Abstract
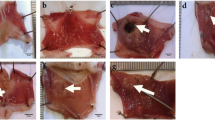
The new agents are needed in treatment of gastric ulcer that have less side effects, adequate efficacy, and no drug interactions. In this study, we aimed to investigate the potential protective effects of humic acid on experimental gastric ulcer. Wistar Albino male rats (n = 48) were randomly divided into 8 groups as follow; Control (without any applications), Humic acid (50 mg/kg), ethanol group (1 mL/rat), and indomethacin group (25 mg/kg). In the treatment groups, both gastric ulcer model and humic acid 50 mg/kg were applied. In addition, famotidine the antiulcer drug was used as positive control. All medications were administered by oral gavage. Levels of ADAM10 and ADAMTS12 in gastric mucosa were determined by ELISA method. Hematoxylin-Eosin (H&E) staining, iNOS, and PCNA immunohistochemical staining were performed for histopathological investigations. Apoptosis was demonstrated by using the TUNEL method. In addition, the levels of inflammatory cytokines (TNF-α, IL-6, IL-10) and caspase-3 gene were determined by qRT-PCR. ADAM10 and ADAMTS12 levels significantly increased in the treatment groups compared to the ulcer groups (p < 0.05). The experimental groups showed mucosal erosion, bleeding, leukocyte infiltration and edema. Treatment with humic acid and famotidine was found to suppress iNOS activity, thereby decreasing proinflammatory activity and preventing damage to the gastric mucosa, while reducing the number of apoptotic cells. IL-6, IL-10, TNF-α and caspase-3 levels were significantly decreased in the treatment groups compared to damaged gastric mucosa. As a result, humic acid may be defined as a potential protective agent with its anti-inflammatory effect in gastric ulcer.








Similar content being viewed by others
REFERENCES
Acharya, S.B., Frotan, M.H., Goel, R.K., et al., Pharmacological actions of Shilajit, Indian J. Exp. Biol., 1988, vol. 26, pp. 775–777. PMID: 3248832.
Almasaudi, S.B., El-Shitany, N.A., Abbas, A.T., et al., Antioxidant, anti-inflammatory, and antiulcer potential of manuka honey against gastric ulcer in rats, Oxid. Med. Cell Longev., 2016, vol. 2016, art. 3643824.https://doi.org/10.1155/2016/364382
Antonisamy, P., Duraipandiyan, V., Aravinthan, A., et al., Protective effects of friedelin isolated from Azima tetracantha Lam. against ethanol-induced gastric ulcer in rats and possible underlying mechanisms, Eur. J. Pharmacol., 2015, vol. 750, pp. 167–175. https://doi.org/10.1016/j.ejphar.2015.01.015
Arab, H.H., Salama, S.A., Omar, H.A., et al., Diosmin protects against ethanol-induced gastric injury in rats: novel anti-ulcer actions, PLoS One, 2015, vol. 10, art. e0122417. https://doi.org/10.1371/journal.pone.0122417
Aziz, R.S., Siddiqua, A., Shahzad, A., et al., Oxyresveratrol ameliorates ethanol-induced gastric ulcer viadownregulation of IL-6, TNF-α, NF-ĸB, and COX-2 levels, and upregulationof TFF-2 levels, Biomed. Pharmacother., 2019, vol. 110, pp. 554–560. https://doi.org/10.1016/j.biopha.2018.12.002
Bansal, V.K. and Goel, R.K., Gastroprotective effect of Acacia nilotica young seedless pod extract: role of polyphenolic constituents, Asian Pacif. J. Trop. Med., 2012, vol. 5, pp. 523–528. https://doi.org/10.1016/S1995-7645(12)60092-3
Baraka, A.M., Guemei, A., and Gawad, H.A., Role of modulation of vascular endothelial growth factor and tumor necrosis factor-alpha in gastric ulcer healing in diabetic rats, Biochem. Pharmacol., 2010, vol. 79, pp. 1634–1639. https://doi.org/10.1016/j.bcp.2010.02.001
Caldas, G.F.R., Oliveira, A.R.D.S., Araújo, A.V., et al., Gastroprotective and ulcer healing effects of essential oil of Hyptis martiusii Benth. (Lamiaceae), PLoS One, 2014, vol. 9, art. e84400. https://doi.org/10.1371/journal.pone.0084400
