§LENA Consortium: Labeling of Enalapril from Neonates up to Adolescents Consortium
Background: Plasma renin activity (PRA) has gained relevance as
prognostic marker in adults with heart failure. The use of PRA as a clinically
meaningful parameter in children and children with heart failure requires a
thorough knowledge of the factors that influence PRA to correctly assess PRA
levels. We aim to evaluate the influence of age, heart failure and
angiotensin-converting enzyme inhibitor (ACEi) on PRA levels in children.
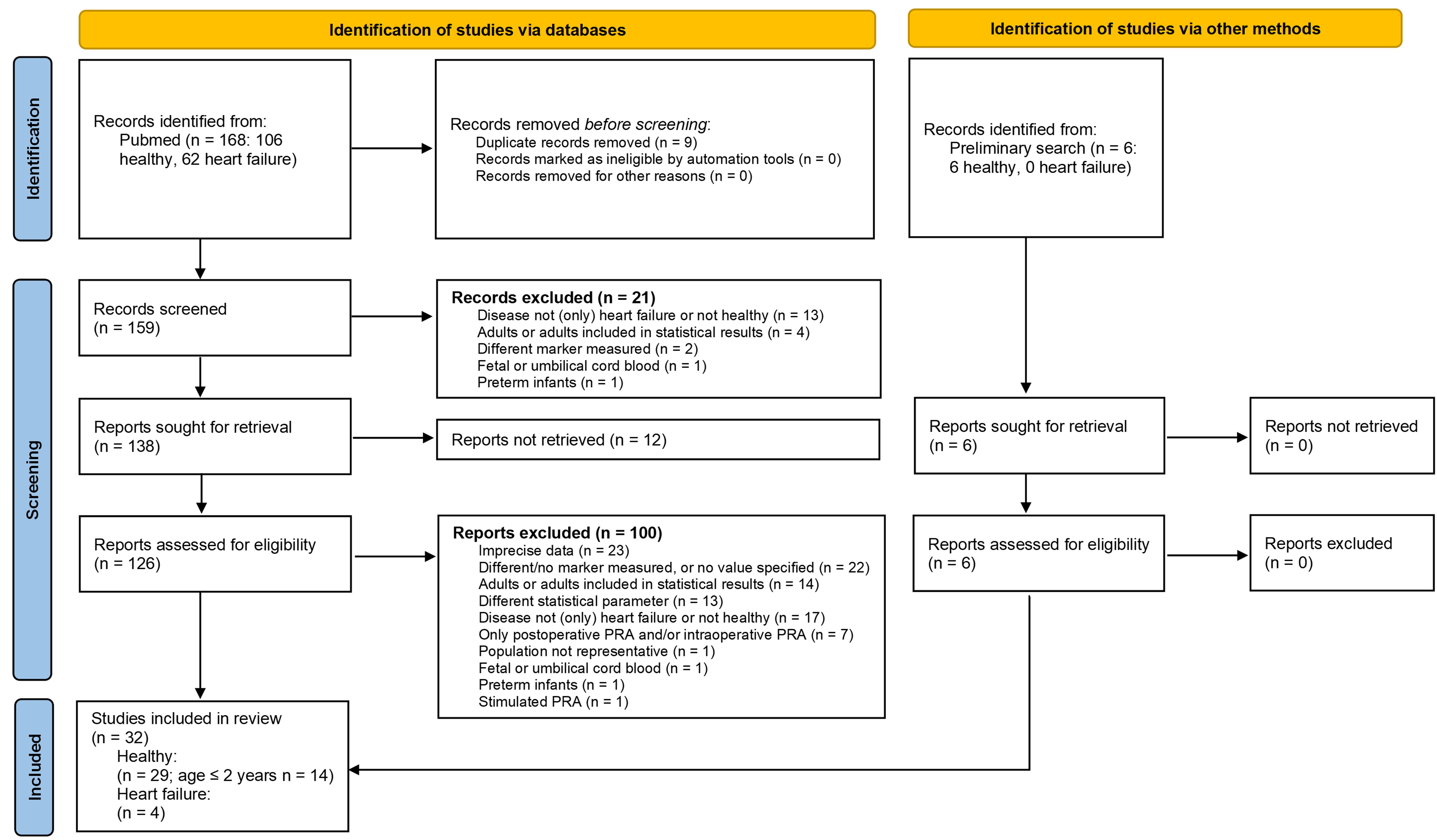
Methods: We conducted a systematic literature search to identify studies
on PRA levels in healthy children and in children with heart failure. In
addition, we analysed PRA data measured before (n = 35, aged 25 days–2.1 years),
4 hours after (n = 34) and within the first 8 days of enalapril treatment (n =
29) in children with heart failure from the European project Labeling of Enalapril from Neonates up to Adolescents (LENA). Results: Age has a profound
effect on PRA levels in healthy children, as PRA levels in the literature are up
to about 7 times higher in neonates than in older children. Children with heart
failure younger than 6 months showed 3–4 times higher PRA levels than healthy
peers in both the literature and the LENA studies. In the LENA studies, the ACEi
enalapril significantly increased median predose PRA by a factor of 4.5 in
children with heart failure after 4.7