Abstract
Background: In non-small-cell lung cancers (NSCLC) with programmed death-ligand 1 (PD-L1) expression on ≥ 50% of tumor cells, first-line treatment with the PD-1 inhibitor pembrolizumab improves survival compared with platinum-doublet chemotherapy. The expression level of PD-L1 is the most accurate and well-analyzed predictive biomarker of benefit from pembrolizumab, but not all patients respond. It is therefore necessary to identify new biomarkers that select the patients who benefit from this type of treatment.
Patients and methods: In this single center retrospective study, we analyzed the impact of PD-L1 expression levels, Lung Immune Prognostic Index (LIPI), Derived neutrophil to lymphocyte ratio (dNLR) and Platelet-to-Lymphocyte Ratio (PLR) on the clinical outcomes in patients who received commercial pembrolizumab as first-line treatment of NSCLC with a PD-L1 expression of ≥ 50% and negative for genomic alterations in the EGFR, ROS1 and ALK genes.
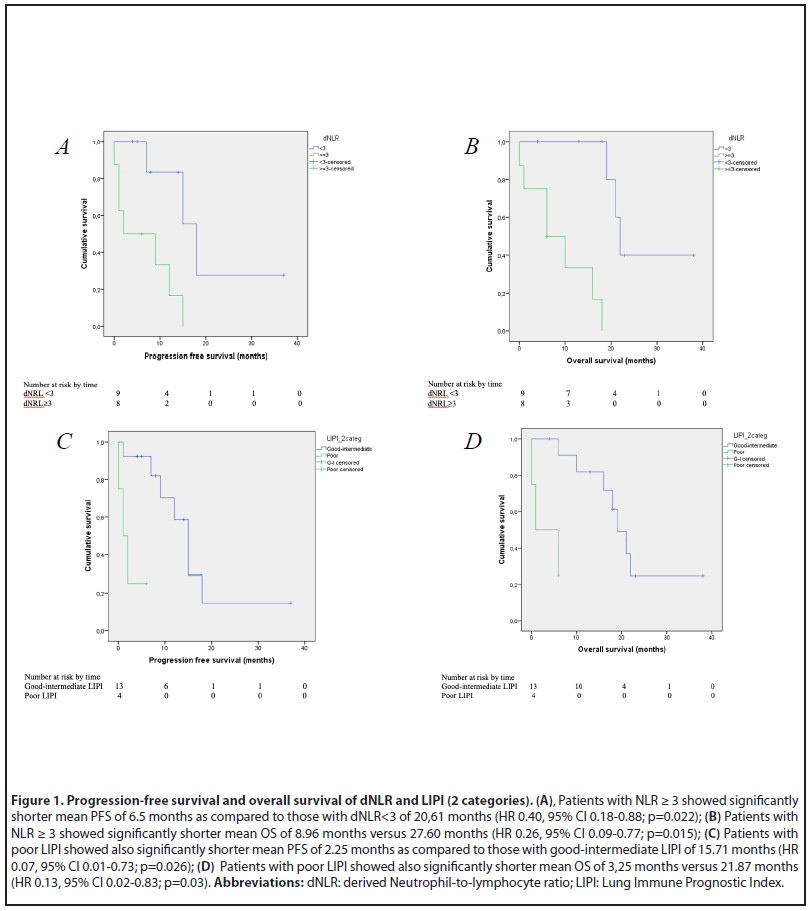
Results: Among 17 patients included in this analysis, the ORR was 41.2% [95% CI 29.3%-53.1%], the mPFS was 12 months (95% CI 6.98–17.01), and the mOS was 18 months (95%CI, 7.58-28.41). Patients with dNLR ≥ 3 showed significantly shorter mean PFS of 6.5 months as compared to those with dNLR <3 of 20.61 months (HR 0.40, 95% CI 0.18-0.88; p=0.022) and shorter mean OS of 8.96 months versus 27.60 months (HR 0.26, 95% CI 0.09-0.77; p=0,015). Patients with poor LIPI showed also significantly shorter mean PFS of 2.25 months as compared to those with good-intermediate LIPI of 15.71 months (HR 0.07, 95% CI 0.01-0.73; p=0.026) and shorter mean OS of 3.25 months vs 21.87 months (HR 0.13, 95% CI 0.02-0.83; p=0.03).
