (2016 Sep) 43(3):451–63. doi: 10.1016/j.pop.2016.04.013
109. Idrose NS, Dharmage SC, Lowe AJ, Lambert KA, Lodge CJ, Abramson MJ, et al. A systematic review of the role of grass pollen and fungi in thunderstorm asthma. Environ Res. (2020) 181:108911. doi: 10.1016/j.envres.2019.108911
110. Ford SA, Baldo BA. A re-examination of ryegrass (Lolium perenne) pollen allergens. Int Arch Allergy Appl Immunol. (1986) 81(3):193–203. doi: 10.1159/000234134
111. Geller-Bernstein C, Portnoy JM. The clinical utility of pollen counts. Clin Rev Allergy Immunol. (2019) 57(3):340–9. doi: 10.1007/s12016-018-8698-8
112. Diette GB, McCormack MC, Hansel NN, Breysse PN, Matsui EC. Environmental issues in managing asthma. Respir Care. (2008) 53(5):602–17. 18426614; 2396450.18426614
113. Staff IA, Taylor PE, Smith P, Singh MB, Knox RB. Cellular localization of water soluble, allergenic proteins in rye-grass (Lolium perenne) pollen using monoclonal and specific IgE antibodies with immunogold probes. Histochem J. (1990) 22(5):276–90. doi: 10.1007/BF01387183
114. Ferguson BJ. Environmental controls of allergies. Otolaryngol Clin North Am. (2008) 41(2):411–7; viii–ix. doi: 10.1016/j.otc.2007.11.006
115. Dallongeville A, Le Cann P, Zmirou-Navier D, Chevrier C, Costet N, Annesi-Maesano I, et al. Concentration and determinants of molds and allergens in indoor air and house dust of French dwellings. Sci Total Environ. (2015) 536:964–72. doi: 10.1016/j.scitotenv.2015.06.039
116. Burr ML, Matthews IP, Arthur RA, Watson HL, Gregory CJ, Dunstan FDJ, et al. Effects on patients with asthma of eradicating visible indoor mould: a randomised controlled trial. Thorax. (2007) 62(9):767–72. doi: 10.1136/thx.2006.070847
118. Respiratory Protection for Residents Reentering and/or Cleaning Homes that Were Flooded [Internet]. [Cited 2023 May 4]. Available at: https://www.cdc.gov/disasters/disease/respiratory.html
119. Rudert A, Portnoy J. Mold allergy: is it real and what do we do about it? Expert Rev Clin Immunol. (2017) 13(8):823–35. doi: 10.1080/1744666X.2017.1324298
120. Wood RA, Chapman MD, Adkinson NF, Eggleston PA. The effect of cat removal on allergen content in household-dust samples. J Allergy Clin Immunol. (1989) 83(4):730–4. doi: 10.1016/0091-6749(89)90006-7
121. Gore R, Bishop S, Durrell B, Curbishley L, Woodcock A, Custovic A. HEPA air filtration units in homes with cats: can they reduce personal exposure to cat allergen? Clin Exp Allergy. (2003) 33:765–9. doi: 10.1046/j.1365-2222.2003.01678.x
122. Choi YJ, Seong S, Lee K, Lee K, Seo H, Oh JW. Effects of mechanical washing and drying on the removal of pet allergens. Allergy Asthma Proc. (2022) 43(5):e25–e30. doi: 10.2500/aap.2022.43.220029
123. Gøtzsche PC, Hammarquist C, Burr M. House dust mite control measures in the management of asthma: meta-analysis. Br Med J. (1998) 317(7166):1105–10; discussion 1110. doi: 10.1136/bmj.317.7166.1105
124. Gøtzsche PC, Johansen HK. House dust mite control measures for asthma. Cochrane Database Syst Rev. (2008) 2008(2):CD001187. doi: 10.1002/14651858.CD001187.pub3
125. Macdonald C, Sternberg A, Hunter P. A systematic review and meta-analysis of interventions used to reduce exposure to house dust and their effect on the development and severity of asthma. Cien Saude Colet. (2008) 13(6):1907–15. doi: 10.1590/S1413-81232008000600026
