You are here
Cyanide removal from aqueous solution by oxidation with hydrogen peroxide in the presence of activated alumina-supported copper catalyst
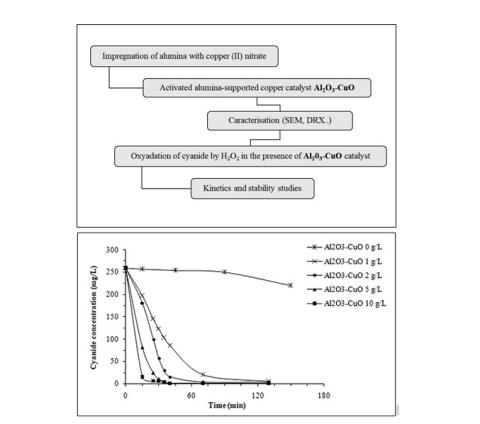
Cyanide compounds are widely used in some electroplating, chemical and metallurgical industries. They are often found in their liquid discharges. This work highlights the performance of an activated alumina-supported copper catalyst in the removal of cyanide by oxidation with hydrogen peroxide in aqueous solution. The influence of catalyst dose, initial molar ratio of hydrogen peroxide/cyanides, temperature, and catalyst reuse was studied. The activated alumina-supported copper significantly enhanced the reaction rate showing a good catalytic activity. The efficiency of cyanide elimination was increased after 30 minutes of oxidation from 48% to 98% by increasing the catalyst dose from 1 to 10 g/L. Rising the temperature from 30°C to 40°C promoted cyanide removal. The catalyst can be recycled four times and show good stability. The kinetics of cyanide oxidation was revealed to be pseudo-first order with regarding cyanides. The rate constants as well as the activation energy were determined.