Conclusions: Among patients with NSCLC and PD-L1 expression of ≥ 50% treated with first-line pembrolizumab, clinical outcomes are significantly improved in NSCLCs with a dNLR <3 and good-intermediate LIPI. These findings are similar to other published studies.
Keywords
Biomarkers, Pembrolizumab, PD-L1 ≥ 50%, Lung immune prognostic index (LIPI), Derived neutrophil to lymphocyte ratio (dNLR), Platelet-to-lymphocyte ratio (PLR) and non-small cell lung cancer (NSCLC)
Introduction
Lung cancer is the leading cause of cancer death worldwide. In 2020, a total of 19 million cancer patients were diagnosed, of which 11.4% were lung cancer, causing 18% of all cancer deaths [1]. In 2020 in Spain, 29,638 cases were estimated [2].
Before the introduction of immunotherapy (IT), platinumbased chemotherapy has been the standard treatment for these patients, although with modest responses and with relatively short intervals until disease progression [3-5].
The emergence of programmed death pathway-1 (PD- 1) inhibitors in the first-line treatment of advanced nonsmall cell lung cancer (NSCLC) has revolutionized patient management. Programmed cell death ligand 1 (PD-L1) is to date the prognostic and predictive biomarker with the greatest potential for the selection of NSCLC patients treated with IT.
Based on the results of the KEYNOTE-001 study, in which patients with NSCLC with PD-L1 immunohistochemical expression ≥ 50% treated with pembrolizumab (PD-1 inhibitor) had better results compared to those with lower levels of PDL1 [6], the KEYNOTE-024 study was performed, a randomized phase III trial evaluating the efficacy of pembrolizumab compared to double platinum chemotherapy for first-line advanced NSCLC with a PD-L1 expression level of ≥ 50% [7]. In the pembrolizumab arm of this study, the overall response rate (ORR) was 44.8%, median progression-free survival (PFS) was 10.3 months, and median OS (mOS) was 30.0 months, all higher to the control arm [7,8].
The benefit in first line treatment was also demonstrated in the population with NSCLC with PD-L1 ≥ 1% in the KEYNOTE-042 [9] study with pembrolizumab and in the IMPOWER 110 study with atezolizumab [10].
EMPOWER-Lung 1 is a Phase 3 study which compared firstline cemiplimab monotherapy with investigator’s choice chemotherapy. In the intention to treat (ITT) and prespecified PD-L1 ≥ 50% populations cemiplimab showed superior mOS (hazard ratio (HR), 0.57; 95% CI: 0.42–0.77; P=0.0002) and mPFS (HR, 0.54; 95% CI: 0.43–0.68; P<0.0001) versus chemotherapy, despite high crossover rate, providing rationale for cemiplimab as a new treatment option for this patient population [11].
Currently, double platinum plus pembrolizumab chemotherapy is one of the standard options for advanced NSCLC in the first-line setting, regardless of PD-L1 expression levels according to the KEYNOTE-189 and KEYNOTE-407 studies [12,13]. However, questions remain as to whether pembrolizumab monotherapy or pembrolizumab plus chemotherapy should be used in patients with NSCLC and a PD-L1 level ≥ 50%.
Current meta-analysis assessed the efficacy of first-line anti-PD-(L)1 monotherapy compared to platinum-based chemotherapy in patients with advanced NSCLC with high PD-L1 expression (≥ 50%) [14]. 2,111 patients were included. In direct comparisons, IT showed a significant improvement in PFS (pooled HR=0.69, 95% CI 0.52 to 0.90, p=0.007), OS (pooled HR=0.69, 95%: 0.61 to 0.78; p<0.001) and ORR (combined hazard ratio (RR)=1.354, 95% CI: 1.04–1.762, p=0.024).
PD-L1 expression is a reliable biomarker for estimating the possible benefits of PD-1/L1 therapies [15]. Tumor PD-L1 predicts ORR, PFS and OS. The main problem is that not all PD-L1 positive patients will benefit from IT. There should be a focus on identifying patients who may be good candidates for this type of treatment through biomarkers, and on effectively controlling adverse reactions [16]. Neutrophils, platelets, macrophages and regulatory T cells, are commonly associated with tumor progression and poor prognosis [17,18]. Frequently used parameters like Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) found in regular clinical practice, demonstrated strong prognostic markers associated with worse OS in multiple tumor types including NSCLC in the pre-immunotherapy era [19,20]. Limited studies suggested that high NLR and PLR predict poor response to nivolumab and pembrolizumab as a second line treatment [21-24].