126. Arroyave WD, Rabito FA, Carlson JC, Friedman EE, Stinebaugh SJ. Impermeable dust mite covers in the primary and tertiary prevention of allergic disease: a meta-analysis. Ann Allergy Asthma Immunol. (2014) 112(3):237–48. doi: 10.1016/j.anai.2014.01.006
127. Leas BF, D’Anci KE, Apter AJ, Bryant-Stephens T, Lynch MP, Kaczmarek JL, et al. Effectiveness of indoor allergen reduction in asthma management: a systematic review. J Allergy Clin Immunol. (2018) 141(5):1854–69. doi: 10.1016/j.jaci.2018.02.001
128. Levy ML, Bacharier LB, Bateman E, Boulet LP, Brightling C, Buhl R, et al. Key recommendations for primary care from the 2022 global initiative for asthma (GINA) update. NPJ Prim Care Respir Med. (2023) 33(1):7. doi: 10.1038/s41533-023-00330-1
129. van Boven FE, Arends LR, Braunstahl GJ, van Wijk RG. A reintroduction of environmental mite allergen control strategies for asthma treatment and the debate on their effectiveness. Clin Exp Allergy. (2019) 49(4):400–9. doi: 10.1111/cea.13340
130. van Boven FE, de Jong NW, Braunstahl GJ, van Wijk R G, Arends LR. A meta-analysis of baseline characteristics in trials on mite allergen avoidance in asthmatics: room for improvement. Clin Transl Allergy. (2020) 10:2. doi: 10.1186/s13601-019-0306-3
131. Woodcock A, Forster L, Matthews E, Martin J, Letley L, Vickers M, et al. Control of exposure to mite allergen and allergen-impermeable bed covers for adults with asthma. N Engl J Med. (2003) 349(3):225–36. doi: 10.1056/NEJMoa023175
132. Dharmage S, Walters EH, Thien F, Bailey M, Raven J, Wharton C, et al. Encasement of bedding does not improve asthma in atopic adult asthmatics. Int Arch Allergy Immunol. (2006) 139(2):132–8. doi: 10.1159/000090388
133. de Vries MP, van den Bemt L, Aretz K, Thoonen BPA, Muris JWM, Kester ADM, et al. House dust mite allergen avoidance and self-management in allergic patients with asthma: randomised controlled trial. Br J Gen Pract. (2007) 57(536):184–90. 17359604; 2042544.17359604
134. Terreehorst I, Hak E, Oosting AJ, Tempels-Pavlica Z, de Monchy JGR, Bruijnzeel-Koomen CAFM, et al. Evaluation of impermeable covers for bedding in patients with allergic rhinitis. N Engl J Med. (2003) 349(3):237–46. doi: 10.1056/NEJMoa023171
135. Miller JD. Analyzing environmental control studies by the achieved decrease in exposure. J Allergy Clin Immunol: in Practice. (2020) 8(7):2456. doi: 10.1016/j.jaip.2020.02.045
136. Halken S, Høst A, Niklassen U, Hansen LG, Nielsen F, Pedersen S, et al. Effect of mattress and pillow encasings on children with asthma and house dust mite allergy. J Allergy Clin Immunol. (2003) 111(1):169–76. doi: 10.1067/mai.2003.5
137. Sweet LL, Polivka BJ, Chaudry RV, Bouton P. The impact of an urban home-based intervention program on asthma outcomes in children. Public Health Nurs. (2014) 31(3):243–52. doi: 10.1111/phn.12071
138. Carter MC, Perzanowski MS, Raymond A, Platts-Mills TA. Home intervention in the treatment of asthma among inner-city children. J Allergy Clin Immunol. (2001) 108(5):732–7. doi: 10.1067/mai.2001.119155
139. Ehnert B, Lau-Schadendorf S, Weber A, Buettner P, Schou C, Wahn U. Reducing domestic exposure to dust mite allergen reduces bronchial hyperreactivity in sensitive children with asthma. J Allergy Clin Immunol. (1992) 90(1):135–8. doi: 10.1016/S0091-6749(06)80024-2
