Academic Editor: Graham Pawelec
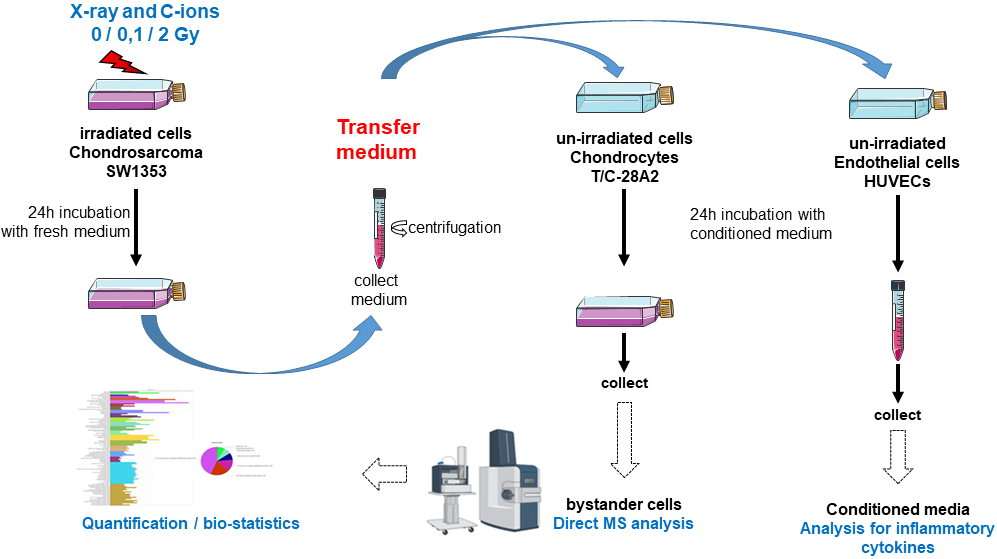
Background: Radiation-induced bystander effects are induced changes in cells that were not themselves directly irradiated but were in the vicinity of a radiation path. Such effects, which occur in the microenvironment of an irradiated tumor, remain poorly understood and depend on the cell type and irradiation quality. This study aimed to evaluate bystander effects in non-irradiated chondrocytes that received conditioned medium from irradiated chondrosarcoma cells. Methods: SW1353 chondrosarcoma cells were irradiated with X-rays and carbon ions, each at 0.1 Gy and 2 Gy, and the conditioned media of the irradiated cells were transferred to T/C-28A2 chondrocytes and Human Umbilical Venous Endothelial Cells (HUVECs). The whole proteome of bystander chondrocytes was analyzed by label-free mass spectrometry, and a comparative study was performed by dose and irradiation quality. HUVECs were evaluated for inflammatory cytokine secretion. Results: The bystander response of chondrocytes to X-ray irradiation primarily affected the protein translation pathway (DHX36, EIF3B, EIF3D, EIF3M, EIF5, RPL6, RPLP0, RPS24, SYNCRIP), IL-12 (AIP, BOLA2, MIF, GAS6, MIF, PDGFRB) and the oxidative stress pathway (MGST3, PRDX2, PXDN, SOD2, TXN, TXNL1). Following carbon-ion irradiation, the G1/S pathway (PCBP4, PSMD12, PSME, XIAP) and mitotic G2 DNA damage checkpoint pathway (MRE11, TAOK1, UIMC1) were engaged. Changes in the regulation of chromosome separation (BCL7C, BUB3, CENPF, DYNC1LI1, SMARCA4, SMC4) were associated with only low-dose X-ray and carbon-ion irradiation. Modification of the protein translation pathway represented at least 30% of bystander effects and could play a role, possibly along with stress granules, in reduction in cellular metabolism to protect proteins. Stress granules were significantly enriched according to an interaction map. Conclusions: All these accessions corresponded to a window of the proteins modulated in response to the bystander effect. Our chondrosarcoma model clarified the nature of the bystander response of chondrocytes and may suggest several interesting new mechanisms that are specific to particular irradiation doses and qualities.