The purpose of this retrospective study is to evaluate the predictive and prognostic performance of NLR, PLR, PD-L1 expression levels, Lung immune prognostic index (LIPI) and their dynamics in patients with PD-L1 expression of ≥ 50% NSCLC treated with pembrolizumab as a first line.
Patients and Methods
Study population
We retrospectively analyzed data from patients with NSCLC PD-L1 ≥ 50% treated with at least one dose of pembrolizumab in the first-line setting in the period between November 2017 and February 2021 in the Hospital Universitario de Torrejón (Madrid, Spain). Patients were included if they had given their consent to institutional review board-approved medical record review protocols at each institution and had advanced NSCLC without driver mutations (EGFR, ALK and ROS-1).
Clinical outcomes
The ORR and PFS were determined by radiologists using Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1. PFS was defined as the time from the start of pembrolizumab to the date of disease progression or death. Patients who were alive without disease progression were censored on the date of their last disease assessment. OS was defined as the time from the start of IT to death. Patients who were still alive at the time of data analysis were censored at the date of last contact.
PD-L1 testing assessment
PD-L1 expression was assessed in formalin-fixed tumor samples at a central laboratory with the use of a rabbit anti-human PD-L1 antibody (clone 28–8; Epitomics Inc, Burlingame, CA). Tumor samples were obtained by coreneedle or excisional biopsy or from tissue resected at the time the metastatic disease was diagnosed. We used the tumor proportion score (TPS). This score is the number of PDL1 positive tumor cells, divided by all tumor cells, and then multiplied by 100. Predefined expression levels were defined by TPS ≥ 50%.
dNLR, LIPI and PLR analysis
Complete blood cell counts and LDH levels at baseline before ICI treatment (within 5-7 days before the first treatment) were extracted from electronic medical records. Derived neutrophil to lymphocyte ratio (dNLR) was calculated by division of absolute neutrophil and lymphocyte counts measured in peripheral blood before start of pembrolizumab treatment. PLR was calculated by division of thrombocytes and lymphocytes accordingly.
The LIPI was developed on the basis of dNLR greater than 3 and LDH greater than ULN (upper limit of normal), characterizing 3 groups (good, 0 factors; intermediate, 1 factor; poor, 2 factors). The LIPI analysis was also performed, dividing it into 2 categories (good-intermediate versus poor). The cutoff for dNLR was greater than 3 (according to the cutoff from the largest published study with immune check points inhibitors (ICIs) in patients with cancer [25]), and the ULN for LDH was defined according the limit of our center (423 UI/L).
Statistical analysis
The software IBPM SPSS statistics version 26 was used for statistical analyses. Frequency tables, chi-squared tests, and two-sided Fisher`s tests were carried out at first place to correlate categorical data. Survival analyses were performed using the Kaplan-Meier method and the log-rank test. All P-values are 2-sided and CIs are at the 95% level, with significance pre-defined to be at the 0.05 level. Association between prognostic factors and survival outcomes were modelled using Cox proportional hazards regression. The association between different variables were calculated by logistic regression.
Results
Patients and tumor characteristics
Seventeen patients were included. Patients’ and tumor characteristics are summarized in Table 1. The majority of patients had adenocarcinoma histology (82.4%) and were former (52.9%) or current smokers (41.2%) with only a minority of never smokers (5.9%). 14 patients (82.4%) were Eastern Cooperative Oncology Group-Performance status (ECOG-PS) <2 and 3 patients (17.6%) were 2. Central nervous system (CNS) was the most common metastatic site (29.4%), followed by bone (23.5%). No patient had liver metastases. All patients were eligible for the examination of the tumor PD-L1 expression, of which 9 patients (52.9%) had more than 90% expression. All patients were EGFR, ALK and ROS-1 wild-type. Median dNLR was 2.6 and median PLR was 192. Good LIPI (17.6%), intermediate LIPI (58.8%) and poor LIPI (23.5%) were observed.