140. Kanchongkittiphon W, Gaffin JM, Phipatanakul W. The indoor environment and inner-city childhood asthma. Asian Pac J Allergy Immunol. (2014) 32(2):103–10. 25003723; 4110514.25003723
141. Yucel E, Suleyman A, Hizli Demirkale Z, Guler N, Tamay ZU, Ozdemir C. ‘Stay at home’: is it good or not for house dust mite sensitized children with respiratory allergies? Pediatr Allergy Immunol. (2021) 32(5):963–70. doi: 10.1111/pai.13477
142. Wood RA, Eggleston PA, Rand C, Nixon WJ, Kanchanaraksa S. Cockroach allergen abatement with extermination and sodium hypochlorite cleaning in inner-city homes. Ann Allergy Asthma Immunol. (2001) 87(1):60–4. doi: 10.1016/S1081-1206(10)62324-1
143. Morgan WJ, Crain EF, Gruchalla RS, O’Connor GT, Kattan M, Evans R, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. (2004) 351(11):1068–80. doi: 10.1056/NEJMoa032097
144. Rabito FA, Carlson JC, He H, Werthmann D, Schal C. A single intervention for cockroach control reduces cockroach exposure and asthma morbidity in children. J Allergy Clin Immunol. (2017) 140(2):565–70. doi: 10.1016/j.jaci.2016.10.019
145. DiMango E, Serebrisky D, Narula S, Shim C, Keating C, Sheares B, et al. Individualized household allergen intervention lowers allergen level but not asthma medication use: a randomized controlled trial. J Allergy Clin Immunol Pract. (2016) 4(4):671–679.e4. doi: 10.1016/j.jaip.2016.01.016
146. Gergen PJ, Mortimer KM, Eggleston PA, Rosenstreich D, Mitchell H, Ownby D, et al. Results of the national cooperative inner-city asthma study (NCICAS) environmental intervention to reduce cockroach allergen exposure in inner-city homes. J Allergy Clin Immunol. (1999) 103(3 Pt 1):501–6. doi: 10.1016/S0091-6749(99)70477-X
147. Kc B, S K, T Z, S B. Face masks suitable for preventing COVID-19 and pollen allergy. A study in the exposure chamber. Allergo J Int. (2021) 30(5):176–82. doi: 10.1007/s40629-021-00180-8
148. Upper respiratory tract microbiota is associated with small airway function and asthma severity | BMC Microbiology | Full Text. Available at: https://bmcmicrobiol.biomedcentral.com/articles/10.1186/s12866-023-02757-5 (Cited May 24, 2023).
149. German JA, Harper MB. Environmental control of allergic diseases. Am Fam Physician. (2002) 66(3):421. 12182518.12182518
150. Sauni R, Uitti J, Jauhiainen M, Kreiss K, Sigsgaard T, Verbeek JH. Remediating buildings damaged by dampness and mould for preventing or reducing respiratory tract symptoms, infections and asthma. Cochrane Rev J. (2013) 8(3):944–1000. doi: 10.1002/ebch.1914
151. Kercsmar CM, Dearborn DG, Schluchter M, Xue L, Kirchner HL, Sobolewski J, et al. Reduction in asthma morbidity in children as a result of home remediation aimed at moisture sources. Environ Health Perspect. (2006) 114(10):1574–80. doi: 10.1289/ehp.8742
152. Gangneux JP, Bouvrais M, Frain S, Morel H, Deguen S, Chevrier S, et al. Asthma and indoor environment: usefulness of a global allergen avoidance method on asthma control and exposure to molds. Mycopathologia. (2020) 185(2):367–71. doi: 10.1007/s11046-019-00417-9
153. Shirai T, Matsui T, Suzuki K, Chida K. Effect of pet removal on pet allergic asthma. Chest. (2005) 127(5):1565–71. doi: 10.1378/chest.127.5.1565