Chang, X., Luo, F., Jiang, X., et al., Protective activity of salidro side against ethanol-induced gastric ulcer via the MAPK/NF-휅B pathway in vivo and in vitro, Int. Immunopharmacol., 2015, vol. 28, pp. 604–615. https://doi.org/10.1016/j.intimp.2015.07.031
Chow, D.K. and Sung, J.J., Non-NSAID non-H. pylori ulcer disease, Best Pract. Res. Clin. Gastroenterol., 2009, vol. 23, pp. 3–9. https://doi.org/10.1016/j.bpg.2008.11.010
DeVault, R.K. and Talley, N.G., Insights into the future of gastric acid suppression, Nat. Rev. Gastroenterol. Hepatol., 2009, vol. 6, pp. 524–532. https://doi.org/10.1038/nrgastro.2009.125
El-Maraghy, S.A., Rizk, S.M., and Shahin, N.N., Gastroprotective effect of crocin in ethanol- induced gastric injury in rats, Chem. Biol. Interact., 2015, vol. 229, pp. 26–35. https://doi.org/10.1016/j.cbi.2015.01.015
Elsaed, W.M., Alahmadi, A.M., Al-Ahmadi, B.T., et al., Gastroprotective and antioxidant effects of fluvoxamine on stress-induced peptic ulcer in rats, J. Taibah Univ. Med. Sci., 2018, vol. 13, pp. 422–431. https://doi.org/10.1016/j.jtumed.2018.04.010
Elshazly, S.M., Abd El Motteleb, D.M., et al., Hesperidin protects against stress induced gastric ulcer through regulation of peroxisome proliferator activator receptor gamma in diabetic rats, Chem. Biol. Interact., 2018, vol. 291, pp. 153–161. https://doi.org/10.1016/j.cbi.2018.06.027
Erin, N., Türker, S., Elpek, Ö., et al., ADAM proteases involved in inflammation are differentially altered in patients with gastritis or ulcer, Exp. Ther. Med., 2018, vol. 15, pp. 1999–2005. https://doi.org/10.3892/etm.2017.5619
Estes, L.L., Fuhs, D.W., Heaton, A.H., et al., Gastric ulcer perforation associated with the use of injectable ketorolac, Ann. Pharmacother., 1993, vol. 27, pp. 42–43. https://doi.org/10.1177/106002809302700111
Eto, K., Puzon-McLaughlin, W., Sheppard, D., et al., RGD-independent binding of integrin α9β1 to the ADAM-12 and -15 disintegrin domains mediates cell–cell interaction, J. Biol. Chem., 2000, vol. 275, pp. 34922–34930. https://doi.org/10.1074/jbc.M001953200
Fisher, A.A. and Le Couteur, D.G., Nephrotoxicity and hepatotoxicity of histamine H2 receptor antagonists, Drug Saf., 2001, vol. 24, pp. 39–57. https://doi.org/10.2165/00002018-200124010-00004
Flower, R.J., The development of COX2 inhibitors, Nat. Rev. Drug Discov., 2003, vol. 2, pp. 179–191. https://doi.org/10.1038/nrd1034
Hsu, S.M., Raine, L., and Fanger, H.X., Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures, J. Histochem. Cytochem., 1981, vol. 29, pp. 577–580. https://doi.org/10.1177/29.4.6166661
Karaboğa, İ., Ovalı, M.A., Yılmaz, A., et al., Gastroprotective effect of apricot kernel oil in ethanol-induced gastric mucosal injury in rats, Biotech. Histochem., 2018, vol. 93, pp. 601–607. https://doi.org/10.1080/10520295.2018.1511064
Kel’ginbaev, N.S., Sorokina, V.A., Stefanidu, A.G., et al., Treatment of long tubular bone fractures with Mumie Assil preparations in experiments and clinical conditions, Eksp. Khir. Anesteziol., 1973, vol. 18, pp. 31–35. PMID: 4271714.
Kurz, T., Hoffjan, S., and Hayes, M.G., Fine mapping and positional candidate studies on chromosome 5p13 identify multiple asthma susceptibility loci, J. Allergy Clin. Immunol., 2006, vol. 118, pp. 396–402. https://doi.org/10.1016/j.jaci.2006.04.036
Laine, L. and Weinstein, W.M., Histology of alcoholic hemorrhagic “gastritis”: a prospective evaluation, Gastroenterology, 1988, vol. 94, pp. 1254–1262. https://doi.org/10.1016/0016-5085(88)90661-0
Lanas, A., Role of nitric oxide in the gastrointestinal tract, Arthritis Res. Ther., 2008, vol. 10, pp. 1–6. https://doi.org/10.1186/ar2465
Laub, R.J., Process for preparing synthetic soil-extract materials and medicaments based thereon, US Patent no. CA2278759A1, 1999.