| Characteristics | Population (n=17) |
|---|---|
| Age | Median 67 years |
| Gender Male Female | 76,5% (13) 23,5% (4) |
| Histology | |
| Non-squamous | 82,4% (14) |
| Squamous | 17,6% (3) |
| Stage | |
| IVA | 17,6% (3) |
| IVB | 82,4% (14) |
| PD-L1 expression (%) | |
| ≥90 | 52,9% (9) |
| >60-<90 | 17,6% (3) |
| ≥50 ≤ 60 | 29,4% (5) |
| No. of metastatic sites | |
| ≥3 | 23,5% (4) |
| <3 | 76,5% (13) |
| CNS metastasis | |
| Yes | 29,4% (5) |
| No | 70,6% (12) |
| Liver metastasis | |
| Yes | 0% (0) |
| No | 100% (17) |
| Smoking history | |
| Nonsmoker | 5,9% (1) |
| Former smoker | 52,9% (9) |
| Currentsmoker | 41,2% (7) |
| Performance status | |
| 0 | 23,5% (4) |
| 1 | 58,8% (10) |
| 2 | 17,6% (3) |
| Previouscorticosteroids | |
| Yes | 70,6% (12) |
| No | 29,4% (5) |
Abbreviations: CNS: Central Nervous System
Table 1: Patient and Tumor characteristics.
Survival outcomes
Median follow-up was 22 months. At the time of analysis, 9 patients (52.9%) had died and 8 (47.1%) were alive. Median OS was 18 months (95%CI, 7.58-28.41) and median PFS was 12 months (95%CI, 6.98-17.01).
Analysis of OS and PFS revealed significant correlations with the LIPI group (2 categories) and dNLR. Higher dNLR and poor LIPI were associated with shorter OS and PFS. Patients with dNLR ≥ 3 before IT showed significantly shorter mean PFS of 6.5 months as compared to those with dNLR<3 of 20.61 months (HR 0.40, 95% CI 0.18-0.88; p=0.022) and shorter mean OS of 8.96 months versus (vs) 27.60 months (HR 0.26, 95% CI 0.09-0.77; p=0.015) (Figure 1A/B). Patients with poor LIPI before IT showed also significantly shorter mean PFS of 2.25 months as compared to those with good-intermediate LIPI of 15.71 months (HR 0.07, 95% CI 0.01-0.73; p=0.026) and shorter mean OS of 3.25 months vs 21.87 months (HR 0.13, 95% CI 0.02-0.83; p=0.03) (Figure 1C/D).
No differences were observed in terms of OS and PFS according to PLR and PD-L1 expression group (Table 2). Patients with high baseline PLR ≥ 200 and with baseline corticosteroid treatment had non-statistically significant trend toward worse OS (22.7 vs 11.2 months and 21 vs 8.3 months; p=0.1 and p=0.25).
| Variable | PFS months | HR PFS; 95% CI; p value | OS months | HR OS; 95% CI; p value |
|---|---|---|---|---|
| PD-L1 expression ≥90 >60-<90 ≥50 ≤ 60 |
14.1 7.5 10.8 |
HR 1.18; 0.57-2.41; p=0.65 | 18 19 13.6 |
HR 1.52; 0.68-3.39; p=0.31 |
| PD-L1 expression 90-100% 50-89% |
14.1 10.4 |
HR 1.33; 0.35-5.07; p=0.68 | 18 15.7 |
HR 1.14; 0.29-4.59; p=0.85 |
| LIPI (3 categories) Good Intermediate Poor | 14.3 16.6 2.2 |
HR 0.31; 0.08-1.3; p=0.1 | 20 21.8 3.2 |
HR 0.23; 0.06-0.94; p=0.04 |
| LIPI (2 categories) Good-Intermediate Poor | 15.7 2.2 |
HR 0.07; 0.01-0.73; p=0.026 | 21.9 3.2 |
HR 0.13; 0.02-0.83; p=0.03 |
| PLR <200 ≥ 200 |
16.6 8.8 |
HR 0.64; 0.38-1.25; p=0.19 | 22.7 11.2 |
HR 0.54; 0.26-1.14; p=0.1 |
| dNLR <3 ≥ 3 |
20.6 6.5 |
HR 0.40; 0.18-0.88; p=0.02 | 27.6 9 |
HR 0.26; 0.1-0.77; p=0.01 |
| Basal corticosteroids No Yes |
15.3 5.8 |
HR 0.53; 0.23-1.21; p=0.13 | 21 8.3 |
HR 0.62; 0.28-1.40; p=0.25 |
| CNS metastasis No Yes |
13.7 7.4 |
HR 0.84; 0.36-1.98; p=0.69 | 19.1 12.5 |
HR 0.96; 0.43-2.17; p=0.93 |
Abbreviation: PFS: Progression Free Survival; OS: Overall Survival; HR: Hazard Ratio; dNLR: Derived Neutrophil-to-Lymphocyte Ratio; LIPI: Lung Immune Prognostic Index; PLR: Platelet-to-Lymphocyte Ratio; CNS; Central Nervous System
Table 2: Univariable analyses of progression free survival and overall survival.