154. Kilburn S, Lasserson T, McKean M. Pet allergen control measures for allergic asthma in children and adults. Cochrane Database Syst Rev. (2001) 2001(1):CD002989. doi: 10.1002/14651858.CD002989
155. Francis H, Fletcher G, Anthony C, Pickering C, Oldham L, Hadley E, et al. Clinical effects of air filters in homes of asthmatic adults sensitized and exposed to pet allergens. Clin Exp Allergy. (2003) 33:101–5. doi: 10.1046/j.1365-2222.2003.01570.x
156. Virtanen T. Immunotherapy for pet allergies. Hum Vaccin Immunother. (2018) 14(4):807–14. doi: 10.1080/21645515.2017.1409315
157. Chan S, Leung D. Dog and cat allergies: current state of diagnostic approaches and challenges. Allergy Asthma Immunol Res. (2018) 10:97. doi: 10.4168/aair.2018.10.2.97
158. Phipatanakul W, Cronin B, Wood RA, Eggleston PA, Shih MC, Song L, et al. Effect of environmental intervention on mouse allergen levels in homes of inner-city Boston children with asthma. Ann Allergy Asthma Immunol. (2004) 92(4):420–5. doi: 10.1016/S1081-1206(10)61777-2
159. Butz AM, Matsui EC, Breysse P, Curtin-Brosnan J, Eggleston P, Diette G, et al. A randomized trial of air cleaners and a health coach to improve indoor air quality for inner-city children with asthma and secondhand smoke exposure. Arch Pediatr Adolesc Med. (2011) 165(8):741–8. doi: 10.1001/archpediatrics.2011.111
160. Vaughan JW, McLaughlin TE, Perzanowski MS, Platts-Mills TAE. Evaluation of materials used for bedding encasement: effect of pore size in blocking cat and dust mite allergen. J Allergy Clin Immunol. (1999) 103(2):227–31. doi: 10.1016/S0091-6749(99)70495-1
161. Lanphear B, Hornung R, Khoury J, Yolton K, Lierl M, Kalkbrenner A. Effects of HEPA air cleaners on unscheduled asthma visits and asthma symptoms for children exposed to secondhand tobacco smoke. Pediatrics. (2011) 127:93–101. doi: 10.1542/peds.2009-2312
162. Grant T, Phipatanakul W, Perzanowski M, Balcer-Whaley S, Peng RD, Curtin-Brosnan J, et al. Reduction in mouse allergen exposure is associated with greater lung function growth. J Allergy Clin Immunol. (2020) 145(2):646–653.e1. doi: 10.1016/j.jaci.2019.08.043
163. Matsui E, Perzanowski M, Peng R, Wise R, Balcer-Whaley S, Newman M, et al. Effect of an integrated pest management intervention on asthma symptoms among mouse-sensitized children and adolescents with asthma: a randomized clinical trial. JAMA. (2017) 317(10):1027–36. doi: 10.1001/jama.2016.21048
Keywords: asthma, allergen, aeroallergen, severe asthma, asthma management
Citation: Pham DL, Le K-M, Truong DDK, Le HTT and Trinh THK (2023) Environmental allergen reduction in asthma management: an overview. Front. Allergy 4:1229238. doi: 10.3389/falgy.2023.1229238
Received: 26 May 2023; Accepted: 12 September 2023;
Published: 6 October 2023.
Edited by:
Lisa A. Miller, University of California, Davis, United StatesReviewed by:
Enrique Fernandez-Caldas, Inmunotek SL, SpainJeffrey D. Miller, Mission: Allergy, Inc., United States
© 2023 Pham, Le, Truong, Le and Trinh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tu H. K. Trinh kim.tu.vn@ump.edu.vn; kim.tu.vn@gmail.com
 Duy Le Pham
Duy Le Pham Kieu-Minh Le3
Kieu-Minh Le3  Huyen T. T. Le
Huyen T. T. Le Tu H. K. Trinh
Tu H. K. Trinh