Lee, J.H., Lee, D.U., and Jeong, C.S., Gardenia jasminoides ellis ethanol extract and its constituents reduce the risks of gastritis and reverse gastric lesions in rats, Food Chem. Toxicol., 2009, vol. 47, pp. 1127–1131. https://doi.org/10.1016/j.fct.2009.01.037
Lemjabbar, H. and Basbaum, C., Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells, Nat. Med., 2002, vol. 8, pp. 41–46. https://doi.org/10.1038/nm0102-41
Li, W., Huang, H., Niu, X., et al., Protective effect of tetrahydrocoptisine against ethanol- induced gastric ulcer in mice, Toxicol. Appl. Pharmacol., 2013, vol. 272, pp. 21–29. https://doi.org/10.1016/j.taap.2013.05.035
Liu, Y., Tian, X., Gou, L., et al., Protective effect of L-citrulline against ethanol-induced gastric ulcer in rats, Environ. Toxicol. Pharmacol., 2012, vol. 3, pp. 280–287. https://doi.org/10.1016/j.etap.2012.04.009
Lu, S., Wu, D., Sun, G., et al., Gastroprotective effects of Kangfuxin against water-immersion and restraint stress-induced gastric ulcer in rats: roles of antioxidation, anti-inflammation, and pro-survival, Pharm. Biol., 2019, vol. 57, pp. 770–777. https://doi.org/10.1080/13880209.2019.1682620
Lv, H., Lin, Y., Liu, P., et al., Protective effects and potential underlying mechanisms of sodium copper chlorophyllin against ethanol-induced gastric ulcer in mice, Acta Biochim. Biophys. Sin., 2019, vol. 51, pp. 925–933. https://doi.org/10.1093/abbs/gmz083
Mahmoud, Y.I. and El-Ghffar, E.A.A., Spirulina ameliorates aspirin-induced gastric ulcer in albino mice by alleviating oxidative stress and inflammation, Biomed. Pharmacother., 2018, vol. 109, pp. 314–321. https://doi.org/10.1016/j.biopha.2018.10.118
Moncada-Pazos, A., Obaya, A.J., and Llamazares, M., ADAMTS-12 metalloprotease is necessary for normal inflammatory response, J. Biol. Chem., 2012, vol. 287, pp. 39554–39563. https://doi.org/10.1074/jbc.M112.408625
Mousa, A.M., El-Sammad, N.M., Sherien, K., et al., Antiulcerogenic effect of Cuphea ignea extract against ethanol-induced gastric ulcer in rats, BMC Complement. Altern. Med., 2019, vol. 19, p. 345. https://doi.org/10.1186/s12906-019-2760-9
Muazam, S., Rana, R., Jawed, S., et al., Gastroprotective effect of sagu pearls on diclofenac sodium induced gastric ulcer, J. Islam Int. Med. Coll., 2015, vol. 10, pp. 204–209.
Oztas, E., Ozler, S., Ersoy, A.O., et al., Placental ADAMTS-12 levels in the pathogenesis of preeclampsia and intrahepatic cholestasis of pregnancy, Reprod. Sci., 2016, vol. 23, pp. 475–481. https://doi.org/10.1177/1933719115604730
Pan, J.S., He, S.Z., Xu, H.Z., et al., Oxidative stress disturbs energy metabolism of mitochondria in ethanol-induced gastric mucosa injury, World J. Gastroenterol., 2008, vol. 14, pp. 5857–5867. https://doi.org/10.3748/wjg.14.5857
Park, J.U., Kang, J.H., Rahman, M.A.A., et al., Gastroprotective effects of plants extracts on gastric mucosal injury in experimental Sprague–Dawley rats, BioMed. Res. Int., 2019, vol. 2019, art. 8759708. https://doi.org/10.1155/2019/8759708
Peterson, W.L., Barnett, C., and Walsh, J.H., Effect of intragastric infusions of ethanol and wine on serum gastrin concentration and gastric acid secretion, Gastroenterology, 1986, vol. 191, pp. 1390–1395. https://doi.org/10.1016/0016-5085(86)90192-7
Polo, C.M., Moraes, T.M., Pellizzon, C.H., et al., Gastric ulcers in middle-aged rats: the healing effect of essential oil from Citrus aurantium L.(Rutaceae), Evid. Based Complement. Alternat. Med., 2012, vol. 2012, art. 509451. https://doi.org/10.1155/2012/509451
Savić, J.S., Dilber, S.P., Marković, B.D., et al., Docking studies and α-substitution effects on the anti-inflammatory activity of β-hydroxy-β-arylpropanoic acids, Molecules, 2011, vol. 16, pp. 6645–6655. https://doi.org/10.3390/molecules16086645
Seals, D.F. and Courtneidge, S.A., The ADAMs family of metalloproteases: multidomain proteins with multiple functions, Genes Dev., 2003, vol. 17, pp. 7–30. https://doi.org/10.1101/GAD.1039703
Sen, S., Chakraborty, R., De, B., et al., Plants and phytochemicals for peptic ulcer: an overview, Phcog. Rev., 2009, vol. 3, pp. 270–279.