Response
The tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors (ver.1.1) (RECIST 1.1) and clinical tumor response was assessed every 3 months. At time of data cut-off response assessment to pembrolizumab treatment was available from all patients. ORR was 41.2% [95% CI 29.3%-53.1%], stable disease (SD) 29.4% and disease control rate (DCR = complete response + partial response + SD) 70.6%, whereas 5 patients (29.4%) were primary refractory. Hyperprogression was observed in one patient (5.9%) and pseudoprogression in 2 (11.8%). None of the variables studied (dNLR, PLR, LIPI or PD-L1 expression) were correlated with the ORR.
Discussion
The current study provides the first data on the predictive and prognostic value of dNLR and LIPI in NSCLC PD-L1 ≥ 50% treated with first-line pembrolizumab. dNLR ≥ 3 and LIPI (poor) had significantly shorter OS and PFS.
One of the immune resistance mechanisms described in cancer patients is the inflammation process. It promotes the growth and spread of cancer and activating oncogenic signaling pathways [26]. A peripheral pro-inflammatory status has been associated with worse outcomes in patients with cancer [27-29]. There are numerous routine blood parameters studied as possible inflammatory biomarkers in cancer patients; elevated levels of circulating white blood cells, absolute neutrophil count, absolute platelet count, and lactate dehydrogenase (LDH) level. All associated with poor results in several cancer types [20,29-31].
dNLR can predict a poor response to checkpoint inhibitors and an unfavorable outcome in patients with NSCLC, similar to data published to date [18,32,33].
Zhang et al. conducted a meta-analysis to investigate the prognostic value of NLR and PLR in NSCLC patients who received ICIs. 1845 NSCLC patients from 21 studies were included and three ICIs (nivolumab, pembrolizumab, and atezolizumab) were used [34]. Overall, high NLR was associated with poor OS (HR: 2.50, 95% CI:1.79–3.51, P<0.001) and PFS (HR: 1.77, 95% CI:1.51–2.01, P<0.001). Subgroup analyses were consistent with the pooled results. Similarly, the pooled results for PLR showed that elevated PLR was related to inferior OS (HR: 1.93, 95% CI: 1.51–2.01, P<0.001) and PFS (HR: 1.57, 95%CI: 1.30–1.90, P<0.001). However, the subgroup analysis based on test time indicated that there was no significant correlation between post-treatment PLR and survival outcomes.
The multicentric retrospective study conducted by Petrova et al., evaluated the predictive and prognostic performance of NLR, and PLR in 119 patients with NSCLC PD-L1 positive treated with pembrolizumab as a second line [24]. Patients with NLR>5 before IT showed significantly shorter mean PFS of 6.86 months (95% CI: 5.81- 7.90) as compared to those with NLR ≤ 5 of 18.82 months (95% CI:15.87-21.78; p<0.001). The only independent predictive factor for shorter PFS in the multivariate analysis was NLR>5 (HR: 4.47, 95% CI: 2.20-9.07, p<0.001), and in the OS analysis the independent negative prognostic factors were the presence of bone metastases (HR: 2.08, 95% CI: 1.10-4.94, p=0.030), NLR>5 before chemotherapy (HR: 8.09, 95% CI: 2.35-27.81, p=0.001) and high PLR before chemotherapy (HR: 2.81, 95% CI: 1.13-6.97, p=0.025). Our data suggests that NLR<3 is a potential predictive marker, which may identify patients appropriate for pembrolizumab as a first-line treatment.
In the exploratory analysis of patients with NSCLC treated with cemiplimab, observed that the magnitude of clinical benefit was associated with PD-L1 expression levels. The results for the prespecified PD-L1 ≥ 50% population, showing that OS, PFS and ORR had improved in patients with higher PD-L1 expression levels (three categories ≥ 50 to ≤ 60%, >60 to <90% and ≥ 90%) [11]. According to these findings, PD-L1 expression can be an effective tool in identifying patients who experience the greatest benefit from cemiplimab treatment.
Similar results are obtained by the study carried out by Aguilar et al. [35]. They analyzed in a multicenter retrospective study the impact of PD-L1 expression levels on the ORR, PFS, and OS in patients who received pembrolizumab as first-line treatment of NSCLC with a PD-L1 expression of ≥ 50%. 187 patients were included. The ORR was 44.4% [95% confidence interval (CI) 37.1% to 51.8%], the PFS was 6.5 months (95% CI 4.5–8.5), and the OS was not reached. The expression level of PD-L1 determined the results. Compared with patients with PD-L1 expression of 50%–89% (N=107), patients with an expression level of 90%–100% (N=80) had a significantly higher ORR (60.0% versus 32.7%, P<0.001), a significantly longer median PFS [14.5 versus 4.1 months, HR 0.50 (95% CI 0.33–0.74), P<0.01], and a significantly longer median OS [not reached versus 15.9 months, HR 0.39 (95% CI 0.21–0.70), p=0.002]. The magnitude of PD-L1 expression had no impact on either PFS, OS or ORR in our study. This is probably due to the small number of patients included in the analysis.
466 patients with advanced NSCLC participated in a multicenter retrospective study conducted by Mezquita et al. Case cohort received PD-1/PD-L1 inhibitors while a control cohort was treated with chemotherapy [36]. They analyzed LIPI (based on dNLR>3 and LDH>ULN), characterizing 3 groups (good, intermediate and poor). A dNLR>3, LDH>ULN and LIPI were independently associated with OS and PFS with LIPI. Disease control rate was also correlated with dNLR>3 and LDH>ULN. Our study provides similar data, with a clear worse prognosis for patients with poor LIPI, both in terms of PFS and OS (p<0.05).
Li et al. investigated the relationship between pretreatment LIPI and the prognosis of patients receiving first-line PD-1/ PD-L1 inhibitors plus chemotherapy in advanced/metastatic small cell lung cancer [37]. One hundred patients were included. 64% were LIPI good, 11% were LIPI por, and the remaining 25% were LIPI intermediate. The LIPI good group had better PFS (median: 8.4 vs 4.7 months, p=0.02) and OS (median: 23.8 vs 13.3 months, p=0.0006) than the LIPI intermediate/poor group. Multivariate analysis showed that pretreatment LIPI intermediate/poor was an independent risk factor for OS (HR: 2.34; 95%CI, 1.13, 4.86; p=0.02). Subgroup analysis showed that pretreatment LIPI good was associated with better PFS and OS in males, extensive disease (ED), PD-1 inhibitor treatment, smokers, and liver metastasis (p<0.05).
Our data could help to select in the future those patients who benefit the least from treatment with pembrolizumab monotherapy. It serves as the basis for future studies evaluating, for example, the role of chemo-immunotherapy instead of immunotherapy alone in that subset of patients in whom the administration of a single drug is possibly insufficient.
Several limitations were identified in our study. First, it is a single-center, retrospective study with a small sample size, although all consecutive patients with advanced NSCLC with PD-L1 ≥ 50% treated with first-line pembrolizumab during the mentioned period were included. Furthermore, the predictive value of NLR was not compared with other potential predictive markers such as tumor mutational burden or microsatellite instability. The small number of patients and events in our cohort did not allow for a multivariate analysis.
However, the correlation of high dNLR (≥ 3) and LIPI (poor) and worse prognosis was statistically significant and also appears clinically significant.
Conclusions
If the predictive value of dNLR and LIPI were demonstrated in prospective studies, they could become tools that help us select patients with PD-L1 NSCLC ≥ 50% in the first line of treatment, either pembrolizumab in monotherapy or the combination of chemotherapy-IT, with the main objective of improving the prognosis of our patients. These findings are similar to other published studies and could have implications for treatment selection as well as for clinical trial interpretation and design.