Somerville, R.P., Longpre, J.M., Apel, E.D., et al., ADAMTS7B, the full-length product of the ADAMTS7 gene, is a chondroitin sulfate proteoglycan containing a mucin domain, J. Biol. Chem., 2004, vol. 279, pp. 35159–35175. https://doi.org/10.1074/jbc.M402380200
Stewartand, D.J. and Ackroyd, R., Peptic ulcers and their complications, Surgery, 2011, vol. 29, pp. 568–574.
Takeuchi, K., Ueshima, K., Hironaka, Y., et al., Oxygen free radicals and lipid peroxidation in the pathogenesis of gastric mucosal lesions induced by indomethacin in rats. Relation to gastric hypermotility, Digestion, 1991, vol. 49, pp. 175–184. https://doi.org/10.1159/000200718
Tamaddonfard, E., Erfanparast, A., Farshid, A.A., et al., Safranal, a constituent of saffron, exerts gastro-protective effects against indomethacin-induced gastric ulcer, Life Sci., 2019, vol. 224, pp. 88–94. https://doi.org/10.1016/j.lfs.2019.03.054
Vinagre, R.M.D.F., Vinagre, I.D.F., Vilar-e-Silva, A.,et al., Helicobacter pylori infection and immune profile of patients with different gastroduodenal diseases, Arq. Gastroenterol., 2018, vol. 55, pp. 122–127. https://doi.org/10.1590/S0004-2803.201800000-21
Wedemeyerand, R.S. and Blume, H., Pharmacokinetic drug interaction profiles of proton pump inhibitors: an update, Drug Saf., 2014, vol. 37, pp. 201–211. https://doi.org/10.1007/s40264-014-0144-0
Wei, J., Richbourgh, B., Jia, T., et al., ADAMTS-12: a multifaced metalloproteinase in arthritis and inflammation, Mediators Inflamm., 2014, vol. 2014, art. 649718 https://doi.org/10.1155/2014/649718
Yan, Y., Shirakabe, K., and Werb, Z., The metalloprotease Kuzbanian (ADAM10) mediates the transactivation of EGF receptor by G protein-coupled receptors, J. Cell Biol., 2002, vol. 158, pp. 221–226. https://doi.org/10.1083/jcb.200112026
Yildirim, F.I.A, Uyanik, Ö., Özyogurtcu, H., et al., Aggravating effect of atorvastatin on indomethacin-induced gastricinjury: focus on PGE2, TNF-a, neutrophils, and iNOS, Prostaglandins Other Lipid Mediators, 2015, vol. 121, pp. 53–62. https://doi.org/10.1016/j.prostaglandins.2015.07.002
Yudina, N.V., Pisareva, S.I., and Saratikov, A.S., Antiulcerogenic activity of phenol. compounds of peat, Khim. Rastit. Syr’ya, 1998, vol. 4, pp. 29–32. NII Article ID (NAID) 10024717260.
Zhao, X., Li, J., Meng, Y., et al., Treatment effects of jinlingzi powder and its extractive components on gastric ulcer induced by acetic acid in rats, Evid. Based Complement. Alternat. Med., 2019, vol. 2019, art. 7365841. https://doi.org/10.1155/2019/7365841
Funding
This study was supported by the Scientific Research Commission of Canakkale Onsekiz Mart University with the project named “Investigation of the Effects of Humic Acid in Different Acute Gastric Ulcer Models in Rats” coded TSA-2018-2592.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. Our study was approved by Canakkale Onsekiz Mart University Animal Experiments Local Ethics Committee with decision 2018/05-02.
Highlights
Humic acid has a healing effect on stomach damage at the histopathological level, Humic acid suppresses iNOS and apoptosis and increases PCNA activity,
Humic acid reduces TNF-a and IL-6 levels that are proinflammatory cytokines in the gastric mucosa, while it increases IL-10 levels which is a member of anti-inflammatory cytokines, Humic acid increases ADAM10 and ADAMTS12 levels, these healing effects of humic acid are as strong as famotidine.
ADAMTS12 was also present in gastric mucosa for the first time, so ADAMTS12 levels were determined in healthy, gastric ulcer models and humic acid treated groups.
About this article
Cite this article
Şehitoğlu, M.H., Öztopuz, Ö., Karaboğa, İ. et al. Humic Acid Has Protective Effect on Gastric Ulcer by Alleviating Inflammation in Rats. Cytol. Genet. 56, 84–97 (2022). https://doi.org/10.3103/S0095452722010091
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452722010091