Conflict of Interest
Luis Cabezón Gutiérrez declares the following conflicts of interest: Advisory role; Boehringer-Ingelheim, Astra Zeneca, Roche and Brystol Myers Squibb. Speakers’ bureau; Roche, Astra Zeneca, Brystol Myers Squibb, Merck Serono, Ipsen Pharma, Lilly and Amgen, Angelini, Grunenthal, Kyowa Kirin, Mudipharma, Pfizer, Roche, Rovi, Leo Pharma and Boehringer Ingelheim.
Sara Custodio-Cabello, Magda Palka-Kotlowska, Silvia María Sanchez and Parham Khosravi-Shahi, declare no conflict of interests.
Authors Contribution
All authors contributed to the study conception and design:
Conceptualization: Luis Cabezón-Gutiérrez; Methodology: Sara Custodio-Cabello, Silvia María Sanchez-Luis and Luis Cabezón-Gutiérrez; Formal analysis and investigation: Sara Custodio-Cabello, Luis Cabezón-Gutiérrez and Parham Khosravi-Shahi; Writing - original draft preparation: Luis Cabezón-Gutiérrez, Sara Custodio-Cabello and Magda Palka- Kotlowska; Writing - review and editing: Luis Cabezón- Gutiérrez and Silvia María Sanchez-Luis; Supervision: Luis Cabezón-Gutiérrez, Parham Khosravi-Shahi and Magda Palka- Kotlowska.
References
2. Las cifras del cáncer en España 2020. Depósito Legal: M-3266- 2020. © 2020. Sociedad Española de Oncología Médica (SEOM). https://seom.org/seomcms/images/stories/recursos/Cifras_del_ cancer_2020.pdf.
3. Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel–carboplatin alone or with bevacizumab for non–small-cell lung cancer. New England Journal of Medicine. 2006 Dec 14;355(24):2542-50.
4. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non– small-cell lung cancer. New England Journal of Medicine. 2002 Jan 10;346(2):92-8.
5. Scagliotti G, Brodowicz T, Shepherd FA, Zielinski C, Vansteenkiste J, Manegold C, et al. Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. Journal of Thoracic Oncology. 2011 Jan 1;6(1):64-70.
6. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. New England Journal of Medicine. 2015 May 21;372(21):2018-28.
7. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non– small-cell lung cancer. New England journal of Medicine. 2016 Nov 10;375(19):1823-1833.
8. Reck M, Rodriguez-Abreu D, Robinson A, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PDL1 tumor proportion score of 50% or greater. J Clin Oncol 2019; 37: 537–546.
9. Lopes G, Wu YL, Kudaba I, Kowalski D, Cho BC, Castro G, et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS)≥ 1%: open-label, phase 3 KEYNOTE-042 study. J Clin Oncol 36: (18 Suppl): LBA4 LBA4, 2018.
10. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of PD-L1– selected patients with NSCLC. New England Journal of Medicine. 2020 Oct 1;383(14):1328-39.
11. Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, et al. LBA52 EMPOWER-Lung 1: phase III first-line (1L) cemiplimab monotherapy vs platinum-doublet chemotherapy (chemo) in advanced non-small cell lung cancer (NSCLC) with programmed cell death-ligand 1 (PD-L1)≥ 50%. Annals of Oncology. 2020 Sep 1;31:S1182-3.
12. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. New England journal of Medicine. 2018 May 31;378(22):2078-92.
13. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non– small-cell lung cancer. New England Journal of Medicine. 2018 Nov 22;379(21):2040-51.
14. Majem M, Cobo M, Isla D, Marquez-Medina D, Rodriguez-Abreu D, Casal-Rubio J, et al. PD-(L) 1 Inhibitors as Monotherapy for the First- Line Treatment of Non-Small-Cell Lung Cancer Patients with High PDL1 Expression: A Network Meta-Analysis. Journal of Clinical Medicine. 2021 Jan;10(7):1365.
15. Salmaninejad A, Valilou SF, Shabgah AG, Aslani S, Alimardani M, Pasdar A, Sahebkar A. PD‐1/PD‐L1 pathway: Basic biology and role in cancer immunotherapy. Journal of Cellular Physiology. 2019 Oct;234(10):16824-37.
16. Cabezón-Gutiérrez L, Custodio-Cabello S, Palka-Kotlowska M, Alonso-Viteri S, Khosravi-Shahi P. Biomarkers of immune checkpoint inhibitors in non-small cell lung cancer: beyond PD-L1. Clinical Lung Cancer. 2021 Sep;22(5):381-389.
17. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. The Lancet Oncology. 2014 Oct 1;15(11):e493-503.
18. Tao H, Mimura Y, Aoe K, Kobayashi S, Yamamoto H, Matsuda E, et al. Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung cancer. 2012 Jan 1;75(1):95-101.
19. Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. British Journal of Cancer. 2012 Aug;107(4):695-9.
20. Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. JNCI: Journal of the National Cancer Institute. 2014 Jun 1;106(6).
21. Park W, Kwon D, Saravia D, Desai A, Vargas F, El Dinali M, Warsch J, Elias R, et al. Developing a predictive model for clinical outcomes of advanced non-small cell lung cancer patients treated with nivolumab. Clinical Lung Cancer. 2018 May 1;19(3):280-8.
22. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017 Sep 1;111:176-81.
23. Russo A, Franchina T, Ricciardi GR, Battaglia A, Scimone A, Berenato R, et al. Baseline neutrophilia, derived neutrophil‐to‐lymphocyte ratio (dNLR), platelet‐to‐lymphocyte ratio (PLR), and outcome in non small cell lung cancer (NSCLC) treated with Nivolumab or Docetaxel. Journal of Cellular Physiology. 2018 Oct;233(10):6337-43.
24. Petrova MP, Eneva MI, Arabadjiev JI, Conev NV, Dimitrova EG, Koynov KD, et al. Neutrophil to lymphocyte ratio as a potential predictive marker for treatment with pembrolizumab as a second line treatment in patients with non-small cell lung cancer. Bioscience Trends. 2020 Feb 29;14(1):48-55.
25. Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, Cappellini GA, et al. Baseline neutrophils and derived neutrophilto- lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Annals of Oncology. 2016 Apr 1;27(4):732-8.
26. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4;144(5):646-74.
27. Zhu L, Li X, Shen Y, Cao Y, Fang X, Chen J, Yuan Y. A new prognostic score based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Onco Targets and Therapy. 2016 Aug 8;9:4879-86.
28. Laird BJ, Fallon M, Hjermstad MJ, Tuck S, Kaasa S, Klepstad P, et al. Quality of life in patients with advanced cancer: differential association with performance status and systemic inflammatory response. Journal of Clinical Oncology. 2016 Aug 10;34(23):2769.
29. McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treatment Reviews. 2013 Aug 1;39(5):534-40.
30. Petrelli F, Cabiddu M, Coinu A, Borgonovo K, Ghilardi M, Lonati V, et al. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncologica. 2015 Aug 9;54(7):961-70.
31. Paramanathan A, Saxena A, Morris DL. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surgical Oncology. 2014 Mar 1;23(1):31-9.
32. Ren F, Zhao T, Liu B, Pan L. Neutrophil–lymphocyte ratio (NLR) predicted prognosis for advanced non-small-cell lung cancer (NSCLC) patients who received immune checkpoint blockade (ICB). Onco Targets and therapy. 2019;12:4235.
33. Passiglia F, Galvano A, Castiglia M, Incorvaia L, Calò V, Listì A, et al. Monitoring blood biomarkers to predict nivolumab effectiveness in NSCLC patients. Therapeutic Advances in Medical Oncology. 2019 Apr;11:1758835919839928.
34. Zhang N, Jiang J, Tang S, Sun G. Predictive value of neutrophillymphocyte ratio and platelet-lymphocyte ratio in non-small cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. International Immunopharmacology. 2020 Aug 1;85:106677.
35. Aguilar EJ, Ricciuti B, Gainor JF, Kehl KL, Kravets S, Dahlberg S, et al. Outcomes to first-line pembrolizumab in patients with non-smallcell lung cancer and very high PD-L1 expression. Annals of Oncology. 2019 Oct 1;30(10):1653-9.
36. Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non–small cell lung cancer. JAMA Oncology. 2018 Mar 1;4(3):351-7.
37. Li L, Pi C, Yan X, Lu J, Yang X, Wang C, et al. Prognostic Value of the Pretreatment Lung Immune Prognostic Index in Advanced Small Cell Lung Cancer Patients Treated With First-Line PD-1/PD-L1 Inhibitors Plus Chemotherapy. Frontiers in Oncology. 2021 Oct 8;11:697